Abstract
Synthesis of the Streptococcus pneumoniae type 3 capsule requires the pathway glucose-6-phosphate (Glc-6-P) → Glc-1-P → UDP-Glc → UDP-glucuronic acid (UDP-GlcUA) → (GlcUA-Glc)n. The UDP-Glc dehydrogenase and synthase necessary for the latter two steps, and essential for capsule production, are encoded by genes (cps3D and cps3S, respectively) located in the type 3 capsule locus. The phosphoglucomutase (PGM) and Glc-1-P uridylyltransferase activities necessary for the first two steps are derived largely through the actions of cellular enzymes. Homologues of these enzymes, encoded by cps3M and cps3U in the type 3 locus, are not required for capsule production. Here, we show that cps3M and cps3U also are not required for mouse virulence. In contrast, nonencapsulated isolates containing defined mutations in cps3D and cps3S were avirulent, as were reduced-capsule isolates containing mutations in pgm. Insertion mutants that lacked PGM activity were avirulent in both immunologically normal (BALB/cByJ) and immunodeficient (CBA/N) mice. In contrast, a mutant (JY1060) with reduced PGM activity was avirulent in the former but had only modestly reduced virulence in the latter. The high virulence in CBA/N mice was not due to the lack of antibodies to phosphocholine but reflected a growth environment distinct from that found in BALB/cByJ mice. The reduced PGM activity of JY1060 resulted in enhanced binding of complement and antibodies to surface antigens. However, decomplementation of BALB/cByJ mice did not enhance the virulence of this mutant. Suppressor mutations, only some of which resulted in increased capsule production, increased the virulence of JY1060 in BALB/cByJ mice. The results suggest that PGM plays a critical role in pneumococcal virulence by affecting multiple cellular pathways.
The polysaccharide capsule of Streptococcus pneumoniae is the single most important virulence factor of this organism. Although 90 distinct capsular polysaccharides have been identified (24), all share the common function of inhibiting complement-mediated phagocytosis of the organism (10, 45). The essential role of the capsule in S. pneumoniae virulence was established through the study of spontaneous mutants, the genetic transfer of capsular serotypes, and the use of a type 3-specific depolymerase to remove the capsule prior to infection (6, 22, 29). Genetic transfer of capsular serotypes indicated linkage of the genes necessary for synthesizing a specific capsular polysaccharide, and recombination experiments confirmed those linkages (4, 18, 37, 38). A role for unlinked genes was indicated by the transfer of the normal capsule phenotype from mutants that produced reduced levels of capsule (28). Recent studies have provided molecular details regarding both the linked genes contained in the capsule loci and the unlinked genes that are also necessary for capsule synthesis. Each of the capsule loci contains a central region of type-specific genes essential for the synthesis of a specific polysaccharide, as well as common, flanking sequences that encode functions involved in the synthesis of essentially all S. pneumoniae capsular polysaccharides (2, 11, 16, 17, 25, 27, 31–34, 36, 46). Many of the capsule genetic loci lack genes encoding the enzymes necessary for precursor sugar synthesis, further indicating a role for unlinked genes in capsule production (27, 31, 34). As described below, genes unlinked to the capsule locus and involved in production of the type 3 polysaccharide have been identified.
Type 3 S. pneumoniae represents one of the most frequently isolated serotypes among invasive pneumococcal strains (40). The type 3 capsule is a linear repeating unit of [3)-β-d-GlcUA-(1→4)-β-d-Glc-(1→]n. Four type 3-specific genes—cps3D, cps3S, cps3U, and cps3M–-are present in the type 3 capsule locus. They are flanked by the common sequences found in all serotypes, but in type 3 these sequences are mutated and are not required for capsule synthesis (2, 11, 16, 17, 48). cps3D encodes a UDP-Glc dehydrogenase that converts UDP-Glc to UDP-GlcUA (1, 16, 17). cps3S encodes the type 3 synthase, a processive enzyme that catalyzes the formation of all the glycosidic linkages necessary to synthesize the type 3 polymer from UDP-Glc and UDP-GlcUA (3, 12, 16, 19). Loss of either of these enzymatic activities results in the inability to synthesize the type 3 polysaccharide and, hence, the nonencapsulated phenotype (16). cps3U and cps3M encode a glucose-1-phosphate uridylyltransferase (Glc-1-P → UDP-Glc) and a protein with homology to phosphoglucomutases (PGMs) (Glc-6-P → Glc-1-P), respectively (11, 16, 17). Although both of these enzymatic functions are necessary for synthesis of precursors in the type 3 pathway, mutations in cps3U or cps3M do not alter capsule production (11, 16, 17). Cps3U has the predicted Glc-1-P uridylyltransferase activity (2), but Cps3M lacks the C terminus found in other PGMs, and no enzymatic activity has been demonstrated (11, 23). Despite the apparent lack of a requirement for cps3U and cps3M, these sequences are found in all type 3 strains thus far examined and are transcribed as part of the type 3 operon cps3DSUM-tnpA-plpA (11). Like cps3M, tnpA and plpA are only partial sequences and are not required for capsule production (11). In addition, insertion mutations that separate cps3UM-tnpA-plpA from cps3DS do not affect mouse virulence (26), indicating that the former also are not required for virulence or that they can be transcribed from promoters other than that upstream of cps3D.
Two genes, pgm and galU, encode most if not all of the PGM and Glc-1-P uridylyltransferase (GalU) activities, respectively, necessary for both the type 3 capsule synthesis pathway and other cellular pathways, including those leading to the teichoic acids (23, 30). Both genes are unlinked to the type 3 capsule locus, and loss of either results in near complete loss of capsule production, as well as growth defects. In contrast, point mutations that reduce but do not eliminate PGM activity can result in reduced capsule production without apparent growth defects. We previously described the PGM mutant JY1060, which contains a single amino acid change that results in approximately 25% of the parental levels of PGM activity and type 3 capsule but does not lead to obvious growth defects during laboratory culture (23). Repair of the point mutation in the JY1060 pgm restores capsule production to parental levels, indicating that this mutation is solely responsible for the mutant phenotype (23). In this report, we describe the effects of mutations throughout the type 3 locus on virulence and show that the cellular PGM plays a critical role in pneumococcal virulence.
MATERIALS AND METHODS
Bacteria and growth conditions.
The S. pneumoniae strains used in these studies are described in Table 1, Fig. 1, and Fig. 2. Insertion-duplication mutations were generated as previously described (47, 49). Restriction or PCR fragments of S. pneumoniae DNA were cloned into the suicide vector pJY4163 or pJY4164 (erythromycin resistance) to target the insertions. The clones were transformed into S. pneumoniae, and the presence of the insertions in erythromycin-resistant isolates was confirmed by Southern blot analysis, as previously described (23). Deletions were first constructed in Escherichia coli DH5α (5) by cloning restriction or PCR fragments that flanked the desired deletion. Clones contained in pJY4163 or pJY4164 were then transformed into S. pneumoniae without selection for the erythromycin resistance marker. Isolates in which deletions were generated as a result of allelic exchange were identified by PCR amplification of pools containing 10 colonies that had been suspended in 200 μl of H2O and lysed by boiling for 5 min. Primers flanking the expected deletion were used for amplification, and isolates containing deletions were identified from the appropriate pools. The deletions were further confirmed by Southern blot analysis. The Cps3D− mutants contain spontaneous point mutations that were localized initially by the ability of restriction fragments from the parent strain to restore full encapsulation and finally by sequencing of the appropriate restriction fragment from the mutant, as previously described (17).
