Abstract
Background
To our knowledge, this is the first meta-analysis of the association of not breastfeeding and the risk of autism spectrum disorder (ASD) based on observational studies.
Purpose
This meta-analysis aimed to evaluate of the association of not breastfeeding and the risk of ASD.
Methods
Three databases (PubMed, Web of Science, and Scopus) were systematically searched until December 2021. Heterogeneity was determined using the chi-square test and its quantity was measured using the I2 statistic. The Begg line regression test was used to assess publication bias. A random-effects model was used to analyze the data. Seven studies were included in this meta-analysis.
Results
The total study population included 3,270 individuals. According to the random-effects model, the estimated odds ratio of the risk of ASD associated with not breastfeeding was 1.81 (95% confidence interval, 1.35–2.27; I2=0%).
Conclusion
The results of the included studies were homogeneous. Our findings showed that not breastfeeding is a risk factor for ASD. These results suggest the importance of breastfeeding in decreasing the risk of ASD in children.
Keywords: Autism spectrum disorders, Breastfeeding, Child, Meta-analysis
Introduction
Autism spectrum disorders (ASD) among children are characterized by impaired social interactions, communication deviance, and the presence of restrictive and repetitive behavior patterns [1]. Previous reviews reported that ASD is predominantly genetic. Genetic factors alone account for 20%–30% of ASD cases, whereas the remaining 70%–80% are due to a complex interaction between environmental risk factors and genetics [2]. Recent meta-analyses have identified the role of advanced parent age, gestational infections, low birth weight, fetal distress, intrauterine growth retardation, small for gestational age, neonatal icterus, preterm labor, cesarean section, preeclampsia, and labor complications in the pathogenesis of ASD [3-5].
Some researchers have suggested that breastfeeding may protect against ASD [6-8], while another study did not show this association [9]. A meta-analysis by Tseng et al. [10] found that children with ASD were significantly less likely to be breastfed than those without ASD. However, they assessed the prevalence of breastfeeding (less versus more breastfeeding) and different breastfeeding durations in children with versus without ASD. Therefore, the present meta-analysis evaluated not breastfeeding versus breastfeeding and the risk of ASD among children based on epidemiological studies.
Methods
We performed the meta-analysis according to the PRISMA (Preferred Reporting Items for Systematic Reviews) criteria.
1. Eligibility criteria
The exposure variable was not breastfeeding, while the outcome of interest was ASD in children aged ≤18 years. Observational studies included cohort, cross-sectional, and case-control studies irrespective of language; publication date; and participant nationality, race, and age. The exclusion criteria were systematic reviews and meta-analyses, case reports and series, controlled trials, letters to the editor, and studies lacking complete data.
2. Information sources and search
We systematically searched the PubMed, Web of Science, and Scopus databases for articles published through December 2021. The search terms were applied in combination: (not breastfeeding, not breastfed, no breast milk, lactation, breastfeeding, breastfed, artificial milk-fed) and (ASD, autism spectrum disorders, or autism). The reference lists of the retrieved articles were manually searched to identify any further studies. We contacted the authors of the included studies for additional data.
3. Study selection
We used EndNote reference management software (Chicago Manual of Style, USA) to merge the results from different databases. Duplicate studies were subsequently excluded. In addition, 2 researchers independently extracted all data, and disagreements between the 2 researchers were resolved by discussion.
4. Data extraction
Data from the included studies were extracted using a data sheet in Stata ver. 13 (StataCorp LLC, College Station, TX, USA). The following data were included: first author, year of publication, country, study design, sample size, control for confounding variables (adjusted, unadjusted), age range, and ASD diagnostic method.
5. Methodological quality
We evaluated study quality using the Newcastle Ottawa Scale (NOS) [11]. Any study could achieve a maximum of 9 NOS stars: 4 for selection quality, 2 for comparability, and 3 for exposure quality. A score ≥7 indicated high quality.
6. Heterogeneity and reporting biases
Interstudy heterogeneity was assessed using the chi-square test [12] and the I2 statistic [13], while publication bias was assessed using Begg [14] line regression test.
7. Summary measures
The association between breastfeeding and the risk of ASD was assessed using odds ratios (ORs) and 95% confidence intervals (CIs). We used fully adjusted ORs to control for potentially confounding factors. A random-effects model was used to analyze the data [15]. Stata ver. 13 was used for the analysis, with a significance level of 0.05.
