Abstract
Quercetin, a well-known flavonoid, has been demonstrated to exert beneficial effects on intestinal functions and gut microbiota in birds. In this study, we investigated the effects of quercetin supplementation on inflammatory responses, intestinal barrier functions and gut microbial community in LPS-challenged laying hens. A total of two hundred eighty-eight 32-wk-old Jingfen No.6 laying hens were randomly assigned to 3 groups, the CON group, the LC group and the LQ group. LQ group was fed with 0.4 mg/kg quercetin and at the end of 12 wk, LC and LQ groups were challenged intraperitoneally with lipopolysaccharide (LPS). After LPS challenge, 8 birds of each group were randomly selected and sampled. LPS challenge induced an obvious intestinal mucosal injury, necrosis and shedding, while quercetin intervention maintained its structure. Quercetin significantly decreased the elevated malondialdehyde contents (P < 0.05), and increased the activity of total antioxidant capacity and glutathione peroxidase (P < 0.05) in intestinal mucosa of LPS-challenged laying hens. Quercetin rescued the LPS-induced decreases in goblet cell density and mucin2 expression levels (P < 0.05). There was a significant decline (P < 0.05) in the mRNA expression of Claudin1 and Occludin in intestinal mucosa of LPS-challenged layers, which could be alleviated (P < 0.05) by dietary quercetin. LPS challenge induced the increased expression levels (P < 0.05) of IL-1β and TLR-4 in intestinal mucosa, while these rises could be reversed (P < 0.05) following dietary quercetin supplementation. LPS challenge induced a shift in gut microenvironment, and quercetin addition could elevate the relative abundance of some short chain fatty acids (SCFA)-producing or health-promoting bacteria such as Phascolarctobacterium, Negativicutes, Selenomonadales, Megamonas, Prevotellaceae, and Bacteroides_salanitronis. In conclusion, dietary quercetin addition ameliorated the LPS challenge-induced intestinal inflammation and improved intestinal functions, possibly associated with its modulation on gut microbiota, particularly the increased population of SCFA-producing bacteria.
Key words: quercetin, inflammatory response, intestinal health, gut microbiota, laying hen
INTRODUCTION
Intestinal inflammation due to intensive metabolism and long-period production are recognized as severe problems responsible for compromised health and poor production performance in poultry (Lilburn and Loeffler, 2015; Feng et al., 2021; Gu et al., 2021). During the laying period, intestinal epithelium of hens is vulnerable to infection and inflammation (Nii et al., 2020), which would further trigger the disruption of barrier functions and immune homeostasis, and exacerbate oxidative stress and gut flora disturbance, thereby negatively affecting host metabolism and production performance (Okumura and Takeda, 2018; Zhang et al., 2022). Besides, age or infection-associated microbial dysbiosis was related to the promotion of mucosal injury and intestinal inflammation (Videnska et al., 2014). Therefore, a healthy intestine and an optimized intestinal microbiome through nutritional intervention is crucial for layer health and extending the laying cycle of commercial flocks.
Quercetin, one of the most well-known flavonoids, has attracted increasing attention as the potential alternative to antibiotics. The alleviation effects of quercetin on inflammatory response and oxidative stress have been extensively explored in vitro and in vivo studies (Saeed et al., 2017; Hai et al., 2020). Quercetin can decrease the production of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) by suppressing the activation of nuclear factor (NF-κB) and c-Jun N-terminal kinase (JNK), thus exerting anti-inflammatory activity in both high-fat diet and lipopolysaccharide-induced mouse models (Overman et al., 2011; Forney et al., 2018). The antioxidative property of quercetin was associated with the ability to scavenge free radicals, enhance the expression levels of endogenous antioxidant enzymes (such as GSH peroxidase, catalase, and SOD) and reduce MDA levels (Xu et al., 2019). Furthermore, the benefits of quercetin have been widely acknowledged in poultry. For example, quercetin supplementation could increase liver Cu-Zn-superoxide dismutase activity, decrease the population of total aerobes and coliforms and elevate Bifidobacteria population in the cecum of laying hens (Liu et al., 2014). It was reported that quercetin intervention could enhance the antioxidant and immunomodulation functions (Saeed et al., 2017; Zhang and Kim, 2020), possibly contributing to the improved growth performance in broilers. A recent study suggested that quercetin ameliorated oxidized oil-induced oxidative stress by promoting the redox balance, and strengthening intestinal morphological structure and barrier functions (Dong et al., 2020). In addition to antibacterial property, quercetin has been demonstrated to interact with gut microbial community (Saeed et al., 2017; Ozdal et al., 2016; Shi et al., 2020). It has been reported that quercetin treatment could inhibit bacterial adhesion to intestinal epithelial cells via depressing focal adhesions (Xue et al., 2019) and prevent E. coli O157: H7-induced inflammasome activation (Xue et al., 2017). Quercetin could modulate gut microbial diversity and richness, and promote the proliferation of beneficial bacteria (Bifidobacterium, Bacteroides, and Lactobacillus) and suppress abundance of pathogens (E. coli and proteobacteria), thus favoring the maintenance of intestinal flora balance (Shabbir et al., 2021). Therefore, quercetin may exhibit beneficial effects on gut environment and modulate intestinal microbiota profile. Dietary quercetin alleviated C. rodentium-induced colitis in mice, which may be partly responsible by the modification of gut microbiota (Lin et al., 2019). However, the ameliorative effects of quercetin on gut health and microbial composition in the intestinal inflammation model of laying hens await further exploration.
Here, we hypothesize that quercetin could alleviate inflammatory damage, maintain intestinal barrier function and modulate gut microbiota of laying hens. Lipopolysaccharide (LPS), a strong inflammatory stimulant, has been widely applied to construct an intestinal inflammation model in diverse species (Zhang et al., 2017; Wang et al., 2019a). Previous studies have demonstrated that LPS would induce intestinal inflammation and oxidative stress, impair intestinal barrier function and disrupt gut morphology (Orsolya et al., 2016; Zhu et al., 2017). In this study, an intestinal inflammation model established by LPS has been used to explain the protective effects of quercetin on inflammatory response, intestinal barrier function and gut microbial community in LPS-challenged laying hens.
MATERIALS AND METHODS
Experimental Design
The trial was conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals, and the regulations of the Animal Ethical and Welfare Committee of Northwest A&F University (Approval No. DK2022061). A total of two hundred eighty-eight 32-wk-old Jingfen No.6 laying hens (Yukou Poultry Co., Ltd., Beijing, China) were randomly assigned to 3 groups with 8 replicates of 12 birds each. Egg production prior to the experiment were similar across all the replicates. Birds were housed in 3-tier battery cages with 3 birds each cage (cage size: 45 cm × 45 cm × 45 cm) and had free access to the water and mash feed. All birds were kept under controlled conditions with temperature between 15 and 24°C and a 16-h light/dark cycle (05:00–21:00). Birds in the control (CON) and LC groups were fed with a basal diet formulated according to NRC (1994) recommendations and Chinese Feeding Standard of Chicken (Table 1), and LQ group was fed a diet supplemented with 0.4 mg/kg quercetin for 12 wk. Quercetin product was obtained from a commercial supply (purity ≥ 97%; Sigma-Aldrich company, St Louis). At the end of the experiment period, all birds except those in CON were challenged with LPS (1 mg/kg body weight; E. coli O55:B5; Sigma-Aldrich, St Louis) through intraperitoneal injection on three consecutive days.
Table 1.
Ingredient and nutrient levels of basal diets (air-dried basis).
| Ingredients, % | Nutrient levels, %b | ||
|---|---|---|---|
| Corn | 64.15 | Metabolizable energy, MJ/kg | 11.20 |
| Soybean meal | 22.70 | Crude protein | 15.80 |
| Soybean oil | 0.65 | Calcium | 3.78 |
| Limestone | 7.50 | Available phosphorus | 0.37 |
| NaCl | 0.15 | Lysine | 0.82 |
| Choline | 0.13 | Methionine | 0.40 |
| DL-Methionine | 0.12 | Methionine+cystine | 0.64 |
| Premixa | 4.60 | ||
| Total | 100.00 |
Premix provided the following per kg of the diet: vitamin A, 12,500 IU; vitamin D3, 4125 IU; vitamin E, 15 IU; vitamin K, 2 mg; thiamine, 1 mg; riboflavin, 8.5 mg; calcium pantothenate, 11 mg; niacin, 32.5 mg; pyridoxine, 8 mg; biotin, 0.5 mg; folic acid, 1.25 mg; vitamin B12, 0.02 mg; Mn, 65 mg; I, 1 mg; Fe, 60 mg; Cu, 8 mg; Zn, 66 mg; phytase, 500 mg.
