Abstract
Successful recognition has been known to produce distinct patterns of neural activity. Many studies have used spectral power or event-related potentials of single recognition-specific regions as classification features. However, this does not accurately reflect the mechanisms behind recognition, in that recognition requires multiple brain regions to work together. Hence, classification accuracy of subsequent memory performance could be improved by using functional connectivity within memory-related brain networks instead of using local brain activity as classifiers. In this study, we examined electroencephalography (EEG) signals while performing a word recognition memory task. Recorded EEG signals were collected using a 32-channel cap. Connectivity measures related to the left hemispheric fronto-parietal connectivity (P3 and F3) were found to contribute to the accurate recognition of previously studied memory items. Classification of subsequent memory outcome using connectivity features revealed that the classifier with support vector machine achieved the highest classification accuracy of 86.79 ± 5.93% (mean ± standard deviation) by using theta (3–8 Hz) connectivity during successful recognition trials. The results strongly suggest that highly accurate classification of subsequent memory outcome can be achieved by using single-trial functional connectivity.
Keywords: memory, recognition, theta, brain connectivity, EEG, fronto-parietal, mutual information
1. Introduction
Successful recognition can be defined as the accurate retrieval of previously encoded information. Researchers have been exploring the neural correlates of successful recognition, as well as ways in which these measures can be used to evaluate memory performance. Such attempts hold significance especially for the criminal justice system. In situations where physical evidence cannot be obtained, eyewitness testimonies serve a significant role in the criminal justice system. However, studies have shown that the testimonies of compliant witnesses without the intention of deception can be prone to error [1], and the consequences of inaccurate eyewitness testimonies can be dire. In fact, eyewitness misidentification is the most common contributing factor to wrongful convictions in the United States [2]. One solution to this inherent problem of inaccurate witness testimonies could be to devise a method to scientifically assess the accuracy of the eyewitness’s memory. Accurate predictions of the eyewitness’s memory performance with neural measures would greatly increase the justice system’s ability to discern which testimonies should be used as evidence.
Electroencephalography (EEG) has been incorporated in exploring the neural correlates of successful recognition. Previous studies have identified multiple event-related potential (ERP) components that show an “old/new effect,” a difference in ERP amplitude between the successful recognition of “old” (witnessed) and “new” (not witnessed) items [3,4]. An example of this would be the late positive complex (LPC), a positive-going ERP component traditionally observed in the parietal regions between 500 and 800 ms. The LPC amplitude evoked by correctly recognized old (hits) items is known to be larger compared to the LPC amplitude evoked by new (correct rejections, CR) test items. This is a left-lateralized phenomenon otherwise known as the parietal old–new effect [5,6], a neural correlate of successful recognition [7,8].
Studies using the parietal old–new effect as a classifier for machine learning algorithms have found the maximum accuracy of this approach to be 57.43% [4]. Along with ERP correlates, rhythmic oscillations in electrical charge are associated with local and global neural interaction and integration [9]. Specifically, previous research showed activity in the theta (3–8 Hz) and gamma (30–50 Hz) frequency bands to be related to successful recognition [10–13]. These studies have shown that it is plausible to incorporate neural measures in predicting one’s subsequent memory outcome.
Recent trends have been shifting toward taking more global, network-level approaches to classify various cognitive functions [14]. Previous EEG-based connectivity studies showed the relations between cognitive emotion regulation strategies and EEG synchronization levels during resting states [15]. In addition, discrete emotional states can be classified by EEG-based graph theoretical network measure [16]. Furthermore, Gupta et al. classified cognitive workload with EEG-based connectivity along with different deep learning algorithms and achieved a classification accuracy of 95.92%. A significant benefit of using brain connectivity measures as classifying features lies in the fact that connectivity measures take into consideration the communication between different brain regions during memory processing. Given the successful application of functional connectivity approaches in the context of memory performance [17], utilizing a network-level approach as such could render a more comprehensive picture of the neural bases of recognition [18]. Brain connectivity approach could be taken to successfully estimate recognition memory performance, even on a single-participant level. Thus, a network-based connectivity approach could be a better suited measure to accurately evaluate a witness’s memory performance.