Results
1. Description of studies
According to our search strategy, 766 references were identified until December 20, 2021. Of them, 189 were duplicates and 566 were excluded by the title and abstract screening. Eleven studies were subjected to full-text review, which eliminated 4 full papers. Therefore, 7 studies were ultimately included in the present meta-analysis (Fig. 1A): 5 with a case-control design [6-9,16] and 2 with a cross-sectional design [17,18]. The total study population was 3,270 individuals. All studies were published in English (Table 1).
Fig. 1.
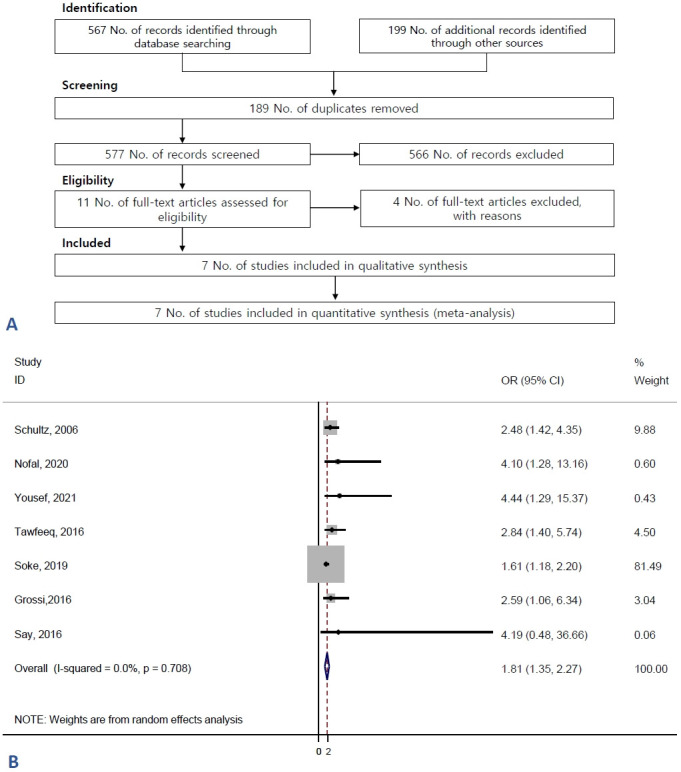
Flow of information through the different phases of the systematic review. (A) Flow of information through the different phases of the systematic review. (B) Forest plot of not breastfeeding and the risk of autism spectrum disorders. OR, odds ratio; CI, confidence interval.
Table 1.
Characteristics of the studies in the present meta-analysis
| Study | Country | Design | Sample | Diagnose method | Child age (mean or range based on year or month) | Estimate | Adjustment | Quality |
|---|---|---|---|---|---|---|---|---|
| Schultz et al., [8] 2006 | USA | Case-control | 984 | Self-report | 2–18 Year | OR | Adjust | High |
| Nofal et al., [16] 2020 | Egypt | Case-control | 144 | DSM | 2–16 Year | OR | Crude | High |
| Yousef et al., [18] 2021 | Egypt | Cross-sectional | 104 | DSM-IV/CARS | 2–5 Year | OR | Crude | Low |
| Tawfeeq et al., [6] 2016 | Iraq | Case-control | 200 | Not reported | 3–15 Year | OR | Crude | Low |
| Soke et al., [17] 2019 | USA | Cross-sectional | 1,549 | DSM-IV-TR | 30–68 Months | OR | Adjust | High |
| Grossi et al., [7] 2016 | Italy | Case-control | 113 | DSM-IV | 12.88 Year in case | OR | Crude | High |
| 9.13 Year in control | ||||||||
| Say et al., [9] 2016 | Turkey | Case-control | 176 | DSM-IV | 3–18 Year | OR | Crude | High |
DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; CARS, Childhood Autism Rating Scale; DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders, Text-Revised; OR, odds ratio.
2. Effects of exposure
Fig. 1B assessed the association between not breastfeeding and the risk of ASD. According to the random-effects model, the estimated OR of the risk of ASD associated with not breastfeeding was 1.81 (95% CI, 1.35–2.27; I2=0%) (Fig. 1B). The results of the included studies were homogenous.
3. Publication bias
Publication bias was assessed using Begg test. The P value for Begg regression among the children with ASD was 0.176. No evidence of publication bias was found among studies reporting an association between breastfeeding and the risk of ASD.
4. Subgroup analysis
We performed a subgroup analysis of the quality of the studies and designs. The pooled results of the cross-sectional and casecontrol designs were OR=1.62 (95% CI, 1.12–2.13) and 2.65 (95% CI, 1.57–3.73), respectively. There was no significant association in studies with a low-quality OR (2.98; 95% CI, 0.91–5.05; Table 2).
