Abstract
The relationships between various diseases and the human gut microbiota (GM) have been revealed. However, the relationships between the human abdominal aortic aneurysm (AAA) and GM remains unknown. The aim of this cross-sectional study was to clarify the association between the human AAA and GM. Stool samples from 30 consecutive patients with AAA before aneurysm repair and those of 30 controls without vascular diseases were analyzed by 16S rRNA gene (V3–4) sequencing using an Illumina MiSeq system and QIIME 2. There was no significant difference in age (75 vs. 75 years) or gender (80% vs. 87% males) between the groups. No significant difference in GM composition was observed in principal coordinate analysis between the two groups, whereas the AAA group showed a significantly lower abundance of Bifidobacterium adolescentis (p<0.01) at the species level than the controls. This study demonstrated that the abundance of B. adolescentis decreased in patients with AAA. This is the first study to show the characteristics of the GM in patients with AAA. Studies are needed to reveal if causal relationships exists between the human AAA and GM.
Keywords: abdominal aortic aneurysm, gut microbiota, Bifidobacterium adolescentis
INTRODUCTION
Abdominal aortic aneurysm (AAA) is a fatal disease in which the abdominal aorta gradually and asymptomatically expands and eventually ruptures. The cause is unknown, and surgery has been the only effective treatment to date. Smoking, male gender, older age, family history of aneurysms, hypertension, and mural thrombosis are associated with aneurysm enlargement. AAA is less prevalent in Hispanic and Asian populations. Diabetes, antibiotic use, and β-blockers are considered to inhibit aneurysm expansion. However, the mechanism by which each of these factors is involved in aneurysm enlargement is unknown [1, 2]. Randomized controlled trials to ascertain the prevention of aneurysm expansion and rupture using antibiotics, such as doxycycline and roxithromycin, and other drugs, such as propranolol and metformin, have been conducted, but there is currently no effective drug treatment for AAA [3, 4]. Research on the pathophysiology of AAA and drug therapy for it has not progressed because there are no animal models that can replicate arteriosclerosis in humans. The AAA animal models reported to date are known to be pathologically and morphologically different from human models of AAA [5]. Therefore, it is necessary to elucidate the pathophysiology of AAA and develop a targeted drug treatment that uses an approach that is different from those used in conventional research.
In total, hundreds of species and 40 trillion microorganisms exist in the gut microbiota (GM) of the human intestinal tract, and they exert various effects on the host directly and indirectly through their metabolites [6,7,8,9]. Since the bacterial 16S ribosomal ribonucleic acid (rRNA) gene analysis method was developed in the 2000s, the relationship between the GM and various diseases has been revealed [10]. Although associations between the GM and cardiovascular diseases, such as hypertension, heart failure, arteriosclerosis, and coronary artery disease, have been reported, nothing is known about associations between the human AAA and GM [11]. Because there are many differences between animal AAA models and humans with AAA, the pathophysiology of AAA remains unclear. Therefore, surgery remains the only treatment option for AAA, as drug treatments are not available [12, 13]. To verify the relationships between the human AAA and GM, we analyzed the fecal GM of patients with AAA based on 16S rRNA genes.
MATERIALS AND METHODS
Patients and controls
Fecal samples were prospectively collected from patients with AAA when they were admitted for surgery at the Jikei University Kashiwa Hospital between May 2019 and May 2020. For the control group, fecal samples were obtained from patients who were 65 years of age or older and did not have an aortic aneurysm or peripheral vascular disease. The controls were examined by blood test, electrocardiogram, ankle-brachial pressure index test, and chest-abdominal CT scan to rule out vascular diseases, such as aortic diseases and peripheral artery disease. All samples were collected under their regular diets. Interviews, physical examinations, plain chest and abdominal computed tomography, and an ankle-brachial pressure index test were performed to exclude aneurysms and peripheral arterial diseases. Patients who had received antibiotics within 3 months of the study were excluded.
