Abstract
Background
The dorsal striatum, a nucleus in the basal ganglia, plays a key role in the execution of cognitive functions in the human brain. Recent studies have focused on how the dorsal striatum participates in a single cognitive function, whereas the specific roles of the caudate and putamen in performing multiple cognitive functions remain unclear. In this paper we conducted a meta-analysis of the relevant neuroimaging literature to understand the roles of subregions of the dorsal striatum in performing different functions.
Methods
PubMed, Web of Science, and BrainMap Functional Database were searched to find original functional magnetic resonance imaging (fMRI) studies conducted on healthy adults under reward, memory, emotion, and decision-making tasks, and relevant screening criteria were formulated. Single task activation, contrast activation, and conjunction activation analyses were performed using the activation likelihood estimation (ALE) method for the coordinate-based meta-analysis to evaluate the differences and linkages.
Results
In all, 112 studies were included in this meta-analysis. Analysis revealed that, of the 4 single activation tasks, reward, memory, and emotion tasks all activated the putamen more, whereas decision-making tasks activated the caudate body. Contrast analysis showed that the caudate body played an important role in the 2 cooperative activation tasks, but conjunction activation results found that more peaks appeared in the caudate head.
Discussion
Different subregions of the caudate and putamen assume different roles in processing complex cognitive behaviors. Functional division of the dorsal striatum identified specific roles of 15 different subregions, reflecting differences and connections between the different subregions in performing different cognitive behaviors.
Keywords: Activation likelihood estimation (ALE), dorsal striatum, functional differentiation, functional magnetic resonance imaging (fMRI), meta-analysis
Introduction
During the brain’s processing of reward information and negative emotions, dopamine release increases in the dorsal and ventral striatum (1,2). The hippocampus in the brain is the key hub of episodic memory, but the insula and dorsal striatum are also related to other forms of memory (3). Similarly, a growing amount of evidence from neuroimaging and neuropsychological studies shows that there are direct and indirect ways to control decision-making behavior in the striatum, and the brain’s choices based on current expected return or actions related to decision making are closely related to the dorsal striatum (4-6). At first, these functions seem unrelated, but the commonality among them all is the dorsal striatum. The striatum is a nucleus in the basal ganglia, and the dorsal striatum, consisting of the caudate and putamen, is the gateway to the basal ganglia. The dorsal striatum receives convergent excitatory afferents from the cortex and thalamus and forms the origins of the direct and indirect pathways that are important in the cortex-basal ganglia-thalamus-cortical circuit (7). The dopaminergic neurons in this circuit are distributed throughout the striatum, which is of great significance to neurological diseases involving abnormal basal ganglia and dopaminergic function, such as Parkinson’s disease and dystonia (8,9). From this viewpoint, it is particularly important to functionally localize the caudate and putamen in the dorsal striatum and explore their differences and connections.
There has been a considerable increase in the number of meta-analyses in neuroimaging field in recent years (10), but studies integrating multiple different activation tasks in the same region to show their coherence remain rare. To better understand the roles of subregions of the dorsal striatum in performing different functions, this study aimed to explore functional partitioning in the caudate and putamen using a meta-analysis of the existing literature. Recent meta-analyses on brain studies have produced substantial findings. For example, using a coordinate-based meta-analysis, Arioli et al. found that the lateral temporal areas and the amygdala are involved in processing social and emotional concepts (11). In 2021, Mas-Herrero et al. used a meta-analysis to integrate brain involvement in music and food responses to identify reward circuits involved in the brain response (12). In the same year, Sorella et al. used a meta-analysis to investigate whether or not anger perception and anger experience rely on similar or different neural mechanisms in the human brain (13). Inspired by these studies, the present study used the activation likelihood estimation (ALE) method to determine commonly activated brain regions by collecting data from multiple studies and used spatial coordinates under reward, emotion, memory, and decision-making tasks (14,15) to discern the functional division of the dorsal striatum.
The idea behind the ALE method is that coordinates reported in the included studies are considered likelihood distributions, which are modeled by a 3-dimensional Gaussian function (16) and from which an ALE value can be calculated for each voxel to estimate the activation probability of each voxel for the task identified throughout the study. Finally, in order to distinguish the real convergence of focus from random clustering, an ALE null-distribution is formed by thousands of iterations of whole-brain coordinates (17). Coordinate-based meta-analyses are not limited by experimental design, task, the number of subjects, or methods of data analysis, and they can maximize the consistency of location information between studies and minimize the subjectivity of analytical methods (18).
In recent years, many researchers have used different methods to map the relationship between the structure and function of the human brain (i.e., a brain atlas) (19). Inspired by the creation of the brain atlas, we used the ALE algorithm to integrate different studies on the striatum in the human brain to create a functional division in this region, with each subregion created by this division being involved in different activation tasks (20). This study had two main aims: (I) to identify the subregions in the striatum of healthy adults involved in processing reward, emotion, memory, and decision-making tasks; and (II) to analyze similarities and differences in the subregions that assume different functions in response to the 4 activation tasks. These activation tasks were chosen because these cognitive functions are widely used by humans and are the most studied in the neuroimaging literature, ensuring there are sufficient activation foci for the meta-analysis. We present the following article in accordance with the PRISMA reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-22-133/rc).
Methods
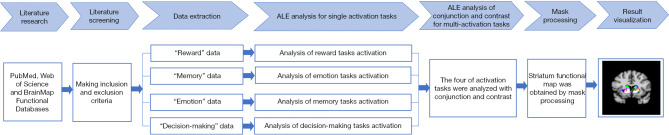
In this study, the functional division of the dorsal striatum was achieved through a literature search, retrieval, screening, and data extraction; ALE analysis of single activation tasks; and ALE conjunction and contrast analyses of multiple activation tasks. Cumulatively, this approach was able to show that different regions assume different functional roles (see Figure 1).
Figure 1.
Methodological flowchart for this study. ALE, activation likelihood estimation.
Data collection
Literature search
A systematic literature search was performed to identify relevant articles in PubMed, Web of Science, and the BrainMap Functional Database (21-23). The BrainMap Functional Database, accessed using Sleuth V3.0.4 (http://www.brainmap.org/sleuth/), contains data of functional and structural neuroimaging experiments based on Talairach coordinates (24) or Montreal Neurological Institute (MNI) space (25), and is widely used for meta-analyses of human brain structure and function research in healthy and diseased individuals.
Specific keyword searches were performed to retrieve literature relating to the reward, memory, emotion, and decision-making paradigms. For the reward paradigm, the PubMed and Web of Science databases were searched using the following keywords: “dorsal striatum” OR “striatum” AND “reward” AND “functional magnetic resonance imaging (fMRI)” NOT “animal”. This search identified 2,970 nonoverlapping studies. In the BrainMap Functional Database, “reward” and “fMRI” were the 2 keywords used, with the experimental environment set to “normal mapping”; this identified another 12 nonoverlapping studies.
For the memory paradigm, the PubMed and Web of Science databases were searched using the keywords “dorsal striatum” OR “striatum” AND “memory” AND “fMRI” NOT “animal”, with 1484 nonoverlapping studies identified. The BrainMap Functional Database was searched using the keywords “memory” and “fMRI”, with the experimental environment set to “normal mapping”; this identified another 22 non-overlapping studies.
