Abstract
Background
Polymyositis (PM) and dermatomyositis (DM) are two common types of idiopathic inflammatory myopathy and can lead to a poor prognosis and quality of life. We designed this cross-sectional study to investigate the abilities of high-frequency ultrasound (HFUS) and shear wave elastography (SWE) to assess muscle properties in patients with PM and DM and to distinguish healthy muscles from diseased muscles with PM and DM.
Methods
A total of 60 patients (26 PM cases and 34 DM cases) and 65 matched healthy volunteers were continuously included in the case and control groups, respectively. For the bilateral deltoid, biceps brachii, rectus femoris, and vastus lateralis, the muscle thickness, echo intensity, and longitudinal shear wave velocity (SWV) of all participants were measured using HFUS and SWE. The intra- and interobserver reliability of SWV measurements of patients with PM and DM and the receiver operating characteristic curve for HFUS and SWE for PM and DM were analyzed.
Results
Patients with PM and DM had significantly decreased muscle thickness and increased muscle echo intensity compared to healthy controls (P<0.001). The patients’ and healthy participants’ deltoid, biceps brachii, rectus femoris, and vastus lateralis thickness was 19.75 and 23.00 mm, 20.45 and 22.80 mm, 18.40 and 20.20 mm, and 20.00 and 22.80 mm, respectively. Except for the biceps brachii, the mean SWV in the longitudinal orientation in patients with PM and DM significantly decreased (P<0.01). The mean SWV of the patients’ and healthy participants’ deltoid, rectus femoris, and vastus lateralis was 2.47 and 2.57 m/s, 1.73 and 1.87 m/s, and 1.57 and 1.77 m/s, respectively. Excellent intra- and interobserver reliability of SWV measurements on the deltoid and rectus femoris of PM and DM patients were found (intraclass correlation coefficient >0.95; P<0.001). The diagnostic performance of echo intensity in lower-extremity proximal muscles for PM and DM was excellent [area under the curve (AUC) >0.9]. The thickness of most muscles displayed moderate diagnostic performance (the AUC ranged from 0.700 to 0.775). The SWV of the vastus lateralis showed a stable performance (AUC =0.741). The combined diagnostic performance of echo intensity and thickness and the combined diagnostic performance of the 3 indicators were relatively high (the AUC ranged from 0.871 to 0.936 and from 0.898 to 0.938, respectively). Muscle thickness and echo intensity showed statistical differences in different disease stages of PM and DM (P'<0.01).
Conclusions
HFUS and SWE may serve as imaging biomarkers for the diagnosis of PM and DM by detecting abnormal muscle thinning, enhanced muscle echo intensity, and reduced muscle SWV.
Keywords: Polymyositis (PM), dermatomyositis (DM), muscle, shear wave elastography (SWE), ultrasound
Introduction
Polymyositis (PM) and dermatomyositis (DM) are idiopathic inflammatory myopathies (IIM), which are group of autoimmune diseases characterized by skeletal muscle inflammation and muscle weakness (1). DM is often accompanied by characteristic dermatological lesions. Early diagnosis and assessment of patients with PM and DM is important because they often have poor prognoses and quality of life (2,3). The main methods for diagnosing and evaluating PM and DM are serological test, electromyography (EMG), muscle biopsy, and imaging. Besides being invasive, the muscle biopsy may lead to a false-negative diagnosis, particularly from non-imaging guided biopsy. A serological test cannot reflect specific muscle involvement, while myogenic lesions suggested by EMG are important indicators of muscle damage, but they are not specific to inflammatory myopathy. Additionally, neither serological test nor EMG can provide muscle morphological information.
Imaging techniques, such as magnetic resonance imaging (MRI) and ultrasound, have variable validity and utility in the setting of IIM (4). MRI is useful in the qualitative assessment of muscle and the determination of the muscle biopsy site and may be useful for IIM subclassification (5-7). However, the feasibility of MRI is limited by cost, accessibility, patient tolerability, and contraindications. Magnetic resonance elastography (MRE) is a relatively new imaging modality for evaluating tissue elasticity, and its feasibility in assessing IIM has been demonstrated (8). However, the protocols still require refinement and validation before being translated for use in the clinical evaluation of IIM (4).
Musculoskeletal ultrasonography has developed rapidly in recent years and showed promising utility in investigating musculoskeletal disorders (9). Muscle echogenicity—the characteristic of tissues to reflect ultrasound waves—is typically used as a sonographic estimate of muscle quality (10). The quantification of muscle echo intensity has been proven to be valuable in assessing muscle status and disease (11,12). Shear wave elastography (SWE), a new ultrasound technology with the ability to evaluate tissue elasticity and stiffness, can superimpose the elastic image on the grayscale image in real time and obtain the shear wave velocity (SWV) of the region of interest (ROI). Young’s modulus is used to represent the mechanical properties of tissues. It is converted from SWV based on the assumptions of constant density, homogeneity, isotropy, and incompressibility using the following equation: E = 3ρc2 (where E is Young’s modules, ρ is the volume density of the tissue, and c is the velocity) (13). However, in anisotropic tissues, such as muscles and tendons, SWV was reported to be more reliable than was Young’s modulus (14,15). SWE has been widely used in the liver, thyroid, and breast and has potential in determining musculoskeletal diseases (16). The feasibility, reliability, and repeatability of SWE in quantifying normal and diseased muscle stiffness have been confirmed (17-20).