TABLE 1.
S. pneumoniae strains
| Straina | Derivation and/or phenotypeb | Reference |
|---|---|---|
| AM124 | WU2 cps3M1; insertion-duplication mutant (independent isolate of MC1092 [11]); Emr Cps+ | This study |
| AM129 | Same as JD900; independent isolate | This study |
| AM130 | Same as JD879; independent isolate | This study |
| AM196 | WU2 ΔtnpA1; Cps+ | This study |
| AM198 | Same as AM196; independent isolate | This study |
| CV1031 | WU2 insertion-duplication mutation downstream of cps3U; Emr Cps+ | This study |
| CV1032 | Same as CV1031; independent isolate | This study |
| CV1034 | WU2 Δcps3UMtnpA1; Cps+ | This study |
| CV1035 | Same as CV1034; independent isolate | This study |
| CV1037 | WU2 Δcps3M2; Cps+ | This study |
| CV1040 | Same as JD879; vector in opposite orientation | This study |
| CV1044 | Same as CV1037; independent isolate | This study |
| CV1047 | WU2 Δcps3U2; Cps+ | This study |
| CV1048 | Same as CV1047; independent isolate | This study |
| CV1058 | WU2 ΔplpA2; Cps+ | This study |
| CV1059 | Same as CV1058; independent isolate | This study |
| GH4531 | WU2 pgm-2; insertion-duplication mutant; Emr Cpsr | 23 |
| GH4533 | Same as GH4531; independent isolate | 23 |
| GH5000 | JY1060 suppressor mutant isolated from i.p. mouse infection; Cpsr | This study |
| GH5001 | JY1060 suppressor mutant isolated from i.p. mouse infection; Cpsr | This study |
| GH5087 | JY1060 suppressor mutant isolated from transformation reaction; Cpsr | 23 |
| GH5088 | JY1060 repaired mutant; Cps+ PGM+ | 23 |
| GH5089 | JY1060 suppressor mutant isolated from transformation reaction; Cpsr | 23 |
| JD611 | WU2 cps3D1L310Z; Cps− | 16, 17 |
| JD619 | WU2 cps3D2E98Z; Cps− | 16, 17 |
| JD770 | WU2 insertion-duplication mutation downstream of cps3S; Emr Cps+ | 16, 17 |
| JD879 | WU2 insertion-duplication mutation downstream of cps3M; Emr Cps+ | 17 |
| JD900 | WU2 cps3U1; insertion-duplication mutant; Emr Cps+ | 16, 17 |
| JD902 | WU2 cps3S1; insertion-duplication mutant; Emr Cps− | 16 |
| JD908 | WU2 insertion-duplication mutation downstream of cps3D; Emr Cps− | 16, 17 |
| JY1060 | WU2 pgm-1K381T; Cpsr PGMr | 23 |
| MC1032 | WU2 plpA1; insertion-duplication mutant; Emr Cps+ | 11 |
| MC1033 | Same as MC1032; independent isolate | This study |
| MC1119 | WU2 insertion-duplication mutation downstream of tnpA; Emr Cps+ | 11 |
| MC1120 | Same as MC1119; independent isolate | 11 |
| MC1057 | Same as AM124; vector in opposite orientation | This study |
| WU2 | Type 3 parent; Cps+ | 9 |
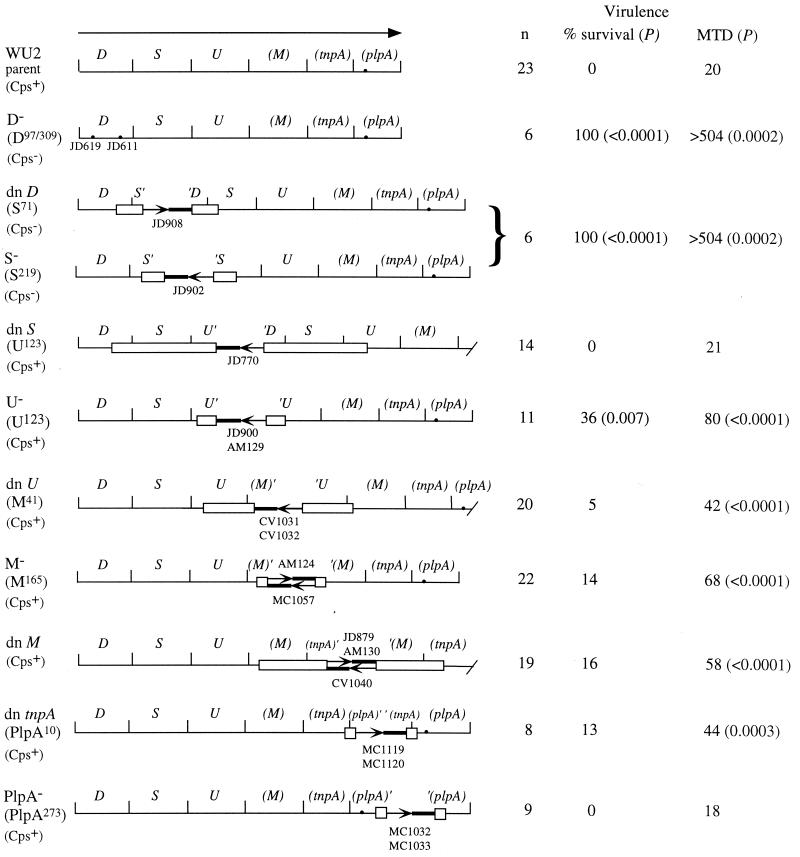
FIG. 1.
Virulence of type 3 point and insertion mutations in BALB/cByJ mice. The type 3 locus is shown at the top (11). The arrow indicates the direction and length of the only known transcript. Parentheses indicate naturally truncated sequences (cps3M at the 3′ end, plpA at the 5′ end, and tnpA at both the 5′ and 3′ ends). plpA also contains a point mutation (·) that causes a frameshift in the remaining partial open reading frame. The partial open reading frame for TnpA is opposite (i.e., right to left) that of all others in the locus. Maps show the structure of each mutant locus, with boxes indicating the region that was used to target insertion-duplication mutations. Arrows within each locus indicate the site of the plasmid insertion, with the orientation indicating the direction of a cat reporter sequence contained in the plasmid. Strain names are indicated above or below the appropriate arrows. Where more than one strain name is given, independent isolates were tested, with approximately equal numbers of mice used for each isolate. The maps are not drawn to scale, but the target fragments are sized proportionately. The amino acid positions of the insertions and effects of the mutations are shown to the left of each map. −, expected loss of function; dn, downstream insertion that results in an intact copy of all sequences. The D−, dn D, and S− mutants are nonencapsulated; all others produce parental amounts of capsule. For virulence studies, BALB/cByJ mice were infected i.v. with 107 bacteria (similar results were obtained with lower doses; the WU2 LD50 is approximately 3 × 105 CFU). The number of mice infected (n) is combined for independent isolates containing the same mutation, none of which differed from each other. The downstream D insertion is polar on S (16); thus, it and the S− mutant are considered together. P values were determined using Fisher's exact test to compare survival (alive versus dead) and the Mann-Whitney two-sample rank test to compare median times to death (MTD; in hours). A time to death of >504 h indicates survival. Bacteria isolated from dead mice retained the expected insertions, as determined by resistance to the antibiotic marker (erythromycin) carried on the plasmid insertion.