For the brain connectivity approach, mutual information (MI) has been adopted for the connectivity analysis in this study. MI is a measure of the amount of dependency between two signals. Compared to linear correlation, MI is a universal measurement, since it could measure nonlinear dependency. The temporal series of mean frequency band signals were used to compute the cross-MI between regions of interest (ROIs). MI values between ROIs can be determined using the probability density function, as follows:
where p(X,Y) is the joint probability distribution function of variables X and Y, and p(X) and p(Y) are the marginal probability distribution functions of X and Y, respectively.
Despite the robust literature suggesting the feasibility of using frequency-specific functional connectivity to classify memory, not many studies to date have taken a machine learning approach in classifying subsequent memory outcome using single-trial functional connectivity. If this approach is successful in improving the accuracy of memory outcome prediction, it may be the key in improving the accuracy of EEG-based eyewitness-memory evaluation methods. Therefore, this study aims to explore the frequency-specific connectivity patterns during the successful recognition of witnessed and non-witnessed items. Moreover, we aim to evaluate whether using single-trial connectivity features as classifiers in a support vector machine (SVM) can yield significantly accurate results in classifying subsequent memory outcome.
2. Methods
2.1. Participants
A total of 40 healthy participants (19 females, 21 males, age = 23.27 years, age range: 19–29 years, SD = 2.60) were recruited by means of advertising. The subjects were rewarded with monetary compensation for their participation. Prior to the procedure, any participants who may have impaired vision was advised to use any visual aid necessary to ensure intact perceptual accuracy. Analysis was restricted to include only those (n = 30) participants whose data fulfilled the analysis criteria (Table 1).
Table 1.
Subject demographics
| Variable | n = 40 | Mean | Standard deviation | % |
|---|---|---|---|---|
| Age | 40 | 23.27 | 2.6 | 100.0 |
| Gender | ||||
| Female | 19 | 47.5 | ||
| Male | 21 | 52.5 | ||
| Handedness | ||||
| Left | 3 | 7.5 | ||
| Right | 37 | 92.5 |
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by Institutional Review Board of the National Forensic Service (906-200228-HR-001-01).
Informed consent: Informed consent has been obtained from all individuals included in this study.
2.2. Experimental design
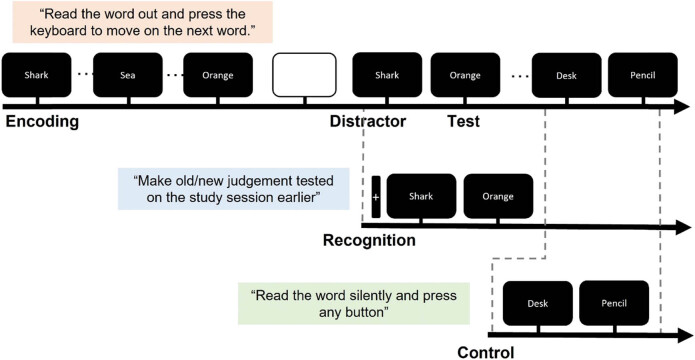
A diagram of the experimental paradigm is displayed in Figure 1. A modified old–new recognition task was used to explore the functional brain connectivity patterns during successful recognition of witnessed items (hit), successful recognition of not witnessed items (CR), and resting state (control). After obtaining informed consent, the participants completed an encoding task during which they studied a list of 120 Korean words for a subsequent memory test. The encoding task was programmed in E-Prime 2.0 (Psychology Software Tools, Inc.). During the encoding task, participants were instructed to read out the word loud that appeared on a 15 in. laptop screen and to press a button to proceed. The task was programmed so that each word appeared five times, without the same word appearing more than twice in a row.
Figure 1.
Example of the timeline of the word recognition memory task. The task was comprised of three successive stages of encoding, distractor, and test phase. The test phase consisted of ten recognition blocks and ten control blocks presented in a randomized order, with an interval of 5,000 ms in between each block. EEG data were collected during the retrieval phase only.
