Abstract
Purpose:
Quantification of dark adaptation (DA) response using the conventional rod-intercept time (RIT) requires very long testing time and may not be measurable in the presence of impairments due to disease such as age related macular degeneration (AMD). The goal of this study was to investigate the advantages of using area under the DA curve (AUDAC) as an alternative to the conventional parameters to quantify DA response.
Methods:
Data on 136 eyes (AMD: 98, normal controls: 38) from an ongoing longitudinal study on AMD was used. DA was measured using the AdaptDx 20-minute protocol. AUDAC was computed from the raw DA characteristic curve at different time points, including 6.5 minutes and 20 minutes (default). Presence of AMD in the given eye was predicted using a logistic regression model within leave-one-out cross-validation framework, with DA response as the predictor while adjusting for age and gender. The DA response variable was either the AUDAC values computed at 6.5 minutes (AUDAC6.5) or at 20 minutes (AUDAC20) cut-off, or the conventional RIT.
Results:
AUDAC6.5 was strongly correlated with AUDAC20 (R = 0.925, p < 0.001). The accuracy of predicting AMD presence using AUDAC20 was 76%, compared to 79% when using RIT, the current gold standard. Additionally, when limiting AUDAC calculation to 6.5-minutes cut-off, the predictive accuracy of AUDAC6.5 was 80%.
Conclusions:
AUDAC can be a valuable measure to quantify the overall DA response and can potentially facilitate shorter testing duration while maintaining diagnostic accuracy.
Introduction
Dark adaptation (DA) is known to be adversely affected in age-related macular degeneration (AMD).1–6 Recent studies have provided evidence that DA can aid in early detection and monitoring of visual function in AMD patients.7, 8 Psychophysical DA measurement typically results in a characteristic curve that plots retinal sensitivity over time in the dark, and the quantification of the DA response is typically performed by extracting key parameters from the DA characteristics such as time to rod-cone break, rod recovery rate, cone sensitivity threshold, and rod sensitivity threshold.9–11
While these traditional DA parameters help understand specific aspects of the DA response, they are not straightforward to derive – for example requiring non-linear regression,12 particularly in the context of using DA measurement as a clinical test. Contemporary DA instruments such as AdaptDx (MacuLogix, Harrisburg, PA) use rod intercept time (RIT) as the outcome measure, which is defined as the time required for the sensitivity to recover by 3 log units. RIT is relatively simpler to derive from the raw data and easier to interpret, with longer times indicating DA impairments. However, it is not possible to determine a valid RIT value in eyes where the sensitivity fails to recover to the preset threshold within the duration of the testing protocol. This scenario is frequently encountered when measuring AMD patients and previous studies have reported that a substantial number of AMD eyes fail to record valid RIT values within the testing time bounds.5, 8, 13 This makes longitudinal follow up of the DA function particularly challenging with RIT as the response measure.
The limitation of RIT as a DA response measure can be addressed, to some extent, by using the area under the DA curve (AUDAC). This is because AUDAC is well-suited to quantify the variation in the underlying DA curves, even in cases where the eyes fail to record valid RIT values for the given testing protocol. For a given measurement protocol, the DA curves are bounded in time and sensitivity threshold. So, the % of area covered by the DA curve can be compared between subjects. Thus, AUDAC can be an alternative way to quantify DA response.
The concept of area under the curve is extensively used in statistics and machine learning.14 However, computing area under the dark adaptation curve as a way to quantify DA response has not been investigated in detail. While it was briefly discussed in the context of evaluation of a mobile application for DA measurement,15 that work did not provide an in-depth analysis of AUDAC over a large data set of DA curves belonging to AMD patients and controls. This work evaluates the utility of AUDAC to quantify DA response. We also investigate the effect of computing AUDAC over shorter DA testing durations on the diagnostic sensitivity and specificity, with the ultimate goal of extracting meaningful information from the DA response measured over shorter duration testing protocols.
Methods
Acquisition of DA data
The DA data were collected as part of a prospective, longitudinal, observational study on AMD. Detailed study procedures including exclusion criteria have been previously described.13 The study followed the tenets of the Declaration of Helsinki, and was approved by the Partners Institutional Review Board (identifier: 00006221). All included participants provided written informed consent.
