Abstract
This systematic review synthesised measurement and prevalence of frailty in COPD and associations between frailty and adverse health outcomes. We searched Medline, Embase and Web of Science (1 January 2001–8 September 2021) for observational studies in adults with COPD assessing frailty prevalence, trajectories, or association with health-related outcomes. We performed narrative synthesis and random-effects meta-analyses. We found 53 eligible studies using 11 different frailty measures. Most common were frailty phenotype (n = 32), frailty index (n = 5) and Kihon checklist (n = 4). Prevalence estimates varied by frailty definitions, setting, and age (2.6–80.9%). Frailty was associated with mortality (5/7 studies), COPD exacerbation (7/11), hospitalisation (3/4), airflow obstruction (11/14), dyspnoea (15/16), COPD severity (10/12), poorer quality of life (3/4) and disability (1/1). In conclusion, frailty is a common among people with COPD and associated with increased risk of adverse outcomes. Proactive identification of frailty may aid risk stratification and identify candidates for targeted intervention.
Subject terms: Chronic obstructive pulmonary disease, Epidemiology
Introduction
An increasing number of people worldwide are living with frailty1. Frailty describes a state of increased vulnerability to decompensation in response to physiological stress2,3. There is growing recognition of the importance of frailty in the management of noncommunicable diseases generally, and chronic respiratory diseases specifically2,4. In the context of chronic obstructive pulmonary disease (COPD) (a condition characterised by progressive decline in pulmonary function and periods of exacerbation, both of which may impact on function and independence), it has been argued that frailty may be a valuable concept to understand individual vulnerability to adverse clinical outcomes5–8.
While, at a conceptual level, frailty describes a state of increased vulnerability to physiological decompensation, there is no single universally accepted operational measure of frailty. Rather, multiple different measures, drawing on different theoretical models of ageing, have been used to identify frailty within individuals2. Populations identified by different frailty definitions only partially overlap9. Frailty is also understood to be dynamic and may worsen or improve within individuals over time.
People with COPD are recognised to have a higher prevalence of frailty than the general population5. However, this prevalence is likely to differ by different frailty definitions as well as in different clinical settings. A previous systematic review has assessed the prevalence of frailty in people with COPD, however this review did not assess the impact of frailty on clinical outcomes5. Moreover, since its publication, a number of further studies focusing specifically on people with COPD, have assessed the prevalence and implications of frailty10,11.
This systematic review aims to (i) review the different measures which have been used to quantify frailty in people with COPD; (ii) summarise the prevalence and trajectories of frailty in people with COPD across a range of settings and frailty definitions; and (iii) quantify the relationship between frailty and clinical outcomes in people with COPD.
Methods
This systematic review of observational studies was carried out according to a pre-specified protocol (PROSPERO CRD42021275574) and is reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines12,13.
Eligibility criteria
Observational studies (cross-sectional or cohort design) meeting the following criteria were included:
Population: adults aged ≥18 years with COPD;
Exposure: frailty (any measure);
Comparison: people with COPD without frailty;
Outcomes: frailty prevalence (primary outcome), transitions in frailty status, mortality, hospitalisation, healthcare utilisation, quality of life, disability, COPD exacerbation, COPD severity, symptoms;
Setting: any (including community, outpatients, inpatients, residential care).
There are a wide range of frailty measures described within the published literature. The earliest of these, and the most widely cited, are the frailty phenotype and the frailty index, however many measures have followed. While each of these seeks to identify individuals at greater risk of physiological decompensation and thus adverse health outcomes, measures vary considerably in their theoretical basis, method of assessment, and their quantification (e.g. categorical or continuous, or describing frailty severity). We included studies using any definition of frailty to allow comparison between different measures. Studies were included if they used a validated frailty measure or provided detailed criteria within the manuscript of the criteria used to identify frailty. We excluded studies that assessed frailty based on a single parameter or proxy measure (e.g. grip strength alone). We excluded studies not published in English, conference abstracts, and grey literature. For synthesis, studies were grouped by frailty definition and setting.
Search strategy
We searched three electronic databases (Medline, Embase and Web of Science Core Collection) using a combination of Medical Subject Headings and keyword searches. The basic search structure was “Frailty” AND “COPD” and was developed by the study authors drawing on previous systematic reviews. Full search terms for Medline are shown in the Supplementary Information and were adapted for the other databases. All search results were exported to Endnote software. Database searches were supplemented by forward citation searches of all eligible studies and hand-searching reference list of relevant papers (included studies and relevant review articles). We did not attempt to contact study authors.
Study selection
We screened titles and abstract of all records identified through database searching. Full texts of all potentially eligible articles were obtained and screened according to our eligibility criteria. Two reviewers, working independently, completed all stages of screening. Agreement was high between reviewers for the title and abstract screening (kappa statistic 97%). Disagreements over eligibility were resolved by consensus, involving a third reviewer if necessary.
Data extraction
Two reviewers, working independently, extracted details of study publication (author, year, journal, location), setting (community, outpatient, inpatient, residential care, etc.), population (recruitment method, eligibility criteria, age, sex, socioeconomic status, comorbidities), frailty definition (including adaptations to the original definition), frailty prevalence and the relationship between frailty and any clinical outcomes listed above.
Quality assessment
The methodological quality of the included studies was assessed using an adaptation of the Newcastle Ottawa Scale (previously adapted for systematic reviews of studies assessing frailty, included in Supplementary Information)14. The initial five questions were used for all studies (cross-sectional or longitudinal) with a further six for longitudinal studies.
Synthesis
We performed a narrative synthesis of frailty prevalence estimates, stratified by study setting and frailty definition. We synthesised studies assessing frailty and clinical outcomes using a combination of narrative synthesis and random effects meta-analysis. Meta-analysis was performed when at least two studies assessed the same outcome, using the same frailty definition, using a similar statistical approach, and when heterogeneity was at an acceptable level (heterogeneity was assessed using the I2 statistic). Where studies were too heterogeneous, a narrative synthesis was performed and summarised using Harvest plots. Harvest plots summarise heterogenous data using bars to represent individual studies placed on a matrix to indicate where the studies showed a positive, negative, or neutral association with a given outcome (we used p < 0.05 to denote statistical significance)15,16. Data processing and analysis was done using R (version 3.6.1).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Results
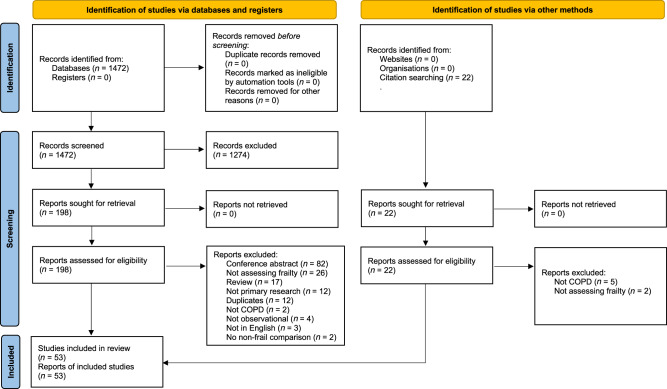
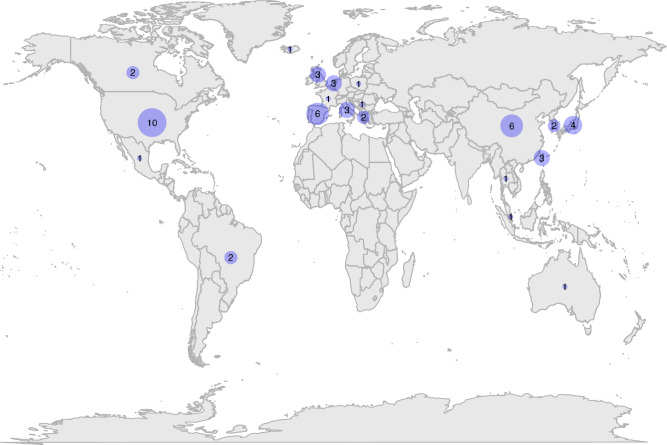
We identified 1402 unique titles and abstracts from electronic database searches, from which we retained 220 for full-text screening and finally identified 53 eligible studies (Fig. 1)10,11,17–67. Sample size (with COPD) ranged from 22 to 8074 (median 192, interquartile range (IQR) 103–149). Study characteristics and quality assessment are summarised in Table 1. Mean study age ranged from 50 to 88 (median 73, IQR 68–75). All studies were from high- or upper–middle-income countries (Fig. 2). COPD was identified using a range of definitions, including spirometry-confirmed/GOLD definition, self-reported, and based on electronic medical records. Studies including only spirometry-confirmed COPD tended to be based in respiratory outpatient departments or hospital inpatients and were often single-centre studies. Most community-based studies used self-reported COPD or relied on electronic medical records (Supplementary Information).
