Abstract
Very little is known about how the host genome influences the composition of the gastrointestinal flora, largely due to the great number and diversity of bacteria present in the flora and the difficulties of using traditional methods of bacterial isolation and identification. We have approached the problem by studying bacterium-derived cellular fatty acids in the stool samples of six mouse strains congenic for the major histocompatibility complex (MHC). The results obtained indicate that the composition of the fecal flora is genetically regulated. In addition to undefined gene loci, MHC alone has a pronounced effect, since mice with different MHC in the same background have significantly different fecal floras. Demonstration of the genetic influence on the gastrointestinal flora opens a new approach to studying the pathogenesis of bacterially induced diseases.
Gastrointestinal flora plays an important role in the health and disease of the host. It is a complex ecosystem with great metabolic activity, consisting in humans of 1.0 to 1.5 kg of bacterial mass, 1011 to 1012 individual bacteria per g, and 400 to 500 different species (14, 17, 18, 36). Most of the bacterial species in the gut are anaerobic, and many of them have not been identified, due to insensitivity of bacterial cultures. The composition of the intestinal flora remains stable over long periods of time, but differences between individuals may be significant, even for those living in close proximity. Several exogenous factors contribute to the stability and changes of the flora. Its content is known to be affected by diet, medical treatment, and stress. Also, in several disease conditions, including Crohn's disease and rheumatoid arthritis, an altered intestinal flora has been reported (5, 9, 14). The only available evidence for a role of other endogenic factors comes from a twin study; by using anaerobic bacterial cultures, it has been shown that fecal floras of monozygotic twins are more similar with each other than those of dizygotic twins (38). Recently we have demonstrated that mice of different inbred strains differ in composition of the fecal flora (37a).
The traditional methods of studying gastrointestinal flora include isolation, identification, and enumeration of different bacterial species. The enormous amount of various bacteria makes isolation and identification of the different species, even in a single stool sample, an extremely laborious task, demanding at least 1 person-year of laboratory work. Even then, the classical culture methods are insensitive, difficult to interpret, and poorly reproducible (35). Some intestinal bacteria do not grow in vitro. Some grow only on selective media, and many prevent growth of other species, making reproducibility of the traditional methods quite poor. For these reasons, classical bacteriological techniques are unsuitable for studies of fecal microecology (7).
Instead of focusing on specific bacterial species, we have used another approach to detect overall differences in the gut flora. This has been made possible by computerized comparison of bacterial cellular fatty acid (CFA) profiles produced by gas-liquid chromatography (GLC) directly from the stool samples. The CFAs are structural components of bacterial cell membranes. They are nonvolatile and contain long-chain fatty acids with a typical composition for each bacterial species (3, 10, 19, 22). Thus, the CFA profile of a stool sample represents all bacteria present in the sample. The GLC method is especially useful when the number of samples is large. It has proved to be considerably more sensitive than traditional methods in detecting microecological changes in the stool (4, 6, 24–29). In the present work, we used GLC to demonstrate that composition of the murine fecal flora is genetically regulated, with a pronounced influence of the major histocompatibility complex (MHC).
MATERIALS AND METHODS
Mice.
Four-week-old male mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Half of the mice were from three congenic strains with an A background genotype and different only in the MHC (H-2) as follows: A.BY (H-2b), A.CA (H-2f), and A.SW (H-2s). The other half were from three congenic strains with a C57BL background as follows: C57BL/10J (H-2b), B10.M (H-2f), and B10.S (H-2s). The strains with the same background genotype differ from each other only in the MHC-encoded genes. From each strain, six to eight mice were used. In the supplying laboratory, the mice were housed in identical environments and fed with autoclaved NIH-31 6% fat diet (Purina Mills, Inc., Richmond, Ind.). The same diet had been in use for 2 years for the previous generations of these mouse strains. In our laboratory, all mice were fed autoclaved R36 diet (Lactamin AB, Södertälje, Sweden), slightly different from the diet given in the supplying laboratory. The mice were housed individually in Macrolon I cages with free access to distilled water. Autoclaved aspen beddings were changed once a week. All mice were handled identically, with only two persons participating throughout the experiment, which was carried out simultaneously with the six mouse strains. In addition, stool samples of four C57BL/B6 germ-free mice obtained from the Laboratory of Medical Microbial Ecology, Department of Cell and Molecular Biology, Karolinska Institute, Stockholm, Sweden, were used. The animal experiments were carried out in concordance with the national and international laws and policies (order no. 1076/85, Government of Finland; EEC Council Directive 86/609, OJL 358, December 1987).