The original study had a cross-sectional design and DA measurements were obtained in 136 eyes of 78 patients (58 AMD patient and 20 control patients). For a subset of these subjects (42 eyes of 27 subjects) 3 year follow up data was also obtained. The study participants included AMD patients and controls above age 50 without any retinal disease. Subjects underwent comprehensive eye exam and individuals with any other confounding eye/vitreoretinal conditions were excluded. AMD was diagnosed and graded using color fundus photos and the AREDS 2 grading scale.16, 17
Dark adaptation testing
Dark adaptation testing was done with dilated eyes (≥ 6 mm) using the standard 20 minute test protocol of the AdaptDx® dark adaptometer.18 Both eyes, if fitting the study criteria, were tested separately for a given individual. The test spot was 5° inferior to fixation. The test session ended if the sensitivity threshold of 3 log unit was crossed or the test time reached 20 minutes. The machine estimated the rod-intercept time (RIT), which was the time required for the sensitivity to reach the 3 log unit threshold within the 20 minutes of testing duration, failing which, RIT value of 20 was assigned by the device. The raw DA characteristics, RIT values, and the fixation error data were exported for analysis. Eyes with total fixation error > 30% were not considered for analysis.
Computation of AUDAC
Based on the raw DA characteristics of each eye, the AUDAC was calculated using standard trapezoid method19 with respect to the 3.0 log unit sensitivity threshold. It covered the region under the DA curve from the start of the DA test (time = 0, sensitivity threshold = 0) till the time when sensitivity threshold of 3.0 log units was achieved (Figure 1A). Data points on the DA curve that were associated with fixation errors were excluded from AUDAC computation. Linear interpolation was used to determine the precise time when the curve crossed the 3.0 log unit sensitivity threshold, failing which, the entire testing time limit was considered for AUDAC calculation. The area thus computed was normalized by the preset testing bounds along the time and sensitivity axes. Larger AUDAC values indicated DA impairments that could be due to delays in recovery of sensitivity, elevated sensitivity thresholds, or both.
Figure 1:
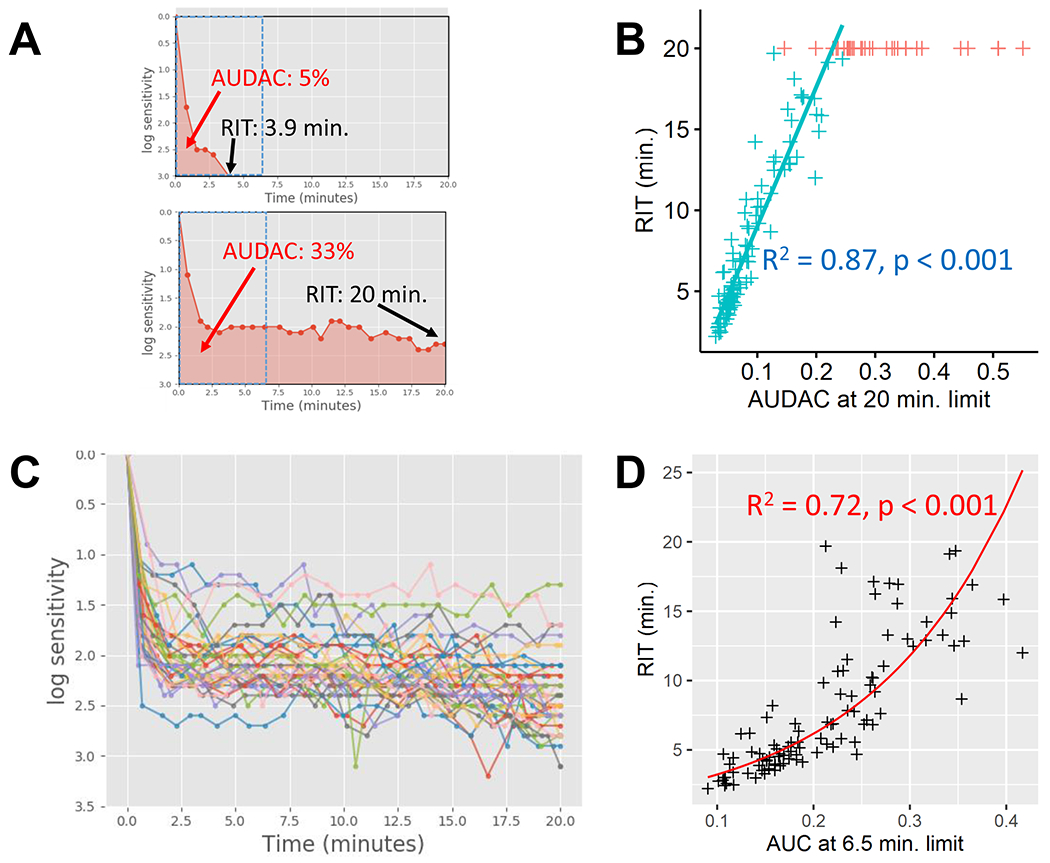
Relationship between AUDAC and RIT. (a) AUDAC can be visualized as the shaded region under the DA curves. In the top curve, the sensitivity recovers faster and a RIT value of 3.9 minutes is recorded. In the bottom curve, the sensitivity does not recover to the RIT criterion within 20 minutes of test duration. AUDAC can quantify DA response in both cases, and larger AUDAC can be indicative of DA impairments. (b) Scatter plot of AUDAC20 vs. RIT. The linear model is fitted to the eyes that recorded RIT < 20 minutes (cyan +). Eyes failing to record a valid RIT (n=35) were assigned a value of 20 (shown in red +) and there is a large variation in their AUDAC values. (c) The DA characteristic curves for the 35 eyes with RIT = 20 minutes. A wide variation can be seen the shapes of the characteristics, which result in varying AUDAC values despite all have RIT = 20 minutes. (d) Scatter plot of AUDAC6.5 vs. RIT, with a log-linear model (log(rit) ~ audac6.5) fitted to the eyes with RIT < 20 minutes. The regression line is shown after taking an exponential of the fit.
