Abstract
Coxiella burnetii, an obligate intracellular bacterium, is the agent of Q fever. The chronic form of the disease is associated with the overproduction of interleukin-10 and deficient C. burnetii killing by monocytes. We hypothesized that the replication of C. burnetii inside monocytes requires a macrophage-deactivating cytokine such as interleukin-10. In the absence of interleukin-10, C. burnetii survived but did not replicate in monocytes. C. burnetii replication (measured 15 days) was induced in interleukin-10-treated monocytes. This effect of interleukin-10 is specific since transforming growth factor β1 had no effect on bacterial replication. C. burnetii replication involves the down-modulation of tumor necrosis factor (TNF) release. First, interleukin-10 suppressed C. burnetii-stimulated production of TNF. Second, the addition of recombinant TNF to interleukin-10-treated monocytes inhibited bacterial replication. Third, the incubation of infected monocytes with neutralizing anti-TNF antibodies favored C. burnetii replication. On the other hand, deficient C. burnetii killing by monocytes from patients with chronic Q fever involves interleukin-10. Indeed, C. burnetii replication was observed in monocytes from patients with Q fever endocarditis, but not in those from patients with acute Q fever. Bacterial replication was inhibited by neutralizing anti-interleukin-10 antibodies. As monocytes from patients with endocarditis overproduced interleukin-10, the defective bacterial killing is likely related to endogenous interleukin-10. These results suggest that interleukin-10 enables monocytes to support C. burnetii replication and to favor the development of chronic Q fever.
Q fever is caused by Coxiella burnetii, an obligate intracellular bacterium. The disease is commonly divided into acute and chronic forms (28). Acute Q fever manifestations are associated with inflammatory and protective immune responses, both indicated by the presence of granuloma (34). Nevertheless, cell-mediated immunity could not lead to the complete eradication of C. burnetii from an infected host (28). The chronic form of Q fever, mainly as endocarditis, sets in several months to years after acute infection, essentially in patients with valvulopathy and, to a lesser extent, in immunocompromised patients (28, 33). Chronic Q fever seems to result from an inefficient protective response to C. burnetii, as demonstrated by the lack of granuloma, the failure of C. burnetii to induce lymphoproliferation, and the presence of high levels of antibodies (Abs) to C. burnetii (23). The deficiency in specific cell-mediated immune response has been associated with the suppressive activity of monocytes and macrophages, in vivo targets of C. burnetii (26). It has been shown that interleukin-10 (IL-10) and transforming growth factor β (TGF-β), two immunoregulatory cytokines, are released by monocytes from patients with Q fever endocarditis and that IL-10 is specifically associated with the occurrence of relapses (6). We and a colleague have also demonstrated that monocytes from patients with Q fever endocarditis exhibit defective killing of C. burnetii (10).
Immunoregulatory cytokines such as IL-10 and TGF-β are involved in increased susceptibility to intracellular pathogens (25). The administration of anti-IL-10 Abs to mice increases their resistance to Mycobacterium avium challenge (11). Transgenic mice with IL-10-secreting T cells are unable to clear the infection with Calmette-Guerin bacillus (29). These cytokines may also account for the severity and/or the reactivation of some infectious diseases. Hence, transcripts for IL-10 are associated with lepromatous leprosy (40). The administration of TGF-β1 exacerbates the progression of experimental pulmonary tuberculosis in guinea pigs (8). In murine tuberculosis, the latency and the control of mycobacterial growth are associated with the production of type 1 cytokines by T cells whereas reactivation results in a shift to IL-10-producing cells (21).
Many mechanisms are used by immunoregulatory cytokines to allow bacterial survival, such as interference with T-cell-mediated adaptative immune responses (12, 27) and/or down-modulation of the microbicidal functions of monocytes/macrophages (12). Indeed, IL-10 down-modulates the anti-M. avium activity of human monocytes and murine macrophages (3) and enables M. avium survival by preventing the apoptosis of infected human macrophages (2). The treatment of monocytes with TGF-β accelerates the intracellular replication of Mycobacterium tuberculosis (19). The specificity or the redundancy of immunoregulatory cytokines is also influenced by the nature of their targets. IL-10 favors the growth of M. avium in murine macrophages but not in human monocytes (36). In the murine model, the inhibition of reactive nitrogen intermediate generation by immunoregulatory cytokines clearly decreases the resistance to infections by intracellular pathogens, but the results are more ambiguous in human cells (14).
Because the overproduction of IL-10 (6) and the defect in C. burnetii killing by monocytes (10) are critically associated with chronic Q fever, we investigated the role of IL-10 in C. burnetii replication. We found that IL-10 stimulated the replication of C. burnetii through the inhibition of tumor necrosis factor (TNF) production. On the other hand, monocytes from patients with chronic Q fever allowed C. burnetii replication but those from patients with acute Q fever did not. IL-10 neutralization by specific Abs inhibited C. burnetii replication in monocytes from patients with chronic Q fever, suggesting that IL-10 plays a major role in Q fever pathophysiology.
MATERIALS AND METHODS
Cells.
