Abstract
Background
In the post-Z0011 era, sentinel lymph node (SLN) status and metastatic burden determine whether axillary management entails conservative sentinel lymph node biopsy (SLNB) or radical axillary lymph node dissection (ALND) in breast cancer patients. However, SLN status and metastatic burden cannot be evaluated preoperatively in clinical practice. This study explored the predictive value of contrast-enhanced ultrasound (CEUS) patterns of SLN to assess the nodal status and metastatic burden in early breast cancer patients.
Methods
A retrospective study was conducted on 88 consecutive patients who were diagnosed with clinical T1-2N0 breast cancer between December 2020 and November 2021 at the Lanzhou University Second Hospital and scheduled for SLNB. Preoperative CEUS was performed to confirm the location and enhancement pattern of the SLN, and the conventional ultrasonic characteristics of the primary breast lesions and SLN were recorded. Intraoperative localized SLN and postoperative pathological results were used as the gold standard for comparison with preoperative ultrasound findings.
Results
CEUS successfully identified at least 1 SLN in 88 patients, with a total of 118 SLNs identified in the entire cohort. Univariate analysis showed that lesion size, blood flow grade, SLN longitudinal diameter, cortical thickness, and enhancement pattern were significant predictive features of SLN metastasis. Further multiple regression analysis indicated that the enhancement pattern of the SLN was an independent risk factor for SLN metastasis, with a sensitivity and a specificity of 84.2% (32/38) and 80.0% (40/50), respectively. Meanwhile, the SLN enhancement pattern could predict the lymph node metastasis burden (P<0.001). In patients presenting with a type I (homogeneous enhancement) or type II (heterogeneous enhancement) SLN, 91.5% (65/71) had ≤2 positive SLNs, whereas in patients with a type III (no enhancement) SLN, 70.6% (12/17) had >2 metastatic nodes.
Conclusions
The contrast-enhanced pattern of the SLN is an independent risk factor for SLN status. Patients presenting with a type I or type II SLN enhanced pattern are unlikely to have high-burden metastases detected at their final surgical treatment and omission of ALND may be appropriate.
Keywords: Contrast-enhanced ultrasound (CEUS), breast cancer, sentinel lymph node, metastatic burden
Introduction
Breast cancer is the most common cancer in women worldwide (1). The status and metastatic burden of axillary lymph nodes (ALN) are critical prognostic factors for breast cancer patients (2). Conservative sentinel lymph node biopsy (SLNB) is the primary approach for ALN staging and is used to determine whether radical axillary lymph node dissection (ALND) should be performed (3). The sentinel lymph nodes (SLN), the first individual or group of lymph nodes drained by the primary tumor, can reflect the involvement of the regional lymphatic system (4). The accurate localization of the SLN is a crucial step in SLNB for breast cancer. It is usually performed intraoperatively using blue dye, radioisotopes, indocyanine green (ICG), or any 2 of these methods combined, with an 89–99.5% identification rate (5). However, there are significant complications associated with this procedure, such as a 7.5% incidence of lymphedema and a 5–8% incidence of sensory and other movement restrictions (6). In addition, several studies have shown that approximately 70% of patients with early-stage breast cancer undergoing SLNB have no detectable metastatic SLN, suggesting that an invasive SLNB may not always be necessary for this group of patients (7).
Recently, based on the research results of the American College of Surgeons Oncology Group (ACOSOG) Z0011 trials, the latest National Comprehensive Cancer Network (NCCN) guidelines for breast cancer recommend the omission of ALND in patients with early breast cancer who have 1–2 metastatic SLNs treated with breast-conserving surgery and postoperative whole-breast segmentation radiotherapy (8-10). These guidelines increase the burden threshold of positive lymph nodes for performing ALND and challenge the role of preoperative imaging methods. To be useful, imaging needs to determine the presence of axillary metastasis and quantify the burden of lymph node metastasis. The SLNB may ideally suit patients with preoperatively predicted low-burden metastases (≤2 metastatic nodes); ALND is the most effective in patients with preoperatively predicted high-burden metastases (>2 metastatic nodes).
Contrast-enhanced ultrasound (CEUS) is a new imaging technique that uses a percutaneous injection of a contrast agent to display the SLN and lymphatic channels in real-time, which has been shown to be a safe and effective method for SLN localization (11,12). However, few studies have explored the clinical utility of CEUS in the post-Z0011 era. Therefore, this study aimed to evaluate the predictive value of preoperative CEUS to assess the SLN status and metastatic burden in early breast cancer patients to assist in the decision-making of axillary surgery. We present the following article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-234/rc).
Methods
Patients
All patients involved in the study were from the Lanzhou University Second Hospital, Lanzhou. Considering the high demand for operational skills and practical experience during the localization of SLN by CEUS, a radiologist with 7 years of ultrasound experience conducted a pilot test on 40 breast cancer patients between May 2020 and November 2020 before commencing the data collection for this study. In the formal study, we enrolled all consecutive patients with newly diagnosed breast cancer between December 2020 and November 2021. Patients were included if they had a lesion <5 cm in size, no abnormal ALNs on the B-mode ultrasound, and had planned to undergo SLNB. Patients were excluded if they had a history of breast surgery or chemoradiation, had an allergic reaction to contrast agents, or failed to detect enhanced lymphatic channels. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval for this retrospective cohort study was granted by the Ethics Committee of Lanzhou University Second Hospital (No. 2022A-256). All participants signed a written informed consent form.