The default testing bounds along time axes were from 0 to 20 minutes, as per the protocol time limit. However, we also explored AUDAC computation for shorter time bounds – reducing the test termination time from the default 20 minute limit to as low as 3.5 minutes, at 0.5 minute intervals. The bounds along the sensitivity axis remained the same throughout (0 to 3.0 log units). Therefore, for a given DA characteristics curve, we could obtain multiple AUDAC values corresponding to different stopping times. To understand how shortening the DA timeline affects AUDAC and its ability to summarize the overall DA response, we chose AUDAC calculated at 6.5 minute time limit (denoted by AUDAC 6.5) for comparison with AUDAC calculated at 20 minute limit (AUDAC 20) and the original RIT value generated by AdaptDx machine. The AUDAC at 6.5 minute time limit was chosen among various potential time endpoints for further detailed analysis because it represents the RIT threshold for normal DA as set in the AdaptDx protocol.
Statistical Analysis
Our first goal was to determine whether the AUDAC can reliably quantify the DA response, which was done by examining the relationship between AUDAC and the existing DA outcome measure (RIT). Here, the relationship of RIT with AUDAC20 and AUDAC6.5 was investigated with a linear and log-linear model, respectively.
The second goal was to explore the relationship between AUDAC values computed at shorter testing times and those obtained for the default 20 minute time limit. For this analysis, we determined the Pearson correlation coefficient between AUDAC6.5 and AUDAC20.
Our third goal was to investigate the ability of AUDAC, particularly AUDAC6.5, to reliably track DA changes in a tested eye between baseline and 3 year follow up measurements. We then evaluated the ability of AUDAC to predict RIT values for eyes that failed to record a valid RIT value, based on the relationship between AUDAC and RIT for eyes with RIT < 20 minutes.
We also investigated the effectiveness of different DA response measures, including RIT, AUDAC20, and AUDAC6.5, in predicting AMD presence (binary outcome – yes/no). A logistic regression model was used with DA response as the predictor, using either AUDAC6.5, AUDAC20, or RIT values, and adjusting for age and gender. Prediction was done in the framework of leave one out cross validation (LOOCV)20 and prediction accuracy, sensitivity, and specificity using different DA response variables were compared.
Finally, the consistency of the trends in DA changes over time (difference between baseline and 3 year) with different DA response measurements were examined via 2×2 tables. We computed the degree of agreement (Cohen’s κ) in the direction of 3 year DA changes between AUDAC6.5, AUDAC20, and RIT to determine whether shortening of the testing time affected the ability to deduce changes over time in the DA response.
Despite having measured DA in both eyes of a large number of the participants, statistical analysis was performed by treating DA response in each eye as independent we are more interested in evaluating and comparing different metrics of DA response for the given eye instead of associating between-subject factors with the DA response. Statistical analysis was done with R and p-values ≤ 0.05 were considered statistically significant.
Results
Baseline DA data was available for 136 eyes of 78 individuals, including AMD patients and controls (Table 1).
Table 1:
Details of the subject population from which baseline DA data were collected.