Stool samples.
Stool samples from the rectum were collected at appropriate intervals and stored immediately at −70°C. Before GLC analysis, the stool samples were processed to separate bacterial mass from other fecal material. First, fibrous material from the diet and eukaryotic cells were allowed to sediment. For this, 100 mg of the fecal sample was suspended in 5 ml physiological saline, gently mixed, and allowed to stand for 2 h at 4°C; thereafter, the sample was remixed and allowed to sediment for 15 min. The supernatant which contains the free fatty acids, fragments of eukaryotic and prokaryotic cell membranes, and whole bacterial cells was then centrifuged (1,000 × g, 15 min) to separate bacterial cells as a pellet. The method effectively separates bacterial mass from other fatty acids present in the feces (23).
For GLC, the separated bacterial mass was saponified and methylated as described elsewhere (3). In brief, the bacterial mass was incubated for 30 min at 100°C with 15% (wt/vol) NaOH in 50% aqueous methanol and then acidified to pH 2.0 with 6 N aqueous HCl in CH3OH. The methylated fatty acids were then extracted with ethyl ether and hexane.
GLC.
GLC analysis was performed with an HP6890 PLUS gas chromatograph (Hewlett-Packard) and an HP-Ultra 2 column of cross-linked 5% phenylmethyl silicone (length, 25 m; inner diameter, 0.2 mm; film thickness, 0.33 μm). Ultra-high-purity helium was used as a carrier gas. The GLC settings were as follows: injection port temperature, 250°C; detector temperature, 300°C; initial column temperature, 170°C, increasing at 5°C/min up to 270°C at 20 min; total analysis time, 25 min; sample volume, 1 μl. The chromatograms obtained are bacterium-derived CFA profiles of the samples analyzed.
Data analysis.
The GLC data analysis is based on computerized comparison of bacterial CFA profiles. Each bacterial species has a typical CFA composition. The CFA profile of bacterial mass in a stool sample represents all bacteria present. Thus, two samples with identical microbial flora yield identical CFA profiles. This type of analysis followed by a cluster analysis reveals which samples are similar to each other and the relative similarity of the samples. A bacterial identification program developed previously (3) was used to analyze the GLC data of stool samples containing several bacterial species. All identified and unidentified peaks of individual fatty acids in the chromatograms were used in the analyses. All samples were compared to each other, and similarity indices (with a scale of 0 to 100) were calculated for each sample pair and between the groups. The calculation program (3) identifies the chromatogram peaks according to the retention time. Those with the same retention time are compared according to the size, reflecting amount of the fatty acid; an index of 100 is given to a pair with the same size, and that of 0 if the other peak is completely missing. A similarity index for a sample pair is obtained by calculating a weighted (according to the sizes of the peaks) average of the peak indices. The same principle is used to calculate a similarity index when two groups (clusters) of samples are compared with each other. An index of 100 indicates complete similarity with same fatty acids (peaks in the chromatogram) found in the same amount in the samples compared; an index of 0 indicates complete dissimilarity.
To calculate the statistical significance of a difference between two groups, the variation of the CFA profiles within each group was compared to the variation between these groups. The variation within a group was determined by calculating the mean ± standard deviation (SD) for all paired comparisons within the group. The variation between two groups was calculated by comparing each CFA profile in one group to all profiles in the other group. The mean ± SD was calculated for all of these comparisons. Finally, the variation between the groups (mean x, SD s, number of comparisons n) was compared to the variation within the groups (x1, s1, and n1 for the first group; x2, s2, and n2 for the second group) by calculating a z value as follows:
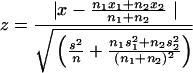 |
The value obtained was used to determine the P value from the z table (1).