Blood from healthy volunteers was collected into EDTA anticoagulant tubes. Monocytes were isolated from peripheral blood mononuclear cells by adherence on glass slides (Lab-Tek chamber; Nalge Nunc Int., Naperville, Ill.) in RPMI 1640 medium containing 25 mM HEPES, 10% fetal calf serum, and 2 mM l-glutamine (Life Technologies, Eragny, France). More than 90% of adherent cells were monocytes (7). Cells were then cultured for 15 days at 37°C in RPMI 1640 supplemented with 10% human AB serum (Sigma Chemical Co., St. Louis, Mo.). L929 cells and HEL cells, provided by the European Collection of Animal Cell Cultures (Sophia Antipolis, France), were cultured in RPMI 1640 and in minimum essential medium containing 10% fetal calf serum and 2 mM l-glutamine, respectively. All culture media were treated with polysulfone filters (10) and checked for the absence of endotoxins by Limulus amebocyte lysate assay (BioWhittaker, Emerainville, France). In some experiments, we studied monocytes from Q fever patients. Informed consent was obtained for each individual, and the study was approved by the Ethics Committee of the Université de la Méditerranée (Marseille, France). The study included 10 patients with ongoing Q fever endocarditis, consisting of 7 men and 3 women (mean age, 43 years; range, 22 to 64 years) and of 8 patients with acute Q fever (6 men and 2 women; mean age, 39 years; range, 32 to 63 years). The diagnosis of Q fever endocarditis was based on modified Duke University criteria, i.e., pathological evidence of endocarditis, positive echocardiogram, positive blood culture, and high titers of immunoglobulin G (IgG) (mean, 10,000; range, 1,600 to 51,200) directed against phase I C. burnetii (15). All these patients had been subjected to valve replacement and medical treatment consisting of doxycycline and chloroquine. Acute Q fever was diagnosed by detection of IgM (mean, 200; range, 100 to 800) and IgG (mean; 1,000; range, 200 to 1,600) specific for phase II C. burnetii (29). These patients were in the early phase (between 1 and 3 months after onset) of the disease, as demonstrated by the high level of specific IgM.
Bacteria.
BALB/c mice were injected intraperitoneally with 108 C. burnetii organisms (Nine Mile strain), as previously described (5). One week later, mice were killed and their spleens were homogenized. Spleen homogenates were added to L929 cell monolayers, and cultures were maintained for five passages. Infected L929 cells were then detached with glass beads and sonicated. Sonicates were spun down at 300 × g for 10 min to remove unbroken cells, and supernatants containing bacteria were centrifuged at 8,000 × g for 10 min. Bacterial pellet was layered on a 25 to 45% linear Renografin gradient and spun down. Purified bacteria were then collected, washed, and suspended in serum-free medium. The concentration of C. burnetii was determined by Gimenez staining. C. burnetii organisms were aliquoted at 109 organisms/ml and stored at −80°C.
Infection procedure.
Monocytes at 5 × 104 cells/ml were pretreated with various doses of human recombinant IL-10 (rIL-10) or rTGF-β1 (R&D Systems, Abingdon, United Kingdom) for 24 h at 37°C and then incubated with C. burnetii at a bacterium-to-cell ratio of 200:1 for 24 h at 37°C. Cells were then washed to remove free bacteria; this time was designated day 0. Infected cells were again cultured with newly added cytokines for 15 days. In some experiments, human rTNF or neutralizing anti-TNF or anti-IL-10 goat Abs (R&D Systems) were included in monocyte cultures. Infection was quantified by indirect immunofluorescence (5). Monocytes were fixed with 1% formaldehyde and permeabilized by 0.1 mg of lysophosphatidylcholine (Sigma)/ml. Cells were then incubated with rabbit anti-C. burnetii serum at a 1/250 dilution in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (Sigma) for 30 min. Fluorescein isothiocyanate-conjugated F(ab′)2 anti-rabbit Ab (Immunotech, Marseille, France) was added to monocytes, which were counterstained with Evans blue. Immunofluorescence results were expressed as an infection index, which is the product of the mean number of bacteria per infected cell and the percentage of infected cells ×100 (10).
Bacterial and cellular viability.
Monocytes, treated or not with cytokines and subsequently infected with C. burnetii, were briefly sonicated, and the homogenates were added to HEL cell monolayers in shell vials (Sterilin, Felthan, United Kingdom) as previously described (9). After 10 days at 37°C, the culture medium was removed and cells were fixed with methanol. Vacuoles containing C. burnetii were revealed by indirect immunofluorescence. Results were expressed as the number of fluorescent vacuoles per shell vial.
The number of adherent monocytes was assessed as described elsewhere (30). Briefly, monocytes were washed with PBS to remove dead cells and 0.2 ml of naphthol blue black (Sigma) was added to each well and incubated for 15 min at room temperature. The number of monocyte nuclei was determined.
Cytokine determination. (i) Immunoassays.
Adherent monocytes (105 cells/assay) were treated with 5 ng of IL-10 or TGF-β1/ml for 24 h and then stimulated by C. burnetii at a bacterium-to-cell ratio of 200:1 in flat-bottom 48-well plates for 24 h. Supernatants were assayed for the presence of TNF, IL-10, IL-1 receptor antagonist (IL-1ra), and TNF-RII. Cytokines were measured by enzyme-linked immunosorbent assay (ELISA) for TNF, IL-10 (Immunotech), IL-1ra, and TNF-RII (R&D Systems), as described by the manufacturers. The limits of detection were 1 pg/ml for TNF and TNF-RII, 4 pg/ml for IL-10, and 15 pg/ml for IL-1ra. The intra- and interassay coefficients of variation of the ELISA kits ranged between 5 and 10%.
(ii) RNA extraction and PCR amplification.
Monocytes (5 × 105 cells/assay) were treated with cytokines for 24 h, and they were stimulated with C. burnetii (bacterium-to-cell ratio, 200:1) for 4 h at 37°C. RNA was extracted using the Trizol method (Life Technologies), and 1 μg was incubated with reverse transcriptase (Superscript; Life Technologies) for 1 h at 37°C. The reaction was stopped by heat inactivation (10 min, 95°C), thereby permitting cDNA generation. cDNA was added to a mixture containing Taq polymerase and specific primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or TNF (7). The mixtures were subjected to 24 cycles of denaturation, annealing (60°C for GAPDH; 65°C for TNF), and extension. PCR products were electrophoresed through 2% agarose gel containing ethidium bromide. TNF transcripts were also quantified with the CytoXpress detection kit (BioSource, Fleurus, Belgium) as previously described (10). Results were expressed as the number of TNF cDNA copies/μg of total RNA.
