Abstract
Background
In participants with pulmonary arterial hypertension, 24 weeks of sotatercept resulted in a significantly greater reduction from baseline in pulmonary vascular resistance than placebo. This report characterises the longer-term safety and efficacy of sotatercept in the PULSAR open-label extension. We report cumulative safety, and efficacy at months 18–24, for all participants treated with sotatercept.
Methods
PULSAR was a phase 2, randomised, double-blind, placebo-controlled study followed by an open-label extension, which evaluated sotatercept on top of background pulmonary arterial hypertension therapy in adults. Participants originally randomised to placebo were re-randomised 1:1 to sotatercept 0.3 or 0.7 mg·kg−1 (placebo-crossed group); those initially randomised to sotatercept continued the same sotatercept dose (continued-sotatercept group). Safety was evaluated in all participants who received ≥1 dose of sotatercept. The primary efficacy endpoint was change from baseline to months 18–24 in pulmonary vascular resistance. Secondary endpoints included 6-min walk distance and functional class. Two prespecified analyses, placebo-crossed and delayed-start, evaluated efficacy irrespective of dose.
Results
Of 106 participants enrolled in the PULSAR study, 97 continued into the extension period. Serious treatment-emergent adverse events were reported in 32 (30.8%) participants; 10 (9.6%) reported treatment-emergent adverse events leading to study discontinuation. Three (2.9%) participants died, none considered related to study drug. The placebo-crossed group demonstrated significant improvement across primary and secondary endpoints and clinical efficacy was maintained in the continued-sotatercept group.
Conclusion
These results support the longer-term safety and durability of clinical benefit of sotatercept for pulmonary arterial hypertension.
Short abstract
This report characterises the longer-term safety and efficacy of sotatercept in adult participants with pulmonary arterial hypertension from the PULSAR open-label extension period https://bit.ly/3QqezKH
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease that results in increased pulmonary artery pressure and right ventricular dysfunction [1]. Pulmonary vascular remodelling is the key underlying driver of PAH, and this stems from an imbalance in anti-proliferative and pro-proliferative signalling pathways that promote hyperproliferation of cells from vessel walls [2]. In PAH, anti-proliferative bone morphogenetic protein receptor type II (BMPR-II)-mediated signalling is reduced, allowing for unchecked pro-proliferative activin signalling through activin receptor type 2A/B (ActRIIA/B). This promotes hyperproliferation of cells from the vessel wall [1, 3, 4].
Currently approved PAH therapies, which act via the prostacyclin, endothelin or nitric oxide pathways, slow disease progression [5, 6] Most recent PAH treatment guidelines recommend that most patients with PAH are managed with a risk-adapted treatment strategy, including use of combination drug therapy [7, 8]. However, many patients do not achieve low-risk status and/or individual treatment goals, and their long-term prognosis remains poor, with an estimated 5–7 year survival of approximately 50% after diagnosis [4, 9, 10]. Thus, new targets and novel therapies are needed that specifically target the underlying cause of PAH to restore vascular wall homeostasis [2, 3, 11].
Sotatercept is a fusion protein that binds to and sequesters select transforming growth factor β superfamily ligands proposed to rebalance anti-proliferative (BMPR-II-mediated) and pro-proliferative (ActRIIA-mediated) signalling. In preclinical models of PAH, sotatercept has been shown to reverse pulmonary arterial wall and right ventricular remodelling [4, 12, 13].
The clinical efficacy and safety of sotatercept on top of background PAH therapy is being evaluated across an extensive clinical trial programme involving participants with PAH, including the phase 2 PULSAR study [1, 14–18]. The 24-week placebo-controlled treatment period of PULSAR demonstrated that compared with placebo, sotatercept treatment significantly reduced pulmonary vascular resistance (PVR) from baseline. Sotatercept also improved 6-min walk distance (6MWD) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels from baseline compared with placebo [1].
Here, we report the results from the open-label extension period of PULSAR and further characterise the longer-term safety and efficacy of sotatercept on top of background PAH therapy in participants with PAH, including those re-randomised to sotatercept after the placebo-controlled treatment period.
Methods
Study design and participants
PULSAR (NCT03496207), a phase 2, multicentre, randomised, placebo-controlled, double-blind study evaluating the safety and efficacy of sotatercept on top of background PAH therapy in participants with PAH, comprised of a 24-week placebo-controlled treatment period followed by an open-label extension period, as previously described [1]. Throughout the placebo-controlled treatment period, stable doses of medications for chronic conditions, including PAH medications, were permitted. If a new medication or dosage of an existing medication was added per the investigator for PAH worsening, discontinuation of study treatment was required.
Participants who completed the placebo-controlled treatment period were able to continue directly into the extension period. Placebo participants were re-randomised 1:1 to sotatercept 0.3 mg·kg−1 or 0.7 mg·kg−1 (placebo-crossed group) through the Interactive Response Technology system; sotatercept participants continued their latest dose (continued-sotatercept group) in a blinded manner. During the extension period, investigators were allowed to substitute, remove or adjust the dose of concomitant medications for any chronic conditions or PAH worsening that were not specifically excluded. The data cut-off date was 8 June 2021. The trial was conducted in accordance with the principles of the Declaration of Helsinki, international ethical guidelines, Good Clinical Practice guidelines, and all applicable laws and regulations. All participants gave written informed consent.
Endpoints
Longer-term safety and tolerability of sotatercept was the primary objective. Safety was assessed as treatment-emergent adverse events (TEAEs) according to Common Toxicity Criteria for Adverse Events version 4.0 (CTCAE v4.0). Tolerability was assessed by TEAEs leading to treatment or study discontinuation. TEAEs of special interest (AESIs) included leukopenia, neutropenia, and thrombocytopenia. Further details on the rationale for the inclusion of these AESIs are provided in the supplementary material.
The primary efficacy endpoint was change from baseline to months 18–24 in PVR, as measured by right heart catheterisation (RHC). Change from baseline to months 18–24 in 6MWD, World Health Organization functional class (WHO FC), and NT-proBNP were secondary endpoints. The study protocol was amended in July 2020 with the third RHC to be performed at month 18. To allow for inclusion of the maximum number of participants, the RHC was performed at the next possible visit, up to month 24, in participants who already passed the month 18 visit. Secondary endpoints were measured on the same day or closest to the third RHC. Additional haemodynamic parameters (cardiac index, cardiac output, pulmonary arterial wedge pressure, mean pulmonary arterial pressure (mPAP) and right atrial pressure (RAP)) were also evaluated.
