Abstract
Several studies have suggested that the gut microbiota affect the health of the host. For example, the Firmicutes/Bacteroidetes (F/B) ratio and the proportion of Akkermansia muciniphila in the microbiota have been closely linked to obesity. In this study, we evaluated the effects of an anti-obesity lignan compound, arctigenin (AG), and burdock sprout extract (GSE), which contains AG, on the gut microbiota of an obese mouse model. C57BL/6J mice were fed high-fat, high-sucrose (HFHS) diets containing AG, GSE, or metformin (MF) for 8 weeks. The composition of the gut microbiota and the cecal content of short-chain fatty acids (SCFAs) were determined using 16S rRNA gene sequencing and high-performance liquid chromatography, respectively. Body weight gain was significantly suppressed in mice treated with AG, GSE, and MF. Analysis of the gut microbiota revealed that the F/B ratio was significantly reduced in the AG- and GSE-treated groups. Furthermore, the copy number of A. muciniphila in the feces was significantly increased in obese mice treated with AG and GSE. In addition, the amount of SCFAs (acetic, propionic, and butyric acids) in the cecal content and their fecal excretions were also significantly increased following AG and GSE treatment. Taken together, these results suggest that AG and GSE prevent obesity by improving the composition of the gut microbiota. Moreover, AG promoted the growth of A. muciniphila in vitro. Thus, AG and GSE may function as novel prebiotic supplements to ameliorate obesity, constipation, and intestinal disorders.
Keywords: arctigenin, obesity, Akkermansia muciniphila, gut microbiota, burdock sprout extract
INTRODUCTION
Recent evaluations have revealed that the gut microbiota affects the health status of the host, with dysregulation in this environment being linked to various disorders. Deterioration of the intestinal microenvironment due to irregularities in diet and lifestyle may induce the development of several lifestyle-related diseases, including chronic inflammatory conditions, impaired glucose tolerance, and obesity [1]. Several studies have reported the link between enterobacteria and obesity in humans and animals. Obese humans have higher Firmicutes/Bacteroidetes (F/B) ratios than healthy people [2], and women with normal rates of weight gain during pregnancy present with higher numbers of Akkermansia muciniphila than those with excessive weight gain during pregnancy [3]. One of the mechanisms by which the gut microbiota contributes to the improvement of obesity is through its metabolic effect; intestinal bacteria produce short-chain fatty acids (SCFAs), which bind to the short-chain fatty acid receptors GPR41 and GPR43, promote energy metabolism and suppress fat accumulation [4]. Therefore, it is likely that specific intestinal microbes may improve obesity by promoting the production of SCFAs.
A. muciniphila was discovered by Derrien et al. in 2004 and is classified as a gram-negative mucin-degrading bacterium belonging to the phylum Verrucomicrobia [5]. Its abundance is low in patients with diabetes and obesity, and it produces both acetic and propionic acids from the mucin of the intestinal mucosa and induces butyric acid production in butyric acid-producing bacteria [6]. These SCFAs contribute to maintaining the health of the host, and a recent study showed that the proportion of A. muciniphila increases in response to a ketogenic diet [7], starvation during fasting [8], and administration of metformin (MF), a therapeutic drug for diabetes [9]. In addition, animal experiments have shown that food-derived ingredients, such as cranberry extract, grape polyphenols, and apple procyanidins, exhibit anti-obesity effects by increasing the proliferation of A. muciniphila within the gut microbiome [10,11,12]. Thus, foods targeting the growth of A. muciniphila may be promising new prebiotic materials.
Arctigenin (AG) is a lignan compound found in burdock (Arctium lappa L.; Japanese name, gobo) [13], and it has various physiological activities, including anti-obesity [14], antidiabetic [15], anti-inflammatory [16], and antioxidant activities [17]. Of these activities, the mechanism underlying its anti-obesity effects has mainly been described in previous studies. AG treatment induces the activation of AMP-activated protein kinase (AMPK) and inhibits its downstream genes, acetyl-CoA carboxylase (ACC), peroxisome proliferator-activated receptor γ, and sterol regulatory element-binding transcription factor 1c (SREBP1c), and it is likely that the downregulation of SREBP1c enhances lipid β-oxidation and suppresses lipid synthesis [18]. However, changes in the gut microbiota after the administration of AG have not been studied. Burdock sprouts, which germinate from burdock seeds, contain approximately 10% AG and are useful as a raw material food for AG. Therefore, we designed this study to evaluate the effects of AG and a burdock sprout extract (GSE) on the gut microbiota of high-fat, high-sucrose (HFHS) diet-induced obese mice and on the growth of A. muciniphila in vitro with the aim of providing some evidentiary support for their use as prebiotic supplements.