Thus far, few studies have used ultrasound elastography to evaluate muscles in patients with inflammatory myopathy; moreover, in the studies that have been conducted, the sample sizes were relatively small, and the conclusions were inconsistent (21). Using strain elastography, Song et al. (22) found increased muscle stiffness in patients with inflammatory myopathy. Using SWE, Alfuraih et al. (23) detected significantly reduced thigh muscle stiffness in patients with active IIM. Given the deficiencies and discrepancies of recent studies, more in-depth studies with larger sample sizes are urgently needed. Our study quantitatively evaluated the thickness, echo intensity, and longitudinal SWV of proximal limb muscles in patients with PM and DM and compared them with those in healthy populations to explore the application value of high-frequency ultrasound (HFUS) and SWE in PM and DM. We aimed to provide objective indicators for the clinical diagnosis and evaluation of PM and DM. We present the following article in accordance with the STARD reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-423/rc).
Methods
This study was approved by the Ethics Committee of West China Hospital of Sichuan University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all participants for publication of this study and any accompanying images.
Study participants
Between January 2018 and January 2020, 60 patients with PM and DM at West China Hospital of Sichuan University were continuously enrolled. The inclusion criteria were in line with the widely accepted Bohan and Peter diagnostic criteria for PM and DM (24) and include (I) symmetrical proximal weakness, (II) elevation of serum muscle enzymes, (III) myogenic damage on EMG, (IV) characteristic pathological changes in muscle biopsy, and (V) characteristic dermatological lesions. The patient was diagnosed with PM if the first 4 criteria were met. DM was diagnosed if the patient had characteristic dermatological lesions and met any 3 of the first 4 criteria. The exclusion criteria were the presence of other skeletal muscle diseases, such as neuromyopathy, infective myositis, or muscular dystrophy; and abnormalities in checked regions, such as trauma, scars, tumors, or a history of surgery. The 60 patients with PM and DM were divided into acute stage (≤6 months) and chronic stage groups according to the duration of their symptoms. For controls, 65 healthy volunteers were continuously recruited during the same period via advertisements near our hospital. Healthy volunteers were included if (I) the muscle strength of their extremities was scored as grade 5 by an experienced rehabilitation physician using manual muscle testing; (II) their age, gender ratio, and body mass index (BMI) were basically matched with the case group; and (III) no muscle disease, arthritis, neuropathy, or other conditions were present that could affect the muscle. Healthy volunteers were excluded if they had abnormalities in checked regions, such as trauma, scars, tumors; or a history of surgery. Participant grouping and general condition assessment were performed by experienced clinicians who did not know the participants’ ultrasound results.
HFUS and SWE examination
The sonographic equipment employed was the Aixplorer (Supersonic Imagine, Aix-en-Provence, France) system with the SuperLinear SL10-2 MHz probe. The superficial musculoskeletal mode was preset. The grayscale imaging gain was set to 50% for the measurement of thickness and acquisition of a grayscale image for the echo intensity measurement. The maximal scale was adjusted to 180 kPa for the measurement of SWV. Considering that PM and DM usually involve the proximal muscles of the extremities, the bilateral deltoid, biceps brachii, rectus femoris, and vastus lateralis were investigated. To obtain images of the deltoid, participants were asked to sit in a neutral position with their forearms on their thighs and palms facing up. We carefully scanned the entire deltoid to determine its extent and measured the muscle thickness, echo intensity, and SWV at 50% of the middle bundle of the deltoid. The biceps brachii was evaluated at the proximal one-third between the axilla and elbow fossa with the participant in a supine position. The rectus femoris and vastus lateralis were assessed at a proximal one-third distance between the anterior superior iliac spine and the proximal border of the patella, with the participants supine with their lower limbs in a neutral position. The position of the patient and probe is shown in Figure 1.
Figure 1.
The position of the patient and probe when scanning was performed in the longitudinal orientation. The deltoid (A), biceps brachii (B), rectus femoris (C), and vastus lateralis (D). When scanning was performed in the transverse orientation, the position was the same, and the probe was rotated 90°.