| Subject categories | Eyes (subj.) | Avg. age ± std. (years) | Female eyes (%) | RIT ≥ 20 min. eyes (%) | RIT avg. ± std. | AUDAC20 avg. ± std. | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| For eyes RIT < 20 min. | Overall | For eyes RIT < 20 min. | Overall | |||||
|
| ||||||||
| Early AMD | 22 | 67.0 ± 9.6 | 14 (63) | 0 | 6.24 ± 3.23 | 6.24 ± 3.23 | 0.072 ± 0.04 | 0.072 ± 0.04 |
| Intermediate AMD | 63 | 69.4 ± 5.1 | 35 (56) | 27 (43) | 11.12 ± 4.74 | 14.93 ± 5.68 | 0.118 ± 0.06 | 0.19 ± 0.1 |
| Late AMD | 13 | 68.6 ± 5.1 | 11 (85) | 7 (54) | 11.1 ± 6.33 | 15.89 ± 6.17 | 0.112 ± 0.05 | 0.273 ±0.17 |
| All AMD | 98 (58) | 68.7 ± 6.4 | 60 (61) | 34 (35) | 9.44 ± 4.98 | 13.11 ± 6.45 | 0.102 ± 0.05 | 0.174 ± 0.12 |
|
| ||||||||
| Controls | 38 (20) | 66.1 ± 7.7 | 16 (42) | 1 (3)* | 5.08 ± 2.5 | 5.48 ± 3.46 | 0.06 ± 0.03 | 0.065 ± 0.04 |
|
| ||||||||
| Overall | 136 (78) | 68.0 ± 6.9 | 76 (56) | 35 (26) | 7.84 ± 4.72 | 10.97 ± 6.71 | 0.086 ± 0.051 | 0.143 ± 0.12 |
Classified as control based on AREDS 2 color fundus photo classification. However this patient showed OCT abnormalities and hyperreflectivity of hyporeflective bands around ellipsoid zone which could explain his delayed DA parameters.
Relationship between AUDAC and RIT
Relationship between AUDAC and RIT was evaluated in eyes with RIT < 20 minutes (n=101). The distribution of AUDAC20 and RIT were long tailed and deviated from normality (AUDAC20: median = 0.063, iqr = 0.054, minimum = 0.029, maximum = 0.244; RIT: median = 5.83, iqr = 6.42, minimum = 2.21, maximum = 19.69). AUDAC20 had a strong linear relationship with RIT values (Figure 1B) in eyes with RIT < 20 minutes (β = 86, p < 0.001, R2 = 0.87). A large variation in the AUDAC values were seen for eyes with RIT ⩾ 20 minutes (n = 35, median = 0.276, iqr = 0.091, minimum = 0.146, maximum = 0.55), as the underlying shapes of the DA characteristics were different in these eyes (Figure 1C). The distribution of AUDAC6.5 was not normal (median = 0.204, iqr = 0.11, minimum = 0.09, maximum = 0.417). Also, unlike AUDAC20, the relationship between AUDAC6.5 and RIT was described by a log-linear model (β = 6.48, p < 0.001, R2 = 0.72), where log(RIT) was linearly associated with AUDAC6.5 (Figure 1D).
AUDAC values for shorter DA test times
AUDAC values at shorter DA test times were strongly correlated with AUDAC20 for the baseline data (Figure 2A). At the lowest sampled stopping time limit of 3.5 minutes, the Pearson correlation coefficient with AUDAC20 was 0.83, which indicates a strong correlation between AUDAC20 and AUDAC calculated at 3.5 minute limit. The correlation coefficient between AUDAC6.5 and AUDAC20 was 0.92. The correlation between AUDAC20 and AUDAC6.5 was also strong in different subject groups (Figure 2B).
Figure 2:
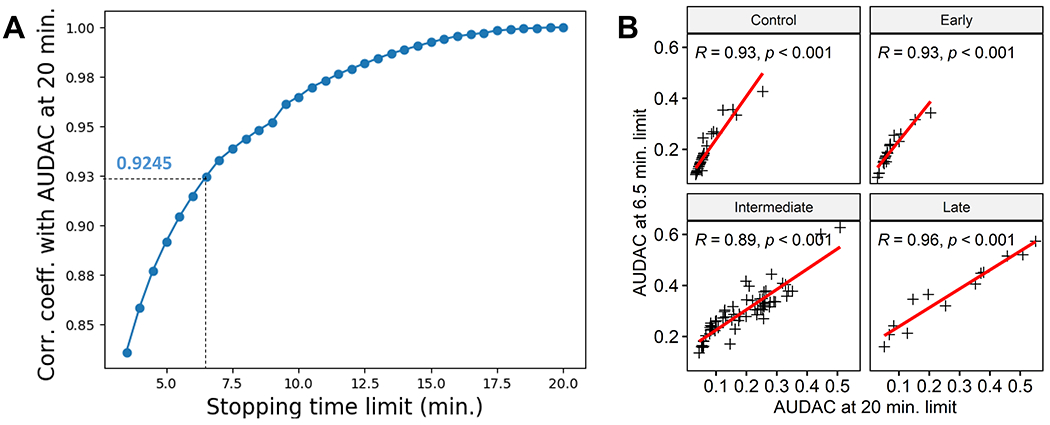
Relationship between AUDAC20 and AUDAC values at shorter stopping time limits. (a) Plot of correlation coefficients with AUDAC20 with different AUDAC values corresponding to different stopping time limits (plotted in the x axis). The minimum correlation coefficient is for 3.5 minute stopping time limit, which still indicates a strong correlation. (b) Correlation between AUDAC20 and AUDAC6.5 for different AMD stages and control subjects. All groups show strong correlation.
