Abstract
Objective:
The purpose of this study was to determine the prevalence of psychiatric diagnoses among a sample of breast reconstruction patients and measure the association between these diagnoses and reconstruction-related, patient-reported outcomes.
Summary of Background Data:
The impact of psychiatric disorders in conjunction with breast cancer diagnosis, treatment, and reconstruction have the potential to cause significant patient distress but remains not well understood.
Methods:
A retrospective review of post-mastectomy breast reconstruction patients from 2007–2018 at Memorial Sloan Kettering Cancer Center was conducted. Patient demographics, comorbidities, cancer characteristics, psychiatric diagnoses, and BREAST-Q Reconstruction Module scores (measuring satisfaction with breast, well-being of the chest, psychosocial, and sexual well-being) at postoperative years 1–3 were examined. Mixed effects models and cross-sectional linear regressions were conducted to measure the effect of psychiatric diagnostic class type and number on scores.
Results:
Of 7414 total patients, 50.1% had at least one psychiatric diagnosis. Patients with any psychiatric diagnoses prior to reconstruction had significantly lower BREAST-Q scores for all domains at all time points. Anxiety (50%) and depression (27.6%) disorders were the most prevalent and had the greatest impact on BREAST-Q scores. Patients with a greater number of psychiatric diagnostic classes had significantly worse patient-reported outcomes compared to patients with no psychiatric diagnosis. Psychosocial (β: −7.29; 95% CI: −8.67, −5.91) and sexual well-being (β: −7.99; 95% CI: −9.57, −6.40) were most sensitive to the impact of psychiatric diagnoses.
Conclusions:
Mental health status is associated with psychosocial and sexual well-being after breast reconstruction surgery as measured with the BREAST-Q. Future research will need to determine what interventions (e.g screening, early referral) can help improve outcomes for breast cancer patients with psychiatric disorders undergoing breast reconstruction.
MINI ABSTRACT
Question:
What is the impact of comorbid psychiatric diagnoses on breast reconstruction patient-reported satisfaction and quality of life outcomes comparing women with a history of psychiatric diagnosis prior to reconstructive surgery versus women without a history?
Findings:
Having any psychiatric diagnosis led to significantly lower patient-reported outcomes. As a patient increased the number of psychiatric diagnosis categories, patient-reported satisfaction and quality of life significantly decreased.
INTRODUCTION
Women with breast cancer can experience significant distress regarding their diagnosis and subsequent treatment, and are at high risk for developing psychiatric disorders during the course of their cancer treatment and into survivorship.1,2 Psychiatric comorbidities, such as depression and anxiety, have a significant impact on breast cancer clinical outcomes. Studies have demonstrated an increased risk of postoperative complications, prolonged hospitalization, non-adherence with cancer treatment, and mortality due to psychiatric comorbidities.3–7 Furthermore, psychiatric disorders can negatively impact patient quality-of-life, sometimes resulting in lasting emotional and social dysfunctions.8,9
Little research has focused specifically on the impact of mental health on breast cancer patients who undergo breast reconstruction.10,11 Given the increasing incidence of breast cancer and rates of survivorship12, it is imperative that clinicians understand the impact of pre-existing psychiatric conditions with breast cancer and reconstruction treatment on quality-of-life outcomes in this growing population.
A recently published study from our group examining patient-reported outcomes from 3,268 postmastectomy reconstruction patients over eight years identified that patients with any psychiatric diagnosis (i.e., ICD-9 or 10 code) were significantly more likely to have lower BREAST-Q satisfaction with breast and physical well-being of chest scores at all examined time points.10 Similarly, a smaller cross-sectional study with 471 patients has found that psychosocial and sexual well-being scores were lower in patients with a psychiatric diagnosis.11 While these studies identified an association between a psychiatric diagnosis and a decrease in BREAST-Q scores, larger, longitudinal studies with more specific categorization of psychiatric class are needed to obtain a deeper understanding.
In this study, we sought to further characterize the impact of mental health on patient-reported outcomes for post-mastectomy reconstruction patients. Our first objective was to quantify the effect of specific classes of psychiatric diagnoses and determine whether particular classes of psychiatric diagnoses have greater effects on BREAST-Q scores by comparing women with a history of psychiatric diagnosis prior to reconstruction versus women with no history of psychiatric diagnosis prior to reconstruction. Our second objective was to determine the impact of a greater number of psychiatric diagnostic classes, on BREAST-Q scores. We hypothesized that over a three-year period, patients with a history of any psychiatric disorder would have lower BREAST-Q scores for all domains, that different psychiatric classes (such as anxiety or depressive disorders) have different effects on scores, and that increasing number of diagnostic classes would be related to BREAST-Q scores decrease.
METHODS:
Data Source and Study Population
An IRB approved study (18–202) was performed to evaluate patient-reported outcomes in post-mastectomy reconstruction patients (a component of routine clinical care). All women who underwent breast reconstruction (immediate or delayed) with implant or autologous tissues between January 2007 and March 2018 at Memorial Sloan Kettering Cancer Center, an academic, National Cancer Institute designated cancer center, were eligible for inclusion. For implant patients, both one- and two-stage reconstructions were included. Autologous flap reconstructions included free transverse rectus abdominis myocutaneous (TRAM), muscle-sparing free TRAM, deep inferior epigastric perforator, and superficial inferior epigastric artery perforator flaps. Patients undergoing therapeutic oncologic and/or prophylactic mastectomy as well as patients having undergone prophylactic mastectomy due to genetic indications were included. All patients were female. The primary exclusion criterion was related to the timing of psychiatric diagnosis. Patients with no history of a psychiatric diagnosis before reconstruction who were later diagnosed with a psychiatric disorder following reconstruction were excluded. Patients with a history of a psychiatric diagnosis and who received an additional new diagnosis following reconstruction were included.
Data Collection and Patient Variables
Demographic data, treatment method, and postoperative outcomes were recorded secondarily and included: age, body mass index (BMI), history of smoking, diabetes, hypertension, history of bariatric surgery, marital status, and insurance type. These data were obtained through chart review of intake notes from the time of a patient’s breast reconstruction consultation. Cancer and surgical-related variables included: malignancy history (none, localized tumor, metastatic), radiation therapy timing (preoperative, postoperative, none), chemotherapy timing (neoadjuvant, adjuvant, none), reconstructive timing (immediate, immediate/delayed, or delayed), and laterality (unilateral or bilateral) plus lymphedema and hormone therapy status.
Classification of Psychiatric Diagnosis
Data was collected and extracted for each patient where psychiatric diagnosis was defined ICD-9: diagnosis codes between ‘290’ and ‘319.99’ or as ICD-10: diagnosis codes such as ‘F#’ and were recorded in patient medical records through clinical evaluation. Diagnosis codes were categorized into classes: anxiety, depressive, substance related, stress and adjustment, schizophrenia and psychotic, bipolar, personality, and other disorders (see Supplementary Table 1). A patient could have multiple diagnoses, some within the same class (e.g., multiple types of depressive diagnoses such as major depressive and depressive episodes) or among different classes (e.g., a depressive and substance-related disorders). The sum of different classes per patient were calculated and classified as number of psychiatric classes. Throughout the paper we will refer to a single ICD-9 or 10 code as “diagnosis” and a group of psychiatric disorders (i.e., depressive, anxiety) as “class”.
Questionnaire
Patient-reported outcomes were assessed via the reconstruction module of the BREAST-Q, which measures (1) satisfaction with breast, (2) psychosocial well-being, (3) physical well-being of the chest and upper body, (4) physical well-being of the abdomen, (5) sexual well-being, and (6) satisfaction with outcome. The BREAST-Q is a validated measure, first developed at our institution in 2007. Memorial Sloan Kettering Cancer Center implementation of the BREAST-Q began in 2009. The BREAST-Q reconstruction module was designed to be completed by patients who undergo either therapeutic or prophylactic mastectomy followed by reconstruction. Values for subscales were converted to summary scores ranging from 0 to 100 via Q-Score software. Higher scores represented superior outcomes, with a difference of 4 points on the Q-Score considered to be clinically significant.13 Satisfaction with breast, physical well-being of the chest, psychosocial well-being, and sexual well-being were included as the primary domains of interest. BREAST-Q data for the current study was classified as preoperative (prior to reconstruction) and postoperative (1–3 years). Patients were included in the patient-reported outcome analysis if they completed a BREAST-Q domain at any of the timepoints of interest.
Statistical Analysis
Baseline demographics, surgical, and cancer characteristics were compared between psychiatric diagnosis cohort and no psychiatric diagnosis cohort with a Student t-test (continuous variables) or Pearson Chi-Square test (categorical variables). Patients were classified as having a psychiatric disorder by the total number of different diagnosed, psychiatric classes per patient, and by class type. Sum of diagnosed classes was defined as number of patients multiplied by the number of diagnosis classes per/patient). Unadjusted and adjusted mixed effects regression models were created to analyze the impact of having any psychiatric diagnosis prior to reconstruction on BREAST-Q domain scores. Per domain, patients with any or all yearly BREAST-Q scores during the postoperative period (1–3 years) were included in the mixed effects models. A subgroup analysis of all patients who completed both a preoperative and 1-year postoperative BREAST-Q was performed to understand the change in score from baseline to one year, examining patients with and without psychiatric diagnoses. Mixed effects modeling was then performed, adjusting for timing of BREAST-Q completion.
