Summary:
The use of genomic data to analyze primary endpoints for clinical trials in diffuse large B-cell lymphomas (DLBCL) significantly improved the development of rational drug combinations for genetically defined patient subsets. Recent genetic mouse models and their ability to recapitulate transitions between germinal center exit and memory B-cell characteristics in DLBCL will accelerate the development of rationale-based clinical trials.
The current clinical method for diagnosing diffuse large B-cell lymphomas (DLBCL) starts with a biopsy collection from a lymph node or an extranodal site and is based on morphology, immunophenotype, FISH analysis, and B-cell clonality analysis. Advances in the understanding of DLBCL biology herald a transition to a molecular genetic classification based on mutational profile, somatic copy-number alterations, and structural variants identified in three large cohorts indicating that seven identified subgroups reflect true biological differences. This new molecular classification, now recognized by the inaugural International Consensus Classification of mature lymphoid neoplasms, comprises of MCD (MYD88, CD79B)/C5, N1 (NOTCH1), A53 (aneuploidy, TP53)/C2, BN2 (BCL6, NOTCH2)/C1, ST2 (SGK1, TET2)/C4, EZB (EZH2, BCL2)/C3+MYC, and EZB (EZH2, BCL2)/C3-MYC, further subclassifying a basic biological division based on cell-of-origin (COO) by gene expression profiling [activated B cell (ABC), germinal center B cell (GCB), and unclassified; ref. 1].
R-CHOP, the current mainstay therapy for DLBCL, fails to cure 35% to 40% of patients. Efforts to utilize the new molecular classification for development of regimens/therapies are ongoing in first-line and relapsed/refractory DLBCL settings. In the first-line setting, learning from the results of the PHOENIX trial (NCT01855750), the ESCALADE trial (NCT04529772) is assessing the impact of addition of acalabrutinib to R-CHOP in younger patients with ABC DLBCL. In addition, there are trials that are, though not selective with a particular subtype of DLBCL, assessing the impact of drugs that have been shown to be of significance in the new molecular classification—namely, Bruton's tyrosine kinase (BTK) inhibitors (NCT03129828), BCL2 inhibitors (NCT02055820), or the combination of BTK and BCL2 inhibitors (NCT04739813, NCT03223610).
Although the introduction of polatuzumab vedotin (antibody–drug conjugate to CD79B) to replace vincristine in R-CHOP (“Pola-R-CHP”; NCT03274492) showed a 6.5% absolute improvement in 2-year progression-free survival (PFS) and challenges standard of care in untreated DLBCL (pending FDA review), it stops short of providing biological insight into this otherwise clinically heterogeneous disease. The PHOENIX trial (NCT01855750) randomized untreated non-GCB DLBCL patients to R-CHOP + ibrutinib or placebo with the underlying rationale that ABC DLBCLs are addicted to BTK-dependent B-cell receptor (BCR) signaling (2). The overall study did not meet its endpoint largely due to increased adverse events in older patients. However, in an a posteriori analysis, younger patients benefited from the addition of ibrutinib, especially those with MCD/C5 and N1 subtypes (2). The lack of prespecified outcomes and small case numbers limit the conclusiveness of genomic analysis-based outcomes. The results highlight the importance of designing clinical trials that utilize genomic data to analyze the primary endpoints, and the need for effective preclinical models based on molecular subtypes to inform development of rationale-based clinical trials.
Genetic mouse models (GEMM) of DLBCL subtypes with more favorable outcomes, including the BN2/C1 and EZB/C3 subtypes, successfully phenocopied human disease (3–5). However, ABC-origin DLBCL models, in particular for the MCD/C5 subtype that is associated with particularly poor clinical outcomes, have been hampered by propensity to terminal plasma cell differentiation.