Sample preparation and microbiota analysis
The collected fecal samples were stored at −80°C. RNA was extracted from their fecal samples, purified using an RNeasy Mini Kit (Qiagen N.V., Venlo, the Netherlands) and was synthesized using a QuantiTect Reverse Transcription Kit (Qiagen N.V.) according to the manufacturer’s instructions, followed by 16S rRNA gene sequencing. PCR amplification and DNA sequencing of the V3–V4 region of the bacterial 16S rRNA gene was performed using an Illumina MiSeq instrument (Illumina, San Diego, CA, USA), as previously described [14]. After removing the sequences consistent with the data from Genome Reference Consortium human build 38 (GRCh38) and the phiX reads from the raw Illumina paired-end reads, the sequences were analyzed using the QIIME 2 software package (version 2017.10). Potential chimeric sequences were removed using DADA2, and 30 and 90 bases were trimmed from the 30 regions of the forward and reverse reads, respectively [15]. UniFrac distances between subjects and four alpha diversity scores (phylogenetic diversity whole tree, Chao1, number of observed species, and Shannon index) were estimated using QIIME 2, as previously described [16].
Statistical analysis
Statistical analyses were performed using EZR ver. 1.50 (Kanda, 2013) or R ver. 3.6.0. Continuous and discontinuous results are expressed as the mean ± standard deviation and median (interquartile range), respectively, unless otherwise stated. A p-value less than 0.05 was considered significant. To assess clinical characteristics among patients with different etiologies, the χ2 test was used to compare the frequency of categorical variables, and the unpaired t-test was used to compare continuous variables. Differences in GM profiles between the groups were analyzed by principal coordinate analysis (PCoA) complemented by a permutational multivariate analysis of variance (PERMANOVA) test for UniFrac distances. After filtering the taxa with a median of 0.5% or more in each group as common taxa in this study, the intergroup differences of the common genera/species in the GM were analyzed by Mann–Whitney U test with the Benjamini–Hochberg procedure for multiple testing to control the false discovery rate [17].
Ethical considerations
The study protocol conformed to the ethical principles of the World Medical Association Declaration of Helsinki, was approved by the Jikei University Institutional Review Board (study number, 30-082(9103)), and was registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN000038284). All study participants provided written informed consent for the use of their samples in the study.
RESULTS
Patient characteristics
Thirty cases were included in both the AAA and control groups. No difference was observed in gender or age (Table 1). The mean aneurysm diameter in the AAA group was 52 mm. Univariate analyses indicated significant differences between the two groups in body mass index (p=0.030) and chronic kidney disease (p=0.012).
Table 1. Comparing the characteristics of patients with abdominal aortic aneurysm (AAA) with those of controls.
| Control | Abdominal aortic aneurysm | p-value | |
|---|---|---|---|
| Mean (standard deviation) or Number (%) | |||
| Number | 30 | 30 | |
| Age (years) | 75 (4.7) | 75 (7.0) | 0.865b |
| Male | 26 (87%) | 24 (80%) | 0.488a |
| Body mass index | 23 (2.1) | 24 (3.6) | 0.030b |
| Current smoker | 4 (14%) | 8 (27%) | 0.219a |
| Diabetes | 4 (13%) | 4 (13%) | 1.000a |
| Hypertension | 18 (60%) | 22 (73%) | 0.273a |
| Dyslipidemia | 23 (77%) | 28 (93%) | 0.071a |
| Coronary artery disease | 4 (13%) | 7 (23%) | 0.317a |
| Chronic kidney disease | 5 (17%) | 14 (47%) | 0.012a |
| Medication | |||
| Calcium channel blocker | 14 (47%) | 18 (60%) | 0.301a |
| Anti-platelet therapy | 5 (17%) | 5 (17%) | 1.000a |
| Gastro-restraint | 4 (13%) | 9 (30%) | 0.117a |
| Steroid | 1 (3%) | 2 (7%) | 0.554a |
| Statin | 8 (27%) | 15 (50%) | 0.063a |
| Beta blocker | 5 (17%) | 9 (30%) | 0.222a |
| Metformin | 2 (7%) | 1 (3%) | 0.554a |
| Serological test | |||
| Total cholesterol (mg/dL) | 202 (31.0) | 197 (37.3) | 0.536b |
| HDL (mg/dL) | 63 (16.8) | 54 (22.3) | 0.115b |
| LDL (mg/dL) | 112 (29.8) | 114 (32.7) | 0.773b |
| Triglyceride (mg/dL) | 163 (136.0) | 164 (72.9) | 0.982b |
| CRP (mg/dL) | 0.16 (0.299) | 0.35 (0.467) | 0.075b |
| eGFR (mL/min/1.73 m2) | 71 (13.1) | 58 (20.0) | 0.001b |
| Aneurysm diameter (mm) | 52 (40–117) | ||
HDL: high-density lipoprotein cholesterol; LDL: low-density lipoprotein cholesterol; CRP: C-reactive protein; eGFR: estimated glomerular filtration rate.
