Abstract
The oral sodium-glucose cotransporter 2 inhibitor, dapagliflozin, is used to treat kidney disease, heart failure, and diabetes in adults, but has not been well studied in pediatrics, and does not have a recognized place in therapy in current practice guidelines. The purpose of this review is to summarize studies that have investigated the efficacy of dapagliflozin in pediatric patients. A systematic review was performed to identify clinical studies of oral dapagliflozin in children 0 to 17 years. Studies were identified through searches of Scopus, Web of Science, PubMed, Google Scholar, Embase, clinical trial registries, research registries, and key journals through August 2022. The Cochrane scoring system was used to assess the methodological quality of the included randomized trials. Five studies were reviewed and included in this analysis. Dapagliflozin at a dose of 5 to 10 mg was utilized in adolescents and young adults with heart failure, chronic kidney disease with proteinuria, type 1 diabetes, or type 2 diabetes. Studies evaluating dapagliflozin in type 1 diabetes evaluated single doses while the other studies monitored long-term use. Dapagliflozin was overall considered to be safe and effective in the studies included in this review, but further studies in larger populations and over extended periods of time are necessary.
Keywords: Dapagliflozin, SGLT-2 inhibitors, Type 1 diabetes mellitus, Type 2 diabetes mellitus, Chronic kidney disease, Heart failure
Highlights
· Dapagliflozin demonstrated efficacy and safety in pediatric patients. Dapagliflozin has been used in pediatric heart failure, kidney disease, and type 2 diabetes. Dapagliflozin’s use in type 1 diabetes should be explored further prior to use.
Introduction
Dapagliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor that works in the renal tubules [1]. SGLT2 is the main cotransporter involved in glucose reabsorption in the kidneys. When SGLT2 is inhibited, glucose is not reabsorbed, but excreted, leading to a total reduction in plasma glucose, hence the use of SGLT2 inhibitors in diabetes. Dapagliflozin has also been found to be both cardioprotective and renoprotective through a similar mechanism – it reduces sodium reabsorption in the renal tubules. This reduces hypertension and hyperfiltration, which preserves glomerular filtration and reduces ischemic damage to the kidneys [2]. Dapagliflozin has therefore not only been approved by the U.S. Food and Drug Administration for diabetes treatment in adults but was also recently approved for treatment of heart failure (HF) and chronic kidney disease (CKD). The general dosing in adults is 10 mg orally once daily.
Diabetes mellitus is a chronic disease that causes increased blood glucose levels due to a reduction in insulin release from pancreatic β-cells [3]. No agents other than insulin are currently recommended for treatment of type 1 diabetes (T1D), while treatment for type 2 diabetes (T2D) in children is limited to 3 medications. If lifestyle changes do not result in a decrease in plasma glucose levels, children can be started on metformin and if additional criteria are met, insulin may be initiated. As a last-line therapy, liraglutide can be added as an adjunctive agent. Metformin and liraglutide are approved for children 10 years or older.
As in adults, dapagliflozin may have benefits in kidney and heart disease, prolonging the time to severe disease. HF in pediatrics involves a range of clinical syndromes ranging from congenital heart disease and cardiomyopathies to structural issues. Treatment varies widely dependent on the underlying etiology, but common pharmacologic treatments include diuretics, antihypertensives, and inotropes [4]. CKD in pediatrics is defined as structural or functional damage to the kidneys for at least 3 months [5]. Other than treating complications (fluid and electrolyte abnormalities, anemia, and bone/mineral disorders), the only treatment is renal replacement therapy when the patient is in end-stage renal disease. The purpose of this review is to determine the safety and efficacy of dapagliflozin in pediatric patients with diabetes, HF, and/or CKD.
Materials and methods
This rapid review was conducted and reported in accordance with the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidelines and other best practices [6,7]. The following databases were utilized and searched from inception: PubMed (MEDLINE), Google Scholar, Scopus, Web of Science, and Embase. For unpublished trials, clinical trial and research registries including the International Standard Randomised Controlled Trials Number Register, ClinicalTrials. gov, European Union Clinical Trials Register, and National Research Registry Archive were searched. Key journals, including Clinical Pediatrics, JAMA Pediatrics, The Journal of Pediatrics, Pediatrics, and American Diabetes Association were manually searched. References of all included trials were reviewed for additional trials. The original search was completed in May 2022 and updated to include articles through August 2022. There were no publication date or language restrictions.
