Abstract
Background:
Cisplatin-induced kidney injury is a major challenge hindering treatment of cancer patients. 30% of patients treated with cisplatin develop acute kidney injury (AKI). Even patients that do not develop AKI are at risk for long term decline in renal function and development of chronic kidney disease (CKD). Despite researcher’s best efforts, no therapeutic agents to treat or prevent cisplatin-induced kidney injury have made it past phase 2 clinical trials.
Summary:
Modeling cisplatin-induced kidney injury in rodents has primarily been done using a single, high dose model of injury. Newer models of injury have utilized repeated, low or intermediate doses of cisplatin to incorporate study of maladaptive repair processes following a renal insult. We believe utilization of all these models is important to understand and treat the diverse types of cisplatin-induced kidney injury patients develop in the clinic. Incorporating comorbidities such as cancer and development of large animal models are also vital to increasing the human relevance of our studies.
Key Messages:
Utilizing multiple dosing models of cisplatin-induced kidney injury, including relevant comorbidities and biological variables, and development of large animal models will increase the translational potential of preclinical studies.
Keywords: cisplatin, AKI, CKD, cancer, porcine models
Introduction
Despite major advances in the field of oncology, drug-induced kidney injury remains a giant hurdle in the treatment of cancer patients. Cisplatin is a prime example of this, with its effectiveness largely hindered by dose-limiting nephrotoxicity. 30% of patients treated with cisplatin develop acute kidney injury (AKI). Even patients that do not develop AKI by clinical standards are at risk for long term decline in renal function and development of fibrosis and chronic kidney disease (CKD). Development of AKI often requires suspension of cisplatin treatment and utilization of less effective alternative therapeutics (such as carboplatin). Many studies have been performed attempting to prevent cisplatin-induced kidney injury, but no therapeutics have made it past phase 2 clinical trials. We believe this is at least in part due to the rodent models being used in preclinical studies [1].
Main Text
Most preclinical studies evaluating cisplatin-induced kidney injury have utilized a single, high dose model of injury. In this model, mice receive a single intraperitoneal injection of 20–25 mg/kg cisplatin and must be euthanized 3–4 days later. These mice develop severe AKI, characterized by large increases in blood urea nitrogen (BUN) and serum creatinine, high levels of tubular apoptosis and necrosis, and severe structural damage. While this model is useful for studying severe, acute responses to injury, it does not mirror the way in which most humans are given cisplatin in the clinic and cannot be used to study chronic effects of cisplatin nephrotoxicity.
To address this, our lab and others developed a repeated, low dose model of cisplatin-induced kidney injury [2]. In this model, mice receive four weekly doses of 7–9 mg/kg cisplatin. With this model, mice do not develop severe AKI, only displaying mild increases in BUN, serum creatinine, and cell death. However, following cisplatin treatment mice have reduced GFR [3] accompanied by development of renal fibrosis and kidney inflammation. Fibrosis is characterized by collagen deposition, myofibroblast accumulation, increased fibronectin protein expression and elevation of Timp-1 and Col1a1 mRNA [2, 4]. Mice treated with this dosing model can also be aged up to 6 months after treatment and show signs of progressive renal fibrosis [2]. Comparing this model to the single, high dose model of cisplatin-induced kidney injury highlights the diversity and interconnectedness of drug-induced kidney pathologies (Fig. 1) [5].
Fig. 1. Models of Cisplatin-Induced Kidney Injury.