MATERIALS AND METHODS
Materials
Powdered GSE was extracted from dried burdock sprouts using hydrated ethanol. The extract was found to contain 13.7% AG as quantified using high-performance liquid chromatography (HPLC). Pure AG (>95.0%) was then obtained from the GSE using the method described by Liu et al. [19]. GSE and AG were produced by our company, Kracie Holdings, Ltd. (GSE, lot no. 170927: AG, lot no. 190422). Metformin hydrochloride was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan).
Feed
Mice were fed a normal diet (ND) or HFHS diet based on their treatment group designation. The ND was based on the AIN-93G diet, and the HFHS feed, F2HFHSD, was manufactured by Oriental Yeast Co., Ltd. (Tokyo, Japan). The test diets containing AG (0.5%), GSE (5%), or MF (0.5%) were prepared using the HFHS feed as the base; their final compositions are described in Table 1.
Table 1. Feed compositions.
| Composition (%) | |||||
|---|---|---|---|---|---|
| Ingredients | ND | HFHS | AG | GSE | MF |
| Test article | - | - | 0.5 | 5 | 0.5 |
| Casein | 20 | 25 | 24.5 | 20 | 24.5 |
| α Corn starch | 13.2 | 14.869 | |||
| Sucrose | 10 | 20 | |||
| Corn starch | 39.749 | - | |||
| Beef tallow | - | 14 | |||
| Lard | - | 14 | |||
| Soybean oil | 7 | 2 | |||
| Powdered cellulose | 5 | 5 | |||
| AIN93 vitamin mix | 1 | 1 | |||
| AIN93G mineral mix | 3.5 | 3.5 | |||
| Choline bitartrate | 0.25 | 0.25 | |||
| t-Butylhydroquinone | 0.001 | 0.006 | |||
| L-Cystine | 0.3 | 0.375 | |||
| Calorie ratio | |||||
| Protein (%) | 19.0 | 18.1 | 17.8 | 15.1 | 17.8 |
| Fat (%) | 16.7 | 53.9 | 54.1 | 55.8 | 54.1 |
| NFE (%) | 64.3 | 28.0 | 28.1 | 29.1 | 28.1 |
| (kcal/100 g) | 377 | 475 | 473 | 473 | 474 |
ND: Normal Diet; HFHS: High-Fat High-Sucrose Diet; AG: HFHS + Arctigenin (0.5%); GSE: HFHS + Burdock Sprout Extract (5%); MF: HFHS + Metformin (0.5%); NFE: Nitrogen Free Extract.
Animal experiments
We purchased 30 five-week-old male C57BL/6J mice from Japan SLC, Inc. (Shizuoka, Japan) and then maintained them on a 12 hr light-dark cycle at 23 ± 3°C and 55 ± 15% humidity. The mice were fed the ND for 1 week and then randomly divided into five groups (n=6/group) based on body weight. The ND group (ND), HFHS group (HFHS diet), AG group (HFHS diet containing 0.5% AG), MF group (HFHS diet containing 0.5% MF), and GSE group (HFHS diet containing 5% GSE) were each fed a mixed feed ad libitum for 8 weeks. Body weight was measured once per week, and food intake was measured twice per week. We then evaluated fecal excretions from each animal for 24 hr in week 6, and fresh feces were collected in week 7. Finally, the liver, fat, and cecum were removed from mice under anesthesia in week 8. These experiments were approved by the Animal Experiment Ethics Committee of Kracie Holdings, Ltd. and were conducted in accordance with all relevant ethical guidelines.
Measurement of fecal excretions
During week 6 of this experiment, each group of mice was transferred to a wire mesh cage and observed for 24 hr while their excreted feces were collected. Once dried, their weights were measured, and the animals were returned to their original cages.
