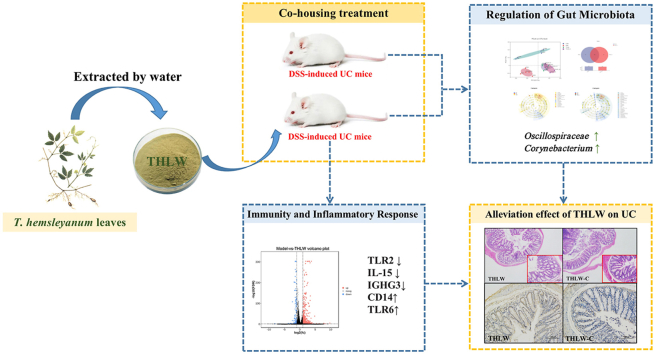
Abstract
Tetrastigma hemsleyanum, a traditional Chinese medicinal plant, possesses various biological activities, including anti-inflammatory and immunomodulatory functions. The purpose of this study was to determine the alleviating effect of the water extract of Tetrastigma hemsleyanum leaves (THLW) on ulcerative colitis (UC) and its relationship with gut microbiota. The administration of THLW significantly decreased the severity of dextran sulfate sodium (DSS)-induced intestinal damage, as demonstrated by the stabilization of body weight and colon length, and decreased disease activity index (DAI) and histological scores. THLW also decreased NF-κB protein expression in colon tissues and reduced the serum levels of IL-6, IL-1β, and TNF-α. Further co-housing experiment confirmed that the anti-UC effect of THLW was possibly by regulating the structure and composition of gut microbiota, including increasing the abundance of Oscillospiraceae, Prevotellaceae and Corynebacterium. Additionally, the expression of genes related to inflammation and immunity was also regulated by THLW treatment as evidenced by transcriptome analysis. These results suggested that the protective effect of THLW on DSS-induced colitis was mediated by alleviating inflammation and modulating the microbiota composition. This work proved the potent protective effects of THLW treatment on colitis and may have potential for UC relief.
Keywords: Water extract of Tetrastigma hemsleyanum leaves, Ulcerative colitis, Anti-inflammatory activity, Gut microbiota, Transcriptome
Graphical abstract
Highlights
-
•
THLW exerted remarkable protective effects on colitis in mice.
-
•
The protective effects of THLW on colitis is related to gut microbiota.
-
•
The anti-UC effect of THLW was associate with regulation of inflammation and immunity pathways.
-
•
THLW treatment have potential for UC relief.
Abbreviations
- CD14
Clusters of differentiation 14
- DAI
Disease activity index
- DEGs
Differentially expressed genes
- DSS
Dextran sulfate sodium
- ELISA
Enzyme-linked immunosorbent assay
- FC
Fold change
- FDR
False discovery rate
- H&E
Hematoxylin-eosin
- IL-6
Interleukin-6
- IL-1β
Interleukin-1β
- IGHG3
Immunoglobulin heavy constant gamma 3
- IBD
Inflammatory bowel disease
- LDA
Linear discriminant analysis
- LEfSe
Linear discriminant analysis effect size
- OTU
Operational taxonomic unit
- PCA
Principal component analysis
- PCoA
Principal coordinate analysis
- PICRUSt
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States
- TNF-α
Tumor necrosis factor-α
- TLR
Toll-like receptor
- THLW
Water extract of Tetrastigma hemsleyanum leaves
- UC
Ulcerative colitis
1. Introduction
Ulcerative colitis (UC) is a relapsing and reoccurring inflammatory bowel disease (IBD) that is associated with various factors, such as micro-environmental factors, genetic susceptibility, abnormal immune responses, and disruption of colon barrier function (Kruidenier et al., 2003). As gut microbiota homeostasis is a key factor in maintaining optimal gastrointestinal health, thus gut microbiota imbalance plays a vital role in the pathogenesis of UC (Gong et al., 2022). According to multiple studies, plant-based foods or traditional Chinese herbs have a curative effect on UC, for instance, modulating gut microbiota (Pan et al., 2020; Sun et al., 2020; Liu et al., 2021).
Tetrastigma hemsleyanum Diels et Gilg. (T. hemsleyanum), a family member of Vitaceae and Tetrastigma obtectum Genus, is a well-known rare folk medicinal plant and is mostly known as “San ye qing” in China. It is widely distributed in Zhejiang, Guangxi, Jiangxi and Hunan provinces (Guo et al., 2019). T. hemsleyanum is known worldwide as a source of phytotherapeutics, and has been used for the treatment of conditions related to inflammatory and immune responses (Ji et al., 2021). Furthermore, whole plants, including tubers, leaves and vines, can be used as medicines and possess various bioactivities, such as anti-tumor (Zhu et al., 2020), anti-inflammatory (Wu et al., 2018; Li et al., 2019; Chu et al., 2019), positive immunomodulatory effects (Ogunrinola et al., 2022), anti-bacterial (Chen et al., 2019) and anti-oxidative stress (Ru et al., 2019) etc. However, due to the lack of systematic research on T. hemsleyanum, the above-ground parts were often discarded, resulting in a low utilization rate of T. hemsleyanum resources. In the recent years, increasing attention has been given to the studies on the above-ground parts of T. hemsleyanum and their biological activities. As a plant with homology of medicine and food, the leaves of T. hemsleyanum have been used as functional tea or dietary supplements for their health benefits, such as improving immune regulation (Sun et al., 2013; Van der Goten et al.). Additionally, the decoction of traditional Chinese medicine using water was widely used in traditional folk medicine, but the anti-UC effect of THLW and its regulatory effect on gut microbiota have not been explored. Thus, the present study focused on the therapeutic effect of THLW on a dextran sulfate sodium (DSS)-induced UC model in BALB/c mice and its relationship with gut microbiota.
2. Materials and methods
2.1. Materials and reagents
T. hemsleyanum leaves were purchased from Ningbo Shengwang Biotechnology Co., Ltd. (Ningbo, China). Dextran sulfate sodium (DSS; MW: 36–50 kDa) was purchased from MP Biochemical (Santa Ana, CA, USA). All enzyme-linked immunosorbent assay (ELISA) kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All other chemicals were of analytical grade.
2.2. Plant material and extract preparation
The THLW was prepared according to our previous study with slight modification (Ru et al., 2019). The powder was ground with a grinder and extracted with distilled water at a ratio of 1:30 (W/W) at 40 °C for 5 h in a BRANSON M8800-C ultrasonic cleaner (Dongguan Binengxin Machinery Co., Ltd., Guangdong, China). The supernatant was collected by centrifugation at 10000 rpm for 10 min and then dried using a freeze dryer (Songyuan, China) and stored at −80 °C until further analysis.
2.3. Experimental ulcerative colitis and treatment
Six-week-old male BALB/c mice were purchased from Hangzhou Hangsi Biological Technology Co., Ltd. (Hangzhou China). The mice were housed in individually ventilated caging systems under a 12-h light/dark cycle at a temperature of 23 °C ± 2 °C and humidity of 55% ± 5%, and allowed free access to sterilized standard rodent chow food and sterilized water. The animal experiment was performed in compliance with the Chinese legislation on the use and care of laboratory animals and was approved by the ethics committee of Zhejiang Pharmaceutical College (NO. ZYLL201905010).