Yearly cross-sectional unadjusted and adjusted linear regression models were used to analyze the influence of having a prevalent psychiatric disorder class on postoperative BREAST-Q domain scores. Specifically, these adjusted models accounted for patients with multiple psychiatric classes. Yearly cross-sectional unadjusted and adjusted linear regression models assessed increasing psychiatric classes (one, two, or three and more classes) on postoperative BREAST-Q domain scores. Given that most patients had an anxiety or anxiety and depressive disorder(s), a sensitivity analysis was conducted to analyze the influence of increasing number of diagnostic classes (one, two, and three or more classes) with class type (anxiety, anxiety and depressive, and anxiety, depressive, and other class). All adjusted models included confounders in the relationship between psychiatric diagnosis to BREAST-Q scores such as: age, BMI, smoking status, race/ethnicity, radiation, and reconstruction modality10. Timing of BREAST-Q responses were adjusted for as part of the mixed-effects analysis. Confounders were selected using a causal inference approach, specifically using directed acyclic graphs (DAGs) to model our causal pathways. The use of DAGs is an established approach for understanding the relationship between exposure and outcome, identifying appropriate variables for modeling, and, conversely, preventing overadjustment with variables that may actually increase bias in the model.14–18 Institutional experience and a review of the literature informed our choice of confounders.10 Our DAG has been included as Supplemental Figure 1. All analyses were conducted using R Statistical Software (R version 4.0.2, packages: tidyverse, ggplot2, nlme). All tests were two sided and an alpha level of 0.05 was considered significant.
RESULTS
A total of 8,515 breast reconstruction patients underwent breast reconstruction during the study period; 7,414 patients were included for final analysis while 1,101 patients did not have a diagnosis prior to reconstruction but were diagnosed at some point following reconstruction were excluded. Of the 7,414 patients, 3,711 (50.1%) patients had at least one psychiatric diagnosis prior to reconstruction (exposure group) and 3,703 (49.9%) patients had no diagnosed psychiatric disorder prior to reconstruction (control group). A flowchart of patients included in the study is shown in Supplemental Figure 2.
Demographics, Surgical, and Cancer-Related Characteristics
Patients with a history of psychiatric diagnosis were on average younger, more likely to be White, more likely to be a former or current smoker, and have hypertension (Table 1a). These patients also had significantly greater BMIs than the no psychiatric diagnosis cohort. Patients with a psychiatric diagnosis were more likely to have a localized tumor or metastatic cancer, require neo- or adjuvant chemotherapy, pre- or postoperative radiation, and hormone therapy. These patients were also more likely to experience lymphedema (Table 1b).
Table 1a.
Demographics of Study Population
| Overall Breast Reconstruction Patients with/without History of Psychiatric Diagnosis (n = 7414) | No History of Psychiatric Diagnosis (n = 3703) | History of Psychiatric Diagnosis (n = 3711) | p value | |
|---|---|---|---|---|
| Age, mean years (SD) | 50.0 (10.2) | 50.4 (10.3) | 49.7 (10.0) | 0.005 |
| Race, n (%) | <0.001 | |||
| White | 6087 (82.1) | 2902 (78.4) | 3185 (85.8) | |
| Black | 607 (8.2) | 358 (9.7) | 249 (6.7) | |
| Asian | 437 (5.9) | 285 (7.7) | 152 (4.1) | |
| Other/Unknown | 283 (3.8) | 158 (4.3) | 125 (3.4) | |
| Ethnicity, n (%) | 0.044 | |||
| Hispanic | 457 (6.2) | 243 (6.6) | 214 (5.8) | |
| Non-Hispanic | 6679 (90.1) | 3305 (89.3) | 3374 (90.9) | |
| Unknown | 278 (3.7) | 155 (4.2) | 123 (3.3) | |
| Smoking Status, n (%) | <0.001 | |||
| Never | 4430 (59.8) | 2544 (68.7) | 1886 (50.8) | |
| Former | 2045 (27.6) | 866 (23.4) | 1179 (31.8) | |
| Current | 539 (7.3) | 39 (1.1) | 500 (13.5) | |
| Unknown | 400 (5.4) | 254 (6.9) | 146 (3.9) | |
| Hypertension, n (%) | 1818 (24.5) | 802 (21.7) | 1016 (27.4) | <0.001 |
| Diabetes, n (%) | 526 (7.1) | 248 (6.7) | 278 (7.5) | 0.183 |
| BMI, mean kg/m2 (SD) | 25.9 (5.3) | 25.8 (5.3) | 26.1 (5.3) | 0.022 |
| History of Bariatric Surgery, n (%) | 31 (0.4) | 14 (0.4) | 17 (0.5) | 0.593 |
| Marital Status, n (%) | <0.001 | |||
| Single | 1278 (17.2) | 544 (14.7) | 734 (19.8) | |
| Married | 5320 (71.8) | 2806 (75.8) | 2514 (67.7) | |
| Separated | 94 (1.3) | 33 (0.9) | 61 (1.6) | |
| Divorced | 517 (7.0) | 224 (6.0) | 293 (7.9) | |
| Life/Domestic Partner | 27 (0.4) | 4 (0.1) | 23 (0.6) | |
| Widowed | 176 (2.4) | 92 (2.5) | 84 (2.3) | |
| Religion | 0.255 | |||
| Christian | 4262 (57.5) | 2150 (58.1) | 2112 (56.9) | |
| Non-Christian | 1171 (15.8) | 564 (15.2) | 607 (16.4) | |
| Other/Unknown | 216 (2.9) | 118 (3.2) | 98 (2.6) | |
| None | 1765 (23.8) | 871 (23.5) | 894 (24.1) | |
| Insurance | <0.001 | |||
| Private | 5870 (79.2) | 2952 (79.7) | 2918 (78.6) | |
| Medicare | 1217 (16.4) | 617 (16.7) | 600 (16.2) | |
| Medicaid | 262 (3.5) | 95 (2.6) | 167 (4.5) | |
| Self-pay | 55 (0.7) | 34 (0.9) | 21 (0.6) | |
| Unknown | 10 (0.1) | 5 (0.1) | 5 (0.1) |
Abbreviations: n Number of patients, SD Standard Deviation
p value for categorical data calculated using Chi-Square test, continuous data calculated using Student’s t-test
Table 1b.
Cancer Characteristics of Study Population
| Overall Breast Reconstruction Patients with/without History of Psychiatric Diagnosis (n = 7414) | No History of Psychiatric Diagnosis (n = 3703) | History of Psychiatric Diagnosis (n = 3711) | p value | |
|---|---|---|---|---|
| Malignancy History, n (%) | <0.001 | |||
| None | 555 (7.5) | 322 (8.7) | 233 (6.3) | |
| Localized Tumor | 6804 (91.8) | 3360 (90.7) | 3444 (92.8) | |
| Metastatic | 55 (0.7) | 21 (0.6) | 34 (0.9) | |
| Chemotherapy, n (%) | <0.001 | |||
| Neoadjuvant | 869 (11.7) | 279 (7.5) | 590 (15.9) | |
| Adjuvant | 2191 (29.6) | 894 (24.1) | 1297 (35.0) | |
| None | 4354 (58.7) | 2530 (68.3) | 1824 (49.2) | |
| Radiation, n (%) | <0.001 | |||
| Preoperative | 549 (7.4) | 257 (6.9) | 292 (7.9) | |
| Postoperative | 1424 (19.2) | 592 (16.0) | 832 (22.4) | |
| None | 5441 (73.4) | 2854 (77.1) | 2587 (69.7) | |
| Reconstruction Method, n (%) | 0.008 | |||
| Autologous | 867 (11.7) | 470 (12.7) | 397 (10.7) | |
| Implant | 6547 (88.3) | 3233 (87.3) | 3314 (89.3) | |
| Timing of Reconstruction, n (%) | 0.058 | |||
| Immediate | 7034 (94.9) | 3535 (95.5) | 3499 (94.3) | |
| Delayed | 361 (4.9) | 161 (4.3) | 200 (5.4) | |
| Immediate/Delayed | 19 (0.3) | 7 (0.2) | 12 (0.3) | |
| Laterality of Reconstruction, n (%) | <0.001 | |||
| Unilateral | 2761 (37.2) | 1488 (40.2) | 1273 (34.3) | |
| Bilateral | 4653 (62.8) | 2215 (59.8) | 2438 (65.7) | |
| Lymphedema, n (%) | 668 (9.0) | 224 (6.0) | 444 (12.0) | <0.001 |
| Hormone Therapy, n (%) | 2738 (36.9) | 1226 (33.1) | 1512 (40.7) | <0.001 |
Abbreviations: n Number of patients, SD Standard Deviation
p value for categorical data calculated using Chi-Square test, continuous data calculated using Student’s t-test
Number and Distribution of Classes
A total of 8,618 psychiatric diagnosis classes (ICD-9 and 10 codes; defined as number of patients multiplied by the number of diagnosis classes per/patient) were grouped into eight different psychiatric classes for the exposed cohort (Table 2). While most of the psychiatric diagnosis patients had only one class (n=1,696, [45.7%]), a large proportion of patients had two different psychiatric classes (30.6%) or three or more different classes (15.8%). No patient had all eight classes. Anxiety disorders were the most common class (n = 3,710 [99.97%]) followed by depressive disorders (n = 2,043 [55.1%]), substance related disorders (n = 1,224 [33%]), and stress and adjustment disorders (n = 1,054 [28.4%]). Supplemental Table 2 demonstrates that, while all patients with psychiatric diagnoses had at least one class present prior to reconstruction, additional/new diagnoses were made in the cohort post reconstruction. Nicotine and tobacco use was the most common substance-related disorder.
