Abstract
The purpose of the present study was to determine the extent of immunologic responses, particularly immunopathologic responses, within the upper and lower respiratory tracts after intranasal immunization using the mucosal adjuvant cholera toxin (CT). BALB/c mice were nasally immunized with influenza virus vaccine combined with CT. The inclusion of the mucosal adjuvant CT clearly enhanced generation of antibody responses in both the nasal passages and lungs. After nasal immunization, antigen-specific immunoglobulin A (IgA) antibody-forming cells dominated antibody responses throughout the respiratory tract. However, IgG responses were significant in lungs but not in nasal passages. Furthermore, parenteral immunization did not enhance humoral immunity in the upper respiratory tract even after a nasal challenge, whereas extrapulmonary lymphoid responses enhanced responses in the lung. After nasal immunization, inflammatory reactions, characterized by mononuclear cell infiltration, developed within the lungs of mice but not in nasal passages. Lowering dosages of CT reduced, but did not eliminate, these adverse reactions without compromising adjuvancy. Serum IgE responses were also enhanced in a dose-dependent manner by inclusion of CT. In summary, there are differences in the generation of humoral immunity between the upper respiratory tract and the lung. As the upper respiratory tract is in a separate compartment of the immune system from that stimulated by parenteral immunization, nasal immunization is an optimal approach to generate immunity throughout the respiratory tract. Despite the promise of nasal immunization, there is also the potential to develop adverse immunopathologic reactions characterized by pulmonary airway inflammation and IgE production.
Immune responses along the respiratory tract are important in the prevention and the pathogenesis of numerous respiratory tract diseases, such as viral and bacterial pneumonias. Importantly, respiratory tract infections have a major health and economic impact (1, 19), and there is a need to improve or develop vaccines to prevent these respiratory diseases. For example, the current parenterally given influenza virus vaccine is generally effective but has a reduced efficacy in the elderly. There are also other respiratory diseases, such as those due to respiratory syncytial virus (RSV) and Mycoplasma pneumoniae, for which a vaccine is unavailable. Many of these infectious agents infect the upper respiratory tract (nasal passages) prior to spreading to the lower respiratory tract (airways and lungs). This suggests that generating immunity against upper respiratory tract infections will decrease the chance of subsequent lower respiratory tract disease. However, studies have shown that parenteral immunization provides variable resistance to infection in the nasal passages, although it is effective against pulmonary infections (10, 24, 26). Thus, to improve effectiveness, vaccination needs to protect against upper respiratory tract infections, decreasing the chance of subsequent lower respiratory tract infections and disease.
The upper respiratory tract is both the initial site of infection and the major locale for antibody (Ab) production after experimental respiratory infections (17, 36, 37). There is also evidence of a significant protective advantage to using the intranasal route of immunization (25). Upper respiratory tract infection of mice with influenza virus was prevented in mice intranasally immunized with inactive influenza virus. In contrast, there was no noticeable protection after systemic immunization as titers of virus recovered from nasal passages were equivalent in naive (unimmunized) and subcutaneously immunized mice. Furthermore, studies clearly show that protection from infection of the upper respiratory tract is primarily due to local immunity, particularly immunoglobulin A (IgA) in mucosal secretions (3, 4, 28, 29). Based on the above-described and other studies (11, 18, 22, 25, 30, 42), we believe that intranasal immunization is an optimal route of administration of vaccines against respiratory tract infections.
Although intranasal immunization with live vaccines is currently under clinical trial (2, 8, 23), there are several major advantages in using inactive vaccine antigens, in contrast to live vaccines, for intranasal immunization. Inactive vaccines could be safely used in individuals immunocompromised due to disease, chemotherapy, or age. Furthermore, in the case of viral vectors for delivery of recombinant vaccines, immune responses against the vector can limit the effectiveness of immunization (41), therefore preventing the widespread utilization of these vectors for use in multiple vaccines. Notably, the ability to immunize with an inactive vaccine allows the use of a wider variety of vaccine antigens, including polysaccharide-protein conjugates that may prove impossible to produce using a viral vector. This should allow the adaptation of this vaccination approach to numerous bacterial and viral respiratory pathogens. Thus, intranasal immunization with inactive vaccines has many attractive features that should result in an extremely powerful approach to preventing respiratory infection and disease.
Understanding the mechanisms involved in generating immunity throughout the respiratory tract and potential problems is critical for intranasal vaccine development and evaluation. The purpose of the present study was to determine the extent of immunologic responses, particularly immunopathologic ones, within the upper and lower respiratory tracts after intranasal immunization and the contribution of systemic lymphoid responses to these immune responses. Immunization with inactive vaccines usually requires the inclusion of an adjuvant, and the most commonly used adjuvant for mucosal (intranasal or oral) immunization is cholera toxin (CT) and its derivatives (18, 30, 42). When CT is intranasally coadministered with an antigen, there is a significant enhancement of both mucosal and systemic immune responses. However, our previous study indicated the potential to develop IgE-associated inflammatory reactions within the lung after intranasal immunization using CT as an adjuvant at a relatively high dosage (35). IgE responses were shown to be enhanced by the high dosage of CT, and simultaneously, a massive infiltration of mononuclear cells was found around pulmonary airways and vessels. Thus, we examined the effects of reducing doses of the mucosal adjuvant CT to augment Ab responses while minimizing IgE-associated inflammatory reactions. In addition, we demonstrate that there is a fundamental difference in immune and inflammatory responses generated in the upper and lower respiratory tracts.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free or virus-free female BALB/c mice were obtained from the Frederick Cancer Research Facility (National Cancer Institute, Frederick, Md.) and Harlan Sprague-Dawley (Indianapolis, Ind.). F344 rats were from breeding colonies of specific-pathogen-free rats, as determined by serologic and cultural tests for rodent virus and bacterial pathogens, at the University of Alabama at Birmingham. Rats and mice were maintained in sterile microisolator cages with sterile food, water, and bedding. All mice and rats were used between 8 and 12 weeks of age.
Prior to experimental manipulation, mice were anesthetized with an intramuscular injection of ketamine-xylazine. For intranasal immunization, mice were allowed to inhale 24 μl of inoculum, which was placed upon the nares. For intraperitoneal immunization, mice were injected with 100 μl of antigen in the lower left quarter of their abdomen. For oral immunization, 100 μl of inoculum was given using an intragastric feeding needle. Serum samples were obtained by retro-orbital bleeding or by laceration of the femoral vein upon sacrifice. Nasal washes were obtained by flushing 1 ml of sterile phosphate-buffered saline (PBS) through the anterior (oral) entrance of the nasal passages using a syringe with a 21-gauge needle and collecting the fluid as it exited the nares. For fecal samples, fecal pellets were collected and dissolved at 1 mg/ml of 0.2% sodium azide in PBS. After centrifugation at 9,000 rpm (5415C microcentrifuge; Eppendorf, Westbury, N.Y.) for 5 min, supernatants were collected and stored at −20°C until use. Urogenital washes were collected by lavaging with two 20-μl washes with PBS.
Cell isolation.
Mononuclear cells were isolated from lungs by a procedure similar to that previously described (37, 38, 41). Lungs were perfused with PBS without magnesium or calcium (HyClone Laboratories, Logan, Utah) to minimize contamination of the final lung cell population with blood cells. The lungs were separated into individual lobes and finely minced. The tissues were suspended in medium containing 300 U of Clostridium histolyticum type I collagenase (Worthington Biochemical Corporation, Freehold, N.J.) per ml and 50 U of DNase (Sigma Chemical Co., St. Louis, Mo.) per ml. The tissues were incubated at 37°C while being mixed on a Nutator (Fisher Scientific, Pittsburgh, Pa.) for 90 to 120 min. During the incubation period, the tissue was vigorously pipetted every 20 min. After incubation, the digestion mixture was passed through a 250-μm nylon mesh to remove undigested tissue. Mononuclear cells were purified from this cell suspension by density gradient centrifugation using Lympholyte M (Accurate Chemicals, Westbury, N.Y.).
Cells from nasal passages were isolated as previously described (37). Briefly, the lower mandibles and skin were removed from the skull. The skull was longitudinally split, and the nasal passages were removed by scraping and transferred to collagenase-DNase digestion medium as used for isolation of lung cells. After about 1 h of incubation at 37°C while being mixed on a Nutator, the tissue was passed through a 250-μm nylon mesh, and the red cells were removed using ACK lysis buffer (15). Spleen cells were isolated by centrifugation of cell suspensions and red cell removal using ACK lysis buffer.
Fluorescent characterization of lymphocyte populations.