aχ2 test, bunpaired t-test.
Microbiota comparisons between fecal samples of the AAA and control groups
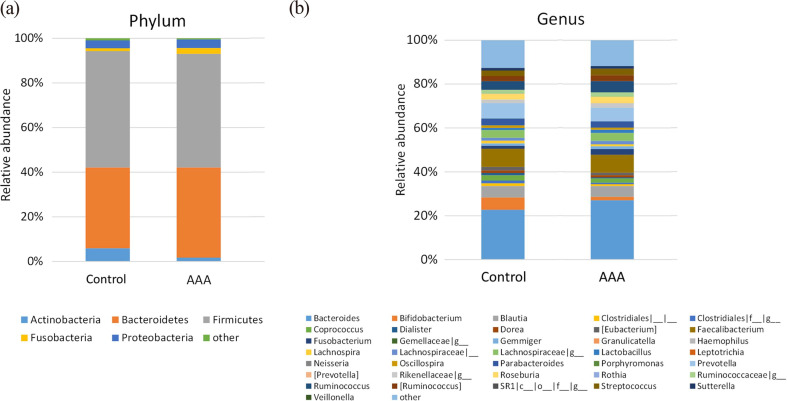
The mean relative abundances of bacteria in fecal samples from patients with AAA revealed the dominance of five phyla (Fig. 1), Firmicutes (50.9%), Bacteroidetes (40.4%), Proteobacteria (3.8%), Fusobacteria (2.6%), and Actinobacteria (1.7%; Fig. 1a), but there was no difference from the controls. Meanwhile, the genus with the highest mean abundance in the AAA group was Bacteroides (27.1%), followed by Faecalibacterium (8.2%), Prevotella (6.2%), and Ruminococcus (5.2%; Fig. 1b).
Fig. 1.
Relative abundances of bacteria at the (a) phylum and (b) genus levels in fecal samples from patients with AAA and controls.
Although five kinds of phyla were dominant at the phylum level (a), the most abundant genus found in both groups was Bacteroides (b). AAA: abdominal aortic aneurysm.