Safety analyses
Safety endpoints are summarised using the safety population and according to participants’ randomised dose. The safety population included all randomised participants who received ≥1 dose of sotatercept and included data from the first time participants received sotatercept until the data cut-off. Overall safety is summarised as number and percentage of all sotatercept-treated participants who reported TEAEs, related TEAEs, AESI, serious TEAEs, related serious TEAEs, and TEAEs leading to treatment and study discontinuation. Number and percentage of all sotatercept-treated participants who reported each TEAE experienced by ≥10% of all sotatercept-treated participants is also summarised.
Time-at-risk exposure-adjusted incidence rate (TAR-EAIR) was calculated for summarised TEAEs. TAR-EAIR was calculated as (number of participants with events/total person-years at risk)×100 for each treatment arm. For participants with events, the person-years at risk was calculated as (first event start date − first sotatercept treatment date + 1)/365.25. For participants without events, the person-years at risk was calculated as (follow-up date − first sotatercept treatment date + 1)/365.25. The most common (≥10%) TEAEs were evaluated by time-period: months 1–6, 7–12, 13–18 and 19–24.
Efficacy analyses
Efficacy was evaluated using two analyses, irrespective of dose: a placebo-crossed efficacy analysis comparing efficacy endpoints at months 18−24 versus baseline within the placebo-crossed group, and a delayed-start efficacy analysis comparing the change from baseline to months 18−24 for efficacy endpoints between the continued-sotatercept and placebo-crossed groups. All efficacy analyses were performed in the full analysis set for the extension period (FAS-E), which comprised all participants who transitioned to the extension period.
The overall type I error rate was two-sided 0.05. The recycle method was used to control the overall type I error rate. The placebo-crossed efficacy analyses were tested first at the two-sided 0.025 level. The gate keeping method was used to sequentially test each efficacy endpoint [19]. If all three efficacy endpoints in the placebo-crossed analyses were statistically significant, then the type I error rate of 0.025 was recycled and each endpoint in the delayed-start efficacy analysis was tested similarly to the placebo-crossed efficacy analyses.
PVR was assessed using analysis of covariance (ANCOVA), with baseline as covariate. Normality was tested with the Shapiro–Wilk test. If normal distribution assumption was violated, a non-parametric Wilcoxon signed-rank test was used. Multiple imputation was used to handle missing data. Further details on standard multiple imputation are provided in the supplementary material. Secondary efficacy endpoints were analysed similarly with ANCOVA and their respective average baseline as covariate. The WHO FC categories were converted to numerical values, category I as 1, II as 2, III as 3 and IV as 4. The numerical values were used in this analysis in order to increase statistical power of the ANCOVA analysis. Several methods were used to handle missing data due to COVID-19, including removing missing participants from the denominator for binary endpoints.
A sensitivity analysis was conducted in both groups to determine the magnitude of change in PVR when adjusted for haematocrit. Haematocrit-adjusted PVR values were determined according to the formula proposed by Vanderpool and Naeije [20].
In order to characterise the difference between sotatercept doses after placebo participants crossed over to sotatercept, aligned analyses were performed by combining the results of change from baseline after 6 months for participants originally randomised to sotatercept treatment arms with the results of change from baseline at month 12 for the placebo-crossed participants after 6 months of sotatercept treatment for each dose. The change from aligned baseline to aligned month 6 in 6MWD and WHO FC for sotatercept 0.7 mg·kg−1 was compared to that of sotatercept 0.3 mg·kg−1.
Role of the funding source
The funder of the study contributed to the study design and data analysis, data interpretation, and writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Participant disposition and baseline characteristics
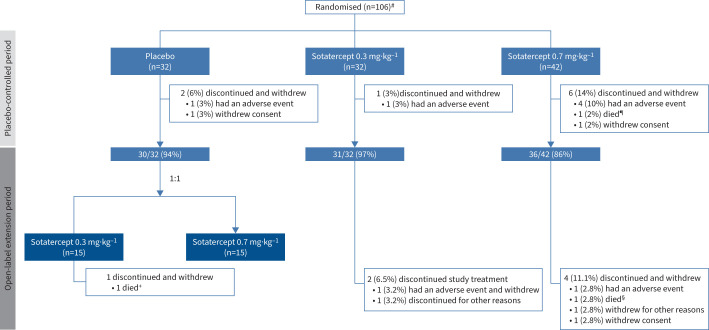
Of 106 participants who were randomised at the start of the study, 97 (92%) completed the placebo-controlled treatment period and entered the open-label extension period. Of these, 31 and 36 continued receiving 0.3 mg·kg−1 and 0.7 mg·kg−1, respectively, and collectively comprise the continued-sotatercept group. 30 participants initially randomised to placebo continued to the extension period and 15 each were re-randomised 1:1 to sotatercept 0.3 mg·kg−1 or 0.7 mg·kg−1; these participants collectively comprise the placebo-crossed group (figure 1).
FIGURE 1.
Participant disposition. Disposition based on randomised dose, not actual dose. #: safety was evaluated in the 104 participants who received at least one dose of sotatercept; ¶: cardiac arrest; +: pulmonary arterial hypertension worsening; §: brain abscess.
Demographic and baseline clinical characteristics were balanced between the continued-sotatercept and placebo-crossed groups and similar to the overall PULSAR population [1]. Participants were predominantly white (92%), female (89%), and were relatively young (mean±sd age 47.6±14.2 years). The most common PAH aetiology was idiopathic (56%), and the mean±sd time since PAH diagnosis to initial enrolment into PULSAR was 7.8±5.6 years. Most participants were receiving either triple (56.7%) or double (36.1%) background PAH therapy, and more than a third (36%) were receiving prostacyclin infusion therapy (table S1).
During the extension period, concomitant medications were adjusted for six participants. Further details on these participants are provided in the supplementary material.
Safety
Safety was evaluated in the 104 participants who had received at least one dose of sotatercept during the study. The most reported (≥10%) TEAEs throughout the trial are listed in table S2. TEAEs were reported in 102 (98.1%) participants, and 72 (69.2%) of these experienced TEAEs considered treatment-related. Serious TEAEs were reported in 32 (30.8%) participants (table 1). Five (4.8%) participants reported six serious TEAEs that were considered related to the study drug: pyrexia, red blood cell increased, systemic lupus erythematosus, ischaemic stroke, pleural effusion and pulmonary hypertension.
