Abstract
Purpose
Four-dimensional computed tomography (4DCT)–ventilation-based functional avoidance uses 4DCT images to generate plans that avoid functional regions of the lung with the goal of reducing pulmonary toxic effects. A phase 2, multicenter, prospective study was completed to evaluate 4DCT-ventilation functional avoidance radiation therapy. The purpose of this study was to report the results for pretreatment to posttreatment pulmonary function test (PFT) changes for patients treated with functional avoidance radiation therapy.
Methods and Materials
Patients with locally advanced lung cancer receiving chemoradiation were accrued. Functional avoidance plans based on 4DCT-ventilation images were generated. PFTs were obtained at baseline and 3 months after chemoradiation. Differences for PFT metrics are reported, including diffusing capacity for carbon monoxide (DLCO), forced expiratory volume in 1 second (FEV1), and forced vital capacity (FVC). PFT metrics were compared for patients who did and did not experience grade 2 or higher pneumonitis.
Results
Fifty-six patients enrolled on the study had baseline and posttreatment PFTs evaluable for analysis. The mean change in DLCO, FEV1, and FVC was –11.6% ± 14.2%, –5.6% ± 16.9%, and –9.0% ± 20.1%, respectively. The mean change in DLCO was –15.4% ± 14.4% for patients with grade 2 or higher radiation pneumonitis and –10.8% ± 14.1% for patients with grade <2 radiation pneumonitis (P = .37). The mean change in FEV1 was –14.3% ± 22.1% for patients with grade 2 or higher radiation pneumonitis and –3.9% ± 15.4% for patients with grade <2 radiation pneumonitis (P = .09).
Conclusions
The current work is the first to quantitatively characterize PFT changes for patients with lung cancer treated on a prospective functional avoidance radiation therapy study. In comparison with patients treated with standard thoracic radiation planning, the data qualitatively show that functional avoidance resulted in less of a decline in DLCO and FEV1. The presented data can help elucidate the potential pulmonary function improvement with functional avoidance radiation therapy.
Introduction
Traditional radiation therapy techniques for locally advanced lung cancer focus on standard lung dose metrics such as mean lung dose, or volume of the lung receiving ≥20 Gy, to reduce the risk of patients developing clinical symptoms.1 Lung dose metrics assume spatially homogeneous lung function without considering potential functional spatial heterogeneity. Approximately 70% of patients with lung cancer demonstrate lung heterogeneity, owing to the tumor itself, possible airway constriction, or underlying comorbidities such as asthma or emphysema.2 To take lung function heterogeneity into account, functional avoidance radiation treatment planning has been proposed.3 In brief, the concept of functional avoidance is to use advanced treatment-planning techniques to prioritize the protection of healthy lung tissue and aims to spare portions of the lung that are higher functioning (where higher-functioning lung is assessed by functional imaging). The hypothesis of functional avoidance is that reducing doses to functional portions of the lung will reduce the probability that patients develop pulmonary adverse effects after treatment.4
Ventilation imaging based on 4-dimensional computed tomography (4DCT) (referred to as 4DCT-ventilation) has been proposed as a means of generating functional images for functional avoidance treatment planning. As part of the treatment-planning process, most patients with lung cancer undergo 4DCT imaging.5 Therefore, generating 4DCT-based ventilation maps has benefit in that it does not require an extra imaging procedure, reduces costs, and reduces imaging-related radiation dose.6,7 4DCT-ventilation has been validated against traditional forms of functional lung imaging, including nuclear medicine planar ventilation-perfusion scans, single-photon emission computed tomography imaging, and more experimental forms of functional lung imaging.8, 9, 10, 11, 12 Retrospective studies showed the feasibility of 4DCT-ventilation functional avoidance in reducing doses to functional portions of the lung without sacrificing tumor dose or exceeding neighboring normal-tissue constraints.13,14 Based on the retrospective data, a 2-institution functional avoidance clinical trial using 4DCT-ventilation was completed.15 The trial reported the primary endpoint of clinical toxicity and found that functional avoidance radiation therapy reduced the rate of grade >2 radiation pneumonitis compared with the historical rate (14.9% vs 25%).
Lung toxic effects caused by radiation are commonly scored via the Common Terminology Criteria for Adverse Events16 or the Radiation Therapy Oncology Group (RTOG) scoring system.1 Radiation pneumonitis can present several clinical challenges: symptoms and radiographic changes may not necessarily progress in parallel, differentiation between pneumonitis and tumor progression is often challenging, more frequent prescription of steroids may result in higher-grade pneumonitis more frequently, and pneumonitis is often not thought of as a quantitative measure.1,17,18
Pulmonary function tests (PFTs) include parameters such as diffusing capacity for carbon monoxide (DLCO), forced expiratory volume in 1 second (FEV1), and forced vital capacity (FVC). PFTs are commonly used in lung fibrosis or chronic obstructive pulmonary disease to monitor disease progression.19, 20, 21 PFTs are also used to assess lung function status for patients with lung cancer treated with chemoradiation.22 A previous systematic review in 2017, which included 7 studies with more recent radiation techniques, found decreases in DLCO and FEV1 after chemoradiation.22 To obtain an objective measure of pulmonary function change, the 4DCT-ventilation functional avoidance study collected PFT data before and after chemoradiation. The purpose of this study was therefore to characterize pretreatment to posttreatment PFT changes for patients treated with 4DCT-ventilation-based functional avoidance radiation therapy.
