Abstract
Rationale
Effective therapies to reduce the severity and high mortality of pulmonary vasculitis and diffuse alveolar hemorrhage (DAH) in patients with systemic lupus erythematosus (SLE) is a serious unmet need. We explored whether biologic neutralization of eNAMPT (extracellular nicotinamide phosphoribosyl-transferase), a novel DAMP and Toll-like receptor 4 ligand, represents a viable therapeutic strategy in lupus vasculitis.
Methods
Serum was collected from SLE subjects (n = 37) for eNAMPT protein measurements. In the preclinical pristane-induced murine model of lung vasculitis/hemorrhage, C57BL/6 J mice (n = 5–10/group) were treated with PBS, IgG (1 mg/kg), or the eNAMPT-neutralizing ALT-100 mAb (1 mg/kg, IP or subcutaneously (SQ). Lung injury evaluation (Day 10) included histology/immuno-histochemistry, BAL protein/cellularity, tissue biochemistry, RNA sequencing, and plasma biomarker assessment.
Results
SLE subjects showed highly significant increases in blood NAMPT mRNA expression and eNAMPT protein levels compared to healthy controls. Preclinical pristane-exposed mice studies showed significantly increased NAMPT lung tissue expression and increased plasma eNAMPT levels accompanied by marked increases in alveolar hemorrhage and lung inflammation (BAL protein, PMNs, activated monocytes). In contrast, ALT-100 mAb-treated mice showed significant attenuation of inflammatory lung injury, alveolar hemorrhage, BAL protein, tissue leukocytes, and plasma inflammatory cytokines (eNAMPT, IL-6, IL-8). Lung RNA sequencing showed pristane-induced activation of inflammatory genes/pathways including NFkB, cytokine/chemokine, IL-1β, and MMP signaling pathways, each rectified in ALT-100 mAb-treated mice.
Conclusions
These findings highlight the role of eNAMPT/TLR4-mediated inflammatory signaling in the pathobiology of SLE pulmonary vasculitis and alveolar hemorrhage. Biologic neutralization of this novel DAMP appears to serve as a viable strategy to reduce the severity of SLE lung vasculitis.
Keywords: eNAMPT, DAMP, SLE, Pulmonary vasculitis, DAH, Pristane
Highlights
-
•
eNAMPT is a highly druggable DAMP and biomarker involved in the pathobiology of SLE.
-
•
eNAMPT/TLR4 inflammatory signaling is intimately linked to pulmonary vasculitis.
-
•
A eNAMPT-neutralizing mAb is highly effective in reducing lupus vasculitis severity.
1. Introduction
SLE is a systemic autoimmune disease with multiorgan involvement and an unpredictable relapsing-remitting course which disproportionately affects women, with African American and Hispanic populations having higher mortality risk [1]. SLE vasculitis is a common manifestation of SLE involving the skin, kidney and lung. Although the exact prevalence of lung involvement in SLE is unknown, many SLE patients exhibit signs of respiratory and pulmonary vascular involvement including vasculitis and diffuse alveolar hemorrhage (DAH) [[2], [3], [4], [5]]. DAH as an acute form of SLE vasculitis (5–10% of patients) represents a catastrophic SLE complication with a 35–50% mortality rate for which current treatments are extremely limited [[6], [7], [8], [9]]. Corticosteroids and immunosuppressive agents, such as methotrexate and cyclophosphamide, and mechanical ventilation remain primary treatments in management of acute immune-mediated lung vasculitis/hemorrhage with subsequent respiratory failure [10]. There remains a serious unmet need for increased understanding of acute SLE lung pathophysiology and for novel effective therapeutics.
Current concepts of SLE vasculitis have highlighted key involvement of dysregulated inflammatory cascades which adversely affect the lung vasculature in SLE. Activation of these evolutionarily-conserved inflammatory pathways presumably occurs via cell-, immune complex-, or autoantibody-mediated mechanisms and/or danger-associated molecular pattern (DAMP) proteins released from injured tissues that bind to pathogen recognition receptors. In addition to SLE, DAMPs have been implicated in autoimmune disorders such as psoriasis, rheumatoid arthritis, and inflammatory bowel disease [11]. In the present study, we explored the potential contribution of the novel DAMP and TLR4 ligand, eNAMPT (extracellular nicotinamide phosphoribosyl-transferase), as a viable therapeutic target in human and preclinical SLE vasculitis. eNAMPT binding to the pathogen-recognition receptor, TLR4 [12] elicits profound NFκB-mediated inflammatory cytokine release and increases vascular permeability [13,14], events implicated in acute human inflammatory lung disorders such as ARDS [15,16] and chronic inflammatory conditions including radiation-induced pneumonitis (27) and fibrosis (39). Plasma eNAMPT levels serve as a novel biomarker in ARDS and pulmonary hypertension [17,18], and NAMPT promoter SNPs confer increased risk of inflammatory disease severity [17,[19], [20], [21], [22], [23]]. Importantly, studies utilizing conditionally-engineered, endothelial cell (EC)-specific NAMPT KO mice, provided compelling support for eNAMPT expression and secretion from vascular endothelial cells as a major driver of acute inflammatory lung injury severity [24] and vascular remodeling in PH [25].
We utilized human SLE blood samples and the murine model of pristane-induced lung vasculitis and DAH to address the participation of eNAMPT/TLR4 signaling in acute autoimmune vascular inflammation and injury. Intraperitoneal (IP) injection of pristane (TMPD, 2, 6, 10, 14-tetramethylpentadecane), a hydrocarbon oil, produces substantial alveolar and perivascular inflammation (capillaritis, small vessel vasculitis), hemorrhage, endothelial cell injury, and infiltration of macrophages, neutrophils, and proinflammatory cytokines over the ensuing 10 days [5,26]. The development of lung damage in the pristane murine model appears relevant to human lupus pulmonary vasculitis as it implicates prominent roles for innate immunity -induced inflammation with heavy monocyte and neutrophil involvement in driving the autoimmune lung pathology [5,27].
Our data indicate that eNAMPT expression is increased in human SLE subjects and pristane-challenged mice with vasculitis. Administration of an eNAMPT-neutralizing humanized mAb significantly reduces the severity of murine pristane-induced vasculitis and alveolar hemorrhage suggesting a potentially novel therapeutic target and biologic intervention for SLE-associated vasculitis and DAH.
2. Materials and methods
2.1. Reagents
The p-NFκB, NFκB, and DOCK1 antibodies were purchased from Cell Signaling Technologies (Danvers, MA); NAMPT antibody from Bethyl Laboratories (Montgomery, TX); β-actin antibody from Sigma-Aldrich (St. Louis, MO); and rabbit secondary antibody from Jackson ImmunoResearch (West Grove, PA), All other reagents were from Sigma-Aldrich. Details of the generation of the eNAMPT-neutralizing humanized mAb, ALT-100 (provided by Aqualung Therapeutics Corporation, Tucson, AZ) have been previously described [28,29].
2.2. Human SLE subjects
Peripheral blood samples from SLE patients (meeting ACR classification criteria for SLE) and healthy controls (HC) (age and sex-matched) were collected via a protocol approved by the local institutional review board. All participants provided informed written consent and the study received prior approval from the institutional ethics review board (IRB protocol #19627). Inclusion included all male and female SLE patients, >18 years of age who meet revised ACR Criteria for SLE [30]. There were no exclusion criteria as all SLE subjects were part of the repository.
2.3. Mouse pristane model
All animal protocols were approved by the fully accredited Institutional Animal Care and Use Committees of the University of Arizona and Cedars Sinai Medical Center and all experiments were performed in compliance with ARRIVE guidelines. Four series of experiments were conducted in wild-type female C57BL/6 mice (6–8 weeks old) with each series consisting of one group of unexposed controls and 3 or 4 groups of mice which were exposed to a single IP injection of 0.5 mL of pristane (2,6,10,14-Tetramethylpentadecane (TMPD), Sigma). In two series of experiments, pristane-exposed mice received either intraperitoneal PBS, isotype IgG control Ab (1 mg/kg), or the eNAMPT-neutralizing humanized mAb, ALT-100 (1 mg/kg) on days 1 and 7 (n = 9 per group). In two additional series of experiments, pristane-challenged mice received either subcutaneously delivered PBS, or 0.4 mg/kg, 1 mg/kg, 4 mg/kg ALT-100 mAb. Mice in each series of experiments were sacrificed at Day 10 post pristane.
2.4. Bronchoalveolar lavage (BAL) analysis
BAL fluid and cells were collected by lavage and centrifuged as described previously for mice [24,31]. BAL albumin and total protein and cell count analysis were performed as we previously described [32].