Examination technique and methods
The same radiologist completed all ultrasound examinations of the patient within 4 hours before the surgery. The patients’ breast and axilla were first scanned in B-mode using an iU22 scanner (Philips Medical Systems, Bothell, WA, USA) equipped with a 3–9 MHz linear probe to record the characteristics of the breast lesion and to confirm that the patient had no suspected metastatic ALNs (a metastatic ALN was defined as a lymph node hilum anomaly or disappearance or cortical thickness ≥3 mm) (13-15). Then, the ultrasound instrument was switched to the contrast mode for CEUS.
The contrast agent (SonoVue, Bracco, Milan, Italy) was mixed with 5.0 mL of saline until a homogeneously-mixed suspension was obtained. The radiologist rapidly injected 2.0 mL of the contrast suspension intradermally at the 3, 6, 9, and 12 o’clock positions in the areola area (0.5 mL at each point) and massaged the injection site for 30 seconds. The probe was then used to locate the visible superficial lymphatic vessels and track the lymphatic vessels to the first enhanced node or group of nodes in the axilla. The two-dimensional and CEUS images were displayed simultaneously on a screen to confirm the architecture that defined SLN (Videos S1,S2 describe our procedure in detail). The SLN enhancement patterns were divided into 3 distinct enhancement patterns: type I (uniform enhancement of the whole lymph node), type II (partial absence of enhancement or diffusion defect in visible lymph nodes), and type III (no enhancement in the lymph node) (16,17). A type I enhancement pattern indicated that there was no SLN metastasis; types II and III patterns indicated the presence of SLN metastasis. In patients with multiple enhanced SLNs, only the lymph nodes with the highest enhancement patterns were analyzed. Two sonographers (with more than 5 years of experience) independently and blindly evaluated the SLN enhancement pattern characteristics. In case of a dispute, a consensus was reached between the 2 examiners after consultation.
Video S1.
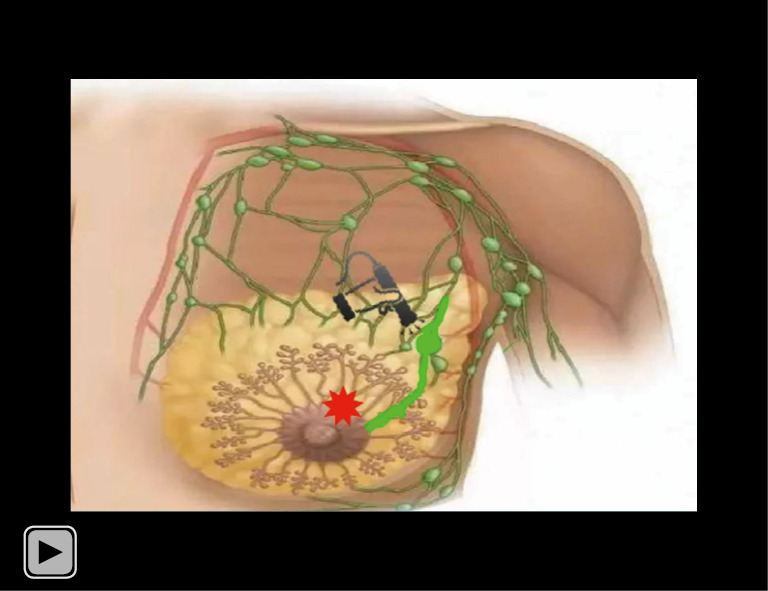
Schematic diagram of the method and process of visualizing SLN by CEUS. SLN, sentinel lymph node; CEUS, contrast-enhanced ultrasound.
Video S2.
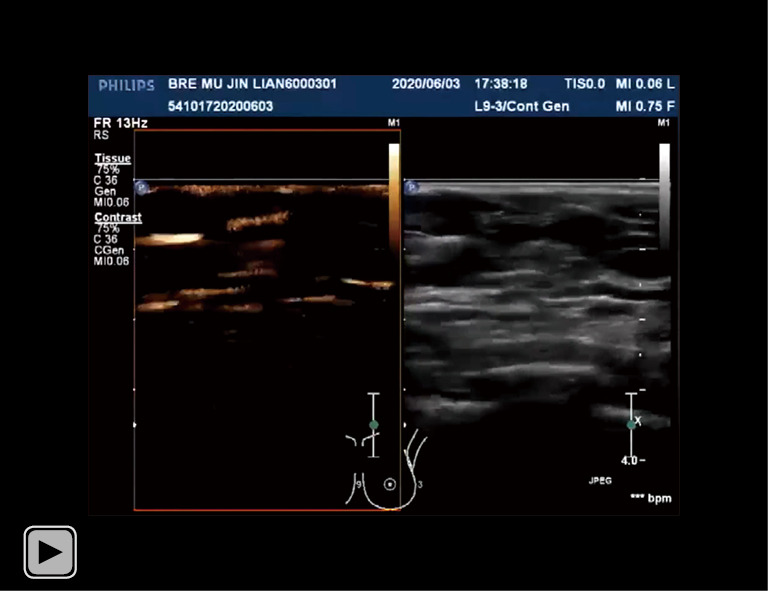
A specific case (CEUS visualization of the superficial lymphatic vessel drainage to the SLN in a patient with early-stage breast cancer). CEUS, contrast-enhanced ultrasound; SLN, sentinel lymph node
Finally, following the previously reported method, the lymphatic drainage pathways and the location of SLNs were marked on the skin surface (16,18). The longitudinal diameter, transverse diameter, aspect ratio, cortical thickness, enhancement pattern, and vertical distance from the skin of the enhanced lymph node were also recorded.