Table 2.
Prevalence of psychiatric disorders by type and number of class(es)
| Number of Classes | Number of Patientsa (n = 3711) | Anxiety Disordersb (n = 3710) | Depressive Disordersb (n = 2043) | Substance Related Disordersb (n = 1224) | Stress and Adjustment Disordersb (n = 1054) | Schizophrenia and Psychotic Disordersb (n = 104) | Bipolar Disorderb (n = 50) | Personality Disordersb (n = 48) | Other Disorderb (n = 270) | Sum of Diagnosis Classesc (N = 8618) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1696 (45.7) | 1085 (64) | 198 (11.7) | 305 (18) | 88 (5.2) | 4 (0.2) | 2 (0.1) | 0 (0) | 14 (0.8) | 1696 (19.7) |
| 2 | 1135 (30.6) | 985 (43.4) | 676 (29.8) | 349 (15.4) | 190 (8.4) | 13 (0.6) | 8 (0.4) | 6 (0.3) | 43 (1.9) | 2270 (26.3) |
| 3 | 585 (15.8) | 553 (31.5) | 514 (29.3) | 252 (14.4) | 333 (19) | 19 (1.1) | 12 (0.7) | 5 (0.3) | 67 (3.8) | 1755 (20.4) |
| 4 | 205 (5.5) | 202 (24.6) | 192 (23.4) | 120 (14.6) | 174 (21.2) | 25 (3) | 9 (1.1) | 14 (1.7) | 84 (10.2) | 820 (9.5) |
| 5 | 68 (1.8) | 67 (19.7) | 67 (19.7) | 49 (14.4) | 57 (16.8) | 28 (8.2) | 16 (4.7) | 13 (3.8) | 43 (12.6) | 340 (3.9) |
| 6 | 20 (0.5) | 20 (16.7) | 20 (16.7) | 20 (16.7) | 19 (15.8) | 13 (10.8) | 3 (2.5) | 8 (6.7) | 17 (14.2) | 120 (1.4) |
| 7 | 2 (0.1) | 2 (14.3) | 2 (14.3) | 2 (14.3) | 2 (14.3) | 2 (14.3) | 0 (0) | 2 (14.3) | 2 (14.3) | 14 (0.2) |
Abbreviations: n Number of patients
Sum of diagnosis classes = number of patients * number of classes
percentages are column percents describing the number of patients per number of classes/total number of patients
percentages are row percents describing the total disorder type/number of classes
percentages are column percents describing the number of classes/total
Mixed Effects Models: The Impact of Psychiatric Diagnosis
During the three-year postoperative period, adjusted models showed significantly lower scores in all four BREAST-Q domains when comparing patients with a diagnosed psychiatric disorder prior to post-mastectomy reconstruction to those who have no psychiatric diagnosis (Table 3). The greatest clinical and significant differences occurred within the psychosocial (β: −7.29; 95% CI: −8.67, −5.91) and sexual well-being (β: −7.99; 95% CI: −9.57, −6.40) domains.
Table 3.
Mixed effects models examining the impact of having any psychiatric diagnosis prior to breast reconstruction on BREAST-Q scores for all BREAST-Q domains
| Unadjusted Model | Adjusted Modela | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | (95% CI) | p | β | SE | (95% CI) | p | ||
| SATBR (n = 2962) | |||||||||
| Psychiatric Diagnosis | −3.78 | 0.62 | (−4.99, −2.56) | <0.001 | −3.46 | 0.63 | (−4.70, −2.23) | <0.001 | |
| PWBC (n = 3214) | |||||||||
| Psychiatric Diagnosis | −4.26 | 0.52 | (−5.27, −3.24) | <0.001 | −3.58 | 0.52 | (−4.61, −2.56) | <0.001 | |
| PSYCH (n = 2949) | |||||||||
| Psychiatric Diagnosis | −7.91 | 0.69 | (−9.25, −6.57) | <0.001 | −7.29 | 0.71 | (−8.67, −5.91) | <0.001 | |
| SEX (n = 2865) | |||||||||
| Psychiatric Diagnosis | −9.10 | 0.79 | (−10.65, −7.55) | <0.001 | −7.99 | 0.81 | (−9.57, −6.40) | <0.001 | |
Abbreviations: n Number of Patients Included, β Beta Coefficient, SE Standard Error, 95% CI 95% Confidence Interval, p p value, SATBR Satisfaction with Breast Domain, PWBC Physical Well-being of the Chest Domain, PSYCH Psychosocial Well-being Domain, SEX Sexual Well-being Domain
Reference cohort for all comparisons = No Psychiatric Diagnosis
Mixed effects model adjusted for fixed effects: age at reconstruction, body mass index (BMI), race/ethnicity, and radiation status. Random effect is patient
Subgroup examination of unadjusted, average score changes from the preoperative to one-year postoperative time period revealed that BREAST-Q scores changes trended similarly for patients who had psychiatric diagnoses and those who did not. (Supplemental Table 3) The mixed effects model (that adjusted for timing of survey response) of the preoperative to one-year postoperative time period, similarly revealed that patients with psychiatric diagnoses had significantly lower scores in all four BREAST-Q domains. (Supplemental Table 4)
Cross-Sectional Linear Regressions: The Most Prevalent Classes
Trends in the adjusted cross-sectional regressions demonstrated that anxiety and depressive classes were the more impactful psychiatric classes over time, amongst all four domains of the BREAST-Q, even after accounting for patients with multiple psychiatric classes (Figure 1, Table 4). Differences in adjusted estimates between patients with anxiety disorders versus no psychiatric disorders, over the three time points, ranged from: satisfaction with breast: −3.25 to −1.94 physical well-being of the chest: −3.95 to −1.43, psychosocial well-being: −4.36 to −3.50, and sexual well-being: −5.37 to −3.84. Adjusted differences comparing patients with depressive disorders versus no psychiatric disorders ranged from: satisfaction with breast: −3.54 to −2.86, physical well-being of the chest: −4.75 to −3.02, psychosocial well-being: −6.76 to −6.51, sexual well-being: −8.19 to −6.80.
Figure 1:
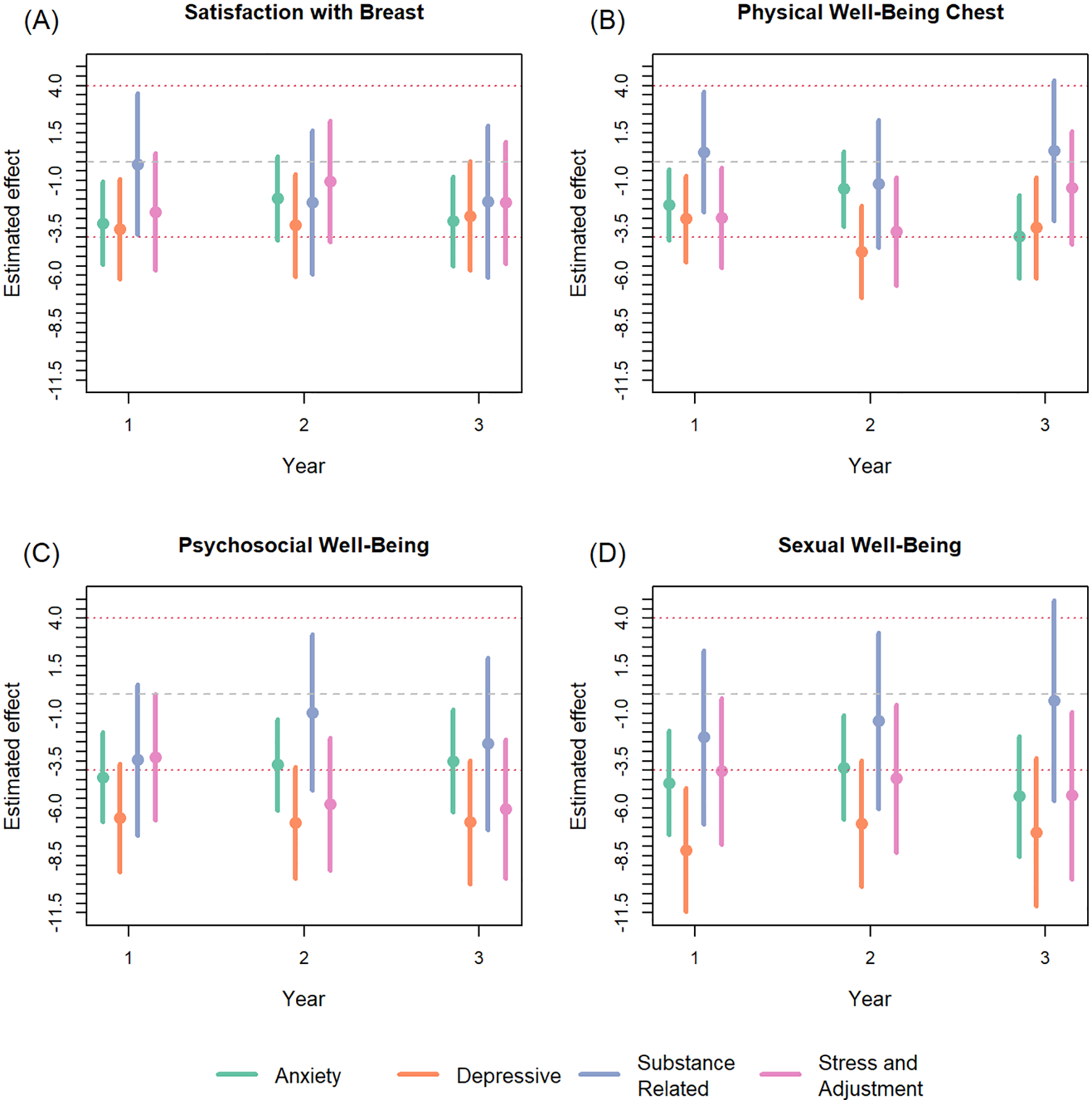
Adjusted cross-sectional regressions examining the impact of psychiatric diagnosis class on BREAST-Q scores per domain for postoperative years 1–3. Dashed lines indicate the range for a 4-point, minimally important clinical difference from 0. Models were adjusted for: age, BMI, smoking status, race/ethnicity, radiation, and reconstruction modality. In addition, models were adjusted for other prevalent psychiatric diagnosis classes.