Prediction using AUDAC
Out of 136 eyes, 35 failed to record a valid RIT value within 20 minutes, so presumably their true RIT values were greater than 20 minutes. With AUDAC20 as the predictor, and using a linear model established earlier with 101 eyes with RIT < 20 minutes, the projected RIT values were > 20 minutes for 33 out of 35 eyes (median = 24.13, iqr = 7.85, minimum = 12.97, maximum = 47.76). With AUDAC6.5 as the predictor, and using the log-linear model, the projected RIT values were > 20 minutes for 12 out of 35 eyes (median = 16.15, iqr = 12.2, minimum = 5.1, maximum = 98.09).
Prediction of AMD presence in an eye based on the DA response was validated with respect to the clinical diagnosis of AMD vs. Control in 136 eyes (98 AMD, 38 Controls). The accuracy rates (correctly predicting AMD presence) using RIT, AUDAC20, and AUDAC6.5 were 78.7%, 76.4%, and 80.1%, respectively (Figure 3). It should be noted that the 78.7% prediction accuracy for RIT was based on our logistic regression model. Instead, if the AdaptDx threshold value for RIT of 6.5 minutes (normal DA if RIT < 6.5 minutes) was used to predict AMD presence, the accuracy was slightly lower at 77.9%. Also, when using the RIT cutoff of 6.5 minutes, the test was less sensitive and more specific.
Figure 3:
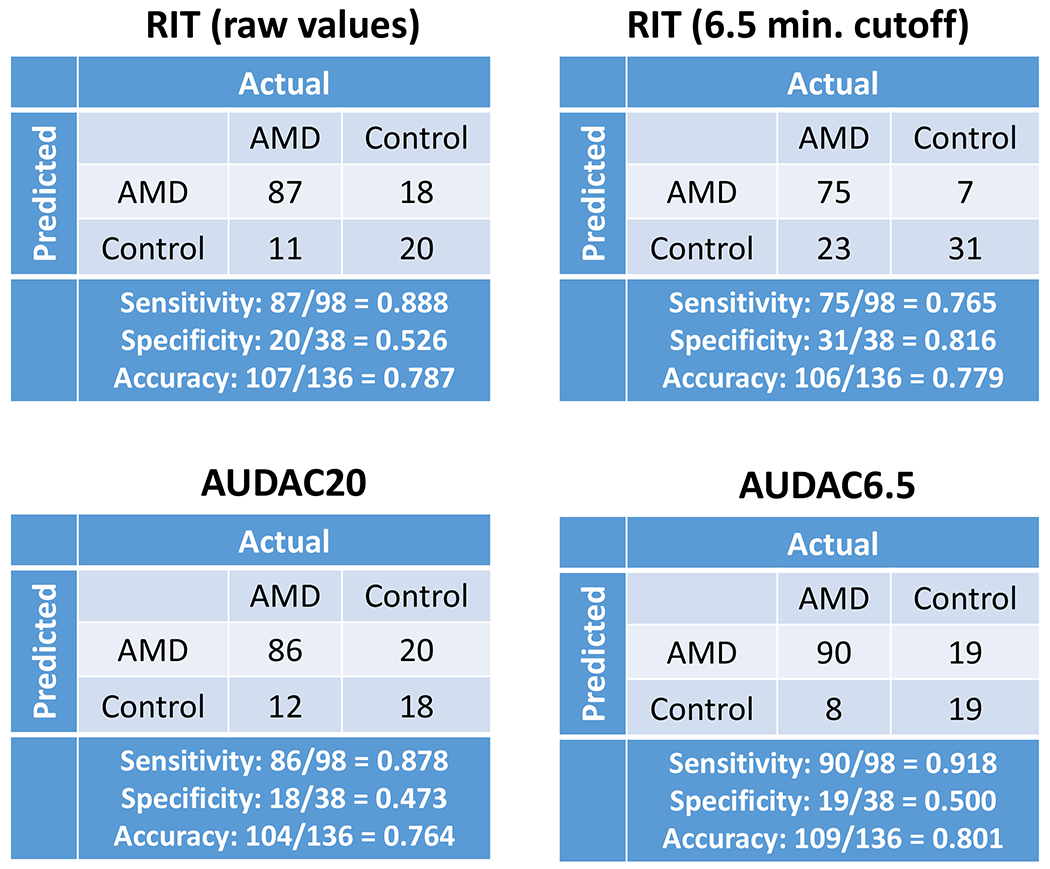
Detection of AMD presence using the DA response quantified with either RIT, AUDAC20, or AUDAC6.5. Also, shown is the 2×2 table for the 6.5 minutes RIT threshold for AMD detection (as per AdaptDx protocol specifications). Similar accuracy levels are seen for all variables, indicating that AUDAC6.5 is doing no worse than conventional measure of RIT for detecting AMD in a given eye.