SLNB and pathological evaluation
The SLNB was performed by surgeons with more than 8 years of surgical experience. In the supine position with general anesthesia, 4 mL of 1% methylene blue (MB) was injected into the areola area at the 3, 6, 9, and 12 o’clock positions, and the injection site was massaged for 10 minutes. Then, 0.1–0.15 mL of ICG was diluted with saline to 1 mL and injected in the same locations described above. SLN was detected by blue-stained lymphatic channels and compared with lymphatic channels and lymph nodes marked by body surface markers. The lymph nodes under the surface marker were first resected, and the depth from those lymph nodes to the skin was measured using a ruler. The remaining fluorescent or blue-stained SLNs were further removed and separately sent to our hospital’s pathology department for freezing and paraffin pathological examination to determine whether they contained cancer cells metastases [classified as isolated tumor cells (≤0.2 mm or <200 cells), micrometastasis (>0.2 and ≤2 mm, or ≥200 cells), and macrometastasis (>2 mm); isolated tumor cells were considered as non-metastases], and the number of SLN metastases. If SLN metastasis was present, ALND was recommended, and the presence and number of additional lymph node (non-SLN) metastases were recorded.
Statistical analysis
The software SPSS 23.0 (IBM Corp., Armonk, NY, USA) and R programming language software (The R Foundation for Statistical Computing, Vienna, Austria) were used to conduct statistical analyses. Continuous variables were expressed as mean ± standard deviation (), and their correlation with SLN metastasis was tested with the independent sample t-test. Categorical variables were represented by the absolute value (n) and relative frequency (%), and their correlation with SLN metastasis was tested with the χ2 test or Fisher’s exact test. Multiple logistic regression was used to analyze the independent factors associated with SLN metastasis. The results were expressed as an odds ratio (OR) and a 95% confidence interval (CI). The Wilcoxon signed-rank test was used to compare the differences between preoperative CEUS and intraoperative MB/ICG identified SLN numbers. Weighted kappa was used to estimate inter-reader variation. A kappa coefficient value >0.75 indicated a good interobserver agreement; P<0.05 was considered statistically significant.
Results
Patient and tumor characteristics
A total of 92 patients underwent preoperative CEUS examinations, 4 of whom were excluded due to failure to detect enhanced lymphatics. The remaining 88 female patients met the eligibility criteria and were ultimately included in this study for analysis (Figure 1). The ages of the patients ranged from 29 to 78 years (mean, 51.67±10.00 years), and 36 were post-menopausal. The diameter of the breast lesions ranged from 0.80 to 4.27 cm (mean, 2.25±0.83 cm). Among the 88 patients, 78 (88.6%) had non-special invasive ductal carcinomas, 4 (4.5%) had mucinous carcinomas, 4 (4.5%) had invasive lobular carcinomas, and 2 (2.4%) had intraductal papillary carcinomas. These breast cancer pathological types were classified into luminal A (32, 36.3%), luminal B (23, 26.2%), human epidermal growth factor receptor 2 (HER2)-enriched (20, 22.7%), and triple-negative (TNBC; 13, 14.8%).
Figure 1.
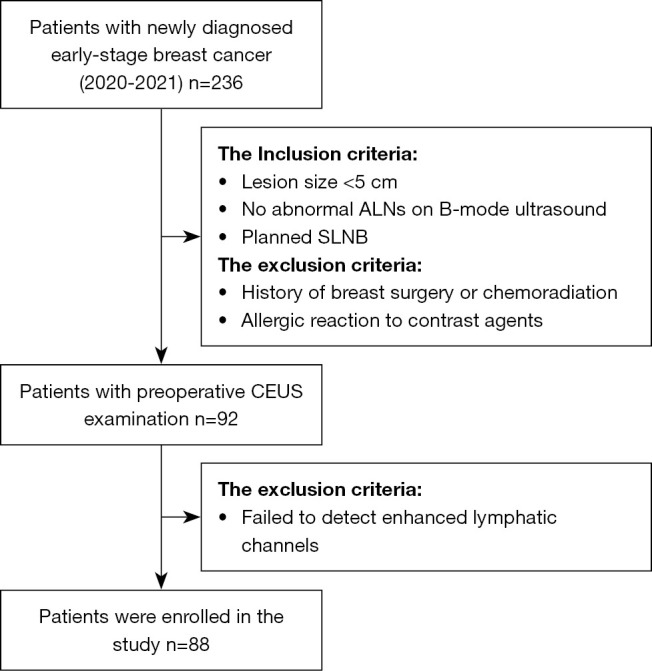
Flow diagram of patient selection. ALN, axillary lymph node; SLNB, sentinel lymph node biopsy; CEUS, contrast-enhanced ultrasound.
All patients first underwent SLNB. Intraoperative pathology showed 50 patients did not have positive SLN, and that the remaining 38 had positive SLN (33 of whom had at least 1 SLN with macrometastases). These 38 patients underwent ALND. The final postoperative pathology of the patients treated with ALND showed that 21 patients had only 1 or 2 positive SLNs with no additional metastatic lymph nodes, and the other 17 patients had more than 2 metastatic lymph nodes (in addition to positive SLN, additional metastatic lymph nodes were found during ALND in 11 cases).