Table 4.
Cross-sectional linear regressions examining the impact of prevalent psychiatric disorder classes on BREAST-Q scores at year 1, 2, and 3 postoperatively for all BREAST-Q domains
| Unadjusted Model | Adjusted Model | Unadjusted Model | Adjusted Model | Unadjusted Model | Adjusted Model | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | (95% CI) | p | β | (95% CI) | p | β | (95% CI) | p | β | (95% CI) | p value | β | (95% CI) | p | β | (95% CI) | p | ||||
| Satisfaction with Breast | Postoperative Year 1 (n = 1360) | Postoperative Year 2 (n = 1258) | Postoperative Year 3 (n = 1032) | ||||||||||||||||||
| Anxiety Disorders | −5.59 | (−7.60, −3.57) | <0.001 | −3.25 | (−5.44, −1.05) | 0.004 | −3.65 | (−5.74, −1.57) | 0.001 | −1.94 | (−4.16, 0.28) | 0.087 | −4.51 | (−6.72, −2.31) | <0.001 | −3.15 | (−5.52, −0.77) | 0.009 | |||
| Depressive Disorders | −6.74 | (−9.15, −4.33) | <0.001 | −3.54 | (−6.19, −0.90) | 0.009 | −5.02 | (−7.56, −2.47) | <0.001 | −3.36 | (−6.08, −0.64) | 0.015 | −5.60 | (−8.26, −2.94) | <0.001 | −2.86 | (−5.73, 0.01) | 0.051 | |||
| Substance Use Disorders | −4.10 | (−7.17, −1.04) | 0.009 | −0.15 | (−3.87, 3.58) | 0.939 | −3.98 | (−7.08, −0.87) | 0.012 | −2.15 | (−5.93, 1.63) | 0.264 | −2.39 | (−5.58, 0.80) | 0.142 | −2.11 | (−6.11, 1.89) | 0.300 | |||
| Stress and Adjustment Disorders | −6.19 | (−9.17, −3.21) | <0.001 | −2.64 | (−5.73, 0.46) | 0.095 | −4.04 | (−7.11, −0.97) | 0.010 | −1.06 | (−4.25, 2.14) | 0.517 | −4.89 | (−8.03, −1.75) | 0.002 | −2.17 | (−5.39, 1.06) | 0.188 | |||
| Physical Well-Being of Chest | Postoperative Year 1 (n = 1362) | Postoperative Year 2 (n = 1253) | Postoperative Year 3 (n = 1030) | ||||||||||||||||||
| Anxiety Disorders | −4.38 | (−6.11, −2.66) | <0.001 | −2.27 | (−4.15, −0.39) | 0.018 | −4.01 | (−5.87, −2.16) | <0.001 | −1.43 | (−3.41, 0.55) | 0.157 | −5.56 | (−7.56, −3.56) | <0.001 | −3.95 | (−6.15, −1.75) | <0.001 | |||
| Depressive Disorders | −5.71 | (−7.77, −3.65) | <0.001 | −3.02 | (−5.28, −0.75) | 0.009 | −6.74 | (−9.00, −4.48) | <0.001 | −4.75 | (−7.17, −2.32) | <0.001 | −6.43 | (−8.84, −4.03) | <0.001 | −3.48 | (−6.14, −0.82) | 0.010 | |||
| Substance Use Disorders | −3.28 | (−5.89, −0.66) | 0.014 | 0.51 | (−2.68, 3.69) | 0.754 | −3.75 | (−6.54, −0.97) | 0.008 | −1.18 | (−4.55, 2.19) | 0.492 | −3.80 | (−6.70, −0.90) | 0.010 | 0.58 | (−3.12, 4.29) | 0.757 | |||
| Stress and Adjustment Disorders | −5.75 | (−8.28, −3.21) | <0.001 | −2.95 | (−5.61, −0.30) | 0.029 | −6.95 | (−9.68, −4.23) | <0.001 | −3.68 | (−6.53, −0.83) | 0.011 | −4.96 | (−7.82, −2.11) | 0.001 | −1.37 | (−4.36, 1.62) | 0.368 | |||
| Psychosocial Well-Being | Postoperative Year 1 (n = 1359) | Postoperative Year 2 (n = 1251) | Postoperative Year 3 (n = 1028) | ||||||||||||||||||
| Anxiety Disorders | −7.90 | (−10.06, −5.75) | <0.001 | −4.36 | (−6.72, −2.00) | <0.001 | −7.32 | (−9.56, −5.07) | <0.001 | −3.70 | (−6.11, −1.2)9 | 0.003 | −7.33 | (−9.79, −4.87) | <0.001 | −3.50 | (−6.21, −0.80) | 0.011 | |||
| Depressive Disorders | −10.18 | (−12.75, −7.62) | <0.001 | −6.51 | (−9.35, −3.66) | <0.001 | −10.22 | (−12.95, −7.49) | <0.001 | −6.76 | (−9.71, −3.80) | <0.001 | −10.76 | (−13.70, −7.83) | <0.001 | −6.73 | (−9.99, −3.47) | <0.001 | |||
| Substance Use Disorders | −7.44 | (−10.72, −4.16) | <0.001 | −3.45 | (−7.44, 0.54) | 0.090 | −5.23 | (−8.62, −1.85) | 0.002 | −0.96 | (−5.07, 3.15) | 0.647 | −6.46 | (−10.02, −2.89) | <0.001 | −2.60 | (−7.15, 1.94) | 0.261 | |||
| Stress and Adjustment Disorders | −8.82 | (−12.01, −5.62) | <0.001 | −3.31 | (−6.64, 0.02) | 0.052 | −10.59 | (−13.89, −7.28) | <0.001 | −5.78 | (−9.26, −2.30) | 0.001 | −10.50 | (−13.99, −7.02) | <0.001 | −6.03 | (−9.70, −2.37) | 0.001 | |||
| Sexual Well-Being | Postoperative Year 1 (n = 1268) | Postoperative Year 2 (n = 1197) | Postoperative Year 3 (n = 992) | ||||||||||||||||||
| Anxiety Disorders | −9.02 | (−11.52, −6.53) | <0.001 | −4.65 | (−7.38, −1.92) | <0.001 | −7.33 | (−9.90, −4.77) | <0.001 | −3.84 | (−6.58, −1.09) | 0.006 | −8.94 | (−11.83, −6.05) | <0.001 | −5.37 | (−8.55, −2.20) | 0.001 | |||
| Depressive Disorders | −12.55 | (−15.50, −9.59) | <0.001 | −8.19 | (−11.46, −4.91) | <0.001 | −10.20 | (−13.31, −7.08) | <0.001 | −6.80 | (−10.14, −3.46) | <0.001 | −11.71 | (−15.23, −8.20) | <0.001 | −7.25 | (−11.13, −3.36) | <0.001 | |||
| Substance Use Disorders | −8.29 | (−12.10, −4.49) | <0.001 | −2.25 | (−6.84, 2.33) | 0.335 | −7.13 | (−10.95, −3.31) | <0.001 | −1.38 | (−6.02, 3.27) | 0.561 | −5.94 | (−10.10, −1.78) | 0.005 | −0.32 | (−5.61, 4.97) | 0.906 | |||
| Stress and Adjustment Disorders | −10.39 | (−14.07, −6.72) | <0.001 | −4.05 | (−7.90, −0.20) | 0.039 | −9.49 | (−13.24, −5.74) | <0.001 | −4.43 | (−8.34, −0.52) | 0.026 | −10.75 | (−14.92, −6.57) | <0.001 | −5.32 | (−9.72, −0.92) | 0.018 | |||
Reference cohort for all comparisons = No Psychiatric Diagnosis
Abbreviations: β Beta Coefficient, 95% CI 95% Confidence Interval, p p value, ref Reference Group, SATBR Satisfaction with Breast Domain, PWBC Physical Well−being of the Chest Domain, PSYCH Psychosocial Well-being Domain, SEX Sexual Well-being Domain
Adjusted models include covariates: age at reconstruction, body mass index (BMI), race/ethnicity, radiation status, other prevalent psychological classes
Cross-Sectional Linear Regressions: Increasing Number of Diagnostic Classes
Trends in the adjusted cross-sectional regressions demonstrated that as the number of psychiatric diagnostic classes increased, patient BREAST-Q scores for all domains decreased when compared to patients with no psychiatric disorder (Figure 2, Table 5). The most sensitive domains, psychosocial and sexual well-being, demonstrated three-year postoperative differences ranging from: one class: −5.40 to −2.91, two classes: −9.10 to −7.05, three or more classes: −15.73 to −14.22, and one class : −5.86 to −3.20, two classes: −12.34 to −7.95, three or more classes: −15.68 to −14.56, respectively.