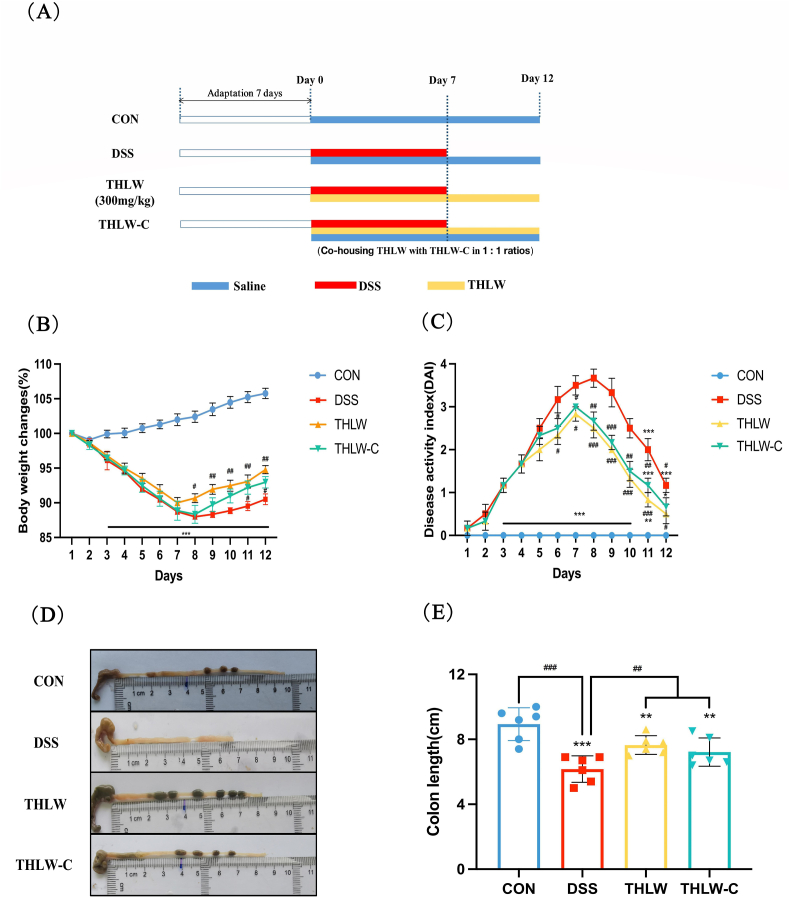
After adaptive feeding for 7 days, the mice were randomly assigned to four groups (n = 9 for each group): normal control group (the CON group), DSS-induced UC model group (the DSS group), water extract of T. hemsleyanum leaves treated UC group (the THLW group) and the co-housed THLW-treated UC group (the THLW-C group). For the co-housing analysis, pairs of age-matched THLW and THLW-C group mice were co-housed in 1:1 ratios in single cages. The mice in all groups except for the CON group were given drinking water with 3% (w/v in distilled water) DSS to induce colitis for 7 days, and then normal drinking water for 6 days of recovery. Mice in the THLW group were gavaged with THLW (dissolved in saline) at 300 mg per kg·BW per day for 12 consecutive days (Fig. 1A). Meanwhile, the mice received an equivalent amount of saline by gavage over the entire experimental period in the control group and DSS group, and all of the mice had a standard diet. Body weight, stool consistency, mouse state and the presence of gross blood in feces and at the anus were recorded daily and used for the disease activity index (Xie et al., 2019).
Fig. 1.
Water extract of Tetrastigma hemsleyanum leaves (THLW) attenuates DSS-induced acute colitis. (A) Experimental design. (B) Body weight changes after DSS induction of colitis. Data are plotted as a percentage of basal body weight. (C) The DAI was calculated during the experiment. (D) Representative images of colon tissues resected on the day of sacrifice. (E) Quantitative results of colon length in different groups. Results are mean ± S.E.M. of six to eight mice in each group. *P < 0.05, **P < 0.01 and ***P < 0.001 versus normal; #P < 0.05, ##P < 0.01 and ###P < 0.001 versus DSS model.
After feces and blood collection, the mice were sacrificed by cervical dislocation and the colons were immediately removed to measure the colon length. The colon was then preserved for histological examination and RNA extraction.
2.4. Histopathological and immunohistochemistry analyses
The colon tissue was fixed in 4% paraformaldehyde for 48 h, then embedded in paraffin, cut into slices, and stained with hematoxylin-eosin (H&E) solution for microscopic observation. The histological score was calculated according to previously reported methods, and the degree of injury was scored according to the degree of inflammatory cell infiltration, mucosal injury and crypt injury (He et al., 2021).
The expression of NF-κB protein in colon tissue was determined by immunohistochemical staining as described previously (Wu et al., 2020). Briefly, the colon sections were treated with 3% hydrogen peroxidase for 10 min and then incubated with NF-κBp65 primary antibody (1:100) overnight at 4 °C, followed by incubation with a secondary antibody at room temperature for 30 min. Finally, the images were observed under an Olympus BH-2 microscope (Tokyo, Japan). Positive staining was brown, and counterstained nuclei were blue. The average optical density of NF-κB (P-p65) translocation in colon tissues was quantified as a percentage of positive areas in each image using Image J software.
2.5. Measurement of inflammatory cytokines
The levels of TNF-α, IL-1β, and IL-6 in serum were detected using enzyme-linked immunosorbent assay (ELISA) kits (Nanjing, China). All procedures followed the manufacturer's instructions.
2.6. Analysis of the gut microbiota
Genomic DNA was extracted from fecal samples using the TIANamp Stool DNA Kit (Tiangen, Beijing, China). After determining the purity of DNA by 1% agarose gel electrophoresis, the MiSeq platform (IIIumina, San Diego, CA, USA) was used to sequence the V3–V4 hypervariable regions of the 16S rRNA gene at Shanghai MajorBio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
QIIME (version 1.9.1) was used to calculate the abundance of operational taxonomic units (OTUs) (based on 97% sequence similarity). These OTUs were imported into taxonomies analysis by RDP algorithm software, and the confidence threshold was set to 0.7. The α-diversity estimator calculations were performed using Mothur (version 1.30.2). Principal coordinate analysis (PCoA) and hierarchical clustering analysis were conducted using the representative sequences of OTUs based on the Bray-Curtis distance. Venn diagram and hierarchical clustering heatmap were conducted using R software (version 3.2.3). Furthermore, the linear discriminant analysis (LDA) effect size (LEfSe) algorithm was used to distinguish the key OTUs of the differential representation among the experimental groups of mice. Additionally, the potential Kyoto Encyclopedia of Genes and Genomes (KEGG) Ortholog functional profiles of microbial communities were predicted with PICRUSt using STAMP (version 2.1.3). All bioinformatics analyses were performed using Majorbio Cloud (http://www.majorbio.com/).
2.7. Transcriptome analysis
Total RNA was extracted from colonic tissues using a TRIzol reagent kit (Invitrogen, Carlsbad, CA, USA). An Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) was used to assess RNA quality and quantity. Then, Oligobads enriched eukaryotic mRNA, which was further fragmented using fragmentation buffer and reversly transcribed into cDNA by the NEBNext Ultra RNA Library Prep Kit for Illumina (NEB #7530, New England Biolabs, Ipswich, MA, USA). The purified double-stranded cDNA fragments were end repaired, and A base were added and ligated to Illumina sequencing adapters. The resulting cDNA library was sequenced using Illumina/Novaseq-6000 by Gene Denovo Biotechnology Co. (Guangzhou, China). Differentially expressed genes (DEGs) with P-value below 0.05 and a fold change of 2.0 were used for transcript analysis, and the KEGG database was used for pathway enrichment analysis.
2.8. Statistical analysis
Data are expressed as the mean ± ± S.E.M. All graphing and statistical analyses were performed using Prism GraphPad software (Version 8.4.0, USA). The multiple-group were compared using one-way ANOVA analysis of variance followed by the least significant difference (LSD) post hoc test, and P < 0.05 was considered statistically significant.
3. Results
3.1. THLW ameliorated DSS-induced acute colitis
The body weights of rats in the CON, DSS, THLW and THLW-C groups are presented in Fig. 1B. Compared with the CON group, the other groups showed significant weight loss on day 3. Moreover, significant differences in body weight appeared in the THLW and THLW-C groups on the 8th and 11th days respectively, compared to the DSS group. Similarly, different degrees of enteritis symptoms resulted in different DAI scores in each group (Fig. 1C). Mice in each group did not show any symptoms in the first two days, but diarrhea and loose feces appeared on day 3, especially in the DSS group. The DAI scores increased significantly under DSS treatment, but a significant decrease appeared in the THLW and THLW-C groups compared with the model group on the 6th day. Additionally, the colon length of THLW-treated and THLW-cohoused colitis mice exhibited less reduction than the DSS group mice (Fig. 1D and E).