The nutrient levels were calculated values.
Sample Collection
One day after the third LPS challenge, 8 birds (one from each replicate) of each group were randomly selected and the intestinal tissue was separated after dissection. The middle portion of jejunum and ileum tissues were fixed in 10% neutral-buffered formalin for histological analysis. Cecal digest were collected in a sterile cryopreservation tube and immediately frozen in liquid nitrogen before storage at −80°C for DNA extraction. The jejunum and ileum tissues were rinsed with cold sterile phosphate buffer saline (PBS) and intestinal mucosa was scraped with sterile slides, and stored in liquid nitrogen before storage at −80°C for antioxidant property and RNA analysis.
Gut Histomorphological Analysis
The fixed jejunal and ileal samples were dehydrated, embedded in paraffin, cut into 5 μm slices and finally stained with hematoxylin and eosin (H&E). Fifteen well-oriented villi and their corresponding crypts were selected to assess the villus height (distance from villus tip to the villus-crypt junction) and crypt depth (distance from villus-crypt junction to the crypt base). Goblet cells in the intestine were stained with periodic acid-Schiff (PAS) stain. The number of goblet cells in each villus were measured and the density of goblet cells was calculated as the number of goblet cells per micrometer of villus height. Histology score was calculated by detection of histopathological changes following previous method (Xie et al., 2022).
Intestinal Mucosal Antioxidant Property Measurement
Intestinal mucosa samples with precooled 0.9% saline were homogenized and centrifuged at 3,000 r/min for 10 min at 4°C. The supernatant was collected for further analysis. Protein concentration in the supernatant was measured by bicinchoninic acid (BCA) assay method with assay kit (Beyotime Biotechnology, Shanghai, China). The activity of catalase (CAT), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) and total antioxidant capacity (T-AOC), and malondialdehyde (MDA) concentration in the mucosal samples were determined with respective assay kits (Nanjing Jiancheng Bioengineering Institute, China).
RNA Extraction and Quantitative PCR
Total RNA was extracted from the jejunum and ileum mucosa with TRIzol reagent (Tiangen, Beijing, China) following the manufacturer's instructions. A spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific, CA) was used to determine the concentration and purity of RNA. After concentration normalization, RNA was reversely transcribed into cDNA using the first-strand synthesis kit (Accurate, Hunan, China). The mRNA expression of target genes was detected using SYBR-Green PCR kits (UE EVERBRIGHT, Suzhou, China) on Bio-rad CFX96 Optics Module (Singapore). Primer sequence in this study is shown in Table 2. The 20-μL PCR reaction system included 10 μL 2 × qPCR Master Mix, 0.4 μL forward primer, 0.4 μL reverse primer, 2 μL cDNA template and 7.2 μL double distilled water. The PCR process was as follows: 95°C for 3 min; 40 cycles at 95°C for 10 s, 60°C for 30 s, 72°C for 30 s. Each sample was detected in triplicate and the relative mRNA expression levels were calculated using β-actin as an internal control by the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Table 2.
Sequences of real-time PCR primers.
| Genes | Primer sequence (5’-3’) | Accession no. |
|---|---|---|
| β-actin | F: ATTGTCCACCGCAAATGCTTC | NM_205518 |
| R: AAATAAAGCCATGCCAATCTCGTC | ||
| IL-1β | F: GTGAGGCTCAACATTGCGCTGTA | NM_204524 |
| R: TGTCCAGGCGGTAGAAGATGAAG | ||
| IL-4 | F: AGACAAATAACAAAACTGAGC | NM_001007079 |
| R: TTGGTGGAAGAAGGTACG | ||
| IL-6 | F: CAAGGTGACGGAGGAGGAC | AJ309540 |
| R: TGGCGAGGAGGGATTTCT | ||
| IL-8 | F: ATGAACGGCAAGCTTGGAGCTG | XM_015301388 |
| R: TCCAAGCACACCTCTCTrCCATCC | ||
| IL-10 | F: GAAGCGCAGCATCTCTGACA | XM_025143715.1 |
| R: GCTGAGGGTGAAGTTTGAGGAA | ||
| MyD88 | F: TCTGGTGACTGTGGAGCAAGGAA | NM_001030962.5 |
| R: CCGCTTGTAGGAAGGCACTAATGG | ||
| TLR4 | F: AGTCTGAAATTGCTGAGCTCAAAT | NM_001030693 |
| R: GCGACGTTAAGCCATGGAAG | ||
| NF-κB | F: GTGTGAAGAAACGGGAACTG | NM_205129 |
| R: GGCACGGTTGTCATAGATGG | ||
| IFN-γ | F: AGCTGACGGTGGACCTATTATT | NM_001007079 |
| R: GGCTTTGCGCTGGATTC | ||
| TNF-α | F: GAGCGTTGACTTGGCTGTC | NM_204627 |
| R: AAGCAACAACCAGCTATGCAC | ||
| ZO-1 | F: TATAGAAGATCGTGCGCCTCC | XM_413773 |
| R: GAGGTCTGCCATCGTAGCTC | ||
| Occludin | F: ACGGCAGCACCTACCTCAA | D21837.1 |
| R: GGGCGAAGAAGCAGATGAG | ||
| Claudin-1 | F: CATACTCCTGGGTCTGGTTGGT | AY750897 |
| R: GACAGCCATCCGCATCTTCT | ||
| Claudin-2 | F: CAAGGACCGAGTGGCAGTG | NM_001277622 |
| R: TTTGATGGAGGGCTGAGGA | ||
| Mucin2 | F: TTCATGATGCCTGCTCTTGTG | XM_421035 |
| R: CCTGAGCCTTGGTACATTCTTGT |
IFN-γ, interferon-γ; IL, interleukin; MyD88, myeloid differentiation primary response 88; NF-κB, nuclear factor kappa B; TLR4, toll-like receptor 4; TNF-α, tumor necrosis factor-α; ZO-1, zonula occludens 1.
DNA Extraction and 16S rRNA Sequencing
The DNA was isolated from the cecal digesta samples using the ZR Feacal DNA Isolation Kit (Zymo Research, Irvine, CA) according to the manufacturer's protocol. The quantity and quality of the extracted DNA were assessed with a NanoDrop ND-1000 instrument (Thermo Scientific, Wilmington, DE), and the absence of degradation was confirmed by 1% agarose gel electrophoresis. The V3-V4 region of the 16S rRNA gene was amplified with 341F (CTAYGGGRBGCASCAG) and 806R (GGACTACNNGGGTATCTAAT) primers and then was sequenced by Novogene (Beijing, China) on Illumina NovaSeq 6000 using NEBNext Ultra Library Prep Kit (New England Biolabs, Ipswich, MA). Sequences with 97% similarity were assigned to the same operational taxonomic units (OTUs). The Venn diagram was used to assess the OTUs content of each group, and alpha diversity indices including PD_whole_tree, ACE, Chao and Shannon, and β-diversity were calculated to evaluate the similarity between groups. Linear discriminant analysis (LDA) combined effect size measurements (LEfSe) analysis were performed to identify the differences in microbial composition between groups. The potential relationship between intestinal function parameters and cecal microbial composition were assessed by Pearson correlation analysis. The sequencing data have been deposited at the National Center of Biotechnology Information (NCBI) Sequence Read Archive (SRA) database (accession number: PRJNA875071).
Statistical Analysis
Data were presented as mean ± SD or mean ± SEM. Data were analyzed using one-way analysis of variance (ANOVA) followed by Duncan's Multiple Range Test using SPSS 27 (SPSS Inc., Chicago, IL). P values < 0.05 were considered statistically significant.