Fig. 1. PRISMA diagram of study identification and screening.
This figure summarises the stages of identification and screening of eligible studies.
Table 1.
Study characteristics and quality assessment.
| Author | Country | Frailty measure | COPD definition | N with COPD | Mean age | Setting | Outcomes |
|---|---|---|---|---|---|---|---|
| Akgun17 | United States | Frailty phenotype | Electronic health records | 182 | 50 | Community | Prevalence |
| Ambagtsheer18 | Australia | Frailty index | Electronic health records | 98 | 88 | Residential care | Prevalence |
| Avila-Funes19 | Mexico | Frailty phenotype | Self report | 814 | 74 | Community | Prevalence |
| Bernabeu-Mora20 | Spain | Edmonton | GOLD criteria | 103 | 71 | Inpatient | Prevalence, hospital readmission, FEV1, dyspnoea |
| Bernabeu-Mora21 | Spain | Frailty phenotype | GOLD criteria | 119 | 67 | Outpatient | Prevalence, frailty transitions, exacerbation |
| Blaum22 | United States | Frailty phenotype | Electronic health records | 230 | 74 | Community | Prevalence |
| Castellana23 | Italy | Frailty phenotype | Self-report | 343 | 74 | Community | Prevalence |
| Chen24 | Taiwan | Frailty phenotype | Self-report | 312 | 73 | Community | Prevalence |
| Chen25 | Taiwan | CFS | Electronic health records | 125 | 77 | Outpatient | Prevalence, exacerbation, dyspnoea |
| Cheong26 | Singapore | Frailty phenotype | Self-report | 239 | 66 | Community | Prevalence |
| Chin27 | Canada | CFS | Hospitalised with exacerbation of COPD | 50 | 72 | Inpatient | Prevalence |
| Crow28 | United States | Frailty phenotype | Self-report | 496 | 71 | Community | Prevalence |
| de Albuquerque29 | Brazil | Frailty phenotype | Self-report | 22 | 74 | Community | Prevalence |
| Dias30 | Brazil | FRAIL | GOLD criteria | 153 | 69 | Outpatient | Prevalence, exacerbation, FEV1, dyspnoea, CAT |
| Fragoso31 | United States | Frailty phenotype | Self-report | 262 | 72 | Community | Prevalence, frailty transitions |
| Fried32 | United States | Frailty phenotype | Self-report | 415 | 72 | Community | Prevalence |
| Gale33 | United Kingdom | Frailty index | GOLD criteria | 520 | 66 | Community | Prevalence, exacerbation, FEV1, dyspnoea, CAT |
| Galizia34 | Italy | Frailty Staging System | Self-report | 489 | 74 | Community | Prevalence, mortality, dyspnoea |
| Gephine35 | France | Frailty phenotype | GOLD criteria | 44 | 66 | Pulmonary rehab | Prevalence, exacerbation, FEV1, dyspnoea, QOL |
| Gu36 | China | Frailty index | Clinician diagnosis of acute exacerbation of COPD | 154 | 80 | Inpatient | Mortality |
| Hanlon37 | United Kingdom | Frailty phenotype | Self-report | 8074 | 58 | Community | Prevalence |
| Hirai38 | Japan | Kihon | GOLD criteria | 201 | 76 | Outpatient | FEV1, dyspnoea, CAT |
| Ierodiakonou39 | Greece | FiND | GOLD criteria | 257 | 65 | Community | Prevalence, exacerbation, FEV1, dyspnoea, CAT |
| Kennedy40 | United States | Frailty phenotype | GOLD criteria | 902 | 68 | Outpatient | Prevalence, mortality, hospital admission, QOL |
| Kim41 | South Korea | Frailty phenotype | Self-report | 83 | 76 | Community | Prevalence |
| Kusunose42 | Japan | Kihon | GOLD criteria | 79 | 75 | Outpatient | Prevalence, dyspnoea, CAT, QOL |
| Lahousse43 | Netherlands | Frailty phenotype | GOLD criteria | 172 | 75 | Community | Prevalence |
| Lahousse44 | Netherlands | Frailty phenotype | GOLD criteria | 172 | 75 | Community | Mortality, FEV1 |
| Lai45 | Taiwan | Frailty phenotype | Medical records | 65 | 82 | Residential care | Prevalence |
| Lee46 | China | Frailty phenotype | Self-report | 236 | 74 | Community | Frailty transitions |
| Limpawattana47 | Thailand | FRAIL | GOLD criteria | 121 | 70 | Outpatient | Prevalence |
| Liotta48 | Italy | Functional geriatric evaluation | Medical records | 218 | 76 | Community | Prevalence |
| Luo10 | China | Frailty phenotype | GOLD criteria | 309 | 86 | Outpatient | Prevalence, mortality, exacerbation, hospital admission, FEV1, dyspnoea, CAT |
| Ma49 | China | Frailty index | Self-report | 205 | 72 | Community | Prevalence |
| Maddocks50 | United Kingdom | Frailty phenotype | GOLD criteria | 816 | 70 | Pulmonary rehab | Prevalence, frailty transitions, exacerbation |
| Medina-Mirapeix51 | Spain | Edmonton | GOLD criteria | 103 | 71 | Inpatient | Prevalence, disability |
| Medina-Mirapeix52 | Spain | Frailty phenotype | GOLD criteria | 137 | 67 | Outpatient | Prevalence, exacerbation, FEV1, dyspnoea, CAT |
| Motokawa53 | Japan | Kihon | Self-report | 6 | 74 | Community | Prevalence |
| Nagorni-Obradovic54 | Serbia | Frailty phenotype | Self-report | 653 | 59 | Community | Prevalence |
| Oishi55 | Japan | Kihon | American Thoracic Society guidelines | 128 | 73 | Outpatient | Prevalence, dyspnoea |
| Park56 | United States | Tilburg | Self-report | 211 | 71 | Community | Prevalence, dyspnoea |
| Park57 | South Korea | Tilburg | Self-report | 417 | 65 | Community | Prevalence, FEV1 |
| Pollack58 | United States | Frailty phenotype | Self-report | 537 | 73 | Community | Prevalence |
| Scarlata59 | Italy | Frailty index | GOLD criteria | 150 | 73 | Outpatient | Prevalence, mortality, exacerbation, FEV1, dyspnoea, CAT |
| Serra-Prat60 | Spain | Frailty phenotype | Self-report | 44 | 80 | Community | Prevalence |
| ter Beek61 | Netherlands | Frailty phenotype | GOLD criteria | 57 | 61 | Pulmonary rehab | Prevalence |
| Uchmanowicz62 | Poland | Tilburg | American Thoracic Society guidelines | 102 | 63 | Outpatient | Prevalence |
| Valenza63 | Spain | Frailty phenotype | American Thoracic Society guidelines | 212 | 73 | Inpatient | Prevalence |
| Veronese64 | Iceland | Frailty phenotype | Self-report | 368 | 76 | Community | Prevalence |
| Warwick65 | Canada | CFS | Hospitalised with exacerbation of COPD | 390 | 68 | Inpatient (ITU) | Prevalence, mortality |
| Xue66 | China | Frailty phenotype | Self-report | 23 | 79 | Outpatient | Prevalence |
| Yee11 | United States | Frailty phenotype | GOLD criteria | 280 | 68 | Outpatient | Prevalence, mortality, exacerbation, hospital admission, FEV1, QOL |
| Zhang67 | China | Frailty phenotype | Self-report | 28 | 75 | Outpatient | Prevalence |
GOLD Global Obstructive Lung Disease, FEV1 Forced expiratory volume in 1 second, CAT COPD Assessment Test, QOL quality of life.