CEUS-identified SLN
The detection rate of the SLN localization on CEUS was 95.7% (88/92). None of the patients who underwent CEUS developed contrast agent-induced adverse reactions or complications within 3 months after the procedure. CEUS successfully identified at least 1 SLN in 88 patients, with a cumulative total of 118 SLNs and an average of 1.23 SLNs per patient. There was no statistical significance between the mean depth of the SLNs (1.14±0.44 cm) identified by CEUS before surgery and the mean depth of SLNs (1.15±0.43 cm) measured intraoperatively (P=0.113). A total of 232 SLNs were identified on the MB/ICG during the SLNB process, with an average of 2.63 SLNs detected per patient. There was a statistically significant difference between the number of SLNs identified by preoperative CEUS and that identified by intraoperative MB/ICG (P<0.001) (Figure 2).
Figure 2.
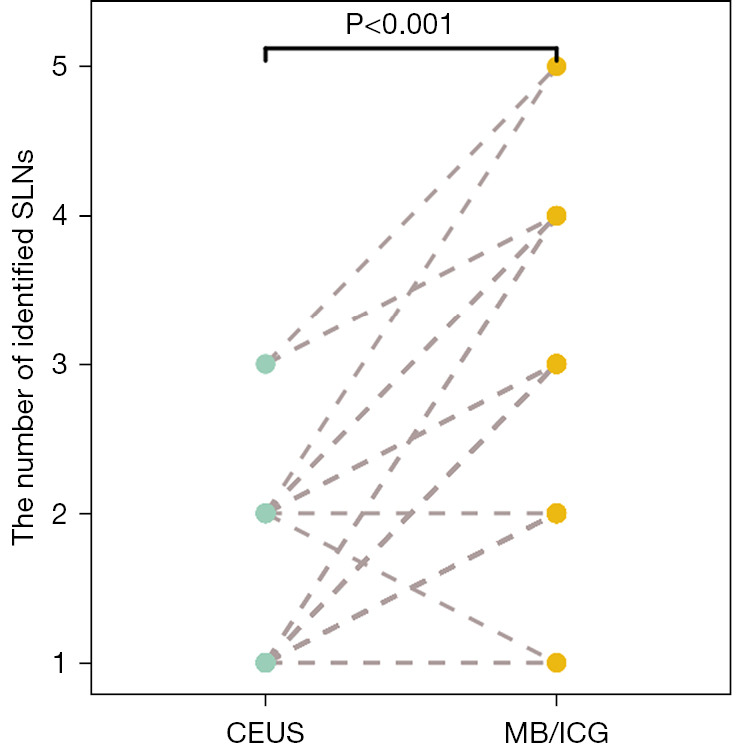
Comparison of the number of SLNs identified by preoperative CEUS and intraoperative MB/ICG. SLN, sentinel lymph node; CEUS, contrast-enhanced ultrasound; MB, methylene blue; ICG, indocyanine green.
Factors associated with sentinel lymph node metastasis
Univariate analysis showed that lesion size (P=0.020), blood flow grade (P=0.028), SLN longitudinal diameter (P=0.004), cortical thickness (P=0.009), and enhancement pattern (P<0.001) were all closely related to SLN status. However, there was no statistical difference between age, menopausal status, microcalcification, molecular classification, transverse diameter, aspect ratio, the vertical distance from the skin of SLN, and SLN status (P>0.05; Tables 1,2). Further multivariate logistic regression analysis indicated that only the enhancement pattern (OR, 18.211; 95% CI: 5.396–61.467; P<0.001) was independently related to SLN metastasis (Table 3).
Table 1. Patient and tumor characteristics vs. the pathology results of SLN.
| Characteristics | Total | SLN negative | SLN positive | P value |
|---|---|---|---|---|
| Age (years) | 51.67±10.00 | 49.70±9.25 | 51.95±10.90 | 0.299 |
| Tumor size (cm) | 2.25±0.83 | 2.05±0.85 | 2.45±0.69 | 0.020 |
| Menopause | 0.283 | |||
| No | 36 (40.9) | 18 (50.0) | 18 (50.0) | |
| Yes | 52 (59.1) | 32 (61.5) | 20 (38.5) | |
| Microcalcification | 0.087 | |||
| No | 53 (60.2) | 34 (64.2) | 19 (35.8) | |
| Yes | 35 (39.8) | 16 (45.7) | 19 (54.3) | |
| Blood flow grade | 0.028 | |||
| 0–1 | 19 (21.6) | 15 (78.9) | 4 (21.1) | |
| 2–3 | 84 (78.4) | 35 (50.7) | 34 (49.3) | |
| Molecular subtype | 0.650 | |||
| Luminal A | 32 (36.4) | 21 (65.6) | 11 (34.4) | |
| Luminal B | 23 (26.1) | 12 (52.2) | 11 (47.8) | |
| HER2 | 20 (22.7) | 10 (50.0) | 10 (50.0) | |
| Triple-negative | 13 (14.8) | 7 (53.8) | 6 (46.2) |
Data are shown as mean ± standard deviation or n (%). SLN, sentinel lymph node; HER2, human epidermal growth factor receptor 2.