TABLE 1.
Overall safety and exposure in all sotatercept-treated participants
| Placebo to sotatercept 0.3 mg·kg−1 (n=15) | Placebo to sotatercept 0.7 mg·kg−1 (n=15) | Continuing sotatercept 0.3 mg·kg−1 (n=32) | Continuing sotatercept 0.7 mg·kg−1 (n=42) | Combined sotatercept 0.3 mg·kg−1 (n=47) | Combined sotatercept 0.7 mg·kg−1 (n=57) | Total (n=104) | |
| TEAEs | 13 (86.7) | 15 (100.0) | 32 (100.0) | 42 (100.0) | 45 (95.7) | 57 (100.0) | 102 (98.1) |
| TEAEs related to treatment | 10 (66.7) | 13 (86.7) | 20 (62.5) | 29 (69.0) | 30 (63.8) | 42 (73.7) | 72 (69.2) |
| AESI # | 5 (33.3) | 1 (6.7) | 5 (15.6) | 7 (16.7) | 10 (21.3) | 8 (14.0) | 18 (17.3) |
| Serious TEAEs | 5 (33.3) | 3 (20.0) | 8 (25.0) | 16 (38.1) | 13 (27.7) | 19 (33.3) | 32 (30.8) |
| Serious TEAEs related to treatment | 2 (13.3) | 0 (0.0) | 1 (3.1) | 2 (4.8) | 3 (6.4) | 2 (3.5) | 5 (4.8) |
| TEAEs leading to treatment discontinuation | 1 (6.7) | 0 (0.0) | 2 (6.3) | 7 (16.7) | 3 (6.4) | 7 (12.3) | 10 (9.6) |
| TEAEs leading to study discontinuation | 1 (6.7) | 0 (0.0) | 2 (6.3) | 7 (16.7) | 3 (6.4) | 7 (12.3) | 10 (9.6) |
| TEAEs leading to death | 1 (6.7) | 0 (0.0) | 0 (0.0) | 2 (4.8) | 1 (2.1) | 2 (3.5) | 3 (2.9) |
| Median exposure, days | 623 | 627 | 788 | 771 | 756 | 737 | 742 |
Safety population (includes all randomised participants who received at least one dose of study treatment). Data are presented as n (%), unless otherwise stated. A treatment-emergent adverse event (TEAE) has start date on or after the first dose of treatment and up to 8 weeks after the last dose of treatment. #: treatment-emergent adverse event of special interest (AESI) is defined as any TEAE of leukopenia, neutropenia or thrombocytopenia.
No serious TEAEs led to treatment or study discontinuation. 10 (9.6%) participants experienced TEAEs leading to treatment or study discontinuation. Three (2.9%) deaths were reported, but none were considered related to study drug by investigators. One participant randomised to sotatercept 0.7 mg·kg−1 died from cardiac arrest during the placebo-controlled treatment period, and two participants died during the extension period: one in the placebo-crossed treatment group due to worsening PAH, and one in the continued-sotatercept treatment group as a result of a brain abscess (figure 1) [1].
AESIs, which included leukopenia, neutropenia, and thrombocytopenia, as defined by the NCI-CTCAE v4·0, occurred in 18 (17.3%) participants (table 1 and table S4). No thrombocytopenia-related bleeding occurred, and no participant received a platelet transfusion. The trend in platelet counts over time was similar regardless of sotatercept dose (0.3 or 0.7 mg·kg−1) (figure S1). 15 (14.4%) participants experienced increased haemoglobin: participants who received sotatercept 0.7 mg·kg−1 had greater changes over time in haemoglobin levels than those on sotatercept 0.3 mg·kg−1 (table S2 and figure S2). 11 (10.6%) participants experienced telangiectasia, which in all cases was non-serious and mostly mild (table S2). All telangiectasias occurred during the extension period and on average, developed after 1.5 years of sotatercept treatment. There were no signs of internal arteriovenous malformations among participants with epidermal telangiectasias.
The number of TEAEs decreased as sotatercept treatment duration increased. During months 1–6, the three most common TEAEs, headache, diarrhoea and nasopharyngitis, occurred in 17.3%, 15.4% and 14.4% of participants, respectively. During months 7–12 these rates dropped to 12.2%, 10.2% and 10.2%, respectively. No participants experienced any of these TEAEs in months 13–18 or 19–24 (table S3).
When adjusting for time-at-risk, exposure-adjusted incidence rates for TEAEs were comparable regardless of sotatercept dose: TAR-EAIRs of serious TEAEs were 17.1 and 21.8 per 100 person-years at-risk in the 0.3 mg·kg−1 and 0.7 mg·kg−1 groups, respectively, and 3.3 and 2.0 per 100 person-years at-risk for treatment-related serious TEAEs (table S4).
61% (63/104) of participants tolerated their randomised dose and needed no dose reductions, although three-quarters experienced at least one dose delay and around half of these were due to logistic problems related to the COVID-19 pandemic. Of the 41 participants who needed dose reductions, 20 were due to TEAEs or AESIs and the rest were related to haemoglobin increases. Of those who needed a dose reduction, 22 (21.2%) participants had their dose increased again. 32 (30.8%) participants had their dose increased from 0.3 mg·kg−1 to 0.7 mg·kg−1 (table S5).
