Abstract
A major target of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the epipharyngeal mucosa. Epipharyngeal abrasive therapy (EAT) is a Japanese treatment for chronic epipharyngitis. EAT is a treatment for chronic epipharyngitis in Japan that involves applying zinc chloride as an anti-inflammatory agent to the epipharyngeal mucosa. Here, we present a case of a 21-year-old man with chronic coughing that persisted for four months after a diagnosis of mild coronavirus disease 2019 (COVID-19), who was treated by EAT. We diagnosed chronic epipharyngitis as the cause of the chronic cough after the SARS-CoV-2 infection. SARS-CoV-2 spike RNA had persisted in the epipharyngeal mucosa of this Long COVID patient. EAT was performed once a week for three months, which eliminated residual SARS-CoV-2 RNA and reduced epipharyngeal inflammation. Moreover, a reduction in the expression of proinflammatory cytokines was found by histopathological examination. We speculate that the virus was excreted with the drainage induced by EAT, which stopped the secretion of proinflammatory cytokines. This case study suggests that EAT is a useful treatment for chronic epipharyngitis involving long COVID.
Keywords: long covid, chronic cough, tnf-α, il-6, chronic epipharyngitis, epipharyngeal abrasive therapy, sars-cov-2 spike rna
Introduction
Some people infected with severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19) might have symptoms that last a long time afterward, known as Long COVID [1]. Long COVID can be defined as the presence of signs and symptoms even after 12 weeks of SARS-CoV-2 infection and beyond [2]. Cough is a frequent symptom of COVID-19 and can persist for months after infection [3,4]. Vagus nerve stimulation by inflammatory cytokines released from inflammatory cells has been speculated as a cause of cough in COVID-19 [3]. The epipharynx, which is the infection target of SARS-CoV-2 [5,6], has dense vagus nerves under the mucosa [7,8], and chronic inflammation of the epipharynx causes coughing [9].
Epipharyngeal abrasive therapy (EAT) is a treatment for chronic epipharyngitis that has been performed mainly by otorhinolaryngologists in Japan since the 1960s and is effective for upper respiratory symptoms, including chronic cough [9]. Drainage of submucosal inflammatory substances is one of the therapeutic mechanisms by abrasion of the epipharyngeal mucosa with a cotton swab coated with anti-inflammatory zinc chloride [10]. In fact, continuous EAT suppresses submucosal inflammatory cytokines in the epipharynx [11]. Here, we report a case in which continuous EAT was effective for a cough that persisted for four months after SARS-CoV-2 infection, based on immunohistological considerations.
Case presentation
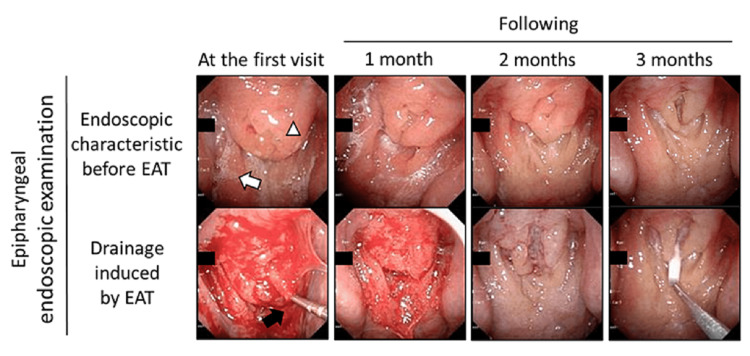
A 21-year-old man presented with chronic coughing that persisted for four months after being diagnosed with mild coronavirus disease 2019 (COVID-19) by an antigen test and clinical symptoms. His past medical history was insignificant. Vital signs, blood tests, chest X-ray, and respiratory function test were normal. A sinus CT scan revealed mucosal thickening of the bilateral maxillary ethmoid and frontal sinus (modified Lund-Mackay score 21/54). Epipharyngeal endoscopic examination showed marked epipharyngeal swelling and a postnasal drip (Figure 1).
Figure 1. Effect of Epipharyngeal abrasive therapy (EAT) in the patient.
Upper panels show endoscopic characteristics before EAT at the first visit and 1, 2, and 3 months. The white triangle indicates swelling of the epipharyngeal mucosa. The white arrow indicates mucus adhesion. Lower panels show bleeding induced by EAT at the first visit and 1, 2, and 3 months. The black arrow indicates a sterile nasal cotton swab containing zinc chloride.