Figure 2:
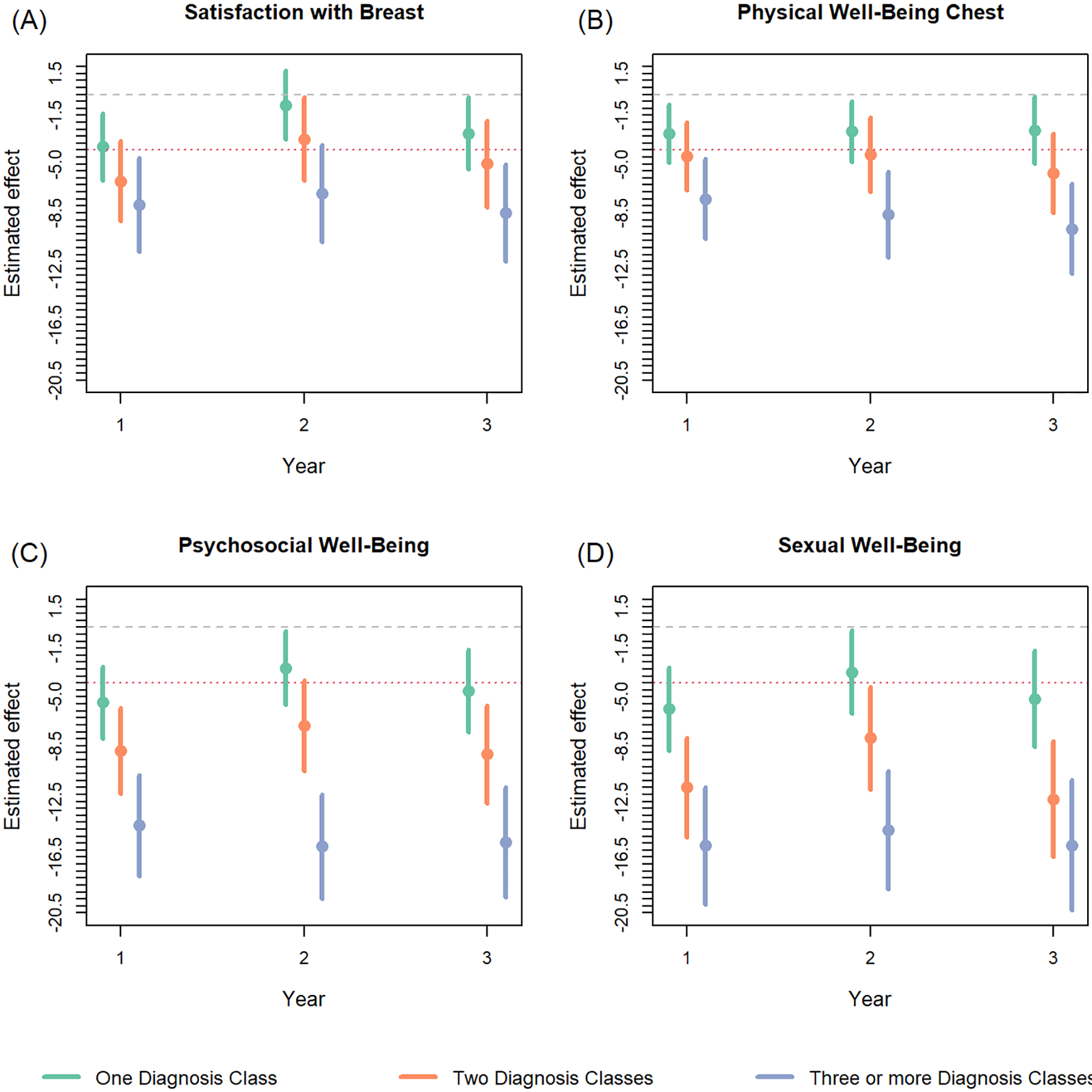
Adjusted cross-sectional regressions examining the impact of number of different psychiatric classes on BREAST-Q scores per domain for postoperative years 1–3. Dashed lines indicate the range for a 4-point, minimally important clinical difference from 0. Models were adjusted for: age, BMI, smoking status, race/ethnicity, radiation, and reconstruction modality.
Table 5.
Cross sectional linear regressions examining the impact of number of psychiatric disorder class(es) on BREAST-Q scores at years 1, 2, and 3, postoperatively for all BREAST-Q domains
| Unadjusted Model | Adjusted Model | Unadjusted Model | Adjusted Model | Unadjusted Model | Adjusted Model | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | (95% CI) | p | β | (95% CI) | p | β | (95% CI) | p | β | (95% CI) | p value | β | (95% CI) | p | β | (95% CI) | p | ||||
| Satisfaction with Breast | Postoperative Year 1 (n = 1344) | Postoperative Year 2 (n = 1242) | Postoperative Year 3 (n = 1013) | ||||||||||||||||||
| Number of Classes | |||||||||||||||||||||
| One | −4.20 | (−6.62, −1.78) | 0.001 | −3.77 | (−6.18, −1.37) | 0.002 | −0.66 | (−3.15, 1.84) | 0.606 | −0.76 | (−3.20, 1.69) | 0.544 | −2.46 | (−5.14, 0.22) | 0.072 | −2.80 | (−5.39, −0.20) | 0.035 | |||
| Two | −7.52 | (−10.38, −4.65) | <0.001 | −6.23 | (−9.09, −3.36) | <0.001 | −3.96 | (−6.95, −0.97) | 0.009 | −3.19 | (−6.17, −0.21) | 0.036 | −4.58 | (−7.73, −1.43) | 0.004 | −4.99 | (−8.08, −1.91) | 0.002 | |||
| Three or More | −9.27 | (−12.58, −5.95) | <0.001 | −7.92 | (−11.28, −4.55) | <0.001 | −7.99 | (−11.46, −4.52) | <0.001 | −7.11 | (−10.57, −3.65) | <0.001 | −8.21 | (−11.71, −4.71) | <0.001 | −8.49 | (−11.96, −5.01) | <0.001 | |||
| Physical Wellbeing of Chest | Postoperative Year 1 (n = 1346) | Postoperative Year 2 (n = 1237) | Postoperative Year 3 (n = 1011) | ||||||||||||||||||
| Number of Classes | |||||||||||||||||||||
| One | −3.34 | (−5.41, −1.26) | 0.002 | −2.82 | (−4.88, −0.75) | 0.007 | −2.55 | (−4.75, −0.34) | 0.024 | −2.64 | (−4.82, −0.46) | 0.018 | −2.65 | (−5.06, −0.23) | 0.032 | −2.56 | (−4.97, −0.15) | 0.037 | |||
| Two | −5.42 | (−7.87, −2.97) | <0.001 | −4.43 | (−6.89, −1.98) | <0.001 | −4.67 | (−7.31, −2.04) | 0.001 | −4.32 | (−6.97, −1.67) | 0.001 | −6.14 | (−8.97, −3.30) | <0.001 | −5.67 | (−8.53, −2.81) | <0.001 | |||
| Three or More | −8.84 | (−11.67, −6.00) | <0.001 | −7.49 | (−10.37, −4.60) | <0.001 | −9.79 | (−12.87, −6.71) | <0.001 | −8.62 | (−11.71, −5.53) | <0.001 | −10.64 | (−13.79, −7.48) | <0.001 | −9.64 | (−12.86, −6.42) | <0.001 | |||
| Psychosocial Wellbeing | Postoperative Year 1 (n = 1343) | Postoperative Year 2 (n = 1235) | Postoperative Year 3 (n = 1009) | ||||||||||||||||||
| Number of Classes | |||||||||||||||||||||
| One | −5.69 | (−8.25, −3.12) | <0.001 | −5.40 | (−7.98, −2.81) | <0.001 | −2.95 | (−5.60, −0.31) | 0.029 | −2.91 | (−5.57, −0.26) | 0.031 | −4.45 | (−7.39, −1.51) | 0.003 | −4.58 | (−7.54, −1.62) | 0.002 | |||
| Two | −9.35 | (−12.38, −6.32) | <0.001 | −8.88 | (−11.95, −5.80) | <0.001 | −7.49 | (−10.68, −4.31) | <0.001 | −7.05 | (−10.30, −3.81) | <0.001 | −9.20 | (−12.65, −5.74) | <0.001 | −9.10 | (−12.61, −5.59) | <0.001 | |||
| Three or More | −15.42 | (−18.93, −11.90) | <0.001 | −14.22 | (−17.85, −10.60) | <0.001 | −16.60 | (−20.28, −12.91) | <0.001 | −15.73 | (−19.49, −11.98) | <0.001 | −15.80 | (−19.64, −11.96) | <0.001 | −15.44 | (−19.40, −11.48) | <0.001 | |||
| Sexual Wellbeing | Postoperative Year 1 (n = 1270) | Postoperative Year 2 (n = 1181) | Postoperative Year 3 (n = 973) | ||||||||||||||||||
| Number of Classes | |||||||||||||||||||||
| One | −6.38 | (−9.34, −3.41) | <0.001 | −5.86 | (−8.85, −2.87) | <0.001 | −3.47 | (−6.51, −0.44) | 0.025 | −3.20 | (−6.21, −0.18) | 0.038 | −5.06 | (−8.53, −1.60) | 0.004 | −5.13 | (−8.60, −1.65) | 0.004 | |||
| Two | −12.48 | (−15.98, −8.98) | <0.001 | −11.49 | (−15.05, −7.94) | <0.001 | −8.89 | (−12.53, −5.25) | <0.001 | −7.95 | (−11.63, −4.27) | <0.001 | −12.39 | (−16.50, −8.29) | <0.001 | −12.34 | (−16.49, −8.19) | <0.001 | |||
| Three or More | −17.30 | (−21.36, −13.23) | <0.001 | −15.68 | (−19.87, −11.49) | <0.001 | −16.13 | (−20.31, −11.94) | <0.001 | −14.56 | (−18.81, −10.32) | <0.001 | −15.87 | (−20.44, −11.31) | <0.001 | −15.65 | (−20.33, −10.97) | <0.001 | |||
Reference cohort for all comparisons = No Psychiatric Diagnosis
Abbreviations: β Beta Coefficient, 95% CI 95% Confidence Interval, p p value, ref Reference Group, SATBR Satisfaction with Breast Domain, PWBC Physical Well-being of the Chest Domain, PSYCH Psychosocial Well-being Domain, SEX Sexual Well-being Domain
Adjusted models include covariates: age at reconstruction, body mass index (BMI), race/ethnicity, radiation status
Sensitivity Analysis: Number of Classes and Most Prevalent Classes
Sensitivity analysis (Supplementary Table 5) showed similar results to the overall cross-sectional regressions with decreasing BREAST-Q scores for all domains as the number of diagnostic classes increased from only one class (of anxiety disorders), two classes (of anxiety and depressive disorders), to three or more classes (anxiety, depressive, and additional disorders).