For the emotion paradigm, the PubMed and Web of Science databases were searched using the keywords “dorsal striatum” OR “striatum” AND “emotion” AND “fMRI” NOT “animal”, identifying 1,604 nonoverlapping studies. The BrainMap Functional Database was searched using the keywords “emotion” and “fMRI”, with the experimental environment set to “normal mapping”, identifying another 36 nonoverlapping studies.
For the decision-making paradigm, the PubMed and Web of Science databases were searched using the keywords “dorsal striatum” OR “striatum” AND (“decision” OR “decision-making”) AND “fMRI” NOT “animal”, with 1,681 nonoverlapping studies identified. Using the search terms (“decision” OR “decision-making”) and “fMRI” in the BrainMap Functional Database, with the experimental environment set to “normal mapping”, another 3 nonoverlapping studies were identified.
Thus, a total of 7,812 nonoverlapping studies were identified in this study.
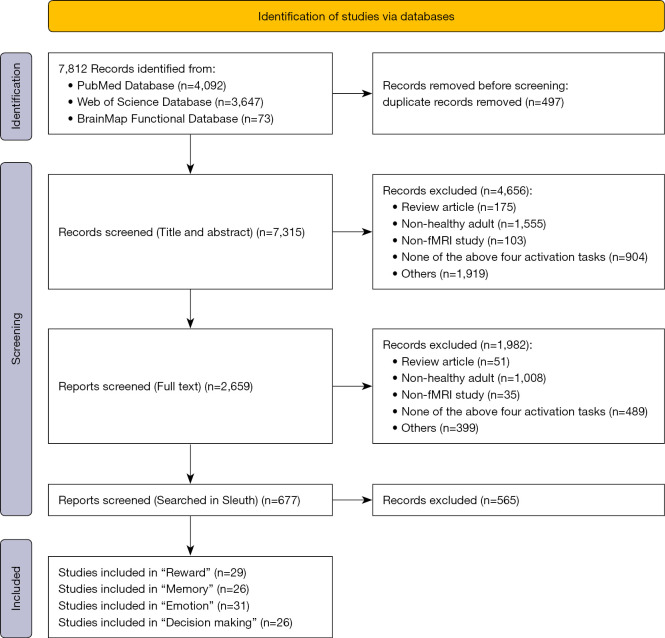
Inclusion and exclusion criteria
Before selecting articles for inclusion in a meta-analysis, comprehensive inclusion criteria must be determined and rigorously applied to minimize bias. In the present study, all studies included were magnetic resonance imaging studies performed in healthy adults aged 14–79 years. The titles, abstracts, and full text of all articles were screened for eligibility (Figure 2). Case reports, pharmacological trials, trials involving clinical populations, neuroimaging studies on children or on patients with certain neurological diseases, and review articles were excluded. Studies were considered eligible for inclusion in the analysis if they met the following criteria: published after 2000; activation reported in standardized 3-dimensional space (Talairach or MNI), with the activation area being the dorsal striatum (caudate, putamen); use of fMRI; the activation task resulting in instantaneous activation; and the activation type reported by the researchers being “reward”, “memory”, “emotion”, and “decision making”. The application of these criteria yielded 29 reward studies (548 subjects, 74 trials), 26 memory studies (463 subjects, 50 trials), 31 emotion studies (658 subjects, 52 trials), and 26 decision-making studies (518 subjects, 50 trials), for a total of 112 studies (Table S1) that were used in the meta-analysis.
Figure 2.
Flowchart showing study inclusion in the meta-analysis. fMRI, functional magnetic resonance imaging.
Data extraction
Data were independently extracted from all included studies by Ba and Wang and included basic information (e.g., article title, author/s, and publication date), participant information (e.g., number of participants, age/mean age, and male:female ratio), experimental materials (e.g., scanned fMRI data) and stimulation methods (e.g., objects, pictures, videos, language, music), study methods (e.g., whether the data coordinates reported are in MNI or Talairach space), and the results (e.g., literature research activation paradigm, as well as activation area).
Data analysis
ALE analysis for single activation tasks
The specific process of ALE analysis for single activation tasks using GingerALE V3.0.2 (http://www.brainmap.org/ale/) software was as follows:
Data organization: the coordinates in all studies included in this analysis were exported to plain text (.txt) files using Sleuth software.
Transformation: all coordinates were transformed into coordinates in common MNI space.
Parameter setting: uncorrected P value thresholds were used to yield reasonable results, with a threshold of P<0.01 and the minimum cluster size set to a volume of 600 mm3.
Mask processing: the object of this study is the dorsal striatum, so the masks of the caudate and the putamen generated by the anatomical automatic labeling (AAL) atlas (19) were used for processing.
Viewing results: Mango (http://www.rii.uthscsa.edu/mango/mango.html) software was used to view the results of the meta-analysis, and the Colin atlas (26) was used with consideration to the statistical coordinate data in MNI space.
ALE analysis of conjunction and contrast for multiple activation tasks
To test for significant differences in the results of analyses of the four single activation tasks, GingerALE software also provides conjunction and contrast meta-analyses. ALE analysis results for the four activation tasks were merged into a single data set before the conjunction and contrast meta-analyses, which resulted in six data sets in total. ALE analysis was performed on the merged data set, and then conjunction and contrast analyses were performed using the ALE threshold images of the merged data set. Conjunction analysis includes any two kinds of conjunction results (e.g., A conjunction B), whereas contrast analysis includes any two kinds of mutual comparisons (e.g., A-B and B-A). As in ALE analysis for single activation tasks, the parameters for contrast and conjunction analyses were a threshold of P<0.01, with 5,000 P value permutations, and a minimum cluster size of 600 mm3.
Functional differentiation of the striatum
Using the specified parameters in the ALE analysis, activation results for single tasks in the whole brain were obtained; the striatum mask in the AAL brain template was used to process the ALE results to obtain 4 functional partitions of the striatum for reward, memory, emotion, and decision-making tasks. Activation areas in the mask were labeled as follows: 1 for reward, 2 for memory, 4 for emotion, and 8 for decision making. Areas of coactivation were also labeled: 3 for reward and memory; 5 for reward and emotion; 6 for memory and emotion; 7 for reward, memory, and emotion; 9 for reward and decision making; 10 for memory and decision making; 11 for reward, memory, and decision making; 12 for emotion and decision making; 13 for reward, emotion, and decision making; 14 for memory, emotion, and decision making; and 15 for reward, memory, emotion, and decision making. Thus, 15 different functional zones were marked in the striatum area.
Results
Analysis of single activation tasks
Reward
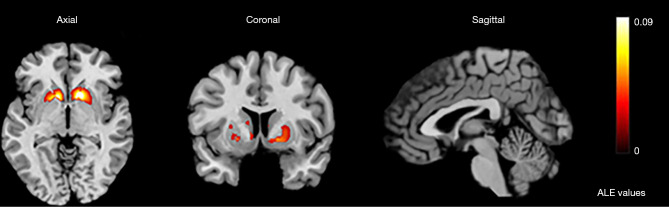
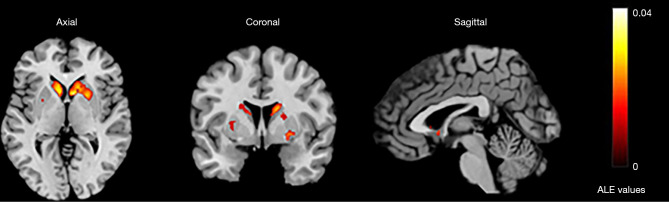
We analyzed activation information in the dorsal striatum under different reward tasks, including 26 monetary rewards, 1 each for food rewards, language and social-type rewards, temperature and taste rewards, and personal preference rewards. The skewed distribution of studies toward monetary reward tasks might have caused inclination in the results. However, these studies showed significant consistency in the caudate and putamen, with a total of 12 clusters activated with the coordinates of the largest cluster located from (–36, –26, –26) to (40, 32, 26) in MNI space and a center coordinate of (2, 7, –5) (see Table S2). The analysis showed significant activation in the caudate on the right side of the brain, whereas activation on the left side was mainly in the caudate body and putamen (Table 1; Figure 3).
