Abstract
The capsular polysaccharides of group B streptococci (GBS) are a primary focus of vaccine development. Immunogenicity and long-lasting protection are best achieved by conjugating polysaccharides to a T-cell-dependent protein antigen. Streptococcal C5a peptidase (SCPB) is a conserved surface protein that is expressed by all streptococcal serotypes tested to date, and it is a possible carrier protein that could itself induce a protective immune response. Clearance of GBS from lungs, mucosal surfaces, or blood probably depends on the opsonophagocytic response of tissue-specific macrophages and polymorphonuclear leukocytes (PMNs). In this study, we examined the potential of antibody directed against SCPB from a serotype II strain to enhance the capacity of mouse bone marrow macrophages (from primary cultures) and human PMNs in whole blood to kill GBS in vitro. Our experiments demonstrated that Streptococcus serotypes Ia, Ib, II, III, and V, preopsonized with anti-SCPB antibody, were killed more rapidly by cultured macrophages and PMNs in whole blood than were nonopsonized GBS. The increased rate of killing was accompanied by an increased macrophage oxidative burst. Furthermore, opsonization was serotype transparent. Immunization with SCPB conjugated to capsular polysaccharide type III produced polysaccharide-specific antibodies. It is interesting that this antiserum promoted serotype-independent killing of streptococci. These data support the use of SCPB in a GBS polysaccharide conjugate vaccine. SCPB not only enhanced the immunogenicity of polysaccharide components of the vaccine, but it might also induce additional serotype-independent protective antibodies.
Group B streptococci (GBS) are a major cause of pneumonia, sepsis, and meningitis in neonates and more recently have become a serious cause of mortality and morbidity in immunocompromised adults (32). Adherence of GBS to a mucosal surface is the first event in colonization and invasion. GBS adhere efficiently to and invade epithelial cells from a variety of tissues (3). Investigation of virulence has, for the most part, focused on the capsular polysaccharides (Cps). Although GBS can bind to various surface receptors present on epithelial cells, including fibronectin, laminin, and cytokeratin 8, neither adhesins nor invasins have been identified for these streptococci. The early actions of macrophages and polymorphonuclear leukocytes (PMNs) determine the outcome of infection. GBS avoid phagocytosis in the absence of opsonic antibody and complement activation (28). Type-specific antibody directed against Cps is opsonic and provides protection in animal models of GBS infection. However, serotype-specific antibody has no effect on heterologous strains.
Development of vaccines against GBS began two decades ago when a correlation between maternal antibody deficiency and increased susceptibility to neonatal infection by GBS was reported (5). Although not directly demonstrated, neonatal resistance to infection by GBS is thought to be associated in part with naturally acquired maternal antibodies to the type-specific Cps. Most healthy newborns have low but measurable antibodies against capsular antigen (8). Immunoglobulin G (IgG) contains antibodies directed against these polysaccharides, which pass into the placenta and are presumed to protect the newborn child from invasive infection by GBS. However, the levels of these antibodies decline rapidly during the first months of life. Virtually nothing is known about the immune response in women who are vaginal carriers of GBS. Vaccine development has focused primarily on the serotype Ia and III Cps because these serotypes are responsible for the majority of neonatal disease. With changing serotype distributions and the emergence of new serotypes, multivalent vaccines for GBS have become an objective. More recently, polysaccharide-protein conjugate vaccines have been tested in an effort to improve immunogenicity and to induce long-term immune memory. Several proteins, including tetanus toxoid (6), alpha C protein (14, 25), Rib protein (25), and beta C protein (27), have been tested as carriers in various animal models. Cps Ia and Ib tetanus toxoid conjugates have been tested in humans (6). These immunogens are well tolerated and induce a vigorous anti-Cps response.
An optimal vaccine would induce an immune response that would limit colonization of the adult vaginal and gastrointestinal tracts and would also protect the neonate. Unfortunately, requirements for colonization have not been investigated. Streptococcal C5a peptidase (SCPB) is a highly conserved surface protein among strains of GBS (34). Enzymatic activity is highly specific for C5a, cleaving the chemotaxin at its PMN binding site (40). Although little is known about the impact of the peptidase on the virulence of GBS, Bohnsack et al. (9) showed that SCPB reduces the acute neutrophil response to infections by GBS in C5a knockout mice supplemented with human recombinant C5a. Based on studies of group A streptococci, there is also reason to believe that SCPB may contribute to the organism's ability to colonize mucosal surfaces. The sequence of SCPB is 98% identical to that expressed by group A streptococci (11). In group A streptococci, the peptidase has been shown to retard clearance of streptococci from the oral mucosa of mice (22). Moreover, mice immunized with recombinant peptidase clear streptococci more rapidly following intranasal challenge (21). Antibody directed toward SCPB can neutralize peptidase activity, but because the protein protrudes from the surface, it could also be opsonic or could induce antibody-dependent killing by macrophages. In this study, we investigated the potential use of SCPB as a stand-alone vaccine antigen and as a protein carrier for polysaccharide vaccines. In our experiments, we used bone-marrow-derived macrophages (BMMs) and PMNs in whole blood to determine whether anti-SCPB enhances the killing of GBS by these phagocytes. SCPB-conjugated Cps type III proved to be a good carrier protein. Antibodies directed against SCPB initialized killing of GBS by mature BMMs and proved to be opsonic in a whole-blood phagocytosis. Overall, experiments suggested that inclusion of SCPB in a polysaccharide vaccine produces another level of protection that is serotype independent.
MATERIALS AND METHODS
Bacterial strains.
GBS M1A00047 (type V), M1A00065 (type III), M1A00063 (type Ib), M1A00070 (type Ia), M1A00071 (type III), and 78–471 (type II) were clinical isolates obtained from P. Ferrieri (Department of Pediatrics, University of Minnesota, Minneapolis, Minn.). Strains were streaked out on blood agar plates, and two or three colonies were picked to start liquid cultures in Todd-Hewitt broth (THB) (Difco Laboratories, Sparks, Md.). For mid-log-phase culture, 0.5 ml of overnight culture was added to 10 ml of THB, and the culture was incubated at 37°C until an optical density at 560 nm (OD560) of 0.5 to 0.6 was achieved.