Placebo-crossed efficacy analysis
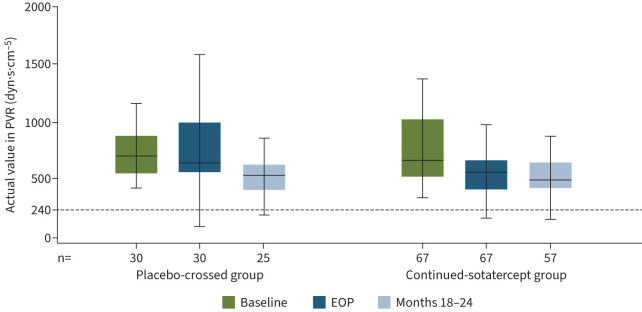
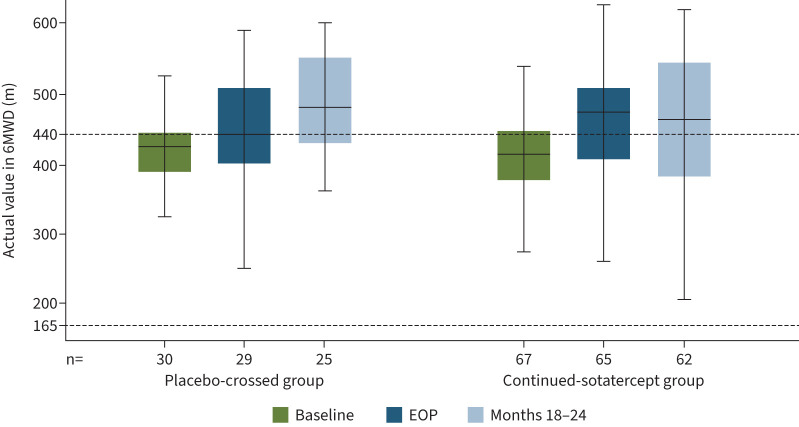
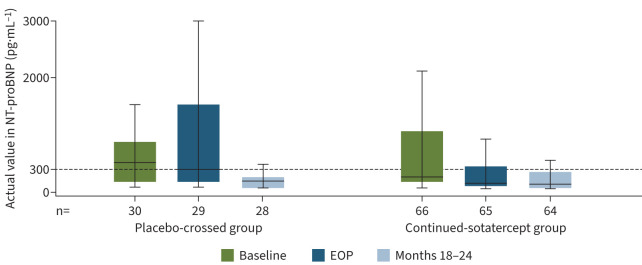
In the placebo-crossed group, statistically significant improvements occurred in all primary and secondary efficacy endpoints from baseline to months 18–24 (table 2). Mean±sd PVR decreased from 802±331 to 583±310 dyn·s·cm−5 (figure 2), mean±sd 6MWD increased from 409±66 to 480±73 m (figure 3), and WHO FC improved from a mean±sd 2.4±0.5 to 1.9±0.6 (all p<0.0001). Mean±sd NT-proBNP decreased from 840±1247 to 363±702 pg·mL−1 (nominal p=0.0004) (figure 4). After adjusting for differences in haematocrit, mean±sd PVR decreased by 297.8±236.0 dyn·s·cm−5 from baseline to months 18–24 (table S6).
TABLE 2.
Summary of efficacy endpoints
| Placebo-crossed (n=30) | Continued-sotatercept (n=67) | ||||||||||
| Baseline | EOP | Months 18–24 | Change from baseline to months 18–24 | p-value | Baseline | EOP | Months 18–24 | Change from baseline to months 18–24 # | Change from EOP to months 18–24 | p-value ¶ | |
| PVR, dyn·s·cm−5 | 802±331 (n=30) | 774±355 (n=30) | 583±310 (n=25) | −223±58+,§ (n=30) | <0.0001+ | 784±372 (n=67) | 564±268 (n=67) | 538±199 (n=57) | −213±254 (n=57) | −3±159 (n=57) | 0.8745 |
| 6MWD, m | 409±66 (n=30) | 439±85 (n=30) | 480±73 (n=25) | 61±13+,§ (n=30) | <0.0001+ | 398±86 (n=67) | 451±96 (n=67) | 458 ±110 (n=62) | 60±81 (n=62) | 7±61 (n=62) | 0.3987 |
| WHO FC, numeric | 2.4±0.5 (n=30) | 2.3±0.5 (n=30) | 1.9±0.6 (n=28) | −0.6±0.7 (n=28) | <0.0001 | 2.4±0.5 (n=67) | 2.1±0.5 (n=67) | 1.9±0.5 (n=63) | −0.4±0.6 (n=63) | −0.2±0.5 (n=63) | 0.0001 |
| NT-proBNP, pg/mL | 840±1247 (n=30) | 1059.2±1334.08 (n=30) | 363±702 (n=28) | −506.2±1190 (n=28) | 0.0004ƒ | 777.4±1051.03 (n=66) | 350±648 (n=67) | 268±457 (n=64) | −470.5±910.44 (n=63) | −76±598 (n=64) | 0.1384 |
All values shown are mean±sd unless otherwise indicated. Baseline refers to month 0 (start of the study). #: all p-values <0.0001; ¶: nominal p-values corresponding to change from end of placebo-controlled treatment period (EOP) to months 18–24; +: multiple imputation; §: values are mean±se; ƒ: nominal p-value. PVR: pulmonary vascular resistance; 6MWD: 6-min walk distance; WHO FC: World Health Organization functional class; NT-proBNP: N-terminal pro-B-type natriuretic peptide.
FIGURE 2.
Actual values of pulmonary vascular resistance (PVR). Data are from the extension period full analysis set. Minimum, lower quartile, median, upper quartile and maximum values are displayed. Outliers were identified and removed from the plots. According to the 2015 European Society of Cardiology/European Respiratory Society guidelines, pulmonary arterial hypertension is characterised by a PVR >240 dyn·s·cm−5 (3 Wood units). EOP: end of placebo-controlled treatment period.
FIGURE 3.
Actual values of 6-min walk distance (6MWD). Data are from the extension period full analysis set. Minimum, lower quartile, median, upper quartile and maximum values are displayed. Outliers were identified and removed from the plots. Threshold values for multiple endpoints have been proposed in the 2015 European Society of Cardiology/European Respiratory Society guidelines to determine prognosis of patients with pulmonary arterial hypertension. According to these guidelines, a threshold value of 6MWD >440 m is considered low risk; 6MWD 165–440 m is considered intermediate risk; and 6MWD <165 m is considered high risk. EOP: end of placebo-controlled treatment period.
FIGURE 4.
Actual values of N-terminal pro-B-type natriuretic peptide (NT-proBNP). Data are from the extension period full analysis set. Minimum, lower quartile, median, upper quartile and maximum values are displayed. Outliers were identified and removed from the plots. Threshold values for multiple endpoints have been proposed in the 2015 European Society of Cardiology/European Respiratory Society guidelines to determine prognosis of patients with pulmonary arterial hypertension. According to these guidelines, NT-proBNP <300 pg·mL−1 is considered low risk. EOP: end of placebo-controlled treatment period.