DISCUSSION
Both the prevalence and impact of psychiatric disorders on patient-reported outcomes in breast cancer patients who undergo breast reconstruction remains poorly understood. In our study, we found that one in two patients had at least one diagnosed psychiatric disorder prior to reconstruction, and over half of patients with psychiatric disorder diagnoses had at least two different classes of disorders prior to reconstruction. Previous studies examining mental health outcomes in breast reconstruction patients focused primarily on the role of surgical reconstruction on mental health post mastectomy. However, these studies fail to consider the trajectory of a patient’s mental health possibly impacted by diagnosis, cancer treatment, and future sequelae of treatment by focusing on one component of the breast cancer treatment process, breast reconstruction. Overall, the high prevalence of psychiatric disorders identified in our study and the impact that such diagnoses have on outcomes supports the importance of screening, early referral, and concurrent mental health treatment in an effort to better serve the needs of our breast cancer patients.
We found that nearly all patients with psychiatric diagnoses history had some type of anxiety disorder and more than half had some type of depressive disorder. This finding is similar to other studies that have examined the prevalence of psychiatric diagnoses in breast cancer patients.19–24 Recent meta-analyses have found that the prevalence of anxiety disorder to be 42% and the prevalence of depression to be 32.2% among breast cancer patients. For every domain and timepoint evaluated, anxiety and depressive disorders had the largest impact on BREAST-Q scores compared to the other classes of psychiatric diagnoses examined. Furthermore, the number of classes of psychiatric diagnoses was inversely related with BREAST-Q score; patients affected by an increasing number of psychiatric classes demonstrated decreasing patient satisfaction and quality-of-life. Previous studies have shown that breast reconstruction patients with psychiatric disorders are more likely to experience decision regret regarding their reconstruction25 and undergo more breast reconstruction revision surgeries.26 Thus, mental health appears to impact both reconstruction-related outcomes (i.e., BREAST-Q scores) and may have more downstream effects years throughout survivorship.9,27
Patient satisfaction and well-being are the measures by which plastic surgeons judge the success of breast reconstruction. In clinical research, these measures have commonly been assessed using BREAST-Q satisfaction with breast and physical well-being domains given the perceived sensitivity of these domains to outcomes. In contrast, psychosocial and sexual well-being domains have not been routinely assessed nor has the potential impact or interaction of one BREAST-Q domain on another been evaluated. For example, quality-of-life factors as measured by the psychosocial well-being domain may explain some percent of satisfaction with breast scores. In our cohort, satisfaction with breast and physical well-being scores were dramatically lower among those with a psychiatric diagnosis compared to those without, suggesting that clinical research and statistical analyses examining BREAST-Q scores must account for a patient’s mental health status. If not, surgeons may draw inaccurate conclusions from patient-reported outcomes research regarding the impact of certain breast reconstruction interventions. More importantly, these results suggest a key opportunity for clinical intervention to improve patient outcomes throughout the cancer treatment process. In other areas of breast cancer care, psychological distress impacts patient decision-making, decision quality, and adherence regarding aspects of their cancer treatment, including chemotherapy, endocrine therapy, and breast surgery.28–33 Our results can serve as additional impetus for improving longitudinal access to psychiatric care. Ideally, concurrent psychiatric care should become routine clinical practice for breast cancer and reconstruction patients given the prevalence of psychiatric disorders in our cohort in addition to psychological distress being termed the “sixth vital sign” in cancer care.34
Through routine BREAST-Q utilization, physicians can better assess and address mental health needs by collaborating with other professionals and referring patients to appropriate mental and sexual health treatment and services. In this study, we found that, while all BREAST-Q domains were affected by psychiatric diagnoses, the psychosocial and sexual well-being domains were the most sensitive to the impact of psychiatric diagnoses. Unlike many other patient-reported outcome measures, the BREAST-Q Reconstruction module was designed specifically for breast reconstruction patients and underwent rigorous psychometric testing to ensure its validity and reliability35,36. At our institution, we routinely administer the BREAST-Q to identify patients who may need additional revision procedures or referrals to physical therapy by trending patient scores over time. Psychosocial and sexual well-being scores can similarly be trended by both plastic surgeons, psychiatrists, and other clinicians involved in the longitudinal treatment of breast cancer patients. Overall, the combination of all BREAST-Q domains provides a comprehensive view of the patient’s quality-of-life where each domain may serve to provide context when interpreting a patient’s other BREAT-Q domain scores.
Breast cancer patients often have multiple contributors to psychological distress, including fear of cancer progression,37 cancer-related intrusive thoughts,37 sleep disturbances,38,39 fatigue,40,41 treatment-related symptoms and side effects (lymphedema, menopausal symptoms),42–45 body image concerns,46,47 and fertility concerns.28,48 Therefore, it is crucial for providers of breast cancer patients to identify those who are affected by psychiatric symptoms, offering evidence-based interventions, pharmacological or non-pharmacological/complementary therapies. Examples of pharmacotherapy interventions, such as SSRIs, anti-psychotics, and anxiolytics, have shown to be effective for treating psychiatric symptoms in breast cancer survivors.49 In addition, non-pharmacological therapies, including cognitive behavioral therapy and other psychotherapies, support groups, and mind body practices (i.e. exercise, meditation) have all had demonstrated efficacy in managing stress and symptoms specific to breast cancer survivors.50–56 Providers who evaluate breast reconstruction patients can use metrics like the BREAST-Q psychosocial and sexual well-being subscales to identify at-risk patients, discuss possible sources of psychological distress, and proactively refer patients to the appropriate treatment pathways.
The current study has both strengths and weaknesses. A key strength is the large cohort size analyzed, which allowed us to conduct robust longitudinal modeling. For the study, an a priori sample size of 394 patients per arm (788 total) was estimated to provide 80% power to measure a four-point clinical difference in BREAST-Q scores with a standard deviation of 20. Therefore, our analyses are adequately powered to detect this difference.