RESULTS
Intestinal Morphology
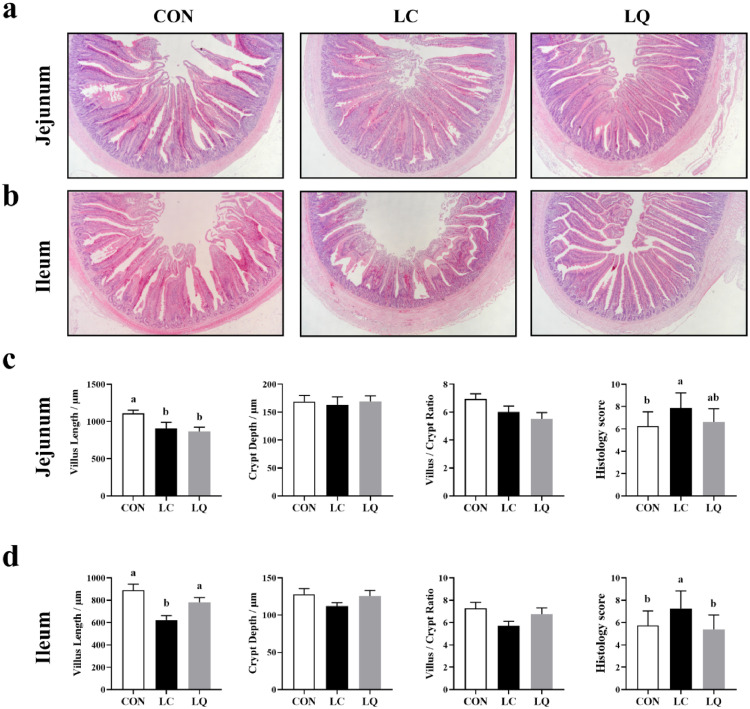
As shown in Figure 1, compared to the CON, LPS challenge induced an obvious intestinal mucosal injury, necrosis and shedding (Figure 1a & b), as evidenced by damaged villi structure in the jejunum and ileum. In contrast, quercetin supplementation attenuated the severity of intestinal injury and shedding. The villus length in the jejunum mucosa of LC and LQ groups was lower (Figure 1c; P < 0.05) than that in the control. Compared to LC group, villus length in the ileum mucosa of LQ group was significantly elevated (P < 0.05). In addition, there was a higher histology score (Figure 1d; P < 0.05) for the severity of jejunum and ileum damage in LC group than that in CON group, whereas quercetin could reverse these adverse effects only in ileum (P < 0.05).
Figure 1.
Effects of dietary quercetin supplementation on gut morphology in LPS-challenged laying hens. Representative photomicrographs of jejunum (a) and ileum (b) with HE staining. Villus length, crypt depth, villus length/crypt depth ratio and histology score of jejunum (c) and ileum (d). CON, laying hens fed with basal diet; LC, laying hens fed with basal diet and challenged with LPS; LQ, laying hens fed with quercetin-supplemented diet and challenged with LPS. Values are presented as mean ± SD (n = 8). a-b Means with different lowercase superscripts represent significant difference (P < 0.05).
Goblet Cell Density and mRNA Expression of Mucin in the Intestinal Mucosa
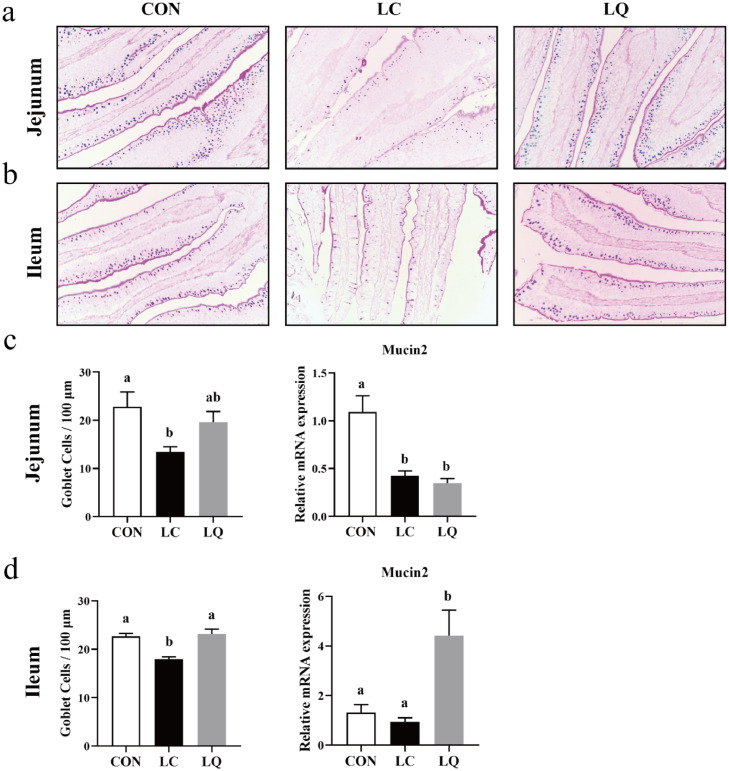
As shown in Figure 2, compared to the CON, LPS induced a significant decrease in the number of goblet cells in jejunum and ileum villus (Figure 2c & d; P < 0.05). Quercetin intervention reversed this decrease in ileum villus (P < 0.05), but not in jejunum (P > 0.05). Interestingly, significant declines (P < 0.05) in mucin2 mRNA expression levels were only observed in jejunum in response to LPS challenge. However, no significant differences (P > 0.05) in mucin2 expression levels in jejunum and a marked elevation (P < 0.05) in mucin2 expression levels in ileum were observed following quercetin intervention.
Figure 2.
Light micrographs represent goblet cells in jejunum (a) and ileum (b) of laying hens with alcian blue and periodic acid Schiff (AB-PAS) staining. Graphs show the number of goblet cells and Mucin2 mRNA expression in jejunum (c) and ileum (d). CON, laying hens fed with basal diet; LC, laying hens fed with basal diet and challenged with LPS; LQ, laying hens fed with quercetin-supplemented diet and challenged with LPS. Values are presented as mean ± SD (n = 8). a-bMeans with different lowercase superscripts represent significant difference (P < 0.05).
Antioxidant Properties of Intestinal Mucosa
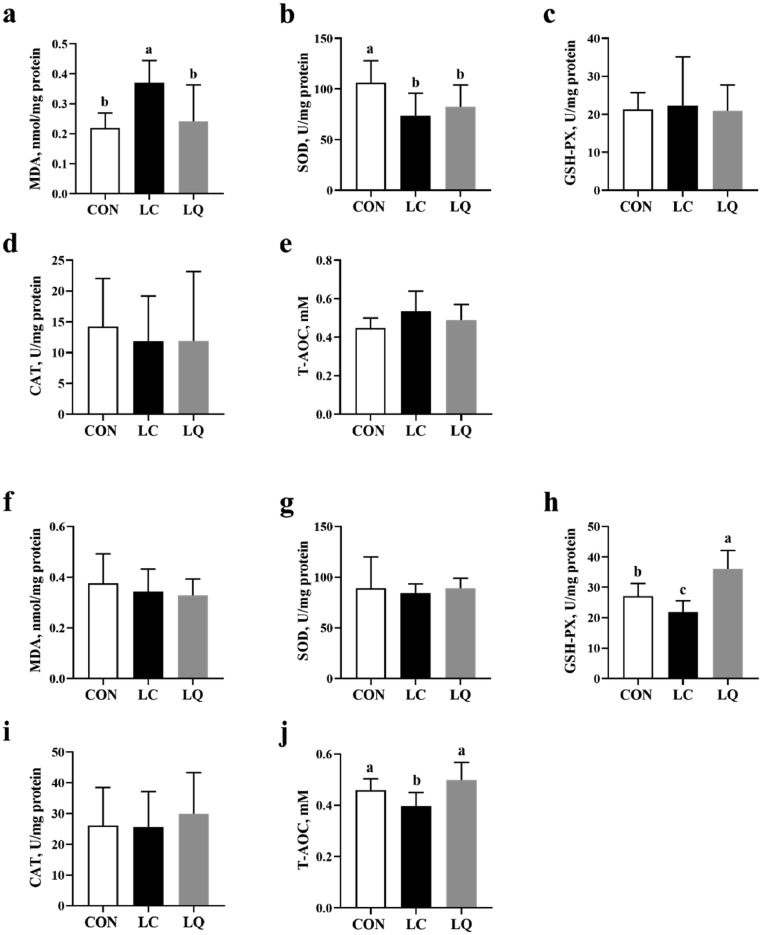
LPS challenge-increased content of MDA in jejunum mucosa compared to the CON was attenuated by quercetin intervention (Figure 3a; P < 0.05). However, LPS-induced decreases in SOD activity of jejunum mucosa compared to the CON were not significantly affected by quercetin (Figure 3b; P > 0.05). The activities of GSH-PX and T-AOC in ileum mucosa were significantly depressed by LPS challenge, while attenuated to normal for T-AOC or even higher levels for GSH-PX by dietary quercetin (Figure 3h and j; P < 0.05).
Figure 3.
Effects of dietary quercetin supplementation on antioxidant properties in the jejunal mucosa (a-e) and ileal mucosa (f-j) of LPS-challenged laying hens. CON, laying hens fed with basal diet; LC, laying hens fed with basal diet and challenged with LPS; LQ, laying hens fed with quercetin-supplemented diet and challenged with LPS. Values are presented as mean ± SD (n = 8). a-cMeans with different lowercase superscripts represent significant difference (P < 0.05).