Similar improvement occurred for some haemodynamic parameters. Mean±sd mPAP and RAP were significantly reduced from baseline to months 18–24: mPAP reduced by 10.7 mmHg, from 53.9±13.72 to 44.1±11.98 mmHg, and RAP reduced by 3.9 mmHg, from 9.6±5.72 to 6.7±3.75 mmHg (both p<0.0001) (table 3). Cardiac index, cardiac output and pulmonary arterial wedge pressure did not significantly change.
TABLE 3.
Change from baseline to months 18–24 in haemodynamic parameters
| Placebo-crossed (n=30) | Continued-sotatercept (n=67) | |||||||
| Baseline | Months 18–24 | Change from baseline | p-value # | Baseline | Months 18–24 | Change from baseline | p-value # | |
| Cardiac index, L·min−1·m−2 | 2.5±0.51 (n=30) | 2.6±0.57 (n=25) | 0.2±0.63 (n=25) | 0.0842 | 2.6±0.60 (n=67) | 2.6±0.67 (n=57) | −0.1±0.66 (n=57) | 0.1926 |
| Cardiac output, L·min−1 | 4.5±0.82 (n=30) | 4.9±1.23 (n=25) | 0.4±1.21 (n=25) | 0.0698 | 4.6±1.07 (n=67) | 4.5±1.07 (n=57) | −0.1±1.19 (n=57) | 0.2934 |
| mPAP, mmHg | 53.9±13.72 (n=30) | 44.1±11.98 (n=25) | −10.7±11.46 (n=25) | <0.0001 | 52.0±12.86 (n=67) | 39.1±10.48 (n=57) | −12.5±9.83 (n=57) | <0.0001 |
| PAWP, mmHg | 10.5±3.01 (n=30) | 11.2±3.63 (n=25) | 0.1±3.85 (n=25) | 0.8663 | 10.3±2.88 (n=67) | 9.9±4.27 (n=57) | −0.3±4.50 (n=57) | 0.5891 |
| RAP, mmHg | 9.6±5.72 (n=29) | 6.7±3.75 (n=23) | −3.9±5.87 (n=23) | <0.0001 | 7.3±3.40 (n=67) | 6.0±3.72 (n=57) | −1.2±3.62 (n=57) | 0.0073 |
All values shown are mean±sd unless otherwise indicated. Data are from the extension period full analysis set. Baseline refers to month 0 (start of the study). #: nominal p-value. mPAP: mean pulmonary arterial pressure; PAWP: pulmonary arterial wedge pressure; RAP: right atrial pressure.
Delayed-start efficacy analysis
At months 18–24, both the continued-sotatercept group and the placebo-crossed group had similar PVR, 6MWD, WHO FC and NT-proBNP measurements, and the magnitude of these improvements was similar from baseline to months 18–24 (table S7).
For the primary endpoint, PVR at months 18–24, the least-squares mean (se) change from baseline was −232.8 (27.8) dyn·s·cm−5 for the continued-sotatercept group and −219.5 (43.7) dyn·s·cm−5 for the placebo crossed group: a between-group difference of −13.3 dyn·s·cm−5 (95% CI −113.2 to 86.70; p=0.7945) (table S7 and figure S3). The changes from baseline to months 18–24 in 6MWD and NT-proBNP were also similar between both groups (p-values 0.8194 and 0.7675, respectively) (table S7, figures S4 and S5).
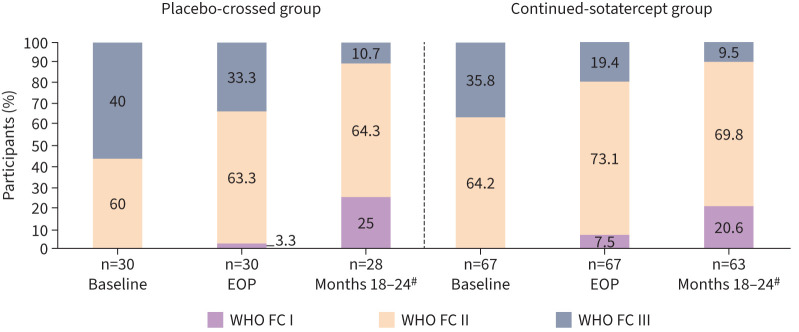
27 of 66 (40.9%) participants in the continued-sotatercept group and 16/29 (55.2%) participants in the placebo-crossed group achieved any improvement in WHO FC from baseline to months 18–24 (p=0.1355) (table S7 and figure S6). Additionally, more participants in both groups were classified as WHO FC I and II at months 18–24 than at baseline (57/63 (90.4%) versus 43/67 (64.2%) in the continued-sotatercept group and 25/28 (89.3%) versus 18/30 (60.0%) in the placebo-crossed group) (figure 5). Overall, 82/91 (90.1%) of the participants were classified as WHO FC I or II at months 18–24. WHO FC data at months 18–24 was missing for six (6.2%) participants; two due to COVID-19 and four due to unknown reasons.
FIGURE 5.
World Health Organization functional class (WHO FC) by visit. Threshold values for multiple endpoints have been proposed in the 2015 European Society of Cardiology/European Respiratory Society guidelines to determine prognosis of patients with pulmonary arterial hypertension. According to these guidelines, WHO FC I/II is considered low risk; WHO FC III is considered intermediate risk; WHO FC IV is considered high risk. #: WHO FC data at months 18–24 were missing for six (6.2%) participants; two due to COVID-19 and four due to unknown reasons. EOP: end of placebo-controlled treatment period.
Participants who were originally randomised to sotatercept experienced significant improvement in all efficacy endpoints from baseline to month 6 (the placebo-controlled treatment period) [1]. These improvements were then maintained throughout the entire extension period (figures 2–4 and figures S3–S6). At the end of the placebo-controlled treatment period, mean±sd PVR was 564±268 dyn·s·cm−5; and was 538±199 dyn·s·cm−5 at months 18–24 (table 2). The same trend was evident after adjusting for haematocrit: PVR was 572.1±278.1 dyn·s·cm−5 at the end of the placebo-controlled treatment period, and 532.6±218.2 at months 18–24 (table S6). Improvements in 6MWD, WHO FC and NT-proBNP were at least maintained from the end of the placebo-controlled period throughout the entire extension period (table 2 and figures 2–5).