2.5. Quantitative lung histology and immunohistochemistry (IHC) analyses
Lungs were fixed overnight in 10% buffered formalin and embedded in paraffin. The embedded tissue was sectioned for routine hematoxylin and eosin staining and imaging (Olympus digital camera, 10x magnification) [33]. For IHC visualization of NAMPT expression in lung tissues, the avidin-biotin-peroxidase method was utilized with a rabbit anti-human NAMPT pAb (1:100 dilution, Bethyl Laboratories) as previously described [12,34,35]. Histological and IHC images were randomly selected for H&E or NAMPT quantification using ImageJ software [36] with additional details provided in the Supplemental Materials and Methods. AF647-conjugated secondary antibodies against rabbit IgG were applied at a dilution of 1:500 for 1 h at room temperature. Secondary antibody given alone without primary antibody was used as negative control for each stain.
2.6. Plasma/serum measurements of human and murine cytokines
Human serum samples were analyzed for levels of eNAMPT, IL-6, Angiopoietin-2 and IL-1RA utilizing a meso-scale ELISA platform (Meso Scale Diagnostics, Rockville, MD) as previously reported [20,37]. Similarly, murine plasma levels of eNAMPT, IL-6, and IL-8 (KC) were measured using the U-Plex MSD platform as we have previously described [23,24,38].
2.7. Flow cytometry
The following conjugated anti-mouse antibodies were used for flow cytometry studies: anti-Ly6G (1A8), anti-CD11b (M1/70), anti-Ly6C (ER-MP20), anti-F4/80 (BM8), anti-CD4 (GK1.5), anti-CD8a (53–6.7), anti-CD11c (3.9), and anti-B220 (RA3-6B2) (eBiosciences). Cells were incubated in CD16/32 (Fc block; BD Biosciences) prior to staining. Cells were acquired on LSR II (BD Biosciences) and analyzed with the FlowJo software (Treestar).
2.8. Biochemical studies
Western blotting of lung homogenates was performed according to standard protocols as previously reported [17,32] with densitometric quantification of lung tissue expression of p-NFκB, NFκB, NAMPT, DOCK1 and β-actin (total protein control).
2.9. Gene expression analysis by Q-PCR
RNA was isolated from SLE subjects and from murine lung tissues and Quantitative PCR (Q-PCR) performed as described previously [38]. In brief, total RNA was extracted from lung using TRIzol reagent (Invitrogen, Carlsbad, CA). RNA was isolated from human blood with the QIAamp RNA Blood Mini Kit (Qiagen). cDNA was synthesized using the Superscript II First-Strand Synthesis kit (Invitrogen). SYBR Green Q-PCR analysis was performed using an Q5 (Applied systems). Gene expression was normalized to 18 S RNA, and the expression level was calculated using the 2-ΔΔCt method. Primer sequences are listed as follows: mNampt Fw: tgtgtaccgaggagacgaca; mNampt Rv: ctgtgccaggctgacatcta; mGapdh Fw: catcaagaaggtggtgaagc; mGapdh Rv: cctgttgctgtagctgtatt; hNAMPT Fw: CACACTGGA GTGGTGTACTAGC; hNAMPT Rv: CCAGGGTAACATCTAGCTGGAA; 18S Fw: GCTTA ATTTGACTCAACACGGGA; 18S Rv: AGCTATCAATCTGTCAATCCTGTC.
2.10. RNA sequencing and Gene expression analysis
Total RNA was extracted from mice lung tissues, quantified (Qubit fluorometer, ThermoFisher Scientific, Waltham, MA), and the RNA quality assessed using the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). RNA was purified using the NEBNext® Poly(A) mRNA Magnetic Isolation Module (New England Biolab Inc, Ipswich, MA), and library construction was performed using the Swift RNA Library Kit (Swift Biosciences, Ann Arbor, MI). Libraries were multiplexed and sequenced using NovaSeq 6000 (Illumina, San Diego, CA).
For data analysis, the raw data was converted to fasq format, reads aligned to the transcriptome using STAR (version 2.6.1) [39] and quantified using RSEM [40], a custom mouse GRCm38 transcriptome. DEseq2 [41] algorithms were used to assess the differential expression between two sample groups and a false discovery rate (FDR) [42] applied to control for multiple testing error. Enriched analysis was conducted applying Gene Ontology (GO) classification, focused on biological process and pathway classification for the statistically-significant DEGs. Unbiased comparison of the gene sets to ConsensusPathDB [43] against KEGG and Reactome pathways databases was conducted as previously reported [23,25,38]. The STRING database was used to construct the interaction networks [44].
2.11. Lung pathological evaluation
The presence of alveolar hemorrhage in lung specimens was initially assessed by gross observation of excised lungs. The percentage of lung involved with hemorrhage was assessed and a DAH score generated in a blinded manner as follows: (0) - lung hemorrhage absent; (1) 0–25% hemorrhagic lung; (2) 25–50% hemorrhagic lung; (3) 50–75% hemorrhagic lung; (4) 75–100% hemorrhagic lung.
2.12. Statistical methods
All data are expressed as means ± SD. Statistical differences were measured using either an unpaired Student's t-test or 2-way analysis of variance (ANOVA) when appropriate with Bonferroni post-hoc test. When the data analyzed was not distributed normally, we used the Mann–Whitney test or Kruskal–Wallis 1-way ANOVA with Dunn's post-hoc test. Data analysis was performed using Prism software version 7.0a (GraphPad, San Diego, CA). A P-value of <0.05 was considered statistically significant. Asterisks in the figures represent the following: *P < 0.05; **P < 0.01; ***P < 0.001, and ****P < 0.0001. eNAMPT levels were correlated with clinical indices by Pearson correlation.
3. Results
3.1. Increased NAMPT mRNA and protein expression in blood from SLE subjects
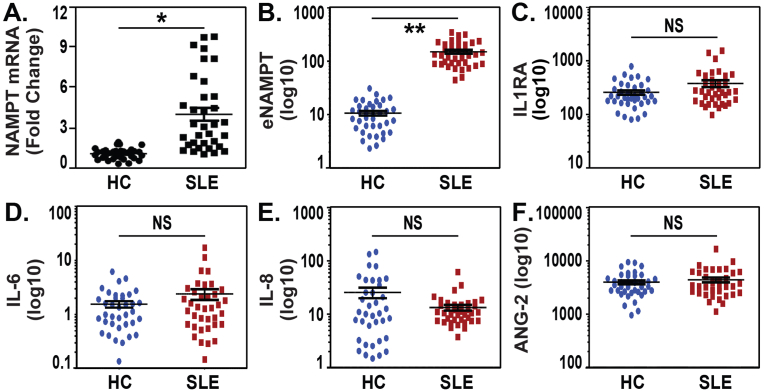
NAMPT mRNA expression was assessed by RT-PCR in whole blood obtained from 38 healthy controls (HCs) and 41 SLE patients (39 female, 2 male) with a mean age of 39 ± 12 years and a mean SLEDAI score of 4.7 ± 4.2 (Table 1). The SLE cohort showed a near 4-fold increase in NAMPT mRNA expression compared to controls (Fig. 1A). Examination of serum levels of eNAMPT, IL-6, IL-8, IL-1RA and ANG-2 from the same SLE cohort revealed that only eNAMPT levels were significantly elevated in SLE subjects when compared to healthy controls (Fig. 1B) with IL-6, IL-8, IL-1RA and ANG-2 levels in SLE subjects comparable to healthy controls (Fig. 1C/D/E/F). We assessed the relationship between eNAMPT mRNA and protein expression levels in blood and the Systemic Lupus Erythematosus Disease Activity Index or SLEDAI. Whereas eNAMPT mRNA expression levels did not show significant correlation with SLEDAI scores (p = 0.2), In contrast, plasma eNAMPT levels strongly trended to a significant relationship between plasma eNAMPT levels and SLEDAI (0.06).
Table 1.
Clinical information on a human SLE cohort (n = 41).
| Mean ± S.D. | Median (Min-Max) | |
|---|---|---|
| Age | 39 ± 12 years | 44 (20–68) |
| Sex Female: Male | 39 : 2 | |
| SLEDAI | 4.7 ± 4.2 | 4 (0–18) |
| Organ Involvement | ||
| Skin Involvement | 5 | |
| Arthritis | 14 | |
| Kidney | 4 | |
| Lupus Headache | 1 | |
| Low Complement | 3 | |
| Mucosal Ulcers | 1 | |
| Vasculitis | 2 | |
| Unknown | 1 | |
| Lab Work | [Ref. Range] | |
| Serum Creatinine mg/dL | 0.7 ± 0.2 [0.59–1.04] | 0.6 (0.5–1.3) |
| Serum Protein g/dL | 7.1 ±0.7 [[6], [7], [8]] | 7.1 (5.9–8.9) |
| Urine Creatinine | 61.9 ± 42.5 | 46 (20.4–122.1) |
| C3 mg/dL | 102.4 ± 36.3 [80–160] | 97 (42–179.7) |
| C4 mg/dL | 22.2 ± 12 [[15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45]] | 18 (5–60) |
| ESR mm/hr | 34.2 ± 21.5 [0–29] | 29 (3–72) |
| CRP mg/dL | 4 ± 4.2 [<1]* | 1 (0.3–15.5) |
| Medications | ||
| Antimalarials | 58% | |
| Steroids (2.5–20 mg) | 58% | |
| Immunosuppressants | 33% |
Fig. 1.