The Myd88cond.p.L252P/wt;Rosa26LSL.BCL2.IRES.GFP/wt;Cd19Cre/wt model combines two frequent lesions of the MCD/C5 subtype, namely an activating Myd88L252P mutation and Bcl2 overexpression. However, this model is limited by its plasma cell phenotype (B220-negative, CD138-positive), which reflects plasmablastic lymphoma rather than MCD/C5 DLBCL (3). The caveat of plasma cell differentiation was addressed in the Prdm1fl/fl;R26LSL.IKK2ca;Cγ1Cre/wt model by conditional deletion of the plasma cell transcription factor Prdm1 combined with activation of the NF-κB pathway via constitutively active IKK2. Lymphomas in this model are IRF4-positive, CD138-negative, and show a transcriptional signature consistent with ABC-DLBCL, all of which are in line with MCD/C5 DLBCL (6). However, alterations in IKBKB (encoding IKK2) are uncommon in ABC-DLBCL and might not fully recapitulate the effects of the defining lesions associated with NF-κB activation in the MCD/C5 subtype.
To further improve MCD/C5 models, Flümann and colleagues refined the original model based on MYD88 mutation/BCL2 gain-of-function by adding either Spib overexpression to antagonize Prdm1 or Prdm1 deletion (3). In addition, Pindzola and colleagues have recently developed similar models with the implementation of up to four concurrent genetic lesions: Myd88L252P, Cd79bY195H, Prdm1 deletion, and Bcl2 overexpression (4). In contrast to Myd88/Bcl2 mice, Myd88/Bcl2/Spib, Myd88/Bcl2/Prdm1, and Myd88/Cd79b/Prdm1/Bcl2 mice recapitulate the MCD/C5 DLBCL subtype more faithfully by preventing (Prdm1 deletion) or reducing (Spib) plasma cell differentiation while maintaining a B220-positive/CD138-negative phenotype and a transcriptional program consistent with ABC-DLBCL (3, 4). Tumor whole-exome sequencing of lymphomas derived from Myd88/Bcl2/Prdm1 mice revealed that these tumors acquired additional lesions in genes commonly mutated in ABC-DLBCL, thus at least partially recapitulating their genetic complexity (3). Given their lymphoma immunophenotype, transcriptional profile, and genotype that accurately replicates human MCD/C5 DLBCL, these models represent a powerful preclinical tool to test novel rationale-based drug combinations. Furthermore, autochthonous mouse models have the benefit of preserving the mouse native immune system, thus maintaining an intact tumor–immune microenvironment. In a proof-of-concept study of their model, Flümann and colleagues tested the combination of ibrutinib and venetoclax in Myd88/Bcl2/Prdm1 mice, which recapitulated known survival benefits from clinical trials for chronic lymphocytic leukemia (CLL) and early-stage trials for patients with DLBCL based on these combinations (NCT04739813, NCT03223610; ref. 7). This combination was subsequently tested in six patients with previously treated, progressive, non-GCB DLBCL, and led to tumor volume shrinkage in five of the six patients. Of note, all responders had BCL2-rearranged tumors, while the only nonresponder was BCL2 negative (3).