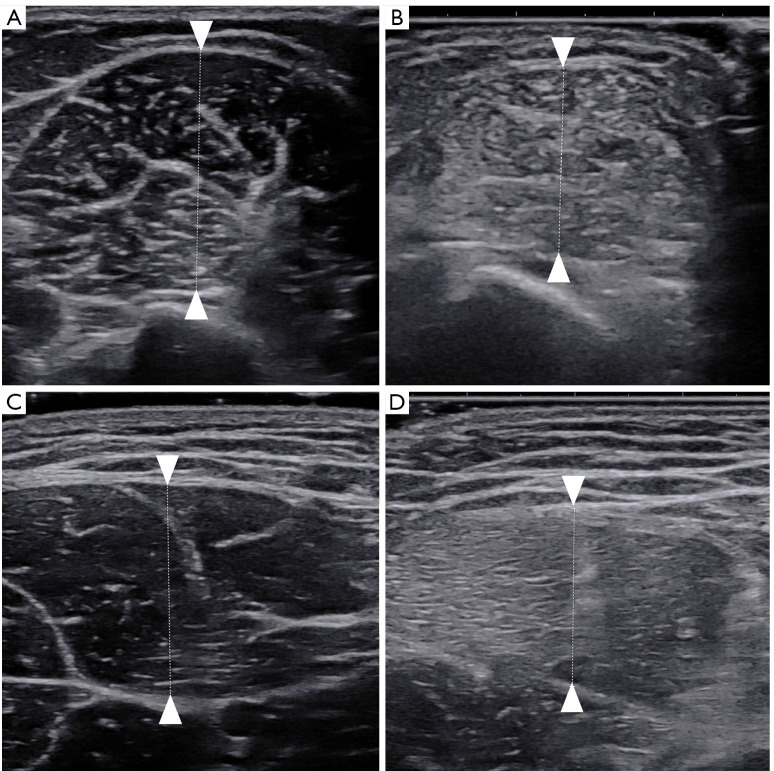
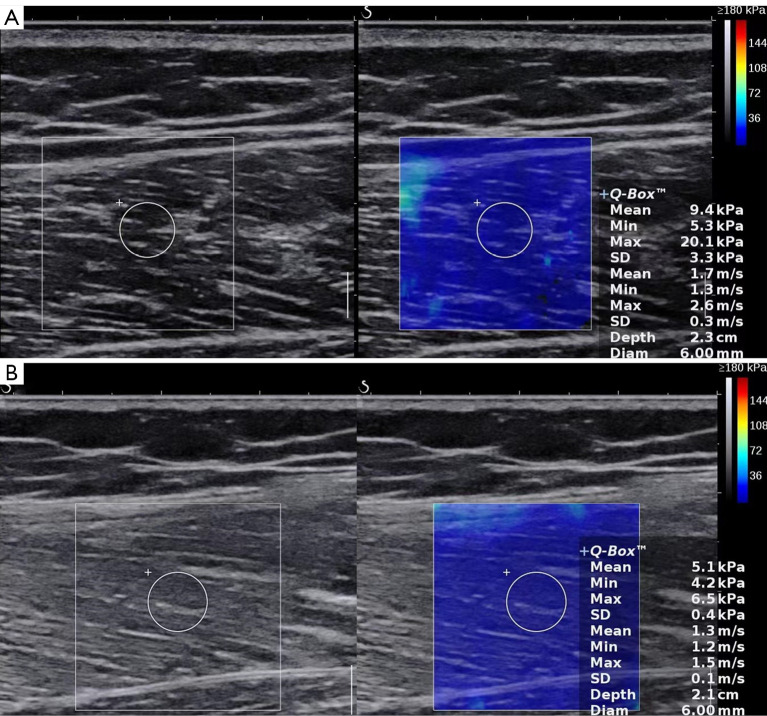
The muscle thickness was measured in the transverse orientation and was measured as the distance between the superficial and deep aponeuroses (Figure 2). The measurements were repeated 3 times, and the average value was calculated and recorded in millimeters. Muscle SWV was measured in the longitudinal orientation. The probe was oriented along the muscle fibers. After switching to the SWE mode, the operator maintained the transducer for a few seconds to obtain a stable color-coded SWE image. The ROI was moved to include the scanned muscle. A circular Q-box was placed at the measured position and fixed to a diameter of 6 mm. The mean SWV value of the muscle automatically generated by the system was recorded. The measurements were repeated 3 times, and the average value was recorded in meters per second (m/s). Figure 3 shows the longitudinal SWE image of the vastus lateralis.
Figure 2.
Measurement of muscle thickness in an HFUS image. The biceps brachii in a healthy control (A) and in a patient with PM or DM (B); the rectus femoris in a healthy control (C) and in a patient with PM or DM (D). HFUS, high-frequency ultrasound; PM, polymyositis; DM, dermatomyositis.
Figure 3.
The mean shear wave velocity of the vastus lateralis in a healthy control (A) and in a patient with PM or DM (B) was 1.7 and 1.3 m/s, respectively. PM, polymyositis; DM, dermatomyositis.
The examination was undertaken by 2 trained sonographers with at least 5 years of experience each in musculoskeletal ultrasound scanning. The 2 sonographers did not know the grouping of participants when performing the ultrasound examination. The participants rested quietly for 30 minutes before scanning and were instructed to relax their muscles in all positions when scanning was being performed. The probe covered with several millimeters of ultrasound gel was placed perpendicularly and smoothly to the skin. Minimal pressure was applied to the transducer. HFUS and SWE acquisitions were repeated 3 times per muscle.
Measurement of echo intensity
Quantitative muscle ultrasound was employed for echo intensity analysis on the saved images. Photoshop CC 2019 (Adobe, San Jose, CA, USA) was used to measure the echo intensity of the grayscale image of muscle in the longitudinal section, in similar fashion to what was done in previous studies (10,25). The sample box size was set to 64×64 pixels and placed in the Q-box of the SWE grayscale image for measurement. The average value of the grayscale was then recorded. The measurements were repeated 3 times.
Statistical analysis
The data analysis was performed using SPSS version 26.0 (IBM Corp., Armonk, NY, USA). The categorical variables are expressed as frequencies. Age and BMI are expressed as the mean ± standard deviation. The disease duration, thickness, echo intensity, and SWV are expressed as the median (quartile). Intra- and interobserver reliability of SWV measurements in the longitudinal orientation was analyzed using the intraclass correlation coefficient (ICC). The difference in the sexual proportion between patients with PM and DM and healthy controls was tested using the chi-squared test. Age and BMI were compared using the t test. Muscle thickness, echo intensity, and SWV were compared using the Mann-Whitney test. Receiver operating characteristic (ROC) curves were plotted to evaluate the performance of HFUS and SWE in discriminating participants with or without PM or DM. The discrimination was quantified with the area under the curve (AUC). The Kruskal-Wallis test was used to compare the control, acute stage, and chronic stage groups. If the differences were statistically significant, we proceeded to use post hoc pairwise comparisons and adjusted the test level according to Bonferroni’s method (P'=P/N; P=0.05; N=3). Spearman correlation coefficients were calculated to correlate thickness, echo intensity, and SWV of muscle with disease duration. All tests were 2-tailed. A P value less than 0.05 and P' value less than 0.05/3 indicated a statistically significant difference.
Results
Patients and characteristics
A total of 60 patients diagnosed with PM or DM participated in this study, including 34 with DM and 26 with PM. A total of 21 patients were in the acute stage and 39 were in the chronic stage. The ratio of men to women in patients with PM and DM was 1:1.9, which was basically consistent with the epidemiological characteristics of PM and DM reported in a previous study (26). No significant difference was found in sex ratio, age, and BMI between patients and healthy controls (P>0.05). The descriptive characteristics were represented and tested for differences as shown in Table 1.