Table 2.
Results of subgroup analysis of not breastfeeding and autism spectrum disorders
| Subgroups | Studies |
||
|---|---|---|---|
| No. of studies | Odds ratio (95% CI) | I 2 | |
| Study design | |||
| Case-control | 5 | 2.65 (1.57, 3.73) | 0% |
| Cross-sectional | 2 | 1.62 (1.12, 2.13) | 0% |
| Quality of the study | |||
| Low | 2 | 2.98 (0.91, 5.05) | 0% |
| High | 5 | 1.75(1.28, 2.22) | 0% |
CI, confidence interval.
5. Study quality
In the evaluation of study quality based on the NOS scale, 6 studies were of high quality and one was of low quality (Table 1).
Discussion
To our knowledge, this is the first meta-analysis based on epidemiologic studies of the association between not breastfeeding versus breastfeeding and the risk of ASD. Our results showed that not breastfeeding was a risk factor for ASD. No heterogeneity was detected among the studies that reported an association between breastfeeding and the risk of ASD. The mechanisms underlying the association between breastfeeding and ASD risk are unclear and warrant further research. Different findings have been reported regarding ASD with different biological factors.
An inadequate intake of “beneficial” omega-3 and omega-6 polyunsaturated fatty acids (PUFA) derived from the mother during pregnancy or lactation may also play an important role in ASD. However, omega-3 and omega-6 PUFA deficiencies have been identified in children with ASD, while the relationship between poor breastfeeding and the risk of ASD has been investigated. Fatty acids are abundant in the colostrum secreted during the first 2–3 days after birth. Breast milk is a rich source of longchain polyunsaturated omega-3 and omega-6 fatty acids considered important in the development of the cognitive, social, and language abilities of children aged 6 months to 3.5 years [19,20]. Other studies reported that the elevated oxytocin levels experienced during infant sucking may protect against the development of ASD [21,22].
A meta-analysis by Ghozy et al. [23] in 2018 showed that breastfeeding decreased the risk of ASD by 58%, while exclusive breastfeeding decreased the risk by 76%. Breastfeeding for 12–24 months resulted in the most significant reduction in the risk of ASD.
Another meta-analysis by Tseng et al. [10] reported that children with ASD (clinical diagnosis or self-reported) were significantly less likely to be breastfed than those without ASD (OR, 0.61; 95% CI, 0.45–0.83; P=0.002). However, they assessed the prevalence (less versus more) and durations in children with ASD versus without ASD. The present meta-analysis evaluated not breastfeeding versus breastfeeding as a risk factor for ASD in children.
This study has some limitations. First, to control for known risk factors for ASD, we used an adjusted form. However, other studies have reported only the unadjusted OR. This may have introduced bias into our study. Second, the sample sizes of the included studies were small. Despite these limitations, our results suggested that not breastfeeding is a risk factor for ASD. Therefore, breastfeeding is necessary to decrease the risk of ASD in children.
In conclusion, our results showed that not breastfeeding was a risk factor for ASD. Therefore, breastfeeding is necessary to reduce the risk of ASD in children. Our findings suggest that the possible association between ASD and not breastfeeding should be added to the list of reasons to provide breastfeeding support.
Key message
This study aimed to determine whether there is an association between not breastfeeding (versus breastfeeding) and the risk of autism spectrum disorders (ASD) among children. We found that the risk of ASD associated with not breastfeeding had an odds ratio of 1.81 (95% confidence interval, 1.35–2.27; I2=0 %). These findings suggest the importance of breastfeeding in decreasing the risk of ASD among children.
Footnotes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This meta-analysis was supported by the Hamadan University of Medical Sciences. The protocol of this paper was supported by Hamadan University of Medical Sciences with Code 140011199609.