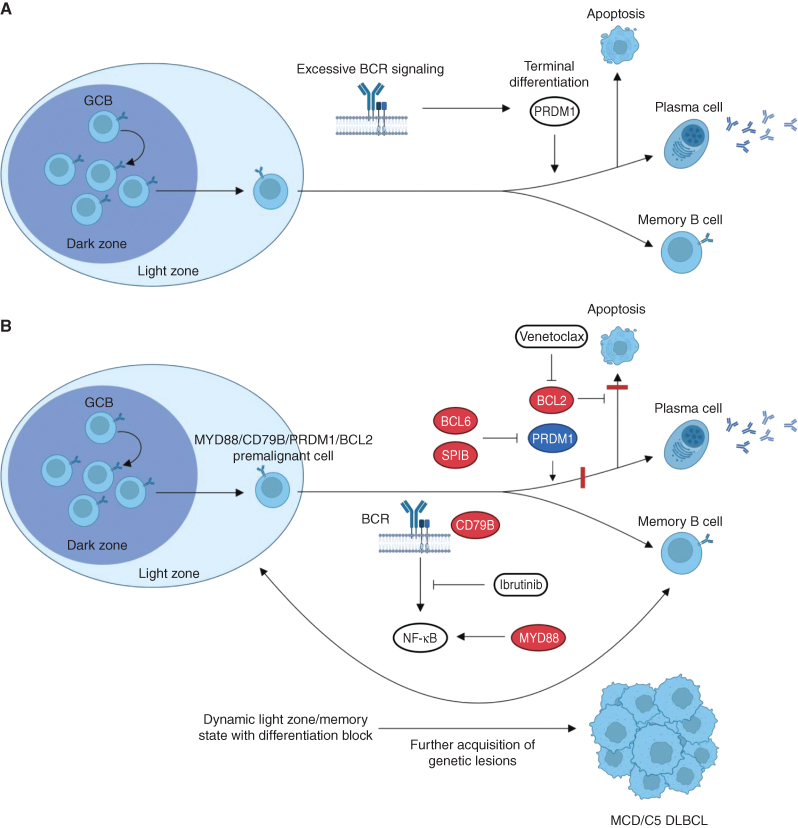
Beyond their use as a preclinical tool for therapeutics, these MCD/C5 DLBCL murine models shed light on the key role of the plasma cell program as a tumor suppressor pathway in DLBCL. Previous studies suggested that hyperactive BCR signaling and BCR-dependent PI3K hyperactivation in autoreactive or premalignant B-cell clones results in activation of PRDM1. PRDM1 activation mediates terminal plasma cell differentiation and cell-cycle arrest as a safeguard to eliminate autoreactive and premalignant B-cell clones (Fig. 1A). Pten-deficient mature B cells are committed to terminal plasma cell differentiation and subsequent apoptosis, and do not aberrantly expand unless they are concomitantly Prdm1 deficient, in which case they also have a propensity for autoreactivity (8). Likewise, hyperactivation of the proximal BCR kinase SYK, reflecting pathologic signaling from an autoreactive BCR or oncogenic BCR signaling in premalignant clones, results in PRDM1-mediated elimination of potentially harmful B-cell clones (9). In light of these observations, the Prdm1 deletion in the Myd88/Bcl2/Prdm1 and Myd88/Cd79b/Prdm1/Bcl2 models enables permissiveness and premalignant B-cell clones to bypass the tumor-suppressive plasma cell program that would otherwise be triggered by aberrant NF-κB activation (MYD88) and oncogenic BCR signaling (CD79B; Fig. 1B; refs. 3, 4).
Figure 1.
Oncogenic and tumor suppressor programs in MCD/C5 DLBCL. A, In wild-type B cells, upon excessive activation of BCR-dependent signaling pathways, as seen with oncogenic alterations or autoreactivity, PRDM1 acts as a tumor suppressor by activating the plasma cell program, leading to terminal differentiation and apoptosis. B,MYD88 and CD79B mutations lead to constitutive activation of the BCR-dependent NF-κB pathway. This excessive pathway activation would normally lead to terminal differentiation and apoptosis, which are respectively prevented by PRDM1 loss (or SPIB overexpression) and BCL2 overexpression. As a result, MYD88/CD79B/PRDM1/BCL2 mutant B cells are blocked in a dynamic state between the germinal center light zone and memory phenotypes and are unable to undergo plasmablastic differentiation. This state primes premalignant B cells to accumulate further genetic lesions and fully transform into MCD/C5 DLBCL. Combination of downregulation of the NF-κB pathway with the BTK inhibitor ibrutinib and reactivation of the apoptotic program with the BCL2 inhibitor venetoclax is therefore a promising therapeutic strategy in MCD/C5 DLBCL, as shown in preclinical studies and early-phase clinical trials. Genes labeled in red represent activating lesions, while genes in blue are inactivating lesions. Red bars represent pathway blocks resulting from genetic lesions. Abbreviations: BCR, B-cell receptor; GC, germinal center.