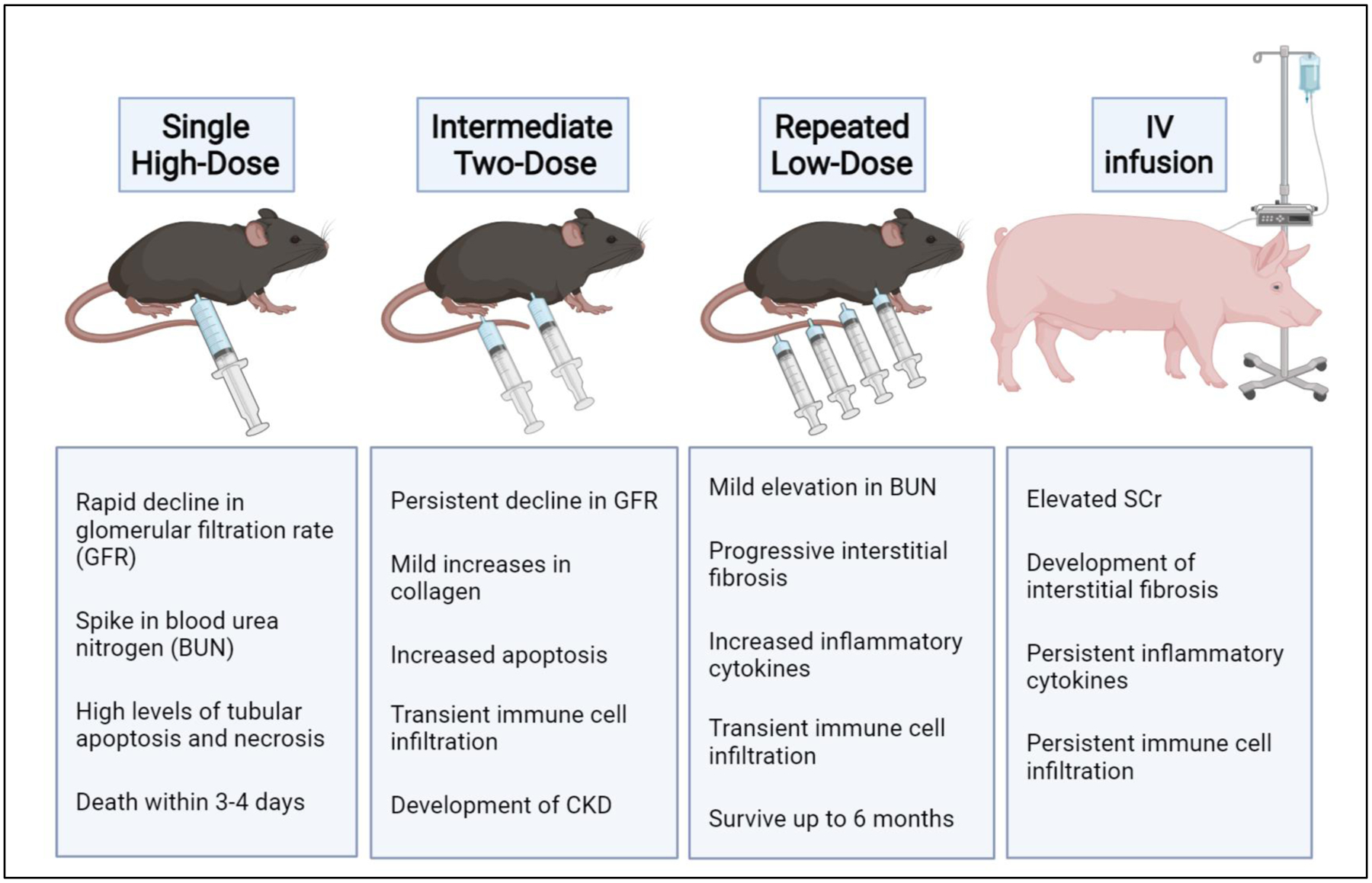
In the Single High-Dose model mice receive intraperitoneal injection of 20–25 mg/kg cisplatin and must be euthanized 3–4 days later. In the Intermediate Two-Dose model or hybrid model of cisplatin-induced kidney injury mice receive two doses of 15 mg/kg cisplatin, two weeks apart. In the Repeated Low-Dose model mice receive four weekly doses of 7–9 mg/kg cisplatin. In the porcine model cisplatin 75 mg/mm2 is administered via slow IV infusion through a surgical port closely mirroring how patient receive cisplatin in the clinic. Created with BioRender.com
Utilization of the repeated, low dose model of cisplatin-induced kidney injury has become more common in recent years. Recent data has been published by several groups highlighting key biological differences from the single, high dose model. We recently published how depletion of kidney resident and not infiltrating macrophage populations reduced development of cisplatin-induced kidney fibrosis [6]. Previously, macrophage depletion was shown to have no effect on AKI development following a single, high dose cisplatin treatment [7]. We have also observed that inhibition of autophagy has opposite effects on kidney injury in the acute and chronic models. Pharmacologic inhibitors of autophagy exacerbate development of cisplatin-induced AKI but reduce levels of cisplatin-induced fibrosis. We believe this protective effect may be due to increased clearance of damaged proximal tubule cells (unpublished data). These results suggest that both resident macrophages and autophagy could be potential therapeutic targets that have been overlooked in the past due to results obtained in the acute dosing model.
Further characterization and study of this repeated, low dose model could lead to identification of many more novel targets to prevent cisplatin nephrotoxicity. Ma et al. recently published snRNA-seq data, characterizing the changes occurring in proximal tubule cells of mice treated with repeated, low doses of cisplatin. They identified a new, “injury/repair” population of proximal tubule cells with increased expression of both Kim-1 and Ki-67. This population of proximal tubule cells is likely to play a major role in the transition from AKI to CKD [8].