Two-color immunofluorescence staining was performed to identify both B-cell and T-cell populations using fluorescein isothiocyanate-labeled anti-murine Ab B220 (Beckman Coulter, Miami, Fla.) and phycoerythrin-labeled anti-murine Ab CD3 (Beckman Coulter). Briefly, 1 × 106 to 2 × 106 cells per tube were incubated with purified 2.4G2 Ab (Fc Block; PharMingen, San Diego, Calif.) for 5 min at 4°C to reduce nonspecific binding of FcII-FcIII receptors prior to fluorescent Ab staining. The cells were incubated for 30 min at 4°C with fluorescent Ab (2 μg/ml). Cells were washed in staining buffer (Mg2+-free and Ca2+-free PBS [HyClone] plus 0.05% sodium azide and 1% fetal bovine serum) and fixed with 4% paraformaldehyde in PBS for 30 min. Cells were then resuspended in staining buffer until analysis. The cells were analyzed using an EPICS XL-MCL flow cytometer (Beckman Coulter). Data collection was done using System 2 software (Beckman Coulter) with further analysis using Expo 2 analysis software (Beckman Coulter). Lymphocyte gates and detector voltages were set using unstained nasal passage, lung, and spleen control cells. The proportion of each cell population was expressed as the percentage of the number of stained cells.
Immunogens and adjuvants.
CT was purchased from List Biological Laboratories, Inc. (Campbell, Calif.). Maurice W. Harmon (Connaught Laboratories, Inc., Swiftwater, Pa.) kindly provided Philippines influenza virus vaccine antigen.
Influenza virus-specific Ab enzyme-linked immunosorbent assay (ELISA).
Falcon Microtest III assay plates (Becton Dickinson, Oxnard, Calif.) were coated with optimal concentrations of influenza virus vaccine (100 μl at 5 μg/ml) in PBS. After overnight incubation at 4°C, the plates were washed three times with PBS–0.05% Tween 20 and blocked with PBS–0.05% Tween 20 supplemented with 10% goat serum (GIBCO BRL, Grand Island, N.Y.) for 2 h at room temperature. Serum, fecal, and urogenital samples were serially diluted with PBS–0.05% Tween 20–10% goat serum, and 100 μl was placed in duplicate into wells of the antigen-coated plates. After overnight incubation at 4°C, the plates were washed four times with PBS–0.05% Tween 20. Secondary Ab (biotinylated anti-mouse Ab stock reagents of 0.5 mg/ml; Southern Biotechnology Associates, Inc., Birmingham, Ala.) was diluted 1:3,000 in PBS–0.05% Tween 20–10% goat serum, and 100 μl was added to the appropriate wells. After 5 h of incubation at room temperature, the plates were again washed four times with PBS–0.05% Tween 20, and a 1:2,000 dilution in PBS–0.05% Tween 20–10% goat serum of horseradish peroxidase (HRP)-conjugated streptavidin (Neutralite avidin; Southern Biotechnology Associates) was added to the wells (100 μl). The plates were incubated at room temperature for 2 h, and the plates were washed twice with PBS–0.05% Tween 20 and twice with PBS. The reaction mixtures were developed at room temperature by addition of 100 μl of 1.1 mM ABTS [2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid)] in 0.1 M citrate-phosphate buffer, pH 4.2, containing 0.01% H2O2 in each of the wells. For serum and fecal samples, endpoint Ab titers were expressed as the reciprocal dilution of the last dilution that gave an optical density (OD) at 415 nm of ≥0.1 U above the OD of negative controls after a 20-min incubation.
To detect antigen-specific IgA Abs in nasal washes, samples were diluted 1:2 in PBS–0.05% Tween 20 containing 10% goat serum and added to the appropriate wells of antigen-coated plates. The reaction mixtures were developed using (3,3′)5,5′-tetramethylbenzidine (TMB; Moss, Inc., Pasadena, Md.) as the substrate, and the OD of the color reaction was read at 630 nm.
Measurement of polyclonal and antigen-specific AFC.
The ELISPOT assays for polyclonal and influenza virus-specific Ab-forming cells (AFC) in lymphoid tissues were based on the method described by Czerkinsky et al. (7), as modified by Simecka et al. (36, 37). Briefly, to determine the number of AFC, irrespective of antigen specificity (polyclonal), 100 μl of polyclonal goat anti-mouse immunoglobulin Ab (2 μg/ml) was added to Millititer HA 96-well filtration plates (Millipore Corporation, Bedford, Mass.). The numbers of influenza virus-specific AFC were determined using plates coated with the optimal concentration of influenza virus antigen (10 μg of protein/ml). After overnight incubation at 4°C, the plates were washed three times with sterile PBS. To minimize nonspecific binding, 100 μl of cell culture medium (RPMI 1640, 10 ml of HEPES, l-glutamine, 5% fetal calf serum [HyClone]) was added to each well, followed by at least 1 h of incubation at 37°C. Twofold dilutions of cells (100 μl/well) were added in triplicate to the plates and incubated overnight at 37°C in a humidified 5% CO2 incubator. The plates were washed with PBS and incubated for 10 min in PBS with 0.5% H2O2 to remove endogenous peroxidase activity. Subsequently, the plates were washed with PBS–0.5% Tween 20.
Biotinylated goat anti-mouse IgM, IgG, or IgA Abs (Southern Biotechnology Associates) were used to reveal the Ab reactions. For IgE, biotinylated anti-IgE monoclonal Abs were used (PharMingen). Biotinylated Abs specific for mouse immunoglobulin classes were placed into the appropriate wells at a 1:3,000 dilution in PBS–10% goat serum. After overnight incubation at 4°C, the wells that contained the monoclonal Abs were reacted with a 1:2,000 dilution of HRP-Neutralite avidin (Southern Biotechnology Associates) for 2 h at room temperature. The plates were then washed with PBS, and substrate was added to each well. The HRP substrate was 25 mg of 3-amino-9-ethylcarbazole (AEC; Sigma Chemical, Co.) per 97 ml in 0.05 M sodium acetate buffer, pH 5.0, plus 0.04% H2O2; AEC was dissolved in 2 ml of N,N-dimethyl formamide. After the substrate reaction, the plates were thoroughly washed with tap water. The spots representing AFC were counted using a stereomicroscope illuminated indirectly with a high-intensity lamp. The AFC were expressed as the absolute number of AFC in each tissue or AFC per 106 cells.
Detection of antigen deposition by luciferase activity.
Mice were intranasally inoculated with 24 μl of luciferase enzyme (Promega, Madison, Wis.). Five minutes after inoculation, whole lungs, nasal passages, tracheas, and esophagus were collected. Tissues were placed in 1 ml of reporter lysis buffer (Promega) for esophagus and tracheas and 3 ml of reporter lysis buffer for lungs and nasal passages. Tissues were homogenized using a Pro 200 homogenizer (Pro Scientific Inc., Monroe, Conn.), and 20 μl of sample was added to 100 μl of luciferase assay substrate (Promega). Luciferase activity in a sample was determined by the amount of luminescence (TD-20e luminometer; Turner, Sunnyvale, Calif.).
Histopathology.
To collect tissues for histologic examination, anesthetized mice were sacrificed by exsanguination by laceration of the femoral artery. The trachea and lungs were removed intact. The lungs were gently inflated with buffered formalin using a 3-ml syringe with a 20-gauge needle. The lungs were subsequently fixed in buffered formalin, and individual lung lobes were processed for paraffin embedding, sectioning, and hematoxylin-eosin staining. Each lung lobe was examined for histopathologic changes by light microscopy.
Determination of total IgE Ab in sera.
Total serum IgE was measured using an ELISA. Plates were coated with capture anti-IgE monoclonal Ab (PharMingen) at 2 μg/ml. After blocking with 10% rat serum in PBS, serial dilutions of murine IgE standard (PharMingen) or serum samples in 3% bovine serum albumin in PBS were added to the wells in duplicate. After overnight incubation at 4°C, biotinylated anti-IgE monoclonal Ab (PharMingen) was added to the wells at a concentration of 4 μg/ml and incubated for 4 h at room temperature. The reaction was revealed using Neutralite avidin-HRP (Southern Biotechnology Associates), followed by addition of the substrate TMB. After addition of 0.25 M HCl to stop the reaction and enhance sensitivity, absorbance readings (450 nm) were obtained from the individual serum samples and were converted to micrograms of IgE per milliliter by reference to a standard curve produced using dilutions of a standard preparation of murine IgE for each assay. The detection limits for these assays were 125 ng/ml.
PCA assay for IgE Abs.