3.2. THLW suppressed histological damage of colon tissues and pro-inflammatory cytokine secretion
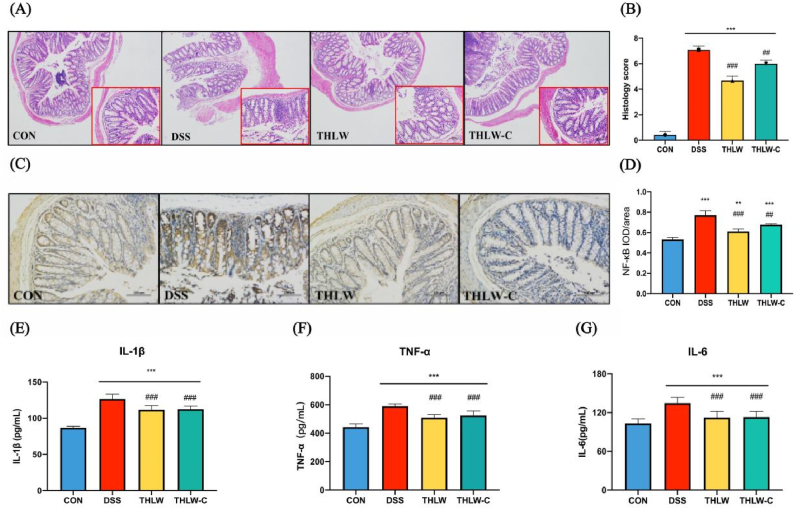
The therapeutic effects of THLW were evaluated at the histological level, as shown in Fig. 2A. Compared with the CON group, the epithelial integrity of the colonic mucosa in the DSS group disappeared, the crypt structures were damaged, and the mucosa and submucosa were infiltrated by a large number of immune cells, resulting in a higher histological score (Fig. 2B). Both THLW and THLW-C could effectively protect the integrity of the crypt, as the histological scores of the colon tissue were significantly lower than those of the DSS group (P < 0.05), but there was no significant difference between these two groups.
Fig. 2.
Bioactive influence of water extract of Tetrastigma hemsleyanum leaves (THLW) on histopathological changes and inflammation in colon adjacent tissues from DSS-induced UC mice: (A) Representative histological photos of each group ( × 50 and × 200). (B) Comparison of histopathologic scores between groups. (C) Immunohistochemical staining of the NF-κB (P-p65) translocation in colon tissues. (D) The average optical density (AOD) of NF-κB (P-p65) translocation in colon tissues. (AOD = IOD/Area), IOD: Integratedoption density. (E) Comparison of the levels of IL-1β in serum between groups. (F) Comparison of the levels of TNF-α in serum between groups. (G) Comparison of the levels of IL-6 in serum between groups. Results are mean ± S.E.M. of six to eight mice in each group. *P < 0.05, **P < 0.01 and ***P < 0.001versus normal; #P < 0.05, ##P < 0.01 and ###P < 0.001 versus DSS model.
To further verify the protective effects of THLW against intestinal inflammation, P-p65 (a subunit of NF-κB) protein expression in colon tissues was detected by immunohistochemistry staining. Immunohistochemical analysis showed that the expression of P-p65 was increased in the DSS group but significantly decreased in both the THLW and THLW-C groups (Fig. 2C and D). Moreover, the levels of several typical pro-inflammatory cytokines (including IL-6, IL-1β, and TNF-α) in serum were also detected and exhibited a significant increase after DSS treatment. Both the THLW and THLW-C groups decreased these three pro-inflammatory cytokines to varying degrees, and the effect of THLW was better than that of the THLW-C group (Fig. 2E–G).
3.3. Gut microbiota analysis
3.3.1. Analysis of the gut microbiota diversity
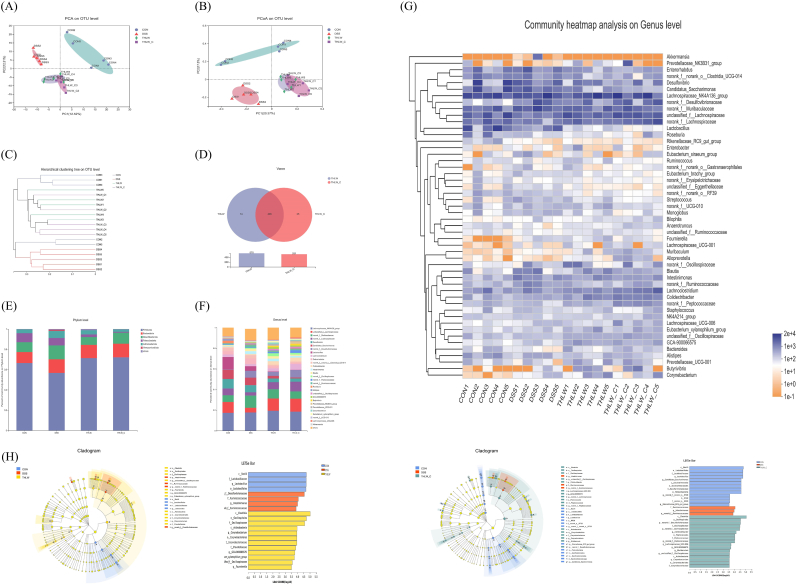
The Illumina MiSeq high-throughput sequencing platform was used to analyze the fecal microbes of mice in different groups. In total, 1282,201 sequences of the V3–V4 region of the 16S rRNA were detected from 20 fecal samples. The average sequence number was 64,110, with a minimum of 56,351 and a maximum of 71,618, while the average length was 412 bp. After removal of the low-quality reads, a total of 889,338 OTUs at a 97% similarity level were identified. The α-indices at the operational taxonomic unit (OTU) level of each sample are shown in Table 1. The THLW group showed the highest Shannon index, representing the characterization of community diversity. The THLW-C group showed the lowest Chao1 and ACE indices, indicating the lowest richness of the gut microbiota among all groups. In the β-diversity analysis, multivariate statistical methods such as principal component analysis (PCA) and principal coordinate analysis (PCoA) were used to analyze the similarity of community composition of the different groups (Fig. 3A and B). The loading plots of various colors indicate different groups and are divided into different zones according to the composition of the community. Altogether, the results showed that the composition of the intestinal flora changed significantly after DSS and THLW treatment, while samples in the THLW and THLW-C groups were more similar. Consistently, the results of the hierarchical clustering tree showed that the OTU levels of the THLW and THLW-C groups were similar and closest to each other (Fig. 3C). To further investigate the high similarity in composition between the THLW and THLW-C groups, a Venn diagram showed that 496 OTUs were identified as core microbiota in the two groups, accounting for approximately 85% of each compartment (Fig. 3D).
Table 1.
Total number of sequences and alpha-diversity indices (at the operational taxonomic unit level) of cecal microbiota.
| Group | Coverage | Shannon | Simpson | Ace | Chao |
|---|---|---|---|---|---|
| CON | 1.00 ± 0.00 | 3.94 ± 0.20ab | 0.06 ± 0.02 | 449.43 ± 6.58ab | 452.68 ± 6.48ab |
| DSS | 1.00 ± 0.00 | 3.87 ± 0.13a | 0.06 ± 0.01 | 478.47 ± 5.82b | 479.61 ± 6.41b |
| THLW | 1.00 ± 0.00 | 4.41 ± 0.08b | 0.03 ± 0.00 | 451.88 ± 13.34b | 469.01 ± 16.82b |
| THLW_C | 1.00 ± 0.00 | 4.17 ± 0.08ab | 0.04 ± 0.01 | 409.01 ± 12.96a | 414.13 ± 15.72a |
Note: a–d Different letters mean significant difference (P < 0.05). Data are expressed as mean ± S.E.M. (n = 6). The abbreviation of groups used in the figure were: normal control group (CON), DSS-induced UC model group (DSS), water extract of Tetrastigma hemsleyanum leaves treated UC group (THLW) and the co-housed THLW-treated UC group (THLW-C).
Fig. 3.
Gut microbiota analysis upon THLW treatment in colitis mice. (A) Principal component analysis (PCA). (B) Principal coordinate analysis (PCoA). (C) Hierarchical clustering tree. (D) Venn diagram of differentially abundant bacteria at the OTU level. (E) Community column diagram at the phylum level. (F) Community column diagram at the genus level. (G) Community heatmap at the genus level. (H and I) Bacterial taxa differentially enriched in different groups determined using the linear discriminant analysis (LDA) effect size (LEfSe) algorithm.