Isolation of BMMs.
Tibias and femurs were aseptically removed from euthanized 8-week-old female strain CD1 mice and dissected free of adherent tissue. The bone ends were cut and marrow was flushed with BMMO medium (Dulbecco modified Eagle medium [DMEM], 10% fetal calf serum [FCS], 5% heat-inactivated horse serum, 20% L-cell-conditioned supernatant, penicillin-streptomycin, l-glutamine, and sodium pyruvate). A single-cell suspension was produced by gently forcing suspensions through an 18-g needle. Suspensions containing 2.5 × 105 to 5 × 105 cells per well were seeded into 24-well plates. Cells were maintained in BMMO medium at 37°C with 5% CO2 and fed every 3 to 4 days. Cell viability was evaluated by trypan blue exclusion (Sigma Chemical Co., St Louis, Mo.).
Measurement of CD11b, CD14, and F4/80 antigen expression.
BMMs were characterized by flow cytometry using fluorescein isothiocyanate (FITC) anti-CD11b (PharMingen, San Diego, Calif.), phosphetidylethanoamine (PE) anti-CD14 (PharMingen), biotinylated-F4/80 (Becton Dickenson Immunocytometry Systems, San Jose, Calif.), and FITC-avidin (Jackson Immunoresearch Laboratories, Inc., West Grove, Pa.). CD14 is predominantly expressed by cells of myeloid origin and is regarded as a specific marker for macrophage. F4/80 is a monoclonal antibody directed specifically against mouse macrophages. CD11b is the alpha chain of Mac-1 integrin (CD11b/CD18), which is expressed at various levels on granulocytes, macrophages, dendritic cells, natural killer cells, and B cells. A suspension containing 5 × 105 cells was fixed with 2.5% paraformaldehyde for 15 min, blocked with 10% FCS in phosphate-buffered serum (PBS)-Ca2+ for 30 min at 37°C, then incubated with 5 μl of FITC-labeled anti-mouse CD11b, 1 μl of phosphatidylethanolamine-labeled anti-mouse CD14, or 5 μl of biotinylated F4/80 followed by 1 μl of FITC-streptavidin in PBS with 10% FCS in the dark for 30 min. The cells were then washed with PBS-Ca2+ twice and resuspended into 0.5 ml of PBS-Ca2+ for use in fluorescence-activated cell sorter (FACScan; Becton Dickenson) analysis. Data were analyzed using CellQuest software (Becton Dickenson). Nonspecific-esterase (NSE) (with α-naphthyl acetate as a substrate) activity of BMMs was also tested with a staining kit, using α-naphthyl-acetate as substrate to form colored dyes with diazonium salts when hydrolyzed by NSE (Sigma). When cells were stained for NSE activity, more than 95% of the cells stained positive.
Killing of GBS by BMMs.
GBS from mid-log phase (OD560 of 0.5 to 0.6) were preincubated with 15% normal heat-inactivated rabbit serum (pooled serum from Gibco Laboratories, Grand Island, N.Y.) or various heat-inactivated rabbit hyperimmune sera (anti-SCPB, anti-Cps, and anti-SCPB-Cps III) in PBS at room temperature for 1 h. Macrophages were infected for 10 min at 37°C with 105 GBS cells in DMEM with 10% FCS. At this time (zero time point of the assay), the culture supernatants were removed, and macrophages were washed three times with PBS-Ca2+. One milliliter of DMEM was added to each well, and the plate was incubated in 37°C for an additional 60 min. To quantify the macrophage-associated GBS at different times postinfection (0, 30, and 60 min), the macrophages were washed with PBS-Ca2+ and then lysed with 1 ml of sterile water. Serial dilutions of lysates from each well were prepared, and 0.1 ml of each dilution was plated on Todd-Hewitt agar plates. The number of CFU was determined after overnight incubation at 37°C. The percentage of killing was calculated by using the number of GBS associated with macrophages at the zero time point as 100%.
Whole-blood phagocytosis assay (23).
GBS from the mid-log phase (OD560, 0.5 to 0.6) were washed with PBS and diluted to a concentration of approximately 1,000 CFU/50 μl. Diluted streptococci (1,000 CFU) and 100 μl of hyperimmune serum (described previously) were added to 850 μl of heparinized whole blood from healthy human donors and incubated at 37°C on a rotator for 3 h. To quantify GBS survival, samples of 100 μl were taken at 0, 30, 90, and 180 min after incubation. Serial dilutions were prepared, and 100 μl of each dilution was plated on Todd-Hewitt agar with sheep blood. The number of CFU per milliliter was determined after overnight incubation at 37°C.
GBS association with PMNs by flow cytometry.
Assays were carried out according to the method described by Ji et al. (22). Mid-log-phase biscarboxyethyl-carboxyfluoresceia-pentaacetoxy-methylester (BCECF-AM)-labeled GBS were incubated with 1 ml of whole blood in the presence of various antisera for 20 min at 37°C in the dark. At times 0 and 20 min, 100 μl of samples were immediately mixed with 2 ml of ice-cold FACScan lysing solution (Becton Dickenson). Cells were washed three times with PBS-Ca2+ and resuspended in 0.5 ml of PBS-Ca2+ for flow cytometric analysis. The gate for PMN analysis was based on the size and granularity of cells, and 10,000 PMN events were recorded. PMNs were fluorescent only when associated with GBS. The zero time point was used as the negative control.
Oxidative burst of BMMs by flow cytometry.
The oxidative burst of BMMs was measured using dihydrorhodamine 123 (DHR 123, Molecular Probes, Eugene, Oreg.). This primarily nonfluorescent dye becomes fluorescent upon oxidation to rhodamine by reactive-oxygen species produced during the respiratory burst of macrophages. DHR was added to infected macrophages at a final concentration of 10 μg/ml, and this mixture was incubated at 37°C in the dark for 30 min. Macrophages were then washed three times with PBS-Ca2+ and scraped off the well and resuspended in 0.5 ml of PBS-Ca2+. To estimate reactive-oxygen production, fluorescence intensities of 5,000 or 10,000 cells were recorded. Uninfected macrophages that were incubated with DHR served as negative controls.