All identified clinical studies indexed to the search term dapagliflozin, filtered to include studies involving patients age 0 to 17 years, were included. The primary outcome of the studies needed to be an evaluation of the efficacy of dapagliflozin in pediatrics. Studies were excluded if they focused on adults, were not full articles, or were non-human studies. The revised Cochrane risk-of-bias tool was used to assess the quality of included trials [8]. A single reviewer conducted the literature search, selected articles for inclusion, and performed risk-ofbias assessment; all steps were checked by a second reviewer.
A single reviewer extracted the following data using a specific data extraction form: trial design, setting, country, number of patients, age, intervention, measures of the effectiveness and safety of dapagliflozin, and results. Due to the small number of studies identified for each indication, a meta-analysis was not conducted. The authors have no relevant conflicts of interest to report.
Results
Five studies, comprising 3 randomized controlled trials, a pilot study, and a prospective observational study, were included (Fig. 1). An overview of the studies is provided in Table 1. All articles were English full-text articles, and the risk of bias was deemed to be low to intermediate (Fig. 2). Indications for dapagliflozin use included T2D (n=1), T1D (n=2), CKD (n=1), and HF (n=1). Patients included were aged 3 months to 24 years. Safety results were reported in all studies. Three studies evaluated dapagliflozin administered daily for extended periods and 2 studies evaluated dapagliflozin administered in a single time frame of around 24 hours.
Fig. 1.
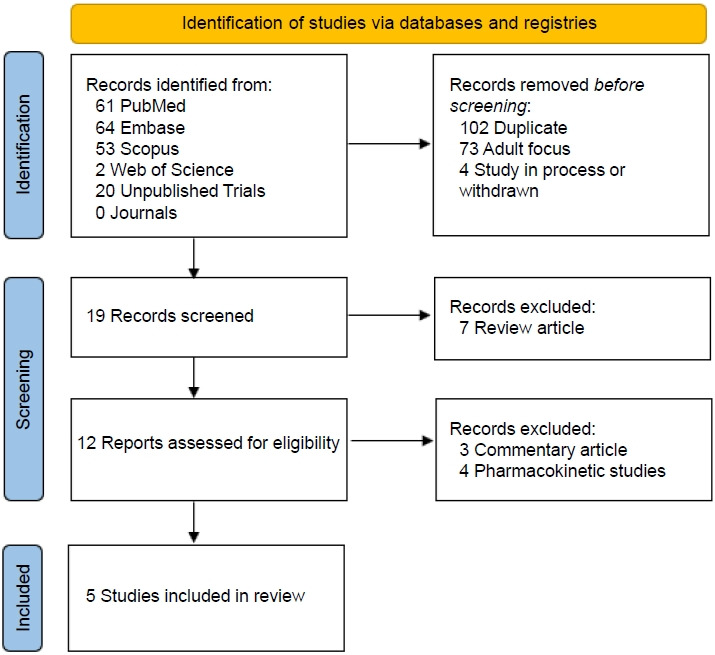
PRISMA (Preferred Reporting Items for Systematic Review and Meta- Analysis) 2020 flow diagram. Summary of the results of the literature search of primarily the PubMed, Embase, and Google Scholar databases using systematic methods.6) Four clinical studies and 1 observational study were identified for inclusion.
Table 1.
