Abstract
Objectives
Aerosols formed during dental treatments have a huge risk for the spread of bacteria and viruses. This study is aimed at determining which part of the working area and at what size aerosol is formed and ensuring more effective use of HEPA-filtered devices.
Materials and methods
Anterior tooth preparation was performed by one dentist with one patient. Particle measurements were made using an airborne particle counter and were taken at four different locations: the chest of the patient, the chest of the dentist, the center of the room, and near the window. Three groups were determined for this study: group 1: measurement in a 24-h ventilated room (before the tooth preparation, empty room), group 2: measurement with the use of saliva ejector (SE), and group 3: measurement with the use of saliva ejector and HEPA-filtered extra-oral suction (HEOS) unit.
Results
The particles generated during tooth preparation were separated according to their sizes; the concentration in different locations of the room and the efficiency of the HEOS unit were examined.
Conclusions
The present study showed that as the particle size increases, the rate of spread away from the dentist’s working area decreases. The HEPA-filtered extra-oral suction unit is more effective on particles smaller than 0.5 microns. Therefore, infection control methods should be arranged according to these results.
Clinical relevance
The effective and accurate use of HEPA-filtered devices in clinics significantly reduces the spread of bacterial and viral infections and cross-infection.
Keywords: HEPA-filtered extra-oral suction, COVID-19, Aerosol, Infection control, Tooth preparation
Introduction
Aerosols consist of liquid particle gas surrounding them, while bioaerosols produced by the human can contain pathogenic microorganisms like bacteria or viruses and may be contaminated with germs, saliva, or blood [1]. And also, aerosol particles that are smaller than 10 μm remain airborne for a long period and enter the nasal passages and serve as carriers of respiratory while aerosol with particles in the range of 10–20 μm remains in the air for up to 30 min, which leads to transmission of SARS-CoV-2 and herpetic virus diseases. In dental practice, the use of high-speed turbine handpieces and ultrasonic scalers produces droplets and aerosols from the saliva and blood of patients [1, 2]. Major dental treatments, such as prosthetic dental preparation (PDP) procedure, lead to the spread of aerosol and aerosol exposure to the dentist and dental professionals’ oronasal and ocular mucosa membrane and could cause a viral infection. Diffused aerosol could cause cross-infection using contamination of clinical settings [3].
The dentist, dental assistant, and clinical settings are exposed to these aerosols. Until today, various measures have been taken to reduce the risk of infection of dentists, dental assistants and patients, which are regular ventilation of the treatment room, the use of masks and face shields, the use of rubber dam, and the application of antiseptic mouthwash before treatment. These measures are routinely practiced and proven to reduce the risk of infection [3]. With COVID-19, more repressive measures have been taken. It has been proven that many cases of SARS-CoV and MERS-CoV are associated with hospital-acquired transmission and result from the application of aerosol-generating procedures [4]. According to the available epidemiological data, COVID-19 is more infectious than SARS-CoV and MERS-CoV [5]. Therefore, standard precautions and infection control mechanisms had to be modified during this pandemic. The use of N95 masks, the use of disposable overalls, and the arrangement of the units in a single room where many dental units are together in one clinic are the changes that come with COVID-19. The use of high-efficiency air filters with HEPA filters has also increased with the COVID-19. Providing effective aspiration during dental treatments is one of the most effective methods to prevent aerosol formation. One of the aims of the study is to evaluate the effectiveness of HEPA-filtered extra-oral suction, which is an easy-to-use and compact device.
There is no standard value for aerosol concentration that can be allowed for effective infection control in dental clinics. However, some values can be said for a clinic in the ideal conditions. In air filter systems, there should be less than 0.5 bacteria-carrying particles in 1 m3 of air passing through the final filter. During dental procedures, there should be an average of 10 or fewer bacteria-carrying particles in 1 m3 of air in the area 30 cm away from the dental treatment zone. In the area of the clinic, which is 3 × 3 m from the working area, the number of bacteria-carrying particles in each cubic meter of air should not be more than 20 [6, 7]. The amount of particles in the air can be determined with particle counters, before and after treatment. Studies have shown that bacterial aerosols formed during dental treatments are much higher than before treatment [3, 8]. However, the bacterial contamination level of these aerosols varies according to the treatment options, working area, and particle size. Although droplets that occur during dental treatments are mainly directed to the patient’s chest area and the dentists’ face, aerosols can be widely found in the clinic. Bacterial aerosols have also been shown to spread beyond the treatment environment [3]. Aerosol intensity also varies among dental procedures. Dental calculus cleaning with hand tools does not cause aerosol formation. The use of high-speed handpiece working with water is always considered to be the process that generates the largest amount of aerosols. Therefore, anterior tooth preparation, which is the most aerosol-generating procedure, was applied in this study. During this treatment, the effectiveness of the HEPA-filtered device was evaluated according to six different particle sizes in four different regions. Thus, it will be determined which part of the working area and what size aerosol is formed and more effective use of HEPA-filtered devices will be ensured.
Materials and methods
This study was conducted in a single-chair dental unit in a dental clinic at a university dental hospital (Gaziantep University, School of Dentistry, Gaziantep, Turkey). Anterior tooth preparation was performed by one dentist on one volunteer. Gaziantep University Ethics Committee approved this study as exempt; it was not considered a human subject research. Particle measurements were made using an airborne particle counter with a flow rate of 2.83 l per minute, Fluke 983 Particle Counter, according to the ISO 14644–1 (Cleanrooms & Association Controlled Environment Part 1 Classification of Air Cleanliness) standard. It was performed by reading the measurement values by pressing the reading mode on the device. The particle counter measured the concentration of particles (PM values) with sizes 0.3 μm, 0.5 μm, 1.0 μm, 2.0 μm, 5.0 μm, and 10.0 μm. Measurements were taken at four different locations: chest of the patient, chest of the dentist, the center of the room, and near the window. The window and door were kept closed during tooth preparation. Ten measurements were taken at 1-min intervals from each location. One dentist completed anterior tooth preparation (tooth no. 11, 12, 13) with high-speed handpiece, with only saliva ejector. Then, the preparation of other teeth (tooth no. 21, 22, 23) was completed with saliva ejector and extra-oral suction device.
HEPA (high-efficiency particulate air)–filtered extra-oral suction unit (External Oral Suction Device; GS-E1000; GREELOY) was used in reducing particles generated during anterior tooth preparation. Three groups were determined for the present study: group 1: measurement in a 24-h ventilated room (before the tooth preparation, empty room), group 2: measurement with the use of saliva ejector (SE), and group 3: measurement with the use of saliva ejector and HEPA-filtered extra-oral suction (HEOS) unit. First, measurements were made from four locations in a 24-h ventilated room, with the window and door of the room closed. Then, the patient was seated on the dental chair, the dentist positioned on the patient’s right side, and the saliva ejector was positioned contralaterally at the back of the oral cavity. The assistant was not present in the room. A total of forty measurements were taken from four locations during anterior tooth preparation; also, the door and window were kept closed during the process. In group 3, the preparation of the remaining tooth of the same patient was performed while working with the SE and HEOS unit. The HEOS unit was located on the patient’s left side and close to the mouth. The HEOS unit was used at maximum vacuum capacity during anterior tooth preparation. Particle measurements were made from four designated locations, with the window and door of the room closed. A total of forty measurements were recorded.
Two-way ANOVA was performed to evaluate the effects of location and groups on PM values for each micron. LSD test was performed as a post hoc analysis when the main or interaction effect was significant according to two-way ANOVA. Mean ± SD was given for numerical variables. Statistical analysis was performed with SPSS for Windows version 24.0 and a P value < 0.05 was accepted as statistically significant.
Results
The particles generated during tooth preparation were separated according to their sizes; the concentration in different room locations and the efficiency of the HEOS unit were examined. For different PM values, two-way ANOVA results are presented in Table 1.
Table 1.
Two-way ANOVA results for different PM values
| 0.3 μm | 0.5 μm | 1 μm | 2 μm | 5 μm | 10 μm | |
|---|---|---|---|---|---|---|
| P value | P value | P value | P value | P value | P value | |
| Corrected model | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Intercept | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Location | 0.001 | 0.530 | 0.074 | 0.100 | 0.005 | 0,024 |
| Group | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| Location * group | 0.001 | 0.417 | 0.032 | 0.078 | 0.014 | 0.033 |
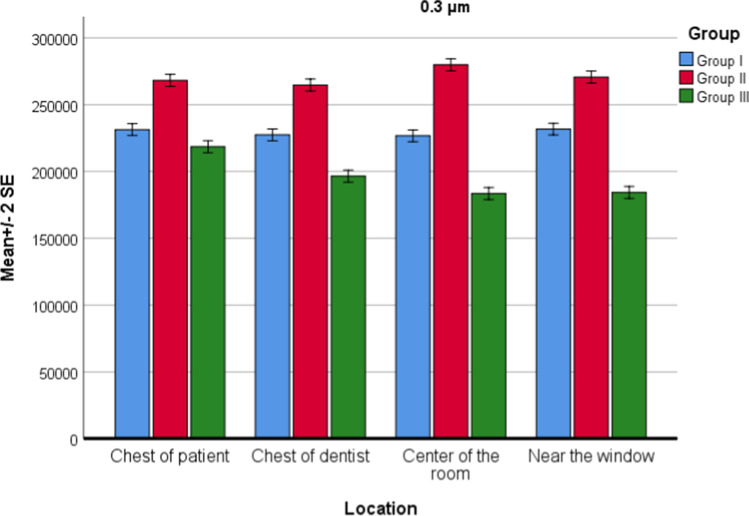
For PM0.3 values, there was a statistically significant effect of both location (P = 0.001) and groups (P = 0.001) on PM values. The interaction of group and location was also significant (P = 0.001). According to the post hoc test for interaction, there was no statistically significant difference between the locations measured in group 1 for PM0.3 values. There was a statistically significant difference between group 1 and group 2 in all four locations (P = 0.001). The HEOS unit reduced the particle concentration to 0.3 microns in all four locations. There was a statistically significant difference in all four regions between the PM0.3 values in group 1 and group 2, and there was an apparent increase (Fig. 1).
Fig. 1.
Location and group interaction for PM0.3 values
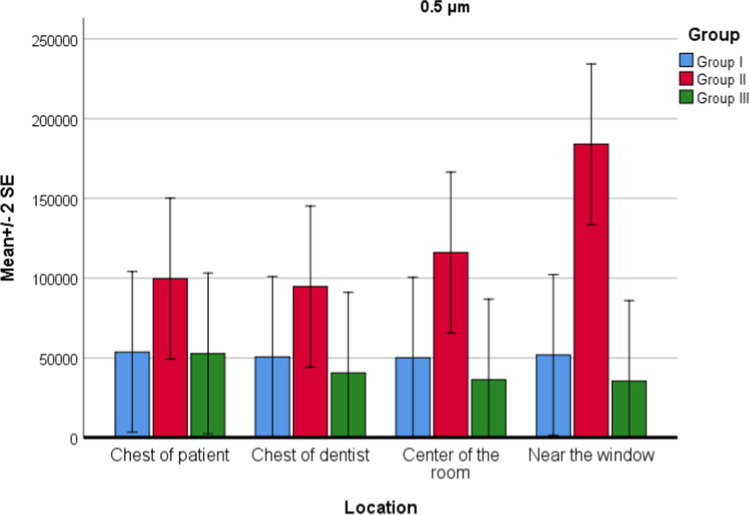
For PM0.5 values, there was no difference between locations (P = 0.530), but significant difference between groups (P = 0.001) was found. Interaction of group and location was not statistically significant (P = 0.417). There was a statistically significant difference between group 1 and group 2 (P = 0.001). In group 2, PM0.5 values increased apparently. There was a statistically significant difference between group 2 and group 3 (P = 0.001). In group 3, PM0.5 values decreased apparently (Fig. 2). There was no significant difference between group 1 and group 3 (P = 0.574).
Fig. 2.
Location and group interaction for PM0.5 values
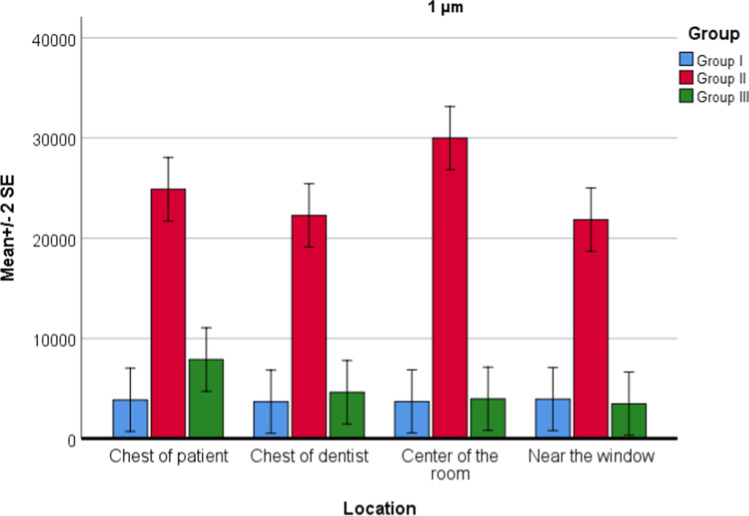
For PM1.0 values, there was no difference between locations (P = 0.074), but significant difference between groups (P = 0.001) was found. Interaction of group and location was also statistically significant (P = 0.032). There was a statistically significant difference between group 1 and group 2 (P = 0.001). In group 2, PM1.0 values were increased. There was a statistically significant difference between group 2 and group 3 (P = 0.001). In group 3, PM1.0 values were decreased (Fig. 3). There was no significant difference between group 1 and group 3. In group 3, the highest decrease in PM1.0 value was observed in the center of the room.
Fig. 3.
Location and group interaction for PM1.0 values
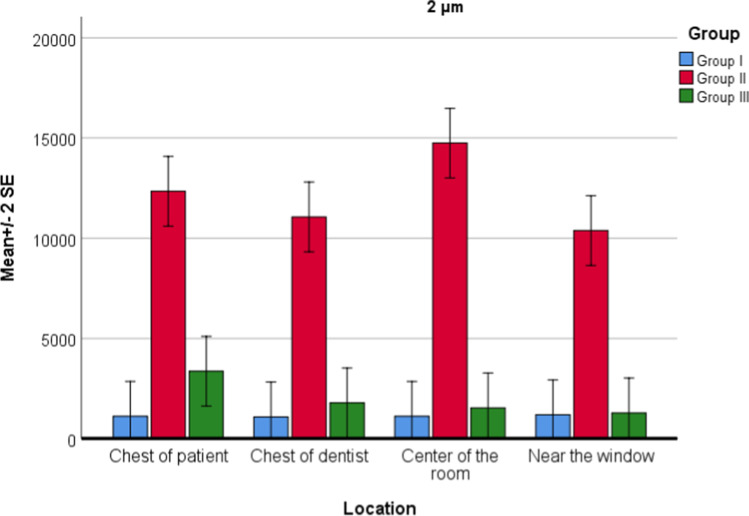
For PM2.0 values, there was no difference between locations (P = 0.100), but a significant difference between groups (P = 0.001) was found. Interaction of group and location was not statistically significant (P = 0.078). There was a statistically significant difference between group 1 and group 2 (P = 0.001). In group 2, PM2.0 values were increased. There was a statistically significant difference between group 2 and group 3 (P = 0.001). In group 3, PM2.0 values were decreased (Fig. 4). There was no significant difference between group 1 and group 3 (P = 0.162).
Fig. 4.
Location and group interaction for PM2.0 values
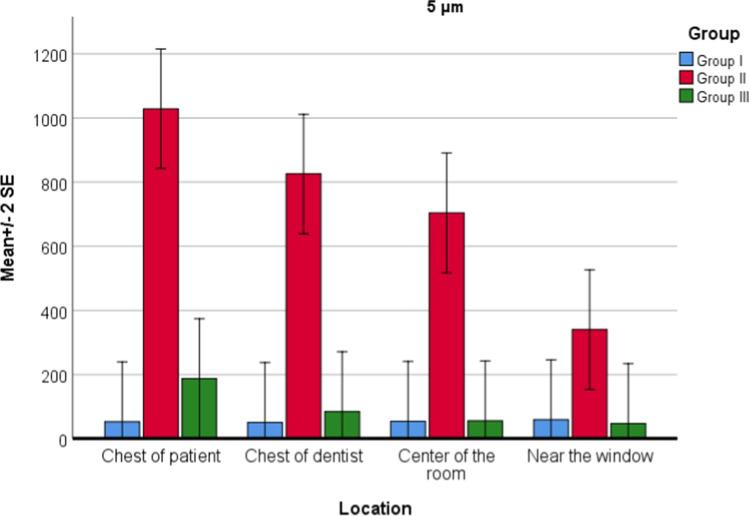
For PM5.0 values, there was statistically significant effect of both location (P = 0.005) and groups (P = 0.001) on PM values. Interaction of group and location was also significant (P = 0.014). According to post hoc test for interaction, in group 1, there was no statistically significant difference between locations. In group 2, there was a statistically significant difference between locations; the maximum PM5.0 value is the chest of patient, chest of the dentist, the center of the room, and near the window, respectively. In group 3, there was no statistically significant difference between locations. There was a statistically significant difference between group 2 and group 3 in all four locations (P = 0.001). The HEOS unit reduced particles of 5 microns in all four locations. There was a statistically significant difference in all four locations between group 1 and group 2, and there was a significant increase (Fig. 5).
Fig. 5.
Location and group interaction for PM5.0 values
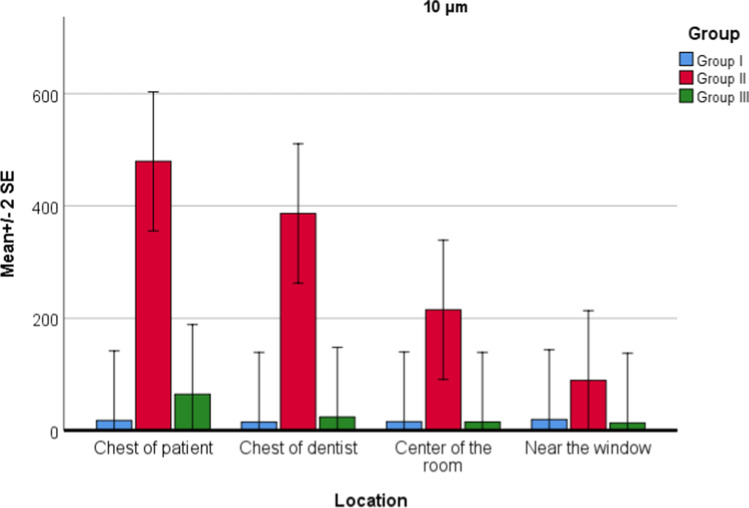
For PM10.0 values, there was a statistically significant effect of both location (P = 0.024) and groups (P = 0.001) on PM values. Interaction of group and location was also significant (P = 0.033). According to the post hoc test for interaction, there was no statistically significant difference between the locations in group 1. There was a statistically significant difference between the locations in group 2; maximum PM10.0 value is the chest of patient, chest of dentist, center of the room, and near the window, respectively. In group 3, there was no statistically significant difference between locations. There was a statistically significant difference between group 2 and group 3 in all four locations (P = 0.001). The HEOS unit reduced particles of 10 microns in all four locations. There was a statistically significant difference in all four locations between group 1 and group 2, and there was a significant increase (Fig. 6).
Fig. 6.
Location and group interaction for PM10.0 values
When the general descriptive statistics were examined, PM0.3 value averages were listed as group 3 with the lowest, followed by group 1 and group 2. In group 3, the highest decrease was seen in PM10.0 and PM5.0 values compared to group 2. This was followed by PM2.0, PM1.0, PM0.5, and PM0.3, respectively. According to these findings, the HEOS unit was more effective as the particle size increases. When the HEOS unit was running, the mean values of the 0.3- and 0.5-micron particles decreased compared to the first measurement in the empty room, and the mean value of the 1.0-, 2.0-, 5.0-, and 10.0-micron particles decreased in group 3 compared with group 2, but increased compared with group 1.
Discussion
With COVID-19, many precautions have been taken to reduce the risk of contamination during dental treatment. Extra-oral suction with HEPA filter is one of the measures taken. HEPA filters were used in hygienic applications, such as hospitals, pharmaceutical factories, and food and beverage production facilities, before and started to be used during dental treatments with COVID-19.
Many studies have been conducted on aerosols, droplets, and splatter contamination during dental treatments [9–19]. In some studies, dental manikin, phantom teeth, or extracted teeth have been used [12, 14, 16, 17]. However, more aerosols are generated during dental treatment with live patient, and these aerosols contain saliva, blood, dental plaque, and tooth debris [17]. Therefore, in the present study, all measurements have been made while performing routine dental preparation in a specific dental room with live patients. The majority of studies used open culture plates, which measured droplets and aerosols fallen onto the surface [9, 12–16]. However, small particles may remain suspended in the air for many hours. Particle counters or air samplers can detect both aerosol and airborne droplets before they fall to the ground. In the present study, a particle counter was used, which measures the concentration of particles in the air. It has been reported that particles with a size of 0.5–10 μm are more likely to spread infection [18]. Particle sizes of 0.3, 0.5, 1, 2, 5, and 10 microns were measured in this study. In addition, the rate of dispersal of particles smaller than 5 microns throughout the room was very high. Therefore, analysis was performed according to particle sizes in four different room parts. Yang et al. reported that the highest level of aerosol occurred in the chest area of the dentist during the aerosol-generating procedure and the extra-oral suction system reduced it to the baseline level [11]. According to the results of our study, particles larger than 2 microns were formed mainly in the chest of the patient region, and the HEOS unit reduced the particle level in this region but it did not reduce it to the baseline level. Ahmed and Jouhar [16] reported that maximum splatter and aerosol produced immediately after the procedure were found at the dentist zone, followed by the assistant zone. This finding is inconsistent with the previous study in which investigators found more splatter in the assistant zone compared to the dentist zone [19]. These studies were performed on a mannequin and used filter paper disc and different dental procedures were applied. In our study, particles smaller than 2 microns were more common as they moved away from the dentist working area.
It was determined that the HEOS unit was effective in reducing particles smaller than 2 microns in every location of the room. However, Senpuku et al. [9] reported that protected areas were limited to the left and posterior sides of the dental chair head when a right-handed dentist and dental hygienist performed scaling. In many studies, it was measured in which region the contamination was more intensive. It was observed that more contamination occurred mainly in the dentist working area and around the patient [18–20]. However, in our study, aerosols formed during tooth preparation were examined separately according to particle size. The density of small particles was high, even at the farthest point of the room where the preparation was made.
In a systematic review, airborne droplets and aerosols generated during eight different dental procedures were examined [19]. It has been reported that the procedures which cause the greatest level of contamination are ultrasonic scaling and high-speed air rotors. Slow-speed handpiece, extractions, and prophylaxis with pumice were rated moderate contamination. Air–water syringe and hand scaling were rated lower contamination. In the present study, anterior tooth preparation was performed. The efficiency of the saliva ejector is greater during the preparation of the posterior teeth; more scattering is observed during the preparation of the anterior teeth. Therefore, in this study, the anterior tooth preparation, which causes the most aerosol splatter and contamination, was preferred.
The limitation of this study can be described as priority particle sizes between 0.3 and 10 microns were measured in this study. During dental procedures, aerosols and droplets larger than 10 microns are formed, and viruses spread the disease in these droplets. Another limitation is that the effectiveness of the device in different dental procedures has not been compared.
Conclusion
The present study showed that as the particle size increases, the rate of spread away from the dentist working area decreases. Small size particles may easily spread all over the room. Therefore, infection control methods should be arranged according to these results. HEPA-filtered extra-oral suction devices effectively prevent the spread of dental aerosols, droplets, and infectious diseases. Therefore, using HEPA-filtered suction devices may help reduce the risk of COVID-19 in dental treatment.
Author contribution
ND formed the hypothesis and idea of the research. IK provided the organization for the design and execution of the study. IK and IKK prepared the experimental environment and made the measurements. All of the authors participated in the preparation of the manuscript and they have approved the final version.
Funding
HEPA-filtered extra oral suction (HEOS) unit was obtained by Gaziantep University Scientific Research Governing Unit.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nermin Demirkol, Email: dt_nerminhamdemirci@hotmail.com.
Irem Karagozoglu, Email: dtiremkaragozoglu@hotmail.com.
Ipek Kulekci Kocer, Email: ipekkocer1@gmail.com.
References
- 1.Veena H, Mahantesha S, Joseph PA, Patil SR, Patil SH. Dissemination of aerosol and splatter during ultrasonic scaling: a pilot study. J Infection Public Health. 2015;8(3):260–265. doi: 10.1016/j.jiph.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Shihama K, et al. Evidence of aerosolised floating blood mist during oral surgery. J Hosp Infect. 2009;71(4):359–364. doi: 10.1016/j.jhin.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Leggat PA, Kedjarune U. Bacterial aerosols in the dental clinic: a review. In Int Dent J. 2001;51(1):39–44. doi: 10.1002/j.1875-595X.2001.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 4.Chowell G, Abdirizak F, Lee S, Lee J, Jung E, Nishiura H, Viboud C. Transmission characteristics of MERS and SARS in the healthcare setting: a comparative study. BMC Med. 2015;13(210):1–12. doi: 10.1186/s12916-015-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J. Pathogenicity and transmissibility of 2019-nCoV—a quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22(2):69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arısoy, P. (2019). Diş hekimliğinde çapraz enfeksiyonlar ve kontrolü. Ankara Üniversitesi Diş Hekimliği Fakültesi Dergisi 46(3):187–195
- 7.CDC Guidelines for infection control in dental health-care settings. Morb Mortal Wkly Rep. 2003;52:RR-17. [PubMed] [Google Scholar]
- 8.Maghiouth A, Yousef Y, Bagieh N. Qualitative and quantitative analysis of bacterial aero- sols. J Contemp Dent Prac. 2004;4(91):1. [PubMed] [Google Scholar]
- 9.Senpuku H, Fukumoto M, Uchiyama T, Taguchi C, Suzuki I, Arikawa K. Effects of extraoral suction on droplets and aerosols for infection control practices. Dent J (Basel) 2021;9(7):80. doi: 10.3390/dj9070080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makhsous S, Segovia JM, He J, Chan D, Lee L, Novosselov IV, Mamishev AV. Methodology for addressing infectious aerosol persistence in real-time using sensor network. Sensors (Basel) 2021;21(11):3928. doi: 10.3390/s21113928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang M, Chaghtai A, Melendez M, Hasson H, Whitaker E, Badi M, Sperrazza L, Godel J, Yesilsoy C, Tellez M, Orrego S, Montoya C, Ismail A. Mitigating saliva aerosol contamination in a dental school clinic. BMC Oral Health. 2021;21(1):52. doi: 10.1186/s12903-021-01417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavis SE, Hines SE, Dyalram D, Wilken NC, Dalby RN. Can extraoral suction units minimize droplet spatter during a simulated dental procedure? J Am Dent Assoc. 2021;152(2):157–165. doi: 10.1016/j.adaj.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Junevicius J, Surna A, Surna R. Effectiveness evaluation of different suction systems. Stomatologija. 2005;7(2):52–57. [PubMed] [Google Scholar]
- 14.Shahdad S, Patel T, Hindocha A, Cagney N, Mueller JD, Seoudi N, Morgan C, Din A. The efficacy of an extraoral scavenging device on reduction of splatter contamination during dental aerosol generating procedures: an exploratory study. Br Dent J. 2020;11:1–10. doi: 10.1038/s41415-020-2112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noordien N, Mulder-van Staden S, Mulder R. In vivo study of aerosol, droplets and splatter reduction in dentistry. Viruses. 2021;13(10):1928. doi: 10.3390/v13101928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed MA, Jouhar R. Dissemination of aerosol and splatter in clinical environment during cavity preparation: an in vitro study. Int J Environ Res Public Health. 2021;18(7):3773. doi: 10.3390/ijerph18073773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King TB, Muzzin KB, Berry CW, Anders LM. The effectiveness of an aerosol reduction device for ultrasonic scalers. J Periodontol. 1997;68(1):45–49. doi: 10.1902/jop.1997.68.1.45. [DOI] [PubMed] [Google Scholar]
- 18.Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dental Assoc. 2004;135(4):429–37 . doi: 10.14219/jada.archive.2004.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Innes N, Johnson IG, Al-Yaseen W, Harris R, Jones R, Kc S, McGregor S, Robertson M, Wade WG, Gallagher JE. A systematic review of droplet and aerosol generation in dentistry. J Dent. 2021;105:103556 . doi: 10.1016/j.jdent.2020.103556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Revathi B, Muralidharan N (2019) Evaluation of extent of aerosols around dental chair during dental treatments. Drug Invent Today 12(10):146–148