RESULTS
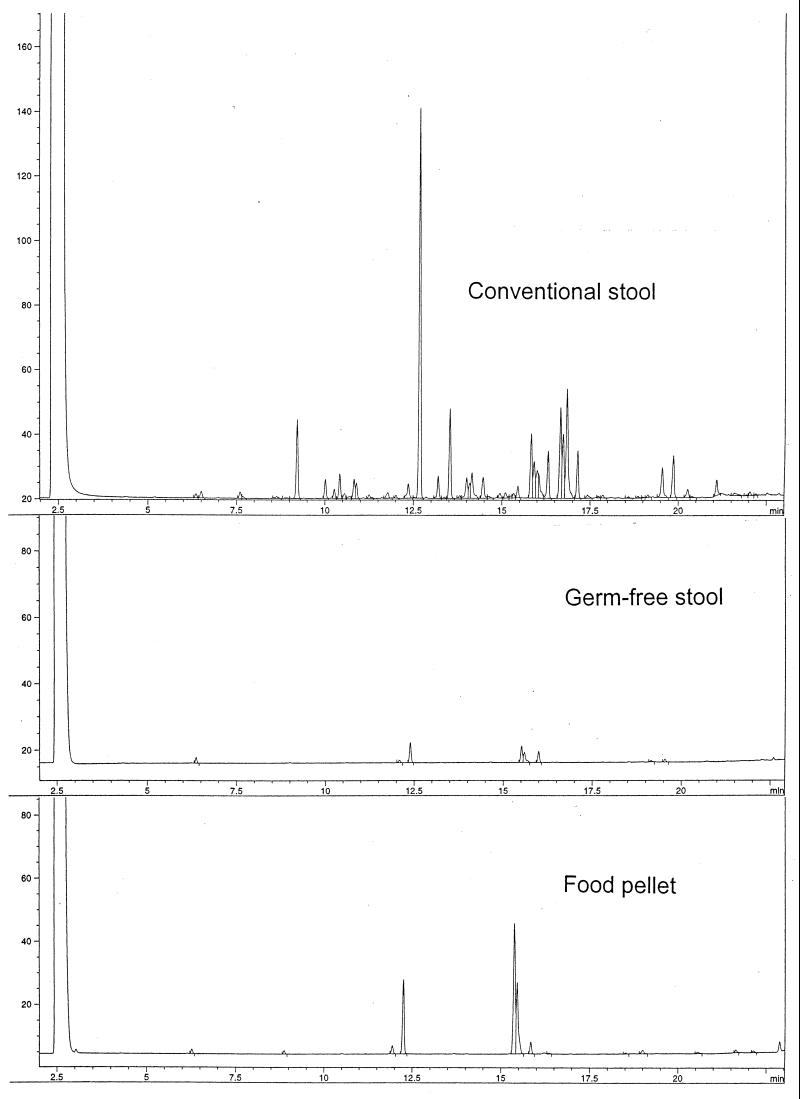
Figure 1 presents stool CFA profiles of a conventionally housed mouse and a germ-free mouse from the samples processed identically. To exclude any eukaryotic contribution, the soluble material and the fibrous parts derived from the diet and host cells were removed from the stool samples (23), and only a few negligible peaks were observed in the CFA profile of the germ-free stool. However, the sterile food pellets appeared rich in gram-positive bacteria when stained. The same, though to a considerably lesser extent, was observed for the germ-free stools. In the chromatographic analysis, the food pellets yield exactly the same few peaks as the germ-free stool samples (Fig. 1), indicating that the small peaks in the chromatogram of the germ-free stool are derived from the dead bacteria present in the sterile food pellets. On these bases, we conclude that only fatty acids derived from bacteria in the stool are included in the GLC analyses.
FIG. 1.
GLC analysis of CFAs from stool samples of a conventionally housed mouse and a germ-free mouse. The samples were processed identically. Each peak represents a fatty acid. Also presented is the CFA profile of sterile food pellets used for the germ-free mice, processed similarly to the stool samples. When stained, both the sterile food pellets and germ-free stool were found to contain gram-positive bacteria.