The encoding task was followed by a recognition task after a 30 min delay. During the recognition task, the participants were situated in a comfortable chair 60 in. away from a 24 in. monitor. STIM2 (Compumedics, Neuroscan) was used to program the task and to record the behavioral responses. The recognition task consisted of ten recognition blocks and ten control blocks presented in a randomized order, with an interval of 5,000 ms in between each block. During the recognition blocks, participants viewed a fixation cross for 14–16 s, followed by the presentation of six previously studied words (old) and six novel words (new). Each word was presented on the screen for 2,800 ( 200) ms. The participants were instructed to make a judgment whether they remember studying the word during the previous study session (old) or not (new). “Old” responses were made by pressing the left button with the left thumb, and “new” responses were made by pressing the right button with the right thumb. The control blocks followed the same temporal structure, except that the participants were presented with unstudied words (new) and were instructed to read the presented words silently without making recognition judgments, and to press any button. The control task was designed as such to physically mimic the recognition task (i.e., button pressing, visual/semantic processing of the presented words, etc.) without evoking recognition. The test session took on average 30 min to complete. EEG data were collected during the test phase only.
2.3. EEG equipment and data collection
EEG signals were obtained through the SynAmps2 System (Compumedics, Neuroscan) in an electrically shielded, sound-attenuated room. Recorded EEG signals were collected using a 32-channel Quick-Cap, which had 30 Ag/AgCl electrodes (FP1, FP2, F7, F3, FZ, F4, F8, FT7, FC3, FCZ, FC4, FT8, T7, C3, CZ, C4, T8, TP7, CP3, CZ, CP4, TP8, P7, P3, PZ, P4, P8, O1, O2, and OZ) mounted on it according to the international 10–20 system and two reference electrodes placed at the opposite lateral mastoids. EEG data were sampled at 500 Hz. To assure the collection of high-quality data, impedance for the previously mentioned channels was kept below 5 kΩ. Horizontal and vertical eye movements were monitored with electrooculogram (EOG) electrodes. Horizontal electrooculogram (hEOG) electrodes were placed above and below the left eye, and vertical electrooculogram (vEOG) electrodes were placed near the outer canthi of each eye. Impedance for the EOG channels was kept below 10 kΩ.
The EEG and EOG signals were baseline corrected and bandpass filtered between 0.3 and 30 Hz. Ocular artifacts were corrected by applying the covariance method with the data from both hEOG and vEOG channels. The data for each were then epoched from −200 to 1,400 ms with reference to stimulus onsets. Extracted epochs were grouped and labeled by trial type (i.e., old, new, and control).
2.4. Frequency-specific EEG connectivity analysis
Subsequent memory outcome was extracted using features from the frequency-specific connectivity across multiple memory-related brain areas and memory-related frequency bands. We applied continuous wavelet transformation for the time–frequency analysis. We focused on theta (3–8 Hz) and gamma (30–50 Hz) frequency bands, as they are shown in previous research to correlate with recognition activity. We normalized these power values by the baseline activity before the stimulus onset of −100 to 0 ms. EEG signals after the stimulus onset at 0–500 ms were used as an “test” phase for subsequent connectivity analysis. Considering the time it takes the subject to perceive and recognize the stimuli, the retrieval phase was defined as 500 ms post-stimulus. We include data from regions involved in the memory-related network that were selected previous memory-related studies (i.e., frontal, temporal, parietal, and occipital). Then we used MI, which evaluates the amount of information about one signal that is contained in another signal [19], as our connectivity measure. For the connectivity analysis, we calculated the time-frequency cross-MI [20]. Therefore, following the continuous wavelet transformation, we obtained the mean value of each frequency band (theta and gamma). After cross-MI values were calculated using retrieval samples, we examined the differences in EEG connectivity between correctly recognized and correctly rejected trials.
2.5. Subject classification using single-trial connectivity features
We used a linear SVM in MATLAB for the classification of memory success. The most informative connectivity values within the top 20% of the t-statistics were selected as the features of successful retrieval for each frequency band (Figure 2). The most informative power was selected as a candidate feature for SVM learning to identify the optimal classifier modified from a previous study [21]. SVM group classification analyses were performed using the Statistics Toolbox in Matlab software (version R2018b; MathWorks Inc., Natick, MA, USA). Nonlinear radial basis function kernel (sigma = 2) and constant soft margin (cost = 1) were applied for the SVM training. In the SVM training procedure, the decision boundary formulated using a candidate feature set was optimized to maximize the group classification accuracy. This was achieved by incorporating 80% of trials randomly selected from the total trials [22]. All SVM procedures, testing, and iterative group classifier performance evaluation (with random permutation of subjects into training and testing sets for cross-validation) were repeated 10,000 times per candidate feature set. The classification performance of individual EEG signal was evaluated by five-fold cross-validation with 100 repetitions.