Fig. 2. Map showing the location of the included studies.
The numbers on the map indicate the number of included studies from each country.
Quality assessment
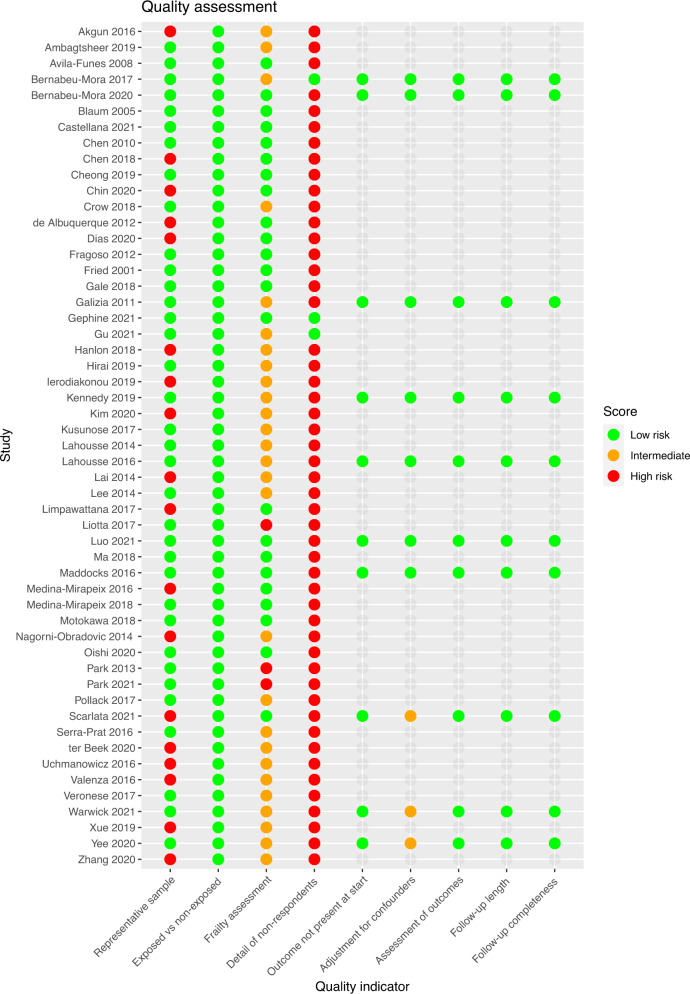
The results of the quality assessment for each study are shown in Fig. 3, with additional detail in Supplementary Information. While many studies were judged somewhat representative (e.g. through consecutive non-selective recruitment of a clinical population, or use of a representative community-based sample), many of these studies still had exclusion criteria with the potential to influence the prevalence of frailty in these studies. For example, many studies excluded people with cognitive impairment (sometimes not specifying the criteria or cut-off used to judge this), people with ‘impaired mobility’ or with some specific long-term conditions (e.g. neurological conditions such as stroke or Parkinson’s disease). While these resulted in few overall exclusions and may be justified given the demands of the study (e.g. in terms of physical assessments), given the associations between these features and frailty it is likely that these criteria would selectively exclude people living with frailty from these studies. This, in turn, may influence prevalence estimates.
Fig. 3. Quality assessment of the included studies.
Coloured dots indicate the quality assessment for each domain based on the Newcastle Ottawa quality assessment scale (full details in given in the supplementary material).
Many studies adapted existing frailty criteria based on their available data. However, frailty measures used were well described in most studies. Very few studies provided any detail of differences between participants with and without missing data or between non-responders and those with complete follow-up.
In the longitudinal studies assessed, all adjusted for age and sex in analyses and all but one adjusted for other relevant covariates (e.g. COPD severity). Length and completeness of follow-up was judged adequate for outcome assessment (Supplementary Table 2).
Frailty measurement
The included studies used a total of 11 different frailty measures. The most common was the frailty phenotype32 (32 studies) followed by the frailty index68,69 (5 studies), Kihon checklist70 (4 studies), Clinical Frailty Scale71 (3 studies), Tilburg frailty indicator72 (3 studies), FRAIL scale73 (2 studies), Edmonton frailty indicator74 (2 studies), FiND75 (1 study), Study for Osteoporotic Fractures frailty score76 (1 study), Frailty staging system77 (1 study) and Functional Geriatric Evaluation78 (1 study). One study assessed used three different measures38. These definitions are summarised in Table 2. While the frailty phenotype was the most commonly used measure, 27 of the 32 studies using this measure made some adaptation to the original frailty phenotype criteria.
Table 2.
Frailty measures used in the included studies.
| Frailty measure | Components | Range and categorisation | Number of included studies |
|---|---|---|---|
| Frailty phenotype32 | 5 components (unintentional weight loss, exhaustion, low grip strength, slow walking pace, low physical activity) |
1–2 criteria: pre-frail ≥3 criteria: frail |
32 |
| Frailty index68,69 | Count of health-related deficits (≥30, type and number of chosen deficits may vary between studies). Total present divided by number of possible deficits |
Range 0–1 Sometimes categorised (threshold for frailty varies (e.g. 0.2, 0.24) |
5 |
| Kihon checklist70 | Self-administered checklist (components: activities of daily living, exercise, falling, nutrition, oral health, cognition, depression) |
Unweighted sum of components. Range 0–25. Pre-frail (4–7), Frail (≥8) |
4 |
| Clinical frailty scale71 | Clinical tool based on functional status |
Ranges 1 (very fit) to 9 (terminally ill). Some dichotomise as ≥5 = frail |
3 |
| Tilburg frailty indicator72 | 15 questions across 3 domains (physical, psychological and social) Responses combined into unweighted sum |
Range 0–15 ≥5 indicates frailty |
3 |
| FRAIL scale73 | 5 components (weight loss, fatigue, weakness, ambulation, illness/comorbidity) |
1–2 criteria: pre-frail ≥3 criteria: frail |
2 |
| Edmonton frailty scale74 | 9 components: cognition, general health, functional independence, social support, medication, nutrition, mood, continence and functional performance |
Score 0–17. Mild (7–8), moderate (9–10) and severe frailty (≥11) |
2 |
| FiND75 | Self-administered frailty screening tool. 5 items: difficulty walking 400 m, difficulty climbing stairs, weight loss, exhaustion and low physical activity | Classed as disability (difficult walking or climbing stairs), frailty (no difficulty with walking or stairs but other deficits present) or robust (no deficits) | 1 |
| Study of Osteoporotic Fracture frailty indicator76 | 3 components (weight loss, chair stand, exhaustion) |
1 component: pre-frail 2–3 components: frail |
1 |
|
Frailty staging system77 |
7 components (disability, mobility, cognition, vision, hearing, continence, social support) |
Range 0–7. Mild (1) moderate (2–3) or severe frailty (≥4) |
1 |
| Functional geriatric assessment78 | Multidimensional evaluation (physical, mental and functional status, socio/economic resources, environment) |
Scored from −108 to 101 Robust (>50), frail (≤50, ≥10) and very frail (<10) |
1 |
Table adapted from Hanlon et al.14.