Table 1. Activation results under the reward task.
| Cluster No. | MNI peak coordinate | ALE value | Brain region | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 1 | 12 | 12 | –4 | 0.085461 | Right cerebrum, sublobar, caudate, gray matter, caudate head |
| 1 | 14 | –2 | 12 | 0.0298301 | Right cerebrum, sublobar, caudate, gray matter, caudate body |
| 1 | –10 | 2 | 12 | 0.026592 | Left cerebrum, sublobar, caudate, gray matter, caudate body |
| 1 | –26 | 4 | 4 | 0.0243451 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
MNI, Montreal Neurological Institute; ALE, activation likelihood estimation.
Figure 3.
The largest activation cluster for the reward task centered at (2,7,–5) in Montreal Neurological Institute space. ALE, activation likelihood estimation.
Memory
There were 26 relevant articles included in the activation study of memory tasks, including 9 working memory, 2 vocabulary memory, 3 emotional memory, and 1 each of episodic and working memory, episodic memory, semantic memory, declarative memory, reward memory, vocabulary and perceptual memory, selective memory, source memory, learning memory, confirmatory memory, precise and distorted memory, and spatial location memory. The results of the whole-brain analysis showed that the memory task activated 12 clusters (Table S3). Activation peaks were found in the dorsal striatum in the 2 larger clusters and were more significant on the right side of the brain. The largest cluster center was located at (21,4,–4) in MNI space (Table 2; Figure 4).
Table 2. Activation results under the memory task.
| Cluster No. | MNI peak coordinate | ALE value | Brain region | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 1 | 10 | 12 | –10 | 0.0303841 | Right cerebrum, sublobar, caudate, gray matter, caudate head |
| 1 | 12 | 8 | –2 | 0.026199 | Right cerebrum, sublobar, caudate, gray matter, caudate head |
| 1 | 20 | 8 | 0 | 0.024628 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| 1 | 22 | 4 | 16 | 0.0220977 | Right cerebrum, sublobar, caudate, gray matter, caudate body |
| 1 | 28 | –2 | 8 | 0.019593 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| 1 | 30 | –8 | –10 | 0.016469 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| 1 | 16 | 12 | 16 | 0.0133372 | Right cerebrum, sublobar, caudate, gray matter, caudate body |
| 2 | –20 | 6 | 0 | 0.0332396 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| 2 | –14 | 0 | 12 | 0.0249166 | Left cerebrum, sublobar, caudate, gray matter, caudate body |
| 2 | –20 | 12 | –12 | 0.0186555 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
MNI, Montreal Neurological Institute; ALE, activation likelihood estimation.
Figure 4.
The largest activation cluster for the memory task centered at (21,4,–4) in Montreal Neurological Institute space. ALE, activation likelihood estimation.
Emotion
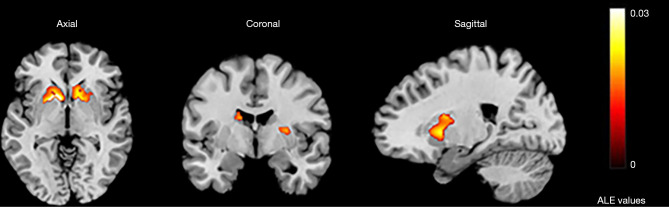
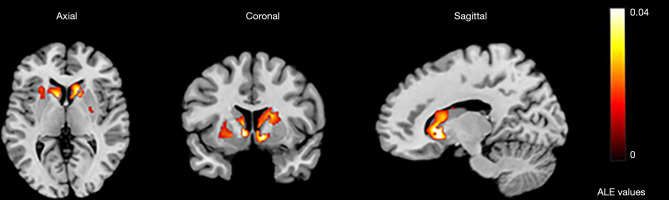
In all, 31 studies studying activation by emotional tasks were included. All selected studies contained emotions such as pleasure, neutrality, sadness, disgust, fear, and pain, and the main ways to stimulate these emotions were pictures, movies, music, smells, facial expressions, and vocabulary. ALE analysis indicated that 8 clusters were activated by the emotion tasks (Table S4). The largest activation cluster was located from (–36, –34, –24) to (36, 32, 22) in MNI space, with the center coordinate at (2, 2, –1). There were 22 peaks found in the largest activation clusters, but there was no obvious activation peak in the cluster in the caudate head region (Table 3; Figure 5).
Table 3. Activation results under the emotion task.
| Cluster No. | MNI peak coordinate | ALE value | Brain region | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 1 | 10 | 8 | 2 | 0.044895 | Right cerebrum, sublobar, caudate, gray matter, caudate body |
| 1 | –10 | 8 | 6 | 0.034102 | Left cerebrum, sublobar, caudate, gray matter, caudate body |
| 1 | 14 | 6 | 16 | 0.034102 | Left cerebrum, sublobar, caudate, gray matter, caudate body |
| 1 | –10 | 16 | 2 | 0.030288 | Left cerebrum, sublobar, caudate, gray matter, caudate body |
| 1 | 22 | 8 | 0 | 0.024583 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| 1 | –20 | 10 | –10 | 0.019019 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| 1 | –24 | –2 | 6 | 0.01578 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| 6 | –32 | –22 | 2 | 0.018084 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
MNI, Montreal Neurological Institute; ALE, activation likelihood estimation.
Figure 5.
The largest activation cluster for the emotion task centered at (2,2,–1) in Montreal Neurological Institute space. ALE, activation likelihood estimation.
Decision making
Decision making involves choosing between different solutions. The ALE algorithm analyzed 4 intertemporal, 3 rewarding, 7 risky, 9 economic, 1 preference, 2 action, and 1 exploratory decision-making studies, showing that 18 clusters were activated in the brain. The largest activated cluster was located from (–2, –14, –20) to (34, 28, 26) in MNI space with central coordinates of (14, 8, 2) and 7 peaks (Table S5). The peaks in the largest clusters tended to converge in the right brain rather than in the left brain. Compared with other functions, decision-making tasks activated both sides of the brain more evenly (Table 4; Figure 6).