Statistical analysis.
Results were expressed as the means ± the standard errors (SE) and were compared using analysis of variance. Differences were significant at P < 0.05.
RESULTS
IL-10 induces the replication of C. burnetii in monocytes.
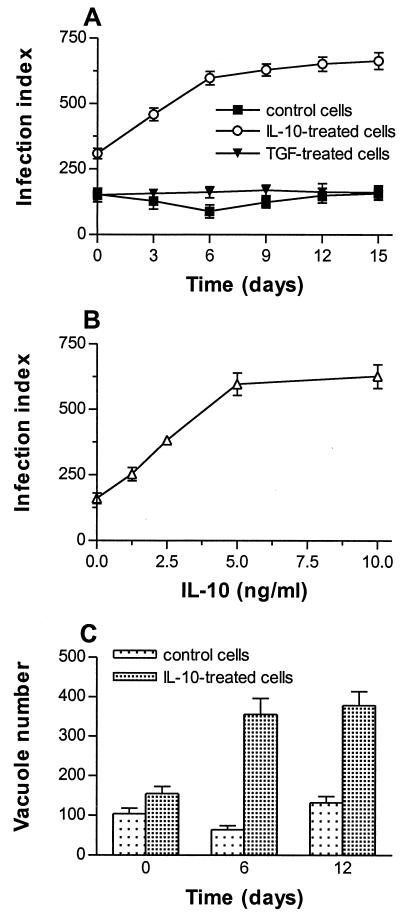
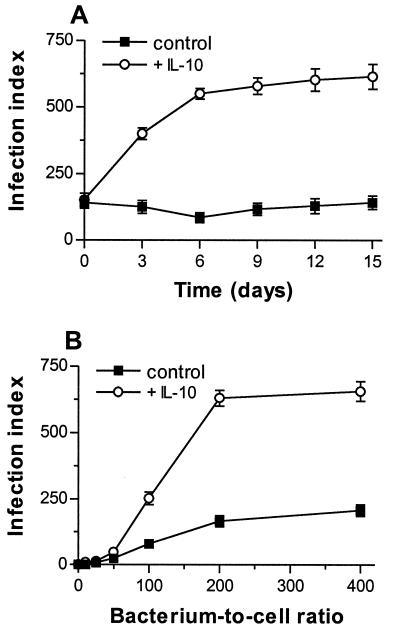
Monocytes were pretreated for 24 h with IL-10 at 5 ng/ml, the concentration that inhibited more than 80% of TNF release by lipopolysaccharide-stimulated monocytes (data not shown). Then, monocytes were infected with C. burnetii at a bacterium-to-cell ratio of 200:1 for 24 h (day 0), leading to substantial and reproducible infection of monocytes (10). In the absence of IL-10, 75% of cells were infected by about two bacteria per cell (Fig. 1A). Infection slowly decreased from day 0 to day 6 (40% inhibition) and then steadily increased; it reached the initial value after 15 days. In IL-10-treated monocytes, the infection index at day 0 was significantly higher than in untreated cells (P < 0.007). It increased from day 0 to day 6 and reached a plateau between day 9 and day 15: almost all monocytes were infected with about six bacteria per cell. The differences between IL-10-treated monocytes and control monocytes were significant (P < 0.002) for each incubation time. The increase in C. burnetii infection of monocytes depended on IL-10 doses. It was observed with 1.25 ng of IL-10/ml and reached a plateau with 5 to 10 ng of IL-10/ml (Fig. 1B).
FIG. 1.
Effect of IL-10 on C. burnetii replication. (A) Monocytes were pretreated with IL-10 or TGF-β1 (5 ng/ml) for 24 h and then infected with C. burnetii at a bacterium-to-cell ratio of 200:1 for 24 h (designated day 0). Bacteria were revealed with rabbit immune serum and fluorescein isothiocyanate-conjugated anti-rabbit F(ab′)2 Ab. The infection index was measured every 3 days; the results are expressed as the mean ± SE of five experiments. (B) Monocytes were pretreated with different doses of IL-10 and infected with C. burnetii as described above. The infection index was measured after 12 days; the results are expressed as the mean ± SE of three experiments. (C) Infected monocytes were sonicated and dilutions of homogenates containing bacteria were added to HEL cell monolayers. Bacteria were revealed by indirect immunofluorescence. Results are expressed as the mean number ± SE of fluorescent vacuoles per shell vial and represent three experiments conducted in triplicate.
The increase in the infection index resulted from C. burnetii replication. Bacterial viability was assessed by measuring C. burnetii-containing vacuoles in HEL cells. In control monocytes, the number of vacuoles per shell vial decreased up to 6 days and then increased; it reached a value similar to the initial value after 12 days (Fig. 1C). In IL-10-treated monocytes, the number of vacuoles at day 0 was higher than in control cells; it was markedly enhanced after 6 days and remained high after 12 days. Because the measurements of infection index and bacterial viability were correlated, only the determination of infection index was used in subsequent experiments.
The replication of C. burnetii mediated by IL-10 was not affected by the magnitude of bacterial uptake. First, monocytes were incubated with C. burnetii at a bacterium-to-cell ratio of 200:1 for 24 h and treated with IL-10 (at 5 ng/ml) and then the infection index was assessed (Fig. 2A). IL-10 elicited an increase (3.5 times) in infection index, which was maximum after 6 days, as for IL-10-pretreated monocytes. Second, IL-10-pretreated monocytes were incubated with C. burnetii at different bacterium-to-cell ratios and then treated with IL-10 for 12 days (Fig. 2B). The effect of IL-10 was observed in monocytes infected with C. burnetii at a bacterium-to-cell ratio of 100:1 and became maximum for bacterium-to-cell ratios of 200:1 and 400:1. IL-10 had to be added after infection to act on C. burnetii replication, since its removal after 24 h diminished bacterial replication by 40%. In all subsequent experiments, cytokines were added to the culture medium after monocyte infection. These results show that C. burnetii survives in resting monocytes and that IL-10 induces bacterial replication.