To study the influence of MHC, 10 stool samples were collected from each mouse at intervals of 1 to 4 weeks. CFA profiles of the samples appear considerably homogeneous when the samples collected at each time point are compared within the mouse strains (Fig. 2). Comparisons between strains congenic for the MHC and carrying different H-2 genotypes (b, f or s) reveal an effect of the MHC on the fecal flora. When mice with different H-2 genotypes in the A background are compared, statistically significant differences in the CFA profiles are observed on all occasions throughout the study, from 5 to 24 weeks of age, the only exception being at 11 weeks of age (Table 1). Similar results are observed when mice with the C57BL background and s genotype are compared to those with b or f genotype in the same background, revealing statistically significant differences on 17 occasions out of 20. In the comparison between the b and f genotypes in the C57BL background, the differences are statistically significant for the samples collected at 5, 7, and 9 weeks of age.
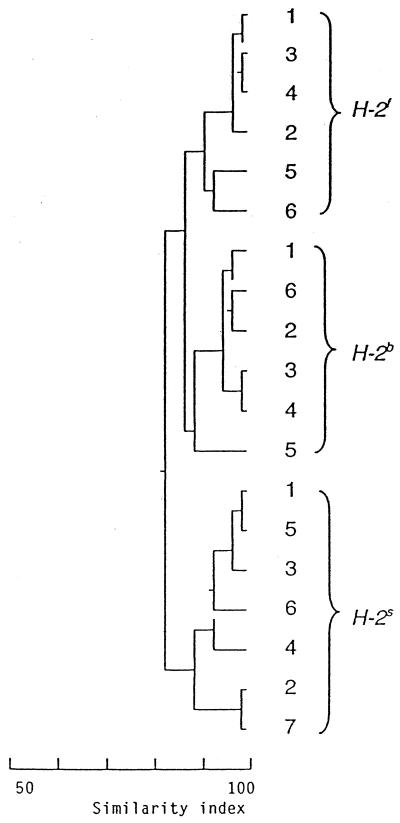
FIG. 2.
Example of cluster analysis with CFA profiles from stool samples of A.CA (H-2f), A.BY (H-2b), and A.SW (H-2s) mice, taken at 7 weeks of age. Each figure within the H-2 genotypes indicates a sample from one mouse. All 19 samples are compared to each other and clustered according to similarity. An index of 100 indicates complete similarity with the same fatty acids (peaks in the chromatogram) found in the same concentrations in the samples compared; an index of 0 indicates complete dissimilarity. Stool CFA profiles from mice with different H-2 genotypes form separate clusters revealing considerable homogeneity within each genotype. Position of the vertical lines on the scale indicates similarity between the samples or between the clusters. Differences between the clusters (H-2 genotypes) are statistically significant (P ≤ 0.001).
TABLE 1.
Comparison of fecal floras from mice with different H-2 genotypes in the same background and from mice with the same H-2 genotype in a different background
| Mice
|
Mean similarity indexa
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Background | H-2 | 5 | 6 | 7 | 8 | 9 | 11 | 13 | 15 | 19 | 24 |
| A | b vs s | 87.4* | 73.7*** | 80.8*** | 68.2*** | 83.8*** | 83.6*** | 85.8*** | 86.6*** | 87.8** | 83.8*** |
| b vs f | 81.3*** | 71.3*** | 85.5*** | 54.9*** | 86.4*** | 81.2*** | 86.4*** | 81.6*** | 88.0** | 87.4** | |
| f vs s | 79.6*** | 78.5* | 81.4*** | 81.8*** | 86.3*** | 88.7 | 90.8* | 85.4** | 88.3** | 89.1* | |
| C57BL | b vs s | 85.8 | 80.2*** | 87.5*** | 88.3 | 80.8*** | 88.1*** | 86.4*** | 85.8*** | 87.3*** | 90.5*** |
| b vs f | 76.4** | 88.8 | 85.4*** | 89.0 | 86.3*** | 90.1 | 90.8 | 92.7 | 90.3 | 92.6 | |
| f vs s | 76.3* | 87.1* | 84.4** | 93.3 | 84.1*** | 85.1*** | 89.4*** | 89.0*** | 89.3*** | 90.2*** | |
| A vs C57BL | b | 79.3*** | 63.6*** | 72.6*** | 64.4*** | 81.2*** | 73.9*** | 82.3*** | 81.8*** | 83.4*** | 85.2*** |
| f | 68.2*** | 80.3 | 73.9*** | 89.6*** | 84.1*** | 85.8*** | 89.3*** | 86.1*** | 88.9* | 88.2*** | |
| s | 77.9*** | 80.3*** | 71.9*** | 75.9*** | 71.2*** | 77.2*** | 84.1*** | 81.5*** | 83.0*** | 87.8*** | |
For each index, CFA profiles of stool samples collected from two strains of mice at the indicated age (weeks) are compared. An index of 100 indicates complete similarity with the same fatty acids (peaks in the chromatogram) found in the same concentration in the samples compared; an index of 0 indicates complete dissimilarity. Each group with a given H-2 genotype consists of six to eight mice. *, P < 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
The results obtained indicate that MHC-encoded genes have a clear-cut contribution to the composition of the murine fecal flora. However, it is apparent that the genes outside the MHC also have an effect, since fecal floras of mice with the same H-2 genotype in a different background differ significantly from each other. This is revealed by all three H-2 genotypes used; there is only one exception among the 30 comparisons made (Table 1). Therefore, it is understandable that mice which differ in both MHC and background have significantly different intestinal floras; only three exceptions occurred among the 60 comparisons made (Table 2).