Proximal tubule damage due to repeated, low dose cisplatin treatment has gained attention as a potential therapeutic target. One study found that the repeated dosing of cisplatin caused cumulative DNA damage in proximal tubule cells. Furthermore, these damaged cells remained in the kidney in the chronic phase of cisplatin-induced kidney injury. This study points to these “proinflammatory failed-repair” proximal tubule cells as key promoters of the AKI to CKD transition [9]. Likewise, recently published data demonstrated that p53 proximal tubule specific genetic deletion and global pharmalogical inhibition reduced development of cisplatin-induced fibrosis [10]. These studies highlight the role of sublethally injured proximal tubule cells in promoting cisplatin-induced kidney injury. In the single, high dose model of injury this population has not been identified, likely because most of the injured proximal tubule cells undergo apoptosis or necrosis.
Other groups have also developed an intermediate or hybrid model of cisplatin-induced kidney injury, whereby mice receive two doses of 15 mg/kg cisplatin, that are given two weeks apart. This model incorporates a higher level of toxicity while still providing insights on the effects of a repeat dose. Using this hybrid model, it was found that a kidney-targeted renalase agonist reduced development of cisplatin-induced chronic kidney disease by reducing populations of injured and stressed proximal tubule cells as well as reducing inflammation [11]. The hybrid model may allow for the study of both cisplatin-induced acute injury and the recovery processes following that injury. While we primarily focus on mouse models in this review, injury models have also been developed in rats, using both single and repeated injections of a range of cisplatin doses. While the dosing scale is slightly different, due to rats being more sensitive to cisplatin nephrotoxicity, the injury responses in the kidney are similar.
Altogether, these new data suggest that proximal tubule injury and differentiation into a failed repair state is a key driver of chronic cisplatin-induced kidney injury. We believe these injured proximal tubule cells promote profibrogenic activity of kidney resident macrophages driving development of cisplatin-induced fibrosis. Unresolved proximal tubule cell damage also appears to promote loss of kidney function and development of CKD. Strategies to protect proximal tubule cells from injury or to promote clearance of “failed-repair” proximal tubule cells deserve significant attention moving forward.
Future studies should also focus on increasing the translational relevance of cisplatin-induced kidney injury models. The addition of relevant comorbidities is important as most studies employ young male mice and rats. All patients receiving cisplatin have cancer and this is an imperative comorbidity to consider. Our lab has found that mice with cancer show signs of kidney injury and development of fibrosis even without cisplatin treatment (unpublished data in subcutaneous tumors in syngeneic mice as well as in genetically engineered mouse models). In these models, development of cancer outside of the kidney appears to increase markers of inflammation, injury, and fibrosis in the kidney. Therefore, cancer may heighten the pathological processes induced by cisplatin in the kidney. Future in depth and mechanistic studies on the mechanism by which tumors alter the kidney both prior to and following cisplatin treatment are needed to identify innovative pharmacological targets. Other relevant biological variables (alone and in combination) to consider modeling include diet (high fat or western diet), sex, aging, various aspects of metabolic syndrome (for example, hypertension, obesity, and/ or insulin resistance), and conditions inducing renal hypoxia.
In addition to including relevant comorbidities, future pre-clinical studies can increase in translational relevance by developing and employing large animal models of cisplatin-induced kidney injury [12]. Our lab and others have pursued development of a porcine model of injury (unpublished data) [13, 14]. The anatomy and physiology of pig kidneys are much closer to humans than that of rodents or even non-human primates. Pigs also display drug handling and metabolism processes that are much closer to what is seen in humans compared to that of mice, making pigs much more relevant for study of drug-induced pathologies. The similarities between pigs and humans have already made them widely used for therapeutic and medical device studies in the field of cardiology. Their larger body size makes it possible to mirror procedures exactly as they are done in humans, such as imaging, biopsies, surgeries, and routes of drug administration. Furthermore, pigs have a much longer lifespan than rodents and display genetic variability among individuals, like humans. The pig genome has also been sequenced and genetically engineered pigs are now available. Unfortunately, the higher cost of using pigs in research over mice can be a limiting factor.