The mRNA Expression of Tight Junction Proteins in the Intestinal Mucosa
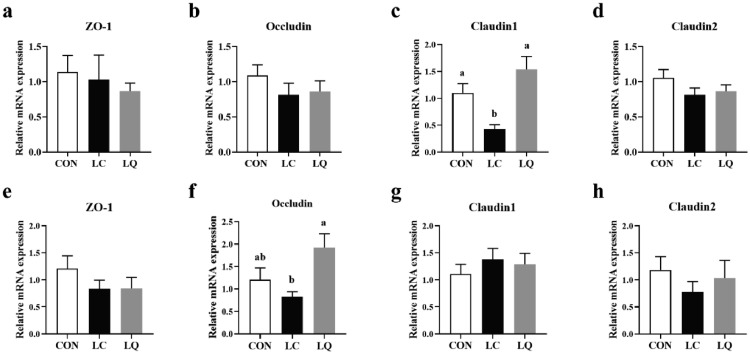
As shown in Figure 4c and f, quercetin intervention significantly alleviated LPS challenge-induced decreases in mRNA expression of Claudin1 in jejunum and Occludin in ileum (P < 0.05). In addition, LPS challenge markedly depressed the expression of Claudin1 in jejunum (P < 0.05), but failed to induce any obvious effects (P > 0.05) on other measurements in both jejunum and ileum.
Figure 4.
Effects of dietary quercetin supplementation on the relative mRNA expression of tight junction proteins in jejunum (a-d) and ileum mucosa (e-h) of LPS-challenged laying hens. CON, laying hens fed with basal diet; LC, laying hens fed with basal diet and challenged with LPS; LQ, laying hens fed with quercetin-supplemented diet and challenged with LPS. Values are presented as mean ± SEM (n = 8). a-bMeans with different lowercase superscripts represent significant difference (P < 0.05).
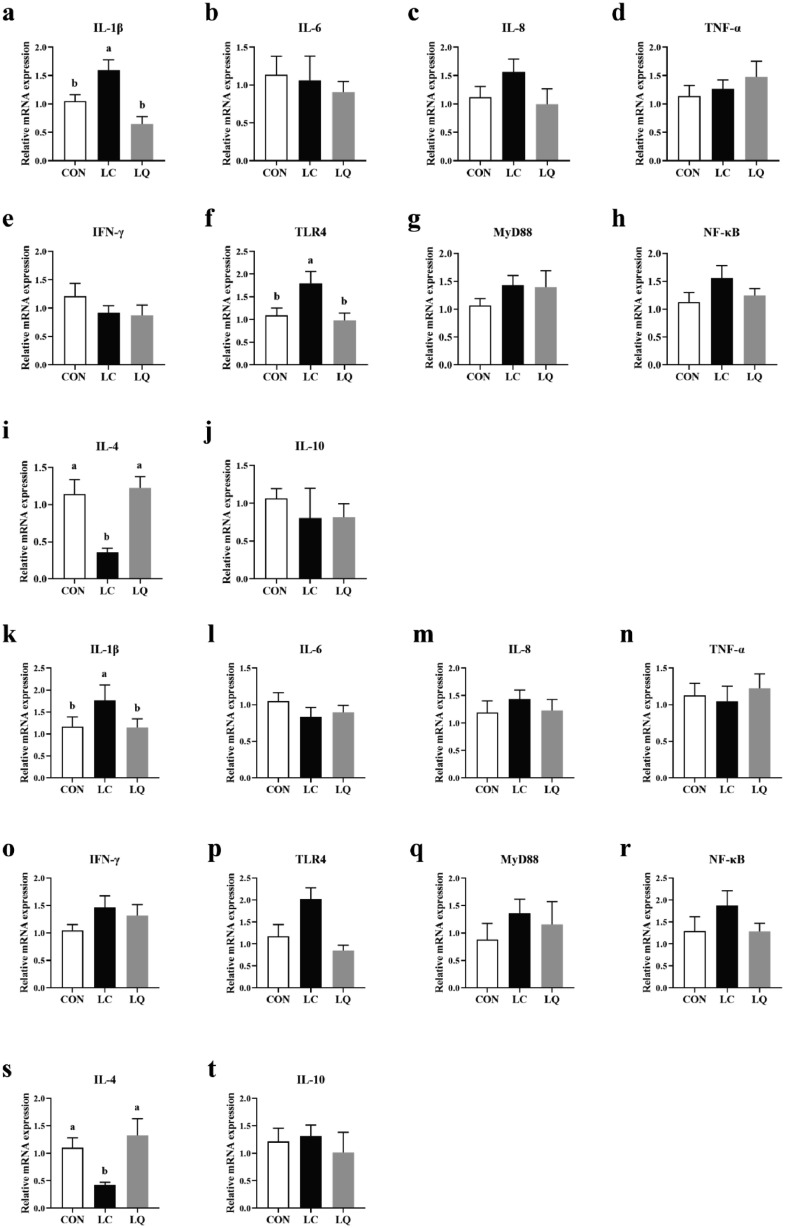
The mRNA Expression of Immune Response-related Genes in the Intestinal Mucosa
As shown in Figure 5, the expression levels of IL-1β in jejunum and ileum mucosa were significantly elevated (P < 0.05) in response to LPS challenge, while these increases could be reversed (P < 0.05) following quercetin supplementation. Similarly, LPS challenge-induced increases in TLR4 mRNA expression in jejunum mucosa compared with the CON were relieved by quercetin intervention (P < 0.05). In addition, LPS treatment resulted in decreased mRNA expression of IL-4 (P < 0.05) in both jejunum and ileum mucosa, but attenuated to normal (P < 0.05) by quercetin supplementation.
Figure 5.
Effects of dietary quercetin supplementation on the relative mRNA expression of immune response-related genes in jejunum (a-j) and ileum mucosa (k-t) of LPS-challenged laying hens. CON, laying hens fed with basal diet; LC, laying hens fed with basal diet and challenged with LPS; LQ, laying hens fed with quercetin-supplemented diet and challenged with LPS. Values are presented as mean ± SEM (n = 8). a-b means with different lowercase superscripts represent significant difference (P < 0.05).
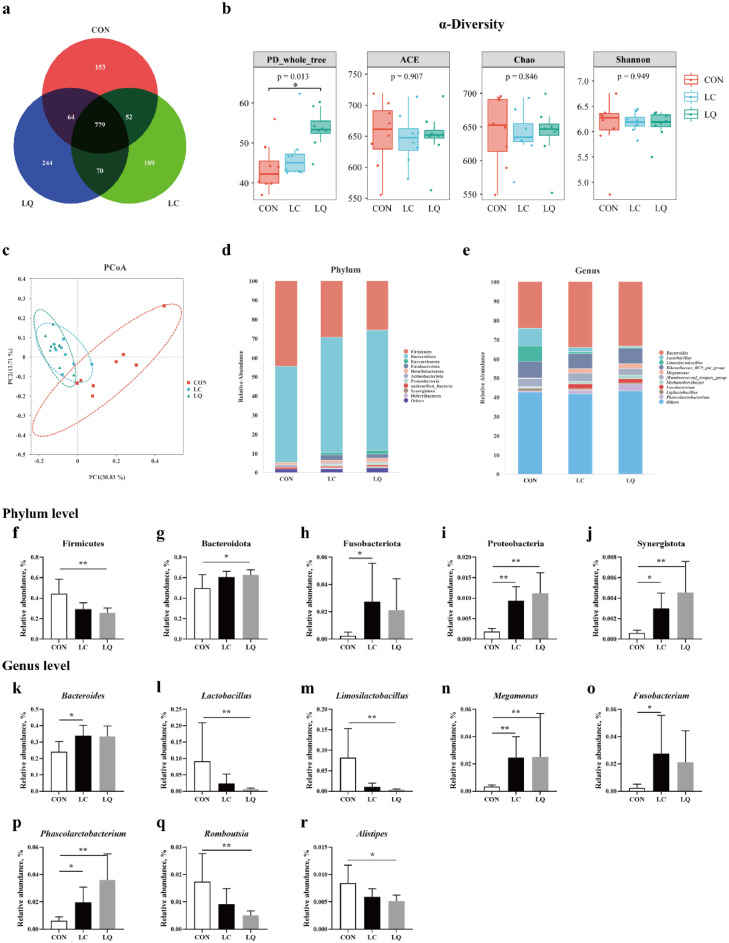
Cecal Microbial Profile
Across all of the samples, 1,295,210 high-quality sequence reads were obtained from the cecal contents, with an average of 53,967 sequences per sample. There were 1,048, 1,090, and 1,137 core OTUs in the CON, LC, and LQ groups, respectively, with 779 core OTUs common in the three groups (Figure 6a). No significant differences (P > 0.05; Figure 6b) in community richness (ACE and Chao) were observed in cecal microbiota, while the community diversity (PD_whole_tree) between cecal microbimes in LQ group was larger (P < 0.05) compared to the CON. Principal co-ordinates analysis (PCoA) showed a separation in the gut microbiota structure among the three groups (Figure 6c). At the phylum level, Firmicutes and Bacteroidota are the mainly dominant phyla, accounting for more than 80% of the whole phyla (Figure 6d). Statistical analysis showed that the relative abundance of Firmicutes and Bacteroidota in the LQ group (Figure 6f and g), and the relative abundance of Fusobacteriota in the LC group (Figure 6h) increased significantly (P < 0.05) compared to the CON group. The abundance of Proteobacteria and Synergistota in the LC and LQ groups were elevated (P < 0.05; Figure 6i and j) compared to the CON group. At the genus level, the cecal microbiota was dominated by Bacteroides, Lactobacillus, Limosilactobacillus, and Rikenellaceae_RC_gut_group (Figure 6e). Statistical analysis indicated that LPS challenge significantly raised (P < 0.05) the relative abundance of Bacteroides and Fusobacterium (Figure 6k and o). The abundance of Lactobacillus, Limosilactobacillus, Romboutsia, and Alistipes in the LQ group decreased significantly (P < 0.05; Figure 6l, m, q, and r) compared to the CON group. The relative abundance of Megamonas and Phascolarctobacterium in the LC and LQ groups were significantly increased (P < 0.05; Figure 6n and p) compared to the CON group.