3.3.2. Composition and structure analysis of the fecal gut microbiota
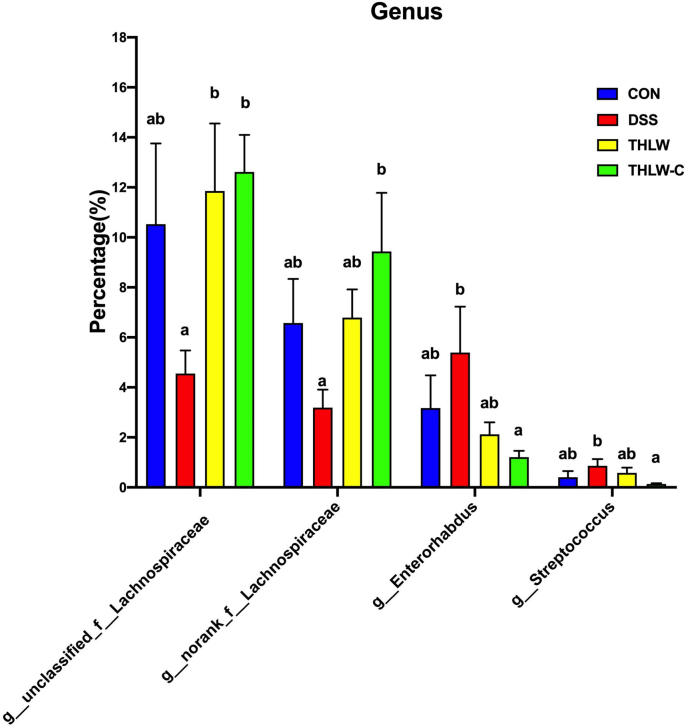
The relative abundance of gut microbiota in each group at the phylum and genus levels is shown in Fig. 3 E and F. At the phylum level, the gut microbiota of each sample was mainly composed of 6 primary bacterial phyla, of which Firmicutes and Bacteroidetes were the two most important phyla. The relative abundance of Firmicutes was decreased after DSS treatment, but recovered to a level similar to that of the CON group after THLW treatment. In addition, the abundance of Verrucomicrobiota in the model group was remarkably higher but decreased after THLW treatment. At the genus level, the DSS treatment significantly inhibited the growth of unclassified_f__Lachnospiraceae (P < 0.05) and remarkably promoted the growth of genera Enterorhabdus and Streptococcus (P < 0.05) compared with the normal group. After THLW treatment, the imbalance of gut microbiota caused by DSS treatment was recovered by preventing the decrease of unclassified_f__Lachnospiraceae (P < 0.05) as well the increase of Enterorhabdus (P < 0.05) and Streptococcus (P < 0.05), as shown in Supplementary Figure 1. These results suggested that THLW could restore the intestinal flora balance in UC mice positively.
The heatmap can distinguish groups with different abundances by clustering and reflect differences in microbial composition in colitis mouse fecal samples with different treatments by color gradient. As shown in Fig. 3G, the heatmap was drawn by clustering analysis of the 50 most abundant bacteria at the genus level. THLW partially inhibited DSS-induced enhancement of bacterial genus, including norank_f__Ruminococcaceae, norank_f__Desulfovibrionaceae, Monoglobus, Akkermansia, and Streptococcus, which were reduced to a level closer to that of the control group.
3.3.3. Analysis of gut microbiota composition and metabolic function changes
The linear discriminant analysis effect size (LEfSe) can be used to identify statistically dominant microorganisms in each group. As shown in Fig. 3H, the CON, DSS and THLW groups had 4, 4 and 12 significantly different taxa at different classification levels (LDA score >3.5), respectively. Among them, c__Bacilli/o__Lactobacillales/f__Lactobacillaceae/g__Lactobacillus were the dominant taxa in the CON group, g__norank_f__Desulfovibrionaceae and f__Ruminococcaceae/g__Intestinimonas were the dominant taxa in the DSS group, and c__Actinobacteria/o__Corynebacteriales/f__Corynebacteriaceae/g__Corynebacterium, c__Clostridia/o__Oscillospirales/f__Oscillospiraceae/g__unclassified_f__Oscillospiraceae and f__Prevotellaceae were the dominant microbes in the THLW group. The THLW-C group showed similar dominant taxa, such as c__Clostridia/o__Oscillospirales/f__Oscillospiraceae/g__unclassified_f__Oscillospiraceae and c__Actinobacteria/o__Corynebacteriales/f__Corynebacteriaceae/g__Corynebacterium (Fig. 3I).
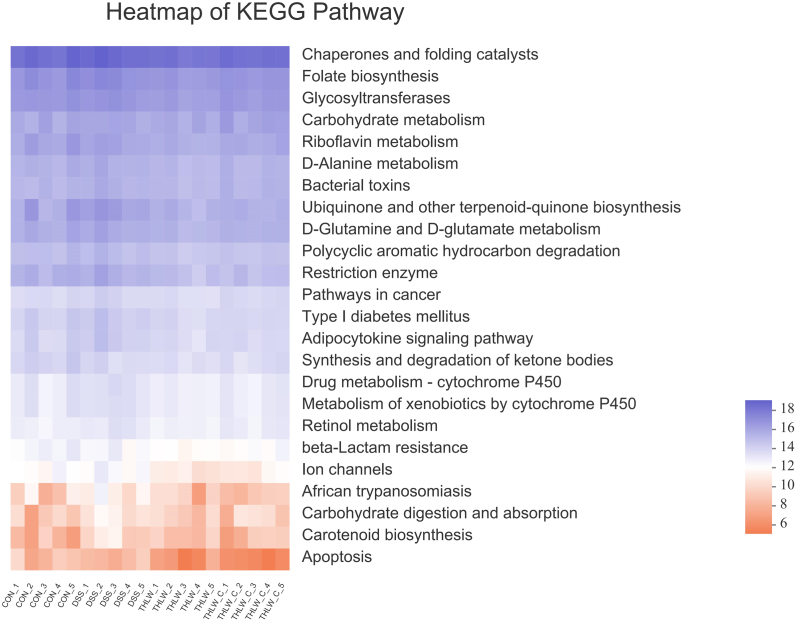
The PICRUSt algorithm was next used to evaluate the functional changes after different treatments and then performed against KEGG to aggregate into different pathways, as shown in Supplementary Table 1. It can be concluded that there were significant differences between the DSS group and the THLW group in 24 KEGG pathways, and the addition of THLW regulated the activities of carbohydrate metabolism, bacterial toxins, apoptosis and so on, providing guidance for our further research (Supplementary Figure 2). In conclusion, these results indicate that THLW regulates the structure, composition and functionality of the gut microbiota in DSS-treated rats, and stabilizes the structure of the gut microbiota similar to that in normal mice, which is beneficial to maintaining health.
3.4. The gene expression profile of the small intestine
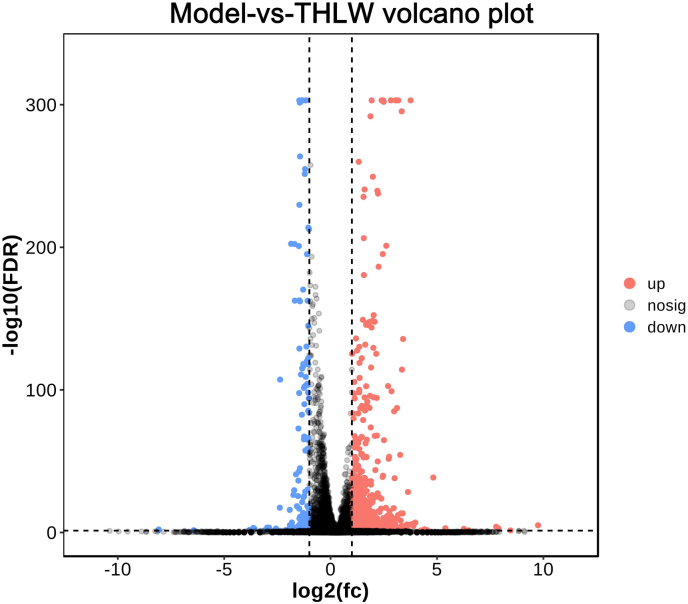
In this study, to determine the anti-colitis effect of THLW, we performed mRNA sequencing analyses using total RNA isolated from small intestine of mice. By analysis of the transcriptome, a total of 621 genes were differentially expressed, including 501 up-regulated genes and 120 down-regulated genes (FDR <0.05 and |log2FC| > 1) (Supplementary Figure 3). Detailed information of each differentially expressed genes (DEGs) are listed in Supplementary Table 2. These 621 genes were enriched in 316 KEGG pathways (Supplementary Table 3). Among them, 7 pathways related to inflammation were screened out and are listed in Table 2. In total, 26 DEGs were screened out from these 7 KEGG pathways, among which 18 were up-regulated and 8 were down-regulated (Table 3). The mRNA-seq analysis revealed that THLW treatment increased the gene expressions (Cp, Atp7a, Sparc, Mmp14, Creb3l3, Loxl2, Gatm, and Il15) which are associated with TNF signaling pathway. Furthermore, THLW supplementation altered NF-kappa B signaling pathway related transcripts such as Ighg1, Cd14, Tlr6, Igha, Ighg3 and Car1. These results support that treatment with THLW leads to the enhancement of immunity and reduction of the inflammatory response in DSS-induced mice (Fig. 2).