Characteristics of the studies included in the review
| Study | Design | treatment target | patient population | Intervention | Control | Outcomes |
|---|---|---|---|---|---|---|
| Liu et al. [9] | Prospective, single-arm, pilot trial | Proteinuric CKD | Age 6–18 years, eGFR less than 60 mL/min/1.73 m2, urinary protein greater than 0.2 g/24-hr, stable on an ACE inhibitor, without history of immunosuppressive therapy or diabetes | Dapagliflozin 5 mg (30 kg or less) or 10 mg (greater than 30 kg) PO daily for 12 weeks | N/A | 22.6% reduction in proteinuria (95% CI 8.3%–36.9%) |
| Mean eGFR 103.8±28.2 vs 109.2±32.0 at baseline (P=0.048) | ||||||
| No discontinuations due to adverse events | ||||||
| Newland et al. 2022 [10] | Retrospective, single-arm, cohort study | Heart failure | Age less than 21 years , left ventricular ejection fraction less than 55% or impaired ventricular filling, symptoms of low cardiac output and/or congestion | Dapagliflozin 0.1-0.2 mg/kg PO daily up to 10 mg per dose | N/A | Mean serum BMP reduced from 222 pg/mL at baseline to 166 pg/mL at a median follow-up of 130 days (P=0.04) |
| Mean eGFR reduced from 118 mL/min/1.73 m2 at baseline to 100 mL/min/1.73 m2 at follow-up (P=0.09) | ||||||
| Biester et al. 2017 [11] | Prospective, double-blind, randomized, crossover trial | Type 1 diabetes mellitus | Age 12–21 years, HbA1c 12.5% or less, insulin total daily dose 0.6–2 units/kg by continuous insulin pump or multiple injections, BMI 18 to 35 kg/m2 (adults) or 10th to 99th percentile (pediatrics) | Dapagliflozin 10 mg PO single dose | Placebo | Mean daily insulin requirement 0.92±0.2 vs. 1.10±0.17 units/kg/day with placebo P<0.001 |
| Mean post-prandial insulin 0.28±0.05 vs. 0.31±0.06 units/kg/day with placebo (P=0.0504 | ||||||
| Mean serum β-hydroxybutyrate 0.17±0.13 vs. 0.11±0.08 mmol/L (P<0.0001) | ||||||
| Biester et al. 2020 [12] | Prospective, double-blind, randomized, crossover trial | Type 1 diabetes mellitus | Age 12–21 years, HbA1c 6.5%–11%, no recent DKA, continuous insulin pump at least 3 months, mean insulin daily dose 0.6- 2 units/kg, BMI 18 to 35 kg/ m2 (adults) or 10th to 99th percentile (pediatrics) | Dapagliflozin 10 mg PO, day 1 at 1900 and day 2 at 0630 | Placebo | Time in blood glucose range (70–180 mg/dL) 68%±7% vs. 50%±13% with placebo (P<0.001) |
| Mean insulin daily dose 31 units±10 units vs. 40 units±13 units (P=0.004) | ||||||
| Mean serum β-hydroxybutyrate level 0.29 vs. 0.16 mmol/L with placebo (P<0.0001) | ||||||
| Tamborlane et al. 2022 [13] | Prospec tive, double-blind, randomized, Phase 3 clinical trial | Type 2 diabetes mellitus | Age 10–24 years, HbA1c 6.5%–11%, FPG 225 mg/dL or less, metformin at least 1,000 mg daily for 8 weeks and/or stable insulin, currently engaged in diet and exercise | Dapagliflozin 10 mg PO daily for 24 weeks (double-blind) plus a 28-week open-label extension | Placebo for 24 weeks plus dapagliflozin during a 28 week open-label extension | Mean HbA1c vs. placebo -0.75% (95% CI, -1.65–0.15) |
| Met treatment goal of HbA1c less than 7%, 25.0% vs 4.0% with placebo (P=0.056) | ||||||
| Adverse events 69% vs. 58% with placebo serious adverse events 5% vs. 9% with placebo |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; PO, orally; N/A, not available; CI, confidence interval; BMP, basic metabolic panel; HbA1c, glycosylated hemoglobin; DKA, diabetic ketoacidosis; FPG, fasting plasma glucose; BMI, body mass index.
Fig. 2.
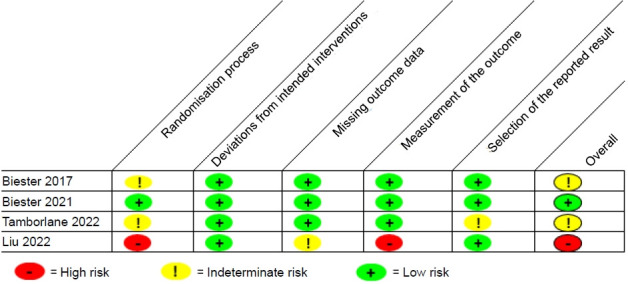
The revised Cochrane risk-of-bias tool was used to quantitatively evaluate each included clinical trial.8) Most were found to have low to intermediate risk of bias.