Increased NAMPT expression in blood from SLE subjects. A. Whole blood was obtained from 38 healthy controls (HCs) and 41 SLE patients (>90% female) (Table 1). NAMPT mRNA expression was assessed by RT-PCR with >4 fold increase in NAMPT mRNA expression observed in SLE subjects compared to HCs. RT-PCR analysis was performed in duplicates and analyzed using ddCt method. *p < 0.01. B/C/D/E/F. Examination of circulating serum levels of eNAMPT, IL-6, IL-8, IL-1RA and ANG2 in 41 SLE subjects revealed that only eNAMPT levels were significantly elevated in SLE subjects compared with 38 HCs with serum levels of IL-6, IL-8, IL-1RA and ANG2 similar to HCs. All data represent mean ± SD. Statistical significance was determined using two tails Student's t-test. **p < 0.001.
3.2. Increased NAMPT mRNA and protein expression in lung tissues and blood from pristane-exposed mice with pulmonary vasculitis/hemorrhage
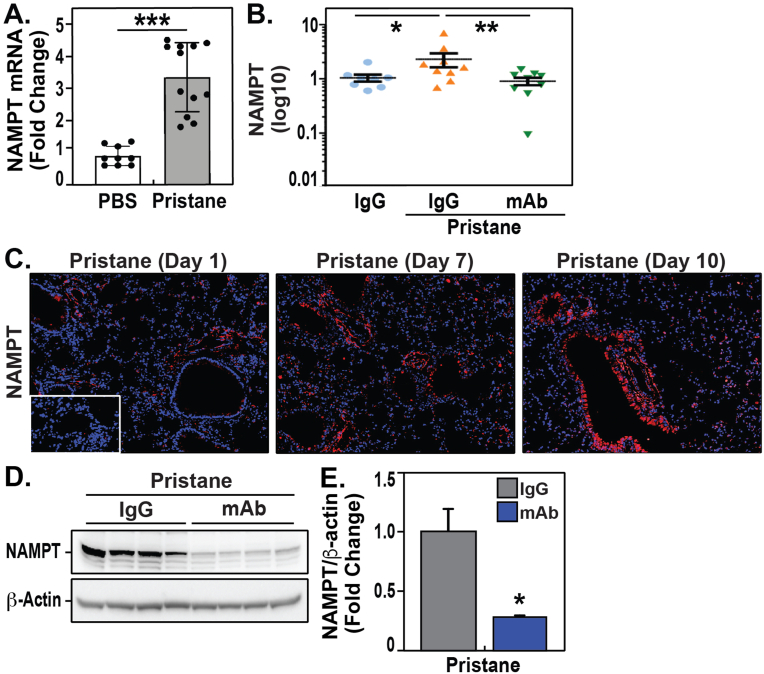
We next examined Nampt mRNA and eNAMPT plasma levels in our preclinical model of pristane-induced SLE vasculitis. Initial studies to determine the level of NAMPT expression in pristane-exposed lung tissues revealed significantly Nampt mRNA expression (∼3-fold increase) compared to non-pristane-exposed control mouse lungs (Fig. 2A). In addition, Day 10 plasma eNAMPT levels were significantly elevated in pristane-exposed, IgG isotype antibody-treated mice (1.55± 0.3 ng/mL, n = 9) compared to non-pristane-exposed, IgG-treated controls (1.01 ± 0.2 ng/mL, n = 9, p < 0.05) (Fig. 2C). NAMPT protein expression in pristane-exposed lung tissues assessed by IHC on day 10 demonstrated significant increases in NAMPT lung tissue protein expression with steady increases in NAMPT expression from Day 1 through Days 7 and 10 (Fig. 2C). Finally, confirming the IHC imaging results, increases in NAMPT expression in pristane-exposed lung homogenates were identified by western blotting (Fig. 2D/E). Taken together, these data suggest that eNAMPT expression is markedly increased in the blood and serum of SLE patients and in the lungs and blood of pristane-challenged mice, a preclinical model of SLE lung vasculitis/hemorrhage.
Fig. 2.
Increased NAMPT mRNA and protein expression in lung tissues and blood from pristane-exposed mice with pulmonary vasculitis/hemorrhage. A. The level of Nampt mRNA expression in pristane-exposed murine lung tissues was examined by RT-PCR. These studies revealed significantly increased Nampt mRNA expression (∼3-fold increase) compared to control, pristane-unexposed mouse lungs. B. Day 10 plasma levels of eNAMPT from IgG- and pristane-exposed mice (n = 9 samples/group). A 3-fold increase in eNAMPT levels was observed in pristane-exposed, IgG-treated mice (1.55± 0.3 ng/mL, n = 9) compared to non-pristane-exposed controls (1.01 ± 0.2 ng/mL, n = 9, p < 0.05). Mice receiving the ALT-100 eNAMPT mAb on Day 1 (1 mg/kg) demonstrated significantly reduced plasma eNAMPT levels. C. NAMPT protein expression in pristane-exposed lung tissues was assessed by IHC on Day 10 (Bethyl Ab, 1:1000 dilution). These studies demonstrated significant increases in NAMPT lung tissue protein expression with steady increases in NAMPT expression from Day 1 through Days 7 and 10. D/E. NAMPT protein expression in pristane-exposed lung homogenates, assessed via western blotting (Bethyl Ab, 1:1000 dilution), showed NAMPT lung tissue expression is markedly increased by pristane challenge and attenuated in mice receiving the eNAMPT-neutralizing ALT-100 mAb. Densitometric analysis was performed using Bio-Rad Image Lab 6.01 software to quantify protein expression normalized to b-actin expression.
3.3. The eNAMPT-neutralizing mAb reduces pristane-induced murine alveolar hemorrhage
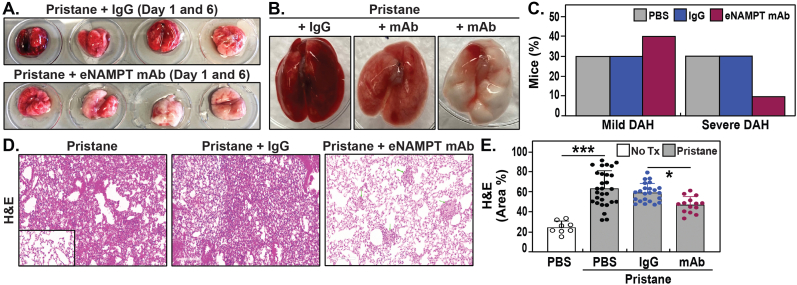
We next assessed whether tissue and blood eNAMPT expression is an active participant in pristane-induced vasculitis/DAH and tested the effects of the ALT-100 mAb eNAMPT-neutralizing antibody in the pristane model (28, 61). Alveolar hemorrhage was absent in control mice that were not exposed to pristane challenge whereas all pristane-exposed mice receiving either PBS or IgG (controls) survived to Day 10 exhibited varying degrees of alveolar hemorrhage (mild to severe) by gross whole lung pathology (Fig. 3A/B). Quantification of the extent of lung involvement with alveolar hemorrhage demonstrated similar severity in both the PBS- and IgG-treated pristane-challenged groups with each groups exhibiting comparable (30%) incidence of mild DAH and severe DAH (Fig. 3C). In contrast, mice receiving the eNAMPT-neutralizing ALT-100 mAb (1 mg/kg, IP) beginning on Day 1 showed substantial reduction in the severity of DAH by gross examination (Fig. 3A/B) and only 10% of mAb-treated mice exhibiting severe lung hemorrhage involvement compared to 30% lung hemorrhage involvement in PBS or IgG-challenged mice (Fig. 3C). Histologic examination of pristane-induced lung inflammatory injury by H&E staining revealed dramatic immune cell and leukocyte infiltration in perivascular area which was similar in both PBS- and IgG-treated mice but significantly reduced in mice treated with the ALT-100 mAb (Fig. 3D), verified by Image J quantification of randomly selected H&E areas (Fig. 3E).
Fig. 3.