Accordingly, the differentiation block in Myd88/Bcl2/Prdm1 mice was more complete than the one in Myd88/Bcl2/Spib mice (3). In contrast to the Myd88/Bcl2 and Myd88/Bcl2/Spib lymphomas, Myd88/Bcl2/Prdm1 mice have a large B1 cell population and lymphomas retain high levels of surface IgM expression, suggesting intensified BCR activity consistent with oncogenic BCR signaling and lack of the PRDM1-driven terminal differentiation safeguard program (3). In addition to PRDM1 and SPIB, TBL1XR1 mutations highlight the importance of plasma cell differentiation as a suppressor of DLBCL lymphomagenesis. These mutations occur in a subset of DLBCL and redirect binding of the SMRT-HDAC3 repressor complex from BCL6 to the memory B-cell transcription factor BACH2, causing a block in plasma cell differentiation in favor of an oncogenic memory B-cell state. Upon antigen reencounter, TBL1XR1-mutant B cells cannot differentiate into plasma cells and are instead prompted to repetitively reenter germinal centers (10). Consistent with this concept, Myd88/Bcl2/Prdm1 and Myd88/Cd79b/Prdm1/Bcl2 mice, prior to the onset of lymphoma, exhibit increased germinal centers and an enlarged GCB pool that is skewed toward memory B cell rather than plasmablastic differentiation (3, 4). In addition to their inability to undergo terminal plasma cell differentiation, premalignant MCD/C5 clones have a competitive advantage over normal B cells. Indeed, the presence of Myd88L252P alone is sufficient to “replace” the requirement of GCBs on T-cell costimulation (via CD40L and IL4) for survival, although it confers dependence of these mutant cells on TLR9 for organization of the MYD88-TLR9-BCR (My-T-BCR) complex that promotes their survival (5). Taken together, these alterations in B-cell dynamics uncover early pathogenic changes and might help identify previously unrecognized vulnerabilities in MCD/C5 DLBCL. Interestingly, in the Myd88/Bcl2/Prdm1 model, lymphomas showed a transcriptional and immunophenotypic signature transitioning between light zone B cells (CXCR4−/CD86+) and memory B-cell characteristics (CD38+/FAS−/IgD−). Flümann and colleagues therefore suggested that clonal evolution towards MCD/C5 DLBCL involves dynamic transition between GC- and prememory stages without fully committing to memory B-cell differentiation, while being unable to enter the plasma cell differentiation program (3). In line with the hypothesis of an atypical transitional prememory B-cell population giving rise to MCD/C5 DLBCL, Venturutti and colleagues have shown that Myd88L252P mice are enriched with a CD11c+ TBX21+ aged/autoimmune memory B-cell (AiBC) subtype, which is also a known driver of human autoimmune diseases. Interestingly, ablation of TBX21, a central AiBC regulator, removed the competitive advantage of Myd88L252P B cells without affecting wild-type cells. TBX21 expression is also selectively featured in a subgroup of aggressive extranodal DLBCL. The authors therefore suggested that AiBC represent a potential premalignant reservoir of cells that are destined to develop MCD/C5 DLBCL (5). In contrast, Pindzola and colleagues observed a predominant expansion of spontaneous splenic GCBs in their Myd88/Cd79b/Prdm1/Bcl2 model, and therefore nominated these cells as a potential cell-of-origin for MCD/C5 DLBCL (4). As for AiBC, spontaneous splenic GCBs have previously been associated with autoimmune diseases (4). It is worth noting that Myd88/Cd79b/Bcl2/Prdm1 mice, unlike Myd88/Bcl2, Myd88/Bcl2/Prdm1, and Myd88/Bcl2/Spib mice, contain a CD79B ITAM mutation, as in approximately half of MCD/C5 DLBCL and frequently cooccurring with MYD88L265P (3, 4). In addition, lymphomas in Myd88/Bcl2/Prdm1 mice were IgG-positive, which only occurs in about 10% of human MCD/C5 DLBCL. The observed differences in these studies might therefore represent biological and molecular heterogeneity within the MCD/C5 cluster that could notably be driven by CD79B mutations and different immunoglobulin isotypes. These findings nevertheless provide valuable new insights in how blockage of plasma cell differentiation, in combination with other genetic lesions, affects B-cell development and primes mutant B cells for malignant transformation.