Table 2.
Information on KEGG pathways related to inflammation.
| Pathway | Pathway ID | Level 1 | Level 2 |
|---|---|---|---|
| IL-17 signaling pathway | ko04657 | Organismal Systems | Immune system |
| TNF signaling pathway | ko04668 | Environmental Information Processing | Signal transduction |
| Toll-like receptor signaling pathway | ko04620 | Organismal Systems | Immune system |
| Inflammatiory bowel disease (IBD) | ko05321 | Human Diseases | Immune diseases |
| NF-kappa B signaling pathway | ko04064 | Environmental Information Processing | Signal transduction |
| Jak-STAT signaling pathway | ko04630 | Environmental Information Processing | Signal transduction |
| T cell receptor signaling pathway | ko04660 | Organismal Systems | Immune system |
Table 3.
Information on regulated differentially expressed genes enriched in inflammation-related KEGG pathways.
| Symbol | Regulation | logFC(THLW/Model) | P Value | FDR | Description | KEGG Pathway |
|---|---|---|---|---|---|---|
| Ighg1 | Down | −1.397182648 | 4.15E-12 | 5.1E-11 | immunoglobulin heavy constant gamma 1 (G1m marker) | ko04064 |
| Car1 | Down | −1.30525813 | 0 | 0 | carbonic anhydrase 1 | ko04064 |
| H2-Eb2 | Down | −1.260062839 | 2.25E-05 | 0.000131689 | histocompatibility 2, class II antigen E beta2 | ko05321 |
| Il22ra2 | Down | −1.108616562 | 3.76E-11 | 4.23E-10 | interleukin 22 receptor, alpha 2 | ko04630 |
| Ighg3 | Down | −1.097243862 | 0.000698432 | 0.003086817 | Immunoglobulin heavy constant gamma 3 | ko04064 |
| Tlr2 | Down | −1.055200874 | 4.06E-125 | 8.18E-123 | toll-like receptor 2 | ko04620; ko05321 |
| Il15 | Down | −1.040448477 | 8.87E-16 | 1.44E-14 | interleukin 15 | ko04668; ko04630 |
| Tnfrsf13c | Down | −1.026043755 | 1.74E-09 | 1.69E-08 | tumor necrosis factor receptor superfamily, member 13c | ko04064; ko04668 |
| Cp | Up | 1.001700289 | 0.000440321 | 0.002025819 | ceruloplasmin | ko04668 |
| Tlr6 | Up | 1.016273522 | 0.002880844 | 0.010969225 | toll-like receptor 6 | ko04620 |
| Tirap | Up | 1.023014566 | 0.002484111 | 0.009619046 | toll-interleukin 1 receptor (TIR) domain-containing adaptor protein | ko04064; ko04620 |
| Dcn | Up | 1.060599264 | 5.85E-30 | 1.95E-28 | decorin | ko04660 |
| Atp7a | Up | 1.095895427 | 2.08E-10 | 2.18E-09 | ATPase, Cu++ transporting, alpha polypeptide | ko04668 |
| Nfatc2 | Up | 1.105035471 | 3.22E-06 | 2.14E-05 | nuclear factor of activated T cells, cytoplasmic, calcineurin dependent 2 | ko04660 |
| Sparc | Up | 1.15146355 | 5.38E-42 | 2.64E-40 | secreted acidic cysteine rich glycoprotein | ko04668 |
| Pla2g4a | Up | 1.268510823 | 5.91E-39 | 2.63E-37 | phospholipase A2, group IVA (cytosolic, calcium-dependent) | ko04620 |
| Cd14 | Up | 1.337139537 | 7.17E-47 | 3.83E-45 | CD14 antigen | ko04064; ko04620 |
| Igha | Up | 1.344523391 | 3.55E-101 | 4.96E-99 | immunoglobulin heavy constant alpha | ko04064 |
| Mmp14 | Up | 1.393287075 | 6.91E-33 | 2.59E-31 | matrix metallopeptidase 14 (membrane-inserted) | ko04668 |
| Chek1 | Up | 1.401545263 | 0.003013397 | 0.011417562 | checkpoint kinase 1 | ko04660 |
| Smpd3 | Up | 1.470146501 | 1.27E-91 | 1.48E-89 | sphingomyelin phosphodiesterase 3, neutral | ko04620 |
| Creb3l3 | Up | 1.488590367 | 5.43E-11 | 6.04E-10 | cAMP responsive element binding protein 3-like 3 | ko04668 |
| Creb3l1 | Up | 1.527901269 | 1.39E-81 | 1.42E-79 | cAMP responsive element binding protein 3-like 1 | ko04668 |
| Loxl2 | Up | 1.604275976 | 5.31E-25 | 1.45E-23 | lysyl oxidase-like 2 | ko04668 |
| Gatm | Up | 1.956441251 | 1.87E-28 | 5.95E-27 | glycine amidinotransferase | ko04668 |
| Pla2g4f | Up | 2.443638412 | 2.41E-41 | 1.16E-39 | phospholipase A2, group IVF | ko04620 |
4. Discussion
UC is a typical type of inflammatory bowel disease (IBD) that is often caused by a combination of genetic predisposition, immune dysfunction and gut microbiota disturbance (J. Hu et al., 2020). Among them, there is increasing evidence that the gut microbiota and the intestinal immune system play a key role in this disease(Yang et al., 2020). Compared with the disadvantages of UC treatment drugs with side effects, increasing attention has been given to the treatment of UC with Chinese herbal medicine, which has been widely used for a long time in many Asian countries and can be used as an alternative for UC patients due to its safe therapeutic effect (Guo et al., 2017). T. hemsleyanum is a medicinal and edible plant that has existed in China since ancient times, and has attracted increasing attention due to its effective effects on inflammatory and immune-reactivity related diseases (Ji et al., 2019, 2021). Our latest study showed that an 80% methanol extract of T. hemsleyanum can effectively inhibit DSS-induced colitis by maintaining the intestinal epithelial barrier and regulating gut microbiota disturbance (Wu et al., 2021). Nevertheless, the beneficial effects of Chinese herbal plants are usually achieved after prolonged extraction in water, while the role of gut microbiota in the THLW-relieved colitis induced by DSS was not fully explained. Therefore, the present study focused on the alleviation effect of THLW on UC that induced by continuous intake of DSS solution, and confirmed the key role of gut microbiota in THLW-relieved colitis by co-housing with the THLW group, further suggesting that intestinal microorganisms may be involved in the regulation of UC inflammation and the immune response.
In this study, administration of THLW alleviated UC caused by DSS, as THLW mitigated the obvious symptoms such as weight loss, diarrhea and hematochezia, colon shortening, and intestinal mucosal and submucosal edema in the DSS-induced UC model. In addition, THLW treatment also reduced inflammatory cell infiltration, crypt loss and epithelial damage resulting from DSS challenge in mice with colitis, thus restoring their protective barrier function. The colonic mucosa of UC is infiltrated by neutrophils and macrophages, which can secrete a large number of inflammatory cytokines, including IL-6, IL-1β and TNF-α etc., all of which are regulated by the NF-κB signaling pathway (Schottelius and Dinter, 2006; Muzes et al., 2012; Feng et al., 2014; Chen et al., 2020). Therefore, controlling the production and release of these inflammatory factors is conducive to the treatment and therapy of colitis. In our study, immunohistochemical analysis showed that THLW treatment effectively decreased the protein expression of the NF-κB subunit (phosphorylated p65) in the colon of DSS-treated mice, accompanied by decreased IL-1β, IL-6, and TNF-α levels in serum. Morever, the chemical profile analysis of THLW by UPLC in our previous study showed that the most abundant component was β-sitosterol, which has been reported to attenuate colitis by inhibiting the NF-κB pathway (Lou et al., 2021; Lee et al., 2012; Feng et al., 2017). These results suggested that the ameliorative effect of THLW on DSS-induced colitis may be closely related to the inhibition of pro-inflammatory cytokines release involving NF-κB.