Methods and Materials
Patients
All patients enrolled provided written informed consent for an institutional review board (IRB)–approved trial (ClinicalTrials.gov identifier: NCT02528942). The trial was open to accrual at University of Colorado and VA Eastern Colorado Health Care System (Aurora, CO, IRB #14-1856) and Beaumont Health System (Royal Oak, MI, IRB# 2016-037). The trial was designed as a phase 2 study with a primary endpoint of grade 2 or higher radiation pneumonitis to be compared against a historical control. For inclusion, patients were required to have pathologically confirmed non-small cell lung cancer or small cell lung cancer, were planned to receive chemotherapy and radiation therapy with a prescription dose of 45 to 66 Gy, and were 18 years of age or older. Patients treated with stereotactic body radiation therapy or patients treated with a prescription dose of less than 45 Gy were excluded. Chemotherapy and/or immunotherapy were given per standard of care. There were no limitations for baseline patient performance status or baseline PFTs. The trial included a 4DCT-ventilation image heterogeneity criterion that required both a quantitative 15% reduction in regional lung function near the tumor and a qualitative ventilation defect, which was scored as a binary “yes” or “no” by the treating radiation oncology team. The quantitative 15% heterogeneity criteria have been described previously and are based on nuclear medicine concepts where the regional function in each lung lobe is estimated using geometric approximations.23, 24, 25 The purpose of the image heterogeneity criterion was that for patients with homogeneous lung function, there are no regions to preferentially spare. For patients with heterogeneous lung function, there are regions more likely to be amenable to preferential functional avoidance. To be eligible for the current analysis, patients had to have completed PFTs both before and after chemoradiation.
Functional imaging and radiation treatment planning
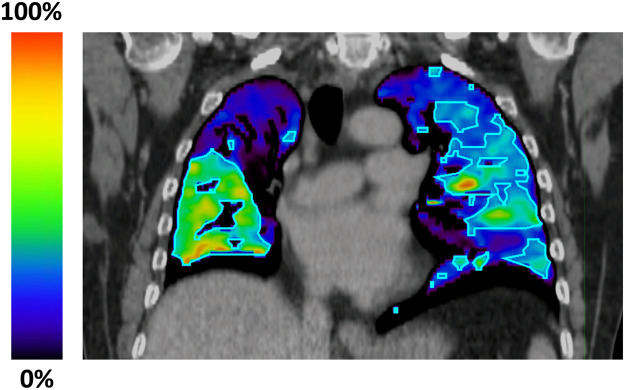
As part of the radiation treatment-planning process, all enrolled patients underwent standard 4DCT imaging using a gated lung CT protocol. Image processing techniques previously described were applied to the 4DCT to generate 4DCT-ventilation images.4,6,8,9,26,27 These processing techniques included contouring the lungs at the end of inspiration and the end of expiration, using deformable registration to link lung voxel elements in each phase of breathing, and applying a density-change–based equation to calculate ventilation in each voxel.6,27,28 A functional lung contour that represented higher-functioning portions of the lung was created to assist in treatment planning using a threshold of 15% or greater function on the 4DCT-ventilation image.24,29 An example of a 4DCT-ventilation image with a functional lung contour is shown in Fig. 1.
Figure 1.
An example of a 4-dimensional computed tomography (4DCT)–ventilation image with a functional lung contour for a patient enrolled on the trial. The bright colors in the 4DCT-ventilation image represent functional portions of the lung, whereas the darker colors represent ventilation defect regions. A functional lung contour (outlined in light blue) that represented higher-functioning portions of the lung was created to assist in treatment planning using a threshold of a 15% or greater function on the 4DCT-ventilation image. The purpose of functional avoidance radiation therapy is to preferentially spare the higher-functioning portions of the lung using treatment-planning techniques, which for this patient included portions of the right lower lobe and left lung.
The radiation oncologist delineated the gross tumor volume, planning target volume, and relevant organs at risk, including the lungs, spinal cord, heart, and esophagus.30 Planning was performed using intensity modulated radiation therapy techniques, and institutions followed Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC), RTOG 0617, NRG LU 001, and RTOG 0538 guidelines and dose constraints.30, 31, 32 The functional avoidance plan was generated by using favorable arc geometry to avoid the functional lung contour and by decreasing doses to the functional lung contour during intensity modulated radiation therapy optimization. Target coverage and standard organ-at-risk dose constraints were not sacrificed to reduce doses to the functional contour, thus ensuring the plans met standard lung cancer radiation therapy criteria.
Pulmonary function testing
The trial's primary outcome of a reduction in grade 2 or higher radiation pneumonitis was reported previously with results showing a 14.9% grade 2 or higher radiation pneumonitis rate for the study cohort, which met the phase 2 study criteria.15 As a secondary trial endpoint, pretreatment and posttreatment PFTs were collected. Patients underwent baseline PFT assessment before chemoradiation with functional avoidance planning and a planned PFT assessment 3 months after treatment. Standard PFT procedures were followed at the 2 enrollment centers and were supervised by the pulmonologists on the protocol. No bronchodilators were used for the PFT assessments. The PFT assessments included DLCO, FEV1, FVC, and FEV1/FVC ratio. The DLCO was recorded in milliliters per millimeter mercury per minute and as a percentage of normal standardized values (based on height, sex, and age).33 The FEV1 was recorded in liters and as a percentage of normal standardized values. The FVC was recorded in liters and as a percentage of normal standardized values. The FEV1/FVC ratio was recorded as the ratio of FEV1 to FVC.