Passive cutaneous anaphylaxis (PCA) assay was used to compare the levels of influenza virus-specific reaginic (IgE) Abs in serum samples (20). Serum was diluted 1:20 with PBS and subsequently serially diluted (1:2) with PBS. Of each diluted sample, 0.1 ml was injected intradermally in the back of ether-anesthetized rats. One microgram of influenza virus antigen was directly injected into the intradermal sites where serum samples had been given the day before. To reveal the reactions, 1 ml of 1% Evans blue was injected intravenously the next day. After 10 min, the last dilution of serum with a positive (blue) reaction was considered the PCA titer.
Statistical analysis.
Data were analyzed by analysis of variance followed by Fisher protected least significant difference multigroup comparison or Mann-Whitney U tests. Analyses were performed using StatView (SAS Institute Inc., Cary, N.C.) computer programs. When appropriate, data were logarithmically transformed prior to statistical analysis and confirmed by a demonstrated increase in the power of the test after transformation of the data. A P value of ≤0.05 was considered statistically significant. If data were analyzed after logarithmic transformation, the antilogs of the means and standard errors of the transformed data were used to present the data and are referred to as the geometric means (± standard errors).
RESULTS
B cells in respiratory tissues of naive mice.
To evaluate T- and B-cell distribution in the upper and lower respiratory tracts, cells from nasal passages and lungs of naive (unimmunized) mice were collected and stained for CD3 and B220 cell surface expression. Using flow cytometry, 78.4% ± 4.6% of the stained lung cells were T cells (CD3+ B220−) while the remaining 21.6% ± 4.6% of stained cells were B cells (CD3− B220+). In the nasal passages, the numbers of T cells and B cells were equivalent (T cells, 52.6% ± 3.8%; B cells, 47.4% ± 3.8%).
The isotypes of plasma cells in upper and lower respiratory tracts were also characterized. Cells from nasal passages, lungs, and spleens were isolated from naive mice, and the numbers of polyclonal IgM-, IgG-, and IgA-producing cells were determined. As shown in Table 1, there were significantly (P ≤ 0.05) more IgA-producing cells than IgM- or IgG-producing cells in nasal passages. Similarly, there were significantly higher (P ≤ 0.05) numbers of IgA-producing cells than of cells producing IgM or IgG in lungs of naive mice. IgM-producing cells were most prevalent in the spleen. There was no significant difference between the numbers of IgG-producing cells found in lungs and those found in spleens. IgE-producing cells were not consistently detected in any tissues, and when found, their numbers were very low (data not shown).
TABLE 1.
Numbers of AFC per 106 cells from naive mice
| Isotype | No. of AFC/106 cellsa
|
||
|---|---|---|---|
| Nasal passage | Lung | Spleen | |
| IgA | 183 (2.0)* | 92 (1.1)* | 1 (1.2) |
| IgG | 5 (3.0) | 11 (1.0) | 12 (1.1) |
| IgM | 15 (1.3) | 2 (2.5) | 34 (1.0)* |
Geometric means (± standard errors) from three experiments (two experiments for lung IgG and IgM) with five mice each. Asterisks indicate the predominant isotype within a tissue (P ≤ 0.05).
Serum Ab responses after intranasal immunization with influenza virus antigen plus CT.
To investigate the adjuvant activity of various doses of CT in developing Ab responses against antigen, anesthetized mice were intranasally immunized on days 0, 7, and 14 with 7.5 μg of hemagglutinin of influenza virus vaccine in combination with 0, 0.1, 0.5, and 2.5 μg of CT. For comparison, one group of mice was immunized intraperitoneally with influenza virus antigen without CT. Serum samples were collected on days 7, 14, and 21 after the primary immunization, and serum Ab titers were determined by endpoint ELISAs for each of the Ab isotypes.
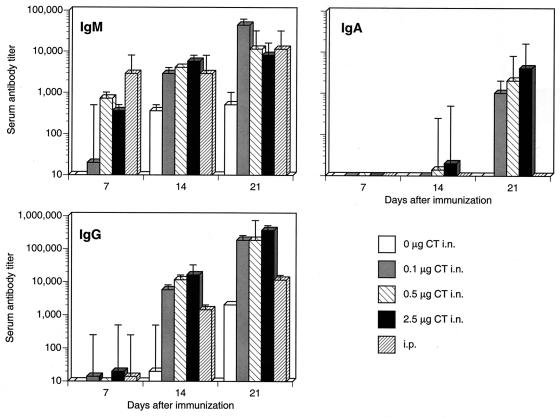
Ab responses in sera were clearly enhanced by inclusion of CT for immunization (Fig. 1). In all immunization groups, Ab responses increased over time (P ≤ 0.05), and as expected, IgM responses appeared earlier than IgG responses, while IgA Ab levels, when present, increased last. In animals intranasally immunized without CT, influenza virus antigen-specific IgG and IgM responses were not detected until 14 days after the primary immunization, and these responses were significantly lower (P ≤ 0.05) than those of mice immunized with antigen combined with all doses of CT. In addition, serum IgA was not detected in mice intranasally immunized without CT. In contrast, mice intranasally immunized with any dose of CT tested developed serum IgM responses earlier (7 days) after immunization, and a similar effect was seen for IgG responses, which also increased over time. However, the higher doses of CT (0.5 and 2.5 μg) resulted in serum IgA responses, albeit with low titers, that were detected earlier (14 days) than those of mice given 0.1 μg of CT. In contrast, mice intraperitoneally immunized with antigen did not develop serum IgA responses, although IgG and IgM responses were significant.
FIG. 1.
Influenza virus antigen-specific serum Ab responses after intranasal (i.n.) immunization with antigen plus various doses of CT. Mice were intranasally immunized weekly with 7.5 μg of influenza virus vaccine in combination with various doses of CT (0.1, 0.5, and 2.5 μg), including antigen alone (0 μg CT i.n.). One group of mice (i.p.) was intraperitoneally immunized with antigen but without CT. Sera were collected by retro-orbital bleeds at the indicated time points (days after immunization). The results are from sera collected from two experiments.
Intranasal immunization induces Ab responses in both the upper and lower respiratory tracts.
To characterize the site(s) of Ab production, we determined the distribution of specific AFC in nasal passages, lungs, and spleens after intranasal immunization using the ELISPOT assay. Influenza virus-specific AFC were found in each of the cell populations (Table 2). However, the inclusion of CT as an adjuvant significantly increased (P ≤ 0.05) the numbers of influenza virus-specific AFC in nasal passages, lungs, and spleens, regardless of Ab isotype. There was, however, no significant difference between the Ab responses obtained using the different doses of CT. In both lung and nasal passage cells, the numbers of antigen-specific IgA AFC were significantly greater than those of IgG AFC while the numbers of IgM AFC were lowest (P ≤ 0.05). Nasal passage cells had the greatest number of antigen-specific IgA AFC (P ≤ 0.05), and lung cells contained higher numbers of IgA AFC than did splenic lymphocytes (P ≤ 0.05). In contrast, the numbers of IgM and IgG AFC were greatest in lung cells, with IgM-producing cells being fewest in nasal passage cells (P ≤ 0.05). Thus, lymphoid responses in the upper respiratory tract are dominated by IgA to an extent even greater than in the lung.
TABLE 2.
Numbers of influenza virus-specific AFC per 106 cells from mice after intranasal immunization using the mucosal adjuvant CT
| Tissue | Isotype | Value for amt of CT (μg)/intranasal immunizationa
|
i.p.b | |||
|---|---|---|---|---|---|---|
| 0 | 0.1 | 0.5 | 2.5 | |||
| Lung | IgM | 6 (3.0)c | 84 (1.42) | 73 (2.8) | 70 (1.6) | 3 |
| IgG | 14 (1.6) | 344 (1.0) | 348 (1.1) | 1,074 (3.0) | 1 | |
| IgA | 12 (1.1) | 2,576 (1.6) | 2,871 (1.9) | 2,965 (1.8) | 0 | |
| Nasal\passages | IgM | 3 (3.5) | 15 (1.3) | 15 (1.5) | 9 (1.6) | 2 (2.2) |
| IgG | 1 (1.6) | 196 (1.8) | 251 (1.8) | 189 (1.4) | 5 | |
| IgA | 32 (1.1) | 3,319 (1.5) | 7,161 (1.4) | 11,940 (1.2) | 0 | |
| Spleen | IgM | 15 (1.0) | 27 (1.1) | 34 (1.2) | 48 (1.2) | 95 (1.7) |
| IgG | 2 (1.2) | 61 (2.8) | 93 (4.6) | 139 (3.0) | 1.3 (1.9) | |
| IgA | 2 (1.9) | 292 (1.3) | 407 (1.9) | 614 (1.2) | 0 | |
Mice were intranasally immunized weekly (four times) with 7.5 μg of influenza virus vaccine in combination with various doses of CT. Six days after the last immunization, the numbers of specific-antibody-producing cells per 106 cells from each of the tissues were determined.