FIG. 2.
Effect of C. burnetii uptake on bacterial replication. (A) Monocytes were infected with C. burnetii at a bacterium-to-cell ratio of 200:1 for 24 h (designated day 0) and then treated with IL-10 at 5 ng/ml. Infection was determined as described in the Fig. 1A legend; the results are the mean ± SE of four experiments. (B) IL-10-pretreated monocytes were infected with C. burnetii at different bacterium-to-cell ratios for 24 h. The infection index was determined after 12 days; the results represent the mean ± SE of three experiments.
TGF-β1 is not active on C. burnetii replication.
Because TGF-β1 has been reported to depress the microbicidal activity of monocytes and macrophages as IL-10 does (37), we wondered if it favors the replication of C. burnetii in monocytes. TGF-β1 (at 5 ng/ml) did not affect the infection index at day 0 and did not stimulate C. burnetii replication (Fig. 1A). However, TGF-β prevented the transient decrease in bacterial number and viability observed after 6 days of monocyte culture. Raising the TGF-β1 doses to 10 ng/ml did not affect C. burnetii replication (data not shown). In another series of experiments, we tested the combination of IL-10 and TGF-β1 on C. burnetii replication (Table 1). The combination of IL-10 and TGF-β1 was more efficient than IL-10 alone in stimulating C. burnetii replication. The synergistic effect of IL-10 and TGF-β1 on C. burnetii replication was not related to increased bacterial uptake at day 0 since their addition to infected monocytes induced the same synergistic effect. Taken together, these results show that IL-10 acts on C. burnetii replication through a specific mechanism.
TABLE 1.
Effect of cytokine combinations on C. burnetii replication
| Cytokine pretreatmenta | Infection index (mean ± SE)b |
|---|---|
| None | 140 ± 25 |
| IL-10 | 543 ± 55 |
| TGF-β1 | 119 ± 23 |
| IL-10 + TGF-β1 | 781 ± 60 |
Monocytes were pretreated with IL-10, TGF-β1 (5 ng/ml), or their combination for 24 h and incubated with C. burnetii at a bacterium-to-cell ratio of 200:1 for 24 h.
The infection index was determined at day 12. Results are expressed as the mean ± SE of three experiments.
IL-10 down-modulates TNF production in C. burnetii-infected cells.
As IL-10 is known to depress the production of inflammatory cytokines such as TNF in monocytes (12), we investigated the effect of IL-10 on C. burnetii-stimulated production of TNF. TNF transcripts were just detectable in unstimulated monocytes and were absent in cells treated with IL-10 or TGF-β1 used as control (Fig. 3A). C. burnetii stimulated the transcription of the TNF gene (Fig. 3B). IL-10 largely down-modulated it whereas TGF-β1 had no effect. These results were confirmed by a quantitative approach. IL-10 decreased the amounts of TNF mRNA by 85% ± 10% (2,300 ± 220 copies per ng of RNA in untreated cells versus 353 ± 75 copies per ng of RNA in IL-10-treated monocytes), whereas the inhibition induced by TGF-β1 did not exceed 20% (1,888 ± 250 copies per ng of RNA). The release of immunoreactive TNF by monocytes was also assessed (Fig. 3C). C. burnetii elicited maximum TNF release after 8 h; it steadily decreased to the value of unstimulated cells after 48 h. IL-10 completely prevented the release of TNF as early as after 8 h of incubation. In contrast, TGF-β1 inhibited TNF release by only 25% ± 7% after 8 h, when TNF was no longer detectable in the presence of IL-10 (Fig. 3C). After 24 h of incubation with C. burnetii, the TNF release was depressed by TGF-β1 by 41% ± 5%. In another set of experiments, monocytes were washed 24 h after C. burnetii stimulation to remove secreted cytokines and were incubated in new culture medium for 48 h. TNF release steadily increased within 48 h but remained lower than that observed without washing. In monocytes pretreated with regulatory cytokines, the recovery of TNF release was lower in IL-10-treated monocytes than in TGF-β1-treated monocytes (data not shown). These results suggest that IL-10 is more potent than TGF-β1 at down-modulating TNF production.
FIG. 3.
Effect of IL-10 and TGF-β1 on TNF production. (A and B) Monocytes were pretreated with IL-10 or TGF-β1 (5 ng/ml) and then incubated in the absence (A) or the presence (B) of C. burnetii (bacterium-to-cell ratio, 200:1) for 4 h. Total RNA was extracted and transcribed in cDNA. After amplification, PCR products were analyzed by agarose gel electrophoresis and ethidium bromide staining. The figure is representative of three experiments. (C) Monocytes were pretreated with IL-10 or TGF-β1 for 24 h and then infected with C. burnetii. Supernatants were assayed for the presence of TNF by ELISA after different times. The results are expressed as the mean ± SE of three experiments.
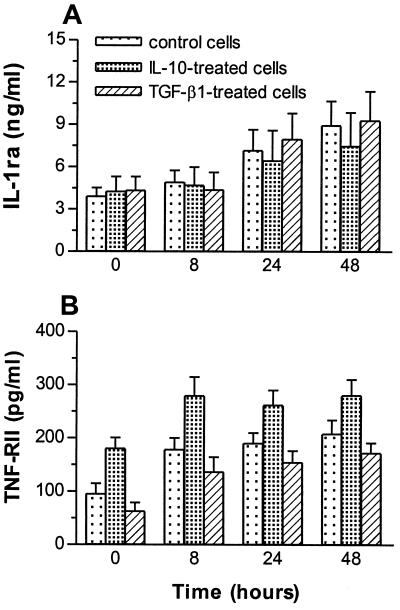
IL-10 upregulates the release of TNF-RII.