PFT quantitative and qualitative analysis
The trial was designed as a phase 2 study with a secondary endpoint of PFT changes for patients treated with 4DCT-ventilation functional avoidance radiation therapy. The purpose of this study was therefore to quantitatively characterize the pretreatment PFT values, posttreatment PFT values, and pretreatment to posttreatment PFT changes. Pretreatment and posttreatment PFT metrics (mean, standard deviation, and range) are presented as both raw values and as percentages based on normal standardized values. The pretreatment to posttreatment PFT changes were calculated by subtracting the posttreatment raw and normal standardized values from the pretreatment raw and normal standardized values.34 The PFT change results are presented with descriptive statistics (mean, standard deviation, and range) and visually using histogram distribution plots.
Although the study was not powered to compare PFT differences in different lung cancer cohorts, it is instructive to qualitatively compare PFT changes with functional avoidance against PFT changes for patients with lung cancer treated with standard thoracic radiation planning. Two studies were used to characterize the historical rates of PFT changes for patients with lung cancer treated with standard thoracic radiation planning: a 2005 review article by Mehta,35 which included 10 studies, and a more recent meta-analysis in 2017, which included 8 studies.22 Mehta reported an FEV1 change from baseline ranging from –20% to 0.6% after chemoradiation for lung cancer.35 Furthermore, a DLCO change from baseline ranging from –27% to –3.5% was reported in the study. The 2017 meta-analysis, which included studies assessing the effects of chemoradiation on patients with lung cancer, showed a FEV1 change ranging from –24% to –1% and a DLCO change ranging from –26% to –10%.22 Results for FVC and FEV1/FVC were not reported in these 2 analyses. Combining the reported DLCO and FEV1 results from the individual studies included in the 2 reviews,22,35 we calculated a mean DLCO change of –15.7% and a mean FEV1 change of –6.4%.
Prior work has demonstrated that PFT metrics can predict severe radiation pneumonitis in patients receiving radiation therapy.36 To further assess the correlation between an objective measure, specifically PFTs, and a clinical outcome, specifically radiation pneumonitis, we evaluated PFT change differences between groups that did or did not experience grade 2 or higher radiation pneumonitis for patients treated with functional avoidance radiation therapy. PFT changes for patients who did or did not experience grade 2 or higher pneumonitis were compared using t test analysis with a significance level of .05.
Results
Patients and treatment
The trial was open to accrual between April 11, 2015, and December 13, 2019. A total of 101 patients were consented to accrue 67 evaluable patients. Of the 67 evaluable patients, 56 patients enrolled on the study had both baseline and posttreatment PFTs available for analysis. Patient, treatment, and clinical characteristics of the study population are provided in Table 1. The median age was 65 years (range, 45-86 years), 36 (64.3%) were female, and 20 (35.7%) were male. The median Karnofsky performance status score was 90 (range, 60-100), 29 (51.8%) had preexisting chronic obstructive pulmonary disease, and 53 (94.6%) were current or former smokers. Among those who currently or previously smoked, the median pack-year history was 37.5 years (range, 1-150 years). Forty-six patients (82.1%) were diagnosed with non-small cell lung cancer, and 43 (76.8%) had stage III disease. Seven patients (12.5%) underwent surgery as part of their treatment, including 6 who received a lobectomy and 1 who received a pneumonectomy. The median dose was 60 Gy (range, 45-66 Gy) delivered in 30 fractions (range, 23-33 fractions). Fifty patients (89.3%) received concurrent chemotherapy, and 17 (30.4%) were treated with immunotherapy. There were no patients on the study who had preexisting interstitial lung disease. With a median follow-up period of 10.6 months (range, 3.0-14.0 months) from the time of treatment, a total of 3 patients (5.4%) experienced regional tumor progression.
Table 1.
Patient, treatment, and clinical characteristics for the study cohort
| Parameter | Number (%) or median (range) |
|---|---|
| Patients | 56 |
| Sex | |
| Female | 36 (64.3) |
| Male | 20 (35.7) |
| Age, y | 65 (45-86) |
| Race | |
| White | 53 (94.6) |
| Black | 2 (3.6) |
| Asian-Pacific | 1 (1.8) |
| KPS index | 90 (60-100) |
| COPD | |
| Yes | 29 (51.8) |
| No | 27 (48.2) |
| Smoking status | |
| Nonsmoker | 3 (5.4) |
| Current smoker | 14 (25) |
| Former smoker | 39 (69.6) |
| Type of lung cancer | |
| NSCLC | 46 (82.1) |
| SCLC | 10 (17.9) |
| Stage | |
| I | 2 (3.6) |
| II | 5 (8.9) |
| III | 43 (76.8) |
| IV | 6 (10.7) |
| Surgery | |
| Yes | 7 (12.5) |
| Lobectomy | 6 (10.7) |
| Pneumonectomy | 1 (1.8) |
| No | 49 (87.5) |
| Chemotherapy | |
| Concurrent | 50 (89.3) |
| Sequential | 2 (3.6) |
| Induction | 4 (7.1) |
| Immunotherapy | |
| Yes | 17 (30.4) |
| No | 39 (69.6) |
| Radiation prescription | |
| Total dose, Gy | 60 (45-66) |
| Number of fractions | 30 (23-33) |
| Fractionation pattern | |
| Daily | 49 (87.5) |
| Twice daily | 7 (12.5) |
Abbreviations: COPD = chronic obstructive pulmonary disease; KPS = Karnofsky Performance Status; NSCLC = non-small cell lung cancer; SCLC = small cell lung cancer.