Mice were intraperitoneally (i.p.) immunized weekly (four times) without CT.
Values are geometric means (± standard errors) of the numbers of influenza virus antigen-specific AFC per 106 viable cells of the specified isotype. Results are taken from two experiments (five mice in each group for each experiment).
To examine the overall contribution of each of the tissues to the Ab response, the results were expressed as the absolute (total) numbers of AFC for each of the tissues (Table 3). Interestingly, the inclusion of CT as an adjuvant resulted in a four- to sixfold increase in the numbers of cells isolated from lungs, but there was little effect on cell numbers collected from nasal passages or spleens. IgA was the major Ab response induced after immunization using CT, followed by IgG (P ≤ 0.05). The total numbers of IgA and IgG AFC were greater in lungs and spleens than in nasal passages (P ≤ 0.05). Total numbers of IgM AFC were greatest in spleens, with the fewest IgM AFC found in nasal passages (P ≤ 0.05). Thus, although the IgA response in the upper respiratory tract is substantial, it was found that the upper respiratory tract was not the major site of Ab responses, as there are significantly higher total numbers in the lungs.
TABLE 3.
Total numbers of influenza virus-specific AFC from each tissue obtained from mice after intranasal immunization using the mucosal adjuvant CT
| Tissue | Isotype | Value for amt of CT (μg)/intranasal immunizationa
|
i.p.b | |||
|---|---|---|---|---|---|---|
| 0 | 0.1 | 0.5 | 2.5 | |||
| Lung | IgM | 18 (2.5)c | 1,188 (2.3) | 1,334 (1.0) | 1,698 (2.1) | 10 |
| IgG | 41 (1.8) | 15,382 (1.1) | 20,091 (1.3) | 26,182 (1.1) | 3 | |
| IgA | 34 (1.0) | 36,392 (2.0) | 52,481 (1.4) | 72,444 (1.8) | 0 | |
| Nasal passages | IgM | 3 (3) | 7.7 (1.3) | 7 (1.9) | 5 (1.1) | 2 (1.9) |
| IgG | 1 (1.6) | 94 (1.8) | 121 (2.4) | 114 (1.0) | 3 (1.1) | |
| IgA | 27 (1.0) | 2,296 (2.2) | 3,656 (2.5) | 3,793 (1.1) | 0 | |
| Spleen | IgM | 927 (1.6) | 1,660 (1.3) | 2,455 (1.3) | 2,399 (1.5) | 3,698 (1.9) |
| IgG | 147 (1.3) | 3,690 (3.4) | 6,607 (5.0) | 7,031 (4) | 662 (2.1) | |
| IgA | 10 (12) | 17,620 (1.6) | 28,973 (2.2) | 31,046 (1.7) | 13 (17) | |
Mice were intranasally immunized weekly (four times) with 7.5 μg of influenza virus vaccine in combination with various doses of CT. Six days after the last immunization, the total numbers of specific-antibody-producing cells in the cells isolated from each of the tissues were determined.
Mice were intraperitoneally (i.p.) immunized weekly (four times) without CT.
Values are geometric means (± standard errors) of the total numbers of influenza virus antigen-specific AFC of the specified isotype obtained from a tissue. Results are taken from two experiments (five mice in each group for each experiment).
Histopathologic changes in lungs result from intranasal immunization with antigen plus CT.
In our earlier studies (35), we found histopathologic changes in lungs and clinical signs in mice given high doses of CT (10 μg of CT for primary immunization and 3.33 μg of CT for secondary immunization). To determine if lower dosages of CT would have the same effect, mice were immunized on days 0 and 7 with 7.5 μg of influenza virus vaccine in combination with 0, 0.1, 0.5, and 2.5 μg of CT. For comparison, one group of mice was immunized intraperitoneally with influenza virus antigen alone (no CT). Three days after the second immunization, clinical signs were noted, and nasal passages and lungs were prepared for histologic examination.
Three days after the second immunization, clinical signs were seen in mice after the secondary intranasal immunization with influenza virus antigen plus 2.5 μg of CT. These mice displayed decreased activity, weight loss, and ruffled fur. No obvious signs were seen for mice of any of the other immunization groups. Because of these clinical signs, we also determined whether histologic changes occurred along the respiratory tract after intranasal immunization with influenza virus antigen plus CT.
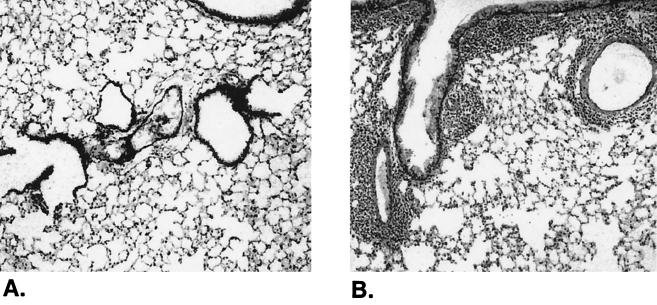
In lungs, infiltration of mononuclear cells was prominent at all doses of CT, but the infiltrates were absent in mice immunized with antigen alone or immunized by the intraperitoneal route. After intranasal immunization with each of the doses of CT, there was a massive accumulation of mononuclear cells around all the airways and blood vessels within the lung (Fig. 2B). However, no alveolar involvement was found at 0.1 or 0.5 μg of CT, but edema, alveolar thickening, and increased cellularity were present in mice immunized using 2.5 μg of CT. In contrast to what occurred in lungs, there was only a minimal inflammatory cell response in the nasal passages of mice given the higher dose (2.5 μg) of CT, but no histologic changes were seen at lower dosages. Immunization with adjuvant alone produced similar inflammatory changes in lungs of mice (data not shown), indicating that the effect was due to a response to CT.
FIG. 2.
Histopathologic changes in lungs of mice intranasally immunized with influenza virus vaccine plus the mucosal adjuvant CT. Mice (n = 4) were intranasally immunized with influenza virus vaccine (7.5 μg) either alone (A) or in combination with CT (0.1 μg) (B) on days 0 and 7. Three days later, lungs were collected for histology and sections were stained with hematoxylin-eosin. There were no histologic changes in lungs of any mice given antigen alone. However, there was a massive infiltration of cells around the airways and blood vessels throughout the lungs of all mice immunized with antigen plus CT.
IgE responses are induced after intranasal immunization with influenza virus antigen plus CT.
We evaluated serum IgE responses in mice immunized with influenza virus vaccine combined with different doses of CT. Mice were intranasally immunized on days 0, 7, and 14 with 7.5 μg of influenza virus vaccine in combination with 0, 0.1, 0.5, or 2.5 μg of CT. For comparison, one group of mice was immunized intraperitoneally with influenza virus antigen alone and no CT. Serum samples were collected on days 7, 14, and 21 after the primary immunization, and total IgE levels in serum samples were determined.
There was an increase in total serum IgE level in mice intranasally immunized with influenza virus antigen (Fig. 3). IgE levels were enhanced in mice immunized using CT as an adjuvant and were highest in sera from mice immunized with 2.5 μg of CT (P ≤ 0.05). The levels of IgE decreased in proportion to the reduction of the doses of CT. However, there was a slight but significant (P ≤ 0.05) increase in the total serum IgE level in mice intranasally immunized with antigen alone (without CT), while there was negligible change in total serum IgE level after intraperitoneal immunization.
FIG. 3.
The effect of intranasal immunization with CT on serum IgE levels (nanograms per milliliter). Mice were intranasally (i.n.) immunized weekly with 7.5 μg of influenza virus vaccine in combination with various doses of CT (0.1, 0.5, and 2.5 μg), including antigen alone (0 μg CT i.n.). One group of mice (i.p.) was intraperitoneally immunized with antigen but without CT. Sera were collected by retro-orbital bleeds at the indicated time points (days after intranasal immunization). The results are from sera collected from two experiments. Unimmunized mice (data not shown) had total serum IgE levels of ≤260 ng/ml.
To examine the production of influenza virus-specific IgE after intranasal immunization, serum samples from two different experiments were evaluated by a modified PCA reaction. In preliminary studies, we demonstrated that influenza virus antigen given by intravenous injection was unable to readily enter the intradermal sites where serum samples were previously injected. To overcome this limitation, the antigen was directly injected into the intradermal sites where serum samples had been given the day before. Using this approach, we found that serum collected from mice 3 days after the second intranasal immunization with 2.5 μg of CT had PCA titers of at least 30 to 40. However, sera from mice intranasally immunized with antigen and lower doses (0.5 or 0.1 μg) of CT had undetectable PCA titers.