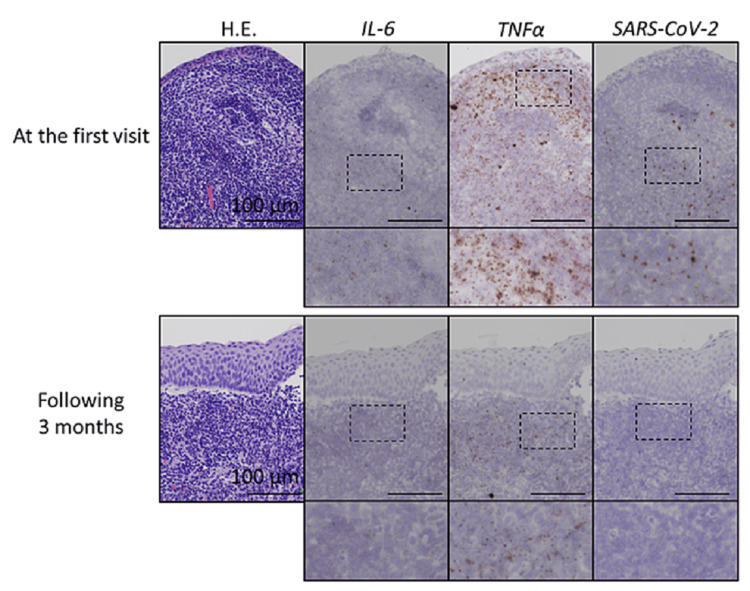
Histopathological examination of the epipharyngeal mucosa revealed hyperplastic lymphoid tissue and high expression of interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α). Additionally, in situ hybridization revealed residual severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike RNA in the epipharynx (Figure 2). Chronic epipharyngitis and chronic sinusitis after SARS-CoV-2 infection were diagnosed as the cause of the chronic cough. Epipharyngeal abrasive therapy, a treatment for chronic epipharyngitis, was performed once a week for three months. Chronic sinusitis was treated with low-dose macrolides, carbocisteine, mometasone furoate, and nasal irrigation. At three months after the start of treatment, histopathological examination showed a reduction in the expression of proinflammatory cytokines. Additionally, SARS-CoV-2 spike RNA persistence was confirmed before treatment had disappeared (Figure 2).
Figure 2. Expression patterns of interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α), and SARS-CoV-2 mRNAs in the epipharynx in the patient.
Expression patterns of IL-6 and TNF-α, and SARS-CoV-2 mRNAs (brown dots) in the epipharynx (Upper panels) at the first visit and (Lower panels) at three months. Magnified images are shown in each lower panel. RNA scope (in situ hybridization system, Advanced Cell Diagnostics, Hayward, CA, USA; IL-6: No. 310371, TNF-α: No. 310421, and SARS-CoV-2: No. 848561) was used following the manufacturer’s guidelines.
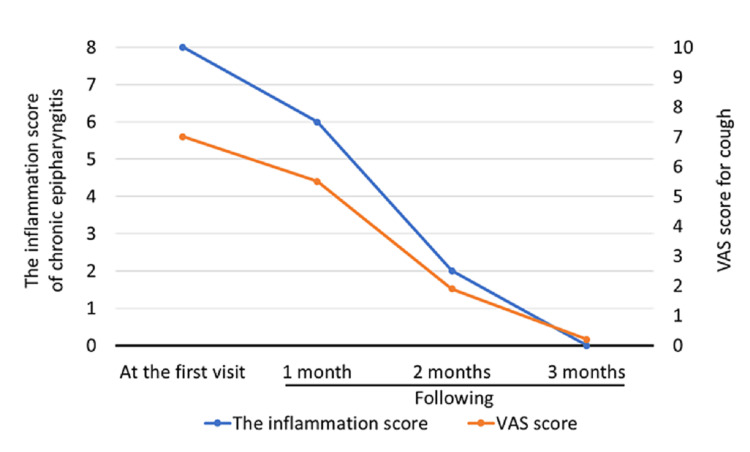
His epipharyngeal inflammation had improved (Figure 3) and the chronic sinusitis had also improved (modified Lund-Mackay score: 21/54→3/54). The visual analog scale (VAS) score for coughing improved from 7.0 to 0.2 (Figure 3).
Figure 3. Improvement of epipharyngeal inflammation and cough by Epipharyngeal abrasive therapy (EAT).
The inflammation of chronic epipharyngitis was scored by the Japan Society of Stomato-pharyngology EAT Review Committee's assessment criteria for 1) Redness of the epipharyngeal mucosa, 2) swelling of the epipharyngeal mucosa, 3) mucus or crust adhesion, and 4) bleeding during abrasion. Each was rated on a three-point scale (0: none; 1: mild-moderate; 2: severe). The visual analog scale (VAS) for coughing was scored from 0 (absence of symptoms) to 10 (highest severity of symptoms).