Because immunoregulatory cytokines may affect monocyte functions through the upregulation of IL-1ra and/or soluble receptors for cytokines (13), we studied their production. Monocytes were pretreated with IL-10 or TGF-β1 and stimulated with C. burnetii, and the release of IL-1ra and TNF-RII was assessed. The release of IL-1ra was increased in response to C. burnetii (Fig. 4A). Pretreatment of monocytes with IL-10 or TGF-β1 did not affect C. burnetii-stimulated IL-1ra release. In contrast, the release of TNF-RII was differently affected by IL-10 and TGF-β1. In the absence of regulatory cytokines, C. burnetii elicited a steady increase in TNF-RII release within 48 h of incubation (Fig. 4B), confirming recent results (16). IL-10 amplified C. burnetii-stimulated release of TNF-RII at 0 and 8 h postinfection (P < 0.05). TGF-β1 did not affect TNF-RII release (Fig. 4B). Clearly, IL-10 upregulated the release of TNF-RII.
FIG. 4.
Effect of immunoregulatory cytokines on the production of IL-1ra and TNF-RII. Monocytes were pretreated with IL-10 or TGF-β1 (5 ng/ml) for 24 h and then stimulated with C. burnetii (bacterium-to-cell ratio, 200:1) for 24 h. Supernatants were assayed for the presence of IL-1ra (A) and soluble TNF-RII (B) by ELISA after different times. The results are expressed as the mean ± SE of three experiments.
TNF is involved in the IL-10-mediated increase in C. burnetii replication.
To correlate the increase in C. burnetii replication and the inhibition of TNF production, we used two approaches. First, monocytes were infected with C. burnetii in the presence of neutralizing Abs directed to TNF for 12 days. The anti-TNF Abs were active, since they neutralized TNF present in supernatants from C. burnetii-stimulated monocytes on cytotoxic bioassay (data not shown). Anti-TNF Abs increased the replication of C. burnetii in a dose-dependent manner (Fig. 5A). Bacterial replication was measurable in the presence of 5 μg of Abs/ml and it became maximum with 10 μg of Abs/ml. Note that C. burnetii replication remained lower than that induced by IL-10. Second, IL-10-pretreated monocytes were infected with C. burnetii in the presence of rTNF for 12 days. As shown in Fig. 5B, IL-10 increased monocyte infection 3.5-fold and the addition of rTNF inhibited the effect of IL-10 in a dose-dependent manner. TNF at 500 pg/ml significantly (P < 0.02) inhibited the IL-10 effect; its inhibitory effect was maximum with 2,000 pg of TNF/ml (P < 0.005), leading to an infection index similar to that of untreated cells. These results show that the modulation of TNF is clearly involved in the replication of C. burnetii mediated by IL-10.
FIG. 5.
Effect of anti-TNF Abs and exogenous TNF on monocyte infection. (A) Monocytes were infected with C. burnetii (bacterium-to-cell ratio, 200:1) and cultured for 12 days in the presence of neutralizing anti-TNF Abs. (B) Monocytes were treated with IL-10 for 24 h, incubated with C. burnetii for 24 h, and cultured for 12 days in the presence of TNF. The infection index at day 12 was expressed relative to day 0. Results are the mean ± SE of four experiments.
Monocyte defect of C. burnetii killing in Q fever involves IL-10.
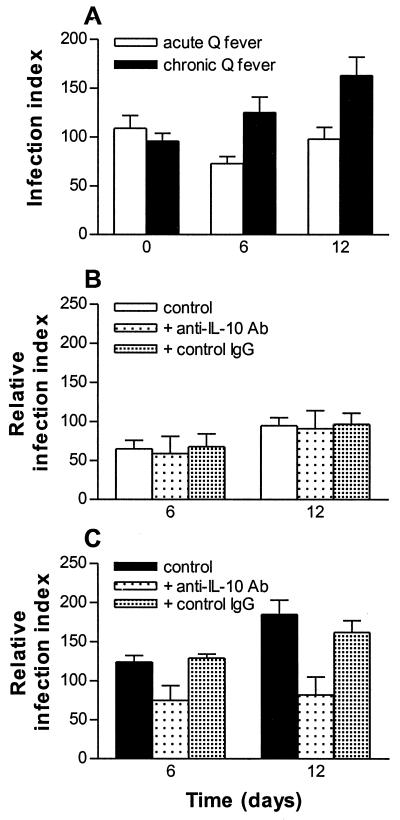
We and a colleague recently showed that monocytes from patients with ongoing Q fever endocarditis are unable to kill C. burnetii (10) and produce more IL-10 than do those from healthy controls (6). As IL-10 stimulates C. burnetii replication, we wondered whether IL-10 is responsible for the defective microbicidal killing of patient monocytes. Of the 18 patients with Q fever investigated, 10 exhibited ongoing endocarditis according to clinical and serological criteria and 8 had acute Q fever. First, we assessed IL-10 release by monocytes (Table 2). It was low in unstimulated monocytes from healthy donors and increased in patients with acute Q fever. IL-10 was significantly (P < 0.03) higher in patients with Q fever endocarditis than in patients with acute Q fever. In response to C. burnetii, the release of IL-10 was similar in healthy donors and in patients with acute Q fever. In contrast, release was increased in patients with Q fever endocarditis. Second, monocytes were incubated with C. burnetii in the presence or the absence of neutralizing anti-IL-10 Abs, and monocyte infection was monitored for 12 days. In patients with acute Q fever, the infection index steadily decreased from day 0 to day 6 and slightly increased at day 12 (Fig. 6A). This pattern was similar to that of infected cells from controls (see Fig. 1A). In patients with Q fever endocarditis, the infection index at day 6 and day 12 was significantly higher than that at day 0 (P < 0.01 and P < 0.002, respectively). When infected monocytes from patients with acute Q fever were cultured with anti-IL-10 Abs (at 10 μg/ml), the infection index at day 6 and day 12 remained unchanged compared to that of untreated cells (Fig. 6B). In contrast, when anti-IL-10 Abs were added to infected monocytes from patients with Q fever endocarditis, the infection index was significantly depressed at day 6 (P < 0.008) and at day 12 (P < 0.001) (Fig. 6C). Thus, the neutralization of IL-10 enabled monocytes from patients with Q fever endocarditis to restrict C. burnetii replication.