The eNAMPT-neutralizing mAb reduces pristane-induced murine vasculitis and alveolar hemorrhage. A/B. Alveolar hemorrhage was absent in control mice that were not exposed to pristane challenge. All pristane-exposed mice receiving either PBS or IgG (controls) survived to Day 10 and exhibited varying degrees of alveolar hemorrhage (mild to severe) by gross whole lung pathology. Representative lung samples are depicted. In contrast, mice receiving the eNAMPT-neutralizing ALT-100 mAb (1 mg/kg) beginning on Day 1 showed substantial reduction in the severity of DAH by gross examination compared with PBS or IgG mice. C. Quantification of the extent of lung involvement with alveolar hemorrhage demonstrated similar severity in both the PBS- and IgG-treated pristane-challenged groups with each groups exhibiting comparable (30%) incidence of mild DAH and severe DAH. In contrast, only 10% of ALT-100 mAb-treated mice exhibited evidence of severe lung hemorrhage compared to 30% of PBS- or IgG-challenged mice. D/E. Histologic examination of pristane-induced lung inflammatory injury by H&E staining (four 20X fields) was examined in randomly selected areas on the H&E sections for each mouse and representative histology images are presented. These studies revealed dramatic immune cell and leukocyte infiltration in perivascular area which was similar in both PBS- and IgG-treated mice but significantly reduced in mice treated with the ALT-100 mAb, verified by Image J quantification of randomly selected H&E areas.
In selected experiments, the efficacy of subcutaneously-delivered ALT-100 mAb (delivered in Day 1 and Day 8) at doses of 0.4 mg/kg, 1 mg/kg, and 4 mg/kg was tested in pristane-exposed mice. Each subQ-delivered ALT-100 mAb dose strongly reduced BAL inflammatory cell numbers with only 1 mg/kg and 4 mg/kg ALT-100 mAb significantly reducing BAL protein levels (Supplemental Fig. 1A/B). Each dose also reduced inflammatory edema and alveolar hemorrhage (1 mg/kg shown in Supplemental Fig. 1C).
3.4. eNAMPT neutralization reduces pristane-induced murine lung inflammation
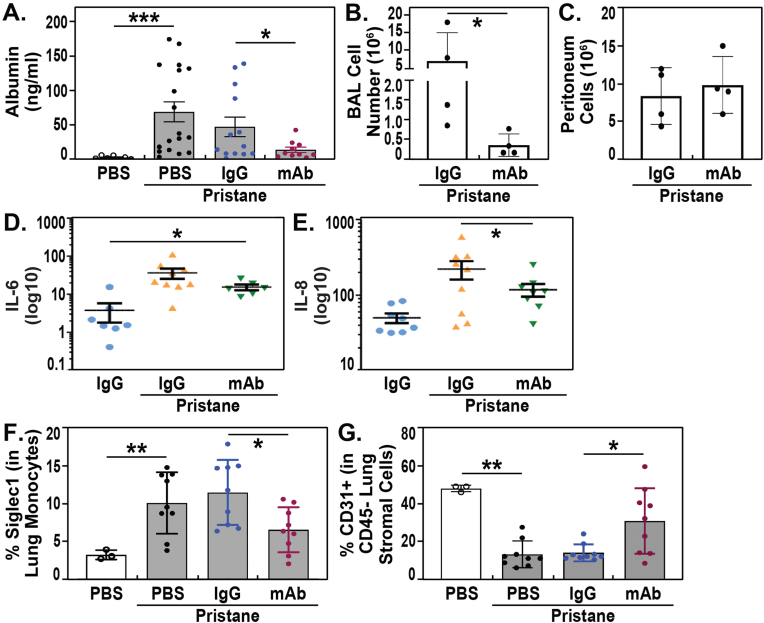
Pristane-exposed mice exhibited significant inflammatory injury reflected by several BAL, tissue and blood indices. For example, BAL protein levels and numbers of BAL inflammatory cells at Day 10 were significantly increased in pristane-exposed, IgG-treated mice compared to unexposed controls (Fig. 4A/B) but significantly reduced in mice receiving the ALT-100 mAb (Fig. 4A/B). In contrast, quantification of peritoneal lavage cells from pristane-challenged mice showed no significant effect of ALT-100 mAb compared to IgG control mice (Fig. 4C). These observations indicate that treatment with the eNAMPT-neutralizing ALT-100 mAb attenuates pristane-induced lung vasculitis with reductions in vascular leak, inflammatory cell influx, NAMPT tissue expression (Fig. 2D/E) and production of proinflammatory cytokines with significant reductions in plasma levels of eNAMPT (Fig. 2B), IL-6 and IL-8 (Fig. 4D/E).
Fig. 4.
eNAMPT neutralization reduces pristane-induced murine lung inflammation. A/B. Pristane-exposed mice exhibited significant inflammatory injury reflected by several BAL, tissue and blood indices. For example, BAL protein levels and numbers of BAL inflammatory cells at Day 10 were significantly increased in pristane-exposed, IgG-treated mice compared to unexposed controls but significantly reduced in mice receiving the ALT-100 mAb. C. In contrast to BAL results, quantification of peritoneal lavage cells from pristane-challenged mice showed no significant effect of ALT-100 mAb compared to IgG control mice. D/E. Levels of proinflammatory cytokines IL-6 and IL-8 were observed in pristane-exposed mice compared to control mice. Consistent with the inflammation-reducing capacity for the ALT-100 mAb and similar to the reductions in plasma levels of eNAMPT (Fig. 2B), reductions in IL-6 and IL-8 were observed in ALT-100 mAb-treated mice. F/G. Inflammatory cell subsets in pristane-exposed lungs were examined by flow cytometry. Significant accumulation of Siglec 1-positive CD11b + Ly6C + monocytes in the lung was observed whereas CD45−CD31+ vascular endothelial cells were significantly reduced by pristane exposure. ALT-100 mAb-treated mice showed normalization of these cellular inflammatory parameters with reduced numbers of Siglec1-positive CD11b + Ly6C + monocytes and increased numbers of lung endothelial cells compared to pristane only or IgG-treated mice.
We further examined inflammatory cell subsets in pristane-exposed lungs by flow cytometry [45,46] and observed significant accumulation of Siglec 1-positive CD11b + Ly6C + monocytes in the lung (Fig. 4F), whereas CD45−CD31+ vascular endothelial cells were significantly reduced by pristane exposure (Fig. 4G). ALT-100 mAb-treated mice showed normalization of these cellular inflammatory parameters with reduced numbers of Siglec1-positive CD11b + Ly6C + monocytes and increased numbers of lung endothelial cells compared to pristane only or IgG-treated mice (Fig. 4F/G).
3.5. eNAMPT neutralization reduces pristane-induced inflammatory signaling and differentially-expressed genes/pathways
To confirm that eNAMPT/TLR4 signaling contributes to lung inflammation, vasculitis and DAH, we performed biochemical and genomic analyses of pristane-exposed lung tissues. Striking increases in the levels of phosphorylated NFκB (standardized against total NFκB) were observed in lung homogenates obtained from pristane-exposed mice being identified by Western blotting (Fig. 5A/B) reflecting strong activation of innate immunity-mediated activation of inflammatory signaling pathways. Importantly, mice receiving the eNAMPT-neutralizing ALT-100 mAb demonstrated near abolishment of the increased levels of phosphorylated NFκB in pristane-exposed mice indicating that the eNAMPT/TLR4 inflammatory signaling pathways is a major driver of the innate immunity response to pristane challenge (Fig. 5A/B). We recently identified the Dock1 protein as a critical regulator of vascular barrier integrity via its requirement for activation of Rac GTPase-mediated cytoskeletal protein rearrangement in vascular endothelial cells [47] with TLR4 activation resulting in marked decreases in Dock1 expression. Western blot analysis of pristane-exposed lung tissue homogenates identified significant reductions in DOCK1 lung expression, which were preserved in mice receiving the eNAMPT-neutralizing ALT-100 mAb (Fig. 5A/C), thus directly linking eNAMPT/TLR4-mediated reductions in DOCK1 to a signaling pathway involved in maintaining vascular barrier integrity.
Fig. 5.
eNAMPT neutralization reduces pristane-induced NFkB inflammatory signaling and differential gene/pathway dysregulation. A/B. Lung tissue homogenates were obtained from IgG-treated and eNAMPT ALT-100 mAb-treated pristane-exposed mice (n = 6/group) and compared to unexposed control mice (n = 4). Western blotting studies revealed striking increases in NFκB phosphorylation/total NFκB levels in lung homogenates obtained from pristane-exposed mice (lanes 5–8) compared to controls (first 4 lanes). Mice receiving the eNAMPT-neutralizing ALT-100 mAb demonstrated near abolishment of the increased levels of phosphorylated NFκB in pristane-exposed mice (lanes 9–12). These studies indicate that the eNAMPT/TLR4 inflammatory signaling pathway is a major driver of the innate immunity response to pristane challenge. A/C. Western blotting studies were also conducted in lung homogenates to examine DOCK1 protein immunoreactivity, a critical regulator of vascular barrier integrity [47]. These studies identified significant reductions in DOCK1 expression, which were restored in mice receiving the eNAMPT-neutralizing ALT-100 mAb. D. RNA sequencing data from pristane-exposed lung tissues (Day 10) from IgG- and ALT-100 mAb-treated mice was analyzed and yielded 447 DEGs (FDR <0.1, FC 2.0), with the top DEGs depicted in the heat map shown, 57 upregulated genes shown in green and 390 down-regulated genes shown in red. E. The STRING Functional protein network depicted shows the interaction of DEGs influenced by ALT-100 mAb treatment in mice exposed to pristane. The edges represent protein-protein associations, line color indicates the evidence the type of interaction (green-gene neighborhood, red-gene fusion, blue-co-occurrence, black-co-expression). The confidence interaction score is 0.15. Six relevant groups of biological-related nodes were identified as detailed in the Results/Discussion. Group #1- Genes involved in TNF, IFN, NF-kappa-B and STAT signaling. Group #2- Genes involved in Interleukin-1 inflammatory signaling and includes the IL-1 receptor antagonist which inhibits IL-1β by binding to its receptor to prevent its association with IL-1RAP for signaling. Group #3- Genes involved in matrix degradation with metalloproteinases which degrade fibronectin, collagens Il6, Socs3 negative reg, and cytokines involved in the Jak/STAT pathway. Group #4- Genes involved in chemotactic/and inflammatory pathways. Group #5- Genes involved in centromere and kinetochore mitotic-related proteins. Group #6- Genes involved in cell cycle control and G1/S-specific related proteins.