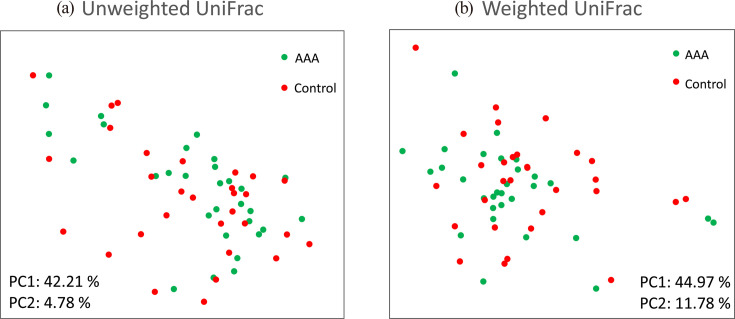
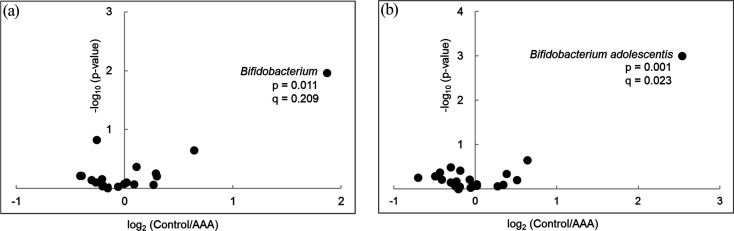
Two-dimensional PCoA plots of UniFrac distances, based on the relative abundances of amplicon sequence variants, revealed no significant difference between the groups in both unweighted (p=0.829 by PERMANOVA, Fig. 2a) and weighted (p=0.402 by PERMANOVA, Fig. 2b) models. The volcano plot based on the common taxa profile at the genus level showed that bifidobacteria (p=0.011, q=0.209) were abundant in the control group compared with the AAA group (Fig. 3a). Among the Bifidobacterium species, Bifidobacterium adolescentis (p=0.001, q=0.023) was significantly more abundant in the control group than in the AAA group (Fig. 3b). Alpha and beta diversity were not significantly different between the two groups.
Fig. 2.
Unweighted (a) and weighted (b) UniFrac principal coordinate analysis (PCoA) of microbiota in the fecal samples of patients with AAA and the controls.
Unweighted and weighted distances are calculated based on the presence or absence and relative abundance of observed bacterial taxa, respectively. Closely spaced plots in the PCoA figure indicate more similar microbiota compositions. AAA: abdominal aortic aneurysm; PC: principal coordinates.
Fig. 3.
Differential abundance of genera (a) and species (b) between the two groups.
Volcano plot of the estimated log2 fold change in taxon abundance between the AAA group and control group. The data of taxa with medians of 0.5% or more in each group were used for the analysis. p-value, Mann–Whitney U test; q-value, Benjamini–Hochberg adjusted p-value. AAA: abdominal aortic aneurysm.
DISCUSSION
Concerning the relationship between the GM and AAA, we hypothesize that trimethylamine N-oxide (TMAO) and lipopolysaccharide (LPS) produced by the GM exacerbate AAA. TMAO and LPS promote atherosclerosis and various cardiovascular diseases [18]. Atherosclerosis is a major risk factor for AAA, and some recent papers reported that TMAO and LPS are involved in the development of AAA in mice. Hu et al. revealed that TMAO induces smooth muscle cell calcification and vascular senescence and promotes AAA formation in mice models [19]. Furthermore, when apolipoprotein E-deficient mice were fed a high-choline diet, the blood TMAO level increased and arteriosclerosis worsened [20]. Xie et al. reported that the abundance of Akkermansia decreased and that the abundances of Odoribacter, Helicobacter, and Ruminococcus increased in a fecal sample of apolipoprotein E-deficient model mice with AAA [11]. However, there have been few studies on the GM of humans with AAA.