Analysis of the amplicons from fecal DNA
During week 7 of evaluation, the animals were again transferred to a wire mesh cage where fresh feces were collected and immediately frozen in liquid nitrogen before being stored at −80°C until use. Frozen feces were then crushed using a Precellys Evolution homogenizer (Bertin Instruments, Montigny-le-Bretonneux, France) and then subjected to DNA extraction and purification using a GENE PREP STAR PI-480 automatic DNA separator and NR-201 kit (Kurabo Industries, Osaka, Japan), as previously described [20]. The V3–V4 regions of the bacterial and archaeal 16S rRNA genes were amplified using prokaryote 16S rRNA primers (Table 2) and prepared for sequencing using the dual index method [20]. These tuned amplicons were then subjected to pair-end sequencing at 2 × 284 bp cycles using the MiSeq (Illumina, San Diego, CA, USA) platform and MiSeq Reagent Kit v3 (600 cycles). Paired-end reads were merged using the fastq-join program under the default settings, and the FASTX-Toolkit was used to extract sequences that met the following criterion: more than 99% of the sequence satisfied a quality value (QV) of 20 or higher. Any chimeric sequences were deleted using USEARCH 6.15, and the Ribosomal Database Project (RDP) Multiclassifier tool (http://rdp.cme.msu.edu/classifier/) [21] was then used to complete the sequence analysis. Bacterial and archaeal species were identified using the Metagenome@KIN ver. 2.2.1 analysis software (World Fusion, Tokyo, Japan) and the TechnoSuruga Lab microbial identification database (DB-BA13.0, TechnoSuruga Laboratory, Shizuoka, Japan). More than 97% of bacteria were extracted [22], and their combined amplicon sequences were analyzed using QIIME 2 (Quantitative Insights into Microbial Ecology version 2, http://qiime.org) ver. 2020.6 [23]. Beta diversity analysis was conducted using the core-metrics-phylogenetic plug-in, which evaluated the weighted UniFrac distances for each sample. Finally, the Emperor tool was used to visualize the principal coordinate analysis (PCoA). All amplicon sequence analyses, quantitative polymerase chain reactions (qPCRs), SCFA measurements, and A. muciniphila growth assays were performed by TechnoSuruga Laboratory Co., Ltd.
Table 2. Primer profiles.
| Target | Primer name | Oligonucleotide sequence (5′–3′) | Experiment |
|---|---|---|---|
| Prokaryote16S rRNA | Pro341F | AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGA-TCTCCTACGGG-AGGCAGCAGCCTACGGGNBGCASCAG | PCR for NGS |
| Pro805R | CAAGCAGAAGACGGCATACGAGATNNNNNNGTGACTGGAGTTCAGACGTGTGCT-CTTCCGATCTGACTACNVGGGTATCTAATCC | ||
| A. muciniphila | Akk-F | CAGCACGTGAAGGTGGGGAC | qPCR |
| Akk-R | CCTTGCGGTTGGCTTCAGAT | ||
PCR: polymerase chain reaction; NGS: next-generation sequencing; qPCR: quantitative polymerase chain reaction.
Quantification of A. muciniphila
The fecal samples used for 16S rRNA gene sequencing were subjected to qPCR to determine the abundance of A. muciniphila. The primers used for A. muciniphila are shown in Table 2, and ATCC® BAA-835TM was used as a standard. Abundance was calculated using 16S rDNA copy number measurements [24] and qPCR was performed on a Rotor-Gene Q quantitative thermal cycler (QIAGEN, Hilden, Germany) using TB Green Premix Ex Taq II (Tli RNaseH Plus, Takara Bio Inc., Kusatsu, Japan). Each reaction mixture (20 µL) contained 20 ng of extracted DNA and 0.2 μM of each primer. The cycling conditions were as follows: initial denaturation at 95°C for 30 sec, followed by 35 cycles at 95°C for 5 sec, 57°C for 20 sec, and 72°C for 20 sec.
SCFA analysis using cecal samples
The SCFA content was evaluated using the method of Mizuno et al. [25]. Briefly, approximately 100 mg of the cecal content was weighed in a sample tube containing zirconia beads, suspended in ultrapure water, and then heat treated at 85°C for 15 min. The sample was then crushed for 45 sec using a FastPrep-24 system (MP Biomedicals, Irvine, CA, USA) and then centrifuged (18,400 × g, 10 min). The supernatant was filtered through a 0.20 μm filter and then introduced into an organic acid analysis system (Prominence™ HPLC system, Shimadzu, Kyoto, Japan). This HPLC system was equipped with a CDD-10A thermal detector (Shimadzu, Kyoto, Japan), and a Shim-pack SCR-102H 300 mm × 8 mm ID column (two in series). We also used a Shim-pack SCR-102H 50 mm × 6 mm ID column as the guard column. We used 5 mM p-toluene sulfonic acid as the mobile phase, and the reaction solution consisted of 5 mM p-toluene sulfonic acid, 100 μM EDTA, and 20 mM Bis-Tris solution. The flow rate and oven temperature were set at 0.8 mL/min and 45°C, respectively.