Table 2. CEUS features of SLN versus the pathology results of SLN.
| Characteristics | Total | SLN negative | SLN positive | P value |
|---|---|---|---|---|
| Longitudinal diameter (cm) | 1.14±0.36 | 1.05±0.28 | 1.27±0.41 | 0.004 |
| Transverse diameter (cm) | 0.61±0.15 | 0.57±1.31 | 0.62±0.14 | 0.130 |
| Distance from the skin (cm) | 1.14±0.44 | 1.18±0.47 | 1.08±0.41 | 0.292 |
| Aspect ratio | 0.139 | |||
| <2 | 50 (56.8) | 25 (50.0) | 25 (50.0) | |
| ≥2 | 38 (43.2) | 25 (65.8) | 13 (34.2) | |
| Cortical thickness | 0.009 | |||
| ≥3 mm | 37 (42.0) | 15 (40.5) | 22 (59.5) | |
| <3 mm | 51 (58.0) | 35 (68.6) | 16 (31.4) | |
| Enhancement pattern | 0.000 | |||
| Type I | 47 (53.4) | 40 (85.1) | 7 (14.9) | |
| Type II–III | 41 (46.6) | 10 (24.4) | 31 (75.6) |
Data are shown as mean ± standard deviation or n (%). CEUS, contrast-enhanced ultrasound; SLN, sentinel lymph node.
Table 3. The results of the multivariate logistic regression.
| Characteristics | B | SE Coeff | Wald | P value | OR (95% CI) |
|---|---|---|---|---|---|
| Constant | −5.034 | 1.410 | 12.744 | 0.000 | |
| Enhancement pattern | 2.902 | 0.621 | 21.863 | 0.000 | 18.211 (5.396–61.467) |
| Tumor size | 0.016 | 0.383 | 0.002 | 0.996 | 1.016 (0.480–2.154) |
| Blood flow grade | 1.028 | 0.844 | 1.484 | 0.223 | 2.797 (0.534–14.636) |
| Longitudinal diameter | 1.839 | 0.948 | 3.762 | 0.052 | 6.291 (0.981–40.341) |
| Cortical thickness | 0.683 | 0.601 | 1.292 | 0.256 | 1.979 (0.610–6.426) |
B, regression coefficients; SE Coeff, standard error of coefficient; OR, odds ratio; CI, confidence interval.
Prediction of lymph node status by enhancement pattern
The mean interobserver kappa value for the evaluation of the CEUS enhancement pattern was 0.934 (95% CI: 0.879–0.990; P<0.001), which indicated a good level of agreement between the 2 sonographers. Among the 88 patients, the contrast enhancement patterns were classified as type I in 46 cases, type II in 24 cases, and type III in 18 cases. Of the patients whose SLN was type I, 40 cases were confirmed pathologically to have no SLN metastases, 5 cases had 1–2 SLN metastases, and 1 case had more than 2 metastatic lymph nodes. For patients whose SLN was type II, 8 cases were confirmed pathologically to have no SLN metastases, 12 cases had 1–2 SLN metastases, and 4 cases had more than 2 metastatic lymph nodes. For patients whose SLN was type III, 2 cases were confirmed pathologically to have no SLN metastases, 4 cases had 1–2 SLN metastases, and 12 cases had more than 2 metastatic lymph nodes. We defined a type I contrast enhancement as the absence of SLN metastasis and types II and III as the presence of SLN metastasis. The sensitivity and specificity of the CEUS evaluation of SLN metastasis were 84.2% (32/38) and 80.0% (40/50), respectively.
Prediction of the nodal metastatic burden by enhancement pattern
The SLN enhancement pattern could predict the lymph node metastasis burden (P<0.001). A proportion of 86.9% (40/46) of patients with type I enhancement had no SLN metastasis, 83.3% (20/24) of patients with type II enhancement had fewer than or equal to 2 SLN metastases, and 66.7% (12/18) of patients with type III enhancement had more than 2 metastatic lymph nodes. For CEUS in the post-Z0011 era, our results found that 91.5% (65/71) of patients had more than or equal to 2 positive SLNs when they presented with a contrast-enhanced pattern of type I or type II, and that 70.6% (12/17) of the patients with type III nodes had a high burden metastasis (>2 metastatic nodes). Figures 3,4 illustrate the clinical utility of the enhancement pattern of SLN.
Figure 3.
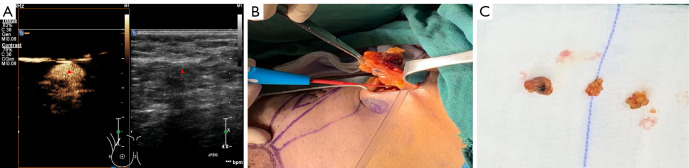
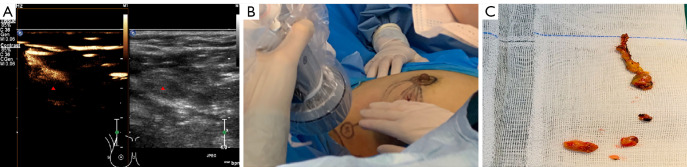
A 52-year-old woman with breast cancer (the triangle represents an SLN). (A) The contrast-enhanced patterns of SLN presented with type I (predicting patients without positive SLN and high-burden metastases). (B) The preoperatively marked SLN showed a blue-stained lymph node during the operation. (C) Three SLNs were identified by intraoperative MB/ICG. All were pathologically negative. SLN, sentinel lymph node; MB, methylene blue; ICG, indocyanine green.
Figure 4.
A 48-year-old woman with breast cancer (the triangle represents an SLN). (A) The contrast-enhanced patterns of SLN with the type III pattern (predicting patients with positive SLN and high-burden metastases). (B) The preoperatively marked SLN showed a fluorescent lymph node during the operation. (C) Four SLNs were identified by intraoperative MB/ICG, of which 3 were pathologically positive. SLN, sentinel lymph node; MB, methylene blue; ICG, indocyanine green.