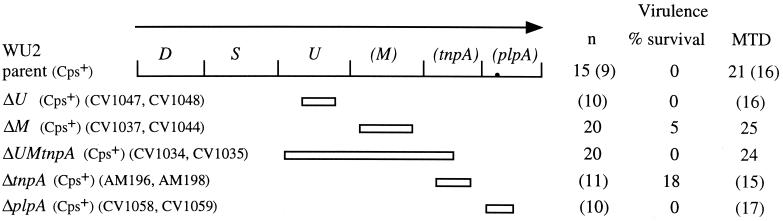
FIG. 2.
Virulence of type 3 deletion mutants in BALB/cByJ mice. The limits of each deletion are shown below the map. All mutants produced parental levels of capsule. Mice were infected i.v. with 107 bacteria. The number of mice infected (n) is combined for independent isolates of the same mutation (indicated by strain names at the left). None of the independent isolates differed from each other. Numbers in parentheses represent a set of experiments that was performed at a later time and that resulted in a shorter median time to death for the parent strain, WU2. The mutants are therefore compared to the WU2 control mice from the appropriate set of experiments. None of the mutants were statistically different from the parent with regard to survival (compared using Fisher's exact test) or median times to death (MTD; in hours; compared using the Mann-Whitney two-sample rank test).
S. pneumoniae strains were grown in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY) at 37°C or on blood agar base no. 2 supplemented with 3% sheep erythrocytes at 37°C in 5% CO2. Medium components and blood were from Difco (Detroit, Mich.) and Colorado Serum Company (Denver, Colo.), respectively. E. coli derivatives were grown in L broth or on L agar. Erythromycin was used at 0.3 μg/ml for S. pneumoniae and at 250 to 300 μg/ml for E. coli.
Mouse infections.
Virulence was assessed in 8- to 12-week-old BALB/cByJ and CBA/N (CBA/CaHN-Btkxid) female mice (Jackson Laboratories, Bar Harbor, Maine). Bacterial cultures were grown to approximately 3 × 108 CFU/ml in THY, serially diluted in lactated Ringer's solution, and injected either intraperitoneally (i.p.) or intravenously (i.v.) in a volume of 0.2 ml. Mice were subsequently monitored for 21 days. Bacteria were recovered from the hearts of dead mice to assess phenotype and test for the presence of the appropriate mutation. For blood clearance assays, mice infected i.v. were bled retro-orbitally and the samples were diluted and plated on blood agar medium to determine the number of CFU per milliliter of blood. Complement depletion was accomplished by i.p. injection of 12.5 U of cobra venom factor (Quidel Corp., San Diego, Calif.) 5 to 8 h prior to infection. C3 concentrations (measured as described below) were reduced to <3% of initial levels 4 h after cobra venom factor administration. This result is consistent with previous reports showing nearly undetectable levels of C3 by 4 h posttreatment and sustained reductions for up to 4 days (41).
Capsule determinations, antibody binding and complement fixation assays.
Capsule production was determined using either an inhibition enzyme-linked immunosorbent assay (ELISA), performed as previously described (11), the Stains-All assay for detecting acidic polysaccharides (39), or an indirect ELISA, performed as described below for the antibody binding assays. For all capsule assays, cultures were grown to a density of 3 × 108 CFU/ml in THY, and the amount of capsule produced was calculated from a standard curve generated using isolated type 3 polysaccharide (American Type Culture Collection).
Antibody binding to the bacterial surface was examined by indirect ELISA. Cultures, grown to 3 × 108 CFU/ml in THY, were heat killed for 20 min at 65°C and pelleted by centrifugation (14,000 × g, 20 min). The cells were suspended in the original culture volume of phosphate-buffered saline (PBS) (140 mM NaCl, 3 mM KCl, 5 mM Na2HPO4, 2 mM KH2PO4 [pH 7.4]), and all samples were adjusted to the same optical density at 600 nm (OD600). Duplicate columns of a Falcon flexible microtiter plate (Fisher Scientific, Pittsburgh, Pa.) were coated with 50 μl of the cell suspension and incubated overnight at 4°C. Plates were washed three times with 0.05% Tween 20 in PBS (PBST) and then blocked with 1% bovine serum albumin (BSA) in PBS for 1 h. Polyclonal antiserum or tissue culture supernatants containing monoclonal antibodies (MAbs) were twofold serially diluted down each column, starting at 1/5,000 for polyclonal antisera and 1/10 for MAbs. Plates were incubated for 45 min, washed three times with PBST, and then incubated with both a goat anti-rabbit (or anti-mouse for MAbs) immunoglobulin-biotin conjugate and a streptavidin-alkaline phosphatase conjugate (Southern Biotechnology, Birmingham, Ala.) at dilutions of 1:1,000 and 1:2,500, respectively. Plates were washed, developed with p-nitrophenyl phosphate (Sigma), and read at OD405. Except as noted, all incubations were at room temperature. Binding is expressed relative to that of JD611, a nonencapsulated type 3 derivative. Results are the means ± standard errors of the means (SEM) for three independent cultures of each strain. The anti-C polysaccharide (teichoic acid), anti-type 19-specific, and anti-type 23-specific polyclonal antisera were obtained from Statens Seruminstitut (Copenhagen, Denmark). MAbs specific for phosphocholine (PC) (140.1C2) and PspA (2A4) (14) were kindly provided by David Briles (University of Alabama at Birmingham). Type 3 capsule was measured using MAb 16.3 (9). Determination of the amount of capsule produced by WU2 and JY1060 using the indirect ELISA gave results comparable to those previously obtained using the inhibition ELISA and the Stains-All assay (23) (data not shown). Thus, the amount of capsule does not affect binding of the bacteria to the microtiter assay plate.