Table 1. Characteristics of the study participants.
| Characteristics | PM and DM (n=60) | Healthy controls (n=65) | P value* | Acute stage (n=21) | Chronic stage (n=39) | P value* |
|---|---|---|---|---|---|---|
| Female/male | 39/21 | 44/21 | 0.750 | 12/9 | 27/12 | 0.349 |
| Age (year) | 44.25±12.51 | 43.80±11.64 | 0.835 | 48.33±10.66 | 42.05±13.00 | 0.063 |
| BMI (kg/m2) | 21.18±2.76 | 21.82±2.03 | 0.146 | 21.04±2.34 | 21.26±2.99 | 0.763 |
| Disease duration (months) | 9 (5, 15) | – | – | 3 (2, 5.5) | 13 (9, 23) | – |
*, a P value below 0.05 indicates a significant difference. Continuous variables were tested using the independent samples t test or the Mann-Whitney test. Categorical variables were tested using the chi-squared test. Data of age and BMI are expressed as the mean ± standard deviation. Disease duration is expressed as the median (upper quartile, lower quartile). BMI, body mass index; PM, polymyositis; DM, dermatomyositis.
Results of HFUS and SWE
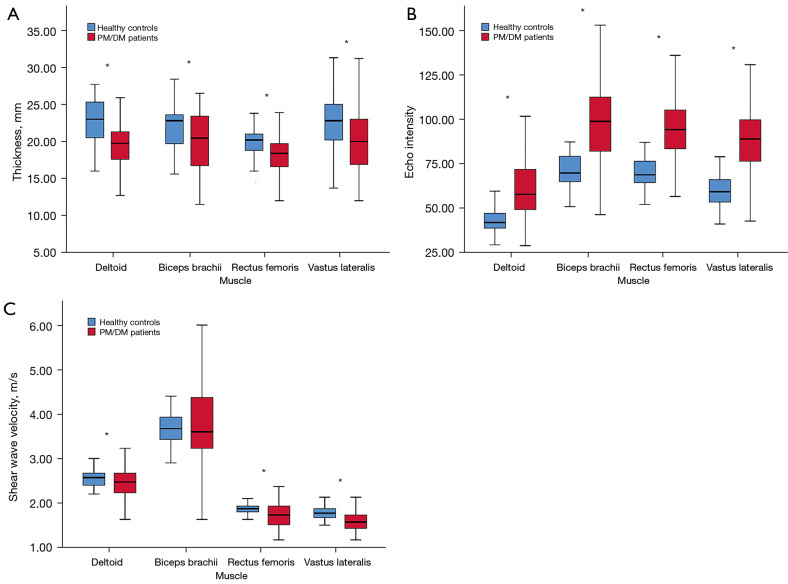
The results showed that the thickness of the deltoid, biceps, rectus femoris, and vastus lateralis decreased in patients with PM and DM (P<0.001), and the echo intensity of all muscles increased (P<0.001).
The SWV values of the deltoid, rectus femoris, and vastus lateralis in patients with PM and DM were lower than those in healthy controls (P<0.01). Although the SWV value of the biceps brachii in PM and DM also decreased, the difference was not statistically significant (P>0.05). Table S1 reports the descriptive data of muscle thickness, echo intensity, and SWV measurements. The clustered boxplots of muscle thickness, echo intensity, and SWV by participant type are shown in Figure 4. Excellent intra- and interobserver reliability of SWV measurements on the deltoid and rectus femoris of the patients with PM and DM were found (ICC >0.95; P<0.001; Table S2).
Figure 4.
Clustered boxplots of muscle thickness (A), echo intensity (B), and shear wave velocity (C) by participant type (healthy controls and PM/DM patients). *, indicates a statistically significant difference. PM, polymyositis; DM, dermatomyositis.
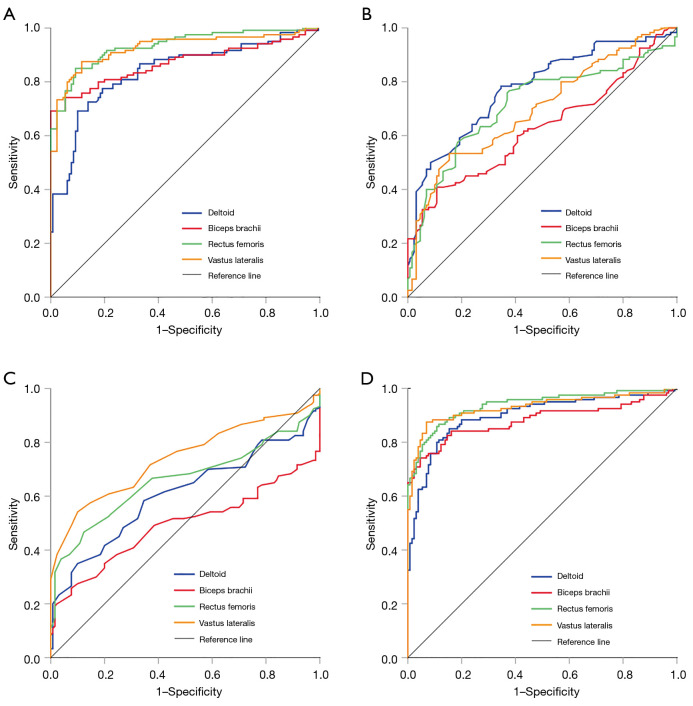
The ROC curves were plotted to evaluate the diagnostic performance of thickness, echo intensity, and SWV for patients with PM and DM (Figure 5). The echo intensity had an excellent level of discrimination. The AUC of the rectus femoris and vastus lateralis was 0.933 [95% confidence interval (CI): 0.902–0.964] and 0.926 (95% CI: 0.891–0.961), respectively. Except for the biceps brachii, the diagnostic performance of the thickness of other muscles was moderate (the AUC ranged from 0.700 to 0.775). The discrimination ability of SWV of different muscles showed variability, among which the SWV of the vastus lateralis showed stable discrimination performance (AUC =0.741). When combining the aforementioned indicators, the combined diagnostic performance of echo intensity and thickness and the combined diagnostic performance of the 3 indicators were relatively high (the AUC ranged from 0.871 to 0.936 and from 0.898 to 0.938, respectively).
