Clonal hematopoiesis with indeterminate potential (CHIP)
With age, cells are exposed to various endogenous and extrinsic factors and accumulate gene mutations. Therefore, they acquire proliferative ability and immortality, which differ from those of conventional normal cells, and develop into cancer cells. Recent developments in next-generation sequencing technology have facilitated the identification of cancer causative genes as well as exploration for gene mutations in normal tissues on a large scale and over time. Hematopoietic cells undergo natural selection under various selection pressures, and clones that match these pressures acquire proliferative dominance and undergo CHIP. CHIP, also called clonal hematopoiesis, has been shown to occur in healthy individuals as well (1). The causes of CHIP include aging, chemotherapy, radiotherapy, and autoimmunity. Recent studies examining variant allele frequency to detect up to 0.03% of clones have demonstrated CHIP in approximately 95% of elderly individuals (2). CHIP is found in approximately 25% of patients with cancer and is characterized by several DNA damage response factors, including TP53, PPM1D, and ATM (3). People with CHIP are more likely to develop myeloid neoplasms (MN); however, myocardial infarction, cerebral infarction, and cancer worsen the quality of life significantly and have been attracting more attention in recent years (4). Further, even if it is possible to diagnose CHIP, no effective therapeutic intervention has been developed to date.
Therapy-related MN
Treatment-related myeloid neoplasms (t-MNs) often develop as myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) 3–7 years after exposure to cytotoxic chemotherapy or radiation therapy. However, in rare cases, they may develop as myeloproliferative neoplasms or lymphocytic leukemia (5). Approximately 90% of all cases are accompanied by characteristic chromosomal structural abnormalities such as deletions and translocations. Development of t-MNs during the treatment of primary malignancies hinders treatment continuation because of decreased white blood cell levels and immunosuppression. Further, many t-MNs are resistant to treatment and have poor clinical prognoses. The reported overall survival rate in t-MNs is approximately 8–14 months and the 5-year survival rate is less than 10% (6). Previously, t-MNs were considered to involve the accumulation of cytotoxic chemotherapy- or radiotherapy-induced gene mutations in hematopoietic stem cells. However, recent genome sequencing studies have shown that t-MNs have a significantly higher frequency of TP53 inherited mutations than those in primary MN; some cases have also been detected as CHIP prior to chemotherapy. Thus, the selection of existing CHIP under chemotherapy and radiation therapy is now shifting to a newly proposed onset model in which the disease develops (7).
Effect of CHIP on clinical prognosis
High-grade serous ovarian cancer is the most common ovarian cancer subtype, accounting for approximately 85% of all ovarian cancer deaths. Regarding changes in the ovarian cancer genome, genome analysis of 316 high-grade serous carcinoma cases in The Cancer Genome Atlas revealed TP53 mutation in 96%, germline BRCA1/2 in 14–15%, and homologous recombination deficiency in more than 50% tumors (8-10). The first-line chemotherapy for ovarian cancer involves a platinum agent; multiple PARP inhibitors (olaparib, niraparib, and rucaparib) for ovarian cancer have also been approved by the FDA. There is a report of meta-analyses of PARP clinical trials for ovarian cancer, gastric cancer, non-small cell lung cancer, prostate cancer, colon cancer, breast cancer, pancreatic cancer, and small cell lung cancer and melanoma (11). As the result of 18 randomized controlled trials of 18 PARP inhibitor and placebo (n=7,307), the incidence of MDS and AML was 21/4,533 in the PARP inhibitor group and 3/2,774 in the placebo group (odds ratio =2.63; P=0.026). The current issue of JAMA Oncology included a retrospective study on the risk of t-MNs using peripheral blood cell samples in two clinical trials of rucaparib for ovarian cancer (12): ARIEL2 (single-arm study of rucaparib in patients with platinum-sensitive, relapsed, high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer) and ARIEL3 (double-blind study of rucaparib as switch maintenance following platinum-based chemotherapy in patients with platinum-sensitive, high-grade serous or endometrioid epithelial ovarian, primary peritoneal or fallopian tube cancer) (Figure 1). Of the 491 ARIEL2 participants and 561 ARIEL3 participants, the background factors that showed a statistically significant difference in t-MN onset included the duration of treatment with a PARP inhibitor, number of prior chemotherapy courses, and duration of receiving platinum. Next-generation sequencing analysis of 20 and 44 patients in the t-MN onset and non-onset groups showed that the prevalence of CHIP mutations in TP53 before treatment with an allele frequency of 1% or more was significantly higher in the t-MN onset group than in the non-onset group (45.0% vs. 13.6%, P=0.009). However, the variant allele frequency level of TP53 in CHIP did not differ between the t-MN onset and non-onset groups. Furthermore, the exposure period of the platinum product and CHIP of TP53 were correlated (14 vs. 11.1 months, P=0.02). The harboring status of germlines BRCA1, BRCA2, RAD51C, and RAD51D, which are homologous recombination-related genes and causative genes of hereditary tumors, as well as the long-term administration period of rucaparib, were both correlated with t-MN onset. In both groups, the platinum agent was highly effective, and simultaneously, its efficacy was strongly correlated with its exposure period. As these clinical and molecular characteristics are not independent, the authors indicated that it is difficult to extrapolate their individual contributions to the overall risk of t-MNs. A strong correlation is often seen between the parameters of genomic data and clinical data in medical statistics, where the amount of information analysis has been increasing in recent years. Bolton et al. used MSK-IMPACT analysis data and clinical information, including CHIP clone data, age, treatment history, and therapeutic drug use, from 24,439 patients with 57 cancer types, and confirmed the association between CHIP and t-MN in patients with cancer. They further validated continuous data from 525 patients and constructed a quantified model of the absolute risk of AML/MDS after breast cancer diagnosis (13). The main genomic alterations detected in CHIP are broadly classified into two types: single-nucleotide variants (SNVs) and copy number variants (CNVs). However, as the two variant types have been examined separately in previous studies, the relationship between SNV and CNV in CHIP remains unclear. Recently, a combined analysis of SNV and CNV has been reported in peripheral blood samples from 11,234 subjects registered with Biobank Japan (1). The results showed that the abnormal variants were detected in 40% of the subjects and that both variant types were observed in 7% of the subjects (16% in positive cases). These results verified a statistically significant coexistence, suggesting a cooperative relationship between the two variant types. SNVs and CNVs frequently coexist in genes such as DNMT3A, TET2, JAK2, and TP53, and these are considered to undergo biallelic loss. Biallelic loss in these genes is a known finding in hematopoietic tumors, but this study revealed that they exist from the CHIP stage.