Complement (C3) binding was determined using the method of Gordon et al. (21) with some modifications. Cultures were grown to 3 × 108 CFU/ml in THY, pelleted by centrifugation, washed in one-half the original culture volume with gelatin Veronal buffer (0.1% gelatin, 1.8 mM sodium barbital, and 3.1 mM barbituric acid [pH 7.4]; Sigma), and resuspended in 180 μl of gelatin Veronal buffer per 1 ml of original culture. Each sample was divided in half and mixed with 10 μl of either pooled mouse serum or heat-inactivated (56°C, 30 min) mouse serum. Following incubation at 37°C for 30 min, samples were centrifuged and washed three times with an equal volume of PBS. Duplicate wells of microtiter plates were coated with 50 μl of washed cells/well, and plates were incubated overnight at 4°C. All subsequent incubations were done at room temperature. The plates were washed three times with PBST, blocked with 1% BSA–PBS for 1 h, and incubated with a 1/500 dilution of goat anti-mouse C3 conjugated to horseradish peroxidase (ICN Pharmaceuticals, Inc., Aurora, Ohio) in 1% BSA–PBS for 1 h. After three washes with PBST, the samples were developed with ABTS [2,2′-azino-di-(3-ethylbenzthiazoline sulfonic acid)] substrate, and the OD415 was read. A C3 standard curve was generated using the normal mouse serum amyloid P component standard from Calbiochem-Novabiochem Corp. (La Jolla, Calif.). Values for the S. pneumoniae samples were normalized to numbers of CFU per milliliter, which were determined at each step of the procedure. Results are expressed as the means ± SEM for five replicates and were compared using the unpaired Student's t test.
RESULTS
Virulence of type 3 mutants in immunologically normal mice.
S. pneumoniae strain JD770 (dn S in Fig. 1) contains an insertion-duplication mutation that separates cps3U, cps3M, tnpA, and plpA from cps3D and cps3S (16, 17). This strain is indistinguishable from its type 3 parent, WU2, in terms of capsule production and virulence in BALB/cByJ mice (26), suggesting that either the downstream sequences are not necessary for these phenotypes or they can be transcribed from promoters other than that located upstream of cps3D. To further examine the requirement in mouse virulence for genes in the type 3 locus, we used additional mutants containing insertion-duplication or point mutations, as shown in Fig. 1. The Cps3D and Cps3S mutants are nonencapsulated but can be restored to full encapsulation by repair of the mutations (16, 17) (data not shown). Isolates containing mutations affecting either cps3D or cps3S (D−, dn D, and S− in Fig. 1) were not, in the absence of reversion of the mutations, able to cause death by the i.p. or i.v. routes of infection (shown for the i.v. infections in Fig. 1). Revertants, isolated from mice that died following i.p. infection with high inocula of these mutants, were encapsulated and, in the case of cps3S mutants, erythromycin sensitive due to loss of the insertion plasmid.
Insertion mutations in the type 3 locus that do not inactivate cps3D or cps3S also do not alter capsule production under standard laboratory culture conditions (11, 16) (data not shown). Insertions located in the cps3UM-tnpA region (U−, dn U, M−, dn M, and dn tnpA in Fig. 1) did, however, result in extended times to death following i.v. infection, and insertions in cps3U (U− in Fig. 1) resulted in an increased overall survival (Fig. 1). No differences were observed for these mutants following i.p. infection (data not shown). The isolates containing downstream insertions (dn U, dn M, and dn tnpA in Fig. 1) were constructed as controls for polar effects of the insertions in cps3U, cps3M, and tnpA, respectively. Each of these constructs contained intact copies of all the genes in the type 3 locus, suggesting that the alterations in virulence observed with all of the insertion mutations in this region may have been the result of polar effects on downstream sequences. However, insertions in plpA (PlpA− in Fig. 1), the most downstream sequence in the operon, did not alter virulence. To eliminate any effects of inserted DNA, we examined mutants containing deletions of the cps3UM-tnpA-plpA region. As with the insertion mutations, deletions of these sequences did not alter capsule production (data not shown). In contrast to the insertion mutants, however, we observed no alterations in virulence with the deletion mutants (Fig. 2). Thus, the effects of the insertion mutations appear to be unrelated to any functions encoded by the disrupted genes. Although we do not know the reason for the effects of the insertions, alterations in transcript stability that affect Cps3D and Cps3S expression are a possible explanation.
Virulence of PGM mutants in immunologically normal mice.
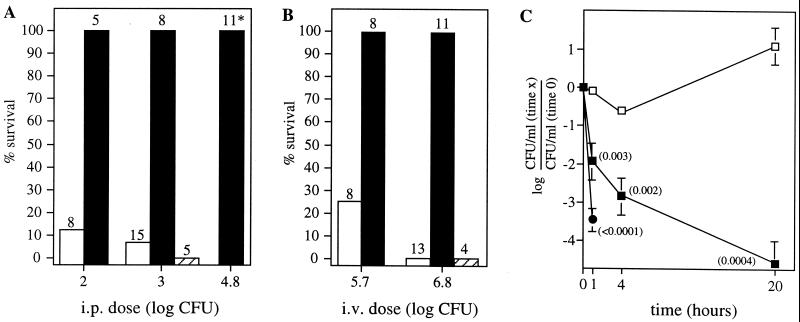
Our previous studies indicated that the majority of PGM activity necessary for capsule synthesis during laboratory culture is derived from the cellular PGM and not from Cps3M (23). The above results indicated that Cps3M function also is not required during infection in the mouse. To examine the requirement for the cellular PGM during virulence, BALB/cByJ mice were infected with either the type 3 WU2 parent or the JY1060 PGM mutant. The latter contains a single point mutation in pgm that results in production of 25% of the parental level of capsule (23). As shown in Fig. 3A and B, JY1060 was essentially avirulent by either the i.p. or i.v. route. Repair of the JY1060 pgm mutation, which restores parental levels of capsule production (23), also restored virulence (Fig. 3A and B, data for GH5088). Following i.v. infection, the number of JY1060 organisms remaining in the blood was rapidly reduced, although not to the extent observed with nonencapsulated strains (Fig. 3C). Mutants GH4531 and GH4533, in which pgm is insertionally inactivated, produce less than 10% of the parental level of capsule and exhibit severe growth defects during laboratory culture (23). They, too, were essentially avirulent in BALB/cByJ mice (survival of 88% [7 of 8] and 100% [4 of 4] at i.p. doses of 2 × 104 and 2 × 106 CFU, respectively; P < 0.002 compared with WU2 at a dose of 103 CFU). Bacteria recovered from the one mouse that died were fully encapsulated and erythromycin sensitive, indicating loss of the pgm insertion mutation.
FIG. 3.
Virulence of the PGM mutant JY1060 in BALB/cByJ mice. Mice were infected i.p. (A) or i.v. (B) with either the type 3 parent (WU2, □), the PGM mutant (JY1060, ▪), or the repaired PGM mutant (GH5088, ▨). The i.p. and i.v. LD50s for WU2 are approximately 50 and 3 × 105 CFU, respectively. The total number of mice infected at each dose is indicated above the bar. Results for GH5088 were not significantly different from those for WU2 by either route of infection but were different from those for JY1060 (P = 0.0008 [i.p.] and 0.0007 [i.v.]; compared using Fisher's exact test). JY1060 was significantly different from WU2 at all doses (i.p., P values were <0.005 [102], <0.0001 [103], and <0.0001 [6 × 104, compared to WU2 at 103]; i.v., P values were 0.007 [5 × 105] and <0.0001 [6 × 106]). At the i.p. dose of 6 × 104 CFU (*), 7 of 11 mice infected with JY1060 died. However, the bacteria recovered from these mice did not exhibit the small colony morphology characteristic of JY1060, and none of the mice were considered to have died from JY1060. (C) Blood clearance following i.v. infection with 6 × 106 bacteria. Mice were bled at 1 min (time zero), 1 h, 4 h, and 20 h postinfection. Results are the means ± SEM. P values (in parentheses) were determined using Student's t test by comparison to WU2 data at each time point. □, WU2 (n = 5); ▪, JY1060 (n = 3); ●, the nonencapsulated strains JD611 and JD908 combined (n = 6). The nonencapsulated strains were undetectable at 4 and 24 h.