Discussion
The epipharynx, located behind the nasal cavity, is responsible for mucosal immunity through both innate and acquired immunity as a component of mucosa-associated lymphoid tissue (MALT) [5,11,12]. Meanwhile, the epipharyngeal ciliated epithelium is a major target of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) because of its high expression of viral entry factors angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) [6]. Chronic epipharyngitis is considered a residual immune response after upper respiratory tract infections including coronavirus disease 2019 (COVID-19) and causes various upper respiratory tract symptoms [13]. In fact, it has been reported that >90% of Long COVID patients suffer from moderate-to-severe chronic epipharyngitis [13]. Endoscopic findings such as mucosal redness, swelling, and mucus adhesion are important for the diagnosis of chronic epipharyngitis [13,14].
Epipharyngeal abrasive therapy (EAT) is a Japanese treatment for chronic epipharyngitis, which abrades the epipharyngeal mucosa with a cotton swab containing zinc chloride with anti-inflammatory effects [6,9,11,13-15]. Continuous EAT down-regulates the expression of viruses entry factors via squamous metaplasia and reduces major proinflammatory cytokines such as interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) in patients with chronic epipharyngitis [6,11,15]. As a consequence, EAT improves various upper respiratory tract symptoms including a cough associated with chronic inflammation of the epipharynx [9]. Furthermore, it has been reported that the reduction of inflammation of the epipharynx by EAT is also effective for the systemic symptoms of Long COVID [13]. However, the mechanism of residual epipharyngeal inflammation and the efficacy of EAT in Long COVID patients remains unclear [16].
Although it has been reported that SARS-CoV-2 spike RNA persistence in the human body at autopsy [17], this is the first report that SARS-CoV-2 RNA persists in the epipharyngeal mucosa of a Long COVID patient. SARS-CoV-2 antigen persistence in infected tissues affects host immune responses [18] indicating that viral RNA persistence in the epipharynx may be a mechanism for protracted epipharyngitis in Long COVID patients. More interestingly, EAT eliminated residual SARS-CoV-2 RNA and improved epipharyngeal inflammation. We speculate that the viral antigen was excreted along with the drainage induced by EAT and the elimination of the cause of inflammation suppressed the secretion of proinflammatory cytokines, which are closely related to driving cough via neuroimmune interactions [3]. Gabapentin and pregabalin, which are neuromodulators, are considered to be effective in controlling chronic refractory cough in Long COVID [3], whereas we suggest that EAT is a potential novel treatment in terms of removing the cause of cough rather than symptomatic treatment.
As a result, EAT suppressed the inflammation related to persistent viral RNA after infection resolution and the host counter-response, including overproduction of cytokines such as IL-6 and TNF-α, which are presumed to be the cause of Long COVID, in the epipharynx [19]. Chronic cough is a frequent symptom in Long COVID patients and it has been reported that about 10% of their non-hospitalized patients had cough over four months after SARS-CoV-2 infection [20]. Because the epipharynx is anatomically visible only by endoscopic examination, inflammation is often overlooked. In Long COVID patients without lower respiratory tract disorders, who suffer from a chronic cough, it is necessary to focus on upper respiratory tract disorders including chronic epipharyngitis. We propose that EAT is useful as a treatment for chronic epipharyngitis involving Long COVID.
Conclusions
Epipharynx is a major target of SARS-CoV-2. Severe inflammation and SARS-CoV-2 spike RNA persisted in the epipharynx of a Long COVID patient. Epipharyngeal abrasive therapy (EAT), a treatment for chronic epipharyngitis, was effective for a chronic cough involving Long COVID. EAT suppresses the expression of proinflammatory cytokines in the epipharynx that cause coughing. Surprisingly, EAT cleared residual SARS-CoV-2 RNA in the epipharynx. As a result, EAT may be effective in improving upper respiratory tract symptoms in Long COVID patients.