Liu et al. [9] published an investigator-initiated, prospective pilot study of pediatric Chinese patients with proteinuric CKD. Their objective was to investigate the antiproteinuric effect and safety of dapagliflozin in children with proteinuric CKD. Patients were included if they were aged 6–18 years and had an estimated glomerular filtration rate (eGFR) >60 mL/min/1.73 m2. Patients were excluded if they had diabetes or an untreated urinary tract infection (UTI). Dapagliflozin was given once daily dosed at 5 mg for patients ≤30 kg and 10 mg for weight >30 kg. Over a 6-month period, 15 patients were screened and 9 met inclusion criteria with 1 patient lost to follow-up after 4 weeks. Five of the 9 patients were male and the mean age was 10.4 years (range, 6.4–14.2 years). Mean eGFR was 104.9 mL/min/1.73 m2 (range, 60.8–163.6 mL/min/1.73 m2) [9]. Results were reported for baseline (n=9), 4 weeks (n=9), and 12 weeks (n=8). From baseline to 4 weeks, there was a 33.3% reduction in proteinuria (95% CI, 23.1%–43.4%). Twenty-four-hour proteinuria (g/m2) was 2.1 (1.4–2.6) at baseline, 1.4 (0.9–2.1) at 4 weeks (P<0.05), and 1.5 (1.2–2.2) at 12 weeks (P<0.05), with a total reduction of 22.6% (95% CI, 8.3%–36.9%). eGFR (mL/min/1.73 m2) was not significantly changed at 4 weeks (108.2±39.7 weeks vs. 109.2±32.0 weeks, P>0.05) but was slightly lower at 12 weeks (103.8±28.2 weeks vs. 109.2±32.0 weeks, P=0.048). While baseline fasting plasma glucose (FPG) was reported and protocols for monitoring glucose were described, glucose values other than the baseline fasting values were not reported. No patients discontinued dapagliflozin due to an adverse event, though there was one case of asymptomatic bacteriuria. The authors concluded that dapagliflozin was an effective treatment option for proteinuric CKD in children.
Newland et al. [10] reported their experience utilizing a dapagliflozin protocol in pediatric patients with HF. This study included patients younger than 21 years started on dapagliflozin from January 2020 to December 2021 and who received dapagliflozin for at least 30 days. Dapagliflozin was added to the patient's regimen which commonly included sacubitril/valsartan (73.7%), a beta-blocker (71%), a mineralocorticoid receptor antagonist (63.2%), and a loop diuretic (71%). The hospital protocol targeted a dapagliflozin dose of 0.1–0.2 mg/kg once daily with a maximum dose of 10 mg. Thirty-eight cardiac, nondiabetic patients were included in the analysis. Median age was 12.2 years, and the majority of patients (60.5%) were aged 5–17 years, though 15.8% were 18–21 years old and another 5.3% were 3–12 months old. The most common diagnosis was dilated cardiomyopathy (68.4%), followed by single ventricle physiology (18.5%), and diastolic HF (10.5%). Patient data were assessed at baseline and then a median of 130 days (interquartile range, 76–332) later at the last follow-up appointment. Though not statistically significant, more patients were classified as New York Heart Association (NYHA) class I at follow-up (3 vs. 0, P=0.24) and conversely NYHA class IV at follow-up (8 vs. 5, P=0.54). Ejection fraction (EF) increased from a median of 32% at baseline to 37.2% at follow-up (P=0.006) and the number of patients with an EF > 40% increased from 1 to 7 (P=0.04). B-type natriuretic peptide (pg/mL) was reduced from 222 to 166 pg/mL from baseline to follow-up (P=0.04), though this is likely because 63.2% of patients were initiated on dapagliflozin during an inpatient admission and were experiencing an acute exacerbation at that time. Vitals, serum chemistries, and concomitant drug therapy did not change significantly following dapagliflozin treatment, though doses and adjustments of other medications were not addressed. There was a decrease in eGFR (mL/min/1.73 m2) from baseline to follow-up (118–100, P=0.09) and 4 patients experienced an acute kidney injury event. Additionally, 6 patients experienced symptomatic UTI while on dapagliflozin. The authors concluded that dapagliflozin use in pediatric HF patients was well-tolerated and a reasonable addition to guideline-directed medical therapy.