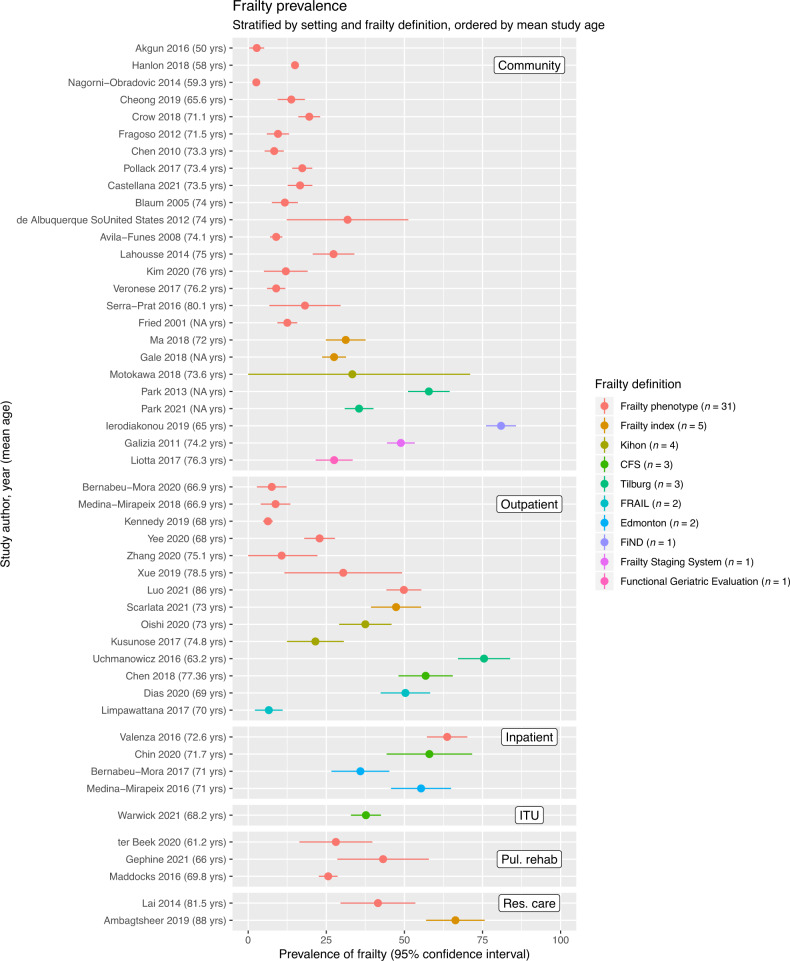
Frailty prevalence
The prevalence of frailty was assessed in 47 studies. These are summarised in Fig. 4, stratified by study setting and ordered by frailty definition and mean age in the included studies. Estimates of prevalence were highly heterogeneous, varying by setting and between frailty definitions. Prevalence ranged from 2.6 to 80.9% in 25 community-based studies and from 6.3 to 75.5% in 14 studies in outpatient settings. Prevalence varied by frailty measure (e.g. generally lower in studies using frailty phenotype). Higher mean age of the sample appeared to be associated with a higher frailty prevalence, while some younger populations (mean age 50–60 years) had considerably lower prevalence estimates, for example among community-based studies using the frailty phenotype (Fig. 4). However, even among community-based studies using the same measure there was heterogeneity in prevalence estimates among samples of a similar age, suggesting that additional factors (such as population demographics or adaptations in the way frailty deficits were specified) also influenced the prevalence of frailty. In other settings, prevalence ranged from 35.9 to 63.7% in hospital inpatients (4 studies), from 41.5 to 66.3% in residential care settings (2 studies) and 25.6 to 43.2% in populations recruited at the commencement of pulmonary rehabilitation programmes (3 studies). Some of the highest frailty estimates were from studies in which the original frailty definition was adapted based on available data and the cut-point for identifying frailty was selected by the study authors (rather than being based on externally validated criteria). This may explain some of the notably high prevalence estimates and limits the generalisability of these studies.
Fig. 4. Plot showing the prevalence of frailty in each of the included studies.
Studies are stratified by setting (community, outpatient, inpatient, intensive care (ITU), pulmonary rehabilitation and residential care). Within strata, studies are ordered by frailty measure (colour) and mean age (descending order on y-axis). Points indicate the prevalence estimate, lines indicate 95% confidence intervals.
Within-person frailty trajectories
Four studies assessed longitudinal changes in frailty over time. These varied in their setting, aim, and design. Two studies focused exclusively on people with COPD. Bernabeu-Mora et al. (n = 119) found that higher baseline muscle strength and lower exacerbation frequency were associated with improvement, while higher baseline dyspnoea was associated with worsening frailty status. Maddocks et al (n = 816) found that people living with frailty were more likely not to complete pulmonary rehabilitation due to exacerbations or hospital admissions; however, of those who did complete, 71/115 (61.3%) no longer met the criteria for frailty (74 pre-frail, 7 robust). People with baseline frailty also experienced greater improvements in dyspnoea, physical activity and health status following pulmonary rehabilitation than people who were not living with frailty at baseline.
Of the two studies of frailty trajectories among general populations (not limited to people with COPD), COPD was associated with worsening frailty status among women who were robust at baseline (adjusted odds ratio (OR) 2.66, 95% confidence interval (CI) 1.10–6.44); however, they did not find the same association in men (adjusted OR 0.61, 95% CI 0.34–1.11). COPD defined by obstructive spirometry was associated with worsening frailty status compared to people with no airway obstruction over 3 years follow-up (adjusted OR 1.58; 95% CI 1.17–2.13). Baseline frailty status was also associated with the development of respiratory impairment (defined as obstructive or restrictive pattern of spirometry) among people with no respiratory impairment at baseline (adjusted OR 1.42; 95% CI 1.11–1.82).
Frailty and clinical outcomes
Studies assessing the relationship between frailty and clinical outcomes (either cross-sectionally or prospectively) are summarised in Fig. 5. Detail of each of the results displayed in Fig. 5 (including analysis and effect sizes) is summarised in the Supplementary Information. These are explored in detail below.
Fig. 5. Harvest plot showing the relationship between frailty and clinical outcomes in the included studies.
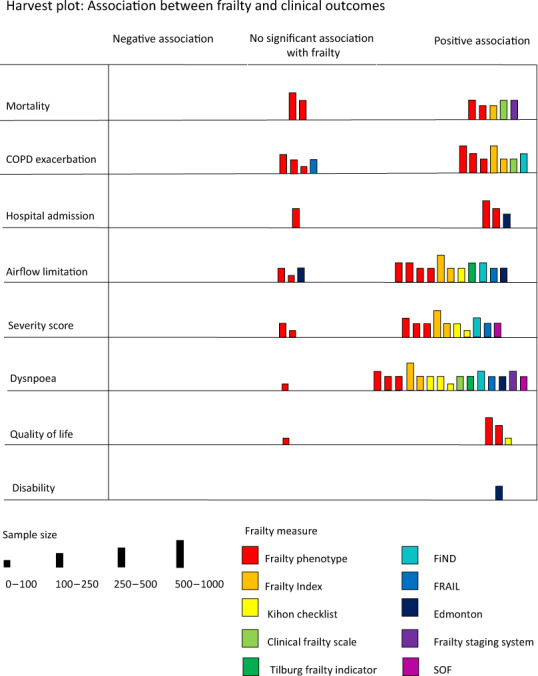
Each bar represents a single study. Colour is used to indicate which frailty measure is used. Height of the bar indicates study sample size. The position of the bar on the matrix indicates the relationship between frailty and the outcome in question (positive association between frailty and the outcome, no significant association, or negative association between frailty and the outcome [i.e. frailty is protective]).
Mortality
Eight studies assessed the relationship between frailty and all-cause mortality in people with COPD.