Figure 2.
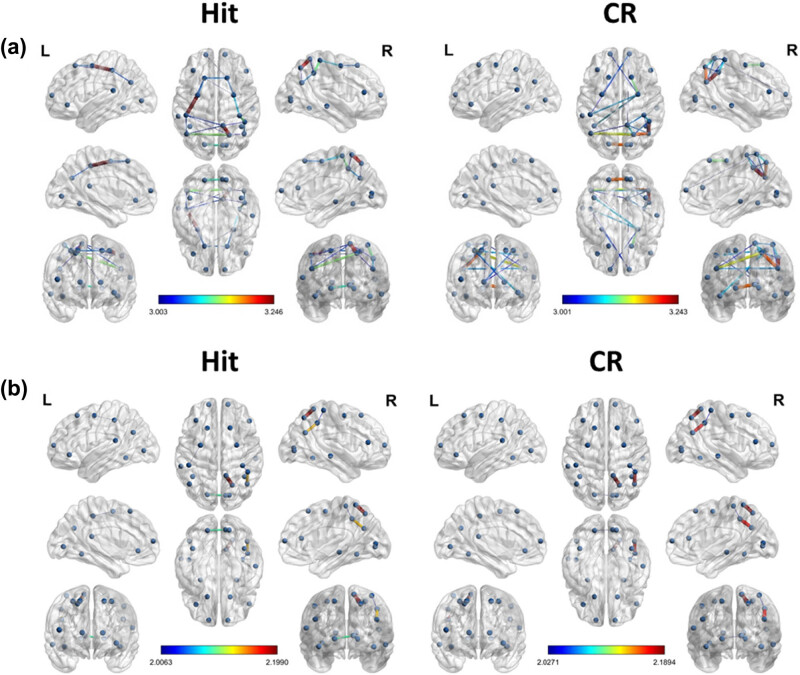
Result of the t-test for the brain connectivity of MI (hit vs CR, p < 0.05). The nodes selected in this study are PFC, DLPFC, VLPFC, FC, TC, PC, and OC. (a) Theta band brain connectivity of MI during hit (left) and CR (right) conditions. (b) Gamma band brain connectivity of MI during hit (left) and CR (right) conditions.
3. Results
3.1. Behavioral results
On an average, participants correctly remembered 84 ± 0.09% (mean ± standard deviation) of all trials, indicating that they were able to effectively encode materials and that we obtained sufficient trials for both remembered and forgotten conditions (Table 2).
Table 2.
Average (%) behavioral results of the test session
| Mean | Standard deviation | |
|---|---|---|
| Accuracy | ||
| Hit | 0.73 | 0.12 |
| CR | 0.95 | 0.06 |
| Miss | 0.27 | 0.12 |
| False alarm | 0.05 | 0.06 |
| Response time (ms) | ||
| Hit | 650.21 | 64.10 |
| CR | 579.95 | 44.75 |
3.2. Frequency-specific EEG feature selection
Table 3 shows the selected features for each frequency band, as well as the t-statistics values and regions of the chosen connectivity. The most informative connectivity values within the top 20% of the t-statistics were selected as the features for correctly recognized (hit) condition and control trials (CR) for each frequency band. The correctly recognized condition presented bigger theta frequency band connectivity in regions including the left hemispheric fronto-parietal region (i.e., PFC-PC) and right temporo-parietal region during successful recognition phase. On the contrary, correctly rejected new trials showed a significant increase in right hemispheric temporo-occipital connectivity.
Table 3.