Pulmonary function testing
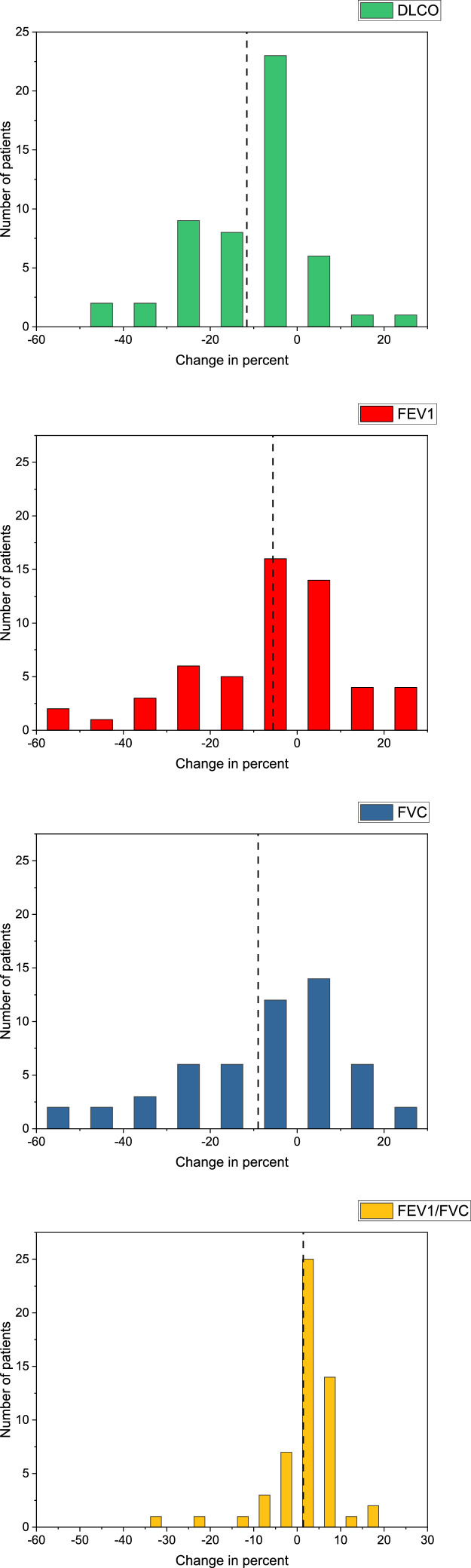
Pulmonary function tests were obtained at baseline and a planned 3 months (median, 3.1 months; range, 0.5-6.0 months) after radiation therapy. A total of 52 patients (92.9%) had PFTs performed 3 ± 1.5 months from the time of radiation therapy treatment completion. The mean, standard deviation, and range for DLCO, FEV1, FVC, and FEV1/FVC both pretreatment and posttreatment are shown in Table 2. The mean change in DLCO was –1.4 ± 5.1 mL/mm Hg/min (range, –14.6 to 14.5 mL/mm Hg/min) and –11.6% ± 14.2% of the predicted (range, –50.0% to 27.0%) (Table 3). The mean change in FEV1 was –0.2 ± 0.5 L (range, –2.1 to 1.3 L) and –5.6% ± 16.9% of the predicted (range, –59.0% to 24.0%). The mean change in FVC was –0.3 ± 0.6 L (range, –2.5 to 0.7 L) and –9.0% ± 20.1% of predicted (range, –61.0% to 27.0%). The mean change in FEV1/FVC was 1.7 ± 8.0 (range, –32.0 to 19.0). The PFT change results are presented visually, using histogram distribution plots, in Fig. 2.
Table 2.
Pretreatment and posttreatment PFT metrics for the study cohort
| PFT metric | Mean ± SD | Range |
|---|---|---|
| DLCO* | ||
| Pretreatment | ||
| Raw | 15.8 ± 6.0 | 2.8-30.1 |
| Predicted, %† | 64.8 ± 16.3 | 39.0-100.0 |
| Posttreatment | ||
| Raw | 14.3 ± 5.9 | 6.0-39.1 |
| Predicted, %† | 52.6 ± 15.6 | 27.0-95.0 |
| FEV1‡ | ||
| Pretreatment | ||
| Raw | 2.1 ± 0.7 | 1.1-3.7 |
| Predicted, %† | 76.0 ± 19.8 | 36.0-123.0 |
| Posttreatment | ||
| Raw | 1.9 ± 0.7 | 1.0-4.4 |
| Predicted, %† | 71.0 ± 15.7 | 31.0-106.0 |
| FVC‡ | ||
| Pretreatment | ||
| Raw | 3.2 ± 0.9 | 1.7-5.3 |
| Predicted, %† | 88.9 ± 15.8 | 58.0-131.0 |
| Posttreatment | ||
| Raw | 2.9 ± 1.0 | 1.4-5.7 |
| Predicted %† | 80.3 ± 17.1 | 45.0-118.0 |
| FEV1/FVC ratio | ||
| Pretreatment | 65.6 ± 12.9 | 41.0-100.0 |
| Posttreatment | 67.3 ± 11.6 | 41.0-89.0 |
Abbreviations: DLCO = diffusing capacity for carbon monoxide; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; PFT = pulmonary function test; SD = standard deviation.
DLCO raw values are recorded in milliliters per millimeter mercury per minute.
Predicted percentage takes into account patient height, sex, and age.
FEV1 and FVC raw values are recorded in liters.
Table 3.