Production of SCPB antibody and conjugate.
Affinity-purified SCPB (full-length SCPB expressed by Escherichia coli) was used to produce anti-SCPB serum. Rabbits were immunized at days 1 and 28 with SCPB emulsified in RIBI R-730 (monophosphoryl lipid A plus trehalose dicorynomycolate plus cell wall skeleton) adjuvant (Corixa Corp., Hamilton, Mont.). A 1.0-ml dose was given as follows: 0.05 ml administered intradermally at six sites, 0.1 ml administered intramuscularly at three sites on each hind leg, and 0.1 ml administered subcutaneously in the neck region. Serum was obtained from blood taken from the marginal ear veins of rabbits 38 to 42 days after the first injection of antigen. The rabbit anti-SCPB titer was 600,000.
Recombinant SCPB protein was expressed by E. coli as a glutathione S-transferase-SCPB fusion protein from the pGEX-4T-1 vector supplied by Pharmacia Biotech Inc. and purified by affinity chromatography (10, 21). Serotype III Cps was purified according to the method of Deng et al. (13). Purified SCPB was coupled by reductive amination to purified polysaccharide that was first oxidized with periodate using the method reported by Wessels et al. (39). Swiss Webster strain mice were injected subcutaneously at weeks 0, 2, and 4 with SCPB-Cps III conjugate with 10 mg of aluminum phosphate as adjuvant. CRM97-Cps III conjugates were produced in the same way. The CRM97 nontoxigenic form of diphtheria toxin was isolated from Corynebacterium diphtheriae strain C7 (β197), as previously described (30, 37).
Quantitation of antibody.
Anti-Cps Ia, anti-Cps II, anti-Cps V, anti-Cps III, and anti-SCPB-Cps III sera were obtained from Wyeth-Lederle Vaccines. For anti-Cps titers, polystyrene 96-well microtiter plates (Greiner Labortechnik, Frickenhausen, Germany) were coated for 90 min at 37°C, then incubated overnight at 4°C with a mixture of equal concentrations (0.1 to 1.0 μg/well) of GBS polysaccharide and methylated human serum albumin (Sigma) in PBS containing 0.02% NaN3. All subsequent incubation steps were performed at room temperature. Coated plates were incubated for 2 h with threefold serial dilutions (1:50 to 1:109,350) of mouse, rabbit, or control sera in PBS with 0.1% Brij 35, 0.02% NaN3, and 5% (vol/vol) fetal bovine serum. Polysaccharide-specific antibody was detected with a 2-h incubation of alkaline phosphatase conjugated to goat anti-mouse IgG (heavy chain-specific, 1:1,000 dilution; Southern Biotechnology, Birmingham, Ala.) or goat anti-rabbit IgG (Fc fragment-specific, 1:2,000; Jackson ImmunoResearch) in the buffer described above, followed by incubation for 1-h with p-nitrophenyl phosphate (Sigma) in diethanolamine buffer. Tris-buffered saline containing 0.1% Brij 35 was used as a wash buffer between each step. Titers were reported as the reciprocal of the dilution that gave an OD405 of 0.1. The method for quantitation of anti-SCPB titers was previously described (21). Antibody titers were as follows: anti-Cps Ia, 19,000,000; anti-Cps II, 4,963,853; anti-Cps III, 4,337,703; anti-Cps V, 4,953,996; and anti-SCPB-Cps III, 204,000 (SCPB) and 268,000 (type III).
RESULTS
SCPB is an effective immunogenic carrier of GBS type III polysaccharide.
The immunogenicity of polysaccharides can be greatly enhanced by coupling them to a protein carrier. Mice were immunized with a SCPB-Cps III conjugate to determine whether this GBS surface protein will enhance the IgG response to the Cps. Mice were immunized with three subcutaneous injections of antigen at 0, 2, and 4 weeks. They developed high IgG titers of both anti-Cps III and anti-SCPB antibodies (Table 1). A vigorous booster response following the second and third injection was observed. Immunization with CRM97-Cps conjugate also produced a vigorous anti-Cps III antibody titer, but titers were significantly lower than those in animals immunized with the SCPB conjugate. As expected, immunization of mice with pure type III polysaccharide mixed with pure CRM97 protein produced IgG anti-Cps III titers of less than 100.
TABLE 1.
Immunogenicity of SCPB-Cps III conjugate vaccine in micea
| Week | Titers of antibodies in sera after immunization of mice with
|
|||
|---|---|---|---|---|
| SCPB-Cps conjugate
|
CRM97-Cps III conjugate | Cps III and free CRM97 | ||
| Anti-SCPB | Anti-Cps | |||
| 0 | <50 | <50 | <50 | <50 |
| 2 | 1,341 | 292 | <95 | <50 |
| 4 | 63,155 | 31,997 | 4,704 | 67 |
| 6 | 79,323 | 64,562 | 19,345 | 46 |
Values represent data from pooled equal volumes of sera from five Swiss Webster strain mice. Mice were immunized with 2.5 μg of Cps III, mixed CRM97-Cps III conjugate, or SCPB-Cps III conjugate at weeks 0, 2, and 4. ELISA was used to measure specific IgG. All immunogens were mixed with aluminum phosphate.
Characterization of primary mouse BMMs.
Recently, Valentin-Weigand et al. (37) and Cornacchione et al. (12) demonstrated that GBS not only invade the macrophage cell line J744 but also persist for an extended period within these cells. Prior opsonization of GBS with human serum containing anti-GBS antibodies did not affect bacterial entry but significantly reduced the intracellular survival of GBS. Since this cell line lacks many of the characteristics of native macrophages isolated from animals, primary BMMs were produced to study the effects of antibody on macrophage killing. BMMs were isolated from the tibias and femurs of CD1 mice and cultured in the presence of 20% L-cell culture supernatant, which contains modest amounts of granulocyte-macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor. These growth factors induce bone marrow precursors to mature into phagocytically active macrophage populations. BMM primary cultures used here were estimated to contain 70 to 80% of CD11b-positive cells, 35 to 47% of CD14-positive cells, and 10% of F4/80-positive cells (Fig. 1B). Double-labeling assays showed that all CD14-positive cells were also CD11b positive (data not shown). More than 98% of bone marrow cells were determined to be viable by trypan blue exclusion. Thus, primary cultures routinely contained 35 to 47% mature, phagocytically active macrophages. BMM cultures are known to contain cells in various stages of maturity and at different levels of activation. These results are similar to those reported by other investigators (19, 30).