Results of the t-test the hit and CR conditions
| Hit | CR | ||||||
|---|---|---|---|---|---|---|---|
| Band | Feature set | p-Value | Side | Band | Feature set | p-Value | Side |
| Theta | PFC PC | 0.003* | L | Theta | TC OC | 0.0084* | R |
| TC PC | 0.0041* | L | OC OC | 0.0090* | LR | ||
| Central OC | 0.0091* | R | TC PC | 0.0075* | R | ||
| Gamma | Central OC | 0.0077* | R | Gamma | Central OC | 0.0087* | R |
| TC OC | 0.0085* | R | TC OC | 0.0094 | R | ||
| OC OC | 0.015* | LR | |||||
*p < 0.05, **p < 0.01. PFC, prefrontal cortex; DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; TC, temporal cortex; PC, parietal cortex; OC, occipital cortex, L, left; R, right.
The gamma frequency band also showed increased connectivity in right hemispheric central-occipital regions for both correctly recognized and correctly rejected conditions. On the other hand, a significant change in connectivity was not found in the left hemispheric region in the gamma frequency band for neither condition. Overall, correct recognition was related to the connectivity of the left fronto-parietal network for the theta frequency, whereas the correctly rejected new condition was found to be related to the connectivity of right temporo-occipital regions.
3.3. Classification accuracy
Table 4 describes the individual classification accuracy for each frequency band. The SVM binary classifier achieved the highest mean classification accuracy of 86.79 5.93% (mean ± standard deviation) for the theta band.
Table 4.
Individual subject classification accuracy
| Subject | Recognition (0–500 ms) (%) | ||
|---|---|---|---|
| Theta (3–8 Hz) | Gamma (30–50 Hz) | Number of trials (hit/CR) | |
| Sub1 | 89.09 | 60.81 | 48/58 |
| Sub2 | 89.32 | 57.63 | 44/59 |
| Sub3 | 90.70 | 51.21 | 49/59 |
| Sub4 | 84.23 | 51.13 | 47/57 |
| Sub5 | 90.11 | 68.25 | 45/54 |
| Sub6 | 95.50 | 59.25 | 43/59 |
| Sub7 | 90.68 | 49.06 | 42/56 |
| Sub8 | 85.85 | 59.79 | 55/58 |
| Sub9 | 88.82 | 53.88 | 46/60 |
| Sub10 | 90.59 | 55.63 | 46/51 |
| Sub11 | 85.33 | 43.88 | 57/58 |
| Sub12 | 83.73 | 42.94 | 51/60 |
| Sub13 | 95.86 | 53.38 | 55/54 |
| Sub14 | 78.25 | 53.25 | 40/55 |
| Sub15 | 81.22 | 50.30 | 44/60 |
| Sub16 | 90.45 | 48.75 | 40/59 |
| Sub17 | 73.30 | 57.07 | 47/58 |
| Sub18 | 92.17 | 50.13 | 51/60 |
| Sub19 | 84.41 | 51.13 | 55/54 |
| Sub20 | 81.77 | 52.06 | 40/55 |
| Sub21 | 81.95 | 50.14 | 53/59 |
| Sub22 | 90.27 | 52.00 | 55/60 |
| Sub23 | 86.85 | 45.75 | 42/59 |
| Sub24 | 88.23 | 50.69 | 44/60 |
| Sub25 | 95.44 | 39.07 | 47/58 |
| Sub26 | 77.50 | 52.38 | 42/53 |
| Sub27 | 94.23 | 51.69 | 47/59 |
| Sub28 | 91.91 | 49.78 | 47/50 |
| Sub29 | 75.57 | 45.80 | 56/53 |
| Sub30 | 80.50 | 43.93 | 50/56 |
| Average | 86.79 | 51.69 | 47.6/57.03 |
| SD | 5.93 | 5.85 | 5.10/2.83 |
4. Discussions
The purpose of this study was to investigate the feasibility of incorporating single-trial connectivity measures as classifiers in predicting one’s future memory performance. Our results revealed that connectivity measures retrieved from time domain EEG data can in fact be used in classifying subsequent memory outcome (i.e., hit vs CR). Furthermore, we were able to predict subsequent memory outcome with the single-trial theta band connectivity feature with a prediction accuracy of 86.79 ± 5.93%. Such results give strong support for the feasibility of single-trial EEG connectivity as a classification feature and achieved a higher classification accuracy than the previous studies that used local brain activities as classifiers (Table 5).