Changes in PFT metrics following radiotherapy for the study cohort
| PFT metric | Mean ± SD | Range |
|---|---|---|
| DLCO change* | ||
| Raw | –1.4 ± 5.1 | –14.6 to 14.5 |
| Predicted, %† | –11.6 ± 14.2 | –50.0 to 27.0 |
| FEV1 change‡ | ||
| Raw | –0.2 ± 0.5 | –2.1 to 1.3 |
| Predicted, %† | –5.6 ± 16.9 | –59.0 to 24.0 |
| FVC change‡ | ||
| Raw | –0.3 ± 0.6 | –2.5 to 0.7 |
| Predicted, %† | –9.0 ± 20.1 | –61.0 to 27.0 |
| FEV1/FVC ratio change‡ | 1.7 ± 8.0 | –32.0 to 19.0 |
Abbreviations: DLCO = diffusing capacity for carbon monoxide; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; PFT = pulmonary function test; SD = standard deviation.
DLCO raw values are recorded in milliliters per millimeter mercury per minute.
Predicted percentage takes into account patient height, sex, and age; change metrics were calculated by subtracting the posttreatment raw and predicted percentage values from the pretreatment raw and predicted percentage values.
FEV1 and FVC raw values are recorded in liters.
Figure 2.
Histogram distribution plots for diffusing capacity for carbon monoxide (DLCO, in green), forced expiratory volume in 1 second (FEV1, in red), forced vital capacity (FVC, in blue), and the FEV1/FVC ratio (in yellow) change. The plots assess the percentage change for the separate pulmonary function test (PFT) metrics from pretreatment to posttreatment. Change metrics were calculated by subtracting the posttreatment percentage predicted values from the pretreatment percentage predicted values. The dashed line in each histogram represents the mean for the specific PFT metric.
A total of 10 patients in the cohort (17.9%) experienced grade 2 or higher radiation pneumonitis, at a median of 6.0 months (range, 0-7.0 months) after radiation treatment. For patients with grade 2 or higher radiation pneumonitis, the mean change in DLCO was –15.4% ± 14.4% (range, –45.0% to 4.0%), and for patients with grade <2 radiation pneumonitis, the mean change in DLCO was –10.8% ± 14.1% (range, –50.0% to 27.0%) (P = .37) (Table 4). For patients with grade 2 or higher radiation pneumonitis, the mean change in FEV1 was –14.3% ± 22.1% (range, –59.0% to 13.0%), and for patients with grade <2 radiation pneumonitis, the mean change in FEV1 was –3.9% ± 15.4% (range, –47.0% to 24.0%) (P = .09).
Table 4.
Pretreatment to posttreatment PFT change for patients with and without grade 2 or higher RP
| PFT metric | Mean ± SD | Range | P value |
|---|---|---|---|
| DLCO | |||
| Grade 2 or higher RP | –15.4 ± 14.0 | –45.0 to 4.0 | .37 |
| Less than grade 2 RP | –10.8 ± 14.1 | –50.0 to 27.0 | |
| FEV1 | |||
| Grade 2 or higher RP | –14.3 ± 22.1 | –59.0 to 13.0 | .09 |
| Less than grade 2 RP | –3.9 ± 15.4 | –47.0 to 24.0 |
Abbreviations: DLCO = diffusing capacity for carbon monoxide; FEV1 = forced expiratory volume in 1 second; PFT = pulmonary function test; SD = standard deviation; RP = radiation pneumonitis.
Numbers reported represent change metrics, which were calculated by subtracting the posttreatment predicted percentage values from the pretreatment predicted percentage values. P values were obtained using unpaired t test analysis with a significance level of .05.
Discussion
4DCT-ventilation functional avoidance is a promising technique to reduce the probability of patients developing pulmonary toxicity after radiation treatment without burdening the patient with an extra imaging procedure.15 To our knowledge, the presented work is the first study to characterize PFT changes for patients treated on a 2-institution, prospective functional avoidance clinical trial where PFT data collection was prospectively acquired. Our data show mean changes in DLCO, FEV1, and FVC of –11.6%, –5.6%, and –9.0%, respectively. Based on previous studies, 2 clinically pertinent PFT metrics relevant to patients with lung cancer treated with radiation therapy are pretreatment to posttreatment DLCO and FEV1 changes.22,35 Although a statistical analysis was not possible, our results can be qualitatively compared against PFT changes presented in the literature for patients treated with standard thoracic radiation therapy techniques. Averaging the results from 2 large review studies, we calculate a mean DLCO change of –15.7% and a mean FEV1 change of –6.4%.22,35 Compared with historical results, our data show less of a decline in DLCO (–11.6% with functional avoidance and –15.7% historically) and FEV1 (–5.6% with functional avoidance and –6.4% historically) for patients treated with functional avoidance.