Figure 6.
Dietary quercetin intervention modulated cecal microbial composition in the LPS-challenged laying hens. (a) Venn diagram of core operational taxonomic units in the cecal digesta. (b) The α diversity parameters (PD_whole_tree, ACE, Chao, and Shannon) of cecal microbiota. (c) Principal coordinate analysis (PCoA) on cecal microbiota. Relative abundance of cecal microbial composition at the phylum (d) and genus (e) level. Relative abundance of cecal microbial community members at the phylum (f-j) and genus (k-r) level. CON, laying hens fed with basal diet; LC, laying hens fed with basal diet and challenged with LPS; LQ, laying hens fed with quercetin-supplemented diet and challenged with LPS. Values are presented as mean ± SD (n = 8). * means P < 0.05.
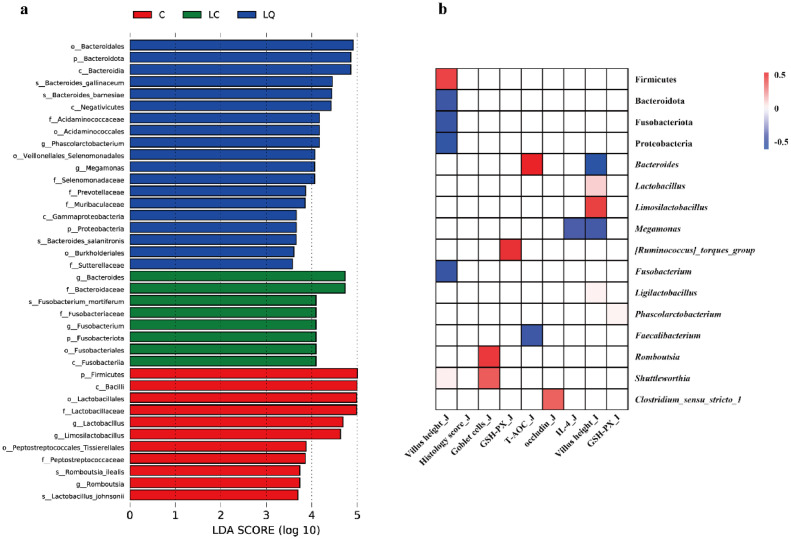
Differentially abundant cecal bacterial taxa in LPS-challenged laying hens in response to quercetin intervention were identified by LEfSe analysis (P < 0.05, LDA > 3.5; Figure 7a). Bacteroidota, Phascolarctobacterium, Megamonas and Proteobacteria showed marked enrichment in the LQ group by LEfSe, while Bacteroides and Fusobacteriota (Fusobacterium) were enriched in the gut microbiota of the LC group. In addition, Firmicutes, Lactobacillus, Limosilactobacillus, and Romboutsia were more abundant in the CON group. Pearson correlation analysis further confirmed that the modifications of gut microbiota were associated with LPS treatment and dietary quercetin intervention (Figure 7b). For example, villus height in the jejunum mucosa was negatively correlated with Bacteroidota, Fusobacteriota, Proteobacteria, and Fusobacterium, while was positively correlated with Firmicutes. Goblet cells in the jejunum mucosa was positively associated with Romboutsia and Shuttleworthia. GSH-PX contents and occludin in the jejunum mucosa was positively associated with [Ruminococcus]_torques_group and Clostridium_sensu_stricto_1, respectively, and Megamonas showed a negative correlation with IL-4. T-AOC was positively correlated with Bacteroides, whereas negatively correlated with Faecalibacterium. Villus height in the ileum mucosa was negatively associated with Bacteroides and Megamonas, while positively associated with Lactobacillus, Limosilactobacillus and Ligilactobacillus. GSH-PX contents in the ileum mucosa exhibited a positive relationship with Phascolarctobacterium.
Figure 7.
Dietary quercetin intervention regulated cecal microbial composition in the LPS-challenged laying hens. (a) Linear discriminant analysis (LDA) combined effect size measurements (LEfSe) analysis of cecal microbiota. (b) Pearson correlation between cecal microbiota and intestinal parameters. The intensity of the colors represents the degree of association, with red representing a significantly positive correlation and blue a significantly negative correlation (P < 0.05). The white indicates no significant correlation (P > 0.05). CON, laying hens fed with basal diet; LC, laying hens fed with basal diet and challenged with LPS; LQ, laying hens fed with quercetin-supplemented diet and challenged with LPS.
DISCUSSION
Recent studies have reported the beneficial effects of dietary quercetin on intestinal functions and health, subsequently favoring production performance in broilers and laying hens under normal conditions (Liu et al., 2014; Saeed et al., 2017). However, there are no reports concerning the regulatory mechanism of quercetin on intestinal microbiota and intestinal functions in laying hens under intestinal inflammatory conditions. Thus, we constructed an intestinal inflammation model by intraperitoneal LPS, with a focus on the regulatory effects of quercetin on intestinal barrier functions, inflammatory response and gut microbial community. This study may provide novel insights into the quercetin-mediated alleviation of intestinal inflammation and facilitate the application of quercetin in feed additives in poultry industry.
In this study, LPS challenge caused damages on the intestinal morphology of laying hens, while quercetin alleviated the injury to ameliorate intestinal morphology and integrity. In addition, LPS successfully stimulated the expression of IL-1β, while quercetin intervention could reverse these adverse effects, as supported by suppression of IL-1β expression and the attenuated intestinal damage. Quercetin was reported to reduce the cytotoxicity and exert anti-inflammatory effects via inhibiting NF-κB pathway and the release of pro-inflammatory cytokines (Saeed et al., 2017). In this study, the declined expression of pro-inflammatory cytokines was consistent with the decreased expression of TLR-4 following quercetin intervention. However, in this study, quercetin had no remarkable effects on the downstream signaling molecules MyD88 and NF-κB. The anti-inflammatory property of quercetin was associated with the inhibition of I-κB phosphorylation and NF-κB translocation, and future studies should be carried out to determine the underlying mechanism at the protein level. Thus, quercetin alleviated the LPS-induced intestinal inflammation, possibly conducing to remission of intestinal injury and the maintenance of mucosal integrity.
LPS can elevate the production of ROS intermediates (like lipid peroxides and nitric oxides) to accelerate ROS and MDA accumulation in tissues (Tian et al., 2022), which was regarded as the key factor causing intestinal oxidative stress (Hou et al., 2014). Consistently, we found that LPS increased jejunum MDA contents and decreased the activity of SOD in jejunum, and decreased the activity of GSH-PX and T-AOC in ileum. The T-AOC reflects the capacity of non-enzymatic antioxidant defense system and antioxidant enzyme, and the decreased levels of T-AOC implied an imbalance between antioxidant and oxidative systems (Gao et al., 2021). Thus, the increased levels of MDA and the inhibition of antioxidant enzyme activity indicated intestinal oxidative stress following LPS treatment. In contrast, quercetin intervention reduced MDA levels in jejunum and increased the activity of GSH-PX and T-AOC in ileum of LPS-challenged laying hens. Similar to our results, quercetin was proved to inhibit the production of ROS, enhance antioxidant capacity and relieve the oxidative injury in endothelial or intestinal epithelial cells (Tian et al., 2019; Jia et al., 2021). Furthermore, quercetin can modulate intracellular redox status by scavenging ROS, increasing intracellular GSH levels and enhancing expression of anti-apoptotic proteins in a Nrf2-dependent regulation manner (Xu et al., 2019). Therefore, the current results indicated that quercetin intervention alleviated intestinal damage and oxidative stress in LPS-challenged laying hens.