Within-eye DA changes over time
Ability of AUDAC to track changes in the DA response over time was evaluated with the 3 year follow up data for 42 eyes of 27 individuals, of which 29 were AMD and 13 controls, at baseline. At 3 year, 5 of the baseline control subjects had developed AMD, and 4 AMD patients had progressed to subsequent stages. At baseline, 11 eyes did not achieve a valid RIT value within 20 minutes of testing and therefore changes in their RIT values at 3 year were not available. On the other hand, changes in AUDAC over 3 year could be computed for all 42 eyes.
There was a good agreement in the direction of DA changes over 3 years between AUDAC20 and AUDAC6.5, followed by AUDAC20 and RIT and AUDAC6.5 and RIT (Figure 4). When comparing AUDAC20 and AUDAC6.5, the 3 year change was consistent for 36 out of 42 eyes (87%), with Cohen’s κ = 0.61 (p < 0.001). Lower, but significant consistency between RIT and AUDAC20 (agreement = 77%, κ = 0.47, p = 0.009), and RIT and AUDAC6.5 (agreement = 74%, κ = 0.34, p = 0.04) was seen for 3y changes in the DA response.
Figure 4:
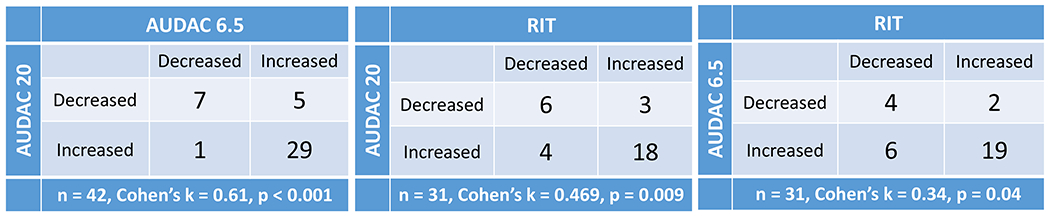
2x2 tables showing the number of eyes where the DA response measures increased or decreased at 3 year relative to the baseline values. The diagonal elements show the number of agreements and off-diagonal elements show disagreement between the two quantities being compared. Here, agreement means that the difference in the DA response between baseline and 3 year follow up was in the same direction (positive or negative) for the two measures being compared. (Left) AUDAC6.5 and AUDAC20 values both increased at 3 year relative to baseline in 29 eyes, and both decreased in 7 eyes (87% agreement). In 6 eyes, the two measures trended in the opposite direction (off-diagonal elements). (Center) RIT values and AUDAC20 values increased at 3 year for 18 eyes and decreased for 6 eyes. In 7 eyes, the changes in RIT and AUDAC20 were not consistent. Only eyes with RIT < 20 minutes at baseline were considered for this table (n=31). (c) AUDAC6.5 and RIT values both increased at 3 year for 19 eyes, both decreased in 4 eyes, and trended in opposite direction for 8 eyes.
Discussion
In this work, we investigated the utility of AUDAC as a DA response measure and for the first time showed that: 1) AUDAC had strong association with RIT, 2) AUDAC values computed for shorter DA test times were correlated with AUDAC computed at the 20 minute default stopping time, 3) AUDAC computed for shorter DA testing times could track longitudinal changes in DA , and 4) AUDAC was able to predict with high sensitivity other parameters of DA response (RIT) and the presence of AMD in a given eye.
These results have 2 main implications. First, AUDAC is a reliable way to quantify DA response, which is particularly relevant for eyes that reach the test ceiling RIT value at baseline. With AUDAC it is possible to obtain a quantifiable measurement of their DA response and to evaluate their progression over time. Therefore, using AUDAC can facilitate easier longitudinal follow up of DA changes in retinal disease such as AMD with impaired DA where current RIT measurements can reach a ceiling value. Second, using AUDAC as the measure of DA response, we can potentially reduce the DA testing time without losing essential information, particularly diagnostic sensitivity. Thus, with AUDAC as the outcome, we can measure DA over a shorter duration and still obtain a reliable measure of the DA response that is similar to what would be obtained from an extended testing protocol. Shortening of the DA testing time could improve the feasibility of DA testing for patients and clinicians, who currently tend to view it as tedious, labor intensive, and burdensome test procedure.