Biester et al. [11,12] published 2 blinded, placebo-controlled, crossover pilot studies looking at patients aged 12–21 years old with T1D. The first was conducted in 2017 and aimed to assess the safety, efficacy, and pharmacokinetics of a single dose of dapagliflozin 10 mg as an adjunct to insulin in patients with different levels of glycemic control [11]. Thirty-nine patients were screened and 33 patients who met age requirements and were diagnosed with T1D with an HbA1c of 12.5% or less were enrolled. These patients were then randomized to receive dapagliflozin or placebo and crossed over after a washout period. Forty-eight hours prior to the study period, insulin pumps were converted to neutral protamine hagedorn insulin. Patients fasted for 10 hours, and were then given dapagliflozin/placebo and a standard 6-mL/kg liquid meal. At baseline, 11 patients each had a HbA1c value ranging from 5.5%–7.5%, 7.6%–9.0%, and 9.1%–12.5%. The daily insulin requirement was significantly lower in the group given dapagliflozin (0.92±0.2 units/kg/day vs. 1.10±0.17 units/kg/day, P<0.001) regardless of baseline HbA1c. Total post-prandial insulin after the standardized meal was not significantly different between groups, but the pooled data showed slightly less post-prandial insulin administered to the dapagliflozin group than the control group, though this finding was not statistically significant (0.28±0.05 units/kg/day vs. 0.31±0.06 units/kg/day, respectively, P=0.0504). Adverse events were minimal and did not differ between groups, with major complaints being headache and UTI. Beta-hydroxybutyrate levels were significantly higher in those patients who received dapagliflozin than those who did not (0.17±0.13 mmol/L vs. 0.11±0.08 mmol/L, respectively, P<0.0001) for all HbA1c groups. While this did not clinically lead to more diabetic ketoacidosis (DKA) cases, there was a trend in that direction. This study showed that adjunct therapy with dapagliflozin in patients with T1D may offer benefits such as increased time in glucose range, but that dapagliflozin also increases β-hydroxybutyrate levels.
The objective of the second Biester study published in 2020 was to investigate the effects of dapagliflozin on glucose levels under full closed-loop conditions.12) Patients were included if they had been diagnosed with T1D for at least 1 year and were on insulin pump therapy for at least 3 months. A total of 30 patients were included in the study; these patients were monitored for a 1-week lead-in period during which their baseline insulin needs and glucose levels were monitored. Patients were monitored for 27 hours overnight on 2 separate occasions with a 30-day washout in-between. Dapagliflozin 10 mg was given at 1900 on day 1 and 0630 on day 2. A standardized meal was administered on day 1 and 2 liquid meals were given on day 2.
Over a 10-month period, 34 patients were screened and 30 met inclusion criteria and completed the study in full [11]. At baseline, patients had a median age of 17 years (range, 12–20 years) and the majority (63%) of patients were female. Fifteen patients were enrolled in both age subgroups due to the 10-month study period: 12–17 years and ≥ 18 years. Average HbA1c at baseline was 8.4% (6.6%–10.4%). The primary outcome of time in the blood glucose range of 70–180 mg/ dL (over 24 hours) was significantly higher with dapagliflozin. During the initial observational period, patients who did not receive dapagliflozin spent an average of 50%±13% time-inrange compared to 68%±7% with dapagliflozin (P<0.001). The percentage of time above range was significantly reduced with dapagliflozin (48%±13% vs. 30%±6%, P<0.001). Dapagliflozin treatment resulted in a significant reduction in total daily insulin dose by 22% (40 units±13 units vs. 31 units±10 units, P=0.004). This was likely driven by a reduction in the number of boluses as opposed to a reduction in basal insulin. In terms of safety, there were no episodes of DKA, though β-hydroxybutyrate levels were significantly higher in those patients who received dapagliflozin than those who did not (0.29 mmol/L vs. 0.16 mmol/L, respectively, P<0.0001). The authors of this study concluded that the addition of dapagliflozin can maximize the percentage of time-in-range during insulin therapy.
Tamborlane et al. published a multicenter, placebocontrolled, double-blind, randomized, phase 3 study with an open-label safety extension that took place in 30 centers across 5 countries [13]. Most patients were from North America (12 centers), but other locations included Hungary, Israel, Mexico, and Russia. The objective of the study was to evaluate the efficacy and safety of dapagliflozin as an add-on therapy in children and young adults with T2D receiving standard of care treatment. Patients were included if they were 10–24 years old with T2D, an HbA1c of 6.5%–11%, and FPG ≤225 mg/dL. Patients were excluded if they had T1D or any genetic disorders that cause insulin resistance or diabetes.
One hundred eighty-six patients were initially screened and 80 of these patients who met inclusion criteria received placebo in a 4-week lead-in period [12]. Eight patients were lost during this phase, therefore 72 patients were randomized to receive dapagliflozin 10 mg (n=39) or placebo (n=33) for the 24-week double-blind period. All patients were then consolidated and received dapagliflozin 10 mg (n=33 from the dapagliflozin group, n=27 from the placebo group) for 28 weeks. Finally, there was a 4-week follow-up period to assess safety. Most patients were 10–15 years old (41% in the dapagliflozin group vs. 42% in the placebo group), about a third of both groups comprised 16-17-year-olds (33% in the dapagliflozin group vs. 30% in the placebo group), and the remainder of patients were between the ages of 18–24 years (26% in the dapagliflozin group vs. 27% in the placebo group). Most patients were female (62% in the dapagliflozin group vs. 58% in the placebo group). Mean HbA1c and FPG at baseline were 7.95%±1.59% and 156.0±55.7 mg/dL in the dapagliflozin group, respectively, and 7.85%±1.19% and 167.0±63.2 mg/dL in the placebo group, respectively. Twentyeight patients (72%) in the dapagliflozin group and 16 (48%) in the placebo group were white. Medications at baseline were different between groups: insulin only (18% vs. 15%), metformin only (44% vs. 61%), and metformin + insulin (38% vs. 24%) in the dapagliflozin and placebo groups, respectively.