Efficacy: aligned analyses
At aligned month 6, the sotatercept 0.3 mg·kg−1 group and sotatercept 0.7 mg·kg−1 group had similar 6MWD and WHO FC measurements, and the magnitude of these improvements was similar from aligned baseline to aligned month 6 (table S8).
Discussion
The open-label extension of the phase 2 PULSAR study enrolled adults with PAH who had previously received sotatercept or placebo on top of background PAH therapy during the trial's initial 24-week placebo-controlled treatment period [1]. The longer-term safety profile of sotatercept was generally consistent with that observed during the initial 24-week placebo-controlled treatment period. Among participants who originally received placebo, significant improvements in PVR, 6MWD, WHO FC and NT-proBNP were achieved at months 18–24 compared with baseline after being randomised to sotatercept. Improvement across all endpoints was maintained in those with longer-term use. About 90% of the participants were classified as WHO FC I or II at months 18–24.
Notably, 97/106 (92%) participants continued into the extension period, highlighting the tolerability of sotatercept and the desire to receive therapy among this heavily treated participant population. This high continuation rate compares favourably with clinical trials evaluating other PAH therapies, in which discontinuation rates of up to 32% have been reported [21–25].
The safety profile of sotatercept was consistent with that observed during the placebo-controlled treatment period, as well as in clinical trials evaluating sotatercept in other patient populations [1, 4, 26–28]. Fewer TEAEs occurred over time with longer exposure to sotatercept, and no additional safety signals were identified in participants who transitioned from placebo to sotatercept, regardless of dose [29]. In addition, incidence rates of TEAEs following extended treatment were similar with both sotatercept doses, indicating a consistent safety profile, and further supporting investigation of 0.7 mg·kg−1 in the phase 3 development programme. No serious TEAEs considered related to sotatercept led to treatment or study discontinuation, and no deaths were considered related to sotatercept. Most participants required no dose reduction during the study, and around half of those who did, had their dose subsequently increased. This suggests that most participants tolerated their initial randomised dose.
Consistent with the placebo-controlled treatment period, thrombocytopenia and increased haemoglobin remained the most common haematological adverse events [1]. The observed increases in haemoglobin levels were consistent with the erythropoietic effects of sotatercept seen in previous clinical trials, and were expected [26–28, 30]. Since anaemia is a frequent complication of PAH reported in up to 50% of patients with PAH [29], patients with PAH who have lower haemoglobin levels could receive sotatercept without unacceptable erythropoietic effects; indeed, they may benefit from the concurrent treatment of underlying anaemia, but this needs further exploration [29]. Telangiectasia occurred as a new event in 11% of participants; however, all cases were mild and further observation is required to determine the clinical significance of this adverse event. Therefore, telangiectasia has been added as an AESI for all studies in the phase 3 development programme of sotatercept.
During the extension period, significant improvements in PVR, 6MWD, WHO FC and NT-proBNP were achieved at months 18–24 compared with baseline in previous placebo participants, while improvement across all endpoints was maintained in those with longer-term use. The magnitude of these improvements in the placebo-crossed treatment group during the extension period was consistent with that of the continued-sotatercept treatment group during the placebo-controlled treatment period. These improvements were also consistent with the initial results from the phase 2 SPECTRA study: a single-arm, open-label, multicentre exploratory study of sotatercept on top of background therapy in adults with WHO FC III [14, 31]. The similarity in magnitude of improvement between the continued-sotatercept and placebo-crossed groups, despite a 6-month delay in treatment among the latter, is unsurprising given the characteristics of this participant population, who had an average time from diagnosis to enrolment of 8 years and were heavily treated with background PAH therapies.
The data presented here have some limitations. First, the population for this study was self-selected from the placebo-controlled treatment period and therefore open to selection bias. However, it should be noted that any potential bias was minimised by the high retention rate of eligible participants. Other limitations include the unblinded nature of an open-label study, which could influence patient responses to assessments, and the absence of a placebo arm as a comparator. However, the NT-proBNP and central haemodynamics should not be affected by the open-label nature of this extension period.
While this trial provides data up to a maximum sotatercept treatment period of 24 months, continued evaluation is needed to determine whether the clinical benefits and safety are maintained for longer. This will be assessed in the SOTERIA study (NCT04796337), a long-term follow-up study to evaluate the tolerability of sotatercept in adults with PAH who have completed prior sotatercept studies. The phase 3 clinical development programme of sotatercept will continue to provide more data on its safety and efficacy. Moreover, it will establish the potential value of sotatercept on top of background PAH therapy across a spectrum of patients with PAH, including those who are more recently diagnosed as well as those who are at higher risk, including patients classified as WHO FC IV.
In conclusion, the longer-term safety profile of sotatercept was generally consistent with that observed during the initial 24-week placebo-controlled period. Further observation is required to determine the clinical significance of mild telangiectasia, which occurred as a new event in 11% of participants. Treatment with sotatercept in the PULSAR open-label extension period suggests a clinical benefit in participants re-randomised from placebo to sotatercept treatment, in alignment with the initial results from the placebo-controlled treatment period. In those who continued sotatercept for 24 months, the initial benefits were maintained. These results support the longer-term safety and durability of clinical benefit of sotatercept in patients with PAH.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01347-2022.Supplement (573.2KB, pdf)
Central illustration. The proposed mechanism of action of sotatercept involves rebalancing growth promoting and growth inhibiting signalling. PAH is associated with reduced antiproliferative BMPR-II–Smad1/5/8 signalling, shifting the balance towards proproliferative activin–Smad2/3 signalling, which leads to pulmonary vascular remodelling. Sotatercept acts to sequester excess ActRIIA ligands, thereby reducing ActRIIA–Smad2/3 signalling to rebalance growth promoting and growth inhibiting signalling. Outliers were identified and removed from the plots. Minimum, lower quartile, median, upper quartile and maximum values are displayed. *: values are mean±standard deviation. †: PAH associated with simple, congenital systemic-to-pulmonary shunts ≥1 year following repair. 6MWD: 6-min walk distance; ALK: activin-like kinase; ActRIIA/B: activin receptor type IIA/B; BMP: bone morphogenetic protein; BMPR-II: BMP receptor type II; CTD: connective tissue disease; FC: functional class; NT-proBNP: N-terminal pro-B-type natriuretic peptide; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; PVR: pulmonary vascular resistance; WHO: World Health Organization. ERJ-01347-2022.Visual_Abstract (79.2KB, jpg)
Shareable PDF
Acknowledgements
We thank the patients and families who participated in the PULSAR trial; the investigators and their research teams who collaborated on the trial; current and former personnel at Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, including Marcie Fowler, Musa Mutyaba and Robert Gerber; and Ana Maria Rodriguez de Ledesma of InterComm International, for medical writing assistance (funded by Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA).