Figure 5.
ROC curves of muscle echo intensity, thickness, and shear wave velocity in the diagnosis of PM and DM. ROC curves of echo intensity (A) of the deltoid, biceps brachii, rectus femoris, and vastus lateralis. ROC curves of thickness (B); shear wave velocity (C); and the combination of echo intensity, thickness, and shear wave velocity (D). ROC, receiver operating characteristic; PM, polymyositis; DM, dermatomyositis.
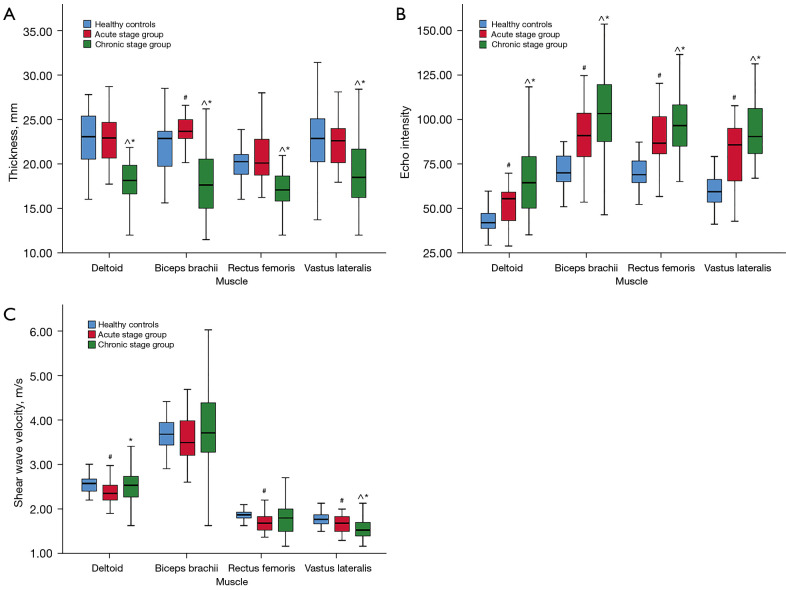
Compared with the control and acute stage groups, muscle thickness of the chronic stage group was significantly decreased (P'<0.01). Correlation analysis results suggested a low to moderate negative correlation between muscle thickness and disease duration in patients with PM and DM (the r value ranged from –0.307 to –0.528; P<0.01). Compared with the control group, the muscle echo intensity of the patients with PM and DM in both the acute stage and chronic stage groups was significantly increased (P'<0.01). The muscle echo intensity of the chronic stage group was higher than that in the acute stage group (P'<0.01). Except for the biceps, SWV in the acute stage group was lower than that in the control group (P’<0.01). Except for the vastus lateralis, there was no significant difference in SWV between the chronic stage and control groups. The clustered boxplots in Figure 6 graphically represent the results for the various muscles of the 3 groups. The descriptive data are provided in Table S3.
Figure 6.
Clustered boxplots of muscle thickness (A), echo intensity (B), and shear wave velocity (C) by grouping type (healthy controls, acute stage group and chronic stage group). #, Comparison between the healthy controls and the acute stage group, statistically significant difference. ^, Comparison between the healthy controls and the chronic stage group, statistically significant difference. *, Comparison between the acute stage group and the chronic stage group, statistically significant difference.
Discussion
This study was novel in simultaneously quantifying the muscle thickness, echo intensity, and SWV in patients with PM and DM. HFUS and SWE could detect abnormal muscle thinning, enhanced muscle echo intensity, and reduced muscle SWV in these patients. Differences between patients and healthy controls suggested the altered properties of diseased muscles.
The results regarding the decreased thickness of muscles in PM and DM suggested the presence of muscle atrophy. Early research similarly found muscle atrophy and thinning in IIM (27). Previous studies have shown that many muscle diseases usually manifest as increased muscle echogenicity (11), reflecting pathological changes such as muscle fibrosis and fatty infiltration (27,28). Through quantitative evaluation and analysis, this study found that the muscle echo intensity was significantly enhanced in patients with PM and DM compared with healthy controls, which was consistent with the results of previous studies (27,29,30). These findings suggest that the ultrasound might perform well in diagnosing PM and DM. Reimers et al. (27) found that different types of inflammatory myopathy exhibited varying degrees of echo enhancement in different muscles. Through histopathological examination, they proposed that muscles with fatty infiltration have higher echo intensity than those muscles without fatty infiltration. By quantifying muscle echo intensity, Albayda et al. (30) found that the echo intensity of muscles in patients with inclusion body myositis, PM, and DM was higher than that in normal participants, and they proposed that muscle echo intensity has the ability to differentiate IIM subtypes and identify subclinical muscle involvement. Our study similarly indicated that a quantitative musculoskeletal ultrasound may provide more clinically useful information than conventional ultrasound.