References
- 1.Fayyad J, Sampson NA, Hwang I, Adamowski T, Aguilar-Gaxiola S, AlHamzawi A, et al. The descriptive epidemiology of DSM-IV Adult ADHD in the World Health Organization World Mental Health Surveys. Atten Defic Hyperact Disord. 2017;9:47–65. doi: 10.1007/s12402-016-0208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383:896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- 3.Jenabi E, Bashirian S, Asali Z, Seyedi M. Association between small for gestational age and risk of autism spectrum disorders: a meta-analysis. Clin Exp Pediatr. 2021;64:538–42. doi: 10.3345/cep.2020.01956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jenabi E, Bashirian S, Khazaei S. Association between neonatal jaundice and autism spectrum disorders among children: a meta-analysis. Clin Exp Pediatr. 2020;63:8–13. doi: 10.3345/kjp.2019.00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenabi E, Karami M, Khazaei S, Bashirian S. The association between preeclampsia and autism spectrum disorders among children: a meta-analysis. Korean J Pediatr. 2019;62:126–30. doi: 10.3345/kjp.2018.07010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tawfeeq WF, Mukhaiser MH, Al-Hemiary NJ. Risk factors for autism in Baghdad city. Al-Kindy Coll Med J. 2016;12:95–102. [Google Scholar]
- 7.Grossi E, Veggo F, Narzisi A, Compare A, Muratori F. Pregnancy risk factors in autism: a pilot study with artificial neural networks. Pediatr Res. 2016;79:339–47. doi: 10.1038/pr.2015.222. [DOI] [PubMed] [Google Scholar]
- 8.Schultz ST, Klonoff-Cohen HS, Wingard DL, Akshoomoff NA, Macera CA, Ji M, et al. Breastfeeding, infant formula supplementation, and Autistic Disorder: the results of a parent survey. Int Breastfeed J. 2006;1:16. doi: 10.1186/1746-4358-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Say GN, Karabekiroğlu K, Babadağı Z, Yüce M. Maternal stress and perinatal features in autism and attention deficit/hyperactivity disorder. Pediatr Int. 2016;58:265–9. doi: 10.1111/ped.12822. [DOI] [PubMed] [Google Scholar]
- 10.Tseng PT, Chen YW, Stubbs B, Carvalho AF, Whiteley P, Tang CH, et al. Maternal breastfeeding and autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Neurosci. 2019;22:354–62. doi: 10.1080/1028415X.2017.1388598. [DOI] [PubMed] [Google Scholar]
- 11.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. Ontario: Ottawa Hospital Research Institute; 2009. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet] [cited 2020 Nov 5]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 12.Higgins JPT, Green S. London: The Cochrane Collaboration; 2008. Cochrane handbook for systematic reviews of interventions Version 5.0.0 [Internet] [updated 2008 Feb; cited 2020 Nov 5]. Available from: https://handbook-5-1.cochrane.org/v5.0.0/ [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Nofal HA, AbdAllah AM, Hamed MS. Epidemiological, clinical and psychometric aspects of Autism spectrum disorder among children in Zagazig University hospital. Egypt Fam Med J. 2020;4:80–95. [Google Scholar]
- 17.Soke GN, Maenner M, Windham G, Moody E, Kaczaniuk J, DiGuiseppi C, et al. Association between breastfeeding initiation and duration and autism spectrum disorder in preschool children enrolled in the study to explore early development. Autism Res. 2019;12:816–29. doi: 10.1002/aur.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yousef AM, Roshdy EH, Fattah NRA, Said RM, Atia MM, Hafez EM, et al. Prevalence and risk factors of autism spectrum disorders in preschool children in Sharkia, Egypt: a community-based study. Mid East Curr Psychiatry. 2021;28:1–14. [Google Scholar]
- 19.Sauenvald TU, Demmelmair H, Fidler N, Koletzko B. In: Short and long term effects of breast feeding on child health. advances in experimental medicine and biology, vol 478. Koletzko B, Michaelsen KF, Hernell O, editors. Boston (MA): Springer; 2002. Polyunsaturated fatty acid supply with human milk; pp. 261–70. [Google Scholar]
- 20.Al-Farsi YM, Al-Sharbati MM, Waly MI, Al-Farsi OA, Al-Shafaee MA, AlKhaduri MM, et al. Effect of suboptimal breast-feeding on occurrence of autism: a case-control study. Nutrition. 2012;28:e27–32. doi: 10.1016/j.nut.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Shafai T, Mustafa M, Hild T, Mulari J, Curtis A. The association of early weaning and formula feeding with autism spectrum disorders. Breastfeed Med. 2014;9:275–6. doi: 10.1089/bfm.2013.0104. [DOI] [PubMed] [Google Scholar]
- 22.Carter CS. Developmental consequences of oxytocin. Physiol Behav. 2003;79:383–97. doi: 10.1016/s0031-9384(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 23.Ghozy S, Tran L, Naveed S, Quynh TTH, Helmy Zayan A, Waqas A, et al. Association of breastfeeding status with risk of autism spectrum disorder: a systematic review, dose-response analysis and meta-analysis. Asian J Psychiatr. 2020;48:101916. doi: 10.1016/j.ajp.2019.101916. [DOI] [PubMed] [Google Scholar]