FIG. 1.
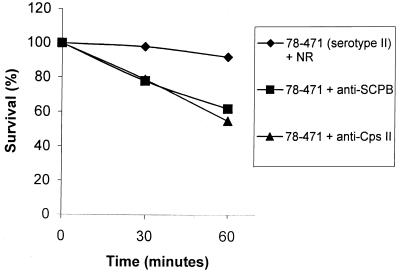
Killing of unopsonized and opsonized GBS strain 78–471 (serotype II) by BMMs. Streptococci were preincubated with various sera, including normal rabbit serum, anti-SCPB serum, and ant-Cps II serum, for 1 h at room temperature. Then 105 streptococci were added to BMMs for 10 min at 37°C. Data are from a single experiment but are representative of those from three independent experiments. Percentage of survival was calculated by using the number of GBS associated with macrophages at the zero time point as 100%.
Survival of GBS associated with mouse macrophages.
To analyze the time course of survival of GBS associated with macrophages, macrophages were exposed to 105 GBS for 10 min to allow for adherence and internalizing of bacteria. The multiplicity of infection was approximately 1 in all experiments. Viability of macrophage-associated GBS was determined at 0, 30, and 60 min after removal of nonadherent bacteria. GBS rapidly adhered to BMMs. By 10 min after initial exposure, 10 to 30% of inoculated streptococci were associated with macrophages. The rate of association depended on the streptococcal strain and varied somewhat with different batches of macrophages. To normalize for this variation the number of CFU associated with the monolayer at 0 min was considered 100%. The kinetics of GBS survival are shown in Fig. 1. The capacity of BMMs to kill GBS was antibody dependent (Fig. 1). Following preincubation with 15% normal rabbit serum, 90% of strain 78-471 (serotype II) or strain M1A-00063 (serotype Ib) survived after 60 min (Fig. 1 and 2). After preincubation with 15% anti-SCPB or anti-Cps II, only 50 to 60% of type II streptococci survived. The survival of other serotypes, including Ia, Ib, II, III, and V strains, after infection of BMMs is shown in Table 2. In the absence of specific antibodies, from 0 to 14% of streptococci were killed after 60 min. When preopsonized with 15% rabbit polyclonal anti-SCPB or homologous anti-Cps antibodies in PBS for 1 h, 38 to 65% of GBS were killed after 60 min. The differences in the capacity of BMMs to kill streptococci that were preopsonized with anti-SCPB or anti-Cps antibodies were not significant. Rabbit anti-SCPB enhanced killing of Ia, Ib, II, III, and V serotypes by macrophages (Table 2).
FIG. 2.
Killing of unopsonized and opsonized GBS strain MIA-00063 (serotype Ib), by BMMs. Conditions and calculations are the same as those in Fig. 1.
TABLE 2.
Anti-SCPB-induced killing of GBS by macrophages in heterologous serotypes
| Strains | Serotype | GBS killed (mean % ± SEM) by BMMs in:
|
||
|---|---|---|---|---|
| Normal serum | Anti-SCPB | Anti-Cpsa | ||
| MIA-00070 | Ia | 14 ± 2.5 | 50 ± 2.3 | 65 ± 3.2 |
| O90R | Ia | 12 ± 1.8 | 50 + 3.1 | (ND) |
| M1A-00063 | Ib | 8.5 ± 2.1 | 48 ± 1.6 | (ND) |
| 78–471 | II | 4 ± 0.3 | 38 ± 1.7 | 45 ± 1.3 |
| M1A-00065 | III | 4 + 0.5 | 48 ± 2.5 | 62 ± 2.5 |
| M1A-00071 | III | 0 ± 4.2 | 55 ± 5.0 | 65 ± 6.4 |
| M1A-00047 | V | 0 ± 2.3 | 42 ± 0.9 | 48 ± 2.5 |
Anti-Cps is the percent of streptococci that were preincubated with homologous antisera, which include anti-Cps Ia, anti-Cps II, anti-Cps III or anti-Cps V, respectively. ND, not done.
In each experiment, the number of streptococci that associate with BMM was measured. Unexpectedly, preincubation with anti-SCPB or anti-Cps antibody did not increase the initial adherence of GBS to macrophages. For example, incubation of MIA-00065, a serotype III strain, with normal rabbit, anti-SCPB, or anti-Cps III sera resulted in 19.5, 18.8, and 25.3% association of streptococci with BMM, respectively (unpublished data).
Because SCPB enhanced the antibody response to type III polysaccharide, an experiment was performed to determine whether SCPB that was chemically conjugated to type III polysaccharide induced antibody that promoted killing of heterologous serotypes. Rabbit anti-Cps III did not induce killing of M1A-00063, a serotype Ib strain (Fig. 2). However, serum from a rabbit that was immunized with SCPB-Cps III polysaccharide conjugate efficiently promoted killing of this Ib strain by BMMs. The experiment was repeated with a serotype V strain (Table 3). Serum directed against the SCPB, conjugated to type III polysaccharide antigen, induced BMMs to kill both Ib and V serotypes of GBS. In contrast, serum from a rabbit immunized with type III polysaccharide antigen alone did not promote killing of the heterologous serotypes Ib and V strains.
TABLE 3.
Increased killing of GBS by macrophages with anti-SCPB-type III Cps conjugate
| Strains | Serotype | GBS killed (mean ± SEM) by BMMs in:
|
||
|---|---|---|---|---|
| Normal serum | Anti-SCPB-Cps III | Anti-Cps III | ||
| MIA-00071 | III | 12 ± 2.5 | 59 ± 4.6 | 57 ± 6.7 |
| MIA-00063 | Ib | 9.5 ± 3.5 | 58 ± 5.4 | 0 ± 3.5 |
| MIA-00047 | V | 10.2 ± 2.6 | 54 ± 2.5 | 0 ± 4.8 |
Oxidative burst of macrophages during the killing of GBS.