Trillions of bacteria colonize in the gastrointestinal tract and are involved in nutrient absorption and immune system regulation (Wang et al., 2020). Changes in the diversity and structure of the gut microbiota, result in inflammatory responses and immune imbalance (Prasain and Barnes, 2020). Therefore, the gut microbiota is closely associated with the pathology development and therapy of UC (Sokol et al., 2017). DSS modeling, commonly used for colitis, often leads to loss of microbial diversity and even imbalance of gut microbiota structure (Sun et al., 2020; X.J. Hu et al., 2020; Xu et al., 2020). Consistent with previous studies, the DSS group in this study also showed dysbiosis of gut microbiota, including Firmicutes reduction and Bacteroidetes increase, accompanied by loss of microbiota diversity. THLW had a positive regulatory effect on the gut microbiota of mice with colitis induced by DSS, which proved that the THLW group was markably increased in the abundance of Oscillospiraceae, which contributed to the increase of Clostridia class. It has been proven that Oscillospiraceae can contribute to the production of valeric acid, which is positively correlated with the anti-inflammatory response(Maya-Lucas et al., 2019). Another dominant family response to THLW treatment is a strictly anaerobic bacterium, Prevotellaceae, which has been reported to be highly expressed in the healthy gut by 16S rRNA gene pyrosequencing (Suchodolski et al., 2012), which is in accordance with our study. Furthermore, the increased abundance of Prevotellaceae and Corynebacterium, which are negatively correlated with intestinal permeability and inflammation (Monk et al., 2016; Mailing et al., 2018), in THLW-treated mice also supports the potential of THLW to reduce intestinal inflammation. In addition, the community composition and dominant microbes (Oscillospiraceae and Corynebacterium) of the co-housing group were similar to those of the THLW group, which confirmed that THLW exerted pharmacological activity on DSS-induced acute UC in mice, and the possible mechanism may be related to the improvement of intestinal function by balancing the micro-environment of the gut microbiota.
The immune system is also a key interface between microorganisms and the inflammatory response, because it can quickly recognize and respond to pathogenic microorganisms. Toll-like receptors (TLRs) are one of the most important pathogen pattern recognition receptors for microorganism-derived pathogenic molecules(Aderem and Ulevitch, 2000; Akira and Sato, 2003). However, pathogenic bacteria in the gut can be identified by TLR, which stimulates the inflammatory response of the intestinal mucosa and improves the development of UC. Clusters of differentiation 14 (CD14) is a glycoprotein expressed on the surfaces of monocytes and macrophages that acts as a pattern recognition receptor and contributes to TLR-induced cell activation (Antal-Szalmas, 2000). Both TLRs and CD14 are important pattern recognition molecules in the immune system. Previously, several studies have reported TLR/CD14 gene polymorphisms involved in the pathogenetic mechanisms of UC (Obana et al., 2001; Gazouli et al., 2005; Wang et al., 2007; Frolova et al., 2008), but there are no consistent results. Based on our results of transcriptional analysis, THLW has been found to alter the expression of the genes involved in inflammation and immune response compared with the DSS-induced colitis model (Van der Goten et al., 2014). Among the seven selected KEGG pathways related to be inflammation or immunity, THLW down-regulated TLR2, which plays important roles in the process of UC (Toiyama et al., 2006; Le et al., 2012; Fan and Liu, 2015). Moreover, TLR6 was up-regulated after THLW treatment, which is consistent with the report of Pierik et al. (2006), who reported that TLR2 and TLR6 genes were positively and negatively correlated with colitis, respectively, suggesting that TLR2 and its co-receptor TLR6 are involved in the immune response to pathogens during IBD development. NF-κB, as an inducible transcription factor downstream of the TLR signaling pathway, plays an important role in the regulation of inflammation in cooperation with TLRs genes. Growing evidence suggests that inhibition of the TLR2/NF-κB signaling pathway can alleviate intestinal inflammation and contribute to the treatment of colitis (Yu et al., 2011; Wang et al., 2015; Zhang et al., 2020). IL-15 is another important mediator of immune responses in the intestine and plays a pivotal role in the pathogenesis of IBD (Sakai et al., 1998; Liu et al., 2000). While immunoglobulin heavy constant gamma 3 (IGHG3) is also commonly highly expressed in UC (Lawrance et al., 2001), the downregulation of these genes by THLW may also alleviate colitis.
5. Conclusions
Taken together, the present study demonstrated that THLW treatment can effectively improve DSS-induced colon injury and reduce intestinal inflammation by regulating the structure and composition of gut microbiot, as well as enhancement of immunity and reduction of the inflammatory response, thereby alleviating colitis. Thus, THLW has been shown to have the potential to relieve colitis and may be used as a beneficial dietary supplement in patients with intestinal dysfunction or colitis, but the optimal dose and effective components still need further research.
CRediT authorship contribution statement
Jing Wang: and, Conceptualization, Validation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Project administration, Funding acquisition, All authors have read and agreed to the published version of the manuscript. Wen Cao: Conceptualization, Methodology, All authors have read and agreed to the published version of the manuscript. Tao Ji: Methodology, All authors have read and agreed to the published version of the manuscript. Minjie Zhao: and, Formal analysis, Writing – original draft, Investigation, All authors have read and agreed to the published version of the manuscript. Tao Liu: Validation, Investigation, Visualization. Junhao Wu: Investigation, Data curation, All authors have read and agreed to the published version of the manuscript. Fengqin Feng: Writing – review & editing, All authors have read and agreed to the published version of the manuscript. Aicun Zhou: Supervision, All authors have read and agreed to the published version of the manuscript. Xin Peng: and, Supervision, Funding acquisition, All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research study was supported by the Ningbo Public Welfare Project (2021S029), and Opening Project of Zhejiang Provincial Key Laboratory of Resources Protection and Innovation of Traditional Chinese Medicine (2021E10013).
Handling Editor: Dr. Quancai Sun
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2022.100426.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
The information of gut microbiota functions predicted by PICRUSt which aggregated into different pathways
The detailed information of all differentially expressed genes (DEGs) between DSS and THLW groups screened out in this study
The information of all KEGG pathways in which the differentially expressed genes (DEGs) enriched in this study
Supplementary Fig. 1.
Changes in the composition of gut microbiota at the genus level. Data are expressed as the mean ± S.E.M. (n = 5). Statistical significance was determined using one-way ANOVA. Different letters at each day indicate significant differences at the P < 0.05 level using Duncan's multiple range test.
Supplementary Fig. 2.
PICRUSt prediction of the significantly different metagenomic function against KEGG database.
Supplementary Fig. 3.
Volcano charts of gene regulations by comparison between DSS and THLW groups.