Table 5.
Talairach coordinates of ROI
| Area | Brodmann area | x | y | z |
|---|---|---|---|---|
| ‘O2’ | 18R | 15.445 | −80.241 | 1.209 |
| ‘O1’ | 18L | −17.944 | −79.578 | 1.471 |
| ‘Oz’ | 17R | 9.722 | −80.832 | 5.779 |
| ‘Pz’ | 7R | 20.152 | −64.938 | 52.17 |
| ‘P4’ | 39R | 43.234 | −63.01 | 29.262 |
| ‘CP4’ | 40R | 43.95 | −44.701 | 44.334 |
| ‘P8’ | 37R | 39.008 | −51.835 | −10.207 |
| ‘C4’ | 1R | 36.017 | −34.877 | 63.832 |
| ‘T8’ | 21R | 56.124 | −24.4 | −6.911 |
| ‘P7’ | 37L | −40.675 | −51.795 | −9.88 |
| ‘P3’ | 39L | −45.702 | −63.239 | 29.779 |
| ‘CPz’ | 5R | 10.565 | −50.376 | 65.651 |
| ‘FC4’ | 6R | 26.821 | −3.144 | 55.585 |
| ‘C3’ | 2L | −46.592 | −34.283 | 48.439 |
| ‘Fz’ | 8L | −18.278 | 22.911 | 55.919 |
| ‘F4’ | 8R | 16.811 | 23.184 | 56.088 |
| ‘F8’ | 45R | 46.494 | 33.754 | 14.35 |
| ‘T7’ | 42L | −58.73 | −35.916 | 17.141 |
| ‘FC3’ | 6L | −28.722 | −3.387 | 55.569 |
| ‘FP2’ | 10R | 13.505 | 59.285 | 10.292 |
| ‘F7’ | 47L | −35.645 | 35.003 | −4.961 |
| ‘FP1’ | 10L | −16.038 | 59.012 | 9.959 |
Previous studies have explored the role of local brain activities, which is the activation of specific brain regions, linked to recognition in the medial temporal lobe and prefrontal cortex [23–25] and throughout the parietal cortex [26–28]. Recent studies have implied the engagement of the rather widely distributed cortical network and the significance of its integrated roles in the memory retrieval, possibly explaining the improved classification accuracy that we observe in our results. In regard of functional connectivity approaches, increased connectivity in core regions such as prefrontal cortex, precuneus, and occipital cortex is related to successful memory retrieval [17]. King et al. and Jun et al., also exhibited that memory (i.e., recollection)-dependent wide-spread functional connectivity of seed regions within an essential memory network comprised of the angular gyrus, medial prefrontal cortex, hippocampus, middle temporal gyrus, and posterior cingulate cortex is related with behavioral performance [29,30]. These studies illustrate the importance of incorporating the brain activity of multiple regions when classifying memory performance. However, these studies used brain activity in limited areas (e.g., spectral power or ERP of specific regions) as a classification feature.
The present study suggests that subsequent memory outcome prediction accuracy can be improved by using functional connectivity rather than local brain activity, in cases where single-trial classification is performed using scalp EEG data. By using single-trial EEG connectivity features, we were able to achieve a mean accuracy better than above 80% for the classification of subsequent memory outcome. Such improved accuracy could be attributed to the fact that our approach reflects the way in which relevant brain regions communicate during information retrieval.
We found anterior and posterior connectivity of the memory-related network in correctly recognized conditions, in that the connectivity of the left hemispheric fronto-parietal distinctly increased, while that of the right hemispheric network decreased during correctly recognized trials. These results converge with previous fMRI studies that show left-lateralized fronto-parietal brain connectivity for correctly recognized items [26,31,32]. Such left lateralization can be observed in other neural substrates of successful recognition as well. Some attribute such lateralization tendencies of the parietal old/new effect to the verbalization of stimuli. However, the parietal old/new effect was left-lateralized even when facial images were presented instead of words, indicating that the lateralization of the effect was dependent on the region, especially, the parietal cortex [26].