Intestinal inflammation and oxidative stress are recognized as the main driving force leading to mucosal barrier dysfunction (Wang et al., 2020). The integrity of tight junctions determines the intestinal selective permeability, which is critical for intestinal barrier function against bacterial invasion and toxic molecules into gastrointestinal tract. Consistent with the destroyed intestinal integrity, LPS stimulation reduced the expression of claudin1 in ileum mucosa. The protective effects of quercetin on the intestinal barrier have been discovered via directly upregulating the expression of tight junction proteins or via assembly of claudin, ZO2 and occluding (Hai et al., 2020). However, in this study, it was surprising that quercetin intervention failed to relieve these adverse effects. In broiler chickens fed with oxidized oil, quercetin alleviated the oxidative stress-induced decreased expression of ZO1 in ileal mucosa in a dose-dependent manner (Dong et al., 2020). This ineffectiveness may be related to the inclusion levels of quercetin and the method of establishing intestinal inflammation in this study, which probably led to severe damage exceeding the prophylactic effects of quercetin. Intestinal mucins secreted by goblet cells have been demonstrated to maintain the integrity of intestinal epithelial cells and regulate intestinal inflammation. The present data showed that LPS reduced the density of goblet cells accompanied with the declined mucin2 expression, while quercetin intervention could rescue this LPS-induced decrease. It has been found that quercetin could stimulate Mucin2 secretion through PLC/PKCα/ERK1-2 pathway in intestinal goblet cell-like cells and Caco-2 cells (Damiano et al., 2018). Similar findings were reported in broilers fed with oxidized oil, in which quercetin alleviated oxidative stress-induced decreased mRNA expression of mucin2 in the ileal mucosa (Dong et al., 2020). Besides, oxidative stress and apoptosis in goblet cells could disrupt mucin2 expression and ultimately destroy intestinal mucus barrier (Zhao et al., 2019), as indicated by the similar tendency in the goblet cell number and mucin2 expression levels in this study. These findings indicated that quercetin ameliorated LPS-induced impairment of intestinal barrier functions, possibly associated with the alleviation of inflammation and oxidative stress.
Intestinal microbiota plays a critical role in nutrient digestion, maintaining the intestinal epithelial barrier and immunity (Yadav and Jha, 2019). Quercetin has been demonstrated to regulate intestinal microbial composition (Shi et al., 2020; Shabbir et al., 2021) and thus the relief of adverse effects in LPS-challenged laying hens were speculated to be associated with the modulation of gut microbiota by quercetin. Loss of gut diversity has been linked to several pathological states such as inflammatory bowel disease and infection (Iszatt et al., 2019), whereas the enhancement of microbial diversity may promote a robust and stable ecosystem for gut functions (Lozupone et al., 2012). In this study, PD Whole tree was increased in the LQ group, indicating that quercetin intervention enhanced the intestinal microbiota diversity, which might be associated with the restoration of gut health and immunity disturbed by intestinal inflammation. The control birds were enrichment of some commensal bacteria like Lactobacillus, Romboutsia and Lachnospiraceae_NK4A136, potentially beneficial for intestinal health, as supported by the positive relationship between Lactobacillus and villus height, and Romboutsia and the density of goblet cells in this study. In contrast, LPS challenge enriched some potentially harmful microbes such as Bacteroides (Bacteroidaceae) and Fusobacterium (Fusobacteriota), whose overabundance is recognized as a general marker of gut dysbiosis, fostering the development of IBD in humans or severe diarrhea in animals (Gevers et al., 2014; Shrestha et al., 2020; Zhao et al., 2022). Similarly, this study indicated a negative correlation between villus height and the abundance of Bacteroidota (Bacteroides) and Fusobacterium. Bacteroidaceae (Bacteroides) and Fusobacterium_mortiferum (Fusobacterium) were identified to be related to many kinds of disease, such as multibacterial sepsis and chronic inflammatory bowel disease (Kollarcikova et al., 2019; Humbel et al., 2020). Besides, a moderate elevation in the abundance of Synergistota, Megamonas, and Phascolarctobacterium was observed following LPS challenge, and these bacteria were believed to play an important role in the pathogenesis of several diseases including inflammatory bowel disease (Bai et al., 2022; Huang et al., 2022). Quercetin induced a further increased abundance of these bacteria, which were suspected to be associated with the relief of intestinal inflammation presumably due to their ability to generate SCFA. Thus, it was reasonable to speculate that the abundance of these bacteria may be the critical factor determining their effects and governing the fate of intestine inflammation, which still needs further investigation. LPS challenge induced a shift in gut microenvironment with increased susceptibility to infection, and quercetin could elevate the relative abundance of some SCFA-producing or health-promoting bacteria such as Phascolarctobacterium, Negativicutes, Selenomonadales, Bacteroides_salanitronis, Prevotellaceae, and Megamonas. SCFAs are the critical metabolites of gut microbiota, which serve as the major energy source for colonocytes, and participate in the regulation of intestinal homeostasis and various physiological functions (Ríos-Covián et al., 2016). Acidaminococcus, Megamonas, B. salanitronis, and Selenomonadales have been demonstrated to utilize amino acids or carbohydrates to produce acetic and butyric acids, which may exert anti-inflammatory activity and gut health-promoting effects, thus negatively associated with several intestinal diseases (Vital et al., 2013; Vargas et al., 2017; Han et al., 2021). Besides, Phascolarctobacterium and Prevotellaceae are acetate and propionate-producing bacteria, which may be associated with reduced inflammation and protection of intestinal mucosa barrier via decreasing the concentrations of LPS-binding protein and C-reactive protein (Zhang et al., 2018; Pei et al., 2021). Similar findings were also discovered in quercetin and vitamin E-fed birds with an intestinal enrichment of Prevotellaceae (Amevor et al., 2022). In contrast, an increase in Prevotellaceae was regarded as the most remarkable characteristics of the microbial dysbiosis and was associated with the generation of pro-inflammatory cytokines in inflammatory bowel disease patients (Scher et al., 2013; Wang et al., 2018). Further research is needed to clarify the contradictory roles of Prevotellaceae in inflammatory responses in healthy and diseased individuals.
Furthermore, quercetin could elevate cecal abundance of the beneficial microbes like Gammaproteobacteria, Selenomonadales and Negativicutes, which may conduce to the balance of gut microbiota and intestinal functions (Shin et al., 2015; Warner et al., 2016; Yu et al., 2020; Patterson et al., 2022). B. salanitronis, B. gallinaceum, and B. barnesiae, previously isolated from healthy chickens, were enriched in quercetin-fed birds, which were assumed to play a critical role in the digestive system (Lan et al., 2006; Irisawa et al., 2016). The exact functions of Muribaculaceae and Burkholderiales in modulating inflammatory responses are still not clear. During inflammatory and healing phases in mice DSS Colitis, the abundance of Muribaculaceae was decreased (Osaka et al., 2017) and its family members can interact with innate and adaptive immune responses via Immunoglobulin A (IgA) coating (Bunker et al., 2015). As for Burkholderia, its increased abundance was accompanied by the enhanced gut structure and production performance, and the relief of S. pullorum-induced intestinal inflammation in poultry (Li et al., 2019; Wang et al., 2019b). It implied a positive effect of Burkholderia on intestinal health and functions in birds. Therefore, quercetin intervention could modulate the bacterial community, especially enrichment of SCFA-producing bacteria, which may conduce to the attenuation of intestinal inflammation in LPS-challenged laying hens.
In summary, this study demonstrated that quercetin intervention could alleviate LPS-induced inflammatory responses and assist to maintain intestinal functions, which may be partly responsible by the modulation of gut microbial composition, particular the enrichment of SCFA-producing bacteria. These findings provide novel insights into the quercetin-mediated attenuation of intestinal inflammatory disorders in poultry.
ACKNOWLEDGMENTS
This study was supported by China Agriculture Research System of MOF and MARA (CARS-40), the Scientific Startup Foundation for Doctors of Northwest A and F University (No. 2452022022), and Agricultural Science and Technology Innovation Project of Shaanxi.
DISCLOSURES
The authors have declared that they have no conflicts of interest.