The dapagliflozin group had an HbA1c change of -0.25% (95% CI, -0.85% to 0.34%) and the placebo group had an HbA1c change of 0.5% (95% CI, -0.18 to 1.17), a betweengroup difference of -0.75% (95% CI, -1.65% to 0.15%); P=0.10 [12]. The study authors performed a sensitivity analysis using a per protocol population that excluded those patients who required glycemic rescue for FPG >240 mg/dL or who discontinued the study (n=24 in the dapagliflozin group vs n=26 patients in the placebo group). The between-group difference in HbA1c from baseline to 24 weeks was -1.13% (95% CI -1.99 to -0.26; P=0.012). There were no differences among subgroups. Safety outcomes were reported for the intentionto-treat (ITT) population. More patients in the dapagliflozin group experienced at least one adverse event during the randomized period than those in the placebo group (69% vs. 58%, respectively). Of those patients who received placebo in the randomized study period and received dapagliflozin during the following 28 weeks, 15% (4 of 27) experienced at least one adverse event. The most common adverse events were headache, nasopharyngitis, UTI, nausea, and diarrhea. The authors concluded that this study provided evidence for the efficacy and safety of dapagliflozin as an additional treatment option in an adolescent population with T2D.
Discussion
Dapagliflozin demonstrated efficacy and safety in the clinical studies reviewed here; however, there were significant differences and major considerations in the studies evaluated. It is notable that dapagliflozin has been studied in several disease states; however, the small number of patients treated with dapagliflozin within each disease state limits the immediate routine use of dapagliflozin in practice. Liu et al. evaluated proteinuria in pediatric patients with CKD [9]. This study had several limitations, one being that it was a pilot study and another being the limited number of patients included. Unfortunately, blood glucose readings were not reported even though these values were collected, but no hypoglycemic or hypoglycemic symptoms were reported. Newland et al. [10] performed an observational study with several limitations, most notably lack of a control group. This study did not have clearly defined objectives or exclusion/inclusion criteria; furthermore, patients with diabetes were excluded and all causes of HF were included. This led to a wide range of cardiac etiologies, making the findings less applicable to a specific cardiac diagnosis. Another limitation was the utilization of concomitant drug therapies and adjustment of these therapies during the study period, making it difficult to ascertain which benefits or side-effects were due to dapagliflozin. Lastly, FPG, HbA1c, and β-hydroxybutyrate monitoring was not performed. Biester et al. [11,12] evaluated the use of dapagliflozin in patients with T1D at 2 separate time periods and while the overall findings were positive, these studies were performed in a strictly controlled environment. Patients were given the same meals and monitored in the hospital for short durations. While this is appropriate for initial assessment of the efficacy and safety of medications in a new patient population, this is not generalizable to actual practice. The study by Tamborlane et al. [13] evaluated dapagliflozin in a much more generalizable way, though a high percentage of patients were lost to follow-up over the 60-week study period. This was exacerbated by patients who discontinued the study drug due to lack of glycemic control or required glycemic rescue, resulting in a modified ITT. Inclusion of these patients with increased FPG or HbA1c levels would likely have affected the study outcomes. There were also major differences between groups in the baseline characteristics of race and treatment. More patients in the dapagliflozin group had insulin in their regimen, which could indicate more severe diabetes at baseline, but p-values were not reported for baseline characteristics.
Dapagliflozin was efficacious at reducing proteinuria, increasing the percentage of time-in-range for glucose levels, reducing HbA1c, and increasing left ventricular ejection fraction. In addition to these larger studies that were reviewed in detail above, multiple case reports and poster presentations have evaluated the addition of dapagliflozin to diabetes regimens in pediatrics. Roman et al. [14] evaluated the use of dapagliflozin in 3 female adolescents with T1D. After 12 months, insulin dose and body mass index (BMI) were reduced in all 3 study participants, though the largest reduction was seen at 6 months. In a case report published by Kordonouri et al. [15], a 16-yearold boy with T1D experiencing insulin resistance was started on dapagliflozin 5 mg once daily. He required multiple daily injections of insulin and continued to gain weight despite this. Upon initiation of dapagliflozin, his glycemic control improved and his insulin needs were reduced.