Discussion
Our study shows that CEUS is a feasible preoperative localization method for SLN, with a detection rate of 95.7% (88/92). However, consistent with other studies, the average number of SLNs per patient identified by CEUS (1.23) was significantly less than that of SLNs identified by MB/ICG (2.63) (19,20). This finding may be related to the inherent properties of commonly used intraoperative SLN tracers, such as MB. The dye molecules used during the SLNB are relatively small and can easily diffuse into secondary lymph nodes or other lymph nodes surrounding the SLN, resulting in the inability to identify true SLN from non-SLN. The number of dissected SLNs is an important risk factor for false-negatives rates during SLNB (21). Although there is currently no consensus on the number of SLNs that should be resected, the larger the number of lymph nodes removed intraoperatively, the higher the risk of developing complications post-surgically (8). Translymphatic CEUS makes it easier to identify true SLNs by displaying them in real-time, and it can avoid damaging draining lymphatic channels by guiding the selection of surgical incisions, which could potentially reduce the false-negative rates of SLNB (19). In addition, our findings demonstrate that the enhancement pattern of CEUS-localized SLN can assist in preoperative prediction of nodal status and metastatic burden, which would be more in line with the concept of SLN.
Specifically, we first evaluated the preoperative risk factors for SLN metastasis in breast cancer patients. The results showed that the only independent risk factor for SLN metastasis was the enhancement pattern on CEUS. ALN breast cancer metastases are usually sequential and are rarely jump metastases (22). When tumor cells invaded lymph nodes at an early stage, substantial changes were observed in lymph node size but not in the structure. The typical lymph node metastatic features include lymph node enlargement, thinning of the medulla, and cortical thickening (23). Therefore, when the appearance of lymph nodes on B-mode ultrasound does not meet the criteria to be considered suspicious, it is impossible to determine whether metastasis has occurred with high sensitivity. The contrast injection used during the CEUS examination makes it easier to identify both normal and metastatic SLNs (24). Considering its high sensitivity to the diagnosis of SLN, we focused on the enhancement pattern of SLN to predict the lymph node burden of breast cancer patients.
We defined the type I enhancement pattern of SLN visualized by CEUS as having no SLN metastasis, with an overall pathological concordance rate of 86.9% (40/46). In contrast, SLN that presented with a type III enhancement pattern was defined as SLN metastasis, with an overall pathological concordance rate of 88.9% (16/18). These data show that types I and III enhancement patterns could be used to discriminate positive from negative SLNs. Another important finding of this study was that more than half of the patients presenting with a type III enhancement pattern had more than 2 metastatic lymph nodes, and some had additional non-SLN metastases. We speculate that this phenomenon may be related to the dynamic pathological process of lymph node invasion (Video S1). Specifically, if the SLN is not invaded and its lymphatic drainage is unobstructed, the contrast agent can move rapidly along the lymphatic vessels to the edge of the SLN and gradually accumulate in the central area, inducing an enhancement of high homogeneity throughout the lymph node (type I). However, if the SLN is invaded, tumor cells will first proliferate via the lymphatic vessels and marginal sinuses. When this proliferation invades localized or multiple lymphatic vessels, a heterogenous enhancement pattern can be observed (type II). The primary input lymphatic vessels can be blocked as the tumor cells continue to proliferate. The tumor eventually destroys the internal structure of the entire lymph node, and the contrast agent does not enter the lymph node (type III) (25-27). These enhancement patterns, which are generally consistent with pathological features of lymph node invasion, could be used by clinicians to preoperatively screen for patients who would truly benefit from SLNB and support the decision to perform more extensive axillary surgery.
Additionally, our results showed that the type II enhancement pattern was cross-linked in differentiating benign and malignant SLNs. The concordance rate with pathology results was only 66.7% (16/24), and the false-positive rate was high. Lymph node inflammation, lymphatic sinus dilatation, and lymphatic vessel reflux appeared as a type II enhancement pattern (28-30). Therefore, the value of the type II enhancement pattern in distinguishing between benign and malignant SLNs needs further study. However, it cannot be ignored that 83.3% (20/24) of patients with a type II enhancement pattern in this study had fewer than 2 SLNs metastases at the definitive surgical treatment. Similar findings were also noted in the study of Zhao et al. (31). When patients with the type II enhancement pattern were combined with those who had the type I enhancement pattern, 91.5% (65/71) of patients were found to have fewer than or equal to 2 positive SLNs at the final surgical treatment. These patients met the Z0011 trial criteria to exempt them from ALND.
The present study had some limitations. First, a low-frequency linear probe (3–9 MHz) was used for all ultrasound examinations. Although this probe provides the best SLN visualization on the Philips iU22 scanner (18,19), a higher resolution ultrasound probe could further improve the reliability of our results when initially recruiting patients using B-mode ultrasound. Second, the surface markers were used in the CEUS identification SLN procedure. Among the patients we recruited, the body position of 1 patient at the time of preoperative CEUS was different from that of the surgical position. The body marks of the lymphatic channel and SLN were inconsistent with the blue-stained lymphatic channel, and the lymph node was neither a fluorescent nor blue-stained SLN, so the patient was excluded from this study. However, previous studies have shown that when patients remained supine, the pathway of lymphatic channels labeled on the body surface was consistent with the MB method (the identification rate was 98.2%, and the consistency rate was 95.8%) (18,32). This supports the imaging technique used in our study. Finally, the sample size in this study was small, limiting the generalizability of the research findings. Therefore, a large, multi-center study is needed to confirm our research results.