References
- Aderem A., Ulevitch R.J. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406(6797):782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Akira S., Sato S. Toll-like receptors and their signaling mechanisms. Scand. J. Infect. Dis. 2003;35(9):555–562. doi: 10.1080/00365540310015683. [DOI] [PubMed] [Google Scholar]
- Antal-Szalmas P. Evaluation of CD14 in host defence. Eur. J. Clin. Invest. 2000;30(2):167–179. doi: 10.1046/j.1365-2362.2000.00610.x. [DOI] [PubMed] [Google Scholar]
- Chen L., Yao M., Fan X., Lin X., Xiao J.B. Dihydromyricetin attenuates streptozotocin-induced liver injury and inflammation in rats via regulation of NF-κB and AMPK signaling pathway. eFood. 2020;1(2):188–195. doi: 10.2991/efood.k.200207.001. [DOI] [Google Scholar]
- Chen X., Tao L., Ru Y., Weng S., Qiu B. Antibacterial mechanism of Tetrastigma hemsleyanum Diels et Gilg's polysaccharides by metabolomics based on HPLC/MS. Int. J. Biol. Macromol. 2019;140:206–215. doi: 10.1016/j.ijbiomac.2019.08.097. [DOI] [PubMed] [Google Scholar]
- Chu Q., Jia R.Y., Chen M., Li Y.L., Yu X., Wang Y.X., Chen W., Ye X., Liu Y.Y., Jiang Y., Zheng X.D. Tetrastigma hemsleyanum tubers polysaccharide ameliorates LPS-induced inflammation in macrophages and Caenorhabditis elegans. Int. J. Biol. Macromol. 2019;141:611–621. doi: 10.1016/j.ijbiomac.2019.09.039. [DOI] [PubMed] [Google Scholar]
- Fan Y., Liu B. Expression of Toll-like receptors in the mucosa of patients with ulcerative colitis. Exp. Ther. Med. 2015;9(4):1455–1459. doi: 10.3892/etm.2015.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Guo C., Zhu Y., Pang L., Yang Z., Zou Y., Zheng X. Baicalin down regulates the expression of TLR4 and NF-kB-p65 in colon tissue in mice with colitis induced by dextran sulfate sodium. Int. J. Clin. Exp. Med. 2014;7(11):4063–4072. [PMC free article] [PubMed] [Google Scholar]
- Feng S., Dai Z., Liu A., Wang H., Chen J., Luo Z., Yang C.S. β-Sitosterol and stigmasterol ameliorate dextran sulfate sodium-induced colitis in mice fed a high fat Western-style diet. Food Funct. 2017;8(11):4179–4186. doi: 10.1039/c7fo00375g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolova L., Drastich P., Rossmann P., Klimesova K., Tlaskalova-Hogenova H. Expression of Toll-like receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients with inflammatory bowel diseases: upregulated expression of TLR2 in terminal ileum of patients with ulcerative colitis. J. Histochem. Cytochem. 2008;56(3):267–274. doi: 10.1369/jhc.7A7303.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazouli M., Mantzaris G., Kotsinas A., Zacharatos P., Papalambros E., Archimandritis A., Ikonomopoulos J., Gorgoulis V.G. Association between polymorphisms in the Toll-like receptor 4, CD14, and CARD15/NOD2 and inflammatory bowel disease in the Greek population. World J. Gastroenterol. 2005;11(5):681–685. doi: 10.3748/wjg.v11.i5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L., Hu L., Wang H., Chen R., Wang J. Protective effect of feruloylated oligosaccharides on dextran sulfate sodium-induced ulcerative colitis in rats. Food Frontiers. 2022 doi: 10.1002/fft2.140. [DOI] [Google Scholar]
- Guo B.J., Bian Z.X., Qiu H.C., Wang Y.T., Wang Y. Biological and clinical implications of herbal medicine and natural products for the treatment of inflammatory bowel disease. Ann. N. Y. Acad. Sci. 2017;1401(1):37–48. doi: 10.1111/nyas.13414. [DOI] [PubMed] [Google Scholar]
- Guo W., Yang Z., Hou Z., Hou Z., Qi Z., Sun Y., Ding X., Ma K., Hu S., Hu J. A comprehensive review of a Chinese folk herbal species Tetrastigmae hemsleyanum with multiplicity of pharmacological effects. Chin. Tradi. Med. J. 2019;|1(2):1–19. [Google Scholar]
- He X.L., Liu J.Y., Long G.H., Xia X.H., Liu M. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside attenuates ischemia/reperfusion-induced brain injury in rats by promoting angiogenesis. Biomed. Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111420. [DOI] [Google Scholar]
- Hu J., Huang H., Che Y., Ding C., Cao L. Qingchang Huashi Formula attenuates DSS-induced colitis in mice by restoring gut microbiota-metabolism homeostasis and goblet cell function. J. Ethnopharmacol. 2020;266(6) doi: 10.1016/j.jep.2020.113394. [DOI] [PubMed] [Google Scholar]
- Hu X.J., Xu N., Yang X., Hu X., Zheng Y.L., Zhang Q. Nigella A ameliorates inflammation and intestinal flora imbalance in DSS induced colitis mice. Amb. Express. 2020;10(1):179. doi: 10.1186/s13568-020-01114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji T., Ji W.W., Wang J., Chen H.J., Peng X., Cheng K.J., Qiu D., Yang W.J. A comprehensive review on traditional uses, chemical compositions, pharmacology properties and toxicology of Tetrastigma hemsleyanum. J. Ethnopharmacol. 2021;264 doi: 10.1016/j.jep.2020.113247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W.W., Peng X., Lou T., Wang J., Qiu W. Total flavonoids from Tetrastigma hemsleyanum ameliorates inflammatory stress in concanavalin A-induced autoimmune hepatitis mice by regulating Treg/Th17 immune homeostasis. Inflammopharmacology. 2019;27(12):1297–1307. doi: 10.1007/s10787-019-00599-0. [DOI] [PubMed] [Google Scholar]
- Kruidenier L., Kulper I., Lamers C., Verspaget H.W. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J. Pathol. 2003;201(1):28–36. doi: 10.1002/path.1409. [DOI] [PubMed] [Google Scholar]
- Lawrance I.C., Fiocchi C., Chakravarti S. Ulcerative colitis and Crohn's disease: distinctive gene expression profiles and navel susceptibility candidate genes. Hum. Mol. Genet. 2001;10(5):445–456. doi: 10.1093/hmg/10.5.445. [DOI] [PubMed] [Google Scholar]
- Le D., Li J., Liu Y., Yue W.X., Luo X.T. Toll-like receptor 2 monoclonal antibody or/and Toll-like receptor 4 monoclonal antibody increase counts of Lactobacilli and Bifidobacteria in dextran sulfate sodium-induced colitis in mice. J. Gastroenterol. Hepatol. 2012;27(1):110–119. doi: 10.1111/j.1440-1746.2011.06839.x. [DOI] [PubMed] [Google Scholar]
- Lee I.A., Kim E.J., Kim D.H. Inhibitory effect of β-sitosterol on tnbs-induced colitis in mice. Planta Med. 2012;78(9):896–898. doi: 10.1055/s-0031-1298486. [DOI] [PubMed] [Google Scholar]
- Li Y., Chu Q., Liu Y., Ye X., Jiang Y., Zheng X. Radix Tetrastigma flavonoid ameliorates inflammation and prolongs the lifespan of Caenorhabditis elegans through JNK, p38 and Nrf2 pathways. Free Radic. Res. 2019;53(5):562–573. doi: 10.1080/10715762.2019.1613534. [DOI] [PubMed] [Google Scholar]
- Liu Z.J., Geboes K., Colpaert S., D'Haens G.R., Rutgeerts P., Ceuppens J.L. IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J. Immunol. 2000;164(7):3608–3615. doi: 10.4049/jimmunol.164.7.3608. [DOI] [PubMed] [Google Scholar]
- Liu H.F., Liang J.X., Xiao G.S., Ma L.K., Wang Q. Dendrobine suppresses lipopolysaccharide-induced gut inflammation in a co-culture of intestinal epithelial Caco-2 cells and RAW264.7 macrophages. eFood. 2021;2(2):92–99. doi: 10.2991/efood.k.210409.001. 2666-3066. [DOI] [Google Scholar]
- Lou T.L., Ji T., Peng X., Ji W.W., Yuan L.X., Wang J., Li S.M., Zhang S., Shi Q.Y. Extract from tetrastigma hemsleyanum leaf alleviates pseudomonas aeruginosa lung infection: network pharmacology analysis and experimental evidence. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.587850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailing L.J., Allen J.M., Pence B.D., Rytych J., Sun Y., Bhattacharya T.K., Park P., Cross T., Mccusker R.H., Swanson K.S. Behavioral response to fiber feeding is cohort-dependent and associated with gut microbiota composition in mice. Behav. Brain Res. 2018;359 doi: 10.1016/j.bbr.2018.09.012. [DOI] [PubMed] [Google Scholar]
- Maya-Lucas O., Murugesan S., Nirmalkar K., David Alcaraz L., Hoyo-Vadillo C., Luisa Pizano-Zarate M., Garcia-Mena J. The gut microbiome of mexican children affected by obesity. Anaerobe. 2019;55:11–23. doi: 10.1016/j.anaerobe.2018.10.009. [DOI] [PubMed] [Google Scholar]