Some limitations include half of patients not completing the BREAST-Q for each domain, possibly resulting in non-response bias. Our cross-sectional regressions may result in underreporting of the true impact of psychiatric disorders on BREAST-Q scores since healthier patients may be more likely to respond. We may have overestimated the number of patients with active psychiatric disorders when using ICD-9 and ICD-10 codes to identify patients with psychiatric disorders. It is possible that these codes could have been entered into the electronic health record without confirmation of the diagnosis by a psychiatrist or the patient may not have had active disease at the time of BREAST-Q assessment. Patients with anxiety and adjustment disorders, especially, share symptoms and may be classified as having one or the other at different timepoints. Non-psychiatrists may also use these two categories interchangeably. Also, there is no information about functioning (axis 5) which could provide more information about the impact of the psychiatric diagnoses. Future research can further parse out the impact of active psychiatric illness on patient-reported outcomes, ideally with the use of a screening tool (e.g. State-Trait Anxiety Inventory57 for anxiety or the Patient Health Questionnaire58 for depressive symptoms). This dataset and analysis included only women who underwent mastectomy with reconstruction. Future research should also examine the impact of psychiatric diagnoses on women with lumpectomy or mastectomy only. Lastly, this is a single urban institution analysis with a population centered mainly in the northeastern United States and with access to a comprehensive cancer center. The exact numbers and prevalence may lack generalizability though this study can serve as a concept applicable to other geographic areas or populations with less access to comprehensive centers (i.e. uninsured or underinsured patients).
CONCLUSIONS
Psychiatric disorders are common in post mastectomy breast reconstruction patients, with more than half of the patients in our cohort having at least one psychiatric diagnosis. Such diagnoses impacted BREAST-Q scores for every domain and timepoint evaluated. Psychosocial and sexual well-being were most sensitive to the effects of psychiatric disorders. Physicians should recognize that mental health is an important determinant of patient satisfaction and quality-of-life after breast reconstruction, and should routinely utilize all domains of the BREAST-Q in clinical practice to screen and identify patients who may need additional mental health care.
Supplementary Material
Supplemental Figure 1: Directed Acyclic Graph used to identify possible confounders in the causal mechanism between exposure to psychiatric illness prior to reconstructive surgery (as measured by a diagnosis of psychiatric illness) and patient well-being following post-mastectomy reconstruction (as measured by BREAST-Q patient reported outcome measures). Red variables indicate confounders.
Flow chart of patient inclusion into the current study.
Source of Funding:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30CA008748.
Footnotes
Financial disclosures:
Babak Mehrara is a consultant for PureTech Corporation. Andrea Pusic is a co-developer of the Q-PROM portfolio (including the BREAST-Q). As such, she may recieve royalties when these PROMs are used in for-profit, industry-sponsored clinical trials. The remaining authors declare no conflicts of interest.
REFERENCES
- 1.Carreira H, Williams R, Muller M, Harewood R, Stanway S, Bhaskaran K. Associations Between Breast Cancer Survivorship and Adverse Mental Health Outcomes: A Systematic Review. J Natl Cancer Inst. Dec 1 2018;110(12):1311–1327. doi: 10.1093/jnci/djy177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avis NE, Levine BJ, Case LD, Naftalis EZ, Van Zee KJ. Trajectories of depressive symptoms following breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev. 2015;24(11):1789–1795. doi: 10.1158/1055-9965.EPI-15-0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iglay K, Santorelli ML, Hirshfield KM, et al. Diagnosis and treatment delays among elderly breast cancer patients with pre-existing mental illness. Breast Cancer Res Treat. Nov 2017;166(1):267–275. doi: 10.1007/s10549-017-4399-x [DOI] [PubMed] [Google Scholar]
- 4.Haskins CB, McDowell BD, Carnahan RM, et al. Impact of preexisting mental illness on breast cancer endocrine therapy adherence. Breast Cancer Res Treat. Feb 2019;174(1):197–208. doi: 10.1007/s10549-018-5050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahlgren-Rimpilainen AJ, Arffman M, Suvisaari J, et al. Excess mortality from breast cancer in female breast cancer patients with severe mental illness. Psychiatry Res. Apr 2020;286:112801. doi: 10.1016/j.psychres.2020.112801 [DOI] [PubMed] [Google Scholar]
- 6.Paredes AZ, Hyer JM, Diaz A, Tsilimigras DI, Pawlik TM. The Impact of Mental Illness on Postoperative Outcomes Among Medicare Beneficiaries: A Missed Opportunity to Help Surgical Patients? Ann Surg. Sep 1 2020;272(3):419–425. doi: 10.1097/SLA.0000000000004118 [DOI] [PubMed] [Google Scholar]
- 7.Fox JP, Philip EJ, Gross CP, Desai RA, Killelea B, Desai MM. Associations between mental health and surgical outcomes among women undergoing mastectomy for cancer. Breast J. May-Jun 2013;19(3):276–84. doi: 10.1111/tbj.12096 [DOI] [PubMed] [Google Scholar]
- 8.Muzzatti B, Bomben F, Flaiban C, Piccinin M, Annunziata MA. Quality of life and psychological distress during cancer: a prospective observational study involving young breast cancer female patients. BMC Cancer. Aug 13 2020;20(1):758. doi: 10.1186/s12885-020-07272-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reyes-Gibby CC, Anderson KO, Morrow PK, Shete S, Hassan S. Depressive symptoms and health-related quality of life in breast cancer survivors. J Womens Health (Larchmt). Mar 2012;21(3):311–8. doi: 10.1089/jwh.2011.2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson JA, Allen RJ Jr., Polanco T, et al. Long-term Patient-reported Outcomes Following Postmastectomy Breast Reconstruction: An 8-year Examination of 3268 Patients. Ann Surg. Sep 2019;270(3):473–483. doi: 10.1097/SLA.0000000000003467 [DOI] [PubMed] [Google Scholar]
- 11.Mehta SK, Sheth AH, Olawoyin O, et al. Patients with psychiatric illness report worse patient-reported outcomes and receive lower rates of autologous breast reconstruction. Breast J. Oct 2020;26(10):1931–1936. doi: 10.1111/tbj.13936 [DOI] [PubMed] [Google Scholar]
- 12.Society AC. Breast Cancer Facts & Figures 2019–2020. American Cancer Society, Inc. ; 2019. [Google Scholar]
- 13.Voineskos SH, Klassen AF, Cano SJ, Pusic AL, Gibbons CJ. Giving Meaning to Differences in BREAST-Q Scores: Minimal Important Difference for Breast Reconstruction Patients. Plast Reconstr Surg. Jan 2020;145(1):11e–20e. doi: 10.1097/PRS.0000000000006317 [DOI] [PubMed] [Google Scholar]
- 14.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Medical Research Methodology. 2008/10/30 2008;8(1):70. doi: 10.1186/1471-2288-8-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haider AH, Bilimoria KY, Kibbe MR. A Checklist to Elevate the Science of Surgical Database Research. JAMA Surg. Jun 1 2018;153(6):505–507. doi: 10.1001/jamasurg.2018.0628 [DOI] [PubMed] [Google Scholar]
- 16.Horowitz NS, Miller A, Rungruang B, et al. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182. J Clin Oncol. Mar 10 2015;33(8):937–43. doi: 10.1200/JCO.2014.56.3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asgari MM, Warton EM, Neugebauer R, Chren MM. Predictors of patient satisfaction with Mohs surgery: analysis of preoperative, intraoperative, and postoperative factors in a prospective cohort. Arch Dermatol. Dec 2011;147(12):1387–94. doi: 10.1001/archdermatol.2011.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Haddad BJS, Jacobsson B, Chabra S, et al. Long-term Risk of Neuropsychiatric Disease After Exposure to Infection In Utero. JAMA Psychiatry. Jun 1 2019;76(6):594–602. doi: 10.1001/jamapsychiatry.2019.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dausch BM, Compas BE, Beckjord E, et al. Rates and Correlates of DSM-IV Diagnoses in Women Newly Diagnosed with Breast Cancer. Journal of Clinical Psychology in Medical Settings. 2004/09/01 2004;11(3):159–169. doi: 10.1023/B:JOCS.0000037610.60644.d6 [DOI] [Google Scholar]
- 20.Hashemi SM, Rafiemanesh H, Aghamohammadi T, et al. Prevalence of anxiety among breast cancer patients: a systematic review and meta-analysis. Breast Cancer. Mar 2020;27(2):166–178. doi: 10.1007/s12282-019-01031-9 [DOI] [PubMed] [Google Scholar]
- 21.Heo J, Chun M, Oh YT, Noh OK, Kim L. Psychiatric comorbidities among breast cancer survivors in South Korea: a nationwide population-based study. Breast Cancer Res Treat. Feb 2017;162(1):151–158. doi: 10.1007/s10549-016-4097-0 [DOI] [PubMed] [Google Scholar]
- 22.Kissane DW, Clarke DM, Ikin J, et al. Psychological morbidity and quality of life in Australian women with early-stage breast cancer: a cross-sectional survey. Med J Aust. Aug 17 1998;169(4):192–6. doi: 10.5694/j.1326-5377.1998.tb140220.x [DOI] [PubMed] [Google Scholar]