In the present study, we found that B. adolescentis was significantly less abundant in the AAA group. Bifidobacteria are major commensal bacteria that are naturally found in the human intestinal tract and are known to have intestinal regulation properties and protective effects against metabolic syndrome [21, 22]. Approximately ten species, including Bifidobacterium bifidum, Bifidobacterium breve, Bifidobacterium longum, B. adolescentis, Bifidobacterium pseudocatenulatum, and Bifidobacterium catenulatum, inhabit the human intestine. B. adolescentis has been reported to strengthen the intestinal barrier, regulate lipid metabolism, regulate immunity and anti-inflammatory action, and regulate other intestinal bacteria [23,24,25,26,27]. B. adolescentis has also been reported to be associated with interleukin 6 receptor susceptibility; however, it is not known how this affects the host [27]. Furthermore, studies have attempted to ascertain the utility of B. adolescentis as a supplement for the treatment of metabolic syndrome and fractures [24, 25]. Metformin has been discussed as a target for AAA drug therapy, but it has been reported that metformin increases the abundance of B. adolescentis and improves glucose tolerance [28]. Wang et al. reported that bifidobacteria reduced plasma TMAO and plasma and cecal trimethylamine concentrations in mice [29]. Therefore, we considered the possibility that bifidobacteria directly exert anti-inflammatory action or suppress AAA by affecting other bacteria. This is the first study to compare the GM of patients with AAA with that of non-AAA controls. Further research is needed to determine the function of these bacteria in the pathogenesis of AAA.
Our study had some limitations. There were differences in the backgrounds of the patients, and renal failure was associated with the GM and may have been a confounding factor. It has been reported that renal dysfunction progresses when an aortic aneurysm expands; however, the mechanism is unknown [30]. Since AAA is an advanced state of arteriosclerosis, it may cause impaired blood flow to the kidneys. Although renal dysfunction is associated with AAA, it is not known if it is confounded by the GM. In addition, it is not possible to compare the enlargement rate and diameter of AAAs with those of other aneurysms. In the future, it will be necessary to accumulate cases and conduct additional studies. Further, it is known that the GM is associated with racial differences. Since this study only included Japanese people, caution is required in the interpretation of its findings [31]. It is also known that bacterial action differs at the strain level. In this study, detailed bacterial species identification and strain level analyses could not be performed. In the future, metagenomic and metabolomic analyses will be required.
In this study, the relative abundance of B. adolescentis was found to be low in stool samples from patients with AAA. Since B. adolescentis is involved in the regulation of lipid metabolism and in immunomodulation and anti-inflammatory responses it has been suggested that these processes may be related to the etiology of AAA. Further research is needed to understand the relationship between the human AAA and GM.
DATA AVAILABILITY
All de-identified participant data will be shared. No additional documents will be shared. Data will be available upon publication and for at least 5 years and will be shared upon submission of a legitimate request to the corresponding author. Data sharing will be at the patient level, with the patients completely de-identified. The information will be available for inclusion in meta-analyses and other investigations that are deemed appropriate by the corresponding author.
AUTHOR CONTRIBUTIONS
EI, NT, AH, T Odamaki, JX, SK, YN, T Ohkusa, NS, and T Ohki contributed to the study conception and design. EI, HN, AH, and T Odamaki prepared materials and collected data. EI, HN, AH, and T Odamaki performed the statistical analysis. EI and YN wrote the first draft of the manuscript. All authors read and approved the final manuscript.
FUNDING
The Department of Microbiota Research is supported by Morinaga Milk Industry Co., Ltd. through a donation course. This work was supported by a Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (KAKENHI Grant Number 19K18194), the Japan Heart Foundation Research Grant, and a grant from the Japan Arteriosclerosis Prevention Fund.
CONFLICT OF INTEREST
T Ohki received advisory fees from W. L. Gore and Boston Scientific Corporation. AH, T Odamaki and JX are employees of Morinaga Milk Industry Co., Ltd. The other authors do not have conflicts of interest or financial ties to disclose.