It should be noted that shorter testing protocols do exist (for example AdpatDx 6.5 minute short duration protocol),21 but they are primarily intended for screening purposes and not for long-term follow up of DA response. In fact, previous DA studies involving AMD patients used 20 minute or 40 minute measurement protocols, and even that was sometimes not sufficient to record a valid RIT value in many eyes.5, 8, 13 One possible alternative to improve the chances of expediting recovery of sensitivity in AMD patients is to move the test spot away from the center and toward the periphery of macula, such that moving the test spot from 5° to 12° allowed recovery in intermediate AMD patients within 20 minutes.22 On the other hand, AUDAC value from the same short duration protocol (6.5 minutes) and measured at the same eccentricity of 5° not only offers higher diagnostic sensitivity than RIT, but can also reliably track changes in the dark adaptation response over time.
Measurement of DA in eyes diagnosed with AMD can aid in understanding the effects of retinal changes on photoreceptor functioning.2–4, 7 With AUDAC, we can extract the underlying variations in the shapes of the DA curves that convey information about delays and impairments in the DA response. In that sense, the measure of AUDAC summarizes changes in the DA curve along both the time and sensitivity axes. Also, AUDAC is relatively simple to calculate as it does not require non-linear curve fitting, or any other kind of parameter estimation processes. Given that DA responses tend to be noisy or highly impaired, particularly in AMD patients, estimation of the traditional DA parameters can become challenging. With AUDAC, we can directly use the raw data obtained from the measurements, and therefore even highly impaired DA characteristics can be quantified with relative ease.
There are a few limitations of AUDAC based on the data presented in this paper. One drawback of reducing overall testing time is that it leads to the apparent loss of information, which can affect how various other parameters of the DA response characteristics are predicted using AUDAC. For example, using AUDAC computed from full 20 minute test time was better at predicting RIT values than with AUDAC6.5. Another limitation of AUDAC is that there is an upper limit to how much variation we can see between eyes that fail to reach the RIT criterion. This is because AUDAC is bounded by the time and sensitivity limits of the underlying protocol. The analysis was done by treating each eye as independent, and in future correlation between the two eyes of the same participants will be included in the statistical analysis involving AUDAC as more data become available. Finally, utility of AUDAC was so far evaluated based on DA measured with the AdaptDx instrument only, so further investigations are needed about the broad applicability of AUDAC as the DA response metric. RIT has been used as an outcome measure in the Medmont dark-adapted chromatic perimeter (Medmont Ltd International, Victoria, Australia),23 and therefore it is possible that the findings presented in this paper might also be applicable to this device.
In conclusion, AUDAC can represent a useful measurement of DA response measure and may allow shortening of the DA testing time without significantly compromising the elicited information. Furthermore, it can be a valuable tool for tracking changes in DA response over time in patients whose DA response cannot be measured using conventional parameters. AUDAC can be a widely applicable DA response measure allowing faster measurement that can potentially impact clinical practice and clinical trial endpoints.
Synopsis.
Using area under the dark adaptation curve as the response measure can help addresses the challenges in quantification of the dark adaptation function when it is highly impaired, and lead to potential measurement efficiencies.
Acknowledgements
We thank Nandini Wappukondur at MEEI for helping us with data acquisition and retrieval during pandemic related research shutdown.
Funding Information
This work was funded in part by NIH grant EY029847, the Miller Retina Research Fund (Mass. Eye and Ear) (Award#: NA), the Champalimaud Vision Award (Award#: NA), the unrestricted departmental Grant from Research to Prevent Blindness, Inc. New York (Award#: NA), and the Commonwealth Unrestricted Grant for Eye Research (Award#: NA).
Footnotes
Disclosures
Shrinivas Pundlik & Gang Luo have a financial interest in EyeNexo, LLC, a company developing smartphone applications for eye measurement. Their interests were reviewed and are managed by Schepens Eye Research Institute and Partners HealthCare in accordance with their conflict of interest policies.