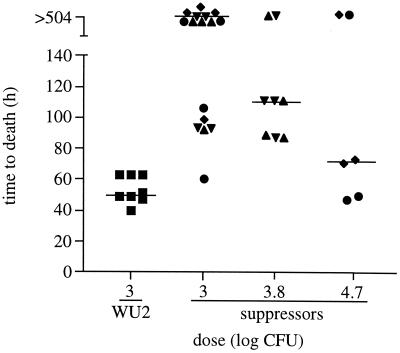
As noted in Fig. 3, infection of mice with high doses of JY1060 resulted in death of some of the animals. Isolation of bacteria from these animals yielded colonies that were intermediate in size between JY1060 and its type 3 parent, WU2. We previously found that second site suppressor mutations, at least some of which were located outside pgm, could restore capsule production to levels between that of JY1060 and WU2 (23). In i.p. infections, we tested the virulence of two of the previously characterized suppressor mutants (GH5087 and GH5089) and two of the isolates (GH5000 and GH5001) obtained from mice that died following i.p. infection with JY1060. Each of the suppressor mutants, except GH5000, produced an amount of capsule between that of JY1060 and WU2 (Table 2). The amount of capsule produced by GH5000 was not significantly different from that produced by JY1060 (Table 2). Nonetheless, the virulence of GH5000 was, like that of the other suppressor mutants, increased over that observed with JY1060. At doses of 1 × 103 to 5 × 104, an overall survival of 47% (n = 30) was observed with the suppressor mutants, whereas 100% survival (n = 19) was observed for JY1060-infected mice in this dose range (P < 0.0001; compare Fig. 3A and Fig. 4). The full level of parental virulence was not restored, however, as the suppressor mutants and the WU2 parent exhibited differences in overall survival and median times to death (Fig. 4). Suppressors with enhanced virulence were not identified using the pgm insertion mutants GH4531 and GH4533. As noted above, mice that succumbed to infection with these isolates had lost the insertion plasmid. In addition, suppressors that were identified as isolates with enhanced growth during laboratory culture did not have increased virulence (data not shown).
TABLE 2.
Capsule production
| Straina | Amt of capsule (n)b |
Pc
|
% WU2 | |
|---|---|---|---|---|
| WU2 | JY1060 | |||
| WU2 | 47.8 ± 1.2 (9) | <0.0001 | 100 | |
| JY1060 | 9.6 ± 1.2 (9) | <0.0001 | 20 | |
| GH5087 | 34.4 ± 1.4 (5) | <0.0001 | <0.0001 | 72 |
| GH5089 | 22 ± 0.5 (2) | <0.0001 | 0.0013 | 46 |
| GH5000 | 10.6 ± 0.45 (2) | <0.0001 | NS | 22 |
| GH5001 | 40.1 ± 2.9 (2) | 0.024 | <0.0001 | 84 |
Capsule production by WU2, JY1060, GH5087, and GH5089 is consistent with that previously reported (23).
Values are expressed in micrograms per milliliter of culture per OD600 of the starting culture and are means ± standard errors. Values were determined for washed cells using the Stains-All assay. n, number of independent samples tested.
P values were determined by comparison to WU2 or JY1060, as indicated, using a two-tailed, unpaired t test. NS, not significant.
FIG. 4.
Virulence of JY1060 suppressor mutants. BALB/cByJ mice were infected i.p. with WU2 (▪) or the suppressor mutants GH5000 (♦), GH5001 (●), GH5087 (▴), and GH5089 (▾). A time to death of >504 h indicates survival. Overall survival, compared using Fisher's exact test, was significantly different (P = 0.0064) for WU2 and the suppressors at the dose of 103 CFU. Median times to death (indicated by the bars and compared using the Mann-Whitney two-sample rank test) were significantly different at the doses of 1 × 103 and 6 × 103 CFU (P = 0.0002 for each, compared to the 103-CFU dose of WU2).
Virulence of PGM mutants in immunodeficient mice.
CBA/N mice express the X-linked immunodeficient (XID) phenotype, which is the counterpart of the human X-linked agammaglobulinemia (42). CBA/N mice respond poorly to polysaccharide antigens, including the capsular polysaccharides and PC components of the S. pneumoniae cell surface (44). Due in part to the lack of innate antibodies to PC, they are highly susceptible to infections with S. pneumoniae (8, 9). In contrast to the results obtained with the immunologically normal BALB/cByJ mice, the lethality of JY1060 in CBA/N mice was not significantly different from that observed with WU2. Median times to death were, however, extended for the mutant following both i.v. and i.p infection (Fig. 5). In contrast, the pgm insertion mutant GH4531 was avirulent in CBA/N mice (80% survival [8 of 10] at a dose of 105 CFU i.p.). Bacteria recovered from the two mice that died were fully encapsulated and erythromycin-sensitive, indicating loss of the pgm insertion mutation.
FIG. 5.
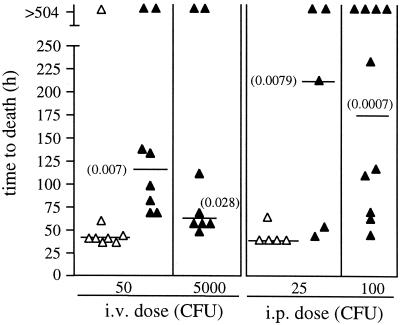
Virulence of JY1060 in XID mice. CBA/N mice were infected i.v. and i.p. at the indicated doses with either WU2 (▵) or JY1060 (▴). A time to death of >504 h indicates survival. Numbers in parentheses are P values for comparison of median time to death with WU2 at the lower dose, as determined using the Mann-Whitney two-sample rank test. There were no significant differences in overall survival.
Interaction of JY1060 with complement and antibodies to surface antigens.
In in vitro assays using serum from BALB/cByJ mice as a source of complement, approximately sevenfold more cell-bound C3 was detectable on the JY1060 mutant than on the WU2 type 3 parent (means ± SEM = 0.25 ± 0.074 versus 0.038 ± 0.013 fg of C3/CFU; P = 0.022). Identical results were obtained using serum from CBA/N mice, suggesting that the enhanced binding of (or accessibility to) C3 was not due to anti-PC-mediated complement activation.
Decomplementation of BALB/cByJ mice with cobra venom factor prior to infection reduced the numbers of both WU2 and JY1060 required for lethal i.v. infection (Fig. 6). Even in the absence of complement, however, the mutant exhibited only low virulence (50% lethal dose [LD50] of >105, compared to <103 for the parent [Fig. 6]).