We also analyzed RNA sequencing data from pristane-exposed lung tissues to further validate the histologic and biochemical findings implicating eNAMPT/TLR4 inflammatory cascade signaling as the mechanism of action for ALT-100 mAb efficacy in pristane-induced lung vascular injury. Analyses of differentially-expressed genes (DEGs) between control mice and pristane-exposed lung tissues (Day 10) identified 604 DEGs (FDR 0.05, FC 2.0) with Gene Ontologies (GO) that were associated with inflammatory molecular functions including IL-8, Toll-like receptors, and chemokine/cytokine activity (Table 2). The top biological pathways are presented in (Table 3) and include TP53 transcription regulation, activation of Ima kinases, and matrix metalloproteinases. DEGs derived from comparisons of pristane-exposed lung tissues from IgG-treated mice and mice receiving the ALT-100 mAb identified 447 DEGs (FDR <0.1, FC 2.0), with 57 upregulated and 390 down-regulated genes with the top DEGs depicted in a heat map (Fig. 5D). We next created a protein-protein interaction network for the ALT-100-influenced DEGs using the STRING-based interactome (Fig. 5E). This analysis identified six relevant groups of biological-related nodes with Groups #1-#4 predominantly inflammatory in nature. These nodes included TNF, IFN, NF-kappa-B and STAT signaling proteins (#1), Interleukin-1 inflammatory signaling and IL-1 receptor antagonist proteins (#2), matrix degradation and metalloproteinases, IL-6, Socs3 negative regulatory proteins and Jak/STAT pathway cytokines (#3), and chemotactic/inflammatory cytokines (#4). The Group #5 node was comprised of centromere and kinetochore mitotic-related proteins and Group #6 node contained cell cycle control and G1/S-specific-related proteins.
Table 2.
Top Gene ontology “molecular function” terms in pristane-exposed lupus mice.
| GO term ID | Term Description | Strength | False Discovery Rate |
|---|---|---|---|
| GO:0019959 | Interleukin-8 binding | 1.61 | 2.09E-02 |
| GO:0035662 | Toll-like receptor 4 binding | 1.38 | 4.55E-02 |
| GO:0045236 | CXCR chemokine receptor binding | 1.27 | 7.20E-04 |
| GO:0050786 | RAGE receptor binding | 1.25 | 1.68E-02 |
| GO:0008009 | Chemokine activity | 1.17 | 1.63E-09 |
| GO:0004875 | Complement receptor activity | 1.09 | 4.12E-02 |
| GO:0035325 | Toll-like receptor binding | 1.06 | 4.90E-02 |
| GO:0019956 | Chemokine binding | 1 | 7.30E-04 |
| GO:0016493 | C–C chemokine receptor activity | 1 | 7.60E-03 |
| GO:0001848 | Complement binding | 0.98 | 2.63E-02 |
| GO:0005125 | Cytokine activity | 0.77 | 1.89E-10 |
| GO:0004896 | Cytokine receptor activity | 0.68 | 5.20E-03 |
| GO:0008201 | Heparin binding | 0.55 | 1.45E-02 |
Table 3.
Top dysregulated pathways in pristane-exposed lupus mice.
| MMU pathway ID | Term Description | Strength | False Discovery Rate | Source |
|---|---|---|---|---|
| 2980767 | Activation of NIMA Kinases NEK9, NEK6, NEK7 | 1.51 | 1.80E-03 | Reactome |
| 69478 | G2/M DNA replication checkpoint | 1.38 | 2.28E-02 | Reactome |
| 6804116 | TP53 Regulates Transcription of Genes Involved in G1 Cell Cycle Arrest | 1.31 | 1.00E-03 | Reactome |
| 380108 | Chemokine receptors bind chemokines | 1.09 | 1.91E-09 | Reactome |
| 75205 | Dissolution of Fibrin Clot | 1.09 | 2.02E-02 | Reactome |
| WP460 | Blood clotting cascade | 1.08 | 6.50E-04 | Wiki |
| 69273 | Cyclin A/B1/B2 associated events during G2/M transition | 1.07 | 3.80E-04 | Reactome |
| WP441 | Matrix metalloproteinases | 1.05 | 7.59E-05 | Wiki |
| 5686938 | Regulation of TLR by endogenous ligand | 1.05 | 7.50E-03 | Reactome |
| 6791312 | TP53 Regulates Transcription of Cell Cycle Genes | 1.02 | 5.08E-05 | Reactome |
| WP1253 | Type II interferon signaling (IFNG) | 0.95 | 7.20E-04 | Wiki |
| 04657 | IL-17 signaling pathway | 0.91 | 1.23E-08 | KEGG |
| 04610 | Complement and coagulation cascades | 0.9 | 1.27E-08 | KEGG |
| 140877 | Formation of Fibrin Clot (Clotting Cascade) | 0.87 | 4.10E-03 | Reactome |
| mmu04115 | p53 signaling pathway | 0.8 | 1.30E-04 | KEGG |
| mmu04060 | Cytokine-cytokine receptor interaction | 0.77 | 8.20E-16 | KEGG |
The top KEGG prioritized pathways generated by ALT-100 mAb-influenced genes from pristane-challenged mice included rheumatoid arthritis-related pathways, cytokine/cytokine receptor signaling, chemokine signaling, IL-17 signaling, p53 signaling, complement/coagulation cascades and TNFα/Toll-like receptor signaling (Table 4). Together these results are highly consistent with a critical role for the eNAMPT/TLR4 signaling pathway in pristane activation of evolutionarily-conserved inflammatory cascades that contribute to the severity of lung vasculitis and DAH pathobiology.
Table 4.
Top Dysregulated KEGG Pathways in ALT-100 mAb-Treated Mice.
| Pathway ID | Term Description | Strength | False Discovery Rate |
|---|---|---|---|
| mmu 04061 | Viral protein interaction with cytokine and cytokine receptor | 1.06 | 1.23E-10 |
| mmu04060 | Cytokine-cytokine receptor interaction | 0.75 | 2.34E-10 |
| mmu 04657 | IL-17 signaling pathway | 0.97 | 5.59E-08 |
| mmu 04668 | TNF signaling pathway | 0.81 | 2.25E-05 |
| mmu 04110 | Cell cycle | 0.77 | 4.58E-05 |
| mmu 05323 | Rheumatoid arthritis | 0.85 | 5.45E-05 |
| mmu 04610 | Complement and coagulation cascades | 0.82 | 8.70E-05 |
| mmu 04062 | Chemokine signaling pathway | 0.66 | 0.0001 |
| mmu04115 | p53 signaling pathway | 0.85 | 0.00039 |
| mmu 04620 | Toll-like receptor signaling pathway | 0.75 | 0.0006 |
| mmu 04623 | Cytosolic DNA-sensing pathway | 0.79 | 0.0055 |
| mmu 04621 | NOD-like receptor signaling pathway | 0.54 | 0.0057 |
| mmu 05166 | Human T-cell leukemia virus 1 infection | 0.5 | 0.0078 |
| mmu 04064 | NF-kappa B signaling pathway | 0.65 | 0.0109 |
| mmu 05160 | Hepatitis C | 0.55 | 0.0125 |
| mmu 05206 | MicroRNAs in cancer | 0.5 | 0.0375 |
| mmu 05161 | Hepatitis B | 0.49 | 0.04 |
| mmu 05202 | Transcriptional misregulation in cancer | 0.46 | 0.04 |
4. Discussion
Lupus vasculitis occurs in approximately 50% of SLE patients [48] affecting the multiple tissues including the skin (butterfly rash), heart, kidney and lung. Life-threatening lupus vasculitis most commonly seen with active lupus nephritis with diffuse alveolar hemorrhage (DAH) a severe complication affecting ∼5% of SLE patients. DAH, often the initial presenting symptom of SLE, is variably treated with high dose corticosteroids, plasmapheresis, cyclophosphamide, rituximab, or mycophenolate mofetil, however, the paucity of randomized controlled trials specific for lupus vasculitis renders selection of therapeutic as arbitrary. Safe, effective treatments for lupus vasculitis and DAH remains an important unmet need in SLE.