Adverse effects in the 3 main studies were minimal, but dapagliflozin use is not without safety considerations. The most common side-effects reported are UTI, headache, pharyngitis, vomiting, and hyperglycemia [9-13]. As Shamchuk et al. [16] noted in their case report of a 17-year-old patient with T2D initiated on an SGLT-2 inhibitor, hyperglycemia can occur with the potential to cause DKA. SGLT-2 inhibitors reduce the reabsorption of glucose in the kidneys, which reduces blood glucose level; this in turn reduces insulin production, but the body still needs a source of energy and may turn to lipolysis, which can lead to DKA. While this was not seen in the studies reviewed here, Tamborlane et al. [13] excluded patients who required glycemic rescue and therefore could have excluded patients with DKA. Biester et al. [11,12] in both of their studies did see a significant trend toward higher β-hydroxybutyrate in patients that received even a single dose of dapagliflozin. Another study by Newland et al. [17] evaluated the safety of dapagliflozin in 9 patients younger than 21 years with HF started on dapagliflozin 5 mg once daily for 30 days. No adverse events were reported during the short timeframe of the study, but the total daily dose of loop diuretics was decreased in 3 patients.
A key factor that needs to be determined is the ideal dose of dapagliflozin. Tirucherai et al. [18] evaluated pharmacokinetics following a single dose of dapagliflozin 2.5, 5, or 10 mg. Children were aged 10–17 years and the average age was 14.5 years. After oral administration, the tmax was around 1.5 hours and the half-life ranged from 10 to 14 hours. The area under the curve (AUC) seemed to be dose-proportional, with the largest AUC seen in the 10-mg group. Urine glucose excretion showed a similar trend – more glucose was found in the urine in the 5- and 10-mg administration groups than the 2.5-mg administration group. In all groups given dapagliflozin, FPG was lower following administration. Six patients did experience side-effects, but this was not associated with specific doses of dapagliflozin. A model-dose scheme was created by Jo et al. [19] who determined that a dose of 10 mg was appropriate for adolescents aged 13–18 years and 5 mg was appropriate for children aged 6–12 years. The studies addressed in this review article utilized similar dosing schemes.
Dapagliflozin has the potential to change practice recommendations for diabetes and other disease states in children. For pediatric patients that are on guideline-approved therapy for diabetes and whose diabetes remains uncontrolled, dapagliflozin could prove efficacious in reducing insulin requirements, lowering HbA1c, reducing BMI, and improving glucose time-in-range. Additionally, a long-term complication of diabetes is kidney injury, and dapagliflozin has been shown to reduce proteinuria in a small cohort of pediatric patients. DKA should be monitored closely, especially in patients with T1D [11,12]. As dapagliflozin significantly increases β-hydroxybutyrate, which increases the risk of DKA, longer duration trials need to be performed prior to routine use of dapagliflozin in T1D [11,12]. Dapagliflozin may also be beneficial for patients with CKD and HF based on study findings in adults and small trials in pediatrics [9,10,17].
This review article has several limitations including the heterogeneity of the topic and patient population. However, given the limited number of studies of dapagliflozin in adolescents, inclusion of dapagliflozin use in multiple different disease states and age groups was necessary. This may make our findings less applicable to specific populations. Another limitation is that many of the studies also included data for young adults aged > 18 years old, limiting generalizability to younger patients. Furthermore, although the Cochrane riskof-bias tool was utilized and the included trials were found to have only intermediate risk of bias, certain aspects of the studies that were reviewed (e.g., differences in baseline characteristics among group) could have affected their findings and therefore our conclusions. A strength of this review is that risk of selection bias was low as all major published studies were included.
In summary, dapagliflozin was found to be safe and effective in the 5 clinical studies included in this rapid review. Limitations of the included studies were small sample sizes, limited generalizability, and methodological concerns with confounding factors. Larger, high-quality trials are needed to detect clinically significant differences in outcomes of pediatric patients who receive dapagliflozin so as to provide consistent recommendations for the use of this SGLT2 inhibitor in pediatric populations.
Footnotes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study can be provided by the corresponding author upon reasonable request.