Table 4. Activation results under the decision-making task.
| Cluster No. | MNI peak coordinate | ALE value | Brain region | ||
|---|---|---|---|---|---|
| x | y | z | |||
| 1 | 12 | 14 | –6 | 0.039232 | Right cerebrum, sublobar, caudate, gray matter, caudate head |
| 1 | 18 | 10 | 12 | 0.028605 | Right cerebrum, sublobar, caudate, gray matter, caudate body |
| 1 | 18 | 6 | –14 | 0.028543 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| 1 | 30 | –8 | 8 | 0.023354 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| 1 | 8 | –2 | 20 | 0.021768 | Right cerebrum, sublobar, caudate, gray matter, caudate body |
| 2 | –10 | 8 | –6 | 0.039481 | Left cerebrum, sublobar, caudate, gray matter, caudate head |
| 2 | –10 | 12 | 4 | 0.03066 | Left cerebrum, sublobar, caudate, gray matter, caudate body |
| 2 | –26 | 10 | –2 | 0.017975 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| 2 | –26 | 4 | 8 | 0.015567 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| 5 | 32 | –20 | –10 | 0.021232 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| 6 | 28 | 16 | –2 | 0.020071 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| 14 | –20 | –6 | 14 | 0.018954 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
MNI, Montreal Neurological Institute; ALE, activation likelihood estimation.
Figure 6.
The largest activation cluster for the decision-making task centered at (14,8,2) in Montreal Neurological Institute space. ALE, activation likelihood estimation.
Contrast analysis
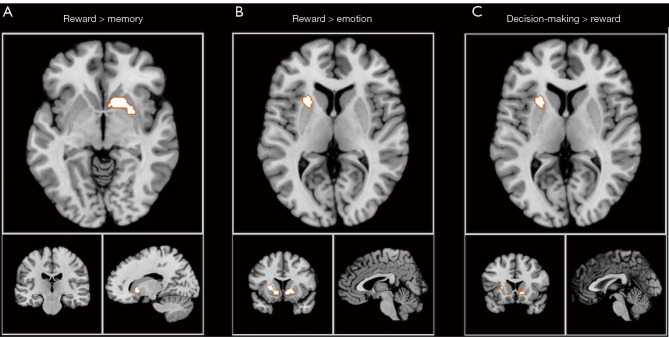
Data in Tables 1-4 indicate that the caudate head and caudate body in the striatum are most activated by decision making, whereas the caudate body is more activated by emotion tasks. To understand differences in activation areas between different tasks, contrast analysis of the 4 activation tasks was performed; the results of whole-brain contrast analysis are presented in Table S6. Contrast analysis of any 2 tasks showed that, in the dorsal striatum, the activation peak was greater in the putamen than that in other tasks, with this being mainly apparent from the reward and decision-making tasks. In the contrast analysis of other tasks, no significant difference was found in the dorsal striatum. The refined contrast analysis results are presented in Table 5 and Figure 7.
Table 5. Contrast analysis of multiple single-activation tasks.
| Contrast | Cluster No. | Activation cluster volume (mm3) | MNI peak coordinate | P value | z score | Brain region | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Reward > memory | 1 | 2,112 | 19.4 | 9.7 | –4.9 | 0.0164 | 0 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| Memory > reward | – | – | – | – | – | – | – | None |
| Reward > emotion | 2 | 2,912 | –16 | 10 | –2 | 0.0004 | 3.352795 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| Emotion > reward | – | – | – | – | – | – | – | None |
| Reward > decision making | – | – | – | – | – | – | – | None |
| Decision making > reward | 1 | 7,52 | 31 | –19 | –7 | 0.004 | 2.65207 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| Memory > emotion | – | – | – | – | – | – | – | None |
| Emotion > memory | – | – | – | – | – | – | – | None |
| Memory > decision making | – | – | – | – | – | – | – | None |
| Decision making > memory | – | – | – | – | – | – | – | None |
| Decision making > emotion | – | – | – | – | – | – | – | None |
| Emotion > decision making | – | – | – | – | – | – | – | None |
MNI, Montreal Neurological Institute.
Figure 7.
Contrast analysis results for (A) reward and memory, (B) reward and emotion, and (C) decision making and reward.
Conjunction analysis
The results of calculating the conjunction of any 2 of the 4 types of activation tasks studied are provided in Table S7; the results for the striatum region are presented in Table 6 and show that the caudate and the putamen have significant agreement in all conjunction analyses. Similarly, the 4 functional paradigms coactivated the core regions of the caudate and putamen, as well as the remaining regions, were also coactivated by different tasks, indicating that the caudate and putamen are involved in functional integration between different functional categories.
Table 6. Conjunction analysis of multiple single activation tasks.
| Conjunction | Cluster No. | Activation cluster volume (mm3) | MNI peak coordinate | ALE value | Brain region | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Reward_conj_memory | 1 | 11,048 | –18 | 8 | 0 | 0.03261438 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| 1 | –24 | 4 | 4 | 0.023548719 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 1 | –12 | 0 | 12 | 0.022341166 | Left cerebrum, sublobar, caudate, gray matter, caudate body | ||
| 1 | –20 | 12 | –12 | 0.018655526 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 2 | 10,240 | 10 | 12 | –10 | 0.030384148 | Right cerebrum, sublobar, caudate, gray matter, caudate head | |
| 2 | 12 | 8 | –2 | 0.026198952 | Right cerebrum, sublobar, caudate, gray matter, caudate head | ||
| 2 | 20 | 8 | 0 | 0.024628002 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 2 | 28 | 0 | 6 | 0.017268842 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 2 | 18 | 4 | 16 | 0.014271136 | Right cerebrum, sublobar, caudate, gray matter, caudate body | ||
| 2 | 18 | 8 | 16 | 0.013320627 | Right cerebrum, sublobar, caudate, gray matter, caudate body | ||
| Reward_conj_emotion | 1 | 25,440 | 10 | 8 | 2 | 0.044894863 | Right cerebrum, sublobar, caudate, gray matter, caudate body |
| 1 | –10 | 8 | 6 | 0.034102 | Left cerebrum, sublobar, caudate, gray matter, caudate body | ||
| 1 | 26 | 0 | –12 | 0.0292304 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 1 | 22 | 8 | 0 | 0.0245828 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 1 | –20 | 10 | –10 | 0.0190189 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 1 | –24 | -2 | 6 | 0.0157796 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| Reward_conj_decision making | 1 | 10,592 | –10 | 12 | 4 | 0.0306597 | Left cerebrum, sublobar, caudate, gray matter, caudate body |
| 1 | –26 | 10 | –4 | 0.0174626 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 1 | –26 | 4 | 8 | 0.0155668 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 2 | 9,512 | 12 | 14 | –6 | 0.0392321 | Right cerebrum, sublobar, caudate, gray matter, caudate head | |
| 2 | 20 | 8 | –14 | 0.0215258 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 2 | 14 | 6 | 14 | 0.0144495 | Right cerebrum, sublobar, caudate, gray matter, caudate body | ||
| 2 | 28 | -2 | 6 | 0.0132864 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 3 | 536 | 26 | 14 | –2 | 0.0150849 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen | |
| 9 | 16 | 28 | 12 | –2 | 0.011703827 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen | |
| Memory_conj_emotion | 1 | 8,568 | –10 | 10 | 0 | 0.026224438 | Left cerebrum, sublobar, caudate, gray matter, caudate head |
| 1 | –18 | 10 | –8 | 0.0178558 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 1 | –24 | -2 | 6 | 0.0157796 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 2 | 5,280 | 12 | 8 | –2 | 0.026199 | Right cerebrum, sublobar, caudate, gray matter, caudate head | |
| 2 | 20 | 8 | 0 | 0.0233723 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 2 | 12 | 16 | –4 | 0.0219033 | Right cerebrum, sublobar, caudate, gray matter, caudate head | ||
| Memory_conj_decision making | 1 | 6,400 | –26 | 10 | –2 | 0.0174461 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen |
| 1 | –26 | 4 | 8 | 0.0155668 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 2 | 6,368 | 10 | 12 | –10 | 0.0303841 | Right cerebrum, sublobar, caudate, gray matter, caudate head | |
| 2 | 12 | 8 | –2 | 0.026199 | Right cerebrum, sublobar, caudate, gray matter, caudate head | ||
| 2 | 28 | –4 | 8 | 0.0168263 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 2 | 20 | 6 | 6 | 0.0165626 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 3 | 720 | 26 | 16 | –2 | 0.0161739 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen | |
| Emotion_conj_decision making | 1 | 8,096 | –8 | 10 | 4 | 0.030274037 | Left cerebrum, sublobar, caudate, gray matter, caudate body |
| 1 | –18 | 10 | –8 | 0.0185376 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen | ||
| 2 | 6,264 | 10 | 8 | 0 | 0.0324197 | Right cerebrum, sublobar, caudate, gray matter, caudate head | |
| 2 | 18 | 8 | 14 | 0.0243084 | Right cerebrum, sublobar, caudate, gray matter, caudate body | ||
| 2 | 14 | 10 | 12 | 0.0241357 | Right cerebrum, sublobar, caudate, gray matter, caudate body | ||
| 4 | 312 | 26 | 14 | –2 | 0.0150849 | Right cerebrum, sublobar, lentiform nucleus, gray matter, putamen | |
| 10 | 24 | –26 | 2 | 6 | 0.0113494 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen | |
| 11 | 16 | –26 | 0 | 8 | 0.0115825 | Left cerebrum, sublobar, lentiform nucleus, gray matter, putamen | |
MNI, Montreal Neurological Institute; ALE, activation likelihood estimation; conj, conjunction.