TABLE 2.
IL-10 secretion by monocytes from Q fever patientsa
| Subjects | IL-10 secretion (pg/ml) with:
|
|
|---|---|---|
| Unstimulated cells | C. burnetii- stimulated cells | |
| Healthy individuals | 47 ± 32 | 699 ± 194 |
| Patients with acute Q fever | 250 ± 164 | 574 ± 362 |
| Patients with Q fever endocarditis | 1,054 ± 219b | 1,552 ± 222b |
Monocytes from 12 controls, 8 patients with acute Q fever, and 10 patients with ongoing Q fever endocarditis were incubated with C. burnetii at a bacterium-to-cell ratio of 200:1 for 24 h. Supernatants were assayed by ELISA for the presence of IL-10.
P of <0.03 represents the comparison between patients with acute Q fever and patients with Q fever endocarditis.
FIG. 6.
Effect of IL-10 on C. burnetii replication in Q fever endocarditis. (A) Monocytes from patients with acute Q fever or ongoing Q fever endocarditis were incubated with C. burnetii at a bacterium-to-cell ratio of 200:1, and the infection index was assessed at days 0, 6, and 12 as described in the legend to Fig. 1A. (B and C) Infected monocytes from patients with acute Q fever (B) or from patients with Q fever endocarditis (C) were cultured in the presence of neutralizing anti-IL-10 Abs or control serum (10 μg/ml), and the infection index was determined after 6 and 12 days relative to day 0.
DISCUSSION
We show here that exogenous IL-10 promotes C. burnetii replication in human monocytes in a specific way since TGF-β1, another potent immunoregulatory cytokine, had no effect on bacterial replication. As C. burnetii organisms do not replicate in resting monocytes, our results provide new insights into mechanisms of microbicidal defect in Q fever endocarditis. An IL-10-mediated increase in C. burnetii uptake is consistent with the ability of IL-10 to increase the expression of monocyte receptors involved in the phagocytosis of microorganisms (12). Nevertheless, the increased bacterial uptake induced by IL-10 was not required for C. burnetii to replicate. Indeed, varying the magnitude of C. burnetii uptake (by varying the bacterium-to-cell ratio) did not affect bacterial replication. In addition, IL-10 induced similar bacterial replication when it was added to monocytes before or after C. burnetii infection. This may be related to the ability of IL-10 added to monocytes before or after infection to enhance the multiplication of Legionella pneumophila (32). On the other hand, the experiments combining IL-10 and TGF-β1 show that they markedly amplified the replication of C. burnetii, confirming the previously reported synergism of IL-10 and TGF-β1 in the prevention of microbicidal activation of macrophages (31).
The effect of IL-10 on C. burnetii replication is likely related to its ability to disarm monocytes. First, IL-10 is known to inhibit the production of reactive oxygen intermediates and reactive nitrogen intermediates (12). However, C. burnetii was unable to induce hydrogen peroxide and peroxynitrite in human monocytes (data not shown). Monocytes from chronic granulomatous disease patients, which do not produce reactive oxygen intermediates, do not allow the replication of C. burnetii (9). Thus, IL-10 cannot induce the replication of C. burnetii in monocytes by interfering with the generation of toxic intermediates. Second, IL-10 has been described as an inducer of IL-1ra, which should be involved in decreased resistance to intracellular organisms (1). Mice lacking endogenous IL-1ra are less susceptible to infection with Listeria monocytogenes (20). The increased IL-1ra gene expression leads to reduced survival in primary listerial infection (22). Extrapulmonary tuberculosis is associated with a high production of IL-1ra by monocytes in response to M. tuberculosis whereas tuberculous pleurisy is associated with a low production (39). We show here that IL-10 did not affect C. burnetii-stimulated production of IL-1ra. This may be related to the lack of IL-10 effect on M. tuberculosis-stimulated production of IL-1ra (35). IL-1ra has a weak effect on the intracellular replication of M. tuberculosis in vitro and on disease susceptibility (39). Hence, the effect of IL-10 on C. burnetii replication does not depend on IL-1ra production by infected monocytes.
Third, IL-10 might down-modulate TNF production in C. burnetii-stimulated monocytes. IL-10 prevented C. burnetii-stimulated expression of TNF mRNA and suppressed TNF release. IL-10 is potent at suppressing TNF release (24) and transcriptional activation of the TNF gene (38). On the other hand, IL-10 upregulated the release of TNF-RII by C. burnetii-infected monocytes. This finding agrees with previous reports that IL-10 increases the release of TNF-RII (24). It may be related to recent reports that chronic Q fever is associated with IL-10 overproduction (6), a defect in C. burnetii killing (10), and an increase in TNF-RII release (16). As soluble TNF-RII is known to antagonize the activity of TNF, it is likely that its upregulation amplifies the monocyte deactivation mediated by IL-10. We provide here evidence that TNF modulation accounts for the effect of IL-10 on C. burnetii replication. Indeed, the addition of exogenous TNF to IL-10-treated monocytes inhibited C. burnetii replication. This differs from L. pneumophila replication, for which the addition of TNF had no effect on bacterial replication (32).