TABLE 2.
Comparison of fecal floras from mice with different H-2 genotypes in a different background (A or C57BL)
|
H-2
|
Mean similarity indexa
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | C57BL | 5 | 6 | 7 | 8 | 9 | 11 | 13 | 15 | 19 | 24 |
| b vs | f | 66.1*** | 66.6*** | 69.1*** | 57.9*** | 81.8*** | 71.6*** | 82.7*** | 80.9*** | 85.3*** | 81.0*** |
| b vs | s | 73.9*** | 63.0*** | 66.9*** | 57.4*** | 75.6*** | 66.6*** | 78.1*** | 73.9*** | 81.2*** | 83.8*** |
| f vs | b | 86.6* | 78.3*** | 81.1*** | 84.2*** | 87.3*** | 86.6*** | 90.6 | 87.3* | 87.7* | 89.9*** |
| f vs | s | 81.4** | 74.4*** | 72.7*** | 88.3*** | 78.5*** | 77.2*** | 86.2*** | 80.7*** | 88.6*** | 89.0*** |
| s vs | b | 82.6*** | 75.7*** | 78.2*** | 80.6*** | 88.2*** | 85.6*** | 88.2*** | 88.8** | 86.7* | 89.2*** |
| s vs | f | 74.1*** | 82.1*** | 75.6*** | 79.4*** | 80.5*** | 81.8*** | 89.6*** | 88.9*** | 87.9 | 89.6 |
See the footnote to Table 1.
All mice, including their mothers and ancestors in the previous 2 years, had been on the same diet in the supplying institution. At arrival in our laboratory, they were all simultaneously changed to a slightly different diet. Therefore, any maternal or environmental contribution to the differences observed between the mouse strains can be excluded. We conclude that MHC-encoded genes have a significant influence on the composition of the fecal flora. In addition, genes outside the MHC also have an impact, as revealed by the comparisons made between mice having the same H-2 genotype in a different background. It is possible that the undefined genes of the C57BL background have a more profound effect than those of the A background, since the differences between b and f genotypes of mice with C57BL background are considerably less frequently significant than the others (Table 1). However, the present data do not allow any conclusions about the relative size of the MHC and non-MHC contributions.
DISCUSSION
The mechanisms behind the effect of MHC on the bacterial flora remain open. Class I and II MHC molecules are highly polymorphic transmembrane glycoproteins of eukaryotic cells; class I molecules are present on the surface of all nucleated cells. The basic structure of MHC molecules consists of domains exhibiting the typical immunoglobulin (Ig) fold, and they function as key elements of the immune response by presenting peptides to T cells. Therefore, the most obvious alternative to explain the influence of MHC on the fecal or intestinal flora would be the immune elimination of certain bacterial species, leading to a restricted colonization. However, very little is known about the effect of MHC on antibacterial responses (14, 37). One should also note that bacterial composition of the murine fecal flora does not necessarily indicate the exact composition of the intestinal flora (31, 36).