Conclusion
The failure to develop agents to treat or prevent cisplatin-induced kidney injury is likely due to limited preclinical modeling. To increase translational potential, studies should incorporate multiple dosing schemes. The single, high dose model provides insight on severe toxic responses to kidney injury, while the repeated, low dose model mirrors development of subclinical injury and development of long-term fibrosis and CKD. The hybrid model allows for study of intermediate cases, allowing for AKI development and recovery. In the clinic, humans can develop cisplatin-induced kidney injury following all these patterns. Therefore, using all three of these models will help us develop agents to treat patients across this spectrum (Fig. 1). Inclusion of relevant comorbidities and biological variables as well as development of large animal injury models will also increase translational potential of preclinical studies. Incorporating these factors will lead to a better understanding of how humans develop cisplatin-induced kidney injury and development of therapeutic strategies to mitigate this injury.
Funding Sources
Support for this work was provided by National Institute of Diabetes and Digestive and Kidney Diseases Grants (R01DK124112 and 3 R01 DK124112-01S1 to L. J. Siskind and F31DK126400 to S. M. Sears).
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- 1.Hukriede NA, Soranno DE, Sander V, Perreau T, Starr MC, Yuen PST, et al. Experimental models of acute kidney injury for translational research. Nat Rev Nephrol. 2022. 2022/02/16. [DOI] [PubMed] [Google Scholar]
- 2.Sharp CN, Doll MA, Megyesi J, Oropilla GB, Beverly LJ, Siskind LJ. Subclinical kidney injury induced by repeated cisplatin administration results in progressive chronic kidney disease. Am J Physiol Renal Physiol. 2018. Jul 1;315(1):F161–F72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black LM, Lever JM, Traylor AM, Chen B, Yang Z, Esman SK, et al. Divergent effects of AKI to CKD models on inflammation and fibrosis. Am J Physiol Renal Physiol. 2018. Oct 1;315(4):F1107–F18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sears SM, Sharp CN, Krueger A, Oropilla GB, Saforo D, Doll MA, et al. C57BL/6 mice require a higher dose of cisplatin to induce renal fibrosis and CCL2 correlates with cisplatin-induced kidney injury. Am J Physiol Renal Physiol. 2020;319(4):F674–F85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sears S, Siskind L. Potential Therapeutic Targets for Cisplatin-Induced Kidney Injury: Lessons from Other Models of AKI and Fibrosis. J Am Soc Nephrol. 2021:ASN.2020101455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sears SM, Vega AA, Kurlawala Z, Oropilla GB, Krueger A, Shah PP, et al. F4/80hi Resident Macrophages Contribute to Cisplatin-Induced Renal Fibrosis. Kidney360. 2022: 10.34067/KID.0006442021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu LH, Oh DJ, Dursun B, He Z, Hoke TS, Faubel S, et al. Increased macrophage infiltration and fractalkine expression in cisplatin-induced acute renal failure in mice. J Pharmacol Exp Ther. 2008. Jan;324(1):111–7. [DOI] [PubMed] [Google Scholar]
- 8.Ma Z, Hu X, Ding HF, Zhang M, Huo Y, Dong Z. Single-Nucleus Transcriptional Profiling of Chronic Kidney Disease after Cisplatin Nephrotoxicity. Am J Pathol. 2022. Apr;192(4):613–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamashita N, Nakai K, Nakata T, Nakamura I, Kirita Y, Matoba S, et al. Cumulative DNA damage by repeated low-dose cisplatin injection promotes the transition of acute to chronic kidney injury in mice. Sci Rep. 2021. 2021/10/22;11(1):20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu S, Hu X, Ma Z, Wei Q, Xiang X, Li S, et al. p53 in Proximal Tubules Mediates Chronic Kidney Problems after Cisplatin Treatment. Cells. 2022;11(4):712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo X, Xu L, Velazquez H, Chen T-M, Williams RM, Heller DA, et al. Kidney-Targeted Renalase Agonist Prevents Cisplatin-Induced Chronic Kidney Disease by Inhibiting Regulated Necrosis and Inflammation. J Am Soc Nephrol. 2022;33(2):342–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, Bayliss G, Zhuang S. Porcine models of acute kidney injury. Am J Physiol Renal Physiol. 2021;320(6):F1030–F44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee Y-J, Li K-Y, Wang P-J, Huang H-W, Chen M-J. Alleviating chronic kidney disease progression through modulating the critical genus of gut microbiota in a cisplatin-induced Lanyu pig model. J Food Drug Anal. 2020. 2020/01/01/;28(1):103–14. [DOI] [PubMed] [Google Scholar]
- 14.Wang S-Y, Zhang C-Y, Cai G-Y, Chen X-M. Method used to establish a large animal model of drug-induced acute kidney injury. Exp Biol Med. 2021;246(8):986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