The notion that IL-10 is required for C. burnetii replication in monocytes provides new insights into the pathophysiology of Q fever. Indeed, monocytes from patients with ongoing Q fever endocarditis are unable to kill C. burnetii, contrary to those from healthy subjects (10) and patients with acute Q fever. They also overproduced IL-10 spontaneously and in response to C. burnetii whereas monocytes from patients with acute Q fever were low producers of IL-10. Incubating monocytes from patients with Q fever endocarditis with C. burnetii in the presence of anti-IL-10 Abs inhibited the replication of C. burnetii. In contrast, neutralizing IL-10 Abs did not interfere with C. burnetii survival by monocytes from patients with acute Q fever. This finding is distinct from the overproduction of IL-10 and TGF-β in tuberculosis, in which only neutralizing anti-TGF-β Abs reduce the intracellular growth of M. tuberculosis (19) and increase lymphocyte responses (18). On the other hand, in human immunodeficiency virus infection, which increases the risk of tuberculosis reactivation, the down-modulation of gamma interferon production induced by M. tuberculosis is partly corrected by neutralizing anti-IL-10 Abs (17). Recently, it has been reported that IL-10 plays an important role in the anergy of patients with pulmonary tuberculosis, which may result in sustained survival of M. tuberculosis (4). Hence, IL-10 produced by monocytes from patients with Q fever endocarditis in an autocrine manner may contribute to the impairment of their microbicidal functions and allow C. burnetii replication. Such a mechanism may account for chronic Q fever relapses, which are associated with sustained high levels of IL-10 (6).
In this report, we show that IL-10 stimulates the replication of C. burnetii in human monocytes. Its effect is associated with the suppression of TNF production. IL-10 may provide a sustained deactivation of monocytes by interfering with the expression of TNF transcripts and by upregulating TNF-RII release. Moreover, IL-10 is involved in the defective killing of C. burnetii by monocytes in Q fever endocarditis. It is likely that IL-10 produced by monocytes during C. burnetii infection allows the replication of C. burnetii via an autocrine loop involving TNF and causes a chronic outcome of the disease.
ACKNOWLEDGMENTS
We thank Jérôme Dellacsagrande and Nathalie Amirayan for technical assistance and Georges Grau for critical reading of the manuscript.
REFERENCES
- 1.Arend W P, Malyak M, Guthridge C J, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 2.Balcewicz-Sablinska M K, Gan H, Remold H G. Interleukin 10 produced by macrophages inoculated with Mycobacterium avium attenuates mycobacteria-induced apoptosis by reduction of TNF-alpha activity. J Infect Dis. 1999;180:1230–1237. doi: 10.1086/315011. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez L E, Kaplan G. Recombinant cytokines for controlling mycobacterial infections. Trends Microbiol. 1995;3:22–27. doi: 10.1016/s0966-842x(00)88864-2. [DOI] [PubMed] [Google Scholar]
- 4.Boussiotis V A, Tsai E Y, Yunis E J, Thim S, Delgado J C, Dascher C C, Berezovskaya A, Rousset D, Reynes J M, Goldfield A E. IL-10-producing T cells suppress responses in anergic tuberculosis patients. J Clin Investig. 2000;105:1317–1325. doi: 10.1172/JCI9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capo C, Lindberg F P, Meconi S, Zaffran Y, Tardei G, Brown E J, Raoult D, Mege J L. Subversion of monocyte functions by Coxiella burnetii: impairment of the cross-talk between αvβ3 integrin and CR3. J Immunol. 1999;163:6078–6085. [PubMed] [Google Scholar]
- 6.Capo C, Zaffran Y, Zugun F, Houpikian P, Raoult D, Mege J L. Production of interleukin-10 and transforming growth factor β by peripheral blood mononuclear cells in Q fever endocarditis. Infect Immun. 1996;64:4143–4147. doi: 10.1128/iai.64.10.4143-4147.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capo C, Zugun F, Stein A, Tardei G, Lepidi H, Raoult D, Mege J L. Upregulation of tumor necrosis factor alpha and interleukin-1β in Q fever endocarditis. Infect Immun. 1996;64:1638–1642. doi: 10.1128/iai.64.5.1638-1642.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai G, McMurray D N. Effects of modulating TGF-beta 1 on immune responses to mycobacterial infection in guinea pigs. Tuber Lung Dis. 1999;79:207–214. doi: 10.1054/tuld.1998.0198. [DOI] [PubMed] [Google Scholar]
- 9.Dellacasagrande J, Capo C, Raoult D, Mege J L. IFN-γ-mediated control of Coxiella burnetii survival in monocytes: the role of cell apoptosis and TNF. J Immunol. 1999;162:2259–2265. [PubMed] [Google Scholar]
- 10.Dellacasagrande J, Ghigo E, Capo C, Raoult D, Mege J L. Coxiella burnetii survives in monocytes from patients with Q fever endocarditis: involvement of tumor necrosis factor. Infect Immun. 2000;68:160–164. doi: 10.1128/iai.68.1.160-164.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denis M, Ghadirian E. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993;151:5425–5430. [PubMed] [Google Scholar]
- 12.De Waal Malefyt R. Role of interleukin-10, interleukin-4, and interleukin 13 in resolving inflammatory responses. In: Gallin J I, Snyderman R, editors. Inflammation: basic principles and clinical correlates. Philadelphia, Pa: Lippincott; 1999. pp. 837–849. [Google Scholar]
- 13.De Waal Malefyt R, Abrams T, Bennett B, Figdor C, De Vries J. IL-10 inhibits cytokine synthesis by human monocytes: an auto-regulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellner J J. The immune response in human tuberculosis: implications for tuberculosis control. J Infect Dis. 1997;176:1351–1359. doi: 10.1086/514132. [DOI] [PubMed] [Google Scholar]