Major determinants of gas exchange in the lung include diffusion of gases, measured by DLCO, as well as gas movement, measured by FEV1.19, 20, 21,35 Typically, DLCO is affected more by local lung dysfunction.35 Our results are in line with previous reviews showing a reduction in PFTs after treatment, with DLCO being most pronounced.22,35 By minimizing irradiation of functional lung, however, the decline in DLCO after treatment appeared to be less than historical rates of DLCO decline for patients treated with standard thoracic radiation therapy techniques. It is possible that with functional avoidance there is a reduction in dose to local pulmonary microvasculature, which functions in gas exchange. For example, even low radiation doses given to large lung volumes in rats can lead to microvascular damage.37 Reduction in FEV1, which reflects airflow obstruction rather than microvascular alteration, was only slightly improved with functional avoidance compared with historical rates.19, 20, 21, 22,35
Grading pneumonitis is commonly used as a marker for measuring lung toxic effects after radiation; however, there are shortcomings to a subjective grading approach.1,17,35 Pneumonitis is defined according to the presence or absence of symptoms such as cough and shortness of breath, radiographic changes, and the treatments required to manage symptoms.1,17 PFT testing can be a beneficial quantitative metric to follow once patients complete treatment for lung cancer, and several studies have attempted to use PFTs as a predictor for pneumonitis.22,35,38 To evaluate whether PFT changes correlated with clinical pneumonitis, our study compared mean PFT changes for patients with and without grade 2 or higher radiation pneumonitis. The mean change in DLCO was –15.4% ± 14.4% for patients with grade 2 or higher radiation pneumonitis and –10.8% ± 14.1% for patients with grade <2 radiation pneumonitis (P = .37). The mean change in FEV1 was –14.3% ± 22.1% for patients with grade 2 or higher radiation pneumonitis and –3.9% ± 15.4% for patients with grade <2 radiation pneumonitis (P = .09). These results align with previous work suggesting that PFT changes can potentially differentiate between patients with and without pneumonitis.22,35,38
With regard to limitations of the current study, the patient population under study was heterogeneous and included both patients with non-small cell lung cancer and small cell lung cancer and once-daily versus twice-daily radiation treatment regimens, and not all patients received immunotherapy after initial chemoradiation. There are several key differences between our study and the studies used for PFT comparison purposes. Many prior studies did not include chemotherapy with radiation, used 3-dimensional conformal therapy planning techniques, had performance status limitations, did not include patients who received immunotherapy, or prohibited surgery as part of the treatment assessment.22,35 Prescription doses used in the Mehta review35 included 45 to 76.9 Gy, and the follow-up was from 1.5 to 38 months. Prescription doses used in the 2017 meta-analysis22 included 54 to 77 Gy in 24 to 58 fractions, and the follow-up was from 1 to 12 months.
Intensity modulated radiation therapy, which was used in the functional avoidance study, has been shown to reduce pulmonary complications compared with 3-dimensional conformal therapy.39,40 The patient cohort used in the functional avoidance work included 17 patients (30%) who received immunotherapy per the results of the PACIFIC trial.41 Immunotherapy, when combined with radiation, has been shown to increase rates of lung toxicity.42,43 In addition, 7 patients (12.5%) underwent surgery as part of their treatment in the study. When patients who underwent surgery were excluded from the analysis, similar PFT changes were noted from pretreatment to posttreatment. Specifically, the mean DLCO change was –11.2% ± 13.6% (range, –45% to 27%), the mean FEV1 change was –5.8% ± 17.5% (range, –59% to 24%), and the mean FVC change was –8.9% ± 19.9% (range, –61% to 27%), thus demonstrating the results obtained were largely unaffected by resection status.
Pulmonary function tests were only assessed once after chemoradiation for patients treated with functional avoidance radiation therapy. It is possible that if further assessment was done after 3 months, continued PFT changes may have occurred, including either decline or improvement. Future studies examining PFT change should look to obtain serial measurements over time to better predict for late pulmonary toxic effects. Uncertainties of 4DCT-ventilation have been described previously and include potential registration inaccuracy and numerical instability.10,44,45 Although this study focused on 4DCT-based methods, it should be noted that other established forms of lung function imaging have been proposed for radiation therapy, including functional avoidance using single-photon emission computed tomography imaging, positron emission tomography–based functional imaging, and magnetic resonance imaging–based perfusion.12,46, 47, 48 Although the inclusion criteria in the study were broad and an appropriate historical control for PFT changes is challenging to determine, the presented PFT changes present seminal data that functional avoidance radiation therapy may mitigate decline in pulmonary function.
Conclusion
The present work quantitatively characterized PFT changes for 56 patients with lung cancer treated on a 2-institution, 4DCT-ventilation functional avoidance radiation therapy protocol. The study found a mean change in DLCO of –11.6% ± 14.2%, a mean change in FEV1 of –5.6% ± 16.9%, and a mean change in FVC of –9.0% ± 20.1%. Patients with grade 2 or higher radiation pneumonitis had a mean FEV1 decline of –14.3% ± 22.1%, whereas patients who did not experience grade 2 or higher radiation pneumonitis had a mean FEV1 decline of –3.9% ± 15.4%. The presented data elucidate the potential pulmonary function improvement with functional avoidance radiation therapy and help refine functional avoidance techniques to reduce toxicity for patients with lung cancer.
Footnotes
Sources of support: This research was funded by National Institutes of Health (NIH) grant R01CA200817 and received oversight support from University of Colorado Cancer Center grant P30CA046934. The 4-dimensional computed tomography ventilation method used in this study was developed under NIH grants R01CA236857, UG3CA247605, R21CA128230, DP2OD007044, K01CA181292, T32CA119930, and R25TCA90301.
Disclosures: Dr Yevgeniy Vinogradskiy, Dr Richard Castillo, and Dr Edward Castillo report a research agreement with MIM Software. No other disclosures were reported.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
ClinicalTrials.gov identifier: NCT02528942.
Contributor Information
Ryan Miller, Email: ryan.miller@jefferson.edu.
Yevgeniy Vinogradskiy, Email: yevgeniy.vinogradskiy@jefferson.edu.