In terms of the lower SWV measurements of muscles in patients with PM and DM (except for the biceps brachii, the differences were statistically significant), our results supported the validity of SWE. The pathological mechanisms for this included muscle fiber damage and surrounding environment change. Inflammation leads to the destruction of extracellular matrix and the increase in inflammatory exudates and intracellular and extracellular water content (23). As the disease progresses, muscle fibers are destroyed, which is accompanied by the loss of contractile properties and muscle atrophy. Alfuraih et al. (23) found that the more severe the muscle edema and atrophy shown by MRI are, the lower the muscle SWV. The fatty infiltration of muscle is another manifestation in the chronic course of IIM. Bachasson et al. (31) proposed that reduced muscle stiffness might be associated with increased muscle fat content. Rosskopf et al. (32) demonstrated that fatty infiltration after muscle injury can impact muscle SWV, but Alfuraih et al. (23) did not find a correlation between the muscle SWV and MRI score of fatty infiltration. Previous studies reported conflicting findings concerning the change in muscle SWV or stiffness in inflammatory myopathy. Alfuraih et al. (23) detected lower thigh muscle stiffness in patients with IIM compared with healthy controls using SWE. McCullough et al. (8) similarly found a noticeable trend of reduced muscle stiffness in patients with myositis using MRE. However, not all studies observed decreased muscle stiffness in inflammatory myopathy. Using strain elastography, Song et al. (22) found increased muscle stiffness in DM and PM. This discrepancy might be related to the differences in elastography modalities and the limitations of strain elastography. Another study demonstrated that transverse-orientation SWE might serve as an imaging biomarker for myositis diagnosis by displaying increased SWV values and a heterogeneous pattern of inflamed muscles (33). Unlike previous studies, the study by Kolb et al. (33) confirmed the value of transverse-orientation SWE in the diagnosis of myositis, but the limitation of their study was that the participants were not distinguished by the type of myositis and muscle. Despite the differences between our findings and those of previous research, our study effectively proved the value of SWE in evaluating PM and DM. The differences in the stages of inflammation and the severity of muscle damage might partly account for the discrepancies between studies. Furthermore, the dermatological lesions of DM might impact the propagation of shear waves through the skin layer to muscle, thus affecting the measurement of muscle SWV. Therefore, the influence of skin on muscle SWV and/or stiffness measurement is worth considering.
The results of the ROC curve analysis indicated that the performance for PM and DM diagnosis of echo intensity in lower-extremity proximal muscles was excellent. Besides distinguishing patients with IIM from the healthy populations, quantified muscle echo intensity was also found to be useful in assessing disease activity, monitoring disease course, and reflecting treatment response (34,35). A significant correlation was found between muscle echo intensity and clinical indicators in juvenile dermatomyositis (34). The thickness of most of the investigated muscles exhibited moderate diagnostic performance in our study, which also supports the validity of using ultrasound imaging in cases of PM and DM. The SWV of the vastus lateralis showed stable performance. Alfuraih et al. (23) concluded that the thigh muscle SWV had a strong diagnostic performance for IIM, but the performance of the biceps brachii was not much better than random chance. Overall, the diagnostic performance of lower-extremity proximal muscles for PM and DM tends to be higher than that of upper-extremity proximal muscles, which might be explained by the different muscle involvement degrees. Therefore, our study proposed that the lower-extremity proximal muscles might be more valuable in the research and diagnosis of PM and DM. However, multiple muscles should be evaluated with ultrasound imaging because of the heterogeneity of myopathy and the variation between individuals. The combination of echo intensity, thickness, and SWV showed strong diagnostic performance in this study, but the AUC values did not improve significantly. As a result, the extent to which the combination of these indicators can improve diagnostic performance remains to be explored.
Significant reduction in muscle thickness in the chronic stage indicated a decrease in muscle mass, suggesting muscle atrophy. Increased echo intensity and changes in SWV in the acute and chronic stages reflected changes in muscle properties resulting from muscle edema, muscle fiber destruction, and fatty infiltration. Fatty infiltration increases with IIM disease duration (5,36), which may partly explain why the echo intensity in the chronic stage is higher than that in the acute stage. Differences in muscle thickness, echo intensity, and SWV between the acute and chronic stage groups suggested differences in the changes of muscles related to disease progression and duration prolongation. This indicated that PM and DM patients might have different clinical symptoms and manifestations at different disease stages. Further research on the relationship between muscle ultrasound performance and clinical indicators will clarify the utility of ultrasonography in evaluating PM and DM.
Several methodological challenges need to be addressed. In anisotropic tissues, such as muscles and tendons, the exact relationship between Young’s modulus and SWV remains unclear. Under pathological conditions, muscle heterogeneity tends to be more prominent. Thus, our study reported SWV rather than elasticity or stiffness. In addition, only the longitudinal-orientation SWV of muscle was measured. Which orientation—transverse or longitudinal—better reflects the muscle properties in inflammatory myopathy is still inconclusive. Combining these two imaging methods may prove useful, as they presumably reflect different aspects of muscle abnormalities. Despite the lack of standardized scoring systems and validation in IIM, HFUS and SWE showed validity compared to other outcome measurements.
Despite our best efforts to standardize the research process, this study still had limitations. First, the measurements of thickness, echo intensity, and SWV could not distinguish between factors that might contribute to the changes in muscle properties, such as muscle fiber damage or extracellular matrix changes. Correlating ultrasound with MRI and histopathology may allow for the elucidation of the exact nature of the changes seen on ultrasound images. Second, this study did not investigate the relationship between muscle SWV values and the severity of muscle damage, which is one of the most relevant potential applications of SWE in IIM. Third, the lack of inclusion of children was also a limitation of this study since DM has a peak incidence in children. Finally, disease activity and treatment were expected to influence the measurement value. Grouping participants according to these conditions may make the study more robust. Current and future studies of our group aim to address these limitations.
Conclusions
Patients with PM and DM showed thinner muscle thickness, higher muscle echo intensity, and lower muscle SWV compared with matched healthy controls. By combining HFUS with SWE, the diseased muscles of patients with PM and DM could be assessed comprehensively in a muscle-specific, noninvasive, efficient, and quantitative manner.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the 1·3·5 Project for Disciplines of Excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (No. 2020HXFH001); and the National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (No. Z2021LC002).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of West China Hospital of Sichuan University. Written informed consent was obtained from the patients for publication of this study and any accompanying images.