An alternative mechanism is an action through bacterial adherence to intestinal epithelial surfaces that is a requisite first step in the colonization process. Bacterial surface molecules, adhesins, recognize proteins or glycoproteins on the epithelial cells. Bacteria unable to adhere are shed. The specificity of the adherence leads to a restricted colonization of the host. As an example, attachment of Helicobacter pylori to human gastric epithelium is selectively mediated by blood group antigen Lewisb (2). Regarding MHC molecules and bacterial adherence, several Ig-binding proteins have been demonstrated on the bacterial surfaces. One type of these, fibrous proteins called curli, have been shown to interact with the Ig-like domains of human class I MHC molecules (21). The effect of different MHC genotypes was not studied. However, Helicobacter felis-induced gastric inflammation, which depends on the bacterial attachment and colonization, varies in severity in congenic mice with different MHC (16). In addition to a twin study on the intestinal flora (38), it has been reported that bacterial flora of the nasal epithelium could be genetically controlled (8). It remains to be established whether MHC influences intestinal colonization through the immune response or by directly affecting bacterial adhesion. The latter possibility could be mediated by MHC molecules as such or by other structures encoded by MHC (15).
Demonstration of the genetic influence on the gastrointestinal flora opens a new perspective to study the pathogenesis of bacterially induced diseases, also including autoimmune and other disorders. For instance, the intestinal flora in patients with newly diagnosed rheumatoid arthritis is significantly different from that in the controls (4), and it is known that rheumatoid arthritis is preferentially associated with certain MHC genotypes (20, 39). Further, cell walls of several bacterial species of the normal human gut flora are capable of inducing experimental chronic polyarthritis (33, 34, 40); on the other hand, degradation products of intestinal bacteria enter the circulation and joint tissue quite often (11–13, 30, 32). Maybe individuals with certain genotypes are intestinally colonized by bacteria which have cell walls capable of inducing arthritis. In the long run, with continuous seeding of bacterial degradation products from the gut, the synovial inflammation is maintained and is followed by erosion, exposure of cartilage antigens, and autoimmunity. It must be remembered that bacteria in the intestinal flora are continuously dividing and degraded, resulting in an enormous amount and variety of bacterial products.
ACKNOWLEDGMENTS
Stool samples of germ-free mice were kindly provided by Elisabeth Norin, Laboratory of Medical Microbial Ecology, Department of Cell and Molecular Biology, Karolinska Institute, Stockholm, Sweden. We thank Pavol Ivanyi and Olli Vainio for comments on the manuscript.
This work was supported by a grant from EVO of Turku University Central Hospital.
REFERENCES
- 1.Altman D G. Practical statistics for medical research. London, England: Chapman & Hall; 1996. [Google Scholar]
- 2.Borén T, Falk P, Roth K A, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 3.Eerola E, Lehtonen O P. Optimal data processing procedure for automatic bacterial identification by gas-liquid chromatography of cellular fatty acids. J Clin Microbiol. 1988;26:1745–1753. doi: 10.1128/jcm.26.9.1745-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eerola E, Möttönen T, Hannonen P, Luukkainen R, Kantola I, Vuori K, Tuominen J, Toivanen P. Intestinal flora in early rheumatoid arthritis. Br J Rheumatol. 1994;33:1030–1038. doi: 10.1093/rheumatology/33.11.1030. [DOI] [PubMed] [Google Scholar]
- 5.Eerola E, Peltonen R. The gastrointestinal system. In: Maddison P J, Isenberg D A, Woo P, Glass D N, editors. Oxford textbook of rheumatology. 2nd ed. Vol. 1. Oxford, England: Oxford University Press; 1998. pp. 255–277. [Google Scholar]