- 15.Fournier P E, Casalta J P, Habib G, Mesana T, Raoult D. Modification of the diagnostic criteria proposed by the Duke endocarditis service to permit improved diagnosis of Q fever endocarditis. Am J Med. 1996;100:629–633. doi: 10.1016/s0002-9343(96)00040-x. [DOI] [PubMed] [Google Scholar]
- 16.Ghigo E, Capo C, Amirayan N, Raoult D, Mege J L. The 75-kD tumor necrosis factor (TNF) receptor is specifically up-regulated in human monocytes during Q fever endocarditis. Clin Exp Immunol. 2000;121:295–301. doi: 10.1046/j.1365-2249.2000.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong J H, Zhang M, Modlin R L, Linsley P S, Lyer D, Lin Y, Barnes P F. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect Immun. 1996;64:913–918. doi: 10.1128/iai.64.3.913-918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch C S, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner J J. Cross-modulation by transforming growth factor β in human tuberculosis: suppression of antigen-driven blastogenesis and interferon γ production. Proc Natl Acad Sci USA. 1996;93:3193–3198. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch C S, Yoneda T, Averill L, Ellner J J, Toossi Z. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor-β1. J Infect Dis. 1994;170:1229–1237. doi: 10.1093/infdis/170.5.1229. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch E, Irikura V M, Paul S M, Hirsh D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc Natl Acad Sci USA. 1996;93:11008–11013. doi: 10.1073/pnas.93.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard A D, Zwilling B S. Reactivation of tuberculosis is associated with a shift from type 1 to type 2 cytokines. Clin Exp Immunol. 1999;115:428–434. doi: 10.1046/j.1365-2249.1999.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irikura V M, Hirsch E, Hirsh D. Effects of interleukin-1 receptor antagonist overexpression on infection by Listeria monocytogenes. Infect Immun. 1999;67:1901–1909. doi: 10.1128/iai.67.4.1901-1909.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izzo A A, Marmion B P. Variation in interferon-gamma responses to Coxiella burnetii antigens with lymphocytes from vaccinated and naturally infected subjects. Clin Exp Immunol. 1993;94:507–515. doi: 10.1111/j.1365-2249.1993.tb08226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joyce D A, Steer J H. IL-4, IL-10 and IFNγ have distinct, but interacting effects on differentiation-induced changes in TNF-α and TNF receptor release by cultured human monocytes. Cytokine. 1996;8:49–57. doi: 10.1006/cyto.1996.0007. [DOI] [PubMed] [Google Scholar]
- 25.Kaufmann S H E. Immunity to intracellular bacteria. In: Paul W E, editor. Fundamental immunology. Philadelphia, Pa: Lippincott; 1999. pp. 1335–1371. [Google Scholar]
- 26.Koster F T, Williams J C, Goodwin J S. Cellular immunity in Q fever: modulation of responsiveness by a suppressor T cell-monocyte circuit. J Immunol. 1985;135:1067–1072. [PubMed] [Google Scholar]
- 27.Letterio J J, Roberts A B. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 28.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray P J, Wang L, Onufryk C, Tepper R I, Young R A. T cell-derived IL-10 antagonizes macrophage function in bacterial infection. J Immunol. 1997;158:315–321. [PubMed] [Google Scholar]
- 30.Nakagara A, Nathan C F. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983;56:261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- 31.Oswald I P, Gazinelli R T, Sher A, James S I. IL-10 synergizes with IL-4 and TGF-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992;148:3578–3582. [PubMed] [Google Scholar]
- 32.Park D R, Skerrett S J. IL-10 enhances the growth of Legionella pneumophila in human mononuclear phagocytes and reverses the protective effect of IFN-γ. J Immunol. 1996;157:2528–2538. [PubMed] [Google Scholar]
- 33.Raoult D. Host factors in the severity of Q fever. Ann N Y Acad Sci. 1990;590:33–38. doi: 10.1111/j.1749-6632.1990.tb42204.x. [DOI] [PubMed] [Google Scholar]
- 34.Raoult D, Marrie T. Q fever. Clin Infect Dis. 1995;20:489–496. doi: 10.1093/clinids/20.3.489. [DOI] [PubMed] [Google Scholar]
- 35.Ruth J H, Bienkowski M, Warmington K S, Lincoln P M, Kunkel S L, Chensue S W. IL-1 receptor antagonist (IL-1ra) expression, function, and cytokine-mediated regulation during mycobacterial and schistosomal antigen-elicited granuloma formation. J Immunol. 1996;156:2503–2509. [PubMed] [Google Scholar]
- 36.Shiratsuchi H, Hamilton B, Toossi Z, Ellner J J. Evidence against a role for interleukin-10 in the regulation of growth of Mycobacterium avium in human monocytes. J Infect Dis. 1996;173:410–417. doi: 10.1093/infdis/173.2.410. [DOI] [PubMed] [Google Scholar]
- 37.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-β. Nature. 1998;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 38.Wang P, Wu P, Siegel M I, Egan R W, Billah M M. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson R J, Patel P, Llewelyn M, Hirsch C S, Pasvol G, Snounou G, Davidson R N, Toossi Z. Influence of polymorphism in the genes for the interleukin (IL)-1 receptor antagonist and IL-1β on tuberculosis. J Exp Med. 1999;189:1863–1873. doi: 10.1084/jem.189.12.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamura M, Uyemura K, Deans R J, Weinberg K, Rea T H, Bloom B R, Modlin R L. Defining protective responses to pathogens: cytokine profiles in leprosy. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]