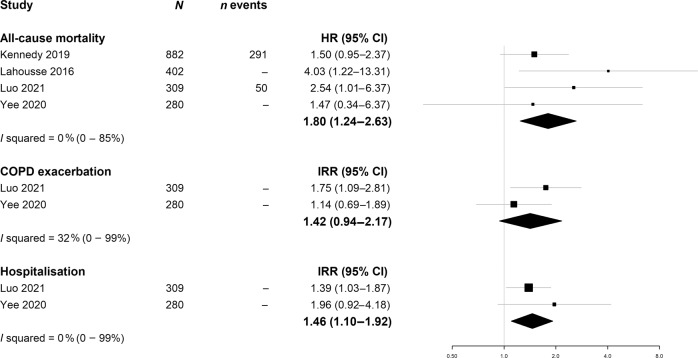
Four of these were outpatient- or community-based studies that used the frailty phenotype definition. The pooled hazard ratio (HR) for mortality associated with frail compared to robust participants was 1.80; 95% CI 1.24–2.62 (Fig. 6). Statistical heterogeneity was low (I2 = 0%) despite variation in study location (2 USA, 1 China, 1 Netherlands), adaptation of the frailty phenotype criteria and covariates included in the adjusted models. However, given the small number of studies included, this assessment of heterogeneity may be imprecise (95% CI 0–85%). One other community study (n = 489) using the Frailty Staging system also showed an association between frailty and mortality. In an outpatient-based study using the frailty index (n = 150), the association between frailty and mortality was not statistically significant (HR 2.1; 95% CI 0.7–5.8).
Fig. 6. Meta-analyses of random-effects meta-analysis of the relationship between frailty phenotype (frailty phenotype definition) and mortality, COPD exacerbation and hospitalisation.
Points indicate hazard ratios (HR) or incidence rate ratios (IRR), respectively. Whiskers show 95% confidence intervals (CI).
Two studies assessed inpatient mortality. One used the Clinical Frailty Scale and assessed survival to discharge from intensive care (n = 390). The other used a Frailty Index based on laboratory measures and compared people surviving to hospital discharge (n = 77) with people who did not (n = 77) using propensity score matching. In both studies, a higher degree of frailty was associated with higher mortality.
Hospitalisation
Three outpatient-based studies assessed the relationship between the frailty phenotype and all-cause hospital admission. Hospitalisation rates among people living with frailty and COPD were high (2 studies reported mean 0.49 and 0.47 exacerbations per person per year, respectively, in people living with frailty compared to 0.20 and 0.21, respectively, in robust participants with COPD). In the two studies assessing the rate of exacerbations, the pooled incident rate ratio (IRR) was 1.46, 95% CI 1.10–1.92. In the remaining study, the adjusted HR was 1.8, 95% CI 1.1–2.9. A fourth study assessed the relationship between frailty (Edmonton frailty indicator) and readmission within 90 days of COPD exacerbation. Severe frailty was strongly associated with readmissions (45% compared to 18% of robust participants, adjusted OR 5.19, 95% CI 1.26–21.50). Taken together, there is consistent evidence that people living with frailty and COPD experience higher rates of hospital admission than robust individuals with COPD.
COPD exacerbation
Eleven studies reported the association between frailty status and COPD exacerbations. Only two of these were prospective studies, both of which used the frailty phenotype definition. On meta-analysing these two studies, incidence of COPD exacerbations was 42% higher in those who were frail compared to those who were not (IRR 1.42, 95% CI 0.94–2.17, see Fig. 6). The remaining 9 studies assessed the unadjusted association between baseline frailty status and exacerbation frequency in the period prior to baseline (typically number of exacerbations, or frequent exacerbations defined as ≥2, in the past year). Six of these nine studies reported an association between frailty and exacerbation frequency.
COPD severity
Fourteen studies assessed the cross-sectional relationship between frailty and either forced expiratory volume in 1 s (FEV1), FEV1 as a percentage of predicted or severity categories based on FEV1. Frailty was associated with a greater degree of airflow limitation in 11/14 of these studies. Seven studies reported frailty prevalence stratified by degree of airflow limitation, showing higher frailty prevalence in participants with more severe airflow limitation (Supplementary Information).
Eight studies, comprising seven different frailty measures, assessed the relationship between frailty and CAT score. All of these reported statistically significant (unadjusted) baseline associations between frailty and higher CAT scores.
Dyspnoea
Of the 16 studies comparing baseline dyspnoea in people with and without frailty, 13 used the modified MRC dyspnoea scale, of which 12 showed greater dyspnoea scores in people living with frailty. Three remaining studies also reported cross-sectional associations between frailty and self-reported dyspnoea. As mentioned above, baseline dyspnoea was also associated with worsening frailty status over 2 years follow-up in the only longitudinal study to assess this outcome.
Quality of life
Four studies assessed the cross-sectional relationship between frailty and quality of life. Three of these, all of which used the short-form 36 quality of life assessment, reported lower quality of life scores in participants with baseline frailty (2 frailty phenotype, 1 Kihon checklist). The remaining study used was small (n = 55) and found no evidence of association between the frailty phenotype and quality of life according to the CCQ.
Disability
A single study assessed trajectories of activities of daily living limitation following hospital admission with COPD exacerbation. Frailty, measured using the Edmonton frailty indicator at the point of admission, was statistically significantly associated with functional decline measured 12 weeks after discharge (adjusted OR 3.97; 95% CI 1.13–13.92).
Discussion
This systematic review summarises the existing literature on the prevalence, trajectories and clinical implications of frailty in people with COPD. The prevalence of frailty varies by setting, age and by method used to identify frailty, ranging between 2.6 and 80.9%. Nonetheless, our findings show that frailty is common among people with COPD. Older populations had a higher prevalence of frailty, as expected; however, frailty remained common in populations of people with COPD including those aged under 65 years. In inpatient settings, the prevalence of frailty was notably higher (between 36 and 64% using a variety of measures), as it was in people living in residential care and in pulmonary rehabilitation.
Our findings demonstrate that people living with COPD and frailty may experience a high frequency of exacerbations, tend to have more severe airflow limitation, higher prevalence of dyspnoea and are at greater risk of readmission following hospital discharge and of mortality. Frailty in people with COPD varies over time.
A previous systematic review, including 27 studies, assessed the prevalence of frailty in COPD but did not synthesise the relationship between frailty and clinical outcomes in COPD5. In this previous review, 13 studies used the frailty phenotype, with a pooled mean prevalence of 19%, 95% CI 14–24%. Heterogeneity of these estimates was high. This previous study also reported that people with COPD had a twofold higher odds of frailty prevalence than people without COPD (odds ratio 1.97, 95% CI 1.53–2.53). Our study builds on these findings by stratifying by setting and also includes several more recent studies that specifically focussed on frailty in COPD (rather than as a subset of a general population). Unlike the previous review, we did not meta-analyse prevalence estimates due to considerable variation in study inclusion criteria (e.g. the exclusion of people with cognitive impairment or mobility difficulty), age range and adaptations of the frailty phenotype criteria. These sources of heterogeneity limit the interpretability of a single pooled prevalence estimate.
The high prevalence of frailty in people with COPD likely represents complex inter-relationships between features of both frailty and COPD79–81. COPD gives rise to a range of extrapulmonary manifestations, such as sarcopenia and fatigue80,81, which may contribute to the manifestation of frailty. Depending on the frailty definition, these factors alone may be sufficient for an individual to be classified as frail (i.e. COPD may cause frailty, in this context)82,83. Conversely, the development of COPD itself involves a complex interplay between environmental exposures and genetic susceptibility84. Accelerated lung ageing has been proposed as one mechanism underlying this process85. Some have argued that the development of COPD should be best understood as an interplay between abnormalities in organ development and maintenance (susceptibility) and the cumulative effect of tissue injury and ageing84,86. This is similar paradigm to the cumulative deficit model of frailty that is operationalised in the frailty index68,69,83. Under this framework, the development of COPD is a manifestation of the same processes underlying frailty, rather than a cause of frailty itself. In summary, depending on the theoretical model used to define frailty, COPD may be understood as both a cause and a consequence of frailty.
While frailty may result from the cumulative effect of acquired deficits, it may improve as well as worsen over time87. This review highlights that COPD may be a risk factor for worsening frailty; however, it also demonstrates that frailty in COPD may be responsive to targeted intervention, specifically pulmonary rehabilitation50. One study in this review also suggested that factors (such as COPD) associated with frailty transitions may vary by gender; however, this study tested a large number of covariates and was also limited the range of comorbidities that were measured; therefore, multiple testing resulting in false positives or residual confounding remain possible alternative explanations42. Sources of variation in frailty trajectories, including underlying long-term conditions and demographic factors such as gender are an important area of future study. The improvement in frailty status with pulmonary rehabilitation was assessed in only one included study. However, these findings are consistent with emerging evidence on interventions targeting frailty in general populations. A recent meta-analysis of 46 interventions showed that interventions incorporating physical activity or nutritional supplementation could improve frailty status in some individuals88.