The upper respiratory tract is a separate compartment of the immune system that can be stimulated by mucosal immunization.
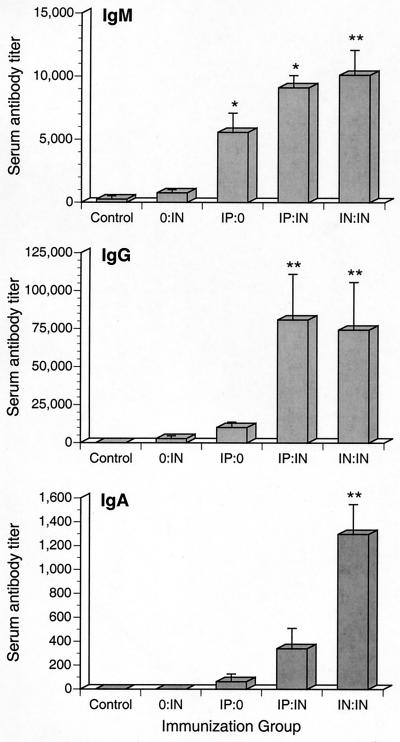
To determine the contribution of systemic immunization in generating Ab responses in respiratory tissues, mice were immunized by one of four protocols as depicted in Table 4. Fourteen days following primary immunization, the levels of influenza virus-specific serum Ab were determined using ELISA. Mice given two immunizations produced higher levels of antigen-specific serum Ab than did other experimental groups. In general, antigen-specific serum IgG and IgM levels were higher than serum IgA levels. Specific IgM Ab levels were significantly (P ≤ 0.05) higher in both IP:IN and IN:IN (designations of experimental groups are explained in Table 4) immunized mice (Fig. 4) than in other immunization groups; however, there was no difference between the levels of serum IgG generated in IP:IN and IN:IN immunized mice. Mice intranasally immunized on days 0 and 7 (IN:IN) produced significantly higher (P ≤ 0.05) levels of serum IgA than did IP:IN immunized mice and other immunization groups.
TABLE 4.
Immunization protocols used in this study
| Day 0a | Day 7 | Designationb |
|---|---|---|
| Intranasal antigen + CT | Intranasal antigen + CT | IN:IN |
| Intraperitoneal antigen + CT | Intranasal antigen + CT | IP:IN |
| Intranasal PBS | Intranasal antigen + CT | 0:IN |
| Intraperitoneal antigen + CT | Intranasal PBS | IP:0 |
| Intranasal PBS | Intranasal PBS | Control |
Day of immunization with 5 μg of influenza virus antigen plus 0.1 μg of CT by either the intranasal or intraperitoneal route. As a control, intranasal inoculation with sterile PBS was done. Seven days after the day 7 immunizations, samples were collected for analysis.
Designations used in the text, tables, and figures to refer to these immunization groups.
FIG. 4.
Serum Ab responses after intranasal and intraperitoneal priming. Immunization groups are designated as described in Table 4. All immunizations were with inactivated influenza virus vaccine plus 0.1 μg of CT. Sera were collected 14 days after primary immunization. The results are from sera collected from three experiments. A single asterisk denotes statistical differences (P ≤ 0.05) from the value for the control, while double asterisks denote differences (P ≤ 0.05) from values for mice given primary immunizations only (0:IN and IP:0).
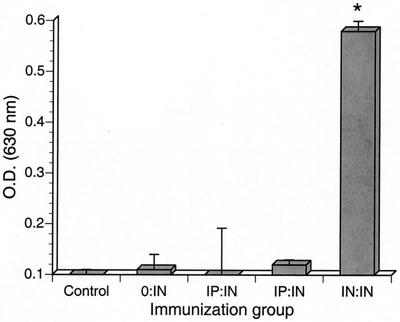
To evaluate IgA production in the upper respiratory tract after immunization, nasal wash samples were collected at 14 days following primary immunization. Mice given primary and secondary intranasal immunizations (IN:IN) produced significantly higher levels of antigen-specific IgA (P ≤ 0.05) than did all other groups (Fig. 5). In contrast, the levels of IgA in nasal washes collected from the other immunized mice (0:IN, IP:0, and IP:IN) were not significantly different from those for unimmunized control mice.
FIG. 5.
Intranasal, but not intraperitoneal, immunization induces antigen-specific IgA production in the upper respiratory tract. Immunization groups are designated as described in Table 4. All immunizations were performed with inactivated influenza virus vaccine plus 0.1 μg of CT. Nasal washes were collected 14 days after primary immunization. The results are from samples collected from three experiments. The single asterisk denotes statistical differences (P ≤ 0.05) from values for all other immunization groups.
To characterize the sites of Ab production, the numbers of cells secreting influenza virus-specific Ab in nasal passages, lungs, and spleens were determined (Table 5). Primary and secondary intranasal immunizations (IN:IN) produced the highest levels of influenza virus-specific IgA AFC in tissues (P ≤ 0.05). In contrast, IP:IN immunized mice had levels of IgA AFC in nasal passages and spleens similar to those of mice given a single intranasal immunization but had significantly higher (P ≤ 0.05) numbers of antigen-specific IgA AFC in lungs. Both IP:IN and IN:IN mice had significantly higher (P ≤ 0.05) numbers of antigen-specific IgG AFC in their lungs than did mice given primary immunizations only or IP:0 or 0:IN immunized mice. However, there was no significant difference between the numbers of antigen-specific IgG AFC found in lungs of IP:IN and IN:IN immunized mice.
TABLE 5.
Numbers of influenza virus-specific AFC per 106 cells from each tissue obtained from mice after intranasal immunization
| Tissue | Isotype | Value for route of immunizationa
|
||||
|---|---|---|---|---|---|---|
| Control | IP:0 | 0:IN | IP:IN | IN:IN | ||
| Nasal passages | IgA | 0b | 2 (2.2) | 63 (1.5) | 157 (1.3) | 3,597 (1.2)* |
| IgG | 0 | 17 (4.7) | 0 | 13 (4.2) | 66 (9.4) | |
| IgM | 0 | 22 (5.2) | 7 (3.1) | 121 (1.4) | 7 (3.1) | |
| Lung | IgA | 0 | 10 (1.7) | 3 (1.2) | 67 (2.6)* | 323 (1.7)* |
| IgG | 0 | 6 (1.7) | 9 (1.2) | 66 (1.4)* | 27 (1)* | |
| IgM | 0 | 3 (1.5) | 5 (1.3) | 17 (2.1)* | 26 (1.1)* | |
| Spleen | IgA | 2 (1.7) | 5 (1.9) | 10 (1.4) | 16 (1.3) | 899 (1.4)* |
| IgG | 2 (1.5) | 26 (2.2) | 10 (1.5) | 69 (1.1) | 265 (1.3)* | |
| IgM | 11 (1.9) | 38 (1.5) | 53 (1.1) | 59 (1.1) | 344 (1.2)* | |
Immunization groups are designated as described in Table 4. All immunizations were performed with inactivated influenza virus vaccine plus 0.1 μg of CT.
Values are geometric means (± standard errors) of numbers of AFC per 106 cells from three experiments (two experiments for lung IgG and IgM) with five mice each. Asterisks indicate values that are significantly different from those for both primary immunization groups (0:IN and IP:0).
To measure influenza virus-specific IgA production in the gastrointestinal tract following immunization, fecal samples were collected from each of the four immunization groups described for the previous experiments, and the levels of antigen-specific IgA were determined. IN:IN immunizations produced significantly higher levels of antigen-specific fecal IgA (P ≤ 0.05) than did all other groups (data not shown). There was no significant antigen-specific fecal IgA in the other groups of mice. Thus, it appears that primary and secondary immunization induces influenza virus-specific mucosal IgA production in the gastrointestinal tract.
Antigen is deposited primarily in the lung after intranasal immunization.
There was a possibility that intranasal immunization could result in mice ingesting antigen and that the fecal IgA production was a result of oral immunization. To determine whether this was indeed a possibility, we measured the distribution of antigen following intranasal inoculation with luciferase enzyme. Similar to our intranasal immunization protocol, mice were intranasally inoculated with 24 μl of luciferase enzyme, and nasal passages, lungs, trachea, and esophagus of each mouse were removed 5 min later. Luciferase enzyme activity was measured in each of these tissues to determine the tissue distribution of its deposition. The majority of enzyme was deposited in the lung (71% ± 14%), but significant amounts of luciferase activity were found in the esophagus (21% ± 9%), trachea (7% ± 3%), and nasal passages (10% ± 4%). Thus, the deposition of luciferase in the esophagus suggests that the fecal IgA responses could be due to an inadvertent oral immunization of the mice following intranasal inoculation.