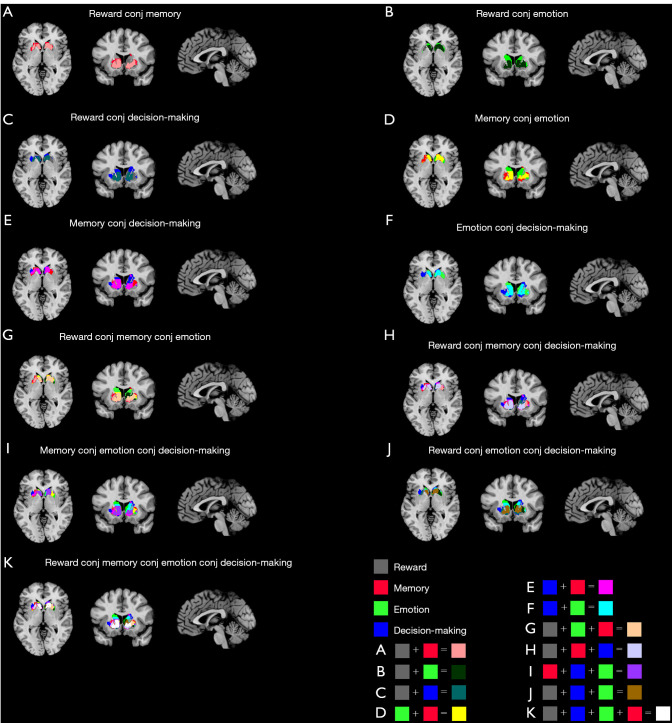
After the conjunction of any 3 of the 4 types of activation tasks, the striatum was divided into 15 functional subregions, with each functional subregion responsible for different cognitive control. Among the functional subregions, the region located in MNI space from (–10, 10, –4) to (15, 10, 2) is jointly activated by reward, memory, emotion, and decision-making functions, with a center coordinate of (–1, 14, –2); this region is part of the caudate head and caudate body.
Figure 8 shows all the conjunction results from 3 different views (axial, coronal, sagittal), and Figure 9 shows the 15 different functional subregions that the dorsal striatum was divided into in the coronal plane. The final mask results for the 15 functional subregions are available online (https://github.com/Isbmm/15-subregions-mask).
Figure 8.
Conjunction activation results of the dorsal striatum under 4 single activation tasks at (–1,14,–2) in Montreal Neurological Institute space. (A-F) Conjunction result of 2 tasks; (G-J) conjunction result of 3 tasks. (K) Final functional differentiation result.
Figure 9.
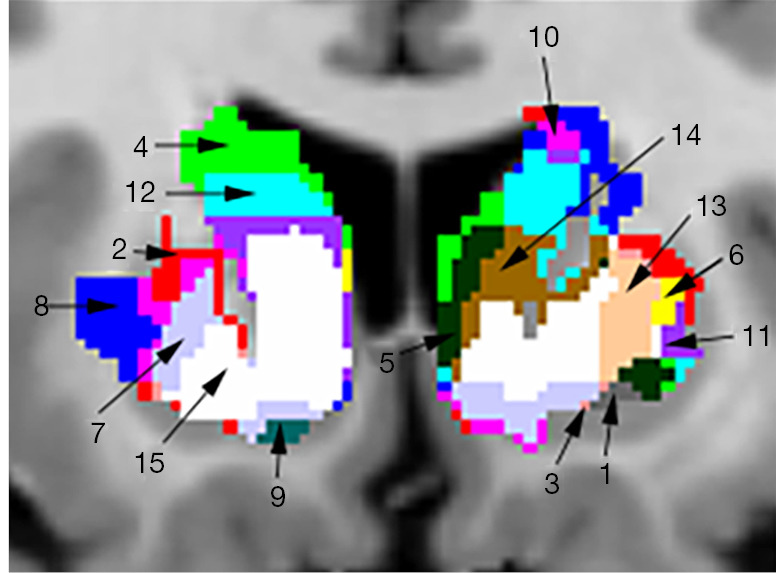
Enlarged view of the dorsal striatum divided into 15 functional subregions.
Discussion
The results of the present study indicate that, based on a large-scale coordinate meta-analysis of published fMRI studies, there is functional differentiation in the dorsal striatum. Using a coordinate-based meta-analysis method we could differentiate the dorsal striatum into four functional areas, which are defined by reward, memory, emotion, and decision-making functions. The activation peaks of reward and memory function were mainly distributed in the caudate, whereas the activation peaks of emotion and decision-making tasks were evenly distributed in the caudate and putamen. In addition to this functional differentiation, the functional categories were subjected to contrast and conjunction analyses to explore the differences and connections between different functional categories. From these analyses, there were obvious differences in the putamen region. However, the result of conjunction activation of any two functions was located in the caudate and putamen, with no significant difference.
Reward is an important motivator for all species and many external factors, such as contextual environment, cognitive value, and reward conditions, can influence individual responses in the face of reward (27). Unidirectional connections from the cortex to the matrix of the corpus striatum initiate the cortex-basal ganglia-thalamus-cortical loop, where the striatum has complementary roles in shaping corticostriatal plasticity to record or anticipate reward (28). Meanwhile, the striatum, as a receptor for cortical and dopamine projections during reward processing, is located at the center of the functional loop that affects the motor and cognitive aspects of behavior (29,30). One study found that when the human brain is rewarded with money, the activation of the right caudate head decreases with decreasing response (31). Similarly, other research showed that during the expectation of primary (32) or secondary rewards (33), the blood oxygen level–dependent response of the dorsal striatum increases. The present study confirms these results in revealing that the dorsal striatum is involved in reward processing. As shown in a comparative study between healthy people and patients with major depressive disorder, decreased reward activation occurs in the caudate, putamen, thalamus, anterior cingulate cortex, and insula (34).