The wide range of frailty definitions available, and lack of consensus over an optimal definition, is well described within the frailty literature. These measures vary considerably in their method of assessment and in their theoretical basis. Our findings highlight that these measures may differ considerably in their prevalence estimates, and therefore choice of measure used to identify frailty is likely to directly influence identification whether used for research or for clinical purposes (e.g. risk stratification). Despite this, the relationship with COPD severity and with adverse outcomes was broadly shared across frailty definitions. Therefore, while populations identified as living with frailty may differ, those measures available do each appear to identify higher risk of adverse outcomes. Choice of measure used to identify frailty should therefore be informed by several factors including practicality of administration and the theoretical basis for identifying frailty. For example, a study seeking to understand how risk factors or physiological parameters affect frailty development may favour a tightly defined physical measure of frailty (e.g. frailty phenotype), whereas studies seeking to assess patients most in need to a multidisciplinary clinical intervention may favour a broader definition incorporating additional domains, such as cognition, nutrition and social vulnerability. In either case, our findings suggest that applying a well-validated frailty measure using explicit cut-offs based on external studies is likely to provide a more reliable frailty assessment.
The high prevalence of frailty among people admitted to hospital with COPD, coupled with the higher rates of hospital readmission, indicate that identifying frailty within respiratory inpatient services may offer opportunities for targeted interventions. However, it is likely that a high proportion of patients would meet the criteria for frailty. It is likely that appropriate clinical response will vary between individuals identified as frail. Individualisation of care, as well as holistic multidisciplinary care, are central tenets of frailty care. There is considerable overlap between these principles and the multidisciplinary care advocated for people with COPD. However, given the high prevalence of frailty, implementing this in practice requires considerable resource and coordination of multiple professionals.
Community prevalence of frailty was lower than among inpatients but still common and associated with adverse events, so proactive identification of frailty may aid risk stratification and identification of individuals for whom limited community resources may be targeted. Ideally, such effort should be integrated into existing systems for the monitoring and management of COPD, to minimise the additional burden on both patients and professionals. Responses to frailty in this context may include advance care planning as well as efforts to maximise function and identify potentially reversible aspects of frailty. Understanding factors that influence trajectories of frailty is an important area for future research, to inform the design and delivery of interventions. Also vital to these efforts would be exploring factors that may facilitate or act as barriers to participation in interventions designed to target frailty.
Strengths of this review include a comprehensive search strategy supplemented by hand-searching reference lists and forward citation searching, followed by duplicate screening and data extraction. However, this review is limited by the exclusion of articles not published in English and of grey literature. As with any systematic review, there is a risk of publication bias. As the number of studies included in meta-analyses was small, assessment of funnel plots to detect publication bias may be unreliable. The included studies themselves are all observational, meaning the observed relationships between frailty and clinical outcomes, or between COPD and frailty trajectories, cannot be assumed to be causal. Many studies defined COPD using either self-report or by coded diagnosis, and there is therefore a risk of some misclassification in COPD in these studies. Spirometry confirmed COPD was mostly limited to studies conducted in respiratory outpatient departments or hospital inpatient settings. While these studies used validated, and therefore more reliable, definitions of COPD most were single centre and the frailty prevalence estimates may not be as generalisable to other settings. Most studies had small sample sizes. Studies from lower–middle-income counties were lacking, limiting the generalisability of prevalence estimates. Furthermore, most of the studies assessing airflow limitation, COPD severity and exacerbations were cross-sectional, further limiting inferences about the consequences of frailty in people with COPD. Most of the included studies contained detailed description of the population, and prospective studies adjusted for relevant potential confounders. However, description of non-responders and loss to follow-up was often limited. Furthermore, the potential confounders included in models assessing prospective outcomes (mortality, hospitalisations and exacerbations) varied widely between studies. Most notably, several did not adjust for severity of airflow limitation. Finally, the included studies were highly heterogenous in terms of inclusion criteria, frailty definitions, setting, and diagnostic criteria for COPD. Therefore, even among studies using the same model of frailty, differences in study populations and adaptation of frailty definition limited the synthesis of prevalence estimates.
Frailty is common in people with COPD and associated with disease severity, symptom burden and with a range of adverse health outcomes. Frailty varies over time and may be responsive to interventions. Proactive identification of frailty may aid risk stratification and identification of individuals for whom interventions may be targeted.
Supplementary information
Acknowledgements
P.H. was funded by a Medical Research Council Clinical Research Training Fellowship (Grant reference MR/S021949/1) entitled ‘Understanding prevalence and impact of frailty in chronic disease and implications for clinical management’. The funder had no role in protocol development.
Author contributions
All authors contributed to the conception and design of the study. P.H., D.M. and F.S.M. developed the data sources and search strategy. P.H., X.G., D.M. and F.S.M. refined the inclusion criteria. P.H., X.G., D.M. and F.S.M. developed the data extraction template, which was piloted by P.H., X.G. and E.M. P.H., X.G. and E.M. carried out screening, quality assessment and data extraction. P.H. performed the analysis with support from J.L. and D.M. P.H. wrote the first draft. All authors critically reviewed this and subsequent drafts. All authors approved the final version of the manuscript for submission. F.S.M. is the guarantor of the review. All authors accept accountability for the accuracy of the findings.
Data availability
Extracted data reported in the manuscript are contained within the Supplementary Information.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: David McAllister, Frances S. Mair.
Supplementary information
The online version contains supplementary material available at 10.1038/s41533-022-00324-5.