The pathogenesis of SLE lung vasculitis is poorly understood but involves complex interactions between the vascular endothelium, inflammatory cells, cytokines, autoantibodies, and immune complexes that result in sterile inflammation, increased vascular permeability, complement/coagulation cascade activation, and leukocyte infiltration into vessels. DAMPs are chemically heterogeneous host-derived molecules that are rapidly released into the extracellular space from damaged extracellular matrices or dying cells under conditions of cellular/organ activation, stress, or injury [49,50]. In the absence of infection, DAMPs potently incite a sterile inflammatory response signal and immune responses via ligation of pattern-recognition receptors (PRRs) which include the Toll-like receptors and cytosolic Nod-like receptors, culminating in nuclear factor κB (NF-κB) activation and pro-inflammatory cytokine secretion. An increasing number of DAMPs have been linked to sterile inflammation and promotion of the maturation/activation of diverse immune cells and production of pro-inflammatory cytokine, events implicated in the pathogenesis of auto-immune diseases including rheumatoid arthritis (RA), SLE, psoriatic arthritis, and systemic sclerosis or scleroderma [51]. Continuous DAMP exposure has been linked the loss of tolerance to self-antigens in these diseases. SLE patients have impaired clearance of apoptotic debris which facilitates extracellular release of HMGB1 and other DAMPs whose activity is implicated in multiple disease phenotypes in SLE, including lupus nephritis and neuropsychiatric lupus. Understanding the mechanisms of sterile inflammation triggered by DAMPs is important in order to generate novel therapeutic auto-immune disease therapeutic strategies that target these molecules. For example, targeting DAMPs with nucleic acid scavengers has been suggested as a SLE therapeutic approach to reduce nucleic acid autoantibodies [52].
We previously utilized multi-species preclinical inflammatory models coupled with genomic–intensive approaches [53,54] to identify eNAMPT as a potent proinflammatory cytozyme that regulates nicotinamide adenine dinucleotide (NAD) biosynthesis [55,56] and serves as a novel innate immunity-activating DAMP [12]. Importantly, we have convincingly demonstrated eNAMPT's DAMP activity stems from high affinity ligation of the PRR, Toll-like receptor 4 (TLR4), to elicit robust NFkB-mediated signaling [12]. In humans and preclinical models, eNAMPT is readily found within areas of sterile inflammation, such as the lungs and blood, with eNAMPT secretion augmented in response to hypoxia, severe bacterial and viral infections, trauma, radiation, and mechanical stress [23,31,38,57,58]. The current study confirms prior studies demonstrating increased circulating eNAMPT levels in patients with SLE [59,60] with SLE study subjects exhibiting both elevated eNAMPT protein and mRNA expression in blood. Pristane-exposed mice demonstrated similar increases in plasma eNAMPT and lung tissue NAMPT expression in a murine model of SLE vasculitis. Studies utilizing the eNAMPT-neutralizing mAb confirmed the involvement of eNAMPT/TLR4 inflammatory cascade activation in SLE-associated lung vasculitis with marked reductions in pristane-induced alveolar hemorrhage, inflammatory lung injury, leukocyte infiltration and preservation of vascular barrier integrity. Activation of the eNAMPT/TLR4 pathway in response to pristane-induced vasculitis is intimately involved in the evoked increases in circulating inflammatory cytokines, and profound loss of lung vascular barrier integrity, events directly linked to SLE mortality [35,[61], [62], [63]].
The precise cellular source of secreted eNAMPT is incompletely understood but is presumed to be highly stimulus- and organ-specific. Previous localizing studies of acute inflammatory lung injury revealed robust spatially-localized NAMPT expression in lung endothelium, epithelium as well as in resident and infiltrating leukocytes [35]. Similar studies in a rat model of hypoxia/sugen-induced pulmonary hypertension confirmed enhanced NAMPT expression in lung endothelial cells (EC) [35]. Utilizing EC-specific conditional NAMPT KO mice, we showed the unequivocal involvement of EC-secreted eNAMPT in driving lung injury severity in both preclinical ARDS and PH models [25,57]. These studies strongly validate the viability of EC-derived eNAMPT as a SLE therapeutic target in lupus vasculitis/DAH and underscore the capacity of an eNAMPT-neutralizing biologic therapy to directly address the unmet need for novel strategies that improve lupus vasculitis mortality. The utility of the eNAMPT-neutralizing mAb in lupus vasculitis is supported by recently published studies detailing the efficacy of eNAMPT/TLR4 pathway targeting in preclinical ARDS [31,34,57,58], pulmonary hypertension [25], radiation pneumonitis [23], radiation fibrosis [38], and prostate cancer [64,65].
We employed FACS cell sorting of pristane-exposed murine lungs to assess the inflammatory components in the SLE model influenced by ALT-100 mAb. Our prior studies highlighted eNAMPT as a PMN chemotaxin [34] and regulator of neutrophil maturation, activity, and life span as well as M2 macrophage polarization [[66], [67], [68]]. Shifting of pristane-treated macrophages to a M1 phenotype has been suggested to be critical in the development of pulmonary vasculitis [69,70]. Interestingly, Siglec 1 expression levels, widely used to assess inflammatory monocyte activation as precursors of resident tissue macrophages, were markedly increased in pristane-exposed mice and reduced in mice receiving ALT-100 mAb.
Our combined biochemical and genomic approaches further confirmed the key role for eNAMPT/TLR4 pathway activation in the development of lung vasculitis in pristane-exposed lung tissues with striking increases in NFkB activation which was significantly attenuated in mice receiving the ALT-100 mAb (Fig. 5). The significant reductions in DOCK1 expression observed in pristane-exposed mice were restored/preserved in eNAMPT mAb-treated mice, a finding that provides insight into the eNAMPT/TLR4 signaling paradigm that produces severe vascular leakage and alveolar hemorrhage, given the now appreciated role of DOCK1 in preservation of vascular integrity [47]. Genomic studies of DEGs and signaling pathways influenced by ALT-100 mAb highlighted signaling pathways previously associated with multiple autoimmune disorders (cytokine/cytokine receptor, chemokine, IL-17, p53, and TNFα/Toll-like receptor, Table 4). The identification of highly significant DEGs in the complement/coagulation cascade (Plaur-Urokinase plasminogen activator receptor, Serpine1-serine proteinase inhibitor) is highly consistent with the known incidence of thrombotic manifestations in SLE patients even when compared with patients with other autoimmune disorders.
5. Conclusion
In summary, these human and preclinical studies are highly consistent with a critical role for the eNAMPT/TLR4 signaling pathway in activation of evolutionarily-conserved inflammatory cascades that contribute to the severity of lupus vasculitis and DAH pathobiology. Our results indicate that IP- and subcutaneously-delivered eNAMPT-neutralizing ALT-100 mAb, known to be an effective biologic therapeutic in preclinical ARDS [31,57,58] pulmonary hypertension [25], and radiation lung injury and fibrosis [23,38], may represent a novel approach to improve SLE-associated lung vasculitis. By dampening pro-inflammatory cytokine production, reducing immune cell infiltration into the lung, and attenuating lung vascular damage and permeability, the ALT-100 mAb may be a candidate for future studies designed to further characterize the therapeutic efficacy of eNAMPT-targeted biologics in SLE subjects.
Author contributions
Conceptualization – JGNG, GT, CJ, Data Curation - GT, SS, NGC, Formal analysis – JGNG, CJ, Funding acquisition – JGNG, CJ, Investigation - GT, DJW, JHS, VRH, DEL, JD, ENM, JMY, Methodology - GT, CLK, SS, NGC, Project administration – JGNG, CJ, MI, DJW, Resources – JGNG, CJ, Software - GT, NGC, Supervision – JGNG, SS, CJ, Validation – SS, CLK, Visualization – SMC, NGC, JHS, Writing-original draft – JGNG, GT, SMC, Writing-reviewing & editing – JGNG, GT, CT, CJ, NGC, CLK, SMC.
Funding
This work was supported by the NIH/NHLBI grants P01HL126609, R01HL094394 and P01HL134610, in addition to the Center for Research in Women's Health Science, Cedars-Sinai.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Joe G.N. Garcia, MD reports financial support and equipment, drugs, or supplies were provided by Aqualung Therapeutics, LLC. Joe G.N. Garcia, MD reports a relationship with Aqualung Therapeutics, LLC that includes: equity or stocks. Joe G.N. Garcia, MD has patent pending to Aqualung Therapeutics, LLC.
Acknowledgements
None.