- Monk J.M., Lepp D., Zhang C.P., Wu W., Zarepoor L., Lu J.T., Pauls K.P., Tsao R., Wood G.A., Robinson L.E., Power K.A. Diets enriched with cranberry beans alter the microbiota and mitigate colitis severity and associated inflammation. JNB (J. Nutr. Biochem.) 2016;28:129–139. doi: 10.1016/j.jnutbio.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Muzes G., Molnar B., Tulassay Z., Sipos F. Changes of the cytokine profile in inflammatory bowel diseases. World J. Gastroenterol. 2012;18(41):5848–5861. doi: 10.3748/wjg.v18.i41.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obana N., Takahashi S., Kinouchi Y., Negoro K., Takagi S., Hiwatashi N., Shimosegawa T. Ulcerative colitis is associated with a promoter polymorphism of lipopolysaccharide receptor gene, CD14. Gastroenterology. 2001;120(5) doi: 10.1080/00365520212504. A458-A458. [DOI] [PubMed] [Google Scholar]
- Ogunrinola O.O., Kanmodi R.I., Ogunrinola O.A. Medicinal plants as immune booster in the palliative management of viral diseases: a perspective on coronavirus. Food Frontiers. 2022;3(1):83–95. doi: 10.1002/fft2.107. [DOI] [Google Scholar]
- Pan G., Liu B., Li S., Han M., Gao L., Xu G., Du Q., Xie L. Kuijieling, a Chinese medicine alleviates DSS-induced colitis in C57BL/6Jmouse by improving the diversity and function of gut microbiota. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 2020;367(13) doi: 10.1093/femsle/fnaa082. [DOI] [PubMed] [Google Scholar]
- Pierik M., Joossens S., Van Steen K., Van Schuerbeek N., Vlietinck R., Rutgeerts P., Vermeire S. Toll-like receptor-1,-2, and-6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflamm. Bowel Dis. 2006;12(1):1–8. doi: 10.1097/01.MIB.0000195389.11645.ab. [DOI] [PubMed] [Google Scholar]
- Prasain J.K., Barnes S. Cranberry polyphenols-gut microbiota interactions and potential health benefits: an updated review. Food Frontiers. 2020;1(4):459–464. doi: 10.1002/fft2.56. [DOI] [Google Scholar]
- Ru Y., Chen X., Wang J., Guo L., Lin Z., Peng X., Qiu B. Polysaccharides from Tetrastigma hemsleyanum Diels et Gilg: extraction optimization, structural characterizations, antioxidant and antihyperlipidemic activities in hyperlipidemic mice. Int. J. Biol. Macromol. 2019;125:1033–1041. doi: 10.1016/j.ijbiomac.2018.11.236. [DOI] [PubMed] [Google Scholar]
- Sakai T., Kusugami K., Nishimura H., Ando T., Yamaguchi T., Ohsuga M., Ina K., Enomoto A., Kimura Y., Yoshikai Y. Interleukin 15 activity in the rectal mucose of inflammatory bowel disease. Gastroenterology. 1998;114(6):1237–1243. doi: 10.1016/s0016-5085(98)70430-5. [DOI] [PubMed] [Google Scholar]
- Schottelius A.J., Dinter H. Cytokines, NF-κB, microenvironment, intestinal inflammation and cancer. Cancer Treat Res. 2006;130(130):67–87. doi: 10.1007/0-387-26283-0_3. [DOI] [PubMed] [Google Scholar]
- Sokol H., Leducq V., Aschard H., Hang-Phuong P., Jegou S., Landman C., Cohen D., Liguori G., Bourrier A., Nion-Larmurier I., Cosnes J., Seksik P., Langella P., Skurnik D., Richard M.L., Beaugerie L. Fungal microbiota dysbiosis in IBD. Gut. 2017;66(6):1039–1048. doi: 10.1136/gutjnl-2015-310746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchodolski J.S., Dowd S.E., Wilke V., Steiner J.M., Jergens A.E. 16S-rRNA gene pyrosequencing reveals bacterial dysbiosis in the duodenum of dogs with idiopathic inflammatory bowel disease. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0039333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Chen H., Kan J., Gou Y., Jin C. Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int. J. Biol. Macromol. 2020;153:708–722. doi: 10.1016/j.ijbiomac.2020.03.053. [DOI] [PubMed] [Google Scholar]
- Sun Y., Li H., Hu J., Li J., Fan Y.W., R Liu X., Deng Z.Y. Qualitative and quantitative analysis of phenolics in Tetrastigma hemsleyanum and their antioxidant and antiproliferative activities. J. Agric. Food Chem. 2013;61(44):10507–10515. doi: 10.1021/jf4037547. [DOI] [PubMed] [Google Scholar]
- Toiyama Y., Araki T.I., Yoshiyama S., Hiro J., Miki C., Kusunoki M. The expression patterns of toll-like receptors in the heal pouch mucosa of postoperative ulcerative colitis patients. Surg. Today. 2006;36(3):287–290. doi: 10.1007/s00595-005-3144-y. [DOI] [PubMed] [Google Scholar]
- Van der Goten J., Vanhove W., Lemaire K., Van Lommel L., Machiels K., Wollants W.-J., De Preter V., De Hertogh G., Ferrante M., Van Assche G., Rutgeerts P., Schuit F., Vermeire S., Arijs I. Integrated mRNA and mRNA expression profiling in inflamed colon of patients with ulcerative colitis. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0116117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Tahara T., Arisawa T., Shibata T., Nakamura M., Fujita H., Iwata M., Kamiya Y., Nagasaka M., Takahama K., Watanabe M., Hirata I., Nakano H. Genetic polymorphisms of CD14 and toll-like receptor-2 (TLR2) in patients with ulcerative colitis. J. Gastroenterol. Hepatol. 2007;22(6):925–929. doi: 10.1111/j.1440-1746.2007.04909.x. [DOI] [PubMed] [Google Scholar]
- Wang L.S., Mo Y.Y., Huang Y.W., Echeveste C.E., Wang H.-T., Chen J., Oshima K., Yearsley M., Simal-Gandaraf J., Battino M., Xiao J., Chen J., Sun C., Yu J., Bai W.B. Effects of dietary interventions on gut microbiota in humans and the possible impacts of foods on patients' responses to cancer immunotherapy. eFood. 2020;1(4):279–287. doi: 10.2991/efood.k.200824.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Liu Y., Dong H., Wu L., Feng X., Zhou Z., Zhao C., Liu H., Wu H. Herb-partitioned moxibustion regulates the TLR2/NF-kappa B signaling pathway in a rat model of ulcerative colitis. Evid. base Compl. Alternative Med. 2015 doi: 10.1155/2015/949065. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Na Y., Zhang Y., Ye Y., Wang F. Radix Tetrastigma hemsleyani flavone exhibits antitumor activity in colorectal cancer via Wnt/β-catenin signaling pathway. OncoTargets Ther. 2018;11:6437–6446. doi: 10.2147/OTT.S172048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Wang X.Y., Xiong X., Deng Z.Y., Peng X., H Xiao L., Jiang L., Sun Y. Bioactives and their metabolites from Tetrastigma hemsleyanum leaves ameliorate DSS-induced colitis via protecting the intestinal barrier, mitigating oxidative stress and regulating the gut microbiota. Food Funct. 2021;12(23):11760–11776. doi: 10.1039/d1fo02588k. [DOI] [PubMed] [Google Scholar]
- Wu Z.C., Zhao Z.L., Deng J.P., Huang J.T., Wang Z.P. Sanhuang Shu'ai decoction alleviates DSS-induced ulcerative colitis via regulation of gut microbiota, inflammatory mediators and cytokines. Biomed. Pharmacother. 2020;125 doi: 10.1016/j.biopha.2020.109934. [DOI] [PubMed] [Google Scholar]
- Xu Z., Chen W., Deng Q., Huang Q., Wang X., Yang C., Huang F. Flaxseed oligosaccharides alleviate DSS-induced colitis through modulation of gut microbiota and repair of the intestinal barrier in mice. Food Funct. 2020;11:8077. doi: 10.1039/D0FO01105C. [DOI] [PubMed] [Google Scholar]
- Xie J.L., Liu Y., Chen B., Zhang G., Ou S., Luo J., Peng X. Ganoderma lucidum polysaccharide improves rat DSS-induced colitis by altering cecal microbiota and gene expression of colonic epithelial cells. Food Nutr. Res. 2019;63 doi: 10.29219/fnr.v63.1559. 1559-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M.M., Yan T., Yu M., Kang J., Gao R.X., Wang P., Zhang Y.H., Zhang H.F., Shi L. Advances in understanding of health-promoting benefits of medicine and food homology using analysis of gut microbiota and metabolomics. Food Frontiers. 2020;1(4):398–419. doi: 10.1002/fft2.49. [DOI] [Google Scholar]
- Yu C., Shan T., Feng A., Li Y., Zhu W., Xie Y., Li N., Li J. Triptolide ameliorates Crohn's colitis is associated with inhibition of TLRs/NF-kappa B signaling pathway. Fitoterapia. 2011;82(4):709–715. doi: 10.1016/j.fitote.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Han D., Yu S., An C., Liu X., Zhong H., Xu Y., Jiang L., Wang Z. Protective effect of Iridoid glycosides of the leaves of Syringa oblata lindl. on dextran sulfate sodium-induced ulcerative colitis by inhibition of the TLR2/4/MyD88/NF-kappa B signaling pathway. BioMed Res. Int. 2020 doi: 10.1155/2020/7650123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R., Xu X., Ying J., Cao G., Wu X. The phytochemistry, pharmacology, and quality control of Tetrastigma hemsleyanum Diels & Gilg in China: a review. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.550497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The information of gut microbiota functions predicted by PICRUSt which aggregated into different pathways
The detailed information of all differentially expressed genes (DEGs) between DSS and THLW groups screened out in this study
The information of all KEGG pathways in which the differentially expressed genes (DEGs) enriched in this study