In vitro A. muciniphila growth assay
A. muciniphila NBRC114322 was obtained from the National Institute of Technology and Evaluation Biological Resource Center (NBRC), Chiba, Japan. Mucin, from porcine stomach, was purchased from FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan. A. muciniphila was cultured under anaerobic conditions using the AnaeroPack system in 2.5 L anaerobic jars (Mitsubishi Gas Chemical Company, Inc., Tokyo, Japan). Once the AnaeroPack was placed in the anaerobic jars, the oxygen level was reduced to <1% in 30 min. Delaney and Onderdonk have shown that the AnaeroPack system is an excellent alternative to established methods for generating an environment for anaerobic incubation [26]. The bacterium was placed on agar plates containing GAM broth, modified “Nissui” (Nissui Pharmaceutical Co. Ltd., Tokyo, Japan), with 1.5% agar (FUJIFILM Wako Pure Chemical Corporation) and 0.1% mucin at 37°C for 48 hr. For experiments, colonies of A. muciniphila were suspended in basal medium and transferred into 10 mL test medium adjusted to 1 × 106 cells/mL in 20 mL glass vials with phenolic resin caps. The basal medium contained (L−1) 0.4 g KH2PO4, 0.53 g Na2HPO4, 0.3 g NH4Cl, 0.3 g NaCl, 0.1 g MgCl2·6H2O, 0.11 g CaCl2, 0.5 mg resazurin, 4 g NaHCO3, 0.25 g Na2S·9H2O, 1 mL alkaline trace element solution, 1 mL acid trace element solution, and 1 mL vitamin solution and was supplemented with 2% (w/v) peptone and yeast extract as described by Xia et al. [27]. The trace element and vitamin solutions were as described previously [28]. The test medium comprised 0.25% mucin or sugars, including N-acetyl-D(+)-glucosamine (FUJIFILM Wako Pure Chemical Corporation), N-acetyl-D-galactosamine (FUJIFILM Wako Pure Chemical Corporation), and glucose (each at 2 g L−1), added to the basal medium [5]. AG and GSE were prepared as filter-sterilized stock solutions and added to the test medium. AG was used as a supplement at 0, 0.1, 1, and 10 μM, and GSE was used at a final concentration of 0.27 µg/mL including 0.1 μM AG. The growth of A. muciniphila was determined spectrometrically by measuring the optical density (OD) at 600 nm (OD600) of samples collected at 0, 24, 48, and 72 hr of incubation.
Statistical analysis
All data are shown as the mean ± standard error of the mean (SEM), and all statistical analyses were performed using Statcel in Excel (3rd edition). Body weight gain curves were compared using a two-way repeated measures ANOVA, and other measurement data were processed using Dunnett’s multiple comparisons test. All results were considered statistically significant at p<0.05.
RESULTS
Body composition of obese mice
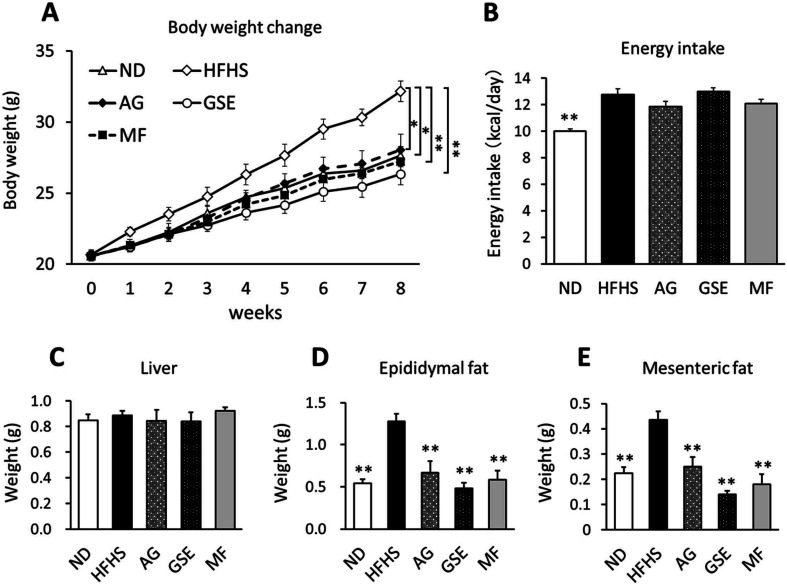
AG, GSE, and MF were administered to HFHS-induced obese mice for 8 weeks. Body weight gain was significantly suppressed in all test samples compared with that in the HFHS group (Fig. 1A). Importantly, the energy intake of the groups did not differ significantly (Fig. 1B). There was no significant difference in liver weight between the groups (Fig. 1C), and both epididymal fat and mesenteric fat were significantly reduced in the AG, GSE, and MF groups when compared with those in the HFHS control (Fig. 1D, 1E).
Fig. 1.