Production of reactive-oxygen intermediates, referred to as an oxidative burst or respiratory burst, is a critical step in the destruction of invading pathogenic bacteria by professional phagocytic cells. The data presented above suggest that ingestion of anti-SCPB-coated streptococci activated a respiratory burst that generated the bactericidal activity. Therefore, following infection with streptococci, the oxidative burst of macrophages was measured by FACS. This was accomplished using DHR 123 as a substrate, which is freely permeable and localizes in the mitochondria of phagocytic cells. Upon oxidation by H2O2 and O2− to rhodamine 123, a measurable bright green fluorescent signal is emitted following excitation by blue light (wave length, 488 nm). In the negative control in which no GBS were added to macrophages, 11% of BMMs elicited background fluorescence. Infection with nonopsonized GBS resulted in 15% of macrophages emitting a fluorescent signal. Exposure of macrophages to GBS that were preopsonized with anti-SCPB or anti-GBS antibodies increased the fraction of fluorescent macrophages to 38 to 42% and also increased the fluorescence intensity (mean fluorescence per macrophage) (Fig. 3). An increase in mean fluorescence indicates increased production of reactive-oxygen intermediates. These results demonstrate that the increased rate of killing of GBS by BMMs is accompanied by an increased macrophage oxidative burst.
FIG. 3.
Oxidative bursts of BMMs after infection with strain O90R. Streptococci were preincubated with various sera, including anti-SCPB and anti-GBS for 1 h at room temperature before the infection of macrophages. DHR 123 was then added to infected macrophages at a final concentration of 10 μg/ml and incubated at 37°C in the dark for 30 min. A total of 10,000 cells was recorded by FACScan. Bars represent cells with fluorescence greater than that of uninfected cells. Counts represent cell numbers.
Killing of GBS by PMNs in whole blood.
Anti-SCPB did not seem to function as an opsonin for macrophage uptake of streptococci in the classical sense. Unlike an opsonin, this antibody did not increase the number of streptococci associated with macrophages, but instead it enhanced the bactericidal activity of these macrophages. This observation prompted us to test whether anti-SCPB antibody influenced the capacity of PMNs in whole human blood to kill GBS. In these experiments specific antibody (anti-SCPB or anti-Cps) was added to whole blood to a final concentration of 10%. Anti-SCPB from a rabbit that was immunized with recombinant SCPB originally from a serotype II strain promoted more rapid killing of a type VI strain (Table 4). Serum from a second rabbit immunized with the same antigen effectively opsonized both type III and type Ia strains (Table 4). Table 4 includes a comparison of the opsonic capacities of anti-SCPB antibody and homologous anti-Cps antibody. Anti-Cps III was a somewhat more efficient opsonin for a serotype III strain than anti-SCPB, but the opsonic capacity of anti-Cps Ia was not significantly different from that of anti-SCPB (Table 4). These comparisons assumed that the specific antibody was in excess. In the absence of specific antibody, the GBS were not completely resistant to phagocytosis (Table 4), but they were gradually killed by PMNs over a 1.5-h incubation period. In the absence of added antibody, opsonization was either IgG independent or caused by low levels of opsonin already present in the blood of volunteer donors or pooled rabbit sera used as a control. Presence of anti-SCPB or anti-Cps, however, resulted in the elimination of GBS from blood at a faster rate. To confirm that GBS actually associated with PMNs, strain MIA-00071 (type III) was labeled with BCECF-AM and incubated with whole blood for 20 min. From 80 to 90% of PMNs were associated with GBS in the presence of anti-SCPB or anti-Cps III, whereas only 20 to 30% of PMNs were associated with GBS in the presence of heterologous anti-Cps II (unpublished data). These results are consistent with those of Herting et al. (16), who reported that PMNs are activated, undergo an oxidative burst, and develop the capacity to kill GBS that have been opsonized with specific antibody.
TABLE 4.
Opsonization of GBS in whole blood
| Duration of incubation of GBS + inoculum (h) | GBS killed (% ± SEM) by inoculum type
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| S2-02288 (serotype VI) + normal serum | S2-02288 + anti-SCPB | MIA-00071 (serotype III) + normal serum | MIA-00071 + anti-Cps II | MIA-00071 + anti-SCPB | MIA-00071 + anti-Cps III | MIA-00070 (serotype Ia) + normal serum | MIA-00070 + anti-SCPB | MIA-00070 + anti-Cps Ia | |
| 0 | 0 ± 5.1 | 0 ± 2.1 | 0 ± 0.8 | 0 ± 0.7 | 0 ± 0.8 | 0 ± 0.8 | 0 ± 0.9 | 0 ± 0.7 | 0 ± 1.0 |
| 0.5 | 15 ± 4.6 | 32.2 ± 3.3 | −2 ± 3.5 | 4.4 ± 0.8 | 26.3 ± 6.8 | 36.2 ± 6.3 | 2.3 ± 2.8 | 1 ± 3.4 | 27.8 ± 0.9 |
| 1.5 | 40.0 ± 5.3 | 84.9 ± 6.1 | 6.2 ± 6.4 | 23.1 ± 7.1 | 56.6 ± 3.6 | 69.9 ± 1.4 | 33.3 ± 2.0 | 80.0 ± 3.1 | 81.6 ± 2.7 |
DISCUSSION
For more than 25 years, GBS have been recognized to be a primary cause of morbidity and mortality in newborns. More recently, physicians in the United States and other developed countries have turned to antibiotic prophylaxis to clear GBS from the vaginal mucosa of pregnant women (4). This approach has reduced the incidence of early-onset disease but has had little impact on late-onset infections. The effectiveness of prophylactic antibiotics may be short term and has the potential to increase antibiotic resistance in this species. Hence, public health officials have enthusiastically stressed the need to develop a vaccine which would protect infants from both early-onset and late-onset disease or, better yet, would eliminate carriage of the bacterium in the vaginal and gastrointestinal tracts. Several factors have delayed that development. First and foremost is the fact that polysaccharide capsules are the only certain virulence determinants to be identified, and these hydrophilic barriers are antigenically variable. Serotypes that are primarily responsible for disease have changed over time (17), but a vaccine which would prevent infection by serotypes Ia, Ib, II, III, and V would eliminate most disease. Another difficulty is the fact that polysaccharides are poorly immunogenic and produce a short-term T-cell-independent response. The immunogenicity of polysaccharide vaccines can be improved by conjugating them to protein carriers. Human trials with GBS Cps-tetanus toxoid conjugates have been shown to increase the frequency of responders and antibody titers and to be free of serious side effects. Trials designed to determine efficacy for prevention of disease in newborns have not yet been performed (6).