Intranasal immunization results in antigen-specific IgA at other mucosal sites.
To determine if IgA responses were due to intranasal but not oral immunization, mice were either intranasally or orally immunized on days 0 and 7 with 5 μg of influenza virus antigen plus 0.1 μg of CT. Seven days later, serum and fecal extracts were collected and assayed for influenza virus-specific IgA production. To examine a mucosal site other than the intestinal tract, we also collected genital tract washes from each of the immunized mice.
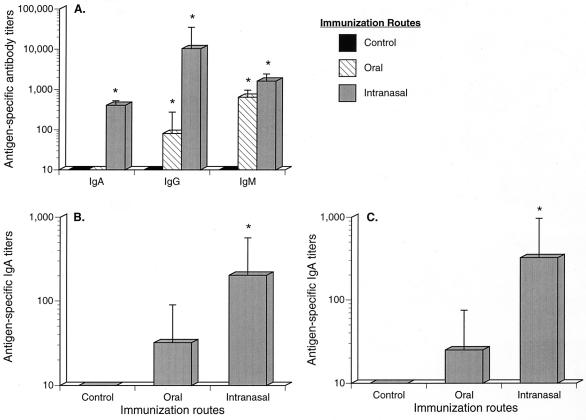
Serum IgA and IgG levels were significantly higher in intranasally immunized animals; however, both intranasally and orally immunized animals generated higher levels of IgM than did control mice (Fig. 6A). In fecal samples, intranasal immunization resulted in significantly higher levels of fecal IgA production than those for control animals (P ≤ 0.05), while oral immunization did not (Fig. 6B). Results were similar for the genital tract washes, where IgA was significantly higher (P ≤ 0.05) in intranasally immunized mice than in control animals. As well, there were low levels of antigen-specific IgA in genital tract washes of orally immunized mice, but these values were not significantly higher than those for unimmunized mice (Fig. 6C).
FIG. 6.
Intranasal, but not oral, immunization induces antigen-specific IgA production in the gastrointestinal and urogenital tracts. Mice were either intranasally or orally immunized on days 0 and 7 with inactivated influenza virus vaccine plus 0.1 μg of CT. Sera (A), fecal samples (B), and urogenital washes (C) were collected 14 days after primary immunization. The results are from three experiments. A single asterisk denotes statistical differences (P ≤ 0.05) from the value for the control.
DISCUSSION
The purpose of the present study was to determine the extent of immunologic responses, particularly immunopathologic responses, within the upper and lower respiratory tracts after intranasal immunization. Intranasal immunization with inactive vaccines has many attractive features, such as use of a wider variety of antigens, that should result in an extremely powerful approach to preventing infection and disease. Despite the promise of intranasal immunization, it is clear that there is a potential for immunopathologic responses. Many studies have used CT as a common adjuvant during intranasal immunization because of its well-known ability to enhance mucosal immune responses via other mucosal routes of inoculation, e.g., oral. However, in a previous study (35), we found that adverse immunologic reactions in the lungs can result from intranasal immunization with tetanus toxoid using relatively high doses of CT as a mucosal adjuvant. IgE responses against the antigen were induced after secondary immunization with antigen plus CT. Along with IgE responses, there was a massive infiltration of mononuclear cells into the lungs of animals. There are many unresolved questions from these studies that need to be addressed, not only to further develop intranasal immunization to prevent infectious diseases but also to understand the generation of immunity along the respiratory tract.
As our previous studies used high doses of CT (10 μg at primary immunization followed by 3.3 μg at secondary immunization) during intranasal immunization (35), we determined whether lowering the dosages of CT could result in retention of adjuvant activity while reducing the adverse IgE-associated inflammatory reactions. Using influenza virus vaccine, we demonstrated that adjuvant activity was significant at doses of 0.1 to 2.5 μg of CT, but obvious clinical signs (loss of activity, weight loss, and ruffled fur) were seen only for mice given 2.5 μg of CT. This indicates that there was a reduction in the severe reactions to CT at lower dosages (0.1 and 0.5 μg). Because of previous suggestions that CT preferentially stimulates Th2 responses (20), which promote IgE production (9, 21), it was expected that lowering the amount of CT would minimize IgE responses. As in our previous studies (35), serum IgE levels were enhanced in mice immunized using CT as an adjuvant. But as expected, this effect was dose dependent, with the total IgE levels being highest in mice immunized with 2.5 μg of CT. In fact, reaginic Ab, as determined by PCA, was readily detected in mice given the highest dose of CT (2.5 μg), while reaginic Ab was not consistently detected in mice given lower doses of CT. However, IgE responses were induced in mice intranasally immunized with antigen alone, and intraperitoneal immunization did not induce significant IgE responses. The difference due to the route of immunization is most likely linked to the preferential distribution of Th2 cells in the lung, whereas nonmucosal lymphoid tissues, such as spleen, have a predominance of Th1 cells (unpublished data). As IgE responses are promoted by production of interleukin-4 (IL-4) by Th2 cells, intranasal immunization should result in Th2 cell activation and subsequent IgE production (9, 21). In contrast, Th1 cells generated after parenteral immunization would be antagonistic to IgE through their production of gamma interferon. Thus, intranasal immunization, although potentially very effective, has the capacity to generate IgE responses with possible serious consequences associated with hypersensitivity reactions, and use of adjuvants can enhance these potentially detrimental responses.
Inflammatory reactions along the respiratory tract may also be a complication of intranasal immunization and use of adjuvants. Previously, we found that severe inflammatory reactions in lung tissue resulted from intranasal immunization (35); however, we did not examine for histopathologic changes in the upper respiratory tract. In the present study, there was, however, only a mild inflammation in the nasal passages associated with the highest dose of CT, and the reaction in the nasal passages was eliminated at the lower doses. Furthermore, the numbers of antigen-specific IgA-producing cells in nasal passages were much higher in mice immunized with the lowest dose of CT (0.1 μg) than in mice given antigen alone, indicating that inflammatory reactions in nasal passages were eliminated while adjuvancy was retained. In lungs, lowering the doses of CT eliminated the histopathologic effects in the alveoli, but in contrast to what occurred in the upper respiratory tract, there was no apparent decrease in the degree of cellular infiltration along the airways and blood vessels of the lung. Interestingly, mice given antigen alone produced IgE responses but lacked histopathologic changes. This suggests that the pulmonary inflammation may not be solely a result of an IgE response and that other immune or inflammatory mechanisms contribute to these reactions.
In support, intranasal immunization was previously shown to prime for delayed-type hypersensitivity reactions (40), which are mediated by Th1 cells (21). Even in a murine model of asthma, inflammatory responses in the lung may result from Th1 cell activity (27) while pulmonary hyperresponses are mediated by Th2 cells and IgE (6). In ongoing studies, we have demonstrated that Th1 cytokines are elicited in lungs after intranasal immunization with influenza virus vaccine antigen and CT, while antigen alone preferentially induced Th2-type responses (unpublished data). In any case, these studies demonstrate that a complication of pulmonary immunization is the potential for inflammatory responses in the lung. Importantly, the presence of cellular infiltration in the lung, but not in nasal passages, suggests that there is a fundamental difference in the generation of immune and inflammatory responses along the upper and lower respiratory tracts.
There is indeed a difference between the immunity in upper and lower respiratory tracts in the contribution of lymphoid responses initiated outside the respiratory tract to these responses. Previously, we demonstrated that adoptive transfer of lymphocytes does not prevent upper respiratory tract infection and disease but does protect the lung from infection (17). Also, we (17) and others (39) demonstrated that intravenously given Ab does not protect against upper respiratory tract infections but does prevent pneumonia. In the present studies, we extended these observations and examined whether prior parenteral (intraperitoneal) immunization can augment development of Ab responses along the respiratory tract. We found that intraperitoneal immunization did not enhance the generation of Ab responses within nasal passages after secondary intranasal immunization, suggesting that lymphocytes do not readily circulate from systemic lymphoid tissues into nasal passages in the absence of disease. This was, however, not true for the lung. Parenteral immunization was able to enhance the development of Ab responses in lungs, demonstrating the ability of extrapulmonary cells to contribute to pulmonary immunity. This is consistent with the histopathologic evidence that shows a cellular infiltration into the lung but not in nasal passages. Also, we consistently recovered more cells in the lungs after intranasal immunization using CT, but similar numbers of cells were recovered from nasal passages whether CT was used or not (data not shown). Thus, Ab responses in the upper respiratory tract are primarily due to lymphoid responses in mucosal tissues. Upper respiratory tract immunity is most likely induced by stimulation of local lymphoid tissues, as oral immunization can also augment immunity in the nasal passages; however, the Ab responses within the lungs are derived from both local and systemic immune systems.