Effects of arctigenin (AG), burdock sprout extract (GSE), and metformin (MF) on the body composition of mice fed a high-fat, high-sucrose (HFHS) diet. Mice were fed a normal diet (ND), HFHS, HFHS + AG (0.5%), HFHS + GSE (5%), or HFHS + MF (0.5%) for 8 weeks. (A) Weight gain curves; (B) average energy intake; and (C) liver, (D) epididymal fat, and (E) mesenteric fat weights. Data are expressed as the mean ± SEM. *p<0.05 versus the HFHS group (n=6). **p<0.01 versus the HFHS group (n=6).
Fecal excretion, cecal weight, and SCFA content
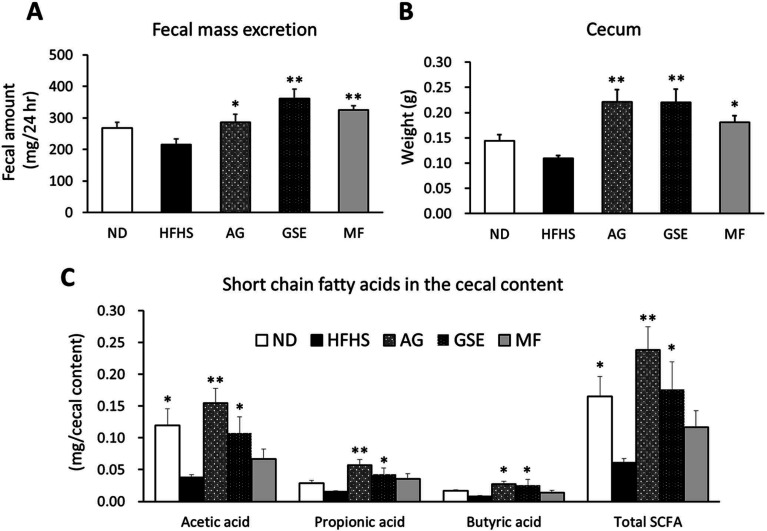
The AG, GSE, and MF groups demonstrated a significant increase in the amount of feces excreted in a 24 hr period (Fig. 2A) and an increased cecal weight at the time of dissection (Fig. 2B) compared with the HFHS group. This increase in cecal weight was primarily due to an increase in the cecal content (data not shown). In addition, acetic acid, propionic acid, and n-butyric acid in the cecal content were also significantly increased in the AG and GSE groups compared with those in the HFHS group (Fig. 2C). In the AG and GSE groups, the contents of acetic acid, propionic acid, and butyric acid were 4.10- and 2.85-fold, 3.77- and 2.83-fold, and 3.44- and 3.25-fold higher, respectively, than those in the HFHS group.
Fig. 2.
Effects of arctigenin (AG), burdock sprout extract (GSE), and metformin (MF) on fecal excretion and intestinal fermentation of mice fed a high-fat, high-sucrose (HFHS) diet. (A) Fecal weight over 24 hr in week 6 of feeding. (B) Cecal weight of mice. (C) The amount of short-chain fatty acids (SCFA) in the cecal content. Data are expressed as the mean ± SEM. *p<0.05 versus the HFHS group (n=6). **p<0.01 versus the HFHS group (n=6).
Changes in the gut microbiota
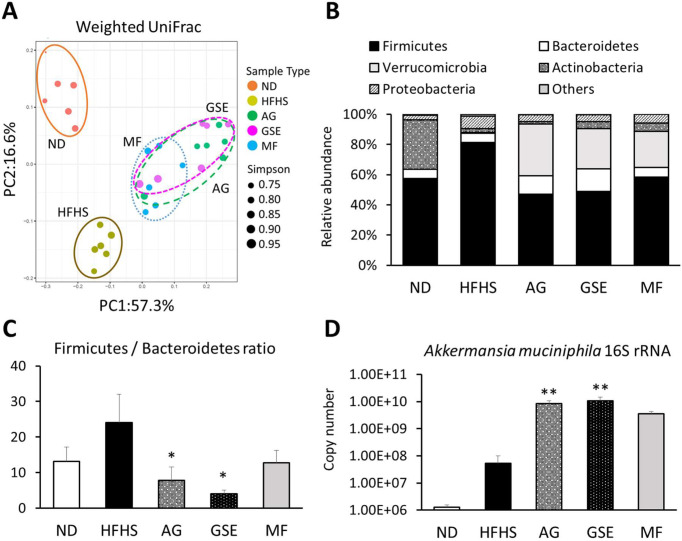
Based on the weighted UniFrac PCoA score plot of the gut microbiota, the first two components (PC1 and PC2) accounted for 73.9% of the cumulative contribution ratio. According to the PCoA, the data were roughly classified into three groups: the ND, HFHS, and sample administration groups (Fig. 3A). Among the sample administration groups, the intestinal floras in the AG and GSE groups were similar to that in the MF group. The composition ratio of Verrucomicrobia increased remarkably in the AG (34.4%), GSE (26.8%), and MF (23.9%) groups compared with that in the HFHS control (0.5%; Fig. 3B). Moreover, the F/B ratio was significantly lower in these groups (Fig. 3C). In addition, the qPCR analysis showed that the A. muciniphila copy number was significantly higher in the AG (156.8-fold) and GSE (195.1-fold) groups than in the control group (Fig. 3D). The other bacterial genera with AG and GSE composition ratios that more than doubled were Bacteroides, Parabacteroides, Parasutterella, Psychrosinus, Butyricicoccus, and Butyrivibrio.