Memory function involves the brain integrating structured information and remembering complex multidimensional information through temporal or spatial associations (35). Evidence from humans and other animals suggests that there are multiple memory systems defined by different neural substrates and functional needs. The memory systems of the hippocampus, amygdala, and dorsal striatum have different roles in memory tasks. Of these brain regions, the dorsal striatum is more important in memory tasks that need to strengthen stimulus response correlation (36). Animal studies have found that the dorsal striatum is important for the gradual, feedback-guided learning that results in habit memory, and an elegant double dissociation was demonstrated in rats after the creation of fornix and caudate lesions in two tasks that appeared to measure declarative memory and habit memory (37). A similar contrast between declarative memory and habit memory was later demonstrated for amnesic patients and patients with Parkinson’ disease (38,39). Although different memory systems are separated, there is still synergistic activation between them. The putamen is mainly associated with the hippocampus in memory tasks, linking the overall events involved in memory (40).
Emotions influence attention, decision making, memory, physiological responses, and social interactions, and the ability to successfully regulate emotions is associated with many important psychological, social, and physical health outcomes (41,42). In the process of emotion perception and regulation, the amygdala and ventromedial prefrontal cortex play a central role in negative emotions, whereas regions such as the striatum and insula cortex play a certain role in the regulation of other emotions (43). The results of the present study are consistent with the findings of related studies on the role of dopamine in human processing of negative emotions. Dynamic molecular imaging technology has been used to explore dopamine release in the striatum during emotion processing, especially in the caudate and putamen (2). However, the present study included not only multiple negative emotion-processing studies, but also studies on positive, neutral, disgust, fear, and other emotions. The results of the present study were not significantly different from those of previous single-imaging studies in the dorsal striatum. Difficulty in emotion regulation is considered to be the core mechanism of mood and anxiety disorders (44). In patients with depression, increases in depression severity are related to the functional connection between the dorsal caudate and the right dorsolateral prefrontal cortex (45).
Decision making is the ability to make an appropriate choice between different action plans, which requires an ability to combine estimates of causal relationships between actions and their consequences or results with their value or utility. Although there is extensive literature linking cognitive functions specific to the prefrontal cortex (46), other studies suggest that these functions depend on reward-related circuitry linking prefrontal, premotor, and sensorimotor cortices with the striatum (47-49). Most evidence suggests that direct and indirect striatal pathways control behavior in an opposing manner (50,51). In one study, significant activation was found in the caudate, putamen, globus pallidus, thalamus, midbrain, and cingulate gyrus in tasks involving economic decision making behind responses to monetary rewards (52). For other types of decision-making processes, such as risk decision making, the putamen, hippocampus, parahippocampal gyrus, and lingual gyrus, but not the caudate, are positively correlated with behavioral preference. The relevant decision-making studies included in the present analysis were primarily focused on risk and economic decision making, and the results showed significant activation in the putamen region.
Contrast analysis was used to examine the significance of differences in the convergence between any two groups of different foci for the four independent tasks. The results showed differences in the processing of different functional information between the caudate and putamen. For example, measurements of anatomical and functional connections in humans, nonhuman primates, and mice have shown that there is a clear link between the caudate and the frontal lobe region responsible for “executive” function (53). The biggest difference between the caudate and putamen in the performance of cognitive function is that the caudate promotes behavior by stimulating correct action schema and selecting appropriate subgoals based on an evaluation of action outcomes, whereas the cognitive function of the putamen is more limited to stimulus response, habit, or learning. The results showed significant differences in the putamen. Different parts of the striatum receive inputs from different cortical regions and project their outputs to the cortex through the thalamus. Meanwhile, the anterior putamen is connected to related areas in the cortex, and the posterior putamen is connected to the primary motor cortex and supplementary motor areas (54). The putamen seems to be the area for the coordination and cross-functional integration of independent functional channels, directing a coordinated behavioral display and modification in response to external and internal stimuli (55). Physiologically, volume changes in the putamen are associated with different neurological and psychiatric disorders, such as bipolar disorder and attention deficit hyperactivity disorder (56,57).
In the context of the functional differentiation of the striatum described above, achieving functional integration is a more critical issue (i.e., conjunction analysis that shows the similarity of the two data sets). Conjunction analysis revealed the overlap of all categories in the caudate and putamen. This overlap may reflect a shared role in information processing between functional systems. This shared role may reflect the multimodal integration between different task categories: on the one hand, with information being transferred between different functional systems; and on the other, with all task categories sharing a common basic functional role (58). Analysis of a large number of different functional categories in this study showed that most of the final conjunction results of all functions converge in the caudate head and putamen. From this point of view, the core of the conjunction outcome converges in subregions in the caudate and putamen. Although the present study only focused on the caudate and putamen, it is important to understand the anatomical structure of the frontostriatal circuit and related disease processes. Studies have shown that the human caudate is interconnected with the prefrontal cortex, inferior and middle temporal gyrus, frontal eye fields, cerebellum, and thalamus; the putamen is interconnected with the prefrontal cortex, primary motor area, primary somatosensory cortex, supplementary motor area, premotor area, cerebellum, and thalamus (59). It can be predicted that the functional partitions into which we have divided the striatum may have important implications for future research on related psychiatric disorders caused by striatal circuit disorders.
Analysis of the functional loss of patients with striatal abnormalities could be used to indirectly validate our functional differentiation. One study confirmed that depression can be safely and effectively treated through repetitive transcranial stimulation therapy (60). This method improves the integrity of frontostriatal connections by inducing striatal dopamine release onto the dorsolateral prefrontal cortex in depressed patients. In another study, transplantation of human embryonic mesencephalic tissue bilaterally into the caudate and putamen led to significant symptomatic relief in patients with Parkinson disease and reduced dependence on levodopa (61). Computed tomography findings in a patient with segmental axial dystonia revealed 3 small areas of encephalomalacia, 1 involving the head of the caudate. In another report, treatment with trihexyphenidyl resulted in significant improvements in dystonia and scoliosis (62). Huntington disease (HD) is characterized by striatal atrophy that starts before the onset of motor symptoms, and treatment with laquinimod results in reduced volume loss in the caudate and other brain regions in patients with early HD (63).
Although the present study provides an idea for the division of the dorsal striatum into different functional areas, we acknowledge several limitations that need to be addressed in future studies. First, because the nucleus accumbens cannot be accurately located in the common brain atlas, the role of the ventral striatum (including the nucleus accumbens) in the functional paradigms we studied was not considered. Second, like many other neuroimaging meta-analysis algorithms, the coordinate-based ALE meta-analysis provides only a measure of reported peak coordinate convergence and not the magnitude of peak activation effects. The coordinate-based meta-analysis requires that each activation cluster exceed the significance threshold set in each study, and does not weight the activation statistics (64). Third, there are considerable differences in stimulus methods, experimental methods, and scanning parameters among neuroimaging studies included in meta-analyses. Although the possible effects of these factors was minimized in the present study during the literature screening process, it is possible that these systematic differences could have led to some discrepancies. Fourth, the striatum is not only involved in reward, memory, emotion, and decision-making paradigms. This area has a greater degree of activation under stimulation tasks such as smell, hearing, language, and learning. Future meta-analyses should include a wider range of stimulus modalities and tasks. Finally, not all studies include voxel-based activation coordinates that would allow them to be included in ALE analysis.