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.01972-2022
This clinical trial was prospectively registered as NCT03496207. Individual participant data and data dictionaries will not be made available.
This study was funded by Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: M. Humbert is a consultant and an advisory committee member for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Aerovate, Altavant, AOP Orphan, Bayer, Ferrer, Janssen, Merck & Co., Inc., Rahway, NJ, USA, MorphogenIX and United Therapeutics, and has received research grants for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Janssen, and Merck & Co., Inc., Rahway, NJ, USA. V. McLaughlin is a consultant for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Aerovate, Altavant, Bayer, Caremark LLC, CiVi Bioharma Inc., Corvista, Gossamer Bio, Janssen, Merck & Co., Inc., Rahway, NJ, USA, and United Therapeutics, has received grant(s) from Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Janssen, Sonovie, United Therapeutics, is involved in CME programs for Impact PH and PHA, and on the board of directors for CiVi Biopharma Inc., and for Clene. J.S.R. Gibbs is a consultant and speaker for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Aerovate, Bayer, Complexa, Janssen, Merck & Co., Inc., Rahway, NJ, USA, MSD, Pfizer and United Therapeutics. M. Gomberg-Maitland is a consultant for Altavant, Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Janssen, and Entelligence Young for Janssen, is part of the advisory committee for United Therapeutics and the data safety monitoring board for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, has received grant(s) and/or reports GW with funds from Altavant, Bayer and United Therapeutics. M.M. Hoeper is a consultant and speaker for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Bayer, GlaxoSmithKline, Janssen, MSD and Pfizer. I.R. Preston is a consultant for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Pfizer, Respira and United Therapeutics, a steering committee member for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and has received grant(s) from Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Bayer, Complexa, Liquidia, PhaseBio, Tenax and United Therapeutics. R. Souza is an advisory committee member for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA. A.B. Waxman is steering committee member for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Altavant, Gossamer Bio, United Therapeutics, an investigator and/or principal investigator for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Aria-CV and United Therapeutics, and has received grant(s) from Acceleron, a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA. H-A. Ghofrani is a consultant for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Bayer, Gossamer Bio, Jansson, MorphogenIX, MSD and Pfizer. P. Escribano Subias has received consulting fees from Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Janssen, MSD and Gossamer Bio, has received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Janssen, MSD, Ferrer, Bayer and AOT, has received support for attending meetings from Janssen and MSD, has received equipment, materials, drugs, medical writing or other services from Janssen, and has participated on data safety monitoring or advisory boards for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway NJ, USA, Janssen, MSD and Gossamer Bio. J. Feldman reports consulting fees from Merck, Aerovate, Altavant, United Therapeutics, Liquidia, Corsair and Janssen. G. Meyer has received payment or honoraria for lectures, presentations, and speakers’ bureaus from Bayer and Janssen and has participated on advisory boards for Bayer and Janssen. D. Montani has received grant(s) from and is a consultant for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Bayer, Chesi, GlaxoSmithKline, MSD and Pfizer. K.M. Olsson is a consultant and speaker for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Bayer, GlaxoSmithKline, Janssen, MSD and Pfizer. S. Manimaran and J. de Oliveira Pena are employees of Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA. D.B. Badesch is a consultant for Pfizer, is a consultant and advisory committee member for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Altavant, Arena, Bayer, Liquidia, Merck & Co., Inc., Rahway, NJ, USA, United Therapeutics, has received research grant for Acceleron Pharma Inc., a wholly owned subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Actelion, Altavant, Arena, Belleraphon, Janssen, Liquidia, Merck & Co., Inc., Rahway, NJ, USA, and United Therapeutics, sits on the data safety monitoring board for United Therapeutics, and is a long-term holder of common stock for Johnson and Johnson.
References
- 1.Humbert M, McLaughlin V, Gibbs JSR, et al. . Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med 2021; 384: 1204–1215. doi: 10.1056/NEJMoa2024277 [DOI] [PubMed] [Google Scholar]
- 2.Tielemans B, Delcroix M, Belge C, et al. . TGFβ and BMPRII signalling pathways in the pathogenesis of pulmonary arterial hypertension. Drug Discov Today 2019; 24: 703–716. doi: 10.1016/j.drudis.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 3.Morrell NW, Aldred MA, Chung WK, et al. . Genetics and genomics of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801899. doi: 10.1183/13993003.01899-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yung LM, Yang P, Joshi S, et al. . ACTRIIA-Fc rebalances activin/GDF versus BMP signaling in pulmonary hypertension. Sci Transl Med 2020; 12: eeaz5660. doi: 10.1126/scitranslmed.aaz5660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisserier M, Pradhan N, Hadri L. Current and emerging therapeutic approaches to pulmonary hypertension. Rev Cardiovasc Med 2020; 21: 163–179. doi: 10.31083/j.rcm.2020.02.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayeux JD, Pan IZ, Dechand J, et al. . Management of pulmonary arterial hypertension. Curr Cardiovasc Risk Rep 2021; 15: 2. doi: 10.1007/s12170-020-00663-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galie N, Channick RN, Frantz RP, et al. . Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801889. doi: 10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galie N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Heart J 2016; 37: 67–119. doi: 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 9.Benza RL, Miller DP, Barst RJ, et al. . An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012; 142: 448–456. doi: 10.1378/chest.11-1460 [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation 2002; 106: 1477–1482. doi: 10.1161/01.CIR.0000029100.82385.58 [DOI] [PubMed] [Google Scholar]