Fig. 3.
Composition of the gut microbiota in obese mice. (A) Principal coordinate analysis (PCoA) for the weighted UniFrac distance in each of the microbial communities in this study. (B) Relative abundance of the notable bacterial phyla. (C) Ratio of the percentage of 16S rRNA gene sequences assigned to Firmicutes versus Bacteroidetes. (D) Absolute abundance of Akkermansia muciniphila in each fecal sample as quantified using qPCR. Data are expressed as the mean ± SEM. *p<0.05 versus the HFHS group (n=6). **p<0.01 versus the HFHS group (n=6).
Growth of A. muciniphila in vitro
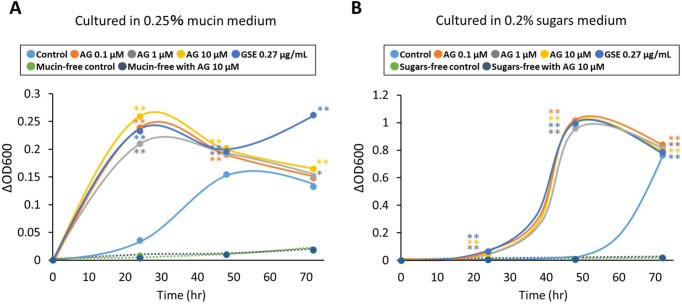
AG supplementation in the test medium, which contained mucin or sugars, significantly increased the change in OD600 compared with each test medium control at all time points after inoculation (Fig. 4A, 4B). In the absence of mucin or sugars in the medium, A. muciniphila failed to proliferate with AG supplementation alone following incubation for 24, 48, and 72 hr.
Fig. 4.
Growth of A. muciniphila in vitro. A. muciniphila was cultured in a medium containing (A) 0.25% mucin or (B) 0.2% sugars and supplemented with 0.1, 1, and 10 μM AG or 0.27 µg/mL GSE including 0.1 μM AG. The OD600 was measured at 0, 24, 48, and 72 hr after inoculation. Data represent the mean change in OD600 of three wells. *p<0.05 versus the control. **p<0.01 versus the control.
DISCUSSION
The lignan compound, AG, has been shown to suppress weight gain and fat accumulation in high-fat diet-induced obese mice [14]. Similarly, we demonstrated that AG and GSE, which contains some AG, exerted anti-obesity effects in HFHS-fed mice. We also revealed that these anti-obesity effects are likely due to an alteration in the gut microbiota and a commensurate increase in SCFA production.
Quantification of A. muciniphila using qPCR revealed that both AG and GSE significantly increased the copy number of A. muciniphila in obese mice. Moreover, they also induced acetic acid, propionic acid, and n-butyric acid production in the cecum. These results indicate that the increased acetic acid and propionic acid production in the AG- and GSE-treated mice is likely because of an increased amount of A. muciniphila, and the increased butyric acid level is considered to be associated with an increase in the composition ratios of n-butyric acid-producing bacteria, Butyricicoccus and Butyrivibrio. In addition, AG and GSE decreased the F/B ratio. The energy uptake efficiency of Firmicutes in the intestinal tract is higher than that of Bacteroidetes, and it has been reported that an increase in the F/B ratio induces obesity [29]. Therefore, it is possible that the anti-obesity effects of both AG and GSE may be aided by their normalization of the F/B ratio in HFHS diet-fed mice. The AG- and GSE-treated mice had similar microbial community diversities, suggesting that the AG content in the GSE is closely associated with its effects on the intestinal flora.