Conclusions
CEUS can efficiently and safely localize SLNs in patients with early-stage breast cancer. Patients with the type I and type II contrast-enhanced pattern of visual SLN (91.5%) had fewer than or equal to 2 SLNs metastases at the final surgical treatment and were more likely to benefit from SLNB without increasing the risk of a second operation for ALND. In contrast, patients with the type III contrast-enhanced pattern of visual SLN (70.6%) had a high nodal burden of axillary disease, increasing the risk of undergoing more extensive axillary surgery. These data can be used as one of the therapeutic implementation resources for patient decision support.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by Gansu Province Science and Technology Plan Project (Nos. 21YF5FA122 and 20JR10FA664) and the Cuiying Science and Technology Innovation Program of Lanzhou University Second Hospital (No. CY2021-ZD-02).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval for this retrospective cohort study was granted by the Ethics Committee of Lanzhou University Second Hospital (No. 2022A-256). All participants signed a written informed consent form.
Footnotes
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-234/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-234/coif). The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.Sun J, Mathias BJ, Laronga C, Sun W, Zhou JM, Fulp WJ, Kiluk JV, Lee MC. Impact of Axillary Dissection Among Patients With Sentinel Node-Positive Breast Cancer Undergoing Mastectomy. J Natl Compr Canc Netw 2021;19:40-7. 10.6004/jnccn.2020.7597 [DOI] [PubMed] [Google Scholar]
- 3.Wetzig N, Gill PG, Espinoza D, Mister R, Stockler MR, Gebski VJ, Ung OA, Campbell I, Simes J. Sentinel-Lymph-Node-Based Management or Routine Axillary Clearance? Five-Year Outcomes of the RACS Sentinel Node Biopsy Versus Axillary Clearance (SNAC) 1 Trial: Assessment and Incidence of True Lymphedema. Ann Surg Oncol 2017;24:1064-70. 10.1245/s10434-016-5669-2 [DOI] [PubMed] [Google Scholar]
- 4.Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol 2014;15:e351-62. 10.1016/S1470-2045(13)70590-4 [DOI] [PubMed] [Google Scholar]
- 5.Guo J, Yang H, Wang S, Cao Y, Liu M, Xie F, Liu P, Zhou B, Tong F, Cheng L, Liu H, Wang S. Comparison of sentinel lymph node biopsy guided by indocyanine green, blue dye, and their combination in breast cancer patients: a prospective cohort study. World J Surg Oncol 2017;15:196. 10.1186/s12957-017-1264-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, Ashikaga T, Weaver DL, Mamounas EP, Jalovec LM, Frazier TG, Noyes RD, Robidoux A, Scarth HM, Wolmark N. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 2010;11:927-33. 10.1016/S1470-2045(10)70207-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, He Y, Wang J, Huo L, Fan Z, Li J, Xie Y, Wang T, Ouyang T. Feasibility of using negative ultrasonography results of axillary lymph nodes to predict sentinel lymph node metastasis in breast cancer patients. Cancer Med 2018;7:3066-72. 10.1002/cam4.1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011;305:569-75. 10.1001/jama.2011.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, et al. Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23-01): a phase 3 randomised controlled trial. Lancet Oncol 2013;14:297-305. 10.1016/S1470-2045(13)70035-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:452-78. 10.6004/jnccn.2020.0016 [DOI] [PubMed] [Google Scholar]
- 11.Nielsen Moody A, Bull J, Culpan AM, Munyombwe T, Sharma N, Whitaker M, Wolstenhulme S. Preoperative sentinel lymph node identification, biopsy and localisation using contrast enhanced ultrasound (CEUS) in patients with breast cancer: a systematic review and meta-analysis. Clin Radiol 2017;72:959-71. 10.1016/j.crad.2017.06.121 [DOI] [PubMed] [Google Scholar]
- 12.Hao Y, Sun Y, Lei Y, Zhao H, Cui L. Percutaneous Sonazoid-enhanced ultrasonography combined with in vitro verification for detection and characterization of sentinel lymph nodes in early breast cancer. Eur Radiol 2021;31:5894-901. 10.1007/s00330-020-07639-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coronado-Gutiérrez D, Santamaría G, Ganau S, Bargalló X, Orlando S, Oliva-Brañas ME, Perez-Moreno A, Burgos-Artizzu XP. Quantitative Ultrasound Image Analysis of Axillary Lymph Nodes to Diagnose Metastatic Involvement in Breast Cancer. Ultrasound Med Biol 2019;45:2932-41. 10.1016/j.ultrasmedbio.2019.07.413 [DOI] [PubMed] [Google Scholar]
- 14.Saffar B, Bennett M, Metcalf C, Burrows S. Retrospective preoperative assessment of the axillary lymph nodes in patients with breast cancer and literature review. Clin Radiol 2015;70:954-9. 10.1016/j.crad.2015.04.019 [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Mei J, Yang Y, Gu R, Zhong J, Jiang X, Liu F, Yong J, Wang H, Shen S, Liang J, Liu Q, Gong C. Specimen number based diagnostic yields of suspicious axillary lymph nodes in core biopsy in breast cancer: clinical implications from a prospective exploratory study. Quant Imaging Med Surg 2021;11:2151-61. 10.21037/qims-20-1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Z, Cheng X, Li J, Jiang J, Jiang Z, Li H, Li T, Zhang Z, Tan B, Lu M. Preliminary study of real-time three-dimensional contrast-enhanced ultrasound of sentinel lymph nodes in breast cancer. Eur Radiol 2020;30:1426-35. 10.1007/s00330-019-06494-0 [DOI] [PubMed] [Google Scholar]