Footnotes
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-423/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-423/coif). The authors have no conflicts of interest to declare.
References
- 1.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003;362:971-82. 10.1016/S0140-6736(03)14368-1 [DOI] [PubMed] [Google Scholar]
- 2.Marie I, Hachulla E, Hatron PY, Hellot MF, Levesque H, Devulder B, Courtois H. Polymyositis and dermatomyositis: short term and longterm outcome, and predictive factors of prognosis. J Rheumatol 2001;28:2230-7. [PubMed] [Google Scholar]
- 3.Ponyi A, Borgulya G, Constantin T, Váncsa A, Gergely L, Dankó K. Functional outcome and quality of life in adult patients with idiopathic inflammatory myositis. Rheumatology (Oxford) 2005;44:83-8. 10.1093/rheumatology/keh404 [DOI] [PubMed] [Google Scholar]
- 4.Paramalingam S, Counsel P, Mastaglia FL, Keen H, Needham M. Imaging in the diagnosis of idiopathic inflammatory myopathies; indications and utility. Expert Rev Neurother 2019;19:173-84. 10.1080/14737175.2019.1572507 [DOI] [PubMed] [Google Scholar]
- 5.Huang ZG, Gao BX, Chen H, Yang MX, Chen XL, Yan R, Lu X, Shi KN, Chan Q, Wang GC. An efficacy analysis of whole-body magnetic resonance imaging in the diagnosis and follow-up of polymyositis and dermatomyositis. PLoS One 2017;12:e0181069. 10.1371/journal.pone.0181069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van De Vlekkert J, Maas M, Hoogendijk JE, De Visser M, Van Schaik IN. Combining MRI and muscle biopsy improves diagnostic accuracy in subacute-onset idiopathic inflammatory myopathy. Muscle Nerve 2015;51:253-8. 10.1002/mus.24307 [DOI] [PubMed] [Google Scholar]
- 7.Yoshida K, Kurosaka D, Joh K, Matsushima S, Takahashi E, Hirai K, Noda K, Ukichi T, Furuya K, Yanagimachi M, Kingetsu I, Fukuda K, Yamada A. Fasciitis as a common lesion of dermatomyositis, demonstrated early after disease onset by en bloc biopsy combined with magnetic resonance imaging. Arthritis Rheum 2010;62:3751-9. 10.1002/art.27704 [DOI] [PubMed] [Google Scholar]
- 8.McCullough MB, Domire ZJ, Reed AM, Amin S, Ytterberg SR, Chen Q, An KN. Evaluation of muscles affected by myositis using magnetic resonance elastography. Muscle Nerve 2011;43:585-90. 10.1002/mus.21923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sconfienza LM, Albano D, Allen G, Bazzocchi A, Bignotti B, Chianca V, et al. Clinical indications for musculoskeletal ultrasound updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) consensus. Eur Radiol 2018;28:5338-51. 10.1007/s00330-018-5474-3 [DOI] [PubMed] [Google Scholar]
- 10.Harris-Love MO, Seamon BA, Teixeira C, Ismail C. Ultrasound estimates of muscle quality in older adults: reliability and comparison of Photoshop and ImageJ for the grayscale analysis of muscle echogenicity. PeerJ 2016;4:e1721. 10.7717/peerj.1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shklyar I, Geisbush TR, Mijialovic AS, Pasternak A, Darras BT, Wu JS, Rutkove SB, Zaidman CM. Quantitative muscle ultrasound in Duchenne muscular dystrophy: a comparison of techniques. Muscle Nerve 2015;51:207-13. 10.1002/mus.24296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaguchi K, Tohara H, Hara K, Nakane A, Yoshimi K, Nakagawa K, Minakuchi S. Factors associated with masseter muscle quality assessed from ultrasonography in community-dwelling elderly individuals: A cross-sectional study. Arch Gerontol Geriatr 2019;82:128-32. 10.1016/j.archger.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 13.Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol 2015;41:1126-47. 10.1016/j.ultrasmedbio.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 14.Royer D, Gennisson JL, Deffieux T, Tanter M. On the elasticity of transverse isotropic soft tissues (L). J Acoust Soc Am 2011;129:2757-60. 10.1121/1.3559681 [DOI] [PubMed] [Google Scholar]
- 15.Alfuraih AM, O'Connor P, Hensor E, Tan AL, Emery P, Wakefield RJ. The effect of unit, depth, and probe load on the reliability of muscle shear wave elastography: Variables affecting reliability of SWE. J Clin Ultrasound 2018;46:108-15. 10.1002/jcu.22534 [DOI] [PubMed] [Google Scholar]
- 16.Taljanovic MS, Gimber LH, Becker GW, Latt LD, Klauser AS, Melville DM, Gao L, Witte RS. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics 2017;37:855-70. 10.1148/rg.2017160116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandenburg JE, Eby SF, Song P, Zhao H, Landry BW, Kingsley-Berg S, Bamlet WR, Chen S, Sieck GC, An KN. Feasibility and reliability of quantifying passive muscle stiffness in young children by using shear wave ultrasound elastography. J Ultrasound Med 2015;34:663-70. 10.7863/ultra.34.4.663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshitake Y, Takai Y, Kanehisa H, Shinohara M. Muscle shear modulus measured with ultrasound shear-wave elastography across a wide range of contraction intensity. Muscle Nerve 2014;50:103-13. 10.1002/mus.24104 [DOI] [PubMed] [Google Scholar]