A. muciniphila not only induces SCFA production but also enhances the integrity of intestinal epithelial cells and the thickness of the mucus layer, thereby promoting intestinal health [30]. AG has also been reported to improve intestinal barrier function in a murine model of inflammatory bowel disease [31]. This effect may be due to an increase in the amount of A. muciniphila as observed in our study. In addition, we revealed that AG directly promoted the growth of A. muciniphila in the presence of mucin or a limited number of sugars, including N-acetylglucosamine, N-acetylgalactosamine, and glucose. Polyphenols, such as epigallocatechin-3-gallate, have been reported to induce the growth of A. muciniphila via co-metabolism with mucin or glucose [27]. AG may have also accelerated the nutrient metabolism in A. muciniphila and adjusted the growth stage from the lag phase to the log phase. The mechanism underlying this growth-stimulating action of AG requires further investigation. Thus, AG is expected to maintain intestinal health through an increase in the amount of A. muciniphila.
The administration of AG and GSE increased the cecal content and 24 hr fecal volume in obese mice. The ingestion of prebiotics, such as inulin and guar gum degradation products, is known to promote intestinal fermentation and increase fecal excretion [32, 33]. This action is associated with improved intestinal smooth muscle contraction and peristaltic activation induced by the SCFAs produced by the intestinal microbiota [34, 35]. Therefore, we suggest that the defecation-promoting effect of AG and GSE is the result of the increased SCFA content in the cecum. This effect was not limited to the GSE treatment group but was also clearly observed when using AG alone. This suggests that AG can act directly on the intestine to improve intestinal function. In addition, a previous pharmacokinetic study revealed that orally administered AG presented the highest distribution in the intestine in rats [36]. Taken together, these findings suggest that AG and GSE may also be useful prebiotic agents for improving bowel movement.
In summary, AG and AG-containing GSE prevent obesity by inducing changes in the gut microbiota, that is, inducing an increase in the amount of A. muciniphila and a decrease in the F/B ratio. Moreover, these materials also upregulate SCFA production in the intestine and promote fecal excretion. These effects are likely to be a direct result of AG. Therefore, AG should be evaluated as a novel prebiotic for improving obesity, constipation, and intestinal health; GSE is likely a useful raw food material for AG consumption.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
REFERENCES
- 1.Hartstra AV, Bouter KE, Bäckhed F, Nieuwdorp M. 2015. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care 38: 159–165. [DOI] [PubMed] [Google Scholar]
- 2.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. 2006. Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023. [DOI] [PubMed] [Google Scholar]
- 3.Santacruz A, Collado MC, García-Valdés L, Segura MT, Martín-Lagos JA, Anjos T, Martí-Romero M, Lopez RM, Florido J, Campoy C, Sanz Y. 2010. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr 104: 83–92. [DOI] [PubMed] [Google Scholar]
- 4.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. 2015. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 7: 2839–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derrien M, Vaughan EE, Plugge CM, de Vos WM. 2004. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 54: 1469–1476. [DOI] [PubMed] [Google Scholar]
- 6.Chia LW, Hornung BVH, Aalvink S, Schaap PJ, de Vos WM, Knol J, Belzer C. 2018. Deciphering the trophic interaction between Akkermansia muciniphila and the butyrogenic gut commensal Anaerostipes caccae using a metatranscriptomic approach. Antonie van Leeuwenhoek 111: 859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. 2018. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173: 1728–1741.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng X, Zhou K, Zhang Y, Han X, Zhao A, Liu J, Qu C, Ge K, Huang F, Hernandez B, Yu H, Panee J, Chen T, Jia W, Jia W. 2018. Food withdrawal alters the gut microbiota and metabolome in mice. FASEB J 32: 4878–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, Escobar JS. 2017. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty Acid-Producing microbiota in the gut. Diabetes Care 40: 54–62. [DOI] [PubMed] [Google Scholar]
- 10.Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy E, Marette A. 2015. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64: 872–883. [DOI] [PubMed] [Google Scholar]
- 11.Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ, Raskin I. 2015. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes 64: 2847–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masumoto S, Terao A, Yamamoto Y, Mukai T, Miura T, Shoji T. 2016. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci Rep 6: 31208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinoda J, Kawagoye M. 1929. Über die Bestandteile des Arctium lappa L. Yakugaku Zasshi 49: 565–575 (in Japanese). [Google Scholar]