References
- 1.O’Caoimh R, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2020;50:96–104. doi: 10.1093/ageing/afaa219. [DOI] [PubMed] [Google Scholar]
- 2.Hoogendijk EO, et al. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 3.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bone AE, Hepgul N, Kon S, Maddocks M. Sarcopenia and frailty in chronic respiratory disease. Chron. Respir. Dis. 2017;14:85–99. doi: 10.1177/1479972316679664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marengoni A, et al. The relationship between COPD and frailty: a systematic review and meta-analysis of observational studies. Chest. 2018;154:21–40. doi: 10.1016/j.chest.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Bousquet, J. et al. Should we use gait speed in COPD, FEV1 in frailty and dyspnoea in both? Eur. Respir. Soc. 48, 315–319 (2016). [DOI] [PubMed]
- 7.Charbek E, Espiritu JR, Nayak R, Morley JE. Frailty, comorbidity, and COPD. J. Nutr. Health Aging. 2018;22:876–879. doi: 10.1007/s12603-018-1068-7. [DOI] [PubMed] [Google Scholar]
- 8.Hanlon P, et al. Frailty in COPD: an analysis of prevalence and clinical impact using UK Biobank. BMJ Open Respir. Res. 2022;9:e001314. doi: 10.1136/bmjresp-2022-001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguayo GA, et al. Agreement between 35 published frailty scores in the general population. Am. J. Epidemiol. 2017;186:420–434. doi: 10.1093/aje/kwx061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo J, Dai Zhang WT, Dou L-Y, Sun Y. Impact of frailty on the risk of exacerbations and all-cause mortality in elderly patients with stable chronic obstructive pulmonary disease. Clin. Interv. Aging. 2021;16:593. doi: 10.2147/CIA.S303852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yee N, et al. Frailty in chronic obstructive pulmonary disease and risk of exacerbations and hospitalizations. Int. J. COPD. 2020;15:1967–1976. doi: 10.2147/COPD.S245505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page MJ, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021;134:103–112. doi: 10.1016/j.jclinepi.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Hanlon, P. et al. Frailty measurement, prevalence, incidence, and clinical implications in people with diabetes: a systematic review and study-level meta-analysis. Lancet Healthy Longev.1, E106–E116 (2020). [DOI] [PMC free article] [PubMed]
- 15.Crowther M, Avenell A, MacLennan G, Mowatt G. A further use for the Harvest plot: a novel method for the presentation of data synthesis. Res. Synth. Methods. 2011;2:79–83. doi: 10.1002/jrsm.37. [DOI] [PubMed] [Google Scholar]
- 16.Ogilvie D, et al. The harvest plot: a method for synthesising evidence about the differential effects of interventions. BMC Med. Res. Methodol. 2008;8:8. doi: 10.1186/1471-2288-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akgun KM, et al. Association of chronic obstructive pulmonary disease with frailty measurements in HIV-infected and uninfected Veterans. AIDS. 2016;30:2185–2193. doi: 10.1097/QAD.0000000000001162. [DOI] [PubMed] [Google Scholar]
- 18.Ambagtsheer, R. C., Beilby, J., Seiboth, C. & Dent, E. Prevalence and associations of frailty in residents of Australian aged care facilities: findings from a retrospective cohort study. Aging Clin. Exp. Res.32, 1849–1856 (2020). [DOI] [PubMed]
- 19.Avila-Funes JA, et al. Frailty among community-dwelling elderly people in France: the three-city study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008;63:1089–1096. doi: 10.1093/gerona/63.10.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernabeu-Mora R, et al. Frailty is a predictive factor of readmission within 90 days of hospitalization for acute exacerbations of chronic obstructive pulmonary disease: a longitudinal study. Therapy. 2017;11:383–392. doi: 10.1177/1753465817726314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernabeu-Mora, R. et al. Frailty transitions and associated clinical outcomes in patients with stable COPD: a longitudinal study. PLoS ONE15, e0230116 (2020). [DOI] [PMC free article] [PubMed]
- 22.Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: the Women’s Health and Aging Studies. J. Am. Geriatr. Soc. 2005;53:927–934. doi: 10.1111/j.1532-5415.2005.53300.x. [DOI] [PubMed] [Google Scholar]
- 23.Castellana F, et al. Physical frailty, multimorbidity, and all-cause mortality in an older population from Southern Italy: results from the Salus in Apulia Study. J. Am. Med. Dir. Assoc. 2021;22:598–605. doi: 10.1016/j.jamda.2020.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Chen C-Y, Wu S-C, Chen L-J, Lue B-H. The prevalence of subjective frailty and factors associated with frailty in Taiwan. Arch. Gerontol. Geriatr. 2010;50:S43–S47. doi: 10.1016/S0167-4943(10)70012-1. [DOI] [PubMed] [Google Scholar]
- 25.Chen PJ, Yang KY, Perng WC, Lin KC, Wang KY. Effect of dyspnea on frailty stages and related factors in Taiwanese men with COPD. Int. J. COPD. 2018;13:2463–2469. doi: 10.2147/COPD.S172694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheong, C. Y. et al. Risk factors of progression to frailty: findings from the Singapore Longitudinal Ageing Study. J. Nutr. Health Aging24, 98–106 (2020). [DOI] [PubMed]
- 27.Chin M, Voduc N, Huang S, Forster A, Mulpuru S. Practical lessons in implementing frailty assessments for hospitalised patients with COPD. BMJ Open Qual. 2020;9:01. doi: 10.1136/bmjoq-2019-000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crow RS, et al. Mortality risk along the frailty spectrum: data from the National Health and Nutrition Examination Survey 1999 to 2004. J. Am. Geriatr. Soc. 2018;66:496–502. doi: 10.1111/jgs.15220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Albuquerque Sousa ACP, Dias RC, Maciel ÁCC, Guerra RO. Frailty syndrome and associated factors in community-dwelling elderly in Northeast Brazil. Arch. Gerontol. Geriatr. 2012;54:e95–e101. doi: 10.1016/j.archger.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Dias LD, Ferreira ACG, da Silva JLR, Conte MB, Rabahi MF. Prevalence of frailty and evaluation of associated variables among COPD patients. Int. J. Chron. Obstruct. Pulm. Dis. 2020;15:1349–1356. doi: 10.2147/COPD.S250299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fragoso CAV, Enright PL, McAvay G, Van Ness PH, Gill TM. Frailty and respiratory impairment in older persons. Am. J. Med. 2012;125:79–86. doi: 10.1016/j.amjmed.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fried LP, et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 33.Gale NS, et al. Frailty: a global measure of the multisystem impact of COPD. Chron. Respir. Dis. 2018;15:347–355. doi: 10.1177/1479972317752763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galizia G, et al. Role of clinical frailty on long-term mortality of elderly subjects with and without chronic obstructive pulmonary disease. Aging Clin. Exp. Res. 2011;23:118–125. doi: 10.1007/BF03351076. [DOI] [PubMed] [Google Scholar]
- 35.Gephine, S., Grosbois, J. M., Saey, D., Maltais, F. & Mucci, P. Effects of home-based pulmonary rehabilitation on physical frailty in people with COPD and chronic respiratory failure. Eur. Respir. J. 56(Supplement 64), 888 (2020).
- 36.Gu J-J, Liu Q, Zheng L-J. A frailty assessment tool to predict in-hospital mortality in patients with acute exacerbations of chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulm. Dis. 2021;16:1093. doi: 10.2147/COPD.S300980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanlon P, et al. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3:e323–e332. doi: 10.1016/S2468-2667(18)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirai K, et al. Comparison of three frailty models and a sarcopenia model in elderly patients with chronic obstructive pulmonary disease. Geriatr. Gerontol. Int. 2019;19:896–901. doi: 10.1111/ggi.13740. [DOI] [PubMed] [Google Scholar]
- 39.Ierodiakonou D, et al. Determinants of frailty in primary care patients with COPD: the Greek UNLOCK study. BMC Pulm. Med. 2019;19:63. doi: 10.1186/s12890-019-0824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy CC, et al. Frailty and clinical outcomes in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2019;16:217–224. doi: 10.1513/AnnalsATS.201803-175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S, Jung HW, Won CW. What are the illnesses associated with frailty in community-dwelling older adults: the Korean frailty and aging cohort study. Korean J. Intern. Med. 2020;35:1004–1013. doi: 10.3904/kjim.2019.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kusunose, M., Oga, T., Nakamura, S., Hasegawa, Y. & Nishimura, K. Frailty and patient-reported outcomes in subjects with chronic obstructive pulmonary disease: are they independent entities? BMJ Open Respir. Res.4, e000196 (2017). [DOI] [PMC free article] [PubMed]
- 43.Lahousse L, et al. Adverse outcomes of frailty in the elderly: the Rotterdam Study. Eur. J. Epidemiol. 2014;29:419–427. doi: 10.1007/s10654-014-9924-1. [DOI] [PubMed] [Google Scholar]
- 44.Lahousse L, et al. Risk of frailty in elderly with COPD: a population-based study. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:689–695. doi: 10.1093/gerona/glv154. [DOI] [PubMed] [Google Scholar]