REFERENCES
- 1.Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, Oderich GS, Patel MS, Schermerhorn ML, Starnes BW. 2018. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg 67: 2–77.e2. [DOI] [PubMed] [Google Scholar]
- 2.Sweeting MJ, Thompson SG, Brown LC, Powell JT, RESCAN collaborators. 2012. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg 99: 655–665. [DOI] [PubMed] [Google Scholar]
- 3.Lindeman JH, Matsumura JS. 2019. Pharmacologic management of aneurysms. Circ Res 124: 631–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golledge J, Moxon JV, Singh TP, Bown MJ, Mani K, Wanhainen A. 2020. Lack of an effective drug therapy for abdominal aortic aneurysm. J Intern Med 288: 6–22. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka H. 2018. Problems and perspectives of abdominal aortic aneurysm research. Curr Drug Targets 19: 1227. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y, Ou C, Chen M. 2019. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest 99: 346–357. [DOI] [PubMed] [Google Scholar]
- 7.Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, Zhu JJ, You C, Chen Q, Zhou L, Pan SY, Zhou HW. 2015. Dysbiosis of gut microbiota with reduced trimethylamine-n-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J Am Heart Assoc 4: e002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu KY, Xia GH, Lu JQ, Chen MX, Zhen X, Wang S, You C, Nie J, Zhou HW, Yin J. 2017. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Sci Rep 7: 1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida N, Emoto T, Yamashita T, Watanabe H, Hayashi T, Tabata T, Hoshi N, Hatano N, Ozawa G, Sasaki N, Mizoguchi T, Amin HZ, Hirota Y, Ogawa W, Yamada T, Hirata KI. 2018. Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation 138: 2486–2498. [DOI] [PubMed] [Google Scholar]
- 10.Liu Z, Li J, Liu H, Tang Y, Zhan Q, Lai W, Ao L, Meng X, Ren H, Xu D, Zeng Q. 2019. The intestinal microbiota associated with cardiac valve calcification differs from that of coronary artery disease. Atherosclerosis 284: 121–128. [DOI] [PubMed] [Google Scholar]
- 11.Xie J, Lu W, Zhong L, Hu Y, Li Q, Ding R, Zhong Z, Liu Z, Xiao H, Xie D, Zheng G, Ye B, Zhong Y, Liu Z. 2020. Alterations in gut microbiota of abdominal aortic aneurysm mice. BMC Cardiovasc Disord 20: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, Dick F, van Herwaarden J, Karkos C, Koelemay M, Kölbel T, Loftus I, Mani K, Melissano G, Powell J, Szeberin Z, de Borst GJ, Chakfe N, Debus S, Hinchliffe R, Kakkos S, Koncar I, Kolh P, Lindholt JS, de Vega M, Vermassen F, Document Reviewers, Björck M, Cheng S, Dalman R, Davidovic L, Donas K, Earnshaw J, Eckstein HH, Golledge J, Haulon S, Mastracci T, Naylor R, Ricco JB, Verhagen H, Esvs Guidelines Committee. 2019. European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg 57: 8–93. [DOI] [PubMed] [Google Scholar]
- 13.Ito E, Toya N, Fukushima S, Nishie R, Akiba T, Ohki T. 2018. Polyester grafts are a risk factor for postimplantation syndrome after abdominal endovascular aneurysm repair: retrospective analysis for polyester graft, excluder, and Endologix Powerlink/AFX. Ann Vasc Dis 11: 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao JZ, Abe F, Osawa R. 2016. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol 16: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13: 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horigome A, Hisata K, Odamaki T, Iwabuchi N, Xiao JZ, Shimizu T. 2021. Colonization of supplemented Bifidobacterium breve M-16V in low birth weight infants and its effects on their gut microbiota weeks post-administration. Front Microbiol 12: 610080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. 2011. Metagenomic biomarker discovery and explanation. Genome Biol 12: R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang WHW, Bäckhed F, Landmesser U, Hazen SL. 2019. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol 73: 2089–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu J, Xu J, Shen S, Zhang W, Chen H, Sun X, Qi Y, Zhang Y, Zhang Q, Guo M, Peng N, Xu B. 2022. Trimethylamine N-Oxide promotes abdominal aortic aneurysm formation by aggravating aortic smooth muscle cell senescence in mice. J Cardiovasc Transl Res [online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jonsson AL, Bäckhed F. 2017. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol 14: 79–87. [DOI] [PubMed] [Google Scholar]