REFERENCES
- Amevor F.L.K., Cui Z.F., Du X.X., Feng J., Shu G., Ning Z.F., Xu D., Deng X., Song W.Z., Wu Y.H., Cao X.Q., Wei S., He J., Kong F.L., Du X.H., Tian Y.F., Benjamin K., Li D.Y., Wang Y., Zhang Y., Zhu Q., Zhao X.L. Synergy of dietary quercetin and vitamin E improves cecal microbiota and its metabolite profile in aged breeder hens. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.851459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J., Wan Z., Zhang Y., Wang T., Xue Y., Peng Q. Composition and diversity of gut microbiota in diabetic retinopathy. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.926926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker J.J., Flynn T.M., Koval J.C., Shaw D.G., Meisel M., Mcdonald B.D., Jabri B., Antonopoulos D.A., Bendelac A. Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity. 2015;43:541–553. doi: 10.1016/j.immuni.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano S., Sasso A., De F.B., Gregorio I., Rosa G., Lupoli G., Belfiore A., Mondola P., Santillo M. Quercetin increases MUC2 and MUC5AC gene expression and secretion in intestinal goblet cell-like LS174T via PLC/PKCα/ERK1-2 pathway. Front. Physiol. 2018;9:357. doi: 10.3389/fphys.2018.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Lei J., Zhang B. Effects of dietary quercetin on the antioxidative status and cecal microbiota in broiler chickens fed with oxidized oil. Poult. Sci. 2020;99:4892–4903. doi: 10.1016/j.psj.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Lu M., Wang J., Zhang H., Qiu K., Qi G.H., Wu S.G. Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J. Anim. Sci. Biotechnol. 2021;12:72. doi: 10.1186/s40104-021-00600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney L.A., Lenard N.R., Stewart L.K., Tara M. Henagan dietary quercetin attenuates adipose tissue expansion and inflammation and alters adipocyte morphology in a tissue-specific manner. Int. J. Mol. Sci. 2018;19:895. doi: 10.3390/ijms19030895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R., Tian S., Wang J., Zhu W. Galacto-oligosaccharides improve barrier function and relieve colonic inflammation via modulating mucosa-associated microbiota composition in lipopolysaccharides-challenged piglets. J. Anim. Sci. Biotechnol. 2021;12:92. doi: 10.1186/s40104-021-00612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers D., Kugathasan S., Denson L.A., B.Yoshiki V.Z., Will V.T., Boyu R., Emma Y.F.S., Dan K., Se J.S., Moran Y., Xochitl C.M., Aleksandar D.K., Chengwei L., Antonio G.L., Daniel M., Yael H., Thomas W., Susan B., Joel R., S.Michael H.Melvin, James M., Robert B., Anne G., Francisco S., David M., Sandra K., Wallace C., Jeffrey H., Curtis H., Rob K., Xavier R.J. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y.F., Chen Y.P., Jin R., Wang C., Wen C., Zhou Y. A comparison of intestinal integrity, digestive function, and egg quality in laying hens with different ages. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai Y., Zhang Y., Liang Y., Ma X., Qi X., Xiao J., Xue W., Luo Y., Yue T. Advance on the absorption, metabolism, and efficacy exertion of quercetin and its important derivatives: absorption, metabolism and function of quercetin. Food Front. 2020;1:420–434. [Google Scholar]
- Han Y., Gong Z.W., Sun G.Z., Xu X., Qi C.L., Sun W.J., Jiang H.J., Cao P.G., Ju H. Dysbiosis of gut microbiota in patients with acute myocardial infarction. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Zhang J., Ahmad H., Zhang H., Xu Z., Wang T. Evaluation of antioxidant activities of ampelopsin and its protective effect in lipopolysaccharide-induced oxidative stress piglets. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Huang H., Peng W., Liu Y., Zhou Y., Xu H., Zhang L., Zhao C., Nie Y. Altered pattern of immunoglobulin A-targeted microbiota in inflammatory bowel disease after fecal transplantation. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.873018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbel F., Rieder J.H., Franc Y., Juillerat P., Scharl M., Misselwitz B., Schreiner P., Begré S., Rogler G., Käne R., Yilmaz B., Biedermann L. Association of alterations in intestinal microbiota with impaired psychological function in patients with inflammatory bowel diseases in remission. Clin. Gastroenterol. Hepatol. 2020;18:2019–2029. doi: 10.1016/j.cgh.2019.09.022. [DOI] [PubMed] [Google Scholar]
- Irisawa T., Saputra S., Kitahara M., Sakamoto M., Sulistiani N., Yulineri T., Achmad D., Moriya O. Bacteroides caecicola sp. nov. and Bacteroides gallinaceum sp. nov., isolated from the caecum of an indonesian chicken. Int. J. Syst. Evol. Microbiol. 2016;66:1431–1437. doi: 10.1099/ijsem.0.000899. [DOI] [PubMed] [Google Scholar]
- Iszatt N., Janssen S., Lenters V., Dahl C., Stigum H., Knight R., Mandal S., Peddada S., Gonzalez A., Midtvedt T., Eggesbo M. Environmental toxicants in breast milk of Norwegian mothers and gut bacteria composition and metabolites in their infants at 1 month. Microbiome. 2019;7:34. doi: 10.1186/s40168-019-0645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H., Zhang Y., Si X., Jin Y., Jiang D., Dai Z., Wu Z. Quercetin alleviates oxidative damage by activating nuclear factor erythroid 2-related factor 2 signaling in porcine enterocytes. Nutrients. 2021;13:375. doi: 10.3390/nu13020375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarcikova M., Kubasova T., Karasova D., Crhanova M., Cejkova D., Sisak F., Rychlik I. Use of 16S rRNA gene sequencing for prediction of new opportunistic pathogens in chicken ileal and cecal microbiota. Poult. Sci. 2019;98:2347–2353. doi: 10.3382/ps/pey594. [DOI] [PubMed] [Google Scholar]
- Lan P.T.N., Sakamoto M., Sakata S., Benno Y. Bacteroides barnesiae sp. nov., Bacteroides salanitronis sp. nov. and Bacteroides gallinarum sp. nov., isolated from chicken caecum. Int. J. Syst. Evol. Microbiol. 2006;56:2853–2859. doi: 10.1099/ijs.0.64517-0. [DOI] [PubMed] [Google Scholar]
- Li C.L., Wang J., Zhang H.J., Wu S.G., Hui Q.R., Yang C.B., Fang R.J., Qi G.H. Intestinal morphologic and microbiota responses to dietary bacillus spp in a broiler chicken model. Front. Physiol. 2019;9:1968. doi: 10.3389/fphys.2018.01968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilburn M.S., Loeffler S. Early intestinal growth and development in poultry. Poult. Sci. 2015;94:1569–1576. doi: 10.3382/ps/pev104. [DOI] [PubMed] [Google Scholar]
- Lin R., Piao M., Song Y. Dietary quercetin increases colonic microbial diversity and attenuates colitis severity in Citrobacter rodentium-infected mice. Front. Microbiol. 2019;10:1092. doi: 10.3389/fmicb.2019.01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.N., Liu Y., Hu L.L., Suo Y.L., Zhang L., Jin F., Feng X.A., Teng N., Li Y. Effects of dietary supplementation of quercetin on performance, egg quality, cecal microflora populations, and antioxidant status in laying hens. Poult. Sci. 2014;93:347–353. doi: 10.3382/ps.2013-03225. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii T., Bungo T., Isobe N., Yoshimura Y. Intestinal inflammation induced by dextran sodium sulphate causes liver inflammation and lipid metabolism disfunction in laying hens. Poult. Sci. 2020;99:1663–1677. doi: 10.1016/j.psj.2019.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura R., Takeda K. Maintenance of intestinal homeostasis by mucosal barriers. Inflammation Regener. 2018;38:5. doi: 10.1186/s41232-018-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsolya P., Erzsébet P., Péter G., Orsolya F., Horacio B. Chlorogenic acid combined with lactobacillus plantarum 2142 reduced lps-induced intestinal inflammation and oxidative stress in ipec-j2 cells. Plos One. 2016;11 doi: 10.1371/journal.pone.0166642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka T., Moriyama E., Arai S., Date Y., Yagi J., Kikuchi J., Tsuneda S. Meta-analysis of fecal microbiota and metabolites in experimental colitic mice during the inflammatory and healing phases. Nutrients. 2017;9:1329. doi: 10.3390/nu9121329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman A., Chuang C.C., McIntosh M. Quercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned media. Int. J. Obes. 2011;35:1165–1172. doi: 10.1038/ijo.2010.272. [DOI] [PubMed] [Google Scholar]