- 6.Grönlund M M, Lehtonen O P, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28:19–25. doi: 10.1097/00005176-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hill M, Drasar B S. The normal colonic bacterial flora. Gut. 1975;16:318–323. doi: 10.1136/gut.16.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoeksma A, Winkler K C. The normal flora of the nose in twins. Acta Leiden. 1963;32:123–133. [PubMed] [Google Scholar]
- 9.Holdeman L V, Good I J, Moore W E C. Human fecal flora. Variation in bacterial composition within individuals and a possible effect of emotional stress. Appl Environ Microbiol. 1976;31:359–375. doi: 10.1128/aem.31.3.359-375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotilainen P, Huovinen P, Eerola E. Application of gas-liquid chromatographic analysis of cellular fatty acids for species identification and typing of coagulase-negative staphylococci. J Clin Microbiol. 1991;29:315–322. doi: 10.1128/jcm.29.2.315-322.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehtonen L, Eerola E, Oksman P, Toivanen P. Muramic acid in peripheral blood leukocytes of healthy human subjects. J Infect Dis. 1995;171:1060–1064. doi: 10.1093/infdis/171.4.1060. [DOI] [PubMed] [Google Scholar]
- 12.Lehtonen L, Eerola E, Toivanen P. Muramic acid in human peripheral blood leucocytes in different age groups. Eur J Clin Investig. 1997;27:791–792. doi: 10.1046/j.1365-2362.1997.1950732.x. [DOI] [PubMed] [Google Scholar]
- 13.Lehtonen L, Kortekangas P, Oksman P, Eerola E, Aro H, Toivanen A. Synovial fluid muramic acid in acute inflammatory arthritis. Br J Rheumatol. 1994;33:1127–1130. doi: 10.1093/rheumatology/33.12.1127. [DOI] [PubMed] [Google Scholar]
- 14.Mackie R I, White B A, Isaacson R E. Gastrointestinal microbiology. 2. Gastrointestinal microbes and host interactions. New York, N.Y: Chapman & Hall; 1997. [Google Scholar]
- 15.The MHC Sequencing Consortium. Complete sequence and gene map of a human major histocompatibility complex. Nature. 1999;401:921–923. doi: 10.1038/44853. [DOI] [PubMed] [Google Scholar]
- 16.Mohammadi M, Redline R, Nedrud J, Czinn S. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infect Immun. 1996;64:238–245. doi: 10.1128/iai.64.1.238-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore W E C, Holdeman L V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974;27:961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore W E C, Holdeman L V. Special problems associated with the isolation and identification of intestinal bacteria in faecal flora studies. Am J Clin Nutr. 1974;27:1450–1455. doi: 10.1093/ajcn/27.12.1450. [DOI] [PubMed] [Google Scholar]
- 19.Moss C W, Nunez-Montiel O L. Analysis of short-chain acids from bacteria by gas-liquid chromatography with a fused-silica capillary column. J Clin Microbiol. 1982;15:308–311. doi: 10.1128/jcm.15.2.308-311.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nepom G T. Major histocompatibility complex-directed susceptibility to rheumatoid arthritis. Adv Immunol. 1998;68:315–332. doi: 10.1016/s0065-2776(08)60563-5. [DOI] [PubMed] [Google Scholar]
- 21.Olsén A, Wick M J, Mörgelin M, Björck L. Curli, fibrous surface proteins of Escherichia coli, interact with major histocompatibility complex class I molecules. Infect Immun. 1998;66:944–949. doi: 10.1128/iai.66.3.944-949.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onderdonk A B, Sasser M. Gas-liquid and high-performance liquid chromatographic methods for the identification of microorganisms. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: ASM Press; 1995. pp. 123–129. [Google Scholar]
- 23.Peltonen R. Studies on faecal microecology with reference to diet, medication and rheumatoid arthritis. Ann Univ Turku. 1994;143:1–96. [Google Scholar]
- 24.Peltonen R, Eerola E. Direct automatic bacterial analysis of rat stools samples; the effects of diet and medical treatment studied by computerised gas-liquid chromatography of bacterial fatty acid. Microb Ecol Health Dis. 1992;5:93–103. [Google Scholar]