Handling editor: M.E. Gershwin
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2022.100181.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Drenkard C., Lim S.S. Update on lupus epidemiology: advancing health disparities research through the study of minority populations. Curr. Opin. Rheumatol. 2019;31:689–696. doi: 10.1097/BOR.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hannah J.R., D'Cruz D.P. Pulmonary complications of systemic lupus erythematosus. Semin. Respir. Crit. Care Med. 2019;40:227–234. doi: 10.1055/s-0039-1685537. [DOI] [PubMed] [Google Scholar]
- 3.Torre O., Harari S. Pleural and pulmonary involvement in systemic lupus erythematosus. Presse Med. 2011;40:e19–e29. doi: 10.1016/j.lpm.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Tselios K., Urowitz M.B. Cardiovascular and pulmonary manifestations of systemic lupus erythematosus. Curr. Rheumatol. Rev. 2017;13:206–218. doi: 10.2174/1573397113666170704102444. [DOI] [PubMed] [Google Scholar]
- 5.Zhuang H., Han S., Lee P.Y., Khaybullin R., Shumyak S., Lu L., et al. Pathogenesis of diffuse alveolar hemorrhage in murine lupus. Arthritis Rheumatol. 2017;69:1280–1293. doi: 10.1002/art.40077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agmon-Levin N., Selmi C. The autoimmune side of heart and lung diseases. Clin. Rev. Allergy Immunol. 2013;44:1–5. doi: 10.1007/s12016-012-8335-x. [DOI] [PubMed] [Google Scholar]
- 7.Badsha H., Teh C.L., Kong K.O., Lian T.Y., Chng H.H. Pulmonary hemorrhage in systemic lupus erythematosus. Semin. Arthritis Rheum. 2004;33:414–421. doi: 10.1016/j.semarthrit.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Kamen D.L., Strange C. Pulmonary manifestations of systemic lupus erythematosus. Clin. Chest Med. 2010;31:479–488. doi: 10.1016/j.ccm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Zamora M.R., Warner M.L., Tuder R., Schwarz M.I. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine (Baltim.) 1997;76:192–202. doi: 10.1097/00005792-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Park M.S. Diffuse alveolar hemorrhage. Tuberc. Respir. Dis. 2013;74:151–162. doi: 10.4046/trd.2013.74.4.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colombo G., Clemente N., Zito A., Bracci C., Colombo F.S., Sangaletti S., et al. Neutralization of extracellular NAMPT (nicotinamide phosphoribosyltransferase) ameliorates experimental murine colitis. J. Mol. Med. (Berl.) 2020;98:595–612. doi: 10.1007/s00109-020-01892-0. [DOI] [PubMed] [Google Scholar]
- 12.Camp S.M., Ceco E., Evenoski C.L., Danilov S.M., Zhou T., Chiang E.T., et al. Unique toll-like receptor 4 activation by NAMPT/PBEF induces NFkappaB signaling and inflammatory lung injury. Sci. Rep. 2015;5 doi: 10.1038/srep13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manago A., Audrito V., Mazzola F., Sorci L., Gaudino F., Gizzi K., et al. Extracellular nicotinate phosphoribosyltransferase binds Toll like receptor 4 and mediates inflammation. Nat. Commun. 2019;10:4116. doi: 10.1038/s41467-019-12055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moschen A.R., Kaser A., Enrich B., Mosheimer B., Theurl M., Niederegger H., et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J. Immunol. 2007;178:1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez Cruz N.E., Maldonado Bernal C., Cuevas Uriostegui M.L., Castanon J., Lopez Macias C., Isibasi A. Toll-like receptors: dysregulation in vivo in patients with acute respiratory distress syndrome. Rev. Alerg. Mex. 2004;51:210–217. [PubMed] [Google Scholar]
- 16.Lynn H., Sun X., Casanova N., Gonzales-Garay M., Bime C., Garcia J.G.N. Genomic and genetic approaches to deciphering acute respiratory distress syndrome risk and mortality. Antioxidants Redox Signal. 2019;31:1027–1052. doi: 10.1089/ars.2018.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X., Sun B.L., Babicheva A., Vandenpool R., Oita R.C., Casanova N., et al. Direct eNAMPT involvement in pulmonary hypertension and vascular remodeling: transcriptional regulation by SOX and HIF2α. Am. J. Respir. Cell Mol. Biol. 2020 doi: 10.1165/rcmb.2019-0164OC. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bime C., Camp S.M., Casanova N., Oita R.C., Ndukum J., Lynn H., et al. The acute respiratory distress syndrome biomarker pipeline: crippling gaps between discovery and clinical utility. Transl. Res. 2020;226:105–115. doi: 10.1016/j.trsl.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adyshev D.M., Elangovan V.R., Moldobaeva N., Mapes B., Sun X., Garcia J.G. Mechanical stress induces pre-B-cell colony-enhancing factor/NAMPT expression via epigenetic regulation by miR-374a and miR-568 in human lung endothelium. Am. J. Respir. Cell Mol. Biol. 2014;50:409–418. doi: 10.1165/rcmb.2013-0292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bime C., Casanova N., Oita R.C., Ndukum J., Lynn H., M S. Camp et al. Development of a biomarker mortality risk model in acute respiratory distress syndrome. Crit. Care. 2019;23:410. doi: 10.1186/s13054-019-2697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bojkova D., Klann K., Koch B., Widera M., Krause D., Ciesek S., et al. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature. 2020;583:469–472. doi: 10.1038/s41586-020-2332-7. [DOI] [PubMed] [Google Scholar]
- 22.Elangovan V.R., Camp S.M., Kelly G.T., Desai A.A., Adyshev D., Sun X., et al. Endotoxin- and mechanical stress-induced epigenetic changes in the regulation of the nicotinamide phosphoribosyltransferase promoter. Pulm. Circ. 2016;6:539–544. doi: 10.1086/688761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia A.N., Casanova N.G., Valera D.G., Sun X., Song J.H., Kempf C.L., et al. Involvement of eNAMPT/TLR4 signaling in murine radiation pneumonitis: protection by eNAMPT neutralization. Transl. Res. 2022;239:44–57. doi: 10.1016/j.trsl.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quijada H., Bermudez T., Kempf C.L., Valera D.G., Garcia A.N., Camp S.M., et al. Endothelial eNAMPT amplifies pre-clinical acute lung injury: efficacy of an eNAMPT-neutralising monoclonal antibody. Eur. Respir. J. 2021;57 doi: 10.1183/13993003.02536-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed M., Zaghloul N., Zimmerman P., Casanova N.G., Sun X., Song J.H., et al. Endothelial eNAMPT drives EndMT and preclinical PH: rescue by an eNAMPT-neutralizing mAb. Pulm. Circ. 2021;11 doi: 10.1177/20458940211059712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chowdhary V.R., Grande J.P., Luthra H.S., David C.S. Characterization of haemorrhagic pulmonary capillaritis: another manifestation of Pristane-induced lupus. Rheumatology. 2007;46:1405–1410. doi: 10.1093/rheumatology/kem117. [DOI] [PubMed] [Google Scholar]
- 27.Jarrot P.A., Tellier E., Plantureux L., Crescence L., Robert S., Chareyre C., et al. Neutrophil extracellular traps are associated with the pathogenesis of diffuse alveolar hemorrhage in murine lupus. J. Autoimmun. 2019;100:120–130. doi: 10.1016/j.jaut.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Garcia J.G., Schaphorst K.L., Verin A.D., Vepa S., Patterson C.E., Natarajan V. Diperoxovanadate alters endothelial cell focal contacts and barrier function: role of tyrosine phosphorylation. J. Appl. Physiol. 2000;89:2333–2343. doi: 10.1152/jappl.2000.89.6.2333. 1985. [DOI] [PubMed] [Google Scholar]
- 29.Ye S.Q., Zhang L.Q., Adyshev D., Usatyuk P.V., Garcia A.N., Lavoie T.L., et al. Pre-B-cell-colony-enhancing factor is critically involved in thrombin-induced lung endothelial cell barrier dysregulation. Microvasc. Res. 2005;70:142–151. doi: 10.1016/j.mvr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Hochberg M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 31.Bermudez T., Sammani S., Song J.H., Hernon V.R., Kempf C.L., Garcia A.N., et al. eNAMPT neutralization reduces preclinical ARDS severity via rectified NFkB and Akt/mTORC2 signaling. Sci. Rep. 2022;12:696. doi: 10.1038/s41598-021-04444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng X., Hassoun P.M., Sammani S., McVerry B.J., Burne M.J., Rabb H., et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am. J. Respir. Crit. Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 33.M. Geraci-Erck, Immunohistochemistry research applications for animal tissue, NSH 32nd Symposium;Wokshop, vol. 82, 1–67.