References
- 1.Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76:S70–S76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinogradskiy Y, Schubert L, Diot Q, et al. Regional lung function profiles of stage I and III lung cancer patients: An evaluation for functional avoidance radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95:1273–1280. doi: 10.1016/j.ijrobp.2016.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marks LB, Spencer DP, Bentel GC, et al. The utility of SPECT lung perfusion scans in minimizing and assessing the physiologic consequences of thoracic irradiation. Int J Radiat Oncol Biol Phys. 1993;26:659–668. doi: 10.1016/0360-3016(93)90285-4. [DOI] [PubMed] [Google Scholar]
- 4.Vinogradskiy Y, Castillo R, Castillo E, et al. Use of 4-dimensional computed tomography-based ventilation imaging to correlate lung dose and function with clinical outcomes. Int J Radiat Oncol Biol Phys. 2013;86:366–371. doi: 10.1016/j.ijrobp.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN guidelines, Non-small cell lung cancer, version 3.2022. Available at: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed April 1, 2022.
- 6.Guerrero T, Sanders K, Castillo E, et al. Dynamic ventilation imaging from four-dimensional computed tomography. Phys Med Biol. 2006;51:777–791. doi: 10.1088/0031-9155/51/4/002. [DOI] [PubMed] [Google Scholar]
- 7.Fuld MK, Blaine Easley R, Saba OI, et al. CT-measured regional specific volume change reflects regional ventilation in supine sheep. J Appl Physiol. 2008;104:1177–1184. doi: 10.1152/japplphysiol.00212.2007. [DOI] [PubMed] [Google Scholar]
- 8.Vinogradskiy Y, Koo PJ, Castillo, et al. Comparison of 4-dimensional computed tomography ventilation with nuclear medicine ventilation-perfusion imaging: A clinical validation study. Int J Radiat Oncol Biol Phys. 2014;89:199–205. doi: 10.1016/j.ijrobp.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo R, Castillo E, McCurdy M, et al. Spatial correspondence of 4D CT ventilation and SPECT pulmonary perfusion defects in patients with malignant airway stenosis. Phys Med Biol. 2012;57:1855–1871. doi: 10.1088/0031-9155/57/7/1855. [DOI] [PubMed] [Google Scholar]
- 10.Kipritidis J, Tahir BA, Cazoulat G, et al. The VAMPIRE challenge: A multi-institutional validation study of CT ventilation imaging. Med Phys. 2019;46:1198–1217. doi: 10.1002/mp.13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathew L, Wheatley A, Castillo R, et al. Hyperpolarized (3)He magnetic resonance imaging: Comparison with four-dimensional x-ray computed tomography imaging in lung cancer. Acad Radiol. 2012;19:1546–1553. doi: 10.1016/j.acra.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Kipritidis J, Siva S, Hofman MS, Callahan J, Hicks RJ, Keall PJ. Validating and improving CT ventilation imaging by correlating with ventilation 4D-PET/CT using 68Ga-labeled nanoparticles. Med Phys. 2014;41 doi: 10.1118/1.4856055. [DOI] [PubMed] [Google Scholar]
- 13.Yaremko BP, Guerrero TM, Noyola-Martinez J, et al. Reduction of normal lung irradiation in locally advanced non-small-cell lung cancer patients, using ventilation images for functional avoidance. Int J Radiat Oncol Biol Phys. 2007;68:562–571. doi: 10.1016/j.ijrobp.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto T, Kabus S, von Berg J, Lorenz C, Keall PJ. Impact of four-dimensional computed tomography pulmonary ventilation imaging-based functional avoidance for lung cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:279–288. doi: 10.1016/j.ijrobp.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Vinogradskiy Y, Castillo R, Castillo E, et al. Results of a multi-institutional phase 2 clinical trial for 4DCT-ventilation functional avoidance thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2022;112(4):986–995. doi: 10.1016/j.ijrobp.2021.10.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) J Natl Cancer Inst. 2014;106:dju244. doi: 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faria SL, Aslani M, Tafazoli FS, Souhami L, Freeman CR. The challenge of scoring radiation-induced lung toxicity. Clin Oncol (R Coll Radiol) 2009;21:371–375. doi: 10.1016/j.clon.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Tucker SL, Jin H, Wei X, et al. Impact of toxicity grade and scoring system on the relationship between mean lung dose and risk of radiation pneumonitis in a large cohort of patients with non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2010;77:691–698. doi: 10.1016/j.ijrobp.2009.05.055. [DOI] [PubMed] [Google Scholar]
- 19.Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2:111–124. doi: 10.1081/copd-200053377. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Peng S, Wei L, Li Z. Relevance analysis of clinical and lung function parameters changing and prognosis of idiopathic pulmonary fibrosis. Int J Clin Exp Med. 2014;7:4759–4769. [PMC free article] [PubMed] [Google Scholar]
- 21.Weinreich UM, Thomsen LP, Brock C, Karbing DS, Rees SE. Diffusion capacity of the lung for carbon monoxide—A potential marker of impaired gas exchange or of systemic deconditioning in chronic obstructive lung disease? Chron Respir Dis. 2015;12:357–364. doi: 10.1177/1479972315601946. [DOI] [PubMed] [Google Scholar]