- Ozdal T., Sela D., Xiao J., Boyacioglu D., Chen F., Capanoglu E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients. 2016;8:78. doi: 10.3390/nu8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson G.T., Osorio E.Y., Peniche A., Dann S.M., Cordova E., Preidis G.A., Suh J.H., Ito I., Saldarriaga O.A., Loeffelholz M., Ajami N.J., Travi B.L., Melby P.C. Pathologic inflammation in malnutrition is driven by proinflammatory intestinal microbiota, large intestine barrier dysfunction, and translocation of bacterial lipopolysaccharide. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.846155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y.L., Chen C.J., Mu Y.L., Yang Y.L., Feng Z., Li B.G., Li H., Li K. Integrated microbiome and metabolome analysis reveals a positive change in the intestinal environment of myostatin edited large white pigs. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.628685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos-Covián D., Ruas-Madiedo P., Margolles A., Miguel G., Clara G.L.R.G., Nuria S. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M., Naveed M., Arain M.A., Arif M., El-Hack M.E., Alagawany M., Slyal F.A., Soomro R.N., Sun C. Quercetin: nutritional and beneficial effects in poultry. World's Poul. Sci. J. 2017;73:355–364. [Google Scholar]
- Scher J.U., Sczesnak A., Longman R.S., Segata N., Ubeda C., Bielski C., Rostron T., Cerundolo V., Pamer E.G., Abramson S.B., Huttenhower C., Littman D.R. Expansion of intestinal prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabbir U., Rubab M., Daliri E.B.M., Chelliah R., Javed A., Oh D. Curcumin, quercetin, catechins and metabolic diseases: the role of gut microbiota. Nutrients. 2021;13:206. doi: 10.3390/nu13010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T., Bian X., Yao Z.X., Wang Y.W., Gao W.N., Guo C.J. Quercetin improves gut dysbiosis in antibiotic-treated mice. Food Funct. 2020;11:8003–8013. doi: 10.1039/d0fo01439g. [DOI] [PubMed] [Google Scholar]
- Shin N.R., Whon T.W., Bae J.W. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Shrestha A., Metzler-Zebeli B.U., Karembe H., Daniel S., Simone K., Anja J. Shifts in the fecal microbial community of Cystoisospora suis infected piglets in response to toltrazuril. Front. Microbiol. 2020;11:983. doi: 10.3389/fmicb.2020.00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R., Yang Z., Lu N.H., Peng Y.Y. Quercetin, but not rutin, attenuated hydrogen peroxide-induced cell damage via heme oxygenase-1 induction in endothelial cells. Arch. Biochem. Biophys. 2019;676 doi: 10.1016/j.abb.2019.108157. [DOI] [PubMed] [Google Scholar]
- Tian S., Wang J., Gao R., Wang J., Zhu W. Early-life galacto-oligosaccharides supplementation alleviates the small intestinal oxidative stress and dysfunction of lipopolysaccharide-challenged suckling piglets. J. Anim. Sci. Biotechnol. 2022;13:70. doi: 10.1186/s40104-022-00711-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas J.E., Snelling T.J., Lorena L.F., David R.Y.R., Carlos G.E., Secundino L. Effect of sunflower and marine oils on ruminal microbiota, in vitro fermentation and digesta fatty acid profile. Front. Microbiol. 2017;8:1124. doi: 10.3389/fmicb.2017.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnska P., Sedlar K., Lukac M., Faldynova M., Gerzova L., Cejkova D., Sisak F., Rychlik I. Succession and replacement of bacterial populations in the caecum of egg laying hens over their whole life. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital M., Penton C.R., Wang Q., Marius V., Christopher E.P., Qiong W., Vincent B.Y., Dion A.A., Mitchell L.S., Hilary G.M., Laura R., Eugene B.C., Gary B.H., Thomas M.S., James R.C., James M.T. A gene-targeted approach to investigate the intestinal butyrate-producing bacterial community. Microbiome. 2013;1:8. doi: 10.1186/2049-2618-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.W., Jia H.J., Zhang H.J., Wang J., Lv H.Y., Wu S.G., Qi G.H. Supplemental plant extracts from flos lonicerae in combination with baikal skullcap attenuate intestinal disruption and modulate gut microbiota in laying hens challenged by Salmonella pullorum. Front. Microbiol. 2019;10:1681. doi: 10.3389/fmicb.2019.01681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Jin X.L., Li Q.Q., Sawaya A.C.H.F., Leu R.K.L., Conlon M.A., Wu L.M., Hu F.L. Propolis from different geographic origins decreases intestinal inflammation and bacteroides spp. Populations in a model of DSS-Induced Colitis. Mol. Nutr. Food Res. 2018;62 doi: 10.1002/mnfr.201800080. [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen Y., Zhang X., Lu Y., Chen H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: a review. J. Funct. Foods. 2020;75 [Google Scholar]
- Wang X.Y., Wang W.J., Wang L.M., Yu C., Zhang G.L., Zhu H.L., Wang C.W., Zhao S.J., Hu C.A.A., Liu Y.L. Lentinan modulates intestinal microbiota and enhances barrier integrity in a piglet model challenged with lipopolysaccharide. Food Funct. 2019;10:479–489. doi: 10.1039/c8fo02438c. [DOI] [PubMed] [Google Scholar]
- Warner B.B., Deych E., Zhou Y.J., Hall-Moore C., Weinstock G.M., Sodergren E., Hoffmann J.A., Linneman L.A., Hamvas A., Khanna G., Rouggly-Nickless L.C., Ndao I.M., Shands B.A., Escobedo M., Sullivan J.E., Radmacher P.G., Shannon W.D., Tarr P.I. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet. 2016;387:1928–1936. doi: 10.1016/S0140-6736(16)00081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S., Zhang H., Matjeke R.S., Zhao J.Y., Yu Q.H. Bacillus coagulans protect against salmonella enteritidis-induced intestinal mucosal damage in young chickens by inducing the differentiation of goblet cells. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Hu M.J., Wang Y.Q., Cui Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules. 2019;24:1123. doi: 10.3390/molecules24061123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y., Du M., Zhu M.J. Quercetin suppresses NLRP3 inflammasome activation in epithelial cells triggered by Escherichia coli O157: H7. Free. Radicals. Biol. Med. 2017;108:760–769. doi: 10.1016/j.freeradbiomed.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Xue Y., Du M., Zhu M.J. Quercetin prevents Escherichia coli O157: H7 adhesion to epithelial cells via suppressing focal adhesions. Front. Microbiol. 2019;9:3278. doi: 10.3389/fmicb.2018.03278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S., Jha R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019;10:2. doi: 10.1186/s40104-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.W., Fu Y.X., Deng Z.Y., Fan Y.W., Li H.Y. Effects of soluble dietary fiber from soybean residue fermented by neurospora crassa on the intestinal flora in rats. Food Funct. 2020;11:7433–7445. doi: 10.1039/d0fo01093f. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wu W.d., Lee Y.K., Xie J.J., Zhang H.F. Spatial heterogeneity and co-occurrence of mucosal and luminal microbiome across swine intestinal tract. Front. Microbiol. 2018;9:48. doi: 10.3389/fmicb.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Pan H.C., Xu Y.J., Wang X.T., Qiu Z.H., Li J. Allicin decreases lipopolysaccharide-induced oxidative stress and inflammation in human umbilical vein endothelial cells through suppression of mitochondrial dysfunction and activation of Nrf2. Cell Physiol. Biochem. 2017;41:2255–2267. doi: 10.1159/000475640. [DOI] [PubMed] [Google Scholar]
- Zhang S., Kim I.H. Effect of quercetin (flavonoid) supplementation on growth performance, meat stability, and immunological response in broiler chickens. Livest. Prod. Sci. 2020;242 [Google Scholar]
- Zhang X., Akhtar M., Chen Y., Ma Z., Liang Y., Shi D., Cheng R., Cui L., Hu Y., Nafady A., Ansari A., Abdel-Kafy E., Liu H. Chicken jejunal microbiota improves growth performance by mitigating intestinal inflammation. Microbiome. 2022;10:107. doi: 10.1186/s40168-022-01299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Jiang L., Fang X.Y., Guo Z.Q., Wang X.X., Shi B., Meng Q.W. Host-microbiota interaction-mediated resistance to inflammatory bowel disease in pigs. Microbiome. 2022;10:115. doi: 10.1186/s40168-022-01303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Qu W., Chen S., Zhang L., Wu D., Chen Z. Bisphenol A inhibits mucin 2 secretion in intestinal goblet cells through mitochondrial dysfunction and oxidative stress. Biomed. Pharmacother. 2019;111:901–908. doi: 10.1016/j.biopha.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Zhu H., Pi D., Leng W., Wang X., Hu C.A.A., Hou Y.Q., Xiong L., Wang C.W., Qin Q., Liu Y.L. Asparagine preserves intestinal barrier function from LPS-induced injury and regulates CRF/CRFR signaling pathway. Innate Immun. 2017;23:546e56. doi: 10.1177/1753425917721631. [DOI] [PubMed] [Google Scholar]