5. Conclusion
The present study verified the feasibility of using single-trial functional connectivity in the retrieval phase by using EEG signals for identification of the subsequent memory outcome. We achieved mean accuracy of better than 80% for the prediction of correctly recognized condition. We found the connectivity of the left fronto-parietal cortex higher in correctly recognized condition, whereas that of the left fronto-parietal cortex was not significant in correctly rejected conditions. For future studies, these results could be valuable in building a classification system for memory prediction that could utilize subsequently remembered and forgotten events. Furthermore, we suggest that future work maximize the effectiveness of classification accuracy by adopting computational implementations of models, which include fusion of memory images in wavelet domain and finest architectures of machine learning using a recurrent neural network [33,34].
Acknowledgements
This research was supported by a grant (2020-06-HR) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2022R1I1A1A01073860).
Footnotes
Author contributions: Y.J., C.P., and K.H. contributed to the study design and S.J., Y.J., and Y.S. wrote the manuscript. Y.J., C.P., and K.H. performed the study. S.J. and Y.J. analyzed the data. C.P. and K.H. obtained funding. All authors contributed to the article and approved the submitted version.
Conflict of interest: Authors state no conflict of interest.
Contributor Information
Soyeon Jun, Email: soy0326@snu.ac.kr.
Keunsoo Ham, Email: ksham@korea.kr.
References
- [1].Wells GL, Memon A, Penrod SD. Eyewitness evidence: improving its probative value. Psychol Sci Public Interest. 2006;7(2):45–75. [DOI] [PubMed]
- [2].Begakis C. Eyewitness misidentification: a comparative analysis between the United States and England. Santa Clara J Int Law. 2017;15(2):3.
- [3].Lefebvre CD, Marchand Y, Smith SM, Connolly JF. Determining eyewitness identification accuracy using event-related brain potentials (ERPs). Psychophysiology. 2007;44(6):894–904. [DOI] [PubMed]
- [4].Ham K, Kim KP, Jeong H. Support vector machine (SVM)-based classification of eyewitness memory using single-trial EEG. Korean J Cognitive Biol Psychol. 2018;30(4):413–9.
- [5].Tsivilis D, Allan K, Roberts J, Williams N, Downes JJ, El-Deredy W. Old–new ERP effects and remote memories: the late parietal effect is absent as recollection fails whereas the early mid-frontal effect persists as familiarity is retained. Front Hum Neurosci. 2015;9:532. [DOI] [PMC free article] [PubMed]
- [6].Wilding EL, Rugg MD. An event-related potential study of recognition memory with and without retrieval of source. Brain. 1996;119(Pt 3):889–905. [DOI] [PubMed]
- [7].Donaldson DI, Curran T. Potential (ERP) studies of recognition memory for faces. Neuroimage. 2007;36(2):488–9. [DOI] [PMC free article] [PubMed]
- [8].Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cogn Sci. 2007;11(6):251–7. [DOI] [PubMed]
- [9].Gold C, Henze DA, Koch C, Buzsaki G. On the origin of the extracellular action potential waveform: a modeling study. J Neurophysiol. 2006;95(5):3113–28. [DOI] [PubMed]
- [10].Burke JF, Ramayya AG, Kahana MJ. Human intracranial high-frequency activity during memory processing: neural oscillations or stochastic volatility? Curr Opin Neurobiol. 2015;31:104–10. [DOI] [PMC free article] [PubMed]
- [11].Long NM, Burke JF, Kahana MJ. Subsequent memory effect in intracranial and scalp EEG. Neuroimage. 2014;84:488–94. [DOI] [PMC free article] [PubMed]
- [12].Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, et al. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex. 2007;17(5):1190–6. [DOI] [PubMed]
- [13].Jun S, Lee SA, Kim JS, Jeong W, Chung CK. Task-dependent effects of intracranial hippocampal stimulation on human memory and hippocampal theta power. Brain Stimul. 2020;13(3):603–13. [DOI] [PubMed]