GBS are known to express several proteins on their surface, which are potential vaccine candidates and/or carrier proteins. Rib, α, and β proteins have been shown to induce protection against systemic infections in rodent models (24, 25). Unfortunately, production of these proteins varies between strains and serotypes, and their roles in virulence are ill defined. Larsson et al. (25) suggested the use of an all-protein vaccine composed of Rib and α. Neither protein alone was found to provide protection against multiple serotypes.
GBS display a C5a peptidase on their surfaces, which is nearly identical to that produced by group A streptococci (10, 18). Preliminary experiments have suggested that the protease may impede clearance of GBS from the lungs (9). The enzyme of the group A streptococci has been more extensively studied and definitively demonstrated to augment virulence of this pathogen. SCPB is known to be produced by all serotypes; however, some type III strains produce an inactive enzyme (35). A polyclonal antibody response to SCPB is expected to induce not only neutralizing antibody but also opsonic antibody because the enzyme protrudes from the bacterial surface. Preliminary experiments in mice indicate that SCPB also binds fibronectin and may serve as an adhesin (O. Cheng et al., unpublished data). Therefore, it is also possible that antibody directed against this protein could reduce the capacity of GBS to colonize mucosal surfaces. Its large size and multidomain character suggest that SCPB could also be an effective carrier when conjugated to purified polysaccharides.
Recruitment of macrophages and PMNs to the site of initial infection is expected to be an important defense at early stages of infection. If antibody directed at SCPB on the bacterial surface would promote more efficient phagocytosis or killing of GBS, then inclusion of this protein could have protective value in a vaccine. The polysaccharide capsules impart resistance to phagocytosis, and antibodies that are directed against a specific polysaccharide are opsonic, but it is not clear that capsules are produced at all stages of infection or that they contribute to the potential of GBS to colonize mucosal surfaces. Marodi et al. (28) observed that although macrophages from cord blood or adult blood were able to ingest GBS in the presence of serum, they were unable to kill the bacteria, but recombinant human gamma interferon (rHuIFNγ)-activated macrophages derived from adult blood were able to kill ingested streptococci. In contrast, macrophages derived from cord blood were not activated by rHuIFNγ. Both types of macrophages could kill GBS when they were activated by recombinant human granulocyte-macrophage colony stimulating factor (28). Activation of mouse peritoneal macrophages by rHuIFNγ or lipopolysaccharides also developed their capacity to destroy internalized GBS (12). Others have shown that uptake of streptococci by mouse macrophages is independent of antibody and complement opsonins and involves direct interaction between the bacteria and CR3 receptors on phagocytes (2). Our experiments confirmed earlier reports that GBS, when ingested by either the peritoneal or the J744 macrophage line in the absence of antibody, are relatively resistant to bactericidal mechanisms (12, 36). Exposure to rabbit anti-SCPB antibody promoted rapid killing of GBS irrespective of their encapsulated state or the serotype of their Cps (Table 1). Anti-SCPB did not significantly increase ingestion of bacteria by BMMs but did promote their killing as efficiently as homologous anti-Cps antibody, even in the absence of complement. Streptococci that associated with BMMs in the absence of specific antibody did not induce reactive-oxygen molecules; however, an oxidative burst was initiated by ingestion of GBS coated with anti-SCPB or anti-Cps. Rabbits immunized with SCPB-Cps III polysaccharide conjugates developed bactericidal antibody for serotype III strains and for other serotypes. The mechanism of killing appears to be similar to antibody-dependent cytotoxicity that has been described for macrophage killing of virus-infected mammalian cells, Legionella pneumophila (20), and Staphylococcus aureus (31). Little is known about the mechanism, but it is assumed that bacterial bound IgG binds to Fc receptors on macrophages, which in turn activates bactericidal activities.
Although serotype Ia strains are opsonized by direct interaction with C1q independently of antibody (26), optimal clearance of streptococci from blood is dependent on PMNs, antibody, and complement opsonins (7, 15). Requirements for antibody and classical complement components for opsonization vary among strains of GBS (33). The capsule has been determined to be primarily responsible for this species capacity to resist phagocytosis by PMNs (38). Most investigators use purified PMNs in the presence of 15% human serum as a source of complement for phagocytosis studies. In our opinion the whole-blood assay, originally described by Lancefield (23), better reflects the native situation, and it was, therefore, used to test whether antiserum directed against SCPB is opsonic. Under these conditions serotype Ia and III were not strictly resistant to phagocytosis and were gradually killed over the 1.5-h incubation period. This could be due to small amounts of GBS antibody in the blood of our donors or the pooled rabbit serum. Alternatively, these strains are partially opsonized by activation of the complement pathway without antibody. Both homologous anti-Cps and anti-SCPB rabbit sera enhanced the rate of phagocytosis. Anti-SCPB serum was able to opsonize both serotype Ia and III strains. Anti-Cps serum served as negative control and, as expected, did not opsonize heterologous serotypes of GBS. Unexpectedly, however, pooled normal rabbit serum was observed to promote both association and phagocytosis of GBS in whole human blood. FACScan experiments showed that 80 to 90% of PMNs were associated with PMNs in the presence of pooled normal rabbit serum in the absence of known specific antibody. This could be due to antibody against GBS or other factors in the pooled rabbit serum that are opsonic. Using the Lancefield phagocytosis method, we found that this serum promoted killing of GBS over a 3-h period, but at a slower rate than either anti-Cps or anti-SCPB hyperimmune serum. Nonopsonic binding of type III GBS to human PMNs has been described (1), but this kind of association did not usually result in ingestion of the bacteria. Overall, these results suggest that transplacental IgG containing anti-SCPB could enhance clearance of GBS from the blood of a neonate and decrease the potential for serious infection.