- 45.Lai HY, Chang HT, Lee YL, Hwang SJ. Association between inflammatory markers and frailty in institutionalized older men. Maturitas. 2014;79:329–333. doi: 10.1016/j.maturitas.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Lee JSW, Auyeung TW, Leung J, Kwok T, Woo J. Transitions in frailty states among community-living older adults and their associated factors. J. Am. Med. Dir. Assoc. 2014;15:281–286. doi: 10.1016/j.jamda.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Limpawattana P, et al. Frailty syndrome in ambulatory patients with COPD. Int. J. COPD. 2017;12:1193–1198. doi: 10.2147/COPD.S134233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liotta G, et al. Assessment of frailty in community-dwelling older adults residents in the Lazio region (Italy): a model to plan regional community-based services. Arch. Gerontol. Geriatr. 2017;68:1–7. doi: 10.1016/j.archger.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Ma L, et al. Prevalence of frailty and associated factors in the community-dwelling population of China. J. Am. Geriatr. Soc. 2018;66:559–564. doi: 10.1111/jgs.15214. [DOI] [PubMed] [Google Scholar]
- 50.Maddocks M, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71:988–995. doi: 10.1136/thoraxjnl-2016-208460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Medina-Mirapeix, F. et al. Patterns, trajectories, and predictors of functional decline after hospitalization for acute exacerbations in men with moderate to severe chronic obstructive pulmonary disease: a longitudinal study. PLoS ONE11, e0157377 (2016). [DOI] [PMC free article] [PubMed]
- 52.Medina-Mirapeix F, et al. Physical frailty characteristics have a differential impact on symptoms as measured by the CAT score: an observational study. Health Qual. Life Outcomes. 2018;16:140. doi: 10.1186/s12955-018-0969-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Motokawa K, et al. Frailty severity and dietary variety in Japanese older persons: a cross-sectional study. J. Nutr. Health Aging. 2018;22:451–456. doi: 10.1007/s12603-018-1000-1. [DOI] [PubMed] [Google Scholar]
- 54.Nagorni-Obradovic LM, Vukovic DS. The prevalence of COPD co-morbidities in Serbia: results of a national survey. NPJ Prim. Care Respir. Med. 2014;24:14008. doi: 10.1038/npjpcrm.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oishi K, et al. A new dyspnea evaluation system focusing on patients’ perceptions of dyspnea and their living disabilities: the linkage between copd and frailty. J. Clin. Med. 2020;9:1–13. doi: 10.3390/jcm9113580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park SK, Richardson CR, Holleman RG, Larson JL. Frailty in people with COPD, using the National Health and Nutrition Evaluation Survey dataset (2003-2006) Heart Lung. 2013;42:163–170. doi: 10.1016/j.hrtlng.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park SK. Frailty in Korean patients with chronic obstructive pulmonary disease, using data from the Korea National Health and Nutrition Examination Survey, 2015 and 2016. Appl. Nurs. Res. 2021;59:151417. doi: 10.1016/j.apnr.2021.151417. [DOI] [PubMed] [Google Scholar]
- 58.Pollack LR, et al. Patterns and predictors of frailty transitions in older men: the Osteoporotic Fractures in Men Study. J. Am. Geriatr. Soc. 2017;65:2473–2479. doi: 10.1111/jgs.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scarlata S, et al. Association between frailty index, lung function, and major clinical determinants in chronic obstructive pulmonary disease. Aging Clin. Exp. Res. 2021;33:2165–2173. doi: 10.1007/s40520-021-01878-z. [DOI] [PubMed] [Google Scholar]
- 60.Serra-Prat M, et al. Factors associated with frailty in community-dwelling elderly population. A cross-sectional study. Eur. Geriatr. Med. 2016;7:531–537. doi: 10.1016/j.eurger.2016.09.005. [DOI] [Google Scholar]
- 61.ter Beek L, et al. Coexistence of malnutrition, frailty, physical frailty and disability in patients with COPD starting a pulmonary rehabilitation program. Clin. Nutr. 2020;39:2557–2563. doi: 10.1016/j.clnu.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 62.Uchmanowicz I, Jankowska-Polanska B, Chabowski M, Uchmanowicz B, Fal AM. The influence of frailty syndrome on acceptance of illness in elderly patients with chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulm. Dis. 2016;11:2401–2407. doi: 10.2147/COPD.S112837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Valenza MC, et al. Physical activity as a predictor of absence of frailty in subjects with stable COPD and COPD exacerbation. Respir. Care. 2016;61:212–219. doi: 10.4187/respcare.04118. [DOI] [PubMed] [Google Scholar]
- 64.Veronese N, et al. Frailty and risk of cardiovascular diseases in older persons: the age, gene/environment susceptibility-Reykjavik Study. Rejuvenation Res. 2017;20:517–524. doi: 10.1089/rej.2016.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warwick, M. et al. Outcomes and resource utilization among patients admitted to the intensive care unit following acute exacerbation of chronic obstructive pulmonary disease. J. Intensive Care Med.36, 1091–1097 (2021). [DOI] [PubMed]
- 66.Xue Q, Qin MZ, Jia J, Liu JP, Wang Y. Association between frailty and the cardio-ankle vascular index. Clin. Interv. Aging. 2019;14:735–742. doi: 10.2147/CIA.S195109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, Y., Li, Y., Chan, P. & Ma, L. Reliability and validity of the self-reported frailty screening questionnaire in older adults. Ther. Adv. Chronic Dis.11, 2040622320904278 (2020). [DOI] [PMC free article] [PubMed]
- 68.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 70.Satake S, et al. Validity of the Kihon Checklist for assessing frailty status. Geriatr. Gerontol. Int. 2016;16:709–715. doi: 10.1111/ggi.12543. [DOI] [PubMed] [Google Scholar]
- 71.Rockwood K, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gobbens RJ, Schols JM, Van Assen MA. Exploring the efficiency of the Tilburg Frailty Indicator: a review. Clin. Interv. Aging. 2017;12:1739. doi: 10.2147/CIA.S130686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J. Nutr. Health Aging. 2012;16:601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526–529. doi: 10.1093/ageing/afl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cesari M, et al. A self-reported screening tool for detecting community-dwelling older persons with frailty syndrome in the absence of mobility disability: the FiND questionnaire. PLoS ONE. 2014;9:e101745. doi: 10.1371/journal.pone.0101745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ensrud KE, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch. Intern. Med. 2008;168:382–389. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 77.Lachs MS, et al. A simple procedure for general screening for functional disability in elderly patients. Ann. Intern. Med. 1990;112:699–706. doi: 10.7326/0003-4819-112-9-699. [DOI] [PubMed] [Google Scholar]
- 78.Scarcella P, Liotta G, Marazzi M, Carbini R, Palombi L. Analysis of survival in a sample of elderly patients from Ragusa, Italy on the basis of a primary care level multidimensional evaluation. Arch. Gerontol. Geriatr. 2005;40:147–156. doi: 10.1016/j.archger.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 79.Bousquet J, et al. Should we use gait speed in COPD, FEV1 in frailty and dyspnoea in both? Eur. Respir. J. 2016;48:315–319. doi: 10.1183/13993003.00633-2016. [DOI] [PubMed] [Google Scholar]
- 80.Barnes P, Celli B. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009;33:1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]
- 81.Natanek SA, et al. Heterogeneity of quadriceps muscle phenotype in chronic obstructive pulmonary disease (COPD); implications for stratified medicine? Muscle Nerve. 2013;48:488–497. doi: 10.1002/mus.23784. [DOI] [PubMed] [Google Scholar]
- 82.Fried LP, et al. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat. Aging. 2021;1:36–46. doi: 10.1038/s43587-020-00017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Howlett SE, Rutenberg AD, Rockwood K. The degree of frailty as a translational measure of health in aging. Nat. Aging. 2021;1:651–665. doi: 10.1038/s43587-021-00099-3. [DOI] [PubMed] [Google Scholar]
- 84.Agustí A, Faner R. COPD beyond smoking: new paradigm, novel opportunities. Lancet Respir. Med. 2018;6:324–326. doi: 10.1016/S2213-2600(18)30060-2. [DOI] [PubMed] [Google Scholar]
- 85.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–180. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- 86.Rutten EP, et al. Various mechanistic pathways representing the aging process are altered in COPD. Chest. 2016;149:53–61. doi: 10.1378/chest.15-0645. [DOI] [PubMed] [Google Scholar]
- 87.Welstead, M., Jenkins, N. D., Russ, T. C., Luciano, M. & Muniz-Terrera, G. A systematic review of frailty trajectories: their shape and influencing factors. Gerontologist61, e463–e475 (2021). [DOI] [PMC free article] [PubMed]
- 88.Travers J, Romero-Ortuno R, Bailey J, Cooney M-T. Delaying and reversing frailty: a systematic review of primary care interventions. Br. J. Gen. Pract. 2019;69:e61–e69. doi: 10.3399/bjgp18X700241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Extracted data reported in the manuscript are contained within the Supplementary Information.