- 11.Huertas A, Tu L, Guignabert C. New targets for pulmonary arterial hypertension: going beyond the currently targeted three pathways. Curr Opin Pulm Med 2017; 23: 377–385. doi: 10.1097/MCP.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 12.Joshi SR, Liu J, Pearsall RS, et al. . Sotatercept analog RAP-011 alleviates cardiopulmonary remodeling and inflammation in a model of heritable PAH arising from BMPR2 haploinsufficiency. Am J Respir Crit Care Med 2021; 203: A3657. [Google Scholar]
- 13.Joshi SR, Liu J, Pearsall RS, et al. . RAP-011, a murine ortholog of ActRIIA-FC (sotatercept), improves pulmonary hemodynamics and restores right ventricular structure and function in a preclinical model of severe angio-obliterative pulmonary arterial hypertension. Circulation 2018; 138: Suppl. 1, 16179. [Google Scholar]
- 14.ClinicalTrials.gov . A Study of Sotatercept for the Treatment of Pulmonary Arterial Hypertension (SPECTRA). Date last accessed: 16 December 2021. https://clinicaltrials.gov/ct2/show/NCT03738150
- 15.ClinicalTrials.gov . A Study of Sotatercept for the Treatment of Pulmonary Arterial Hypertension (STELLAR). Date last accessed: 22 November 2021. https://clinicaltrials.gov/ct2/show/NCT04576988
- 16.ClinicalTrials.gov . Study of Sotatercept in Newly Diagnosed Intermediate- and High-risk PAH Patients (HYPERION). Date last accessed: 22 November 2021. https://clinicaltrials.gov/ct2/show/NCT04811092
- 17.ClinicalTrials.gov . A Study of Sotatercept in Participants with PAH WHO FC III or FC IV at High Risk of Mortality (ZENITH). Date last accessed: 22 November 2021. https://clinicaltrials.gov/ct2/show/NCT04896008
- 18.ClinicalTrials.gov . A Long-term Follow-up Study of Sotatercept for PAH Treatment (SOTERIA). Date last accessed: 22 November 2021. https://clinicaltrials.gov/ct2/show/NCT04796337
- 19.Ouyang J, Zhang P, Carroll KJ, et al. . Comparisons of global tests on intersection hypotheses and their application in matched parallel gatekeeping procedures. J Biopharm Stat 2020; 30: 593–606. doi: 10.1080/10543406.2019.1696355 [DOI] [PubMed] [Google Scholar]
- 20.Vanderpool RR, Naeije R. Hematocrit-corrected pulmonary vascular resistance. Am J Respir Crit Care Med 2018; 198: 305–309. doi: 10.1164/rccm.201801-0081PP [DOI] [PubMed] [Google Scholar]
- 21.Frost AE, Barst RJ, Hoeper MM, et al. . Long-term safety and efficacy of imatinib in pulmonary arterial hypertension. J Heart Lung Transplant 2015; 34: 1366–1375. doi: 10.1016/j.healun.2015.05.025 [DOI] [PubMed] [Google Scholar]
- 22.Ghofrani HA, Grimminger F, Grunig E, et al. . Predictors of long-term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT-2 open-label, randomised, long-term extension trial. Lancet Respir Med 2016; 4: 361–371. doi: 10.1016/S2213-2600(16)30019-4 [DOI] [PubMed] [Google Scholar]
- 23.Galie N, Gaine S, Channick R, et al. . Long-term survival, safety and tolerability with selexipag in patients with pulmonary arterial hypertension: results from GRIPHON and its open-label extension. Adv Ther 2022; 39: 796–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghofrani HA, Galie N, Grimminger F, et al. . Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013; 369: 330–340. doi: 10.1056/NEJMoa1209655 [DOI] [PubMed] [Google Scholar]
- 25.Sitbon O, Channick R, Chin KM, et al. . Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. doi: 10.1056/NEJMoa1503184 [DOI] [PubMed] [Google Scholar]
- 26.Abdulkadyrov KM, Salogub GN, Khuazheva NK, et al. . Sotatercept in patients with osteolytic lesions of multiple myeloma. Br J Haematol 2014; 165: 814–823. doi: 10.1111/bjh.12835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cappellini MD, Porter J, Origa R, et al. . Sotatercept, a novel transforming growth factor beta ligand trap, improves anemia in beta-thalassemia: a phase II, open-label, dose-finding study. Haematologica 2019; 104: 477–484. doi: 10.3324/haematol.2018.198887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raftopoulos H, Laadem A, Hesketh PJ, et al. . Sotatercept (ACE-011) for the treatment of chemotherapy-induced anemia in patients with metastatic breast cancer or advanced or metastatic solid tumors treated with platinum-based chemotherapeutic regimens: results from two phase 2 studies. Support Care Cancer 2016; 24: 1517–1525. doi: 10.1007/s00520-015-2929-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang P, Bocobo GA, Yu PB. Sotatercept for pulmonary arterial hypertension. N Engl J Med 2021; 385: 92–93. doi: 10.1056/NEJMc2107209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Komrokji R, Garcia-Manero G, Ades L, et al. . Sotatercept with long-term extension for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes: a phase 2, dose-ranging trial. Lancet Haematol 2018; 5: e63–e72. doi: 10.1016/S2352-3026(18)30002-4 [DOI] [PubMed] [Google Scholar]
- 31.Waxman A, Risbano M, Frantz R, et al. . The SPECTRA study: a phase 2a single-arm, open-label, multicenter exploratory study to assess the effects of sotatercept for the treatment of pulmonary arterial hypertension (PAH). Am J Respir Crit Care Med 2021; 203: A1187. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01347-2022.Supplement (573.2KB, pdf)
Central illustration. The proposed mechanism of action of sotatercept involves rebalancing growth promoting and growth inhibiting signalling. PAH is associated with reduced antiproliferative BMPR-II–Smad1/5/8 signalling, shifting the balance towards proproliferative activin–Smad2/3 signalling, which leads to pulmonary vascular remodelling. Sotatercept acts to sequester excess ActRIIA ligands, thereby reducing ActRIIA–Smad2/3 signalling to rebalance growth promoting and growth inhibiting signalling. Outliers were identified and removed from the plots. Minimum, lower quartile, median, upper quartile and maximum values are displayed. *: values are mean±standard deviation. †: PAH associated with simple, congenital systemic-to-pulmonary shunts ≥1 year following repair. 6MWD: 6-min walk distance; ALK: activin-like kinase; ActRIIA/B: activin receptor type IIA/B; BMP: bone morphogenetic protein; BMPR-II: BMP receptor type II; CTD: connective tissue disease; FC: functional class; NT-proBNP: N-terminal pro-B-type natriuretic peptide; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; PVR: pulmonary vascular resistance; WHO: World Health Organization. ERJ-01347-2022.Visual_Abstract (79.2KB, jpg)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01347-2022.Shareable (590.3KB, pdf)