Acknowledgments
Kensuke Nishi and Shohei Yoshimoto contributed equally to this work and should be considered co-first authors. Conceptualization, K.N.; methodology, K.N. and Y.S.; validation, S.Y.; formal analysis, S.Y. and S.K.; investigation, T.T.; data curation, K.N.; writing—original draft preparation, K.N.; supervision, T.Y.; project administration, K.N. All authors have read and agreed to the published version of the manuscript.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. The Ethics Committee of Fukuoka Dental College issued approval ID: 552. Institutional Review Board Statement: The study was approved by the Ethics Committee of Fukuoka Dental College (ID: 552; date of approval: 12 July 2021)
References
- 1.Long COVID: an overview. Raveendran AV, Jayadevan R, Sashidharan S. Diabetes Metab Syndr. 2021;15:869–875. doi: 10.1016/j.dsx.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.A clinical case definition of post-COVID-19 condition by a Delphi consensus. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. Lancet Infect Dis. 2022;22:0–7. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Song WJ, Hui CK, Hull JH, Birring SS, McGarvey L, Mazzone SB, Chung KF. Lancet Respir Med. 2021;9:533–544. doi: 10.1016/S2213-2600(21)00125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long lasting symptoms of dyspnea, cough and fatigue after COVID-19 - narrative review of epidemiological studies (Article in Polish) Marciniak E, Górniak A, Hanke W. Med Pr. 2021;72:711–720. doi: 10.13075/mp.5893.01190. [DOI] [PubMed] [Google Scholar]
- 5.The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Gallo O, Locatello LG, Mazzoni A, Novelli L, Annunziato F. Mucosal Immunol. 2021;14:305–316. doi: 10.1038/s41385-020-00359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epipharyngeal abrasive therapy down-regulates the expression of SARS-CoV-2 entry factors ACE2 and TMPRSS2. Nishi K, Yoshimoto S, Nishi S, et al. In Vivo. 2022;36:371–374. doi: 10.21873/invivo.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasopharyngeal reflexes: integrative analysis of evoked respiratory and cardiovascular effects. White SW, McRitchie RJ. Aust J Exp Biol Med Sci. 1973;51:17–31. doi: 10.1038/icb.1973.2. [DOI] [PubMed] [Google Scholar]
- 8.Parkinson disease affects peripheral sensory nerves in the pharynx. Mu L, Sobotka S, Chen J, et al. J Neuropathol Exp Neurol. 2013;72:614–623. doi: 10.1097/NEN.0b013e3182965886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Outcome of an outpatient specialty clinic for chronic epipharyngitis. Mogitate M, Sasaki Y, Komiyama A. https://doi.org/10.1016/j.anl.2020.09.019. Auris Nasus Larynx. 2021;48:451–456. doi: 10.1016/j.anl.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Possible mechanisms underlying epipharyngeal abrasive therapy (EAT) with ZnCl2 solution for the treatment of autoimmune diseases and functional somatic syndrome. Hotta O, Inoue C, Tanaka A, Ieiri N. https://www.longdom.org/open-access/possible-mechanisms-underlying-epipharyngeal-abrasive-therapy-eat-with-znclsub2sub-solution-for-the-treatment-of-autoimm-16808.html Journal of Antivirals & Antiretrovirals. 2017;9:81–86. [Google Scholar]
- 11.Epipharyngeal abrasive therapy (EAT) reduces the mRNA expression of major proinflammatory cytokine IL-6 in chronic epipharyngitis. Nishi K, Yoshimoto S, Nishi S, et al. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23169205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Role of palatine tonsil and epipharyngeal lymphoid tissue in the development of glomerular active lesions (glomerular vasculitis) in immunoglobulin A nephropathy. Hotta O, Ieiri N, Nagai M, Tanaka A, Harabuchi Y. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23020727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epipharyngeal abrasive therapy (EAT) has potential as a novel method for long COVID treatment. Imai K, Yamano T, Nishi S, et al. Viruses. 2022;14 doi: 10.3390/v14050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Exhaled nitric oxide levels are associated with the severity of chronic epipharyngitis and decreased via epipharyngeal abrasion. Mogitate M. https://doi.org/10.1016/j.anl.2022.11.003. Auris Nasus Larynx. 2022 doi: 10.1016/j.anl.2022.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Epipharyngeal abrasive therapy down-regulates the expression of Cav1.2: a key molecule in influenza virus entry. Nishi K, Yoshimoto S, Nishi S, et al. In Vivo. 2022;36:2357–2364. doi: 10.21873/invivo.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long-COVID treatments: why the world is still waiting. Ledford H. Nature. 2022;608:258–260. doi: 10.1038/d41586-022-02140-w. [DOI] [PubMed] [Google Scholar]
- 17.SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Stein SR, Ramelli SC, Grazioli A, et al. Nature. 2022;612:758–763. doi: 10.1038/s41586-022-05542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Postacute COVID-19 is characterized by gut viral antigen persistence in inflammatory bowel diseases. Zollner A, Koch R, Jukic A, et al. Gastroenterology. 2022;163:495–506. doi: 10.1053/j.gastro.2022.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long COVID: an inevitable sequela of SARS-CoV-2 infection. Lai CC, Hsu CK, Yen MY, Lee PI, Ko WC, Hsueh PR. https://doi.org/10.1016/j.jmii.2022.10.003. J Microbiol Immunol Infect. 2022 doi: 10.1016/j.jmii.2022.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long COVID in the Faroe islands: a longitudinal study among nonhospitalized patients. Petersen MS, Kristiansen MF, Hanusson KD, et al. Clin Infect Dis. 2021;73:0–63. doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]