- 17.Sharma N, Cox K. Axillary Nodal Staging with Contrast-Enhanced Ultrasound. Curr Breast Cancer Rep 2017;9:259-63. 10.1007/s12609-017-0258-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Lu M, Cheng X, Hu Z, Li H, Wang H, Jiang J, Li T, Zhang Z, Zhao C, Ma Y, Tan B, Liu J, Yu Y. How Pre-operative Sentinel Lymph Node Contrast-Enhanced Ultrasound Helps Intra-operative Sentinel Lymph Node Biopsy in Breast Cancer: Initial Experience. Ultrasound Med Biol 2019;45:1865-73. 10.1016/j.ultrasmedbio.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 19.Zhu Y, Fan X, Yang D, Dong T, Jia Y, Nie F. Contrast-Enhanced Ultrasound for Precise Sentinel Lymph Node Biopsy in Women with Early Breast Cancer: A Preliminary Study. Diagnostics (Basel) 2021;11:2104. 10.3390/diagnostics11112104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo J, Feng L, Zhou Q, Chen Q, Liu J, Wu C, Luo J, Chen J, Wu H, Deng W. The value of contrast-enhanced ultrasound in determining the location of sentinel lymph nodes in breast cancer. Cancer Imaging 2021;21:28. 10.1186/s40644-021-00397-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Jun Z, Zhi-Cheng G, Xiang Q. Factors that affect the false negative rate of sentinel lymph node mapping with methylene blue dye alone in breast cancer. J Int Med Res 2019;47:4841-53. 10.1177/0300060519827413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jatoi I. Management of the axilla in primary breast cancer. Surg Clin North Am 1999;79:1061-73. 10.1016/S0039-6109(05)70061-X [DOI] [PubMed] [Google Scholar]
- 23.Bedi DG, Krishnamurthy R, Krishnamurthy S, Edeiken BS, Le-Petross H, Fornage BD, Bassett RL, Jr, Hunt KK. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: in vitro sonographic study. AJR Am J Roentgenol 2008;191:646-52. 10.2214/AJR.07.2460 [DOI] [PubMed] [Google Scholar]
- 24.Niu Z, Xiao M, Ma L, Qin J, Li W, Zhang J, Zhu Q, Jiang Y. The value of contrast-enhanced ultrasound enhancement patterns for the diagnosis of sentinel lymph node status in breast cancer: systematic review and meta-analysis. Quant Imaging Med Surg 2022;12:936-48. 10.21037/qims-21-416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sever AR, Mills P, Jones SE, Cox K, Weeks J, Fish D, Jones PA. Preoperative sentinel node identification with ultrasound using microbubbles in patients with breast cancer. AJR Am J Roentgenol 2011;196:251-6. 10.2214/AJR.10.4865 [DOI] [PubMed] [Google Scholar]
- 26.Cox K, Sever A, Jones S, Weeks J, Mills P, Devalia H, Fish D, Jones P. Validation of a technique using microbubbles and contrast enhanced ultrasound (CEUS) to biopsy sentinel lymph nodes (SLN) in pre-operative breast cancer patients with a normal grey-scale axillary ultrasound. Eur J Surg Oncol 2013;39:760-5. 10.1016/j.ejso.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 27.Zhong J, Sun DS, Wei W, Liu X, Liu J, Wu X, Zhang Y, Luo H, Li Y. Contrast-Enhanced Ultrasound-Guided Fine-Needle Aspiration for Sentinel Lymph Node Biopsy in Early-Stage Breast Cancer. Ultrasound Med Biol 2018;44:1371-8. 10.1016/j.ultrasmedbio.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 28.Goldberg BB, Merton DA, Liu JB, Thakur M, Murphy GF, Needleman L, Tornes A, Forsberg F. Sentinel lymph nodes in a swine model with melanoma: contrast-enhanced lymphatic US. Radiology 2004;230:727-34. 10.1148/radiol.2303021440 [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Wang W, Li J, Tang J. Gray-scale contrast-enhanced ultrasonography of sentinel lymph nodes in a metastatic breast cancer model. Acad Radiol 2009;16:957-62. 10.1016/j.acra.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Liu X, He J, Gou B, Luo Y, Deng S, Wen H, Zhou L. Percutaneous contrast-enhanced ultrasound for localization and diagnosis of sentinel lymph node in early breast cancer. Sci Rep 2019;9:13545. 10.1038/s41598-019-49736-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J, Zhang J, Zhu QL, Jiang YX, Sun Q, Zhou YD, Wang MQ, Meng ZL, Mao XX. The value of contrast-enhanced ultrasound for sentinel lymph node identification and characterisation in pre-operative breast cancer patients: A prospective study. Eur Radiol 2018;28:1654-61. 10.1007/s00330-017-5089-0 [DOI] [PubMed] [Google Scholar]
- 32.Shimazu K, Miyake T, Tanei T, Naoi Y, Shimoda M, Kagara N, Kim SJ, Noguchi S. Real-Time Visualization of Lymphatic Flow to Sentinel Lymph Nodes by Contrast-Enhanced Ultrasonography with Sonazoid in Patients with Breast Cancer. Ultrasound Med Biol 2019;45:2634-40. 10.1016/j.ultrasmedbio.2019.07.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as