- 14.Han YH, Kee JY, Park J, Kim HL, Jeong MY, Kim DS, Jeon YD, Jung Y, Youn DH, Kang J, So HS, Park R, Lee JH, Shin S, Kim SJ, Um JY, Hong SH. 2016. Arctigenin inhibits adipogenesis by inducing AMPK activation and reduces weight gain in high-fat diet-induced obese mice. J Cell Biochem 117: 2067–2077. [DOI] [PubMed] [Google Scholar]
- 15.Huang SL, Yu RT, Gong J, Feng Y, Dai YL, Hu F, Hu YH, Tao YD, Leng Y. 2012. Arctigenin, a natural compound, activates AMP-activated protein kinase via inhibition of mitochondria complex I and ameliorates metabolic disorders in ob/ob mice. Diabetologia 55: 1469–1481. [DOI] [PubMed] [Google Scholar]
- 16.Hyam SR, Lee IA, Gu W, Kim KA, Jeong JJ, Jang SE, Han MJ, Kim DH. 2013. Arctigenin ameliorates inflammation in vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing M1 macrophages to M2-like macrophages. Eur J Pharmacol 708: 21–29. [DOI] [PubMed] [Google Scholar]
- 17.Wu RM, Sun YY, Zhou TT, Zhu ZY, Zhuang JJ, Tang X, Chen J, Hu LH, Shen X. 2014. Arctigenin enhances swimming endurance of sedentary rats partially by regulation of antioxidant pathways. Acta Pharmacol Sin 35: 1274–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Y, Li X, Liu Y, Hu Y, Yang R. 2019. Arctigenin improves lipid metabolism by regulating AMP-activated protein kinase and downstream signaling pathways. J Cell Biochem 120: 13275–13288. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Chen K, Schliemann W, Strack D. 2005. Isolation and identification of arctiin and arctigenin in leaves of burdock (Arctium lappa L.) by polyamide column chromatography in combination with HPLC-ESI/MS. Phytochem Anal 16: 86–89. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi S, Tomita J, Nishioka K, Hisada T, Nishijima M. 2014. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS One 9: e105592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73: 5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasai C, Sugimoto K, Moritani I, Tanaka J, Oya Y, Inoue H, Tameda M, Shiraki K, Ito M, Takei Y, Takase K. 2015. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol 15: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu YX, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS, 2nd, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vázquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37: 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. 2013. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110: 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizuno H, Bamba S, Abe N, Sasaki M. 2020. Effects of an alginate-containing variable-viscosity enteral nutrition formula on defecation, intestinal microbiota, and short-chain fatty acid production. J Funct Foods 67: 103852. [Google Scholar]
- 26.Delaney ML, Onderdonk AB. 1997. Evaluation of the AnaeroPack system for growth of clinically significant anaerobes. J Clin Microbiol 35: 558–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia Y, Zhang X, Jiang M, Zhang H, Wang Y, Zhang Y, Seviour R, Kong Y. 2021. In vitro co-metabolism of epigallocatechin-3-gallate (EGCG) by the mucin-degrading bacterium Akkermansia muciniphila. PLoS One 16: e0260757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stams AJ, Van Dijk JB, Dijkema C, Plugge CM. 1993. Growth of syntrophic propionate-oxidizing bacteria with fumarate in the absence of methanogenic bacteria. Appl Environ Microbiol 59: 1114–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krajmalnik-Brown R, Ilhan ZE, Kang DW, DiBaise JK. 2012. Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract 27: 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, de Vos WM, Satokari R. 2015. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol 81: 3655–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao Y, Yue M, Lv C, Yun X, Qiao S, Fang Y, Wei Z, Xia Y, Dai Y. 2020. Pharmacological activation of ERβ by arctigenin maintains the integrity of intestinal epithelial barrier in inflammatory bowel diseases. FASEB J 34: 3069–3090. [DOI] [PubMed] [Google Scholar]
- 32.Koeda T, Hara K, Wada T, Morita T, Arai E. 2015. Effect of inulin containing rice bread on bowel habit in Japanese young healthy adult. Jpn Pharmacol Ther 43: 1731–1737. [Google Scholar]
- 33.Polymeros D, Beintaris I, Gaglia A, Karamanolis G, Papanikolaou IS, Dimitriadis G, Triantafyllou K. 2014. Partially hydrolyzed guar gum accelerates colonic transit time and improves symptoms in adults with chronic constipation. Dig Dis Sci 59: 2207–2214. [DOI] [PubMed] [Google Scholar]
- 34.Mcmanus CM, Michel KE, Simon DM, Washabau RJ. 2002. Effect of short-chain fatty acids on contraction of smooth muscle in the canine colon. Am J Vet Res 63: 295–300. [DOI] [PubMed] [Google Scholar]
- 35.Kamath PS, Phillips SF, Zinsmeister AR. 1988. Short-chain fatty acids stimulate ileal motility in humans. Gastroenterology 95: 1496–1502. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Li X, Ren YS, Lv YY, Zhang JS, Xu XL, Wang XZ, Yao JC, Zhang GM, Liu Z. 2017. Elucidation of arctigenin pharmacokinetics and tissue distribution after intravenous, oral, hypodermic and sublingual administration in rats and beagle dogs: Integration of in vitro and in vivo findings. Front Pharmacol 8: 376. [DOI] [PMC free article] [PubMed] [Google Scholar]