Taken together, these newly developed GEMMs will provide insight into the pathogenesis of DLBCL and accelerate the development of rational drug combinations for genetically defined subsets of patients, including therapies harnessing the immune system, such as immune checkpoint inhibitors, T-cell engagers, and chimeric antigen receptor (CAR) T cells, which have shown efficacy in the Myd88/Bcl2/Prdm1 model (3). In addition to allowing the investigation of different treatments, these models can assist in the prospective identification of molecular markers predicting response to therapy, notably by using coisogenic mice. For instance, it is likely that Myd88/Cd79b/Prdm1 mice would not respond to the combination of ibrutinib and venetoclax as well as Myd88/Bcl2/Prdm1 mice. Similarly, treating Myd88/Cd79b/Prdm1/Bcl2 and Myd88/Bcl2/Prdm1 mice with polatuzumab vedotin could uncover a role of Cd79b mutations in the response to this therapy. In addition, identification of biases in different B-cell populations, such as the AiBC population observed by Venturutti and colleagues, may allow the determination of premalignant states, analogous to monoclonal gammopathy of undetermined significance (MGUS) in multiple myeloma, that might warrant closer monitoring or even prophylactic interventions (5). While the new GEMMs provide important insight into the high-risk MCD/C5 DLBCL subset, similar models can be generated as preclinical models for other DLBCL subtypes, including TP53 to model A53/C2, as well as SGK1 and TET2 for ST2/C4 subtypes of DLBCL. Future advances might therefore allow a more efficient transition from preclinical to clinical studies with a higher success rate of mechanism-based therapies in selected disease subtypes. Beyond the realm of oncology, these advances might also benefit the fields of immunology and autoimmunity, as suggested by the role of AiBC and spontaneous splenic GCBs as both drivers of lymphomagenesis and autoimmune diseases, as well as the role of the plasma cell differentiation program to protect against those two disease states (4, 5).
Authors’ Disclosures
S. Kothari reports other support from Karyopharm, other support from Incyte, other support from Magallan Health, and other support from Radyus Research outside the submitted work. No disclosures were reported by the other authors.
References
- 1. Wright GW, Huang DW, Phelan JD, Coulibaly ZA, Roulland S, Young RM, et al. A probabilistic classification tool for genetic subtypes of diffuse large B cell lymphoma with therapeutic implications. Cancer Cell 2020;37:551–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilson WH, Wright GW, Huang DW, Hodkinson B, Balasubramanian S, Fan Y, et al. Effect of ibrutinib with R-CHOP chemotherapy in genetic subtypes of DLBCL. Cancer Cell 2021;39:1643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flümann R, Hansen J, Pelzer BW, Nieper P, Lohmann T, Kisis I, et al. Distinct genetically determined origins of /-driven aggressive lymphoma rationalize targeted therapeutic intervention strategies. Blood Cancer Discov 2023;4:78–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pindzola GM, Razzaghi R, Tavory RN, Nguyen HT, Morris VM, Li M, et al. Aberrant expansion of spontaneous splenic germinal centers induced by hallmark genetic lesions of aggressive lymphoma. Blood 2022;140:1119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Venturutti L, Rivas MA, Pelzer BW, Flumann R, Hansen J, Karagiannidis I, et al. An aged/autoimmune B-cell program defines the early transformation of extranodal lymphomas. Cancer Discov 2022 Oct 20 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calado DP, Zhang B, Srinivasan L, Sasaki Y, Seagal J, Unitt C, et al. Constitutive canonical NF-kB activation cooperates with disruption of BLIMP1 in the pathogenesis of activated B cell-like diffuse large cell lymphoma. Cancer Cell 2010;18:580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou Z, Zhang L, Wang X, Li X, Li L, Fu X, et al. Ibrutinib combined with venetoclax for the treatment of relapsed/refractory diffuse large B cell lymphoma. Ann Hematol 2021;100:1509–16. [DOI] [PubMed] [Google Scholar]
- 8. Setz CS, Hug E, Khadour A, Abdelrasoul H, Bilal M, Hobeika E, et al. PI3K-mediated Blimp-1 activation controls B cell selection and homeostasis. Cell Rep 2018;24:391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hug E, Hobeika E, Reth M, Jumaa H. Inducible expression of hyperactive Syk in B cells activates Blimp-1-dependent terminal differentiation. Oncogene 2014;33:3730–41. [DOI] [PubMed] [Google Scholar]
- 10. Venturutti L, Teater M, Zhai A, Chadburn A, Babiker L, Kim D, et al. TBL1XR1 mutations drive extranodal lymphoma by inducing a Pro-tumorigenic memory fate. Cell 2020;182:297–316. [DOI] [PMC free article] [PubMed] [Google Scholar]