References
- 1.Owsley C, Huisingh C, Jackson G, et al. Associations between abnormal rod-mediated dark adaptation and health and functioning in older adults with normal macular health. Investigative Ophthalmology and Visual Science. 2014;55(8):4776–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owsley C, Jackson G, Cideciyan A, et al. Psychophysical Evidence for Rod Vulnerability in Age Related Macular Degeneration. Investigative Ophthalmology and Visual Science. 2000;41(1):267–273. [PubMed] [Google Scholar]
- 3.Owsley C, Jackson G, White M, Feist R, Edwards D. Delays in Rod-mediated Dark Adaptation in Early Age-related Maculopathy. Ophthalmology. 2001;108(7):1196–1202. [DOI] [PubMed] [Google Scholar]
- 4.Owsley C, McGwin G Jr, Jackson G, Kallies K, Clark M. Cone- and Rod-Mediated Dark Adaptation Impairment in Age-Related Maculopathy. Ophthalmology. 2007;114(9):1728–1735. [DOI] [PubMed] [Google Scholar]
- 5.Flamendorf J, Agrón E, T.Wong W, et al. Impairments in Dark Adaptation Are Associated with Age-Related Macular Degeneration Severity and Reticular Pseudodrusen. Ophthalmology. 2018;122(10):2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimitrov P, Guymer R, Zele A, Anderson A, Vingrys A. Measuring Rod and Cone Dynamics in Age-Related Maculopathy. Investigative Ophthalmology and Visual Science. 2008;49(1):55–65. [DOI] [PubMed] [Google Scholar]
- 7.Owsley C, McGwin G Jr, Clark ME, et al. Delayed Rod-Mediated Dark Adaptation Is a Functional Biomarker for Incident Early Age-Related Macular Degeneration. Ophthalmology. 2016;123(2):344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen KG, Alvarez JA, Yazdanie M, et al. Longitudinal Study of Dark Adaptation as a Functional Outcome Measure for Age-Related Macular Degeneration. Ophthalmology. 2018;126(6):856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamb TD, Pugh EN Jr. Dark adaptation and the retinoid cycle of vision. Progress in Retinal and Eye Research. 2004;23:307–380. [DOI] [PubMed] [Google Scholar]
- 10.Pugh E Jr RUSHTON’S PARADOX: ROD DARK ADAPTATION AFTER FLASH PHOTOLYSIS. Journal of Physiology. 1975;248:413–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly JM. The Parameters of a Dark Adaptation Model Explained 2016; Available from: https://cran.r-project.org/web/packages/Dark/vignettes/parameter_exp.html.
- 12.McGwin G Jr, Jackson G, Owsley C. Using nonlinear regression to estimate parameters of dark adaptation. Behav Res Methods Instrum Comput. 1999;31:712–717. [DOI] [PubMed] [Google Scholar]
- 13.Laíns I, Miller J, Park D, et al. Structural Changes Associated with Delayed Dark Adaptation in Age-Related Macular Degeneration. Ophthalmology. 2017;124(9):1340–1352. [DOI] [PubMed] [Google Scholar]
- 14.Fawcett T An introduction to ROC analysis. Pattern Recognition Letters. 2006;27:861–874. [Google Scholar]
- 15.Pundlik S, Luo G. Dark adaptation measurement using a smartphone. In: Investigative Ophthalmology & Visual Science (Association for Research in Vision and Opthalmology Annual Meeing) 2018. [Google Scholar]
- 16.Danis RP, Domalpally A, Chew EY, et al. Methods and Reproducibility of Grading Optimized Digital Color Fundus Photographs in the Age-Related Eye Disease Study 2 (AREDS2 Report Number 2). Investigative Ophthalmology and Visual Science. 2013;54(7):4548–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. American Journal of Ophthalmology. 2001;132(5):668–681. [DOI] [PubMed] [Google Scholar]
- 18.AdaptDx. MacuLogix, Inc., Hummelstown, PA. In: User Manual; 2014. [Google Scholar]
- 19.Chiou W Critical evaluation of the potential error in pharmacokinetic studies of using the linear trapezoidal rule method for the calculation of the area under the plasma level-time curve. Journal of Pharmacokinetics and Biopharmaceutics. 1978;6(6):539–546. [DOI] [PubMed] [Google Scholar]
- 20.James G, Witten D, Hastie T, Tibshirani R. An Introduction to Statistical Learning: With Applications in R: Springer Publishing Company, Incorporated; 2014. [Google Scholar]
- 21.Jackson G, Edwrds J. A short-duration dark adaptation protocol for assessment of age-related maculopathy. Journal of Ocular Biology Disease and Informatics. 2008;1:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binns AM, Taylor DJ, Edwards LA, Crabb DP. Determining Optimal Test Parameters for Assessing Dark Adaptation in People With Intermediate Age-Related Macular Degeneration. Investigative Ophthalmology and Visual Science. 2018;59(4):AMD114–AMD121. [DOI] [PubMed] [Google Scholar]
- 23.Flynn OJ, Cukras CA, Jeffrey BG. Characterization of Rod Function Phenotypes Across a Range of Age-Related Macular Degeneration Severities and Subretinal Drusenoid Deposits. Investigative Ophthalmology and Visual Science. 2018;59(6):2411–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]