Conclusions
Using a large-scale meta-analysis, this study has revealed the functional differentiation and integration of the caudate and putamen in the human dorsal striatum. The 15 different subregions identified are involved in the processing of different functions. The observed functional differentiation and integration of the dorsal striatum may constitute the link between these different functional systems and the transmission of information between them. We conducted a comprehensive summary and evaluation of the relevant brain cognitive neuroimaging literature. The ensuing results may benefit future research on depression, Parkinson’s disease, schizophrenia, and other diseases.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors acknowledge the published data available in the public domain for meta-analyses.
Funding: This work was supported by the National Science Foundation of China (No. 61728107), the Science and Technology Planning Program of Henan Province (Nos. 212102310088, 212102210082, 2021GGJS093, 2020GGJS123, and 20A520042), and the Union Project of Medical and Technology Research Program of Henan Province (No. LHGJ20190159).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-22-133/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-22-133/coif). TH serves as an unpaid editorial board member of Quantitative Imaging in Medicine and Surgery. The other authors have no conflicts of interest to declare.
References
- 1.Sorg C, Manoliu A, Neufang S, Myers N, Peters H, Schwerthöffer D, Scherr M, Mühlau M, Zimmer C, Drzezga A, Förstl H, Bäuml J, Eichele T, Wohlschläger AM, Riedl V. Increased intrinsic brain activity in the striatum reflects symptom dimensions in schizophrenia. Schizophr Bull 2013;39:387-95. 10.1093/schbul/sbr184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badgaiyan RD. Dopamine is released in the striatum during human emotional processing. Neuroreport 2010;21:1172-6. 10.1097/WNR.0b013e3283410955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwabe L. Memory under stress: from single systems to network changes. Eur J Neurosci 2017;45:478-89. 10.1111/ejn.13478 [DOI] [PubMed] [Google Scholar]
- 4.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci 2007;27:8161-5. 10.1523/JNEUROSCI.1554-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee AM, Tai LH, Zador A, Wilbrecht L. Between the primate and 'reptilian' brain: Rodent models demonstrate the role of corticostriatal circuits in decision making. Neuroscience 2015;296:66-74. 10.1016/j.neuroscience.2014.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weglage M, Wärnberg E, Lazaridis I, Calvigioni D, Tzortzi O, Meletis K. Complete representation of action space and value in all dorsal striatal pathways. Cell Rep 2021;36:109437. 10.1016/j.celrep.2021.109437 [DOI] [PubMed] [Google Scholar]
- 7.Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron 2008;60:543-54. 10.1016/j.neuron.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simonyan K. Recent advances in understanding the role of the basal ganglia. F1000Res 2019;8:eF1000 Faculty Rev-122. [DOI] [PMC free article] [PubMed]
- 9.Shang S, Wu J, Chen YC, Chen H, Zhang H, Dou W, Wang P, Cao X, Yin X. Aberrant cerebral perfusion pattern in amnestic mild cognitive impairment and Parkinson's disease with mild cognitive impairment: a comparative arterial spin labeling study. Quant Imaging Med Surg 2021;11:3082-97. 10.21037/qims-20-1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulpizio S, Del Maschio N, Fedeli D, Abutalebi J. Bilingual language processing: A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 2020;108:834-53. 10.1016/j.neubiorev.2019.12.014 [DOI] [PubMed] [Google Scholar]
- 11.Arioli M, Gianelli C, Canessa N. Neural representation of social concepts: a coordinate-based meta-analysis of fMRI studies. Brain Imaging Behav 2021;15:1912-21. 10.1007/s11682-020-00384-6 [DOI] [PubMed] [Google Scholar]
- 12.Mas-Herrero E, Maini L, Sescousse G, Zatorre RJ. Common and distinct neural correlates of music and food-induced pleasure: A coordinate-based meta-analysis of neuroimaging studies. Neurosci Biobehav Rev 2021;123:61-71. 10.1016/j.neubiorev.2020.12.008 [DOI] [PubMed] [Google Scholar]
- 13.Sorella S, Grecucci A, Piretti L, Job R. Do anger perception and the experience of anger share common neural mechanisms? Coordinate-based meta-analytic evidence of similar and different mechanisms from functional neuroimaging studies. Neuroimage 2021;230:117777. 10.1016/j.neuroimage.2021.117777 [DOI] [PubMed] [Google Scholar]
- 14.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 2009;30:2907-26. 10.1002/hbm.20718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Mapp 2012;33:1-13. 10.1002/hbm.21186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 2005;25:155-64. 10.1002/hbm.20136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 2002;16:765-80. 10.1006/nimg.2002.1131 [DOI] [PubMed] [Google Scholar]
- 18.Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage 2012;59:2349-61. 10.1016/j.neuroimage.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans AC, Janke AL, Collins DL, Baillet S. Brain templates and atlases. Neuroimage 2012;62:911-22. 10.1016/j.neuroimage.2012.01.024 [DOI] [PubMed] [Google Scholar]
- 20.Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage 2002;16:317-30. 10.1006/nimg.2002.1063 [DOI] [PubMed] [Google Scholar]
- 21.Fox PT, Laird AR, Fox SP, Fox PM, Uecker AM, Crank M, Koenig SF, Lancaster JL. BrainMap taxonomy of experimental design: description and evaluation. Hum Brain Mapp 2005;25:185-98. 10.1002/hbm.20141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laird AR, Lancaster JL, Fox PT. BrainMap: the social evolution of a human brain mapping database. Neuroinformatics 2005;3:65-78. 10.1385/NI:3:1:065 [DOI] [PubMed] [Google Scholar]
- 23.Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE Meta-Analysis Workflows Via the Brainmap Database: Progress Towards A Probabilistic Functional Brain Atlas. Front Neuroinform 2009;3:23. 10.3389/neuro.11.023.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 2000;10:120-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans AC, Collins DL, Mills S, et al. editors. 3D statistical neuroanatomical models from 305 MRI volumes. 1993 IEEE Conf Rec Nuclear Sci Symp Med Imaging Conf 1993. [Google Scholar]
- 26.Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 1998;22:324-33. 10.1097/00004728-199803000-00032 [DOI] [PubMed] [Google Scholar]
- 27.Chiew KS, Braver TS. Positive affect versus reward: emotional and motivational influences on cognitive control. Front Psychol 2011;2:279. 10.3389/fpsyg.2011.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shipp S. The functional logic of corticostriatal connections. Brain Struct Funct 2017;222:669-706. 10.1007/s00429-016-1250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010;35:4-26. 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters SK, Dunlop K, Downar J. Cortico-Striatal-Thalamic Loop Circuits of the Salience Network: A Central Pathway in Psychiatric Disease and Treatment. Front Syst Neurosci 2016;10:104. 10.3389/fnsys.2016.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhingra I, Zhang S, Zhornitsky S, Le TM, Wang W, Chao HH, Levy I, Li CR. The effects of age on reward magnitude processing in the monetary incentive delay task. Neuroimage 2020;207:116368. 10.1016/j.neuroimage.2019.116368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron 2002;33:815-26. 10.1016/S0896-6273(02)00603-7 [DOI] [PubMed] [Google Scholar]