- 21.Haro C, Garcia-Carpintero S, Alcala-Diaz JF, Gomez-Delgado F, Delgado-Lista J, Perez-Martinez P, Rangel Zuñiga OA, Quintana-Navarro GM, Landa BB, Clemente JC, Lopez-Miranda J, Camargo A, Perez-Jimenez F. 2016. The gut microbial community in metabolic syndrome patients is modified by diet. J Nutr Biochem 27: 27–31. [DOI] [PubMed] [Google Scholar]
- 22.Ghadimi D, de Vrese M, Ebsen M, Röcken C, Olaf Frahm S, Zahlten J, Fölster-Holst R, Heller KJ, Bockelmann W. 2021. Study on the additive protective effect of PGLYRP3 and Bifidobacterium adolescentis Reuter 1963 on severity of DSS-induced colitis in Pglyrp3 knockout (Pglyrp3 −/−) and wild-type (WT) mice. Immunobiology 226: 152028. [DOI] [PubMed] [Google Scholar]
- 23.Guo Y, Xie JP, Deng K, Li X, Yuan Y, Xuan Q, Xie J, He XM, Wang Q, Li JJ, Luo HR. 2019. Prophylactic effects of Bifidobacterium adolescentis on anxiety and depression-like phenotypes after chronic stress: a role of the gut microbiota-inflammation axis. Front Behav Neurosci 13: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts JL, Liu G, Darby TM, Fernandes LM, Diaz-Hernandez ME, Jones RM, Drissi H. 2020. Bifidobacterium adolescentis supplementation attenuates fracture-induced systemic sequelae. Biomed Pharmacother 132: 110831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Wang R, Li XF, Wang RL. 2012. Bifidobacterium adolescentis supplementation ameliorates visceral fat accumulation and insulin sensitivity in an experimental model of the metabolic syndrome. Br J Nutr 107: 1429–1434. [DOI] [PubMed] [Google Scholar]
- 26.Wang B, Kong Q, Cui S, Li X, Gu Z, Zhao J, Zhang H, Chen W, Wang G. 2021. Bifidobacterium adolescentis isolated from different hosts modifies the intestinal microbiota and displays differential metabolic and immunomodulatory properties in mice fed a high-fat diet. Nutrients 13: 1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawabata K, Baba N, Sakano T, Hamano Y, Taira S, Tamura A, Baba S, Natsume M, Ishii T, Murakami S, Ohigashi H. 2018. Functional properties of anti-inflammatory substances from quercetin-treated Bifidobacterium adolescentis. Biosci Biotechnol Biochem 82: 689–697. [DOI] [PubMed] [Google Scholar]
- 28.Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernàndez-Real JM, Bäckhed F. 2017. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 23: 850–858. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Guo M, Liu Y, Xu M, Shi L, Li X, Zhao J, Zhang H, Wang G, Chen W. 2022. Bifidobacterium breve and Bifidobacterium longum attenuate choline-induced plasma trimethylamine n-oxide production by modulating gut microbiota in mice. Nutrients 14: 1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsushita K, Kwak L, Ballew SH, Grams ME, Selvin E, Folsom AR, Coresh J, Tang W. 2018. Chronic kidney disease measures and the risk of abdominal aortic aneurysm. Atherosclerosis 279: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto Y, Hamaguchi M, Kaji A, Sakai R, Osaka T, Inoue R, Kashiwagi S, Mizushima K, Uchiyama K, Takagi T, Naito Y, Fukui M. 2020. Intake of sucrose affects gut dysbiosis in patients with type 2 diabetes. J Diabetes Investig 11: 1623–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All de-identified participant data will be shared. No additional documents will be shared. Data will be available upon publication and for at least 5 years and will be shared upon submission of a legitimate request to the corresponding author. Data sharing will be at the patient level, with the patients completely de-identified. The information will be available for inclusion in meta-analyses and other investigations that are deemed appropriate by the corresponding author.