FIG. 6.
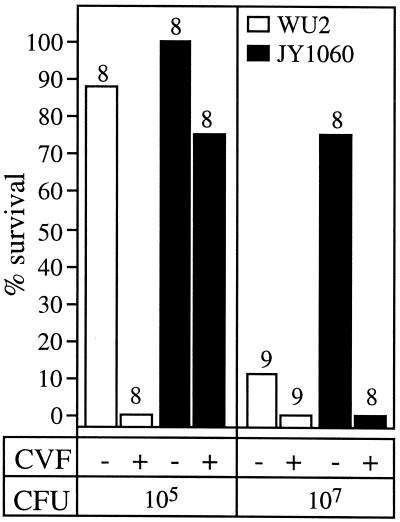
Effect of decomplementation on virulence. BALB/cByJ mice were infected i.v. with WU2 (□) or JY1060 (▪), 5 to 8 h after injection with cobra venom factor (CVF). The total number of mice infected at each dose is indicated above the bar. Statistical significance was calculated using Fisher's exact test. P values at the 105-CFU dose were 0.0014 (WU2 with CVF versus WU2 without CVF) and 0.007 (WU2 versus JY1060, with CVF). At the 107-CFU dose, P values were 0.007 (JY1060 with CVF versus JY1060 without CVF) and 0.015 (WU2 versus JY1060, without CVF). The LD50 of WU2 was reduced to <103 CFU in CVF-treated mice (data not shown).
To determine whether interactions with factors other than complement were altered in the mutant, the ability of antibodies to bind surface components was tested. As a measure of general surface exposure, bacteria were reacted with pneumococcal serotyping antiserum specific for capsule type 19 strains. Because whole cells are used as immunogens for generating these antisera, and because the type 19 polysaccharide itself is weakly immunogenic, the majority of antibodies in these sera are reactive with noncapsular surface components. The anti-type 19 polyclonal antiserum reacted with JD611, a nonencapsulated derivative of the type 3 WU2, at antiserum dilutions of (>2 × 105)-fold. As shown in Fig. 7, the presence of the type 3 capsule largely blocked this binding. A significant increase in antibody binding was observed for JY1060, compared to the type 3 parent. Similar results were obtained using the type 23-specific polyclonal antiserum (data not shown). We had anticipated that at least some of the increase in binding observed with the polyclonal antisera reacted with JY1060 would be due to antibodies to PC, C polysaccharide (teichoic acid), and PspA, three noncapsular immunodominant surface antigens. This did not prove to be the case, however, as JY1060 and the type 3 parent bound similarly low levels of antibodies specific for these cell surface components (Fig. 7). The identity of the surface antigens bound by the polyclonal antisera remains to be determined.
FIG. 7.
Binding of antibodies to surface components. Reactivity of antibodies with whole cells was tested using indirect ELISAs. Binding to WU2 and JY1060 is expressed relative to the nonencapsulated JD611. The anti-type 19 polyclonal antiserum contains a high titer of antibody to noncapsule components and reacts with JD611 at antiserum dilutions of (>2 × 105)-fold. It was used as a source of polyclonal antibody to S. pneumoniae surface antigens. Results are expressed as the means ± SEM (n = 3). Reactivity of WU2 and JY1060 with the anti-type 19 polyclonal antiserum was significantly different (P = 0.001, Student's t test). Binding of the other antibodies or antiserum to WU2 and JY1060 was not significantly different. TA, teichoic acid (C polysaccharide).
DISCUSSION
Although classic studies established an essential role for the capsule in pneumococcal virulence, the nature of mutations resulting in reduced capsule production and virulence has generally not been known. A more recent study used transposon mutagenesis to generate nonencapsulated mutants that proved to be avirulent (43). However, genetic linkage of the transposon to the nonencapsulated phenotype could not be demonstrated (43), and additional mutant phenotypes unrelated to the loss of capsule were later determined (35). As evidenced by recent studies of the genetic basis for capsule expression, capsule production can be altered by mutations that affect capsule-specific genes as well as genes involved in basic cellular processes (23, 30). Using isolates containing defined mutations, we have shown that nonencapsulated mutants altered only in capsule production are avirulent but that avirulence can also occur in strains reduced in capsule production due to alterations in the level of cellular PGM activity. At least some of the alteration in virulence observed with the JY1060 PGM mutant is likely due to the reduction in capsule. However, ongoing studies in our laboratory indicate that mutations affecting the type 3-specific genes and resulting in levels of capsule similar to that in JY1060 cause only modest reductions in virulence (A. D. Magee and J. Yother, submitted for publication). Thus, the avirulence of JY1060 is likely the result of multiple defects arising from reduced PGM activity that are manifested in the animal environment. Such defects may include additional alterations in growth, teichoic acid synthesis, and capsule synthesis that are not apparent during laboratory culture.
Several other observations also suggest that in vivo growth alterations affect the virulence of JY1060. In immunologically normal (BALB/cByJ) mice, JY1060 was avirulent but was cleared from the bloodstream at a lower rate than nonencapsulated strains. Although increased levels of accessible, surface-bound C3b could be responsible for the clearance of JY1060, its virulence was only modestly enhanced in decomplemented mice. Decomplementation should also minimize the protective effects of anti-PC, an important mediator of complement-dependent phagocytosis of S. pneumoniae (7). Thus, a lack of optimal anti-PC activity would appear to be of minimal significance in the host's ability to resist infection with JY1060. Yet JY1060 exhibited high virulence, with only a slight increase in the time required to kill, in the immunodeficient (XID) CBA/N mouse. Taken together, these results suggest that the environments encountered in BALB/cByJ and CBA/N mice are distinctly different in ways other than anti-PC levels. In the CBA/N mouse, the low level of PGM activity of JY1060 is adequate to allow bacterial survival and a high level of growth. In contrast, the lack of PGM activity that occurs in pgm insertion mutants renders S. pneumoniae completely avirulent in both animal environments. Other studies have also suggested that factors other than anti-PC contribute to the high susceptibility of CBA/N mice to pneumococcal infections. The non-XID CBA background has been shown to result in greater susceptibility to lethal intranasal infection with type 2 S. pneumoniae than the BALB background due to reduced neutrophil recruitment in the former (20). The CBA background is, however, only slightly more susceptible than the BALB background to i.v. infection with the type 3 WU2 strain used in our studies (8). Regardless of the basis for the difference, our results further highlight the facts that mouse strains can vary greatly in their susceptibility to pneumococcal infections and that strains of S. pneumoniae producing reduced levels of essential virulence factors resulting in avirulence in one host can be highly virulent in another.
JY1060 suppressor mutants with increased virulence in BALB/cByJ mice were selectively enriched during infection and under conditions that induce genetic competence (23). Three of four suppressor mutants examined produced increased amounts of capsule, compared to JY1060, though none had parental levels. The increase in virulence did not require an increase in capsule production, however, as the fourth suppressor mutant (GH5000) produced the same amount of capsule as JY1060. Thus, either the levels of capsule produced during laboratory culture are not reflective of those produced during infection, or the suppressor mutations compensate for other defects caused by reduced PGM activity. The failure to obtain suppressors with enhanced virulence when using the pgm insertion mutants suggests that most such mutations either occur within pgm or require some level of PGM activity (or the protein itself) to be effective. Determining the respective contributions of capsule and other factors to the virulence of the suppressor mutants awaits identification of the gene(s) affected by the suppressor mutations.