Author contribution
Conceptualization: PMG, RDB; Data curation: PMG, RDB; Formal analysis: PMG, RDB; Methodology: PMG, RDB; Project administration: RDB; Writing - original draft: PMG; Writing - review & editing: PMG, RDB
References
- 1. Farxiga (dapagliflozin) [prescribing information]. Wilmington (DE): AstraZeneca Pharmaceuticals LP; 2021.
- 2.Leoncini G, Russo E, Bussalino E, Barnini C, Viazzi F, Pontremoli R. SGLT2is and renal protection: from biological mechanisms to real-world clinical benefits. Int J Mol Sci. 2021;22:4441. doi: 10.3390/ijms22094441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association Children and adolescents: standards of medical care in diabetes – 2021. Diabetes Care. 2021;44(Suppl 1):S180–99. doi: 10.2337/dc21-S013. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed H, VanderPluym C. Medical management of pediatric heart failure. Cardiovasc Diagn Ther. 2021;11:323–35. doi: 10.21037/cdt-20-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 6.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inform Libr J. 2009;26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 8.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Cui J, Fang X, Chen J, Yan W, Shen Q, et al. Efficacy and safety of dapagliflozin in children with inherited proteinuric kidney disease: a pilot study. Kidney Int Rep. 2022;7:638–41. doi: 10.1016/j.ekir.2021.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newland DM, Law YM, Albers EL, Friedland-Little JM, Ahmed H, Kemna MS, et al. Early clinical experience with dapagliflozin in children with heart failure. Pediatr Cardiol. 2022 Aug 10; doi: 10.1007/s00246-022-02983-0. . [Epub] [DOI] [PubMed] [Google Scholar]
- 11.Biester T, Aschemeier B, Fath M, Frey M, Scheerer M, Kordonouri O, et al. Effects of dapagliflozin on insulin requirement, glucose excretion, and β-hydroxybutyrate levels are not related to baseline HbA1c in youth with type 1 diabetes. Diabetes Obes Metab. 2017;19:1635–39. doi: 10.1111/dom.12975. [DOI] [PubMed] [Google Scholar]
- 12.Biester T, Muller I, von dem Berge T, Atlas E, Nimri R, Phillip M, et al. Add-on therapy with dapagliflozin under full closed loop control improves time in range in adolescents and young adults with type 1 diabetes: the DAPADream study. Diabetes Obes Metab. 2021;23:599–608. doi: 10.1111/dom.14258. [DOI] [PubMed] [Google Scholar]
- 13.Tamborlane W, Laffel L, Shehadeh N, Isganaitis E, Van Name M, Ratnayake J, et al. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol. 2022;10:341–50. doi: 10.1016/S2213-8587(22)00052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roman R, Pereyra M, Ramirez C. Adolescent with type 1 diabetes on insulin and dapagliflozin a SGLT2 inhibitor developed an euglycemic diabetic ketosis. Horm Res Paediatr. 2016;86(Suppl 2):43. [Google Scholar]
- 15.Kordonouri O, Biester T, Fath M, Gottwald I, Datz N, Danne T. Add-on treatment with dapagliflozin, a sodium-glucose co-transporter (SGLT) 2 inhibitor, in type 1 diabetes (T1D) – a case report. Pediatr Diabetes. 2015;16(Suppl 21):124. [Google Scholar]
- 16.Shamchuk A, Doulla M, Jetha M. Possible association between diabetic ketoacidosis and use of sodium-glucose co-transporter 2 inhibitor in a 17-year-old youth with type 2 diabetes. CMAJ. 2021;193:E13858–8. doi: 10.1503/cmaj.202627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newland D, Hong B, Albers E, Friedland-Little J, Kemma M, Hong B, et al. Safety of dapagliflozin in children with heart failure. J Heart Lung Transplant. 2021;40(4 Suppl):682. [Google Scholar]
- 18.Tirucherai G, LaCreta F, Ismat F, Tang W, Boulton W. Pharmacokinetics and pharmacodynamics of dapagliflozin in children and adolescents with type 2 diabetes. Diabetes Obes Metab. 2016;18:678–84. doi: 10.1111/dom.12638. [DOI] [PubMed] [Google Scholar]
- 19.Jo H, Pilla Reddy V, Parkinson J, Boulton DW, Tang W. Model-Informed pediatric dose selection for dapagliflozin by incorporat ing developmental changes. CPT Pharmacometrics Syst Pharmacol. 2021;10:108–18. doi: 10.1002/psp4.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]