- 23.Kissane DW, Grabsch B, Love A, Clarke DM, Bloch S, Smith GC. Psychiatric Disorder in Women with Early Stage and Advanced Breast Cancer: a Comparative Analysis. Australian & New Zealand Journal of Psychiatry. 2004/05/01 2004;38(5):320–326. doi: 10.1080/j.1440-1614.2004.01358.x [DOI] [PubMed] [Google Scholar]
- 24.Pilevarzadeh M, Amirshahi M, Afsargharehbagh R, Rafiemanesh H, Hashemi SM, Balouchi A. Global prevalence of depression among breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat. Aug 2019;176(3):519–533. doi: 10.1007/s10549-019-05271-3 [DOI] [PubMed] [Google Scholar]
- 25.Flitcroft K, Brennan M, Spillane A. Decisional regret and choice of breast reconstruction following mastectomy for breast cancer: A systematic review. Psychooncology. Apr 2018;27(4):1110–1120. doi: 10.1002/pon.4585 [DOI] [PubMed] [Google Scholar]
- 26.Orr JP, Sergesketter AR, Shammas RL, et al. Assessing the Relationship between Anxiety and Revision Surgery following Autologous Breast Reconstruction. Plast Reconstr Surg. Jul 2019;144(1):24–33. doi: 10.1097/PRS.0000000000005696 [DOI] [PubMed] [Google Scholar]
- 27.Park J, Rodriguez JL, O’Brien KM, et al. Health-related quality of life outcomes among breast cancer survivors. Cancer. Apr 1 2021;127(7):1114–1125. doi: 10.1002/cncr.33348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. Apr 10 2014;32(11):1151–6. doi: 10.1200/JCO.2013.52.8877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sella T, Poorvu PD, Ruddy KJ, et al. Impact of fertility concerns on endocrine therapy decisions in young breast cancer survivors. Cancer. Apr 22 2021;doi: 10.1002/cncr.33596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llarena NC, Estevez SL, Tucker SL, Jeruss JS. Impact of Fertility Concerns on Tamoxifen Initiation and Persistence. JNCI: Journal of the National Cancer Institute. 2015;107(10)doi: 10.1093/jnci/djv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandes-Taylor S, Bloom JR. Post-treatment regret among young breast cancer survivors. Psychooncology. May 2011;20(5):506–16. doi: 10.1002/pon.1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheehan J, Sherman KA, Lam T, Boyages J. Association of information satisfaction, psychological distress and monitoring coping style with post-decision regret following breast reconstruction. Psychooncology. Apr 2007;16(4):342–51. doi: 10.1002/pon.1067 [DOI] [PubMed] [Google Scholar]
- 33.Sheehan J, Sherman KA, Lam T, Boyages J. Regret associated with the decision for breast reconstruction: the association of negative body image, distress and surgery characteristics with decision regret. Psychol Health. 2008;23(2):207–19. doi: 10.1080/14768320601124899 [DOI] [PubMed] [Google Scholar]
- 34.Bultz BD, Johansen C. Screening for distress, the 6th vital sign: where are we, and where are we going? Psychooncology. Jun 2011;20(6):569–71. doi: 10.1002/pon.1986 [DOI] [PubMed] [Google Scholar]
- 35.Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. Aug 2009;124(2):345–353. doi: 10.1097/PRS.0b013e3181aee807 [DOI] [PubMed] [Google Scholar]
- 36.Zhong T, McCarthy C, Min S, et al. Patient satisfaction and health-related quality of life after autologous tissue breast reconstruction: a prospective analysis of early postoperative outcomes. Cancer. Mar 15 2012;118(6):1701–9. doi: 10.1002/cncr.26417 [DOI] [PubMed] [Google Scholar]
- 37.Mehnert A, Berg P, Henrich G, Herschbach P. Fear of cancer progression and cancer-related intrusive cognitions in breast cancer survivors. Psychooncology. Dec 2009;18(12):1273–80. doi: 10.1002/pon.1481 [DOI] [PubMed] [Google Scholar]
- 38.Palesh O, Aldridge-Gerry A, Ulusakarya A, Ortiz-Tudela E, Capuron L, Innominato PF. Sleep disruption in breast cancer patients and survivors. J Natl Compr Canc Netw. Dec 1 2013;11(12):1523–30. doi: 10.6004/jnccn.2013.0179 [DOI] [PubMed] [Google Scholar]
- 39.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. Feb 10 2008;26(5):768–77. doi: 10.1200/JCO.2007.14.3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. Feb 2000;18(4):743–53. doi: 10.1200/JCO.2000.18.4.743 [DOI] [PubMed] [Google Scholar]
- 41.Biering K, Frydenberg M, Pappot H, Hjollund NH. The long-term course of fatigue following breast cancer diagnosis. J Patient Rep Outcomes. May 18 2020;4(1):37. doi: 10.1186/s41687-020-00187-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beaulac SM, McNair LA, Scott TE, LaMorte WW, Kavanah MT. Lymphedema and quality of life in survivors of early-stage breast cancer. Arch Surg. Nov 2002;137(11):1253–7. doi: 10.1001/archsurg.137.11.1253 [DOI] [PubMed] [Google Scholar]
- 43.Vassard D, Olsen MH, Zinckernagel L, Vibe-Petersen J, Dalton SO, Johansen C. Psychological consequences of lymphoedema associated with breast cancer: a prospective cohort study. Eur J Cancer. Dec 2010;46(18):3211–8. doi: 10.1016/j.ejca.2010.07.041 [DOI] [PubMed] [Google Scholar]
- 44.Alcorso J, Sherman KA. Factors associated with psychological distress in women with breast cancer-related lymphoedema. Psychooncology. Jul 2016;25(7):865–72. doi: 10.1002/pon.4021 [DOI] [PubMed] [Google Scholar]
- 45.Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. Mar 7 2012;104(5):386–405. doi: 10.1093/jnci/djr541 [DOI] [PubMed] [Google Scholar]
- 46.Boquiren VM, Esplen MJ, Wong J, Toner B, Warner E, Malik N. Sexual functioning in breast cancer survivors experiencing body image disturbance. Psychooncology. Jan 2016;25(1):66–76. doi: 10.1002/pon.3819 [DOI] [PubMed] [Google Scholar]
- 47.Przezdziecki A, Sherman KA, Baillie A, Taylor A, Foley E, Stalgis-Bilinski K. My changed body: breast cancer, body image, distress and self-compassion. Psychooncology. Aug 2013;22(8):1872–9. doi: 10.1002/pon.3230 [DOI] [PubMed] [Google Scholar]
- 48.Ruddy KJ, Gelber S, Ginsburg ES, et al. Menopausal symptoms and fertility concerns in premenopausal breast cancer survivors: a comparison to age- and gravidity-matched controls. Menopause. Jan 2011;18(1):105–8. doi: 10.1097/gme.0b013e3181ef39f8 [DOI] [PubMed] [Google Scholar]
- 49.Grassi L, Caruso R, Hammelef K, Nanni MG, Riba M. Efficacy and safety of pharmacotherapy in cancer-related psychiatric disorders across the trajectory of cancer care: a review. Int Rev Psychiatry. Feb 2014;26(1):44–62. doi: 10.3109/09540261.2013.842542 [DOI] [PubMed] [Google Scholar]
- 50.Carlson LE, Tamagawa R, Stephen J, Drysdale E, Zhong L, Speca M. Randomized-controlled trial of mindfulness-based cancer recovery versus supportive expressive group therapy among distressed breast cancer survivors (MINDSET): long-term follow-up results. Psychooncology. Jul 2016;25(7):750–9. doi: 10.1002/pon.4150 [DOI] [PubMed] [Google Scholar]
- 51.Ferguson RJ, Sigmon ST, Pritchard AJ, et al. A randomized trial of videoconference-delivered cognitive behavioral therapy for survivors of breast cancer with self-reported cognitive dysfunction. Cancer. Jun 1 2016;122(11):1782–91. doi: 10.1002/cncr.29891 [DOI] [PubMed] [Google Scholar]
- 52.Stagl JM, Bouchard LC, Lechner SC, et al. Long-term psychological benefits of cognitive-behavioral stress management for women with breast cancer: 11-year follow-up of a randomized controlled trial. Cancer. Jun 1 2015;121(11):1873–81. doi: 10.1002/cncr.29076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Im EO, Kim S, Yang YL, Chee W. The efficacy of a technology-based information and coaching/support program on pain and symptoms in Asian American survivors of breast cancer. Cancer. Feb 1 2020;126(3):670–680. doi: 10.1002/cncr.32579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atema V, van Leeuwen M, Kieffer JM, et al. Efficacy of Internet-Based Cognitive Behavioral Therapy for Treatment-Induced Menopausal Symptoms in Breast Cancer Survivors: Results of a Randomized Controlled Trial. J Clin Oncol. Apr 1 2019;37(10):809–822. doi: 10.1200/JCO.18.00655 [DOI] [PubMed] [Google Scholar]
- 55.Mann E, Smith MJ, Hellier J, et al. Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial. Lancet Oncol. Mar 2012;13(3):309–18. doi: 10.1016/S1470-2045(11)70364-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schover LR, Jenkins R, Sui D, Adams JH, Marion MS, Jackson KE. Randomized trial of peer counseling on reproductive health in African American breast cancer survivors. J Clin Oncol. Apr 1 2006;24(10):1620–6. doi: 10.1200/JCO.2005.04.7159 [DOI] [PubMed] [Google Scholar]
- 57.CD S State-Trait Anxiety Inventory: Bibliography. . Consulting Psychologists Press;. 1989; [Google Scholar]
- 58.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. Sep 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Directed Acyclic Graph used to identify possible confounders in the causal mechanism between exposure to psychiatric illness prior to reconstructive surgery (as measured by a diagnosis of psychiatric illness) and patient well-being following post-mastectomy reconstruction (as measured by BREAST-Q patient reported outcome measures). Red variables indicate confounders.
Flow chart of patient inclusion into the current study.