With the exception of type 3, none of the capsule loci thus far sequenced contains a PGM homologue (functional or otherwise) that could serve to provide additional Glc-1-P. The syntheses of all S. pneumoniae capsules require UDP-Glc and likely utilize cellular pools of Glc-1-P. Like JY1060 and the pgm insertion mutants, all strains would be expected to have reduced virulence as a result of alterations in PGM activity. A similar situation likely occurs in other streptococci, which also lack PGM homologues in their capsule loci (13, 15).
ACKNOWLEDGMENTS
This study was supported by Public Health Service grants AI28457, T32 AI07051, T32 HL07553, T32 AI07041, and T32 GM08111 from the National Institutes of Health and by the University of Alabama at Birmingham Comprehensive Minority Faculty and Student Development Program.
We thank Melanie Abeyta and Karita Ambrose for assistance with these studies.
REFERENCES
- 1.Arrecubieta C, Garcia E, Lopez R. Demonstration of UDP-glucose dehydrogenase activity in cell extracts of Escherichia coli expressing the pneumococcal cap3A gene required for the synthesis of type 3 capsular polysaccharide. J Bacteriol. 1996;178:2971–2974. doi: 10.1128/jb.178.10.2971-2974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrecubieta C, Garcia E, Lopez R. Sequence and transcriptional analysis of a DNA region involved in the production of capsular polysaccharide in Streptococcus pneumoniae type 3. Gene. 1995;167:1–7. doi: 10.1016/0378-1119(95)00657-5. [DOI] [PubMed] [Google Scholar]
- 3.Arrecubieta C, Lopez R, Garcia E. Type 3-specific synthase of Streptococcus pneumoniae (Cap3B) directs type 3 polysaccharide biosynthesis in Escherichia coli and in pneumococcal strains of different serotypes. J Exp Med. 1996;184:449–455. doi: 10.1084/jem.184.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austrian R, Bernheimer H P, Smith E E B, Mills G T. Simultaneous production of two capsular polysaccharides by pneumococcus. II. The genetic and biochemical basis of binary capsulation. J Exp Med. 1959;110:585–602. doi: 10.1084/jem.110.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1994. [Google Scholar]
- 6.Avery O T, Dubos R. The protective action of a specific enzyme against type III pneumococcus infection in mice. J Exp Med. 1931;54:73–89. doi: 10.1084/jem.54.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briles D E, Forman C, Horowitz J C, Volanakis J E, Benjamin W H, Jr, McDaniel L S, Eldridge J, Brooks J. Antipneumococcal effects of C-reactive protein and monoclonal antibodies to pneumococcal cell wall and capsular antigens. Infect Immun. 1989;57:1457–1464. doi: 10.1128/iai.57.5.1457-1464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briles D E, Horowitz J, McDaniel L S, Benjamin W H, Jr, Claflin J L, Booker C L, Scott G, Forman C. Genetic control of susceptibility to pneumococcal infection. Curr Top Microbiol Immunol. 1986;124:103–120. doi: 10.1007/978-3-642-70986-9_7. [DOI] [PubMed] [Google Scholar]
- 9.Briles D E, Nahm M, Schoroer K, Davie J, Baker P, Kearney J, Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown E J. Interaction of gram-positive organisms with complement. Curr Top Microbiol Immunol. 1985;121:159–187. doi: 10.1007/978-3-642-45604-6_8. [DOI] [PubMed] [Google Scholar]
- 11.Caimano M J, Hardy G G, Yother J. Capsule genetics in Streptococcus pneumoniae and a possible role for transposition in the generation of the type 3 locus. Microb Drug Resist. 1998;4:11–23. doi: 10.1089/mdr.1998.4.11. [DOI] [PubMed] [Google Scholar]
- 12.Cartee R T, Forsee W T, Schutzbach J S, Yother J. Mechanism of type 3 capsular polysaccharide synthesis in Streptococcus pneumoniae. J Biol Chem. 2000;275:3907–3914. doi: 10.1074/jbc.275.6.3907. [DOI] [PubMed] [Google Scholar]
- 13.Chaffin D, Beres S, Yim H, Rubens C. The serotype of type Ia and III group B streptococci is determined by the polymerase gene within the polycistronic capsule operon. J Bacteriol. 2000;182:4466–4477. doi: 10.1128/jb.182.16.4466-4477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crain J M, Waltman II W D, Turner J S, Yother J, Talkington D K, McDaniel L S, Gray B M, Briles D E. Pneumococcal surface protein A (PspA) is serologically variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990;58:3293–3299. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crater D L, Dougherty B A, van de Rijn I. Molecular characterization of hasC from an operon required for hyaluronic acid synthesis in group A streptococci. Demonstration of UDP-glucose pyrophosphorylase activity. J Biol Chem. 1995;270:28676–28680. doi: 10.1074/jbc.270.48.28676. [DOI] [PubMed] [Google Scholar]
- 16.Dillard J P, Vandersea M W, Yother J. Characterization of the cassette containing genes for type 3 capsular polysaccharide biosynthesis in Streptococcus pneumoniae. J Exp Med. 1995;181:973–983. doi: 10.1084/jem.181.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillard J P, Yother J. Genetic and molecular characterization of capsular polysaccharide biosynthesis in Streptococcus pneumoniae type 3. Mol Microbiol. 1994;12:959–972. doi: 10.1111/j.1365-2958.1994.tb01084.x. [DOI] [PubMed] [Google Scholar]
- 18.Ephrussi-Taylor H. Genetic aspects of transformations of pneumococci. Cold Spring Harbor Symp Quant Biol. 1951;16:445–456. doi: 10.1101/sqb.1951.016.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Forsee W T, Cartee R T, Yother J. Biosynthesis of type 3 capsular polysaccharide in Streptococcus pneumoniae: enzymatic chain release by an abortive translocation process. J Biol Chem. 2000;275:25972–25978. doi: 10.1074/jbc.M002613200. [DOI] [PubMed] [Google Scholar]
- 20.Gingles N A, Alexander J E, Kadioglu A, Andrew P W, Kerr A, Mitchell T J, Hopes E, Denny P, Brown S, Jones H B, Little S, Booth G C, McPheat W L. Role of genetic resistance in invasive pneumococcal infection: identification and study of susceptibility and resistance in inbred mouse strains. Infect Immun. 2001;69:426–434. doi: 10.1128/IAI.69.1.426-434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon D L, Rice J, Finley-Jones J J, McDonald P J, Hostetter M K. Analysis of C3 deposition and degradation on bacterial surfaces after opsonization. J Infect Dis. 1988;157:697–704. doi: 10.1093/infdis/157.4.697. [DOI] [PubMed] [Google Scholar]