Several polysaccharide vaccines are in development and all require linkage to a safe protein carrier. Although tetanus and diphtheria toxoids have been used for this purpose (6), overexposure to these antigens as the battery of vaccines increases in number may negatively affect the immune response to the antigen of importance or, even worse, have other negative consequences for vaccinees. As anticipated, immunization with free Cps III antigen failed to induce a significant IgG response to the polysaccharide. In contrast, both CRM97 and SCPB-Cps III conjugates induced strong antibody responses. Furthermore, the SCPB-Cps III conjugate induced functional antibody. Antibody directed against SCPB both is opsonic for PMNs and initiates a macrophage bactericidal response that is serotype independent. Therefore, inclusion of the C5a peptidase in a conjugate vaccine not only promotes a strong T-cell-dependent antibody response to polysaccharide components but also potentially induces another level of protection. Experiments are now in progress to test whether immunization with SCPB protects mice against GBS challenge.
ACKNOWLEDGMENTS
This work was funded by NIAID grant AI20016 and a grant from Wyeth-Lederle Vaccines Inc.
REFERENCES
- 1.Albanyan E A, Vallejo J G, Smith C W, Edwards M S. Nonopsonic binding of type III group B streptococci to human neutrophils induces interleukin-8 release mediated by the p38 mitogen-activated protein kinase pathway. Infect Immun. 2000;68:2053–2060. doi: 10.1128/iai.68.4.2053-2060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antal J M, Cunningham J V, Goodrum K J. Opsonin-independent phagocytosis of group B streptococci: role of complement receptor type three. Infect Immun. 1992;60:1114–1121. doi: 10.1128/iai.60.3.1114-1121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker C J, Edwards M S. Infectious diseases of the fetus and newborn infant. Philadelphia, Pa: The W. B. Saunders Co.; 1995. Group B streptococcal infections; p. 980. [Google Scholar]
- 4.Baker C J, Halsey N A, Schuchat A. 1997 AAP guidelines for prevention of early-onset group B streptococcal disease. Pediatrics. 1999;103:197–198. doi: 10.1542/peds.103.3.701. [DOI] [PubMed] [Google Scholar]
- 5.Baker C J, Kasper D L. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976;294:753–756. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- 6.Baker C J, Paoletti L C, Wessels M R, Guttormsen H K, Rench M A, Hickman M E, Kasper D L. Safety and immunogenicity of capsular polysaccharide-tetanus toxoid conjugate vaccines for group B streptococcal types Ia and Ib. J Infect Dis. 1999;179:142–150. doi: 10.1086/314574. [DOI] [PubMed] [Google Scholar]
- 7.Baltimore R S, Kasper D L, Baker C J, Goroff D K. Antigenic specificity of opsonophagocytic antibodies in rabbit anti-sera to group B streptococci. J Immunol. 1977;118:673–678. [PubMed] [Google Scholar]
- 8.Berg S, Kasvi S, Trollfors B, Pilichowska-Paszkiet J, Fattom A, Tessin I, Lagergard T. Antibodies to group B streptococci in neonates and infants. Eur J Pediatr. 1998;157:221–224. doi: 10.1007/s004310050799. [DOI] [PubMed] [Google Scholar]
- 9.Bohnsack J F, Widjaja K, Ghazizadeh S, Rubens C E, Hillyard D R, Parker C J, Albertine K H, Hill H R. A role for C5 and C5a-ase in the acute neutrophil response to group B streptococcal infections. J Infect Dis. 1997;175:847–855. doi: 10.1086/513981. [DOI] [PubMed] [Google Scholar]
- 10.Chmouryguina I, Suvorov A, Ferrieri P, Cleary P P. Conservation of the C5a peptidase genes in group A and B streptococci. Infect Immun. 1996;64:2387–2390. doi: 10.1128/iai.64.7.2387-2390.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chmouryguina I I, Suvorov A N, Carlson B, Cleary P. Structural and functional similarity of C5a-ase enzymes from group A and B streptococci. Adv Exp Med Biol. 1997;418:757–759. doi: 10.1007/978-1-4899-1825-3_178. [DOI] [PubMed] [Google Scholar]
- 12.Cornacchione P, Scaringi L, Fettucciari K, Rosati E, Sabatini R, Orefici G, von Hunolstein C, Modesti A, Modica A, Minelli F, Marconi P. Group B streptococci persist inside macrophages. Immunology. 1998;93:86–95. doi: 10.1046/j.1365-2567.1998.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng L, Kasper D L, Krick T P, Wessels M R. Characterization of the linkage between the type III capsular polysaccharide and the bacterial cell wall of group B Streptococcus. J Biol Chem. 2000;275:7497–7504. doi: 10.1074/jbc.275.11.7497. [DOI] [PubMed] [Google Scholar]
- 14.Gravekamp C, Kasper D L, Paoletti L C, Madoff L C. Alpha C protein as a carrier for type III capsular polysaccharide and as a protective protein in group B streptococcal vaccines. Infect Immun. 1999;67:2491–2496. doi: 10.1128/iai.67.5.2491-2496.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemming V G, Hall R T, Rhodes P G, Shigeoka A O, Hill H R. Assessment of group B streptococcal opsonins in human and rabbit serum by neutrophil chemiluminescence. J Clin Investig. 1976;58:1379–1387. doi: 10.1172/JCI108593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herting E, Jarstrand C, Rasool O, Curstedt T, Hakansson S, Robertson B. Effect of surfactant on nitroblue tetrazolium reduction of polymorphonuclear leucocytes stimulated with type Ia group B streptococci. Acta Paediatr. 1995;84:922–926. doi: 10.1111/j.1651-2227.1995.tb13793.x. [DOI] [PubMed] [Google Scholar]
- 17.Hickman M E, Rench M A, Ferrieri P, Baker C J. Changing epidemiology of group B streptococcal colonization. Pediatrics. 1999;104:203–209. doi: 10.1542/peds.104.2.203. [DOI] [PubMed] [Google Scholar]
- 18.Hill H R, Bohnsack J F, Morris E Z, Augustine N H, Parker C J, Cleary P, Wu J T. Group B streptococci inhibit the chemotactic activity of the fifth component of complement. J Immunol. 1988;141:3551–3556. [PubMed] [Google Scholar]