Although the contribution of systemic immunity in protection differs between the upper and lower respiratory tract, mucosal immunization is still necessary to develop IgA responses in nasal passages and lungs. Parenteral immunization alone was unable to elicit an IgA response within the respiratory tract. Intranasal immunization readily stimulated IgA production in both the upper and lower respiratory tracts. In fact, it appears that both the upper and lower respiratory tracts are predisposed to producing IgA responses. In support, there was a substantially greater number of spontaneously IgA-producing cells in nasal tissues and lungs of naive (unimmunized) mice than of cells secreting IgG or IgM. Intranasal immunization also resulted in specific IgA in nasal secretions and large numbers of antigen-specific IgA AFC within both nasal tissues and lungs. However, there was a significant number of antigen-specific IgG-producing cells in lungs, whereas IgG responses were minimal in the upper respiratory tract. Furthermore, as parenteral immunization alone did not stimulate IgA responses, it was surprising that parenteral immunization followed by intranasal immunization resulted not only in secondary IgG responses in lungs but also enhanced pulmonary IgA responses. The mechanisms for this are unknown, but studies suggest that migration of antigen-presenting B cells from peripheral lymphoid tissues to the intestines may contribute to generation of mucosal IgA responses after parenteral immunization (5). A similar mechanism may be contributing to humoral immunity, including IgA, within the lung after intraperitoneal immunization. In a previous study (17), we also showed that the initial B-cell response generated in the upper respiratory tract in response to respiratory virus infection is indeed production of IgA. Thus, the microenvironment within both the upper and lower respiratory tracts preferentially promotes IgA production, supporting the idea that mucosal IgA responses are critical to preventing infectious diseases of the upper respiratory tract and the subsequent spread of infections along the airways into the lungs.
We examined the deposition of antigen following intranasal inoculation of luciferase and found that the majority of inoculum was deposited in the respiratory tract. This suggests that the effectiveness of intranasal immunization to stimulate mucosal IgA responses is through deposition of antigen directly on a mucosal inductive site, most likely the nasally associated lymphoid tissue (43, 44). However, antigen-specific IgA responses in the gastrointestinal and genital tracts indicate that IgA-IgB cells, stimulated after intranasal immunization, can migrate to other mucosal sites. Alternatively, it is possible that IgA in serum is being transported directly across mucosal epithelial surfaces or by hepatobiliary transport (16, 31, 32, 34). Although the transport of IgA may be contributing to the IgA at other mucosal sites, there was little or no IgA found in serum at this time point after intranasal immunization using this dose of adjuvant (0.1 μg of CT). Also, IgA responses were found in spleen cells, supporting the idea that IgA-IgB cells can migrate from respiratory tract lymphoid tissues to nonrespiratory tract tissues, which likely include other mucosal sites. This also suggests the possibility of primed IgA-IgB cells migrating from the upper respiratory tract into the lung, thereby bolstering pulmonary immunity against infections found initially in nasal passages. Thus, our data support the efforts to use intranasal immunization to enhance immunity at other mucosal sites (12–14, 33), as well as throughout the respiratory tract.
Despite the promise of intranasal immunization to protect against respiratory disease, our results support our previous observations that there is the potential to develop adverse immunopathologic reactions characterized by pulmonary airway inflammation associated with IgE production (35). In the present studies, lowering dosages of CT did reduce, but not eliminate, these adverse reactions without compromising immunogenicity. The lymphoid infiltration into the lung may be one of CT's mechanisms of adjuvancy. This effect of CT may have not only a quantitative impact on immunity in the lung but, by enhancing recruitment of cells from extrapulmonary sites, also some qualitative effects. However, our data suggest that the microenvironment of the lung may still have some influence on the type of immune responses generated. In contrast to what occurred in lungs, there was little histopathologic evidence of adverse reactions in nasal passages. Furthermore, systemic lymphoid responses did not readily contribute to immunity within the upper respiratory tract. These results verify previous observations that parenteral immunization results in limited immunity and protection from upper respiratory tract infections (10, 24, 26). Importantly, IgA responses were shown to dominate humoral immunity after intranasal immunization throughout the respiratory tract, whereas IgG responses significantly contributed to immunity in lungs, and not in nasal passages. Despite the potential problems, intranasal immunization is an optimal approach to generate mucosal IgA responses in both the upper and lower respiratory tracts and can also provide immunity at other mucosal sites. Thus, approaches need to be developed to avoid potentially serious immunologic or inflammatory reactions. For example, other adjuvants need to be examined not only for their adjuvant activity but also for their potential immunopathology. However, it is possible, given the preferential production of IL-4 by lung cells (unpublished data), that IgE production will be a major component of any response to a soluble antigen given by this route unless approaches are developed to avoid these complications. By understanding the mechanisms of immunity operating in both the lower and upper respiratory tracts, the events leading to the development of IgE responses and recruitment of cells to the lungs can be identified. Future studies can then determine if these responses can be beneficially altered by treatment with recombinant cytokines or other modulators that down regulate IgE production or cellular recruitment, leading to vaccine-adjuvant combinations which induce appropriate protective immune responses.
ACKNOWLEDGMENTS
We thank the other members of the University of Alabama Immunobiology Vaccine Center, Ray Jackson, Mark Hart, and Tony Romeo, for their useful discussions. We also thank Maurice W. Harmon (Connaught Laboratories, Inc.) for kindly providing influenza virus vaccine antigen.
This work was supported by the American Lung Association of Texas (J.W.S.) and Public Health Service grants AI 42075 (J.W.S.) and AI 15128, AI 43197, and AI 18958 (J.R.M.) from the National Institutes of Health. Lisa M. Hodge was supported, in part, by a grant from Advanced Technology Program, Texas Coordinating Board.
REFERENCES
- 1.Barker W H. Excess pneumonia and influenza associated hospitalization during influenza epidemics in the United States, 1970–78. Am J Public Health. 1986;76:761–765. doi: 10.2105/ajph.76.7.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belshe R B, Mendelman P M, Treanor J, King J, Gruber W C, Piedra P, Bernstein D I, Hayden F G, Kotloff K, Zangwill K, Iacuzio D, Wolff M. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine in children. N Engl J Med. 1998;338:1405–1412. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 3.Clements L M, Betts R F, Tierney E L, Murphy B R. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24:157–160. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clements L M, O'Donnell S, Levine M M, Chanock R M, Murphy B R. Dose response of A/Alaska/6/77 (H3N2) cold-adapted reassortment vaccine virus in adult volunteers: role of local antibody in resistance to infection with vaccine virus. Infect Immun. 1983;40:1044–1051. doi: 10.1128/iai.40.3.1044-1051.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coffin S E, Clark S L, Bos N A, Brubaker J O, Offit P A. Migration of antigen-presenting B cells from peripheral to mucosal lymphoid tissues may induce intestinal antigen-specific IgA following parenteral immunization. J Immunol. 1999;163:3064–3070. [PubMed] [Google Scholar]
- 6.Cohn L, Tepper J S, Bottomly K. IL-4-independent induction of airway hyperresponsiveness by Th2, but not Th1, cells. J Immunol. 1998;161:3813–3816. [PubMed] [Google Scholar]