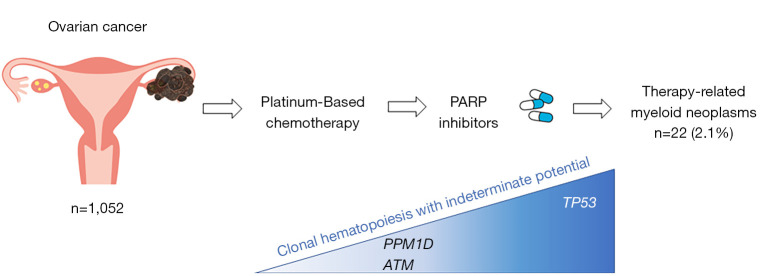
Figure 1.
Outline of this issue. Relationship between therapy-related myeloid neoplasms and clonal hematopoiesis with indeterminate potential after PARP inhibitor treatment for platinum-sensitive ovarian cancer. PPM1D, protein phosphatase magnesium/manganese-dependent 1D; ATM, ataxia telangiectasia mutated; PARP, poly (ADP-ribose) polymerase; TP53, transformation-related protein 53.
With the explosive spread of genomic testing in cancer, CHIP is expected to be increasingly detected in the future. Currently, the presence or absence of CHIP detection does not affect decision-making in cancer treatment. However, research advances are expected to promote discussions on the selection of chemotherapy, administration timing, monitoring methods, early detection, and prevention, with respect to risk stratification in the development of t-MNs.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Translational Medicine. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-2022-65/coif). The authors have no conflicts of interest to declare.
References
- 1.Saiki R, Momozawa Y, Nannya Y, et al. Combined landscape of single-nucleotide variants and copy number alterations in clonal hematopoiesis. Nat Med 2021;27:1239-49. 10.1038/s41591-021-01411-9 [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Briggs AW, Chew WL, et al. Engineering and optimising deaminase fusions for genome editing. Nat Commun 2016;7:13330. 10.1038/ncomms13330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coombs CC, Zehir A, Devlin SM, et al. Therapy-Related Clonal Hematopoiesis in Patients with Non-hematologic Cancers Is Common and Associated with Adverse Clinical Outcomes. Cell Stem Cell 2017;21:374-382.e4. 10.1016/j.stem.2017.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durrani J, Awada H, Kishtagari A, et al. Large granular lymphocytic leukemia coexists with myeloid clones and myelodysplastic syndrome. Leukemia 2020;34:957-62. 10.1038/s41375-019-0601-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNerney ME, Godley LA, Le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer 2017;17:513-27. 10.1038/nrc.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fianchi L, Pagano L, Piciocchi A, et al. Characteristics and outcome of therapy-related myeloid neoplasms: Report from the Italian network on secondary leukemias. Am J Hematol 2015;90:E80-5. 10.1002/ajh.23966 [DOI] [PubMed] [Google Scholar]
- 7.Gillis NK, Ball M, Zhang Q, et al. Clonal haemopoiesis and therapy-related myeloid malignancies in elderly patients: a proof-of-concept, case-control study. Lancet Oncol 2017;18:112-21. 10.1016/S1470-2045(16)30627-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patch AM, Christie EL, Etemadmoghadam D, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015;521:489-94. 10.1038/nature14410 [DOI] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas Research Network . Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609-15. 10.1038/nature10166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konstantinopoulos PA, Ceccaldi R, Shapiro GI, et al. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov 2015;5:1137-54. 10.1158/2159-8290.CD-15-0714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morice PM, Leary A, Dolladille C, et al. Myelodysplastic syndrome and acute myeloid leukaemia in patients treated with PARP inhibitors: a safety meta-analysis of randomised controlled trials and a retrospective study of the WHO pharmacovigilance database. Lancet Haematol 2021;8:e122-34. 10.1016/S2352-3026(20)30360-4 [DOI] [PubMed] [Google Scholar]
- 12.Kwan TT, Oza AM, Tinker AV, et al. Preexisting TP53-Variant Clonal Hematopoiesis and Risk of Secondary Myeloid Neoplasms in Patients With High-grade Ovarian Cancer Treated With Rucaparib. JAMA Oncol 2021;7:1772-81. 10.1001/jamaoncol.2021.4664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolton KL, Ptashkin RN, Gao T, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet 2020;52:1219-26. 10.1038/s41588-020-00710-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as