- 19.Lee SS, Spear S, Rymer WZ. Quantifying changes in material properties of stroke-impaired muscle. Clin Biomech (Bristol, Avon) 2015;30:269-75. 10.1016/j.clinbiomech.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SS, Gaebler-Spira D, Zhang LQ, Rymer WZ, Steele KM. Use of shear wave ultrasound elastography to quantify muscle properties in cerebral palsy. Clin Biomech (Bristol, Avon) 2016;31:20-8. 10.1016/j.clinbiomech.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paramalingam S, Morgan K, Becce F, Diederichsen LP, Ikeda K, Mandl P, Ohrndorf S, Sedie AD, Sharp V, Tan AL, Terslev L, Wakefield RJ, Bruyn GAW, D'Agostino MA, Keen HI. Conventional ultrasound and elastography as imaging outcome tools in autoimmune myositis: A systematic review by the OMERACT ultrasound group. Semin Arthritis Rheum 2021;51:661-76. 10.1016/j.semarthrit.2020.11.001 [DOI] [PubMed] [Google Scholar]
- 22.Song Y, Lee S, Yoo DH, Jang KS, Bae J. Strain sonoelastography of inflammatory myopathies: comparison with clinical examination, magnetic resonance imaging and pathologic findings. Br J Radiol 2016;89:20160283. 10.1259/bjr.20160283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alfuraih AM, O'Connor P, Tan AL, Hensor EMA, Ladas A, Emery P, Wakefield RJ. Muscle shear wave elastography in idiopathic inflammatory myopathies: a case-control study with MRI correlation. Skeletal Radiol 2019;48:1209-19. 10.1007/s00256-019-03175-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344-7. 10.1056/NEJM197502132920706 [DOI] [PubMed] [Google Scholar]
- 25.Ismail C, Zabal J, Hernandez HJ, Woletz P, Manning H, Teixeira C, DiPietro L, Blackman MR, Harris-Love MO. Diagnostic ultrasound estimates of muscle mass and muscle quality discriminate between women with and without sarcopenia. Front Physiol 2015;6:302. 10.3389/fphys.2015.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prieto S, Grau JM. The geoepidemiology of autoimmune muscle disease. Autoimmun Rev 2010;9:A330-4. 10.1016/j.autrev.2009.11.006 [DOI] [PubMed] [Google Scholar]
- 27.Reimers CD, Fleckenstein JL, Witt TN, Müller-Felber W, Pongratz DE. Muscular ultrasound in idiopathic inflammatory myopathies of adults. J Neurol Sci 1993;116:82-92. 10.1016/0022-510X(93)90093-E [DOI] [PubMed] [Google Scholar]
- 28.Hu CF, Chen CP, Tsai WC, Hu LL, Hsu CC, Tseng ST, Shau YW. Quantification of skeletal muscle fibrosis at different healing stages using sonography: a morphologic and histologic study in an animal model. J Ultrasound Med 2012;31:43-8. 10.7863/jum.2012.31.1.43 [DOI] [PubMed] [Google Scholar]
- 29.Botar-Jid C, Damian L, Dudea SM, Vasilescu D, Rednic S, Badea R. The contribution of ultrasonography and sonoelastography in assessment of myositis. Med Ultrason 2010;12:120-6. [PubMed] [Google Scholar]
- 30.Albayda J, Christopher-Stine L, Bingham Iii CO, Paik JJ, Tiniakou E, Billings S, Uy OM, Burlina P. Pattern of muscle involvement in inclusion body myositis: a sonographic study. Clin Exp Rheumatol 2018;36:996-1002. [PMC free article] [PubMed] [Google Scholar]
- 31.Bachasson D, Dubois GJR, Allenbach Y, Benveniste O, Hogrel JY. Muscle Shear Wave Elastography in Inclusion Body Myositis: Feasibility, Reliability and Relationships with Muscle Impairments. Ultrasound Med Biol 2018;44:1423-32. 10.1016/j.ultrasmedbio.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 32.Rosskopf AB, Ehrmann C, Buck FM, Gerber C, Flück M, Pfirrmann CW. Quantitative Shear-Wave US Elastography of the Supraspinatus Muscle: Reliability of the Method and Relation to Tendon Integrity and Muscle Quality. Radiology 2016;278:465-74. 10.1148/radiol.2015150908 [DOI] [PubMed] [Google Scholar]
- 33.Kolb M, Ekert K, Schneider L, Fritz J, Ioanoviciu SD, Henes J, Horger M. The Utility of Shear-Wave Elastography in the Evaluation of Myositis. Ultrasound Med Biol 2021;47:2176-85. 10.1016/j.ultrasmedbio.2021.04.010 [DOI] [PubMed] [Google Scholar]
- 34.Bhansing KJ, Hoppenreijs EP, Janssen AJ, van Royen-Kerkhof A, Nijhuis-Van der Sanden MW, van Riel PL, Pillen S. Quantitative muscle ultrasound: a potential tool for assessment of disease activity in juvenile dermatomyositis. Scand J Rheumatol 2014;43:339-41. 10.3109/03009742.2013.879674 [DOI] [PubMed] [Google Scholar]
- 35.Habers GE, Van Brussel M, Bhansing KJ, Hoppenreijs EP, Janssen AJ, Van Royen-Kerkhof A, Pillen S. Quantitative muscle ultrasonography in the follow-up of juvenile dermatomyositis. Muscle Nerve 2015;52:540-6. 10.1002/mus.24564 [DOI] [PubMed] [Google Scholar]
- 36.Zheng Y, Liu L, Wang L, Xiao J, Wang Z, Lv H, Zhang W, Yuan Y. Magnetic resonance imaging changes of thigh muscles in myopathy with antibodies to signal recognition particle. Rheumatology (Oxford) 2015;54:1017-24. 10.1093/rheumatology/keu422 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as