- 7.Czerkinsky C C, Nilsson L A, Nygren H, Ouchterlony O, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–121. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 8.Doepel L. Novel concepts put to the test in three new AIDS vaccine trials. NIAID News press release (29 Jan.). Bethesda, Md: National Institute of Allergy and Infectious Diseases; 1998. [Google Scholar]
- 9.Finkelman F D, Urban J F, Jr, Beckmann M P, Schooley K A, Holmes J M, Katona I M. Regulation of murine in vivo IgG and IgE responses by a monoclonal anti-IL-4 receptor antibody. Int Immunol. 1991;3:599–607. doi: 10.1093/intimm/3.6.599. [DOI] [PubMed] [Google Scholar]
- 10.Gorse G J, Otto E E, Daughaday C C, Newman F K, Eickhoff C S, Powers D C, Lusk R H. Influenza virus vaccination of patients with chronic lung disease. Chest. 1997;112:1221–1233. doi: 10.1378/chest.112.5.1221. [DOI] [PubMed] [Google Scholar]
- 11.Gruber W C, Darden P M, Still J G, Lohr J, Reed G, Wright P F. Evaluation of bivalent live attenuated influenza A vaccines in children 2 months to 3 years of age: safety, immunogenicity and dose-response. Vaccine. 1997;15:1379–1384. doi: 10.1016/s0264-410x(97)00032-7. [DOI] [PubMed] [Google Scholar]
- 12.Hordnes K, Tynning T, Brown T A, Haneberg B, Jonsson R. Nasal immunization with group B streptococci can induce high levels of specific IgA antibodies in cervicovaginal secretions of mice. Vaccine. 1997;15:1244–1251. doi: 10.1016/s0264-410x(97)00021-2. [DOI] [PubMed] [Google Scholar]
- 13.Hu K F, Ekstrom J, Merza M, Lovgren-Bengtsson K, Morein B. Induction of antibody responses in the common mucosal immune system by respiratory syncytial virus immunostimulating complexes. Med Microbiol Immunol. 1999;187:191–198. doi: 10.1007/s004300050092. [DOI] [PubMed] [Google Scholar]
- 14.Klavinskis L S, Barnfield C, Gao L, Parker S. Intranasal immunization with plasmid DNA-lipid complexes elicits mucosal immunity in the female genital and rectal tracts. J Immunol. 1999;162:254–262. [PubMed] [Google Scholar]
- 15.Kruisbeek A. Isolation and fractionation of mononuclear cell populations. In: Coligan J E, Kruisbeek A, Margulies D, Shevach E, Strober W, editors. Current protocols in immunology. Vol. 1. New York, N.Y: John Wiley & Sons, Inc.; 1999. pp. 3.1.1–3.1.5. [Google Scholar]
- 16.Lamm E M, Robinson J K, Rao C K, Vaerman J P, Kaetzel C S. Epithelial transport of IgA immune complexes. Adv Exp Med Biol. 1991;310:187–191. doi: 10.1007/978-1-4615-3838-7_24. [DOI] [PubMed] [Google Scholar]
- 17.Liang S C, Simecka J W, Lindsey J R, Cassell G H, Davis J K. Antibody responses after Sendai virus infection and their role in upper and lower respiratory tract disease in rats. Lab Anim Sci. 1999;49:385–394. [PubMed] [Google Scholar]
- 18.Liang X P, Lamm M E, Nedrud J G. Cholera toxin as a mucosal adjuvant for respiratory antibody responses in mice. Reg Immunol. 1989;2:244–248. [PubMed] [Google Scholar]
- 19.Lui K J, Kendal A P. Impact of influenza epidemics on mortality in the United States from October 1972 to May 1985. Am J Public Health. 1987;77:712–716. doi: 10.2105/ajph.77.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marinaro M, Staats H F, Hiroi T, Jackson R J, Coste M, Boyaka P N, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, et al. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 21.Mosmann T, Coffman R. TH1 and TH2: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 22.Murphy B R, Clements M L, Johnson P R, Wright P F. The mucosal and systemic immune responses of children and adults to live and inactivated influenza A virus vaccines. In: Strober W, Lamm M E, McGhee J R, James S P, editors. Mucosal immunity and infections at mucosal surfaces. New York, N.Y: Oxford University Press; 1988. pp. 303–318. [Google Scholar]
- 23.Nichol K L, Mendelman P M, Mallon K P, Jackson L A, Gorse G J, Belshe R B, Glezen W P, Wittes J. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults: a randomized controlled trial. JAMA. 1999;282:137–144. doi: 10.1001/jama.282.2.137. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson K G, Baker D J, Chakraverty P, Farquhar A, Hurd D, Kent J, Litton P A, Smith S H. Immunogenicity of inactivated influenza vaccine in residential homes for elderly people. Age Ageing. 1992;21:182–188. doi: 10.1093/ageing/21.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novak M, Moldoveanu Z, Schafer D P, Mestecky J, Compans R W. Murine model for evaluation of protective immunity to influenza virus. Vaccine. 1993;11:55–60. doi: 10.1016/0264-410x(93)90339-y. [DOI] [PubMed] [Google Scholar]
- 26.Powers D C, Sears S D, Murphy B R, Thumar B, Clements M L. Systemic and local antibody responses in elderly subjects given live or inactivated influenza A virus vaccines. J Clin Microbiol. 1989;27:2666–2671. doi: 10.1128/jcm.27.12.2666-2671.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randolph D A, Carruthers C J, Szabo S J, Murphy K M, Chaplin D D. Modulation of airway inflammation by passive transfer of allergen-specific Th1 and Th2 cells in a mouse model of asthma. J Immunol. 1999;162:2375–2383. [PubMed] [Google Scholar]
- 28.Renegar K B, Small P A., Jr Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991;65:2146–2148. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renegar K B, Small P A., Jr Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- 30.Reuman P D, Keely S P, Schiff G M. Similar subclass antibody responses after intranasal immunization with UV-inactivated RSV mixed with cholera toxin or live RSV. J Med Virol. 1991;35:192–197. doi: 10.1002/jmv.1890350309. [DOI] [PubMed] [Google Scholar]
- 31.Russell W M, Brown T A, Mestecky J. Preferential transport of IgA and IgA-immune complexes to bile compared with other external secretions. Mol Immunol. 1982;19:677–682. doi: 10.1016/0161-5890(82)90369-8. [DOI] [PubMed] [Google Scholar]
- 32.Russell W M, Brown T A, Mestecky J. Role of serum IgA. Hepatobiliary transport of circulating antigen. J Exp Med. 1981;153:968–976. doi: 10.1084/jem.153.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russell W M, Hedges S R, Wu H Y, Hook E W, 3rd, Mestecky J. Mucosal immunity in the genital tract: prospects for vaccines against sexually transmitted diseases—a review. Am J Reprod Immunol. 1999;42:58–63. doi: 10.1111/j.1600-0897.1999.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 34.Simecka J. Mucosal immunity of the gastrointestinal tract and oral tolerance. Adv Drug Delivery Rev. 1998;34:235–259. doi: 10.1016/s0169-409x(98)00042-8. [DOI] [PubMed] [Google Scholar]
- 35.Simecka J, Jackson R, Kiyono H, McGhee J. Mucosally induced immunoglobulin E-associated inflammation in the respiratory tract. Infect Immun. 2000;68:672–679. doi: 10.1128/iai.68.2.672-679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simecka J W, Patel P, Davis J K, Cassell G H. Upper respiratory tract is the major site of antibody production in mycoplasmal induced chronic respiratory disease. Reg Immunol. 1989;2:385–389. [PubMed] [Google Scholar]
- 37.Simecka J W, Patel P, Davis J K, Ross S E, Otwell P, Cassell G H. Specific and nonspecific antibody responses in different segments of the respiratory tract in rats infected with Mycoplasma pulmonis. Infect Immun. 1991;59:3715–3721. doi: 10.1128/iai.59.10.3715-3721.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simecka J W, Thorp R B, Cassell G H. Dendritic cells are present in the alveolar region of lungs from specific pathogen-free rats. Reg Immunol. 1992;4:18–24. [PubMed] [Google Scholar]
- 39.Small P A., Jr Influenza: pathogenesis and host defense. Hosp Pract. 1990;25:51–54. doi: 10.1080/21548331.1990.11704033. , 57–62. [DOI] [PubMed] [Google Scholar]
- 40.Tamura S, Miyata K, Matsuo K, Asanuma H, Takahashi H, Nakajima K, Suzuki Y, Aizawa C, Kurata T. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant-combined recombinant nucleoprotein. J Immunol. 1996;156:3892–3900. [PubMed] [Google Scholar]
- 41.van Ginkel F W, Liu C, Simecka J W, Dong J Y, Greenway T, Frizzell R A, Kiyono H, McGhee J R, Pascual D W. Intratracheal gene delivery with adenoviral vector induces elevated systemic IgG and mucosal IgA antibodies to adenovirus and beta-galactosidase. Hum Gene Ther. 1995;6:895–903. doi: 10.1089/hum.1995.6.7-895. [DOI] [PubMed] [Google Scholar]
- 42.Walsh E E. Mucosal immunization with a subunit respiratory syncytial virus vaccine in mice. Vaccine. 1993;11:1135–1138. doi: 10.1016/0264-410x(93)90075-9. [DOI] [PubMed] [Google Scholar]
- 43.Wu H Y, Nguyen H H, Russell M W. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand J Immunol. 1997;46:506–513. doi: 10.1046/j.1365-3083.1997.d01-159.x. [DOI] [PubMed] [Google Scholar]
- 44.Wu H Y, Russell M W. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol Res. 1997;16:187–201. doi: 10.1007/BF02786362. [DOI] [PubMed] [Google Scholar]