- 33.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 2001;21:RC159. 10.1523/JNEUROSCI.21-16-j0002.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord 2013;151:531-9. 10.1016/j.jad.2013.06.039 [DOI] [PubMed] [Google Scholar]
- 35.de Rover M, Petersson KM, van der Werf SP, Cools AR, Berger HJ, Fernández G. Neural correlates of strategic memory retrieval: differentiating between spatial-associative and temporal-associative strategies. Hum Brain Mapp 2008;29:1068-79. 10.1002/hbm.20445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald RJ, White NM. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav Neurosci 2013;127:835-53. 10.1037/a0034883 [DOI] [PubMed] [Google Scholar]
- 37.Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci 1989;9:1465-72. 10.1523/JNEUROSCI.09-05-01465.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foerde K, Shohamy D. The role of the basal ganglia in learning and memory: insight from Parkinson's disease. Neurobiol Learn Mem 2011;96:624-36. 10.1016/j.nlm.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Squire LR, Dede AJ. Conscious and unconscious memory systems. Cold Spring Harb Perspect Biol 2015;7:a021667. 10.1101/cshperspect.a021667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang J, Brashier NM, Egner T. Memory Meets Control in Hippocampal and Striatal Binding of Stimuli, Responses, and Attentional Control States. J Neurosci 2015;35:14885-95. 10.1523/JNEUROSCI.2957-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberton T, Daffern M, Bucks RS. Emotion regulation and aggression. Aggr Violent Behav 2012;17:72-82. 10.1016/j.avb.2011.09.006 [DOI] [Google Scholar]
- 42.Brooke A, Harrison NA. Neuroimaging and emotion. Stress: concepts, cognition, emotion, and behavior. Elsevier, 2016:251-9. [Google Scholar]
- 43.Yang M, Tsai SJ, Li CR. Concurrent amygdalar and ventromedial prefrontal cortical responses during emotion processing: a meta-analysis of the effects of valence of emotion and passive exposure versus active regulation. Brain Struct Funct 2020;225:345-63. 10.1007/s00429-019-02007-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell-Sills L, Barlow DH. Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. Handb Emot Regu 2007:542-59. [Google Scholar]
- 45.Kerestes R, Harrison BJ, Dandash O, Stephanou K, Whittle S, Pujol J, Davey CG. Specific functional connectivity alterations of the dorsal striatum in young people with depression. Neuroimage Clin 2014;7:266-72. 10.1016/j.nicl.2014.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katsuki F, Constantinidis C. Unique and shared roles of the posterior parietal and dorsolateral prefrontal cortex in cognitive functions. Front Integr Neurosci 2012;6:17. 10.3389/fnint.2012.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang JY, Chen L, Luo F, Shi LH, Woodward DJ. Neuronal responses in the frontal cortico-basal ganglia system during delayed matching-to-sample task: ensemble recording in freely moving rats. Exp Brain Res 2002;142:67-80. 10.1007/s00221-001-0918-3 [DOI] [PubMed] [Google Scholar]
- 48.Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature 2002;418:413-7. 10.1038/nature00892 [DOI] [PubMed] [Google Scholar]
- 49.Tanaka SC, Samejima K, Okada G, Ueda K, Okamoto Y, Yamawaki S, Doya K. Brain mechanism of reward prediction under predictable and unpredictable environmental dynamics. Neural Netw 2006;19:1233-41. 10.1016/j.neunet.2006.05.039 [DOI] [PubMed] [Google Scholar]
- 50.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat 2011;5:41. 10.3389/fnana.2011.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakanishi S, Hikida T, Yawata S. Distinct dopaminergic control of the direct and indirect pathways in reward-based and avoidance learning behaviors. Neuroscience 2014;282:49-59. 10.1016/j.neuroscience.2014.04.026 [DOI] [PubMed] [Google Scholar]
- 52.Preuschoff K, Bossaerts P, Quartz SR. Neural differentiation of expected reward and risk in human subcortical structures. Neuron 2006;51:381-90. 10.1016/j.neuron.2006.06.024 [DOI] [PubMed] [Google Scholar]
- 53.Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol 2008;86:141-55. 10.1016/j.pneurobio.2008.09.004 [DOI] [PubMed] [Google Scholar]
- 54.Bozkurt B, Yagmurlu K, Middlebrooks EH, Karadag A, Ovalioglu TC, Jagadeesan B, Sandhu G, Tanriover N, Grande AW. Microsurgical and Tractographic Anatomy of the Supplementary Motor Area Complex in Humans. World Neurosurg 2016;95:99-107. 10.1016/j.wneu.2016.07.072 [DOI] [PubMed] [Google Scholar]
- 55.Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci 2016;18:7-21. 10.31887/DCNS.2016.18.1/shaber [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cortese S. The neurobiology and genetics of Attention-Deficit/Hyperactivity Disorder (ADHD): what every clinician should know. Eur J Paediatr Neurol 2012;16:422-33. 10.1016/j.ejpn.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 57.Roessner V, Overlack S, Schmidt-Samoa C, Baudewig J, Dechent P, Rothenberger A, Helms G. Increased putamen and callosal motor subregion in treatment-naïve boys with Tourette syndrome indicates changes in the bihemispheric motor network. J Child Psychol Psychiatry 2011;52:306-14. 10.1111/j.1469-7610.2010.02324.x [DOI] [PubMed] [Google Scholar]
- 58.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct 2010;214:519-34. 10.1007/s00429-010-0255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leh SE, Ptito A, Chakravarty MM, Strafella AP. Fronto-striatal connections in the human brain: a probabilistic diffusion tractography study. Neurosci Lett 2007;419:113-8. 10.1016/j.neulet.2007.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baeken C, Brem AK, Arns M, Brunoni AR, Filipčić I, Ganho-Ávila A, Langguth B, Padberg F, Poulet E, Rachid F, Sack AT, Vanderhasselt MA, Bennabi D. Repetitive transcranial magnetic stimulation treatment for depressive disorders: current knowledge and future directions. Curr Opin Psychiatry 2019;32:409-15. 10.1097/YCO.0000000000000533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindvall O. Treatment of Parkinson's disease using cell transplantation. Philos Trans R Soc Lond B Biol Sci 2015;370:20140370. 10.1098/rstb.2014.0370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rossi M, Medina Escobar A, Radrizzani M, Tenembaum S, Perandones C, Merello M. Dystonia in a Patient with Autosomal-Dominant Progressive External Ophthalmoplegia Type 1 Caused by Mutation in the POLG Gene. Mov Disord Clin Pract 2016;4:266-9. 10.1002/mdc3.12397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reilmann R, Gordon MF, Anderson KE, Feigin A, Tabrizi SJ, Leavitt BR, Stout JC, Piccini P, Borowsky B, Rynkowski G, Volkinstein R, Savola JM, Hayden MR. The efficacy and safety results of laquinimod as a treatment for huntington disease (LEGATO-HD) (S16.007). Neurology 2019;92:S16.007. [Google Scholar]
- 64.Salimi-Khorshidi G, Smith SM, Keltner JR, Wager TD, Nichols TE. Meta-analysis of neuroimaging data: a comparison of image-based and coordinate-based pooling of studies. Neuroimage 2009;45:810-23. 10.1016/j.neuroimage.2008.12.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as