- [14].Gupta A, Siddhad G, Pandey V, Roy PP, Kim BG. Subject-specific cognitive workload classification using EEG-based functional connectivity and deep learning. Sensors (Basel). 2021;21(20):6710. [DOI] [PMC free article] [PubMed]
- [15].Aydin S. Cross-validated adaboost classification of emotion regulation strategies identified by spectral coherence in resting-state. Neuroinformatics. 2022;20(3):627–39. [DOI] [PubMed]
- [16].Kilic B, Aydin S. Classification of contrasting discrete emotional states indicated by EEG based graph theoretical network measures. Neuroinformatics. 2022;20(4):863–77. [DOI] [PubMed]
- [17].Schedlbauer AM, Copara MS, Watrous AJ, Ekstrom AD. Multiple interacting brain areas underlie successful spatiotemporal memory retrieval in humans. Sci Rep. 2014;4:6431. [DOI] [PMC free article] [PubMed]
- [18].Petrovska J, Loos E, Coynel D, Egli T, Papassotiropoulos A, de Quervain DJ, et al. Recognition memory performance can be estimated based on brain activation networks. Behav Brain Res. 2021;408:113285. [DOI] [PubMed]
- [19].Fraser AM, Swinney HL. Independent coordinates for strange attractors from mutual information. Phys Rev A Gen Phys. 1986;33(2):1134–40. [DOI] [PubMed]
- [20].Jeong J, Gore JC, Peterson BS. Mutual information analysis of the EEG in patients with Alzheimer’s disease. Clin Neurophysiol. 2001;112(5):827–35. [DOI] [PubMed]
- [21].Jun S, Kim JS, Chung CK. Prediction of successful memory encoding based on lateral temporal cortical gamma power. Front Neurosci. 2021;15:517316. [DOI] [PMC free article] [PubMed]
- [22].Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–61. [DOI] [PMC free article] [PubMed]
- [23].Wagner AD, Desmond JE, Glover GH, Gabrieli JD. Prefrontal cortex and recognition memory. Functional-MRI evidence for context-dependent retrieval processes. Brain. 1998;121(Pt 10):1985–2002. [DOI] [PubMed]
- [24].Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends Cogn Sci. 2002;6(2):93–102. [DOI] [PubMed]
- [25].Reber PJ, Wong EC, Buxton RB. Comparing the brain areas supporting nondeclarative categorization and recognition memory. Brain Res Cogn Brain Res. 2002;14(2):245–57. [DOI] [PubMed]
- [26].Guerin SA, Miller MB. Lateralization of the parietal old/new effect: an event-related fMRI study comparing recognition memory for words and faces. Neuroimage. 2009;44(1):232–42. [DOI] [PubMed]
- [27].Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004;24(45):10084–92. [DOI] [PMC free article] [PubMed]
- [28].Wheeler ME, Buckner RL. Functional dissociation among components of remembering: control, perceived oldness, and content. J Neurosci. 2003;23(9):3869–80. [DOI] [PMC free article] [PubMed]
- [29].King DR, de Chastelaine M, Elward RL, Wang TH, Rugg MD. Recollection-related increases in functional connectivity predict individual differences in memory accuracy. J Neurosci. 2015;35(4):1763–72. [DOI] [PMC free article] [PubMed]
- [30].Jun S, Kim JS, Chung CK. Direct stimulation of human hippocampus during verbal associative encoding enhances subsequent memory recollection. Front Hum Neurosci. 2019;13:23. [DOI] [PMC free article] [PubMed]
- [31].Vilberg KL, Moosavi RF, Rugg MD. The relationship between electrophysiological correlates of recollection and amount of information retrieved. Brain Res. 2006;1122(1):161–70. [DOI] [PMC free article] [PubMed]
- [32].Wilding EL. In what way does the parietal ERP old/new effect index recollection? Int J Psychophysiol. 2000;35(1):81–7. [DOI] [PubMed]
- [33].Yadav SP, Zaidi S, Mishra A, Yadav V. Survey on machine learning in speech emotion recognition and vision systems using a recurrent neural network (RNN). Arch Comput Methods Eng. 2022;29(3):1753–70.
- [34].Prakash Yadav S, Yadav S. Fusion of medical images in wavelet domain: a discrete mathematical model. Ingeniería Solidaria. 2018;14(25):1–11.