- 25.Peltonen R, Eerola E, Suomi K, Aho H, Kuusisto M, Toivanen P. Effect of dietary fish powder on intestinal flora and development of arthritis in the pig. Br J Rheumatol. 1993;32:1049–1054. doi: 10.1093/rheumatology/32.12.1049. [DOI] [PubMed] [Google Scholar]
- 26.Peltonen R, Kjeldsen-Kragh J, Haugen M, Tuominen J, Toivanen P, Förre Ö, Eerola E. Changes of faecal flora in rheumatoid arthritis during fasting and one-year vegetarian diet. Br J Rheumatol. 1994;33:638–643. doi: 10.1093/rheumatology/33.7.638. [DOI] [PubMed] [Google Scholar]
- 27.Peltonen R, Ling W H, Hänninen O, Eerola E. An uncooked vegan diet shifts the profile of human fecal microflora: computerized analysis of direct stool sample gas-liquid chromatography profiles of bacterial cellular fatty acids. Appl Environ Microbiol. 1992;58:3660–3666. doi: 10.1128/aem.58.11.3660-3666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peltonen R, Nenonen M, Helve T, Hänninen O, Toivanen P, Eerola E. Faecal microbial flora and disease activity in rheumatoid arthritis during a vegan diet. Br J Rheumatol. 1997;36:64–68. doi: 10.1093/rheumatology/36.1.64. [DOI] [PubMed] [Google Scholar]
- 29.Peltonen R, Toivanen P, Eerola E. Effect of a non-steroidal anti-inflammatory drug, naproxen, on faecal microbial flora. Br J Rheumatol. 1993;32:996–999. doi: 10.1093/rheumatology/32.11.996. [DOI] [PubMed] [Google Scholar]
- 30.Salmi M, Jalkanen S. How do lymphocytes know where to go: current concepts and enigmas of lymphocyte homing. Adv Immunol. 1997;64:139–218. doi: 10.1016/s0065-2776(08)60889-5. [DOI] [PubMed] [Google Scholar]
- 31.Savage D C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 32.Schrijver I A, Melief M-J, Tak P P, Hazenberg M P, Laman J D. Antigen-presenting cells containing bacterial peptidoglycan in synovial tissues of rheumatoid arthritis patients coexpress costimulatory molecules and cytokines. Arthritis Rheum. 2000;43:2160–2168. doi: 10.1002/1529-0131(200010)43:10<2160::AID-ANR3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 33.Severijnen A J, van Kleef R, Hazenberg M P, van de Merwe J P. Cell wall fragments from major residents of the human intestinal flora induce chronic arthritis in rats. J Rheumatol. 1989;16:1061–1068. [PubMed] [Google Scholar]
- 34.Simelyte E, Rimpiläinen M, Lehtonen L, Zhang X, Toivanen P. Bacterial cell wall-induced arthritis: chemical composition and tissue distribution of four Lactobacillus strains. Infect Immun. 2000;68:3535–3540. doi: 10.1128/iai.68.6.3535-3540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon G L, Gorbach S L. Intestinal flora in health and disease. Gastroenterology. 1984;86:174–193. [PubMed] [Google Scholar]
- 36.Tannock G W. Analysis of the intestinal microflora: a renaissance. Antonie Leeuwenhoek. 1999;76:265–278. [PubMed] [Google Scholar]
- 37.Toivanen P, Koskimies S, Granfors K, Eerola E. Bacterial antibodies in HLA-B27+ healthy individuals. Arthritis Rheum. 1993;36:1633–1635. doi: 10.1002/art.1780361122. [DOI] [PubMed] [Google Scholar]
- 37a.Vaahtovuo, J., P. Toivanen, and E. Eerola. Study of murine microflora by cellular fatty acid analysis: effect of age and mouse strains. Antonie Leeuwenhoek, in press. [DOI] [PubMed]
- 38.van de Merwe J P, Stegeman J H, Hazenberg M P. The resident faecal flora is determined by genetic characteristics of the host. Implications for Crohn's disease? Antonie Leeuwenhoek. 1993;49:119–124. doi: 10.1007/BF00393669. [DOI] [PubMed] [Google Scholar]
- 39.Weyand C M, Goronzy J J. Pathogenesis of rheumatoid arthritis. Med Clin North Am. 1997;81:29–55. doi: 10.1016/s0025-7125(05)70504-6. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Rimpiläinen M, Simelyte E, Toivanen P. What determines arthritogenicity of bacterial cell wall? A study on Eubacterium cell wall-induced arthritis. Rheumatology. 2000;39:274–282. doi: 10.1093/rheumatology/39.3.274. [DOI] [PubMed] [Google Scholar]