- 34.Hong S.B., Huang Y., Moreno-Vinasco L., Sammani S., Moitra J., Barnard J.W., et al. Essential role of pre-B-cell colony enhancing factor in ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 2008;178:605–617. doi: 10.1164/rccm.200712-1822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye S.Q., Simon B.A., Maloney J.P., Zambelli-Weiner A., Gao L., Grant A., et al. Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am. J. Respir. Crit. Care Med. 2005;171:361–370. doi: 10.1164/rccm.200404-563OC. [DOI] [PubMed] [Google Scholar]
- 36.Jensen E.C. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat. Rec. 2013;296:378–381. doi: 10.1002/ar.22641. [DOI] [PubMed] [Google Scholar]
- 37.Oita R.C., Camp S.M., Ma W., Ceco E., Harbeck M., Singleton P., et al. Novel mechanism for nicotinamide phosphoribosyltransferase inhibition of TNF-alpha-mediated apoptosis in human lung endothelial cells. Am. J. Respir. Cell Mol. Biol. 2018;59:36–44. doi: 10.1165/rcmb.2017-0155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.A.N. Garcia, N.G. Casanova, C.L. Kempf, T. Bermudez, D.G. Valera, J.H. Song, et al., eNAMPT is a novel DAMP that contributes to the severity of radiation-induced lung fibrosis, Am. J. Respir. Cell Mol. Biol. Vol 66, Iss 5, pp 497–509, May 2022 (2022). [DOI] [PMC free article] [PubMed]
- 39.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li B., Dewey C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cock P.J., Fields C.J., Goto N., Heuer M.L., Rice P.M. The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Res. 2010;38:1767–1771. doi: 10.1093/nar/gkp1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. JR Statist. Soc. B. 1995;57:289–300. Find this article online. [Google Scholar]
- 43.Kamburov A., Stelzl U., Lehrach H., Herwig R. The ConsensusPathDB interaction database: 2013 update. Nucleic Acids Res. 2013;41:D793–D800. doi: 10.1093/nar/gks1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szklarczyk D., Morris J.H., Cook H., Kuhn M., Wyder S., Simonovic M., et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bossaller L., Rathinam V.A., Bonegio R., Chiang P.I., Busto P., Wespiser A.R., et al. Overexpression of membrane-bound fas ligand (CD95L) exacerbates autoimmune disease and renal pathology in pristane-induced lupus. J. Immunol. 2013;191:2104–2114. doi: 10.4049/jimmunol.1300341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tumurkhuu G., Chen S., Montano E.N., Ercan Laguna D., De Los Santos G., Yu J.M., et al. Oxidative DNA damage accelerates skin inflammation in pristane-induced lupus model. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.554725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song J.H., Mascarenhas J.B., Sammani S., Kempf C.L., Cai H., M S. Camp et al. TLR4 activation induces inflammatory vascular permeability via Dock1 targeting and NOX4 upregulation. Biochim Biophys Acta Mol Basis Dis. 2022;1868(12) doi: 10.1016/j.bbadis.2022.166562. Epub 2022 Sep 27. PMID: 36179995. [DOI] [PubMed] [Google Scholar]
- 48.Leone P., Prete M., Malerba E., Bray A., Susca N., Ingravallo G., et al. Lupus vasculitis: an overview. Biomedicines. 2021;9 doi: 10.3390/biomedicines9111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang W., Gilkeson G. Sex Differences in monocytes and TLR4 associated immune responses; implications for systemic lupus erythematosus (SLE) J. Immunother. Appl. 2014;1:1. doi: 10.7243/2055-2394-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Y.W., Tang W., Zuo J.P. Toll-like receptors: potential targets for lupus treatment. Acta Pharmacol. Sin. 2015;36:1395–1407. doi: 10.1038/aps.2015.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alvarez K., Vasquez G. Damage-associated molecular patterns and their role as initiators of inflammatory and auto-immune signals in systemic lupus erythematosus. Int. Rev. Immunol. 2017;36:259–270. doi: 10.1080/08830185.2017.1365146. [DOI] [PubMed] [Google Scholar]
- 52.Olson L.B., Hunter N.I., Rempel R.E., Sullenger B.A. Targeting DAMPs with nucleic acid scavengers to treat lupus. Transl Res. 2022;245:30–40. doi: 10.1016/j.trsl.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grigoryev D.N., Ma S.F., Irizarry R.A., Ye S.Q., Quackenbush J., Garcia J.G. Orthologous gene-expression profiling in multi-species models: search for candidate genes. Genome Biol. 2004;5:R34. doi: 10.1186/gb-2004-5-5-r34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simon B.A., Easley R.B., Grigoryev D.N., Ma S.F., Ye S.Q., Lavoie T., et al. Microarray analysis of regional cellular responses to local mechanical stress in acute lung injury. Am. J. Physiol. 2006;291:L851–L861. doi: 10.1152/ajplung.00463.2005. [DOI] [PubMed] [Google Scholar]
- 55.Revollo J.R., Grimm A.A., Imai S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr. Opin. Gastroenterol. 2007;23:164–170. doi: 10.1097/MOG.0b013e32801b3c8f. [DOI] [PubMed] [Google Scholar]
- 56.Revollo J.R., Korner A., Mills K.F., Satoh A., Wang T., Garten A., et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metabol. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quijada H., Bermudez T., Kempf C.L., Valera D.G., Garcia A.N., Camp S.M., et al. Endothelial eNAMPT amplifies pre-clinical acute lung injury: efficacy of an eNAMPT-neutralising monoclonal antibody. Eur. Respir. J. 2021;57 doi: 10.1183/13993003.02536-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sammani S., Bermudez T., Kempf C.L., Song J.H., Fleming J.C., Reyes Hernon V., et al. eNAMPT neutralization preserves lung fluid balance and reduces acute renal injury in porcine sepsis/VILI-induced inflammatory lung injury. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.916159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung C.P., Long A.G., Solus J.F., Rho Y.H., Oeser A., Raggi P., et al. Adipocytokines in systemic lupus erythematosus: relationship to inflammation, insulin resistance and coronary atherosclerosis. Lupus. 2009;18:799–806. doi: 10.1177/0961203309103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vadacca M., Margiotta D., Rigon A., Cacciapaglia F., Coppolino G., Amoroso A., et al. Adipokines and systemic lupus erythematosus: relationship with metabolic syndrome and cardiovascular disease risk factors. J. Rheumatol. 2009;36:295–297. doi: 10.3899/jrheum.080503. [DOI] [PubMed] [Google Scholar]
- 61.Bajwa E.K., Boyce P.D., Januzzi J.L., Gong M.N., Thompson B.T., Christiani D.C. Biomarker evidence of myocardial cell injury is associated with mortality in acute respiratory distress syndrome. Crit. Care Med. 2007;35:2484–2490. doi: 10.1097/01.ccm.0000281852.36573.22. [DOI] [PubMed] [Google Scholar]
- 62.Bajwa E.K., Yu C.L., Gong M.N., Thompson B.T., Christiani D.C. Pre-B-cell colony-enhancing factor gene polymorphisms and risk of acute respiratory distress syndrome. Crit. Care Med. 2007;35:1290–1295. doi: 10.1097/01.CCM.0000260243.22758.4F. [DOI] [PubMed] [Google Scholar]
- 63.O'Mahony D.S., Glavan B.J., Holden T.D., Fong C., Black R.A., Rona G., et al. Inflammation and immune-related candidate gene associations with acute lung injury susceptibility and severity: a validation study. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun B.L., Sun X., Casanova N., Garcia A.N., Oita R., Algotar A.M., et al. Role of secreted extracellular nicotinamide phosphoribosyltransferase (eNAMPT) in prostate cancer progression: novel biomarker and therapeutic target. EBioMedicine. 2020;61 doi: 10.1016/j.ebiom.2020.103059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun B.L., Tang L., Sun X., Garcia A.N., Camp S.M., Posadas E., et al. A humanized monoclonal antibody targeting extracellular nicotinamide phosphoribosyltransferase prevents aggressive prostate cancer progression. Pharmaceuticals. 2021;14 doi: 10.3390/ph14121322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Audrito V., Serra S., Brusa D., Mazzola F., Arruga F., Vaisitti T., et al. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood. 2015;125:111–123. doi: 10.1182/blood-2014-07-589069. [DOI] [PubMed] [Google Scholar]
- 67.Jia S.H., Li Y., Parodo J., Kapus A., Fan L., Rotstein O.D., et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J. Clin. Invest. 2004;113:1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skokowa J., Lan D., Thakur B.K., Wang F., Gupta K., Cario G., et al. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway. Nat. Med. 2009;15:151–158. doi: 10.1038/nm.1913. [DOI] [PubMed] [Google Scholar]
- 69.Guo Q., Yaron J.R., Wallen J.W., 3rd, Browder K.F., Boyd R., Olson T.L., et al. PEGylated serp-1 markedly reduces pristane-induced experimental diffuse alveolar hemorrhage, altering uPAR distribution, and macrophage invasion. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.633212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lemos F.S., Pereira J.X., Carvalho V.F., Bernardes E.S., Chammas R., Pereira T.M., et al. Galectin-3 orchestrates the histology of mesentery and protects liver during lupus-like syndrome induced by pristane. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-50564-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.