- 22.Griffith F. The significance of pneumococcal types. J Hyg. 1928;27:113–159. doi: 10.1017/s0022172400031879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardy G G, Caimano M J, Yother J. Capsule biosynthesis and basic metabolism in Streptococcus pneumoniae are linked through the cellular phosphoglucomutase. J Bacteriol. 2000;182:1854–1863. doi: 10.1128/jb.182.7.1854-1863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995;33:2759–2762. doi: 10.1128/jcm.33.10.2759-2762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iannelli F, Pearce B J, Pozzi G. The type 2 capsule locus of Streptococcus pneumoniae. J Bacteriol. 1999;181:2652–2654. doi: 10.1128/jb.181.8.2652-2654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly T, Dillard J P, Yother J. Effect of genetic switching of capsular type on virulence of Streptococcus pneumoniae. Infect Immun. 1994;62:1813–1819. doi: 10.1128/iai.62.5.1813-1819.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolkman M A, Wakarchuk W, Nuijten P J, van der Zeijst B A. Capsular polysaccharide synthesis in Streptococcus pneumoniae serotype 14: molecular analysis of the complete cps locus and identification of genes encoding glycosyltransferases required for the biosynthesis of the tetrasaccharide subunit. Mol Microbiol. 1997;26:197–208. doi: 10.1046/j.1365-2958.1997.5791940.x. [DOI] [PubMed] [Google Scholar]
- 28.MacLeod C M, Krauss M R. Control by factors distinct from the S transforming principle of the amount of capsular polysaccharide produced by type III pneumococci. J Exp Med. 1956;97:767–771. doi: 10.1084/jem.97.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacLeod C M, Krauss M R. Relation of virulence of pneumococcal strains for mice to the quantity of capsular polysaccharide formed in vitro. J Exp Med. 1950;92:1–9. doi: 10.1084/jem.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mollerach M, Lopez R, Garcia E. Characterization of the galU gene of Streptococcus pneumoniae encoding a uridine diphosphoglucose pyrophosphorylase: a gene essential for capsular polysaccharide biosynthesis. J Exp Med. 1998;188:2047–2056. doi: 10.1084/jem.188.11.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morona J K, Morona R, Paton J C. Characterization of the locus encoding the Streptococcus pneumoniae type 19F capsular polysaccharide biosynthetic pathway. Mol Microbiol. 1997;23:751–763. doi: 10.1046/j.1365-2958.1997.2551624.x. [DOI] [PubMed] [Google Scholar]
- 32.Morona J K, Paton J C, Miller D C, Morona R. Tyrosine phosphorylation of CpsD negatively regulates capsular polysaccharide biosynthesis in Streptococcus pneumoniae. Mol Microbiol. 2000;35:1431–1442. doi: 10.1046/j.1365-2958.2000.01808.x. [DOI] [PubMed] [Google Scholar]
- 33.Muñoz R, Mollerach M, Lopez R, Garcia E. Characterization of the type 8 capsular gene cluster of Streptococcus pneumoniae. J Bacteriol. 1999;181:6214–6219. doi: 10.1128/jb.181.19.6214-6219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muñoz R, Mollerach M, Lopez R, Garcia E. Molecular organization of the genes required for the synthesis of type 1 polysaccharide of Streptococcus pneumoniae: formation of binary encapsulated pneumococci and identification of cryptic dTDP-rhamnose biosynthesis genes. Mol Microbiol. 1997;25:79–92. doi: 10.1046/j.1365-2958.1997.4341801.x. [DOI] [PubMed] [Google Scholar]
- 35.Neeleman C, Geelen S P, Aerts P C, Daha M R, Mollnes T E, Roord J J, Posthuma G, van Dijk H, Fleer A. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect Immun. 1999;67:4517–4524. doi: 10.1128/iai.67.9.4517-4524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramirez M, Tomasz A. Molecular characterization of the complete 23F capsular polysaccharide locus of Streptococcus pneumoniae. J Bacteriol. 1998;180:5273–5278. doi: 10.1128/jb.180.19.5273-5278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ravin A W. Linked mutations borne by deoxyribonucleic acid controlling the synthesis of capsular polysaccharide in pneumococcus. Genetics. 1960;45:1387–1403. doi: 10.1093/genetics/45.10.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravin A W. Reciprocal capsular transformations of pneumococci. J Bacteriol. 1959;77:296–309. doi: 10.1128/jb.77.3.296-309.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schrager H M, Rheinwald J G, Wessels M R. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J Clin Investig. 1996;98:1954–1958. doi: 10.1172/JCI118998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott J, Hall A, Dagan R, Dixon J, Eykyn S, Fenoll A, Hortal M, Jette L, Jorgensen J, Lamothe F, Latorre C, MacFarlane J, Shales D, Smart L, Taunay A. Serogroup-specific epidemiology of Streptococcus pneumoniae: association with age, sex and geography in 7,000 episodes of invasive disease. Clin Infect Dis. 1996;22:973–981. doi: 10.1093/clinids/22.6.973. [DOI] [PubMed] [Google Scholar]
- 41.Szalai A J, Briles D E, Volanakis J E. Role of complement in C-reactive-protein-mediated protection of mice from Streptococcus pneumoniae. Infect Immun. 1996;64:4850–4853. doi: 10.1128/iai.64.11.4850-4853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas J D, Sideras P, Smith C I, Vorechovsky I, Chapman V, Paul W E. Co-localization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science. 1993;261:355–358. doi: 10.1126/science.8332900. [DOI] [PubMed] [Google Scholar]
- 43.Watson D, Musher D M. Interruption of capsule production in Streptococcus pneumoniae serotype 3 by insertion of transposon Tn916. Infect Immun. 1990;58:3135–3138. doi: 10.1128/iai.58.9.3135-3138.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wicker L S, Scher I. X-linked immune deficiency (xid) of CBA/N mice. Curr Top Microbiol Immunol. 1986;124:87–101. doi: 10.1007/978-3-642-70986-9_6. [DOI] [PubMed] [Google Scholar]
- 45.Winkelstein J A. Complement and the host's defense against the pneumococcus. Crit Rev Microbiol. 1984;11:187–208. doi: 10.3109/10408418409105903. [DOI] [PubMed] [Google Scholar]
- 46.Yother J. Common themes in the genetics of streptococcal capsular polysaccharides. In: Goldberg J B, editor. Genetics of bacterial polysaccharides. Boca Raton, Fla: CRC Press; 1999. pp. 161–184. [Google Scholar]
- 47.Yother J. Genetics of Streptococcus pneumoniae. In: Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Gram-positive pathogens. Washington, D.C.: American Society for Microbiology; 2000. pp. 232–243. [Google Scholar]
- 48.Yother J, Ambrose K D, Caimano M J. Association of a partial H-rpt element with the type 3 capsule locus of Streptococcus pneumoniae. Mol Microbiol. 1997;25:201–204. doi: 10.1046/j.1365-2958.1997.4361798.x. [DOI] [PubMed] [Google Scholar]
- 49.Yother J, Handsome G L, Briles D E. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J Bacteriol. 1992;174:610–618. doi: 10.1128/jb.174.2.610-618.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]