- 19.Hirsch S, Austyn J M, Gordon S. Expression of the macrophage-specific antigen F4/80 during differentiation of mouse bone marrow cells in culture. J Exp Med. 1981;154:713–725. doi: 10.1084/jem.154.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horwitz M A. Phagocytosis of the Legionnaires' disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984;36:27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- 21.Ji Y, Carlson B, Kondagunta A, Cleary P P. Intranasal immunization with C5a peptidase prevents nasopharyngeal colonization of mice by the group A streptococcus. Infect Immun. 1997;65:2080–2087. doi: 10.1128/iai.65.6.2080-2087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji Y, Schnitzler N, DeMaster D, Cleary P P. Impact of M49, Mrp, Enn, and C5a peptidase proteins on colonization of the mouse oral mucosa by Streptococcus pyogenes. Infect Immun. 1998;66:5399–5405. doi: 10.1128/iai.66.11.5399-5405.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancefield R C. Current knowledge of the type specific M antigens of group A streptococci. J Immunol. 1962;89:307–313. [PubMed] [Google Scholar]
- 24.Larsson C, Stalhammar-Carlemalm M, Lindahl G. Experimental vaccination against group B streptococcus, and encapsulated bacterium, with highly purified preparations of cell surface proteins Rib and alpha. Infect Immun. 1996;64:3518–3523. doi: 10.1128/iai.64.9.3518-3523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsson C, Stalhammar-Carlemalm M, Lindahl G. Protection against experimental infection with group B streptococcus by immunization with a bivalent protein vaccine. Vaccine. 1999;17:454–458. doi: 10.1016/s0264-410x(98)00218-7. [DOI] [PubMed] [Google Scholar]
- 26.Levy N J, Kasper D L. Antibody-independent and -dependent opsonization of group B streptococcus requires the first component of complement C1. Infect Immun. 1985;49:19–24. doi: 10.1128/iai.49.1.19-24.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madoff L C, Paoletti L C, Tai J Y, Kasper D L. Maternal immunization of mice with group B streptococcal type III polysaccharide-beta C protein conjugate elicits protective antibody to multiple serotypes. J Clin Investig. 1994;94:286–292. doi: 10.1172/JCI117319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marodi L, Kaposzta R, Nemes E. Survival of group B streptococcus type III in mononuclear phagocytes: differential regulation of bacterial killing in cord macrophages by human recombinant gamma interferon and granulocyte-macrophage colony-stimulating factor. Infect Immun. 2000;68:2167–2170. doi: 10.1128/iai.68.4.2167-2170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orikasa M, Kawase T, Suzuki A. Induction of macrophagic and granulocytic differentiation of murine bone marrow progenitor cells by 1,25-dihydroxyvitamin D3. Calcif Tissue Int. 1993;53:193–200. doi: 10.1007/BF01321837. [DOI] [PubMed] [Google Scholar]
- 30.Porro M, Saletti M, Nencioni L, Tagliaferri L, Marsili I. Immunogenic correlation between cross-reacting material (CRM97) produced by a mutant of Corynebacterium diphtheriae and diphtheria toxoid. J Infect Dis. 1980;142:716–724. doi: 10.1093/infdis/142.5.716. [DOI] [PubMed] [Google Scholar]
- 31.Prokesova L, Dung D H, Trebichavsky I, Formankova E, Stepankova V, John C. Antibacterial activity of human mononuclear leukocytes against Staphylococcus aureus. Folia Microbiol. 1994;39:428–434. doi: 10.1007/BF02814451. [DOI] [PubMed] [Google Scholar]
- 32.Schuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev. 1998;11:497–513. doi: 10.1128/cmr.11.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shigeoka A O, Hall R T, Hemming V G, Allred C D, Hill H R. Role of antibody and complement in opsonization of group B streptococci. Infect Immun. 1978;21:34–40. doi: 10.1128/iai.21.1.34-40.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suvorov A N, Cleary P P, Ferrieri P. C5a peptidase gene from group B streptococci. In: Dunny G, Cleary P, McKay L, editors. Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington, D.C.: American Society for Microbiology; 1991. pp. 230–232. [Google Scholar]
- 35.Takahashi S, Adderson E E, Nagano Y, Nagano N, Briesacher M R, Bohnsack J F. Identification of a highly encapsulated, genetically related group of invasive type III group B streptococci. J Infect Dis. 1998;177:1116–1119. doi: 10.1086/517408. [DOI] [PubMed] [Google Scholar]
- 36.Uchida T. Action and production of diphtheria toxin, with special reference to its analysis using the cross-reacting toxin material. Jpn J Bacteriol. 1974;29:651–653. [PubMed] [Google Scholar]
- 37.Valentin-Weigand P, Benkel P, Rohde M, Chhatwal G S. Entry and intracellular survival of group B streptococci in J774 macrophages. Infect Immun. 1996;64:2467–2473. doi: 10.1128/iai.64.7.2467-2473.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wessels M R, Haft R F, Heggen L M, Rubens C E. Identification of a genetic locus essential for capsule sialylation in type III group B streptococci. Infect Immun. 1992;60:392–400. doi: 10.1128/iai.60.2.392-400.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wessels M R, Paoletti L C, Kasper D L, DiFabio J L, Michon F, Holme K, Jennings H J. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B streptococcus. J Clin Investig. 1990;86:1428–1433. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wexler D, Chenoweth E D, Cleary P. A streptococcal inactivator of chemotaxis: a new virulence factor specific to group A streptococci. Proc Natl Acad Sci USA. 1985;82:179–180. doi: 10.1073/pnas.82.23.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]