Summary
Background
Broad direct-acting antiviral (DAA) access may reduce hepatitis C virus (HCV) incidence through a “treatment as prevention” (TasP) effect. We assessed changes in primary HCV incidence following DAA access among people living with HIV (PLHIV).
Methods
We used pooled individual-level data from six cohorts from the International Collaboration on Hepatitis C Elimination in HIV Cohorts (InCHEHC). Follow-up started from the first recorded negative HCV antibody test date and ended at last negative antibody test or estimated infection date. Follow-up was restricted to 2010–2019. We used segmented Poisson regression to model trends across pre-, limited- (i.e., restrictions on access) and broad-DAA access periods.
Findings
Overall, 45,942 participants had at least one HCV antibody negative result and follow-up between 2010 and 2019. We observed 2042 incident HCV infections over 248,189 person-years (PY). Pooled incidence decreased from 0.91 per 100 PY in 2015 to 0.41 per 100 PY in 2019. Compared to the average pre-DAA period incidence (0.90 per 100 PY), average incidence was similar during the limited-DAA access period (Incidence rate ratio [IRR] = 0.98; 95%CI = 0.87, 1.11), and 52% lower during the broad-DAA access period (IRR = 0.48; 95%CI = 0.42, 0.52). The average annual decline in HCV incidence was 2% in the pre-DAA period; an additional 9% annual decline in incidence was observed during the limited-DAA access period (IRR = 0.91; 95%CI = 0.82, 1.00) and a further 20% decline in the broad-DAA access period (IRR = 0.80, 95%CI = 0.73, 0.89).
Interpretation
Our findings suggest that broad DAA access has a TasP effect on primary HCV incidence among PLHIV. Based on the initial years of DAA availability, the countries in the InCHEHC collaboration are on track to meet the World Health Organization's 80% HCV incidence reduction target for PLHIV by 2030.
Funding
This study was funded by the Australian Government National Health and Medical Research Council (Grant number GNT1132902).
Keywords: HIV, Hepatitis C virus, Elimination, Direct-acting antivirals, Trends, Incidence
Research in context.
Evidence before this study
In 2013, hepatitis C virus (HCV) treatment was transformed by direct-acting antiviral (DAA) therapies that cure ≥95% of patients. This prompted the World Health Organization (WHO) to set ambitious targets to eliminate HCV as a public health threat. HCV infection is more common among people living with HIV (PLHIV) than HIV-negative individuals, and coinfected people experience higher rates of HCV-related mortality than HCV monoinfected people. Therefore, PLHIV are a key population for HCV elimination.
We searched PubMed for English, Spanish and Dutch publications, with no restrictions, to identify relevant publications relating to primary (i.e., first) HCV incidence among PLHIV. We used the search terms (“hepatitis C″ OR “HCV”) AND (“incidence” OR “trends”) AND (“treatment” OR “direct acting antivirals”) AND “HIV”. Relevant publications were also obtained from co-authors of our collaboration. Previous single-country studies have reported decreases in HCV incidence in recent years, and particularly following DAA introduction. In some countries, though, primary HCV incidence was declining before DAA introduction, or trends in incidence have only been analysed during the DAA era. It is thus unclear whether the observed reductions in incidence would have occurred even without DAA introduction. It is important to note that restrictions on DAA reimbursement remain in place in many countries, which may prevent achievement of HCV elimination.
Using multinational cohort data from the International Collaboration on Hepatitis C Elimination in HIV-Coinfection (InCHEHC), we assessed whether countries represented in this collaboration (Australia, France, the Netherlands, Spain and Switzerland) are on track to meet the WHO HCV incidence target for PLHIV, and whether incidence changed after DAA introduction. We assessed whether changes in HCV incidence occurred when DAA reimbursement was restricted, limiting DAA access, and when DAAs were broadly available.
Added value of this study
This is the first formal evaluation of how DAA access affected changes in HCV incidence among PLHIV across countries with different pre-DAA incidence trends. This multinational longitudinal study, with a sample size of 45,942 PLHIV and 248,189 person-years (PY) of follow-up, demonstrated that broad DAA access was associated with a ∼50% reduction in HCV incidence compared to the period without DAAs; no change was observed during the limited-DAA access period. The effect of DAAs on HCV incidence was consistent across countries, with a decline being observed regardless of the underlying HCV epidemic prior to DAA introduction. Our findings indicate that DAAs have a “treatment as prevention” (TasP) effect on primary HCV incidence.
Implications of all the available evidence
Countries that are part of this InCHEHC collaboration are on track to meet the 80% HCV incidence reduction target among PLHIV by 2030, suggesting that previous efforts towards elimination have been successful and should continue. However, HCV incidence in PLHIV remains 10 times higher than the WHO's absolute incidence target to validate HCV elimination among the general population, set at ≤5 per 100,000 PY. Our findings suggest that limited DAA access is unlikely to substantially reduce HCV incidence where HCV transmission is ongoing, even at low levels. Broad DAA access, on the other hand, has an immediate and lasting TasP effect on HCV incidence among PLHIV in the first years of broad DAA availability. Whether DAAs will continue to drive incidence reductions after 2019 and how the COVID-19 pandemic affected incidence trends is largely unknown; hence, continued monitoring is warranted.
Introduction
Around 58 million people are living with the hepatitis C virus (HCV) globally, with an estimated 290,000 annual deaths.1,2 In 2013, HCV treatment was transformed by direct-acting antiviral (DAA) therapies, which cure ≥95% of patients treated.3 In 2016 the World Health Organization (WHO) set ambitious targets to eliminate HCV as a public health threat. Global targets include reducing HCV incidence by 30% in 2020 and by 80% in 2030, compared to 2015. In addition, 2021 WHO guidelines for countries seeking validation of elimination define the incidence target as an absolute annual incidence of ≤5 per 100,000 person-years (PY) in the general population and ≤2 per 100 PY among people who inject drugs (PWID).4
Modelling studies suggest global HCV elimination targets are unlikely to be met by 2030,1 although several high-income countries are on track for the 80% incidence reduction target.5, 6, 7 Access to DAAs may reduce HCV incidence through a “treatment as prevention” (TasP) effect by reducing the number of individuals able to transmit the virus.8,9 A TasP effect is more likely if broad access to DAAs facilitates high coverage, particularly among people with ongoing high-risk behaviours associated with HCV infection. This is supported by modelling studies reporting substantial decreases in HCV prevalence and incidence by 2030 among men who have sex with men (MSM) and PWID with high DAA coverage8,9 However, empirical data on progress towards elimination and the role of DAAs in reducing HCV incidence remain scarce. Moreover, in several countries, access to DAAs was initially restricted to people with severe liver fibrosis and/or based on HIV status.10 In most high-income countries, restrictions have been relaxed and DAAs are now broadly available, but in some, fibrosis and drug abstinence-based restrictions likely continue to impede HCV elimination.11
To validate HCV elimination, retