Abstract
Inflammation and infections such as malaria affect estimates of micronutrient status. Medline, Embase, Web of Science, Scopus and the Cochrane library were searched to identify studies reporting mean concentrations of ferritin, hepcidin, retinol or retinol binding protein in individuals with asymptomatic or clinical malaria and healthy controls. Study quality was assessed using the US National Institute of Health tool. Random effects meta-analyses were used to generate summary mean differences. In total, forty-four studies were included. Mean ferritin concentrations were elevated by: 28·2 µg/l (95 % CI 15·6, 40·9) in children with asymptomatic malaria; 28·5 µg/l (95 % CI 8·1, 48·8) in adults with asymptomatic malaria; and 366 µg/l (95 % CI 162, 570) in children with clinical malaria compared with individuals without malaria infection. Mean hepcidin concentrations were elevated by 1·52 nmol/l (95 % CI 0·92, 2·11) in children with asymptomatic malaria. Mean retinol concentrations were reduced by: 0·11 µmol/l (95 % CI −0·22, −0·01) in children with asymptomatic malaria; 0·43 µmol/l (95 % CI −0·71, −0·16) in children with clinical malaria and 0·73 µmol/l (95 % CI −1·11, −0·36) in adults with clinical malaria. Most of these results were stable in sensitivity analyses. In children with clinical malaria and pregnant women, difference in ferritin concentrations were greater in areas with higher transmission intensity. We conclude that biomarkers of iron and vitamin A status should be statistically adjusted for malaria and the severity of infection. Several studies analysing asymptomatic infections reported elevated ferritin concentrations without noticeable elevation of inflammation markers, indicating a need to adjust for malaria status in addition to inflammation adjustments.
Key words: ferritin, iron, retinol, vitamin A, malaria
Micronutrient deficiencies are a major public health burden, especially in low-income countries, and accurate prevalence estimates are important to guide planning and monitoring of nutritional interventions(1). However, prevalence of micronutrient deficiencies can be incorrectly estimated because certain micronutrient biomarkers are affected by inflammation and infections such as malaria(2). Inflammation is characterised by the acute-phase response to infection, injury or environmental insults. Some acute-phase proteins are also micronutrient markers; for example, serum ferritin, the primary iron storage protein, is a positive acute-phase protein – that is, its concentration increases in response to inflammation – and retinol binding protein (RBP) is a negative acute-phase protein – that is, its concentration decreases in response to inflammation(2,3). Whilst in the absence of inflammation, the concentration of plasma or serum ferritin is positively correlated with the size of the total body iron stores, during inflammation plasma/serum ferritin is raised and does not represent iron stores(2). Infants and young children, as well as women of reproductive age, are at high risk of micronutrient deficiencies due to increased physiological needs(4). They are also at considerably greater risk of contracting malaria, and developing severe disease, than other demographic groups(5). According to the WHO, there were 229 million cases of malaria in 2019(5). More than 90 % of these cases were located in the WHO African region. The presence of parasites can produce a chronic or mild acute-phase response(6). In settings of higher and more holoendemic malaria transmission, more individuals in a population, especially non-pregnant adults, will have some degree of immunity to malaria. Asymptomatic malaria, that is, the presence of parasitaemia in the absence of fever or other malaria-related symptoms, is very common in malaria endemic areas, with some prevalence rates exceeding 50 %(7). There are five well-established malaria parasite species that infect humans, namely Plasmodium falciparum, P. vivax, P. ovale, P. malariae and P. knowlesi. P. falciparum accounts for 99·7 % of infections in sub-Saharan Africa, while P. vivax accounts for 75 % of infections in the Americas(8). Currently used diagnostic methods include microscopy which visualises parasites in stained blood smears, rapid diagnostic tests that detect parasite antigen/s in blood samples;and PCR which identifies the presence of specific malaria genes in a blood sample.
Recently, the WHO published an updated guide on the use of ferritin to assess iron status and the recommended adjustments for inflammation, measured on the basis of C-reactive protein (CRP) and alpha-1-acid glycoprotein (AGP) concentrations in blood serum/plasma(9). Ferritin values may differ by malaria infection status(10) after correcting for inflammation defined by raised CRP and/or AGP, and the updated WHO guidelines mention malaria as a possible factor for adjustment. Other biomarkers are also likely to be affected by malaria. Retinol, the predominant circulating form of vitamin A in the blood, is known to be affected by malaria infection(11). In children aged 6–59 months in Ghana, increasing malaria parasite density was significantly associated with decreasing serum retinol concentrations(11). These reductions have been attributed largely to the inflammatory response. As the measurement of serum retinol requires expensive laboratory equipment, some micronutrient surveys measure its carrier protein, RBP, instead of retinol itself. In young children in Liberia, Larson et al. found a significant added effect of malaria on RBP concentrations and vitamin A deficiency prevalence estimates even after adjusting for CRP and AGP using the regression approach(12). There is also a growing interest in the impact of malaria on hepcidin, the iron regulatory hormone(13,14). Hepcidin seems to be upregulated in malaria infection even in asymptomatic human infection(7). This results in a blockage of iron absorption from the diet and a redistribution of iron into the body, away from the serum.
Taking into account the effect of malaria on micronutrient biomarkers has the potential to significantly modify the estimation of the prevalence of micronutrient deficiencies derived from large population-based surveys such as national micronutrient surveys. This research estimated the effect of malaria on several biomarker values (ferritin, hepcidin, retinol and RBP) by performing a meta-analysis of studies comparing biomarker values in individuals infected with malaria and individuals without malaria infection.
Methods
The protocol of this systematic review has been published on PROSPERO on the 24 September 2021: CRD42021279974. Ethical approval was not needed as the data used in the analysis are fully available in the public domain. We followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting checklist for this systematic review and meta-analysis.
Eligibility criteria
Randomised controlled trials or quasi-randomised controlled trials, prospective observational studies with data collection at multiple time points and cross-sectional studies with a control group that measured selected biomarkers in malaria-infected individuals were eligible. In children and non-pregnant adults, studies that distinguished asymptomatic and clinical malaria cases were included; studies in these populations that combined asymptomatic and symptomatic infections in a single malaria group were excluded. Studies that provided an intervention believed to impact the iron or vitamin A status of the participants were only included if data from a control group, or baseline data, could be extracted. Studies of human participants of any age and sex were eligible. As individuals suffering from severe malaria are often not sampled in large population-based surveys that measure micronutrient status, we decided not to include reports that only recruited participants with severe malaria. However, if individuals with severe malaria were included in papers that meet other selection criteria, they were analysed separately. For similar reasons, studies that recruited individuals based on their being anaemic or having another disease that is likely to affect iron or vitamin A metabolism (sickle cell disease and thalassemia) were not included.
Search strategy
Medline, Embase, Web of Science, Scopus and the Cochrane library were searched in April 2021. The search strategy included the use of Medical Subject Heading (MeSH) terms and text words, with the use of explosion technique. The complete search strategy, which was reviewed by a qualified librarian, is included in supplementary file 1. There was no restriction on the date of publication. Reports written in English, French and Spanish were eligible. Abstracts and unpublished studies were not considered. Two reviewers, FS and LSR, screened each record independently, in a two-stage process: first the reviewers examined titles and abstracts. The full texts were then retrieved and the reviewers examined the full-text reports for compliance with the eligibility criteria. After retrieval of articles from the search, the reference lists of all selected articles were checked for other potentially relevant articles; two additional papers were identified. Disagreements were discussed between the two reviewers until an agreement could be reached. References were managed in EndNote 20 (Clarivate Analytics). The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow chart of identified studies is illustrated in Fig. 1.
Fig. 1.
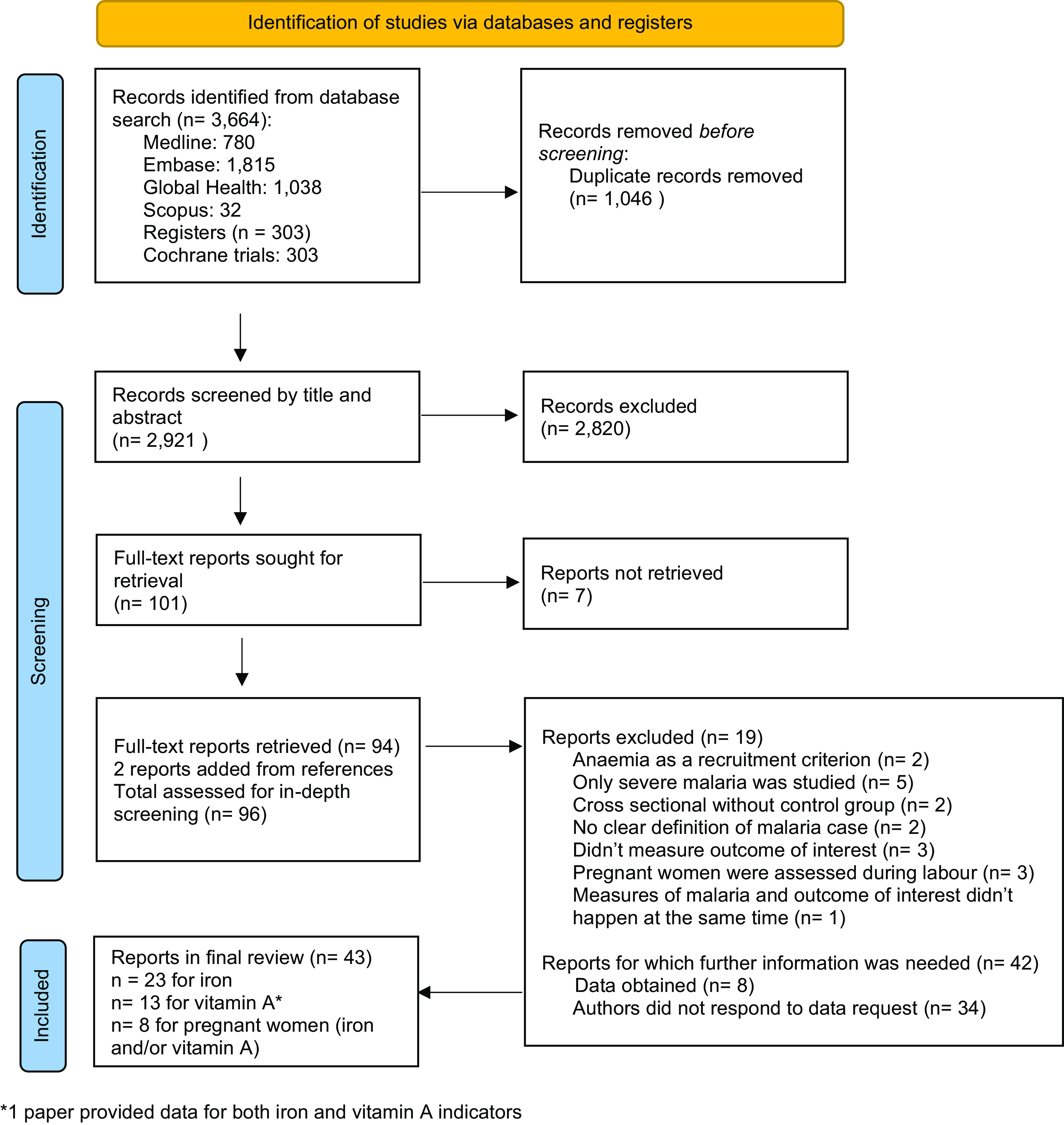
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram of publications screened in a systematic review of the impact of malaria infection on indicators of iron and vitamin A status.
Data extraction and statistical analysis
Data were extracted into MS Excel by FS using a tool which included author name, study design, sample size, population group, age group, year when the study took place, country, malaria endemicity profile, method of diagnosis of malaria, clinical definition of malaria, malaria species, and the summary statistics of ferritin, hepcidin, retinol and RBP. Although Hb and soluble transferrin receptors are sometimes used to describe iron status, we did not include them in our analysis, as Hb is not a specific indicator of iron deficiency and soluble transferrin receptors concentrations are not reported as often as ferritin concentrations. The malaria endemicity profile was defined by the Plasmodium falciparum prevalence rate (PfPR) among children aged 2–10 years, as described in the Malaria Atlas Project(15). The different categories were defined by the WHO(16): PfPR < 1 %: very low intensity, PfPR ≥ 1 % and < 10 %: low intensity, PfPR ≥ 10 and < 35 %: moderate intensity, PfPR ≥ 35 %: high intensity. The data from three clinical groups were included: healthy participants with negative malaria test results, asymptomatic participants with positive malaria test and without clinical sign of illness, and a clinical malaria group who had a positive malaria test and fever. For prospective studies, the biomarker measurement at admission was considered the measurement of the malaria group, and the measure at the final follow-up point was considered the measurement of the control group. We analysed separately three population groups: children, non-pregnant adults and pregnant women. When a study provided data for different malaria species, or for different age groups, the corresponding data were entered into different datasets to allow for subgroup analysis, which explains why there is a greater number of datasets than studies. If the data from the same group were used for two comparisons in the same meta-analysis, we halved the number of participants from this group in each comparison, following the method described in the Cochrane Handbook(17). Authors were contacted if the relevant information was not available in the report. The risk of bias of all included studies was assessed by FS, using the US National Institute of Health quality assessment tool for observational cohort and cross-sectional studies. This tool contains fourteen questions around key concepts for evaluating the internal validity of a study. They are not intended to create a final score but help to assess potential selection, information, measurement and confounding biases. The use of this tool for cross-sectional studies was recommended in a recent review by Ma et al. (18). For the outcomes specified earlier, we reported the mean value of different groups (healthy control group, asymptomatic malaria group and clinical malaria group), as well as the 95 % CI or the standard deviation. We calculated the mean difference with 95 % CI between groups. We also attempted to calculate missing information from other reported measurements, if possible. When the geometric mean was provided, we transformed it into an arithmetic mean using the method explained by Higgins(19). We generated meta-analyses based on the severity of the disease, either asymptomatic or clinical malaria and specific outcomes, such as ferritin or retinol concentrations. We first calculated a summary statistic for each study to describe the observed malaria effect. As our data were continuous, the summary statistic was a difference between means and a 95 % CI. When the data came from prospective studies, we followed the method described in the Cochrane Handbook to impute a standard deviation change from baseline to endline(17). We then used a random effects meta-analysis for combining data, as we anticipated that there may be natural heterogeneity between studies attributable to the different populations and settings. The study weights were equal to the inverse of the variance of each study’s effect estimate according to the methodology developed by DerSimonian and Laird(20). We generated forest plots and we provided a CI, which communicates the precision (or uncertainty) of the summary estimate and a P-value.
When data were available, and when more than one study provided relevant data for meta-analysis, we conducted the following subgroup analyses: species of malaria (falciparum v. vivax), malaria endemicity profile of the country (low v. moderate v. high intensity of transmission, as defined by(15,16)), method of diagnosis of malaria (rapid diagnostic tests v. microscopy or both), age of children (under v. over 5 years old) and design of the study (cross-sectional v. prospective study). Heterogeneity was assessed using the I2 statistic. Both a qualitative (funnel plot) and a quantitative (Egger’s regression test) approach were used to examine potential publication biases. An influence analysis was conducted to determine the effect of removing each included study on the overall effect and 95 % CI using the technique described by Viechtbauer and Cheung(21). The meta-analyses, funnel plot, Egger’s regression test and influence analysis were conducted using RStudio software version 1.3.959(22) with the dmetar (v. 0.0.9000(23)) and meta-packages(24). The workbooks for meta-analysis (version 1.5) developed by Suurmond were used to perform the subgroup analyses(25). A P-value of < 0·05 for meta-analyses was considered statistically significant.
Results
Of 101 full-text reports screened, 43 papers describing 44 studies conducted in 27 countries met selection criteria(7,10,11,13,26–65) (Fig. 1). Twenty-nine studies were conducted in fifteen African countries(10,11,13,26,28–31,38–41,46,47,49–53,55,57,62,63), nine were conducted in Asia(27,32,36,37,42,44,48,56,58), two in Oceania(54,64), three in Europe(43,45,48)(two studied imported cases and one experimental malaria infection) and one in the Americas(35) (Table 1). Among the included studies, twenty-three reported on ferritin and/or hepcidin concentrations in adults and/or children(7,10,13,45–64), thirteen reported on retinol or RBP concentrations in adults and/or children(11,34–44,63), and eight reported on either ferritin, hepcidin, retinol or RBP concentrations in pregnant women(26–33). Eight studies compared the ferritin or retinol level between groups with different severity of malaria(13,30,40,51,52,54,56,64). Thirty-four studies were cross-sectional(11,13,26–34,36–40,42,44,50–55,57–60,62–64), whereas ten were prospective(7,41,43,45–49,56,61). The predominant species of malaria was P. falciparum in forty-one studies(7,11,13,26,28–34,36–43,45–57,59,60,62–64), whereas P. vivax was either predominant or as present as P. falciparum in four studies(27,35,44,58). A broad range of endemic profiles were represented. The pooled sample size for the analysis of children (N 14 330) was larger than for adults (N 985) (Table 2). The risk of bias assessment revealed that the majority of studies had a low or unclear risk of bias (online Supplementary Table 1).
Table 1.
Characteristics of the studies included in the systematic review of the impact of malaria infection on indicators of iron and vitamin a status
| n | Author, publication year | Period the study was conducted | Country | Study design | Study participants | Malaria endemicity profile* | Total cases | Clinical severity | Malaria species | Diagnostic of Malaria | Outcomes measured |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies reporting on iron status indicators | |||||||||||
| 1 | Barffour et al. 2018(10) | 2012–2013 | Zambia | CS nested in a cluster-RCT | Children 4–8 years | Moderate intensity, seasonal transmission; two cross-sectional surveys conducted, one during high and one in low transmission period | 744 | A and control | F | RDT (HRP2) and P† | Ferritin |
| 2 | Beesley et al. 2000(45) | NA | Europe | Prospective study | Adults | Imported cases | 36 | C | F | P | Ferritin |
| 3 | Burte et al. 2013(13) | 2008–2010 | Nigeria | CS as part of a larger prospective study | Children 6 months–12 years | High intensity, year-round | 117 | DC, C, CM, SMA and control | F | P | Ferritin |
| 4 | Castberg et al. 2018(46) | 2014–2015 | Ghana | Prospective study as part of a larger study | Children 1–12 years | Moderate intensity, year-round | 98 | C | F | P | Ferritin, hepcidin |
| 5 | Cercamondi et al. 2010(47) | NA | Benin | Prospective study | Adults (women) | High intensity, seasonal transmission; survey conducted during high transmission season | 23 | A | F | P | Ferritin, hepcidin |
| 6 | de Mast et al. 2010(7) | NA | Indonesia | Prospective study | Children 5–15 years | Low intensity, year-round | 108 | A and control | F and V | P | Ferritin, hepcidin |
| 7 | de Mast et al. 2009(48) | 1999–2003 | Europe | Prospective experimental human malaria infection | Adults | Experimental infection | 5 | C and control | F | P | Ferritin, hepcidin |
| 8 | Diallo et al. 2020(26,65) | 2011–2014 | Burkina Faso | CS surveys included in a prospective study | Pregnant women and infants | High intensity, year-round | Women: 916 | Positive malaria test and control | F | P | Ferritin |
| 9 | Dreyfuss et al. 2000(27) | 1994–1997 | Nepal | CS as part of an RCT | Pregnant women | Very low intensity, year-round | 288 | C and control | V | P | Ferritin |
| 10 | Glinz et al. 2015(49) | NA | Cote d’Ivoire | Prospective study | Children 11–17 years | Moderate intensity, year-round | 17 | A | F | P | Ferritin, hepcidin |
| 11 | Jeremiah et al. 2007(50) | 2005–2006 | Nigeria | CS | Children 1–8 years | High intensity, year-round | 240 | A and control | F | P | Ferritin |
| 12 | Kabore et al. 2020(51) | 2016–2017 | Burkina Faso | CS | Children (over 3 months) and adults | Moderate intensity, seasonal transmission; survey conducted during high transmission season | Children: 722 Adults: 396 |
A and C and control | F | P | Ferritin, hepcidin |
| 13 | Kivibidila et al. 1999(52) | 1991 | DR Congo | CS | Children 6 months–16 years | Moderate intensity | 44 | CM, C and control | F | P | Ferritin |
| 14 | Mockenhaupt et al. 2000(28) | 1998 | Ghana | CS | Pregnant women | High intensity | 530 | Positive malaria test and control | F and one case of P. Ovale | P and PCR | Ferritin |
| 15–18 | Muriuki et al. 2020(53,73–76) | 2013 | Burkina Faso | CS | Children 12 months | High intensity, year-round | 348 | A and control | F | P | Ferritin, hepcidin |
| 2001 | The Gambia | CS as part of a prospective study | Children 2–6 years | Moderate intensity, seasonal transmission; survey conducted during high transmission season | 753 | A and control | F | P | Ferritin, hepcidin | ||
| 2012–2014 | Kenya | CS as part of a prospective study | Children under 5 years | Low intensity, seasonal transmission; survey conducted during high transmission season | 1484 | A and control | F | P | Ferritin, hepcidin | ||
| 2003–2005 | Uganda | CS as part of an RCT | Children under 5 years | Moderate intensity, year-round | 1374 | A and control | F | P | Ferritin, hepcidin | ||
| 19 | Mwangi(29) | 2011–2014 | Kenya | CS as part of an RCT | Pregnant women | Low intensity, year-round | 470 | C and control | F | P | Ferritin |
| 20 | O’Donnell et al. 2009(54) | 1993– 1996 |
Papua New Guinea | CS | Children under 4 years | Low intensity | 495 | C, severe malaria and control | F | P | Ferritin |
| 21 | Odunukwe et al. 2000(55) | NA | Nigeria | CS | Adults | High intensity, year-round | 300 | A and control | F and malariae | P | Ferritin |
| 22 | Phillips et al. 1986(56) | NA | Thailand | Prospective study | Adults | Very low intensity | 23 | C, CM and control | F | P | Ferritin |
| 23 | Righetti et al. 2013(57) | 2010 | Cote d’Ivoire | CS as part of prospective study | Children 6 months–8 years and adults | High intensity, year-round | Children: 246 Adults: 92 |
A and C | F | RDT (HRP2) and P‡ | Ferritin |
| 24 | Saad et al. 2012(30) | 2010 | Sudan | CS | Pregnant women | Low intensity | 64 | Severe, C and control | F | P | Ferritin |
| 25 | Seyrek et al. 2004(58) | NA | Turkey | CS | Adults | Very low intensity | 31 | C and control | V | P | Ferritin |
| 26 | Shulman et al. 1996(31) | 1993 | Kenya | CS | Pregnant women | Low intensity, seasonal. Survey conducted during a low transmission period | 275 | C and control | F | P | Ferritin |
| 27 | Stoltzfus et al. 1997(59) | 1994 | Zanzibar | CS | Schoolchildren 5–19 years | Moderate intensity, year-round | 3605 | A and control | F | P | Ferritin |
| 28 | Stoltzfus et al. 2000(60) | 1996 | Zanzibar | CS | Children under 5 years | Moderate intensity, year-round | 464 | A and control | F | P | Ferritin |
| 29 | Uscategui et al. 2009(61) | 2002 | Colombia | Prospective case study | Children 4–10 years | Very low intensity | 89 | C | F and V | P | Ferritin |
| 30 | Verhoef et al. 2001(62) | 1997 | Kenya | CS | Children 2–36 months | Moderate intensity, seasonal transmission; survey conducted during high transmission season | 318 | A and control | F | P | Ferritin |
| 31 | Wessells et al. 2014(63) | 2009 | Burkina Faso | CS data from an RCT | Children 6–23 months | High intensity, year-round | 437 | A and control | F | ELISA (HRP2) | Ferritin |
| 32 | Wessells et al. 2017(33) | 2014–2015 | Niger | CS as part of an RCT | Pregnant women | Moderate intensity | 787 | Positive malaria test and control | F | ELISA (HRP2) | Ferritin |
| 33 | Williams et al. 1999(64) | 1994 | Vanuatu | CS | Children, mean age: 11 years | Low intensity, seasonal transmission; survey conducted during high transmission season | 115 | A, C and control | F and V | P | Ferritin |
| Studies reporting on vitamin A status indicators | |||||||||||
| 1 | Barffour et al. 2018(34) § | 2012–2013 | Zambia | CS nested in a cluster RCT | Children 4–8 years | Moderate intensity, seasonal transmission; two cross-sectional surveys conducted, one during high and one in low transmission period | 744 | A and control | F | RDT (HRP2) and P† | Retinol |
| 2 | Carmona et al. 2008(35) || | 2002 | Colombia | Prospective case study | Children 4–10 years | Very low intensity | 89 | C | F and V | P | Retinol |
| 3 | Das et al. 1996(36) | 1992–1993 | India | CS | Children 2–11 years | Low intensity | 173 | A, C and severe malaria | F | P | RBP, retinol |
| 4 | Davis et al. 1993(37) | 1991 | China | CS | Adults | Very low intensity | 27 | C and control | F and V | P | Retinol |
| 5 | Diatta et al. 2013(38) | NA | Senegal | CS | Children under 5 years | Low intensity, seasonal transmission; survey conducted during high transmission season | 312 | A and control | F | P | Retinol |
| 6 | Filteau et al. 1993(11) | 1990–1991 | Ghana | CS as part of a randomised double-blind study | Children 6–59 months | High intensity, year-round | 59 | A and control | F | P | Retinol |
| 7 | Inocent et al. 2007(39) | 2004–2005 | Cameroon | CS | Children 0–6 years | High intensity | 116 | C and control | F | P | Retinol |
| 8 | Mfonkeu et al. 2010(40) | 2007 | Cameroon | CS | Children 6 months–14 years | High intensity | 139 | C, MA, CM, CM & MA and control | F | P | Retinol |
| 9 | Nussenblatt et al. 2002(41) | 1998 | Uganda | Prospective study | Children 1–10 years | High intensity, year-round | 273 | C | F | P | Retinol |
| 10 | Raza et al. 2009(42) | 2006–2007 | India | CS | Children 2–5 years | Low intensity | 170 | C and control | F | P | Retinol |
| 11 | Stuetz et al. 2005(32) | 1998–2000 | Thailand | Secondary samples analysis | Pregnant women | Low intensity, year-round | 108 | Positive malaria test and control | F | P | Retinol |
| 12 | Tabone et al. 1992(43) | NA | Europe | Prospective study | Adults | Imported cases | 7 | C | F | P | Retinol, RBP |
| 13 | Thurnman et al. 1991(44) | NA | Thailand | CS | Adults | Very low intensity | 45 | C and control | F, V and mixed | P | Retinol |
| 14 | Wessells et al. 2014(63) | 2009 | Burkina Faso | CS data from an RCT | Children 6–23 months | High intensity, year-round | 437 | A and control | F | ELISA (HRP2) | RBP |
CS, cross-sectional; A, asymptomatic malaria; F, falciparum; RDT, rapid diagnostic test; HRP2, histidine-rich protein 2; NA, non-available; C, clinical-uncomplicated malaria; P, parasitaemia by microscopy; DC, disease control; CM, cerebral malaria; SMA, severe malarial anaemia; V, vivax; RCT, randomised controlled trial; MA, malarial anaemia; RBP, retinol binding protein. ‘Control’ is defined as healthy children with no malaria detected by the study-specific diagnostic test.
Seasonality was defined by the author and the information was not systematically reported. The intensity of transmission was defined by the Plasmodium falciparum prevalence rate (PfPR) among children aged 2–10 years, as described in the Malaria Atlas Project(15). As defined by WHO(16), PfPR < 1 %: very low intensity, PfPR ≥ 1 % and < 10 %: low intensity, PfPR ≥ 10 and < 35 %: moderate intensity, and PfPR ≥ 35 %: high intensity. The PfPR is the proportion of the population found to carry asexual blood-stage parasitaemia, a basis for the classical categorical measures of malaria transmission.
Malaria parasitaemia was detected by RDT and/or microscopy.
Malaria diagnosis was by microscopy-confirmed RDT.
Participants are the same as in ref. 10.
Participants are the same as in ref. 61.
Table 2.
Characteristics of individuals and settings from studies included in the systematic review of the impact of malaria infection on indicators of iron and vitamin A status
(Number and percentages)
| Children | Non-pregnant adults | Pregnant women | Total | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Number of studies included | 27 | 11 | 8 | 46* | ||||
| Number of participants in the included studies | 14 330 | 985 | 3438 | 18 753 | ||||
| Age in years (mean ± sd) of participants in the included studies | ||||||||
| Mean | 6·2 | 29·6 | 24·1 | |||||
| sd | 4 | 9 | 5·1 | |||||
| Estimated malaria transmission intensity during the survey in the included studies† | ||||||||
| Very low (< 1 %) | 2 | 7 % | 4 | 37 % | 1 | 13 % | 7 | 16 % |
| Low (1–10 %) | 7 | 26 % | 0 | 4 | 50 % | 11 | 14 % | |
| Moderate (10–35 %) | 8 | 30 % | 1 | 9 % | 1 | 13 % | 10 | 23 % |
| High (> 35 %) | 10 | 37 % | 3 | 27 % | 2 | 25 % | 15 | 34 % |
| Imported cases treated in Europe or experimental infection | 0 | 3 | 27 % | 0 | 3 | 6 % | ||
| Seasonality of transmission in the included studies | ||||||||
| Holoendemic | 12 | 44 % | 2 | 18 % | 4 | 40 % | 18 | 40 % |
| Seasonal | 6 | 22 % | 2 | 18 % | 1 | 20 % | 9 | 19 % |
| Imported cases treated in Europe or experimental infection | 0 | 3 | 28 % | 0 | 3 | 7 % | ||
| Not specified by authors | 9 | 34 % | 4 | 36 % | 2 | 40 % | 15 | 34 % |
Two reports provided data for children and adults.
As defined in ref. 15 and 16.
Asymptomatic malaria and ferritin concentrations in children and adults
Fifteen studies(7,10,49–51,53,57,59,60,63,64) (twenty-three datasets) analysed the association between malaria and ferritin concentrations in asymptomatic children (4309 children with malaria infection and 6375 control children). Overall, ferritin concentrations were 28·2 µg/l (95 % CI 15·6, 40·9, P < 0·001) greater in children with asymptomatic malaria compared with control groups (Fig. 2). The subgroup analyses did not reveal any differences (Table 3). There was strong evidence of between-study heterogeneity of effect (I2 = 99 %). Heterogeneity was not explained by descriptive study factors (Table 3). The sensitivity analysis showed the stability of the pooled results after the leave-one-out analysis (Supplementary Fig. 1), and all studies had a low or unclear risk of bias (Table 4). The funnel plot and the Egger test did not show significant asymmetry, indicating no significant publication bias for this analysis (Supplementary Fig. 2).
Fig. 2.
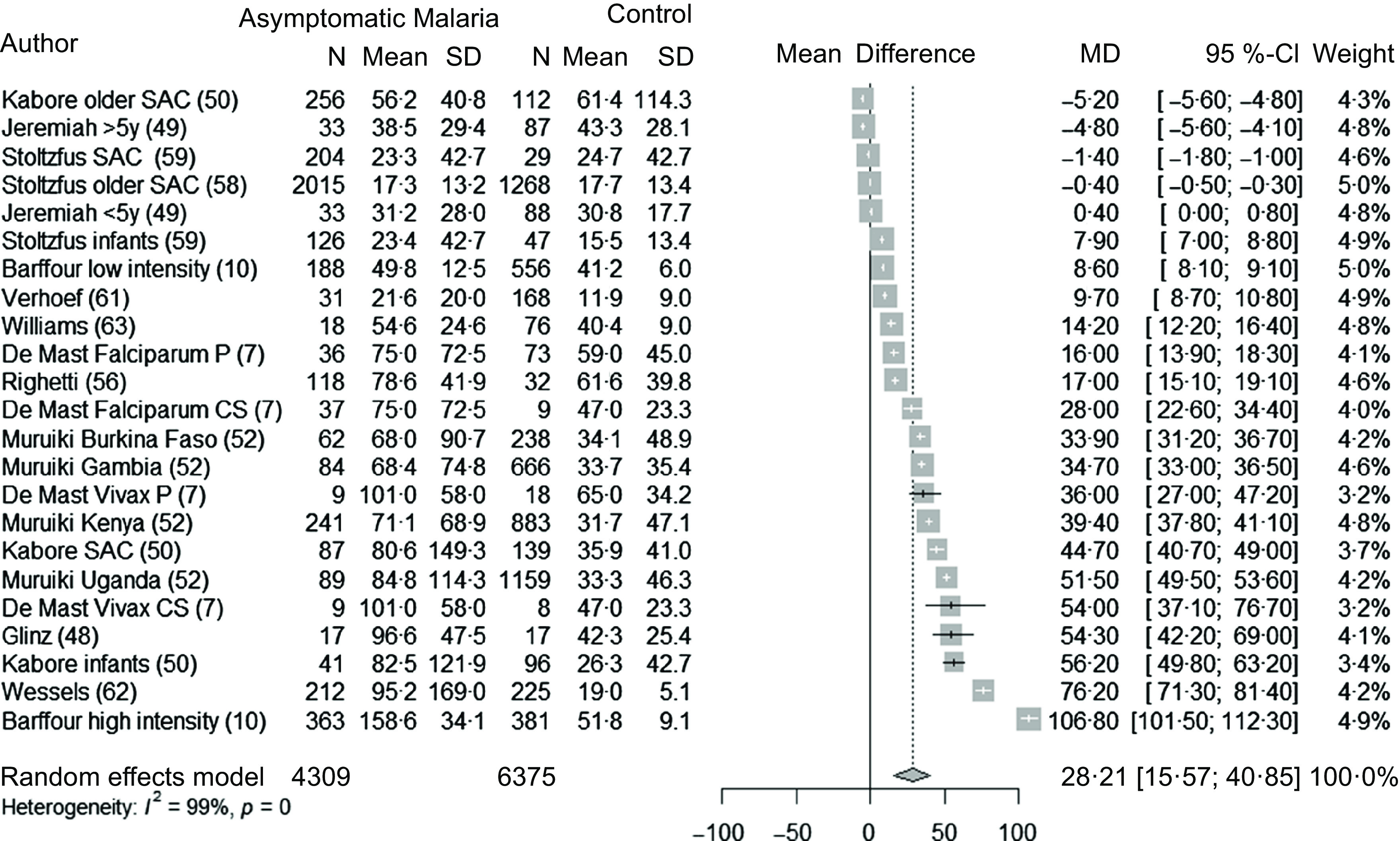
Forest plot of differences in ferritin concentrations (µg/l) between children with asymptomatic malaria and control group, using the random effect model. The grey squares represent the mean difference from each study, while the horizontal line represents the corresponding 95 % CI. The hollow diamond represents the overall pooled effects, while the left and right points of the diamond represent the corresponding 95 % CI. SAC, school age children; P, prospective; CS, cross-sectional; MD, mean difference.
Table 3.
Results of subgroup analyses for ferritin concentration (µg/l) in children with asymptomatic malaria parasitaemia and control group
(Mean difference and 95 % confidence interval)
| Number of datasets | Mean difference | 95 % CI | I 2 for heterogeneity | P subgroup | |
|---|---|---|---|---|---|
| Endemic profile | 0·69 | ||||
| High intensity | 5 | 24·1 | –4·34, 52·4 | 99 % | |
| Moderate intensity | 12 | 28·6 | 9·8, 47·4 | 99 % | |
| Low intensity | 6 | 30 | 18·4, 41·7 | 99 % | |
| Malaria species | 0·06 | ||||
| P. falciparum | 21 | 26·6 | 14·2, 39 | 99 % | |
| P. vivax | 2 | 43·3 | 25·5, 61·1 | 66 % | |
| Age | 0·12 | ||||
| < 60 months | 12 | 30·6 | 16·8, 44·3 | 99 % | |
| > 60 months | 11 | 23·3 | 3·4, 43·2 | 99 % | |
| Diagnostic method | 0·51 | ||||
| Parasitaemia by microscopy | 20 | 25·1 | 14·5, 35·7 | 99 % | |
| RDT or microscopy | 2 | 57·7 | –38·7, 154 | 99 % | |
| Design of the study | 0·50 | ||||
| Cross-sectional | 20 | 26·9 | 13·9, 39·9 | 99 % | |
| Prospective | 3 | 34·7 | 12·9, 56·6 | 96 % |
RDT, rapid diagnostic test detecting histidine-rich protein 2.
Table 4.
Summary of meta-analyses results by biomarker in children and adults with malaria parasitaemia compared with control group
(Mean difference and 95 % confidence interval)
| All studies | Only studies with low or unclear risk of bias | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of datasets | Mean difference | 95 % CI | I2 for heterogeneity | P | Number of datasets | Mean difference | 95 % CI | I2 for heterogeneity | P | |
| Ferritin (µg/l) | ||||||||||
| Children | ||||||||||
| Asymptomatic malaria | 23 | 28·2 | 15·6, 40·9 | 99 % | <0·001 | 23 | 28·2 | 15·6, 40·9 | 99 % | <0·001 |
| Clinical malaria | 9 | 366 | 162, 570 | 91 % | 0·003 | 7 | 334 | 106, 563 | 92 % | 0·01 |
| Adults | ||||||||||
| Asymptomatic malaria | 4 | 28·5 | 8·1, 48·9 | 51 % | 0·02 | 3 | 20·5 | 4·8, 36·3 | 0 % | 0·03 |
| Clinical malaria | 5 | 493 | –219, 1206 | 83 % | 0·13 | 2 | 155 | –758, 1069 | 57 % | 0·28 |
| Pregnant women | 8 | 26·8 | 5·8, 47·7 | 100 % | 0·02 | 7 | 18·1 | 5·6, 30·7 | 99 % | 0·01 |
| Hepcidin (nmol/l) | ||||||||||
| Children | ||||||||||
| Asymptomatic malaria | 12 | 1·52 | 0·92, 2·11 | 96 % | <0·001 | 12 | 1·52 | 0·92, 2·11 | 96 % | <0·001 |
| Clinical malaria | 2 | 10·8 | –18·1, 39·7 | 0 % | 0·13 | 1 | 10·6 | 5·4, 15·8 | NA | <0·001 |
| Adults | ||||||||||
| Asymptomatic malaria | 2 | 0·3 | –10·3, 10·9 | 52 % | 0·78 | 2 | 0·3 | –10·3, 10·9 | 52 % | 0·78 |
| Clinical malaria | 2 | 6·3 | –21·6, 34·3 | 52 % | 0·21 | 2 | 6·3 | –21·6, 34·3 | 52 % | 0·21 |
| Retinol (µmol/l) | ||||||||||
| Children | ||||||||||
| Asymptomatic malaria | 4 | –0·11 | –0·22, −0·01 | 60 % | 0·04 | 3 | –0·10 | –0·18, −0·03 | 21 % | 0·03 |
| Clinical malaria | 6 | –0·43 | –0·71, −0·16 | 97 % | 0·01 | 4 | –0·36 | –0·77, 0·06 | 93 % | 0·07 |
| Adults | ||||||||||
| Clinical malaria | 5 | –0·73 | –1·11, −0·36 | 54 % | 0·005 | 1 | –1·03 | –1·40, −0·66 | NA | <0·001 |
| Pregnant women | 1 | –0·54 | –0·67, −0·41 | NA | <0·001 | 1 | –0·54 | –0·67, −0·41 | NA | <0·001 |
NA, non-applicable.
A greater ferritin concentration was also observed in non-pregnant adults with asymptomatic malaria (28·5 µg/l, 95 % CI 8·1, 48·8, P = 0·02) (Fig. 3). This mean difference was calculated for 234 adults with malaria and 400 control adults, from 4 studies conducted in sub-Saharan Africa in settings with moderate or high intensity of malaria transmission(47,51,55,57). Given the limited number of studies, we did not perform subgroup analyses. The heterogeneity was moderate (51 %) and the sensitivity analysis showed the stability of the pooled result (Supplemental Fig. 3, Supplemental Fig. 4 and Table 4)
Fig. 3.
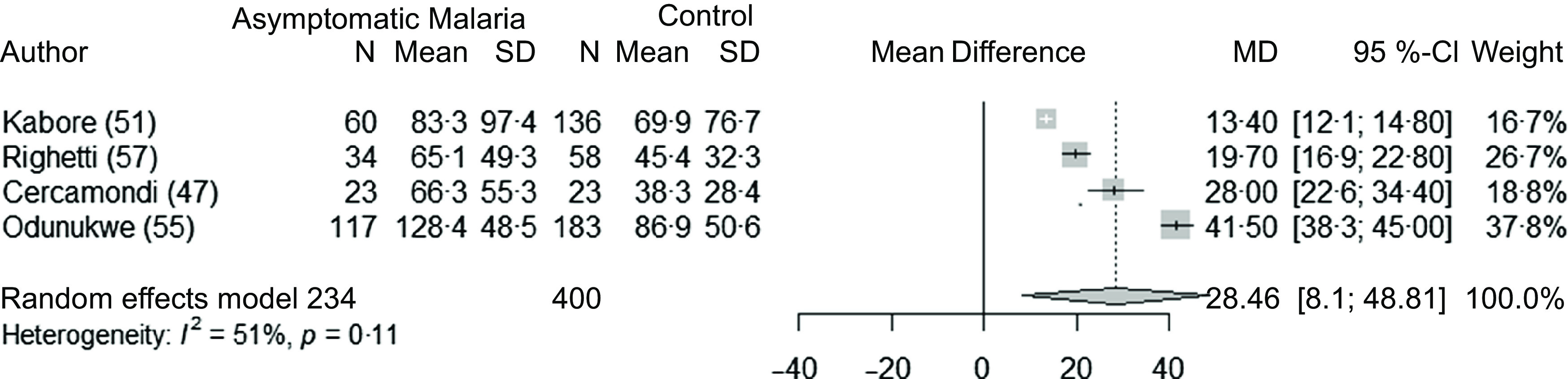
Forest plot for differences in ferritin concentrations (µg/l) between adults with asymptomatic malaria and control group using the random effect model. The grey squares represent the mean difference from each study, while the horizontal line represents the corresponding 95 % CI. The hollow diamond represents the overall pooled effects while the left and right points of the diamond represent the corresponding 95 % CI. MD, mean difference.
Clinical malaria and ferritin concentrations in children and adults
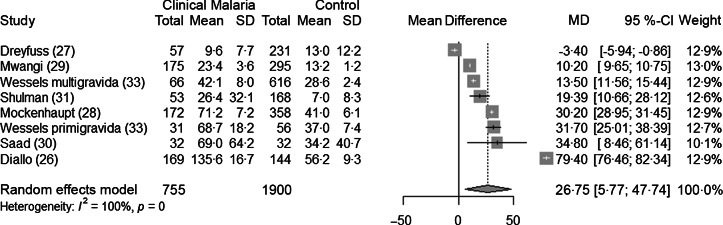
Seven studies(13,46,51,52,54,61,64) (nine datasets) analysed the association between clinical malaria and ferritin concentrations in children (595 children with clinical malaria and 876 healthy, control children). These studies were conducted in Africa, in Oceania and in the Americas. Overall, ferritin concentrations were 366 µg/l (95 % CI 162, 570) P < 0·003) greater in children with clinical malaria compared with control group (Fig. 4). The sub-group analyses showed that the difference in mean ferritin was the greatest in settings with moderate transmission, compared with low transmission (Table 5). The sensitivity analysis showed the stability of the pooled results after the leave-one-out analysis (Supplementary Fig. 5) and after removing the studies with high risk of bias (Table 4). The funnel plot revealed a publication bias for this analysis, with an underreporting of small studies (Supplementary Fig. 6).
Fig. 4.
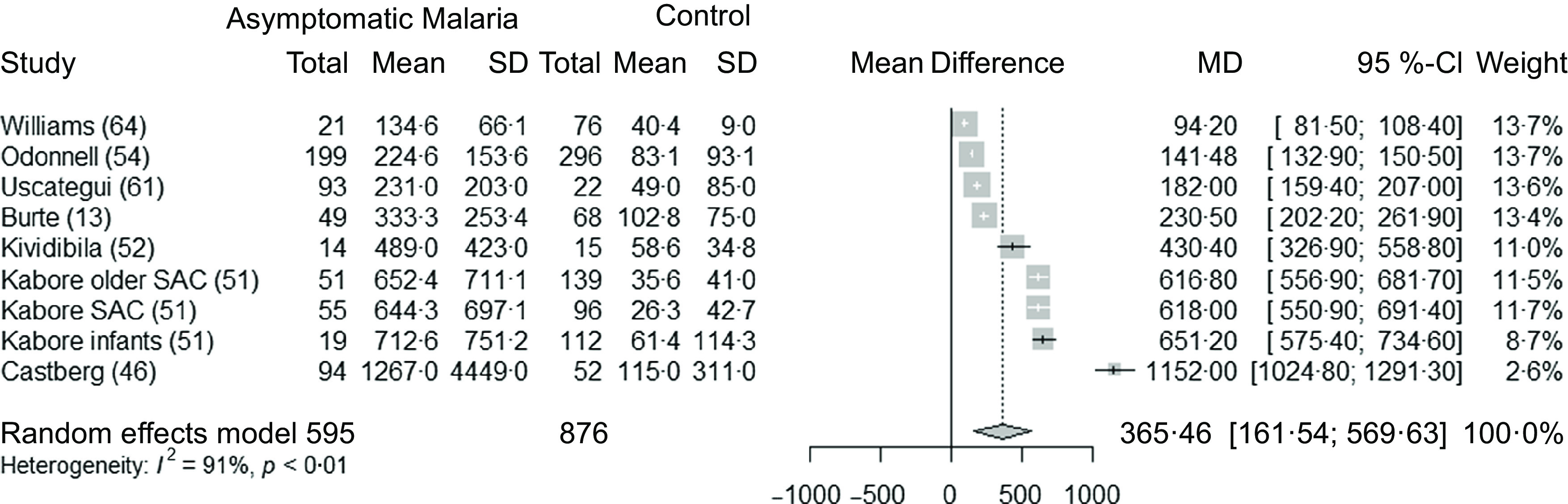
Forest plot for differences in ferritin concentrations (µg/l) in children between clinical malaria and control group using the random effect model. The grey squares represent the mean difference from each study, while the horizontal line represents the corresponding 95 % CI. The hollow diamond represents the overall pooled effects, while the left and right points of the diamond represent the corresponding 95 % CI. SAC, school age children; MD, mean difference.
Table 5.
Results of subgroup analyses for ferritin (µg/l) in children with clinical malaria parasitaemia and control group
(Mean difference and 95 % confidence interval)
| Number of datasets | Mean difference | 95 % CI | I2 for heterogeneity | P subgroup | |
|---|---|---|---|---|---|
| Endemic profile | <0·001 | ||||
| Moderate intensity | 5 | 688 | 457, 919 | 94 % | |
| Low intensity | 3 | 138 | 89, 188 | 96 % | |
| Age | 0·13 | ||||
| <60 months | 4 | 637 | 233, 1042 | 99 % | |
| >60 months | 5 | 304 | 119, 490 | 99 % | |
| Study design | 0·57 | ||||
| Cross-sectional | 7 | 389 | 209, 570 | 99 % | |
| Prospective | 2 | 665 | –290, 1620 | 99 % |
In terms of clinical malaria in adults, 269 adults were included in the analyses of 5 datasets, including an experimental infection and a study of imported cases in Europe. All the studies reported a higher ferritin concentration in the clinical malaria group (107 to 1554 µg/l elevation in the clinical group compared with the control group). In the meta-analysis, the CI was wide and included zero (493 µg/l, 95 % CI −219, 1206).
The elevation of ferritin concentrations in clinical malaria patients increased with greater severity of malaria in all studies that included this analysis, in adults and children (data not shown).
Malaria infection and hepcidin concentrations in children and adults
Seven studies(7,49,51,53) (twelve datasets) were used to analyse the association between asymptomatic malaria parasitaemia and hepcidin concentrations in children. The studies were mainly conducted in Africa apart from one study (four datasets) that was conducted in Indonesia. Hepcidin concentrations were 1·52 nmol/l (95 % CI 0·92, 2·11, P < 0·001) greater in malaria-infected groups compared with controls (Fig. 5). No interaction was reported in the subgroup analyses (Table 6). The heterogeneity was high (I2 = 96 %). The leave-one-out analysis and the sensibility analysis by risk of bias showed the stability of the pooled result (Supplementary Fig. 7 and Table 4). There was no significant publication bias, as assessed by the funnel plot and Egger’s test (Supplementary Fig. 8).
Fig. 5.
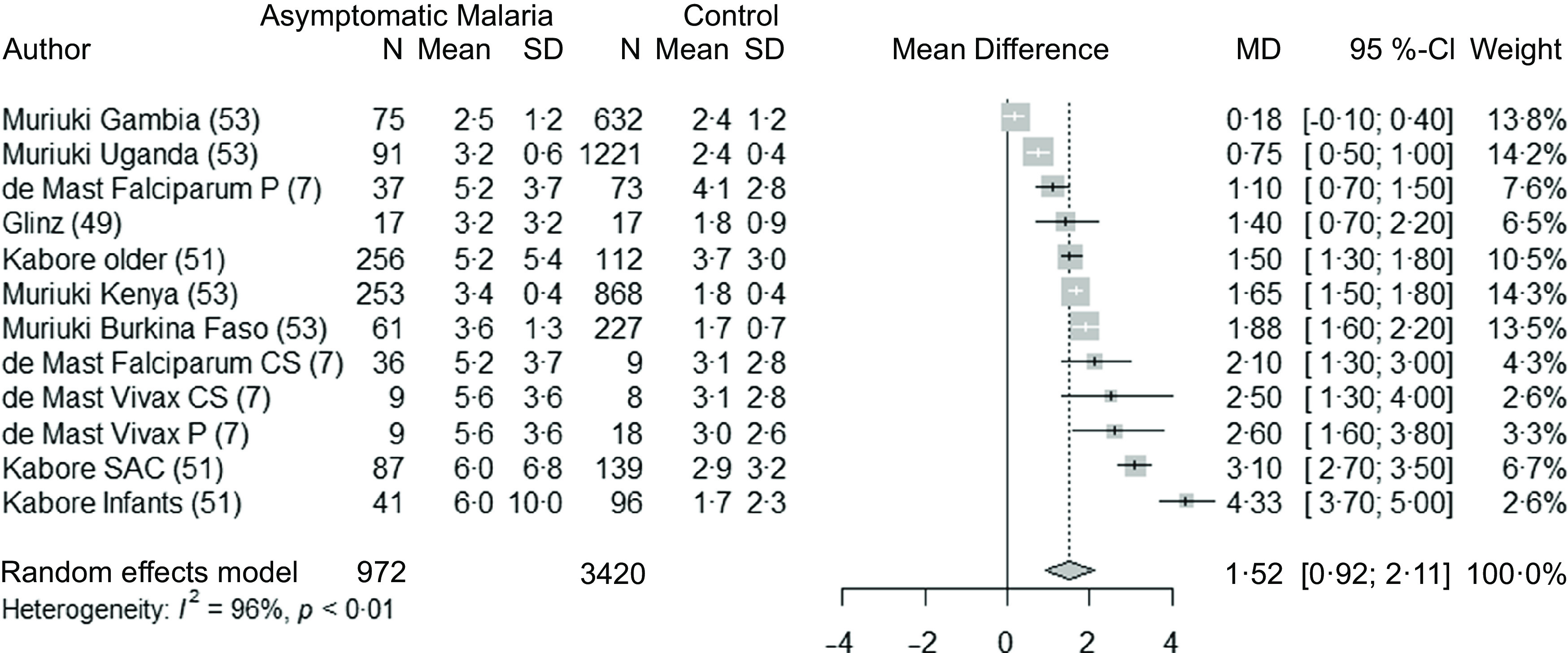
Forest plot for differences in hepcidin concentration (nmol/l) in children between malaria parasitaemia and control groups using the random effect model. The grey squares represent the mean difference from each study, while the horizontal line represents the corresponding 95 % CI. The hollow diamond represents the overall pooled effects, while the left and right points of the diamond represent the corresponding 95 % CI. SAC, school age children; P, prospective; CS, cross-sectional; MD, mean difference.
Table 6.
Results of subgroup analyses for hepcidin concentration (nmol/l) in children with asymptomatic malaria parasitaemia and control group
(Mean difference and 95 % confidence interval)
| Endemic profile | Number of datasets | Mean difference | 95 % CI | I 2 for heterogeneity | P subgroup |
|---|---|---|---|---|---|
| Moderate intensity | 6 | 1·86 | 0·62, 3·1 | 98 % | 0·88 |
| Low intensity | 5 | 1·77 | 1·25, 2·29 | 68 % | |
| Malaria species | 0·12 | ||||
| P. falciparum | 10 | 1·77 | 1·04, 2·5 | 97 % | |
| P. vivax | 2 | 2·56 | 2·46, 2·66 | 0 % | |
| Age | 0·54 | ||||
| <60 months | 6 | 1·95 | 0·74, 3·16 | 98 % | |
| >60 months | 6 | 1·66 | 1·21, 2·11 | 60 % | |
| Design of the study | 0·46 | ||||
| Cross-sectional | 9 | 1·95 | 1·14, 2·76 | 98 % | |
| Prospective | 3 | 1·58 | 0·73, 2·42 | 71 % |
Two studies(47,51) reported on asymptomatic malaria infection in adults and hepcidin concentrations, including 242 adults in total. There was a non-significant elevation in hepcidin in the malaria group of 0·3 nmol/l (95 % CI −10·3, 10·9). Two studies reported concentrations of hepcidin in clinical malaria infection in children(46,51) and reported a non-significant elevation in hepcidin of 10·8 nmol/l (95 % CI −18·1, 39·7) in the malaria group. In adults(48,51), there was also a non-significant elevation of hepcidin of 6·3 nmol/l (95 % CI −21·6, 34·3) in the malaria group.
Malaria infection and ferritin concentrations in pregnant women
Seven studies(26–31,33) analysed ferritin concentrations in pregnant women with or without malaria infection, as defined by a positive parasitaemia. The authors did not systematically report the presence of clinical symptoms. Pregnant women with malaria parasites had greater ferritin concentrations than control pregnant women without parasites (+26·8 µg/l, CI 5·8, 47·7, P = 0·02) (Fig. 6). The subgroup analysis revealed that the mean difference in ferritin was higher in settings with high malaria transmission (Table 7). Heterogeneity was high (I2 = 100 %). The sensitivity analysis showed the stability of the pooled result (Supplementary Fig. 9 and Supplementary Fig. 10). After excluding the study with high risk of bias, the difference in ferritin concentration was lower (+18·1 µg/l, 95 % CI 5·6, 30·7, P = 0·01) (Table 4).
Fig. 6.
Forest plot for differences in ferritin concentrations (µg/l) in pregnant women between malaria and control group using the random effect model. The grey squares represent the mean difference from each study, while the horizontal line represents the corresponding 95 % CI. The hollow diamond represents the overall pooled effects, while the left and right points of the diamond represent the corresponding 95 % CI. MD, mean difference.
Table 7.
Results of subgroup analyses for ferritin concentration (µg/l) in pregnant women with malaria parasitaemia and control group
(Mean difference and 95 % confidence interval)
| Endemic profile | Number of datasets | Mean difference | 95 % CI | I 2 for heterogeneity | P subgroup |
|---|---|---|---|---|---|
| High intensity | 2 | 54·8 | –258, 367 | 99 % | <0·001 |
| Moderate intensity | 2 | 22·3 | –93, 138 | 96 % | |
| Low intensity | 3 | 16·5 | –7·6, 40·6 | 74 % |
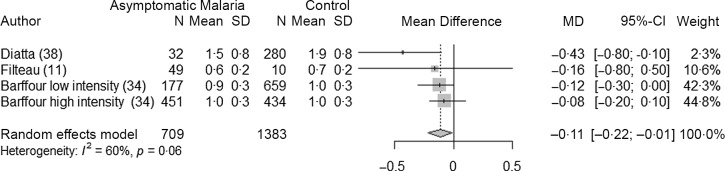
Asymptomatic malaria infection and retinol concentrations in children and adults
Three studies(11,34,38) (four datasets) analysed the association between malaria and retinol concentrations in asymptomatic children (709 children with malaria and 1383 control children). All studies were conducted in Africa and the species involved was always P. falciparum. Overall, retinol concentrations were lower, that is, −0·11 µmol/l (95 % CI −0·22, −0·01, P = 0·04) in children with asymptomatic malaria compared with control group (Fig. 7). We did not perform subgroup analyses because of the limited number of studies. The heterogeneity was moderate (I2 = 60 %) and the sensitivity analysis showed the stability of the pooled result after the leave-one-out analysis, and after removing the studies with high risk of bias (Supplementary Fig. 11, Supplementary Fig. 12 and Table 4).
Fig. 7.
Forest plot for differences in retinol concentration (µmol/l) in children between asymptomatic malaria and control group using the random effect model. The grey squares represent the mean difference from each study, while the horizontal line represents the corresponding 95 % CI. The hollow diamond represents the overall pooled effects, while the left and right points of the diamond represent the corresponding 95 % CI. MD, mean difference.
There were no studies on the associations between asymptomatic malaria and retinol concentrations in adults.
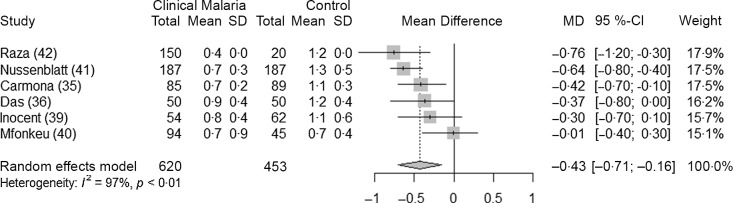
Clinical malaria infection and retinol concentrations in children and adults
Six studies(35,36,39–42) analysed the association between clinical malaria and retinol concentrations in children (620 children with malaria parasitaemia and 453 control children). The analysis showed that retinol concentrations were reduced during an infection (−0·43 µmol/l, 95 % CI −0·71, −0·16, P = 0·01) (Fig. 8). When the studies with high risk of bias were excluded, the mean difference in retinol concentration was no longer statistically significant (Table 4). There were no differences noted in the subgroup analyses (Table 8).
Fig. 8.
Forest plot for differences in retinol concentration (µmol/l) in children between clinical malaria and control group using the random effect model. The grey squares represent the mean difference from each study, while the horizontal line represents the corresponding 95 % CI. The hollow diamond represents the overall pooled effects, while the left and right points of the diamond represent the corresponding 95 % CI. MD, mean difference.
Table 8.
Results of subgroup analyses for retinol concentration (µmol/l) in children with clinical malaria parasitaemia and control group
(Mean difference and 95 % confidence interval)
| Number of datasets | Mean Difference | 95 % CI | I2 for heterogeneity | Psubgroup | |
|---|---|---|---|---|---|
| Endemic profile | 0·55 | ||||
| High intensity | 3 | –0·34 | –0·70, 0·03 | 80 % | |
| Low intensity | 3 | –0·47 | –0·68, −0·27 | 0 % | |
| Age | 0·9 | ||||
| < 60 months | 4 | –0·43 | –0·75, −0·1 | 74 % | |
| > 60 months | 2 | –0·40 | –0·45, −0·35 | 0 % |
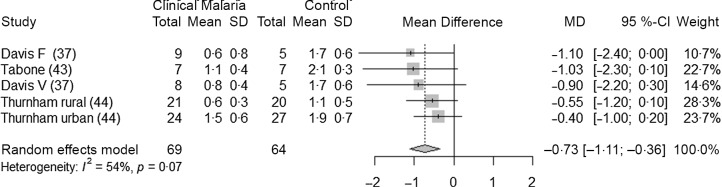
Three studies(37,43,44) (five datasets) analysed the association between clinical malaria and retinol concentrations in adults (69 adults with malaria and 64 control adults). Two of the studies were conducted in Asia, and they were both conducted in settings with very low intensity of transmission. One was conducted in Europe with imported cases, and the author did not specify the endemicity profile of the country of origin. Retinol concentrations were lower in adults with clinical malaria compared with healthy control adults, by 0·73 µmol/l (95 % CI −1·11, −0·36, P = 0·005) (Fig. 9). Due to the limited number of studies, we did not perform any subgroup analyses. The heterogeneity was moderate (I2 = 54 %). The leave-one-out analysis showed the stability of pooled results (Supplementary Fig. 13 and Supplementary Fig. 14). Only one study in this analysis was considered at low risk of bias (Table 4).
Fig. 9.
Forest plot for differences in retinol concentration (µmol/l) in adults between clinical malaria and control group using the random effect model. The grey squares represent the mean difference from each study, while the horizontal line represents the corresponding 95 % CI. The hollow diamond represents the overall pooled effects, while the left and right points of the diamond represent the corresponding 95 % CI. F, falciparum; V, vivax; MD, mean difference.
Only one study(32) reported data on retinol concentrations in pregnant women with malaria. They found a significantly reduced concentration of retinol in pregnant women with malaria (−0·54, 95 % CI −0·67, −0·41, P < 0·001).
Malaria infection and retinol binding protein concentrations in children and adults
One study(63) conducted in Burkina Faso with 262 children found that children with asymptomatic malaria had lower RBP values than the control group, and the mean difference was −0·13 (95 % CI −.17, −0·09, P < 0·001). One study(36) conducted in India with 100 children presented data on RBP concentration in children with clinical malaria and found that children with clinical malaria had lower RBP values than the control group and the mean difference was −1·52 (95 % CI −1·70, −1·35, P < 0·001).
Only one study reported RBP data in adults during clinical malaria(43), and the sample size was too small to report the data (seven patients).
Discussion
We conducted several meta-analyses to estimate associations between malaria infection and nutrition biomarkers by using data from cross-sectional and prospective studies. Although mostly based on data from observational studies, our analyses indicate that malaria infection is associated with increased ferritin and reduced retinol concentrations even in asymptomatic infections, when individuals might not have elevated markers of inflammation.
Association between malaria and iron indicators
The results provide strong and consistent evidence that malaria infection, asymptomatic or symptomatic, is associated with increased ferritin concentrations in children. This result was expected, as ferritin synthesis is highly upregulated by inflammatory cytokines and by infections including malaria(53,66,67). In our analyses, the increase in ferritin concentration during an asymptomatic infection was similar in children and adults, and there were no differences noted in the subgroup analyses. The quality of the evidence for children seems strong as the sample size for analysis is large, the CI is relatively small and the sensitivity analysis did not reveal any significant influencer in the results or publication bias. Moreover, studies included in this analysis were considered of good quality based on the risk of bias analysis. The increase in ferritin concentration did not vary by age group. For residents in malaria-endemic areas, parasitaemia peaks in children younger than 5 years old and subsequently declines in an age-dependent manner(68). In populations living under heavy exposure to malaria and frequent infection, individuals tend to develop partial immunity against the disease earlier in childhood, which may explain why children under 5 years old did not have a greater increase in ferritin than older children. However, this interpretation is limited by the fact that age groups were not particularly well defined and therefore, there might have been differences between age groups that we were not able to observe.
Overall, in the case of an asymptomatic malaria infection, the average increase in ferritin concentration is estimated at c. 28·5 µg/l, across all settings and all groups of population. Considering that the mean value of ferritin concentration in control groups was c. 25 µg/l, this indicates that the ferritin concentration in asymptomatic malaria infection was approximately doubled compared with a control group. As a comparison, based on sixteen datasets included as part of the BRINDA collaboration, Namaste and colleagues have assessed the increase in ferritin concentrations during an inflammation process, based on elevated CRP and/or AGP(66); both markers are elevated in large proportions of children in low- and middle-income countries(69). This definition is imperfect, as inflammation is a complex process than cannot be captured simply by the elevation of these two acute-phase proteins. However, in the absence of other widely available biomarkers of inflammation in population-based surveys, we continue to rely on a definition on inflammation based on these two markers. Namaste reported that ferritin concentration increases from 19·5 µg/l in the reference group to 50·8 µg/l during the early convalescence phase of an inflammatory episode, which in this analysis was defined as when CRP and AGP concentrations are at their highest(66). This represents an increase of about 30 µg/l, which is in the same range as the increase we see during a malaria asymptomatic infection. For women of reproductive age, they report an increase in the same range (c. 30 µg/l).
The recent WHO guidelines on the use of ferritin concentrations to define iron status recommend adjusting for inflammation and indicate that it is possible to adjust for malaria. There are, however, currently no details on why this adjustment should be made or whether some specificities, such as the severity of the infection, the population group or the endemic profile should be considered. An important question for micronutrient surveys is whether elevated acute-phase proteins fully capture the effects of malaria on micronutrient markers, as might be assumed from the similar differences in ferritin due to malaria or CRP plus AGP, or whether we should account for both inflammation and malaria. Several studies included in this review report that not all children with asymptomatic malaria have elevated CRP or AGP. In the study in Burkina Faso in children with asymptomatic malaria, Barffour reported that only half of the children with malaria also had elevated AGP during low malaria season, based on AGP concentrations >1 g/l(10). Righetti found similar results in Cote d’Ivoire. Among children 6–8 years old with asymptomatic malaria, 55 % had neither CRP concentration > 5 mg/l nor AGP concentration > 1g/l(57). In non-pregnant women, this proportion was even higher (65 %). Similarly, de Mast found low circulating concentrations of CRP in Indonesian schoolchildren with asymptomatic parasitaemia, and 84 % of them had CRP concentrations < 5 mg/l, the threshold to define inflammation(7). These findings could be attributed to the use of thresholds for CRP and AGP and might mask a mild elevation in inflammatory markers. Several studies found that even after adjusting for raised CRP and/or AGP with the regression method, ferritin concentrations were higher in children suffering from asymptomatic malaria than in the control group(63,70). In children 6–23 months old in Burkina Faso, Wessells found that after adjusting for acute-phase proteins, children with asymptomatic malaria had greater plasma ferritin concentrations than the control group (23·5 ± 1·5 µg/l v. 11·1 ± 0·8 µg/l; P < 0·001)(63). Muriuki found that children with malaria had greater ferritin concentrations at every decile of CRP, compared with those without malaria. Even in the lowest decile of CRP, the difference in ferritin between children with malaria and without malaria was of about 20 µg/l. They found that malaria parasitaemia also increased ferritin levels independently of increased CRP and/or AGP in multivariable analyses(53). In longitudinal studies looking at ferritin concentrations and inflammatory markers concentrations after a malaria infection, it is notable that, even if CRP and AGP concentrations are slightly elevated during an asymptomatic infection, their concentrations go back to normal rapidly once the malaria infection is cleared, while ferritin concentrations remain elevated for about 1 month after the infection(47,49). Considering this, we can assume that individuals with an asymptomatic malaria infection are either not suffering from inflammation or have elevated CRP and AGP for a period of time that is shorter than the time needed for their ferritin concentration to return to normal.
The highest increase in ferritin is unsurprisingly seen in clinical malaria, even though we could not reach a conclusion on clinical infection and ferritin in adults, due to the high heterogeneity and the small number of adults included. In children, the highest increase in ferritin was observed in the countries with a moderate parasite rate. In pregnancy, there was also a significant increase in ferritin concentrations in pregnant women with malaria, of c. 27 µg/l. The difference in mean concentration was greater in settings with high transmission intensity.
Hepcidin concentrations were also increased during malaria infections. As for the ferritin data, these datasets included children with and without raised CRP or AGP, and we can assume that the increase in hepcidin concentration might be occurring through both an inflammatory(49) and a non-inflammatory pathway, as suggested previously(14,53). Hepcidin reduces iron absorption from the gut and increases iron sequestration, resulting in a decrease of iron in the blood and a decrease in erythropoiesis(49). Considering the high proportion of the population suffering from asymptomatic malaria infections in sub-Saharan Africa, this increase in hepcidin concentration could help to explain why iron deficiency prevalence remains high in population surveys and why iron supplementation and iron fortification programmes have been less effective than expected(71).
Associations between malaria and indicators of vitamin A deficiency
Children with an asymptomatic infection had lower values of serum retinol than the control group. In case of a clinical infection, the reduction was greater. In adults, lower values of serum retinol were also observed in case of a clinical infection. The reductions we observed in children and adults with a clinical infection (respectively 0·43 and 0·73 µmol/l) were greater than the reductions in retinol due to inflammation defined by elevated CRP and/or AGP, reported by the BRINDA collaboration. The BRINDA collaboration reported that inflammation reduces retinol by 0·27 µmol/l in preschool children and by 0·24 µmol/l in women of reproductive age(66). However, the populations are different as BRINDA is more likely to include datasets coming from healthy participants while in these specific analyses, patients were ill and hospitalised due to malaria. Also, in these analyses, not all endemic profiles were represented. These results need to be interpreted with caution as the sample size for these analyses were small. The acute-phase response to either infection or inflammation affects retinol homoeostasis, and substantial vitamin A can be lost in the urine during illness accompanied by high fever(3). There may also be increased tissue retinol uptake due to increased needs for retinol in infection which could decrease plasma retinol concentrations(44). An alternative explanation is that sick children tend to eat less, and malaria could lead to vitamin A deficiency in children who already had low reserves of vitamin A(72).
Implications for large-scale surveys interpretation
The magnitude of change in ferritin and retinol concentrations in the case of an asymptomatic infection is likely to affect the estimations of iron and vitamin A deficiency in population-based surveys. Asymptomatic individuals are often present in these surveys. They are likely to experience an increase in ferritin without a significant elevation of CRP and AGP values, and therefore their ferritin values would not be adjusted by the BRINDA method as it is recommended by WHO. With regard to retinol, even if BRINDA has recommended to adjust for inflammation in children, WHO does not currently recommend any adjustment. Children and adults with asymptomatic infection could be wrongly considered as iron replete or vitamin A-deficient, and the validity of the deficiency prevalence estimates could be affected. Adjusting for malaria is only referred to as a ‘possible adjustment’ in the WHO guidelines on ferritin, and there is no mention of asymptomatic infections. However, we observed that ferritin concentrations were elevated by 28·2 µg/l in asymptomatic children and by 366 µg/l in children with clinical malaria compared with healthy children. Retinol concentrations were reduced by 0·11 µmol/l in asymptomatic children and by 0·43 µmol/l in children with clinical malaria. These data suggest an important difference according to the severity of infection, and this could have important repercussions in the assessment of iron and vitamin A status in populations where different forms of malaria infections co-exist. Even if individuals with clinical malaria are not likely to be included in surveys, people recovering from clinical malaria might be. Applying a single correction factor to all forms of malaria, as is currently recommended in the WHO guidelines, would certainly affect estimates coming from micronutrient surveys and other surveys that assess the iron status of a sample of a population in a malaria endemic setting. More research should be done to confirm whether the study setting should be considered when applying an adjustment, as our data seem to indicate that a clinical infection could have different repercussions on ferritin concentrations depending on the endemic profile. We did not have enough datasets to analyse infections with P. vivax, and it requires further research. In our analyses, the use of different malaria diagnostic methods did not seem to impact the magnitude of the effects.
Limitations
The primary limitation to these analyses is the variability between studies, including the large age range among children in some of the datasets. Another limitation is the population used as a control group, as most studies had different strategies to recruit their control group. We did not investigate whether the children were suffering from hookworm or Schistosoma infections, which could have affected further their iron status. Another limitation is the generalisability of the results related to clinical infections. Most of the studies including clinical infections were conducted at the hospital, whereas in population-based surveys conducted to measure the micronutrient status of a population, participants would be sampled in the community. We also acknowledge that our search strategy, despite being large and inclusive of three languages of publication, did not include grey literature or regional databases, which might have introduced a selection bias in the systematic review. Finally, although there were many studies on the association of ferritin and malaria in children, fewer studies were included in other meta-analyses, and these included studies had higher risk of bias, suggesting caution is required in interpreting these results.
Conclusion
The findings of this systematic review and meta-analysis suggest that malaria infection should be measured and adjusted for in nutritional surveys of populations living in malaria endemic areas, particularly for assessments of iron status. Malaria test results should be reported in population-based surveys, as well as a measure of clinical symptoms in the participant. This will allow more accurate adjustment of serum ferritin concentrations to define individual iron status. Preliminary analyses indicate that retinol concentration also is affected by malaria, but not enough data are currently available to support firm conclusions for children and adults. Further research is needed to develop individualised adjustment methods that can take into account the concentrations of acute-phase proteins and the presence, and severity, of a malaria infection.
Acknowledgements
The authors would like to thank Dr Sujit Rathod for his help regarding statistical methods in epidemiology.
This work was supported, in whole or in part, by the Bill & Melinda Gates Foundation INV-002855. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission.
FS, EJMJ, SF and HH conceptualised the analytical protocol. FS and LSR conducted the literature search. FS conducted the study data analyses with the support of AMDFS was the primary writer. All authors reviewed the content of the manuscript and provided feedback. None of the authors have any conflicts of interest to declare.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0007114522000757.
click here to view supplementary material
References
- 1. Bailey RL, West KP & Black RE (2015) The epidemiology of global micronutrient deficiencies. Ann Nutr Metab 66, 22–33. [DOI] [PubMed] [Google Scholar]
- 2. Suchdev PS, Williams AM, Mei Z, et al. (2017) Assessment of iron status in settings of inflammation: challenges and potential approaches. Am J Clin Nutr 106, 1626S–1633S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tanumihardjo SA, Russell RM, Stephensen CB, et al. (2016) Biomarkers of Nutrition for Development (BOND) – vitamin A review. J Nutr 146, 1816S–1848S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Victora CG, Christian P, Vidaletti LP, et al. (2021) Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet 397, 1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO (2020) World Malaria Report 2020:20 Years of Global Progress and Challenges. Geneva: WHO. [Google Scholar]
- 6. Northrop-Clewes CA (2008) Interpreting indicators of iron status during an acute phase response--lessons from malaria and human immunodeficiency virus. Ann Clin Biochem 45, 18–32. [DOI] [PubMed] [Google Scholar]
- 7. de Mast Q, Syafruddin D, Keijmel S, et al. (2010) Increased serum hepcidin and alterations in blood iron parameters associated with asymptomatic P. falciparum and P. vivax malaria. Haematologica 95, 1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mbanefo A & Kumar N (2020) Evaluation of malaria diagnostic methods as a key for successful control and elimination programs. Trop Med Infect Dis 5, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. WHO (2020) WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations. Geneva: WHO. [PubMed] [Google Scholar]
- 10. Barffour MA, Schulze KJ, Coles CL, et al. (2018) Malaria exacerbates inflammation-associated elevation in ferritin and soluble transferrin receptor with only modest effects on iron deficiency and iron deficiency anaemia among rural Zambian children. Trop Med Int Health 23, 53–62. [DOI] [PubMed] [Google Scholar]
- 11. Filteau SM, Morris SS, Abbott RA, et al. (1993) Influence of morbidity on serum retinol of children in a community-based study in northern Ghana. Am J Clin Nutr 58, 192–197. [DOI] [PubMed] [Google Scholar]
- 12. Larson LM, Addo OY, Sandalinas F, et al. (2017) Accounting for the influence of inflammation on retinol-binding protein in a population survey of Liberian preschool-age children. Matern Child Nutr 13, e12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burte F, Brown BJ, Orimadegun AE, et al. (2013) Circulatory hepcidin is associated with the anti-inflammatory response but not with iron or anemic status in childhood malaria. Blood 121, 3016–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Atkinson SH, Uyoga SM, Armitage AE, et al. (2015) Malaria and Age Variably but Critically Control Hepcidin Throughout Childhood in Kenya. EBioMedicine 2, 1478–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Malaria Atlas Project (2021) Explore Global Malaria Data Using our Custom Mapping Tools. https://malariaatlas.org (accessed November 2021).
- 16. WHO (2018) Malaria Surveillance, Monitoring & Evaluation: a Reference Manual. Geneva: WHO. [Google Scholar]
- 17. Higgins JPT, Thomas J, Chandler J, et al. (2021) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. https://training.cochrane.org/handbook/current (accessed February 2022).
- 18. Ma LL, Wang YY, Yang ZH, et al. (2020) Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res 7, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JP, White IR & Anzures-Cabrera J (2008) Meta-analysis of skewed data: combining results reported on log-transformed or raw scales. Stat Med 27, 6072–6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DerSimonian R & Laird N (2015) Meta-analysis in clinical trials revisited. Contemp Clin Trials 45, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Viechtbauer W & Cheung MW (2010) Outlier and influence diagnostics for meta-analysis. Res Synth Methods 1, 112–125. [DOI] [PubMed] [Google Scholar]
- 22. R Core Team (2020) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
- 23. Harrer M, Cuijpers P, Furukawa T, et al. (2019) dmetar: Companion R Package for the Guide ‘Doing Meta-Analysis in R’. R Package Version 009000. http://dmetar.protectlab.org/ (accessed November 2021).
- 24. Balduzzi S, Rücker G & Schwarzer G (2019) How to perform a meta-analysis with R: a practical tutorial, Evidence-Based Ment Health 22, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suurmond R, van Rhee H & Hak T (2017) Introduction, comparison, and validation of meta-essentials: a free and simple tool for meta-analysis. Res Synth Meth 8, 537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Diallo S, Roberts SA, Gies S, et al. (2020) Malaria early in the first pregnancy: potential impact of iron status. Clin Nutr 39, 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dreyfuss ML, Stoltzfus RJ, Shrestha JB, et al. (2000) Hookworms, malaria and vitamin A deficiency contribute to anemia and iron deficiency among pregnant women in the plains of Nepal. J Nutr 130, 2527–2536. [DOI] [PubMed] [Google Scholar]
- 28. Mockenhaupt FP, Rong B, Gunther M, et al. (2000) Anemia in pregnant ghanaian women: importance of malaria, iron deficiency and haemoglobinopathies. Trans R Soc Trop Med Hyg 94, 477–483. [DOI] [PubMed] [Google Scholar]
- 29. Mwangi MN, Echoka E, Knijff M, et al. (2019) Iron status of kenyan pregnant women after adjusting for inflammation using BRINDA regression analysis and other correction methods. Nutrients 11, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saad AA, Mohamed OE, Ali AA, et al. (2012) Acute-phase proteins in pregnant Sudanese women with severe Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg 106, 570–572. [DOI] [PubMed] [Google Scholar]
- 31. Shulman CE, Graham WJ, Jilo H, et al. (1996) Malaria is an important cause of anaemia in primigravidae: evidence from a district hospital in coastal Kenya. Trans R Soc Trop Med Hyg 90, 535–539. [DOI] [PubMed] [Google Scholar]
- 32. Stuetz W, McGready R, Cho T, et al. (2005) Relation of DDT residues to plasma retinol, α -tocopherol, and beta-carotene during pregnancy and malaria infection: a case-control study in Karen women in northern Thailand. Sci Total Environ 363, 78–86. [DOI] [PubMed] [Google Scholar]
- 33. Wessells KR, Ouedraogo CT, Young RR, et al. (2017) Micronutrient status among pregnant women in zinder, niger and risk factors associated with deficiency. Nutrients 9, 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barffour MA, Schulze KJ, Coles CL, et al. (2018) Comparability of inflammation-adjusted vitamin a deficiency estimates and variance in retinol explained by C-reactive protein and alpha1 - acid glycoprotein during low and high malaria transmission seasons in rural Zambian children. Am J Trop Med Hyg 98, 334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carmona-Fonseca J, Botero AMC, Penuela RMU (2008) State of the skin, mucous membranes, hair and vitamin A in children of endemic malaria zones of Antioquia (Colombia). Iatreia 21, 21–32.
- 36. Das BS, Thurnham DI & Das DB (1996) Plasma α-tocopherol, retinol, and carotenoids in children with falciparum malaria. Am J Clin Nutr 64, 94–100. [DOI] [PubMed] [Google Scholar]
- 37. Davis TM, Garcia-Webb P, Fu LC, et al. (1993) Antioxidant vitamins in acute malaria. Trans R Soc Trop Med Hyg 87, 596–597. [DOI] [PubMed] [Google Scholar]
- 38. Diatta A, Cisse F, Traore S, et al. (2013) Malaria as an underlying cause of Hypovitaminosis a: results of HPLC retinol assessment among preschool children in Senegal rural areas. Int Res J Biochem Bioinform 3, 71–74. [Google Scholar]
- 39. Inocent G, Gustave LL, Issa TS, et al. (2007) Influence of malaria on the serum levels of vitamin A, zinc and calcium of children in Douala-Cameroon. Afr J Biotechnol 6, 871–876. [Google Scholar]
- 40. Mfonkeu JB, Gouado I, Kuate HF, et al. (2010) Biochemical markers of nutritional status and childhood malaria severity in Cameroon. Br J Nutr 104, 886–892. [DOI] [PubMed] [Google Scholar]
- 41. Nussenblatt V, Mukasa G, Metzger A, et al. (2002) Relationship between carotenoids and anaemia during acute uncomplicated Plasmodium falciparum malaria in children. J Health Popul Nutr 20, 205–214. [PubMed] [Google Scholar]
- 42. Raza A, Khan HM, Malik MA, et al. (2009) Serum retinol concentration in patients with acute falciparum malaria in Aligarh, India. J Infect Dev Ctries 3, 865–868. [PubMed] [Google Scholar]
- 43. Tabone MD, Muanza K, Lyagoubi M, et al. (1992) The role of interleukin-6 in vitamin A deficiency during Plasmodium falciparum malaria and possible consequences for vitamin A supplementation. Immunol 75, 553–554. [PMC free article] [PubMed] [Google Scholar]
- 44. Thurnham DI & Singkamani R (1991) The acute phase response and vitamin A status in malaria. Trans R Soc Trop Med Hyg 85, 194–199. [DOI] [PubMed] [Google Scholar]
- 45. Beesley R, Filteau S, Tomkins A, et al. (2000) Impact of acute malaria on plasma concentrations of transferrin receptors. Trans R Soc Trop Med Hyg 94, 295–298. [DOI] [PubMed] [Google Scholar]
- 46. Castberg FC, Sarbah EW, Koram KA, et al. (2018) Malaria causes long-term effects on markers of iron status in children: a critical assessment of existing clinical and epidemiological tools. Malar J 17, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cercamondi CI, Egli IM, Ahouandjinou E, et al. (2010) Afebrile Plasmodium falciparum parasitemia decreases absorption of fortification iron but does not affect systemic iron utilization: a double stable-isotope study in young Beninese women. Am J Clin Nutr 92, 1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Mast Q, van Dongen-Lases EC, Swinkels DW, et al. (2009) Mild increases in serum hepcidin and interleukin-6 concentrations impair iron incorporation in haemoglobin during an experimental human malaria infection. Br J Haematol 145, 657–664. [DOI] [PubMed] [Google Scholar]
- 49. Glinz D, Hurrell RF, Righetti AA, et al. (2015) In Ivorian school-age children, infection with hookworm does not reduce dietary iron absorption or systemic iron utilization, whereas afebrile Plasmodium falciparum infection reduces iron absorption by half. Am J Clin Nutr 101, 462–470. [DOI] [PubMed] [Google Scholar]
- 50. Jeremiah ZA, Uko EK, Buseri FI, et al. (2009) Malarial iron-deficiency anaemia among asymptomatic Nigerian children. J Nutr Environ Med 16, 232–241. [Google Scholar]
- 51. Kabore B, Post A, Berendsen MLT, et al. (2020) Red blood cell homeostasis in children and adults with and without asymptomatic malaria infection in Burkina Faso. PLOS ONE 15, e0242507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kivibidila S, Warrier RP, Ode D, et al. (1999) Lack of difference in iron status assessed by soluble transferrin receptor between children with cerebral malaria and those with non-cerebral malaria. J Trop Pediatr 45, 166–167. [DOI] [PubMed] [Google Scholar]
- 53. Muriuki JM, Mentzer AJ, Webb EL, et al. (2020) Estimating the burden of iron deficiency among African children. BMC Med 18, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. O’Donnell A, Fowkes FJ, Allen SJ, et al. (2009) The acute phase response in children with mild and severe malaria in Papua New Guinea. Trans R Soc Trop Med Hyg 103, 679–686. [DOI] [PubMed] [Google Scholar]
- 55. Odunukwe NN, Salako LA, Okanny C, et al. (2001) Serum ferritin and other haematological measurements in apparently healthy children with malaria parasitaemia in Lagos, Nigeria. West Afr J Med 20, 42–45. [PubMed] [Google Scholar]
- 56. Phillips RE, Looareesuwan S, Warrell DA, et al. (1986) The importance of anaemia in cerebral and uncomplicated falciparum malaria: role of complications, dyserythropoiesis and iron sequestration. Q J Med 58, 305–323. [PubMed] [Google Scholar]
- 57. Righetti AA, Wegmuller R, Glinz D, et al. (2013) Effects of inflammation and Plasmodium falciparum infection on soluble transferrin receptor and plasma ferritin concentration in different age groups: a prospective longitudinal study in Cote d’Ivoire. Am J Clin Nutr 97, 1364–1374. [DOI] [PubMed] [Google Scholar]
- 58. Seyrek A, Kocyigit A & Erel O (2004) Essential trace elements selenium, zinc, copper, and iron concentrations and their related acute-phase proteins in patients with vivax malaria. Biol Trace Elem Res 106, 107–115. [DOI] [PubMed] [Google Scholar]
- 59. Stoltzfus RJ, Chwaya HM, Albonico M, et al. (1997) Serum ferritin, erythrocyte protoporphyrin and hemoglobin are valid indicators of iron status of school children in a malaria-holoendemic population. J Nutr 127, 293–298. [DOI] [PubMed] [Google Scholar]
- 60. Stoltzfus RJ, Chwaya HM, Montresor A, et al. (2000) Malaria, hookworms and recent fever are related to anemia and iron status indicators in 0- to 5-year old Zanzibari children and these relationships change with age. J Nutr 130, 1724–1733. [DOI] [PubMed] [Google Scholar]
- 61. Uscategui RM, Correa AM & Carmona-Fonseca J (2009) Changes in retinol, hemoglobin and ferritin concentrations in Colombian children with malaria. Biomedica 29, 270–281. [PubMed]
- 62. Verhoef H, West CE, Ndeto P, et al. (2001) Serum transferrin receptor concentration indicates increased erythropoiesis in Kenyan children with asymptomatic malaria. Am J Clin Nutr 74, 767–775. [DOI] [PubMed] [Google Scholar]
- 63. Wessells KR, Hess SY, Ouedraogo ZP, et al. (2014) Asymptomatic malaria infection affects the interpretation of biomarkers of iron and vitamin A status, even after adjusting for systemic inflammation, but does not affect plasma zinc concentrations among young children in Burkina Faso. J Nutr 144, 2050–2058. [DOI] [PubMed] [Google Scholar]
- 64. Williams TN, Maitland K, Rees DC, et al. (1999) Reduced soluble transferrin receptor concentrations in acute malaria in Vanuatu. Am J Trop Med Hyg 60, 875–878. [DOI] [PubMed] [Google Scholar]
- 65. Gies S, Roberts SA, Diallo S, et al. (2021) Risk of malaria in young children after periconceptional iron supplementation. Matern Child Nutr 17, e13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Namaste SM, Aaron GJ, Varadhan R, et al. (2017) Methodologic approach for the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. (Review). Am J Clin Nutr 106, 333–347S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. de Mast Q, Nadjm B, Reyburn H, et al. (2008) Assessment of urinary concentrations of hepcidin provides novel insight into disturbances in iron homeostasis during malarial infection. J Infect Dis 199, 253–262. [DOI] [PubMed] [Google Scholar]
- 68. Doolan DL, Dobano C & Baird JK (2009) Acquired immunity to malaria. Clin Microbiol Rev 22, 13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Merrill RD, Burke RM, Northrop-Clewes CA, et al. (2017) Factors associated with inflammation in preschool children and women of reproductive age: biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr 106, 348S–358S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Grant FK, Suchdev PS, Flores-Ayala R, et al. (2012) Correcting for inflammation changes estimates of iron deficiency among rural Kenyan preschool children. J Nutr 142, 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rohner F, Zimmermann MB, Amon RJ, et al. (2010) In a randomized controlled trial of iron fortification, anthelmintic treatment, and intermittent preventive treatment of malaria for anemia control in Ivorian children, only anthelmintic treatment shows modest benefit. J Nutr 140, 635–641. [DOI] [PubMed] [Google Scholar]
- 72. Sanjoaquin MA & Molyneux ME (2009) Malaria and vitamin A deficiency in African children: a vicious circle? Malar J 8, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Atkinson SH, Rockett K, Sirugo G, et al. (2006) Seasonal childhood anaemia in West Africa is associated with the haptoglobin 2–2 genotype. PLoS Med 3, e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tiono AB, Nebie I, Anagnostou N, et al. (2018) First field efficacy trial of the ChAd63 MVA ME-TRAP vectored malaria vaccine candidate in 5–17 months old infants and children. PLOS ONE 13, e0208328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bejon P, Williams TN, Liljander A, et al. (2010) Stable and unstable malaria hotspots in longitudinal cohort studies in Kenya. PLoS Med 7, e1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Elliott AM, Kizza M, Quigley MA, et al. (2007) The impact of helminths on the response to immunization and on the incidence of infection and disease in childhood in Uganda: design of a randomized, double-blind, placebo-controlled, factorial trial of deworming interventions delivered in pregnancy and early childhood (ISRCTN32849447). Clin Trials 4, 42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0007114522000757.
click here to view supplementary material