- 22.Niezink AGH, de Jong RA, Muijs CT, Langendijk JA, Widder J. Pulmonary function changes after radiotherapy for lung or esophageal cancer: A systematic review focusing on dose-volume parameters. Oncologist. 2017;22:1257–1264. doi: 10.1634/theoncologist.2016-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinogradskiy Y, Rusthoven CG, Schubert L, et al. Interim analysis of a two-institution, prospective clinical trial of 4DCT-ventilation-based functional avoidance radiation therapy. Int J Radiat Oncol Biol Phys. 2018;102:1357–1365. doi: 10.1016/j.ijrobp.2018.07.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waxweiler T, Schubert L, Diot Q, et al. A complete 4DCT-ventilation functional avoidance virtual trial: Developing strategies for prospective clinical trials. J Appl Clin Med Phys. 2017;18:144–152. doi: 10.1002/acm2.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker JA, Coleman RE, Grady E, et al. SNM practice guideline for lung scintigraphy 4.0. J Nucl Med Technol. 2012;40:57–65. doi: 10.2967/jnmt.111.101386. [DOI] [PubMed] [Google Scholar]
- 26.Guerrero T, Sanders K, Noyola-Martinez J, et al. Quantification of regional ventilation from treatment planning CT. Int J Radiat Oncol Biol Phys. 2005;62:630–634. doi: 10.1016/j.ijrobp.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Castillo R, Castillo E, Martinez J, Guerrero T. Ventilation from four-dimensional computed tomography: Density versus Jacobian methods. Phys Med Biol. 2010;55 doi: 10.1088/0031-9155/55/16/004. 4661-4485. [DOI] [PubMed] [Google Scholar]
- 28.Castillo E, Castillo R, White B, Rojo J, Guerrero T. Least median of squares filtering of locally optimal point matches for compressible flow image registration. Phys Med Biol. 2012;57:4827–4833. doi: 10.1088/0031-9155/57/15/4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faught AM, Yamamoto T, Castillo R, et al. Evaluating which dose-function metrics are most critical for functional-guided radiation therapy. Int J Radiat Oncol Biol Phys. 2017;99:202–209. doi: 10.1016/j.ijrobp.2017.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancers (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsakiridis T, Hu C, Skinner HD, et al. Initial reporting of NRG-LU001 ( NCT02186847), randomized phase II trial of concurrent chemoradiotherapy (CRT) +/- metformin in locally advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2019;37:8502. [Google Scholar]
- 33.Pellegrino R, Viegi G, Brusasco V, et al. Interpretive strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 34.Borst GR, De Jaeger K, Belderbos JSA, Burgers SA, Lebesque JV. Pulmonary function changes after radiotherapy in non-small-cell lung cancer patients with long-term disease-free survival. Int J Radiat Oncol Biol Phys. 2005;62:639–644. doi: 10.1016/j.ijrobp.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 35.Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: Pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys. 2005;63:5–24. doi: 10.1016/j.ijrobp.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 36.Robnett TJ, Machtay M, Vines EF, McKenna MG, Algazy KM, McKenna WG. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2000;48:89–94. doi: 10.1016/s0360-3016(00)00648-9. [DOI] [PubMed] [Google Scholar]
- 37.Ghobadi G, Bartelds B, van der Veen SJ, et al. Lung irradiation induces pulmonary vascular remodeling resembling pulmonary arterial hypertension. Thorax. 2012;67:334–341. doi: 10.1136/thoraxjnl-2011-200346. [DOI] [PubMed] [Google Scholar]
- 38.Ferro AC, Han R, Voong R, et al. Pulmonary function trends following modern radiation therapy: Association of CTC provider assessment with subacute and late PFT changes. Int J Radiat Oncol Biol Phys. 2018;102:e681–e682. [Google Scholar]
- 39.Yom SS, Liao Z, Helen Liu H, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:94–102. doi: 10.1016/j.ijrobp.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 40.Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: A secondary analysis of the NRG Oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35:56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 42.Nishino M, Sholl LM, Stephen Hodi F, Hatabu H, Ramaiya NH. Anti-PD-1-related pneumonitis during cancer immunotherapy. N Engl J Med. 2015;373:288–290. doi: 10.1056/NEJMc1505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durm GA, Jabbour SK, Althouse SK, et al. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: Hoosier Cancer Research Network LUN 14-179. Cancer. 2020;126:4353–4361. doi: 10.1002/cncr.33083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto T, Kabus S, von Berg J, Lorenz C, Loo BW, Keall PJ. 4D-CT pulmonary ventilation image-guided radiotherapy planning is significantly influenced by deformable image registration algorithms and metrics. Int J Radiat Oncol Biol Phys. 2010;78:S185. [Google Scholar]
- 45.Castillo E, Castillo R, Vinogradskiy Y, Guerrero T. The numerical stability of transformation-based CT ventilation. Int J Comput Assist Radiol Surg. 2017;12:569–580. doi: 10.1007/s11548-016-1509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee E, Zeng J, Miyaoka RS, et al. Functional lung avoidance and response-adaptive escalation (FLARE) RT: Multimodality plan dosimetry of a precision radiation oncology strategy. Med Phys. 2017;44:3418–3429. doi: 10.1002/mp.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siva S, Thomas R, Callahan J, et al. High-resolution pulmonary ventilation and perfusion PET/CT allows for functionally adapted intensity modulated radiotherapy in lung cancer. Radiother Oncol. 2015;115:157–162. doi: 10.1016/j.radonc.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 48.Kumar S, Liney G, Rai R, Holloway L, Moses D, Vinod SK. Magnetic resonance imaging in lung: A review of its potential for radiotherapy. Br J Radiol. 2016;89 doi: 10.1259/bjr.20150431. [DOI] [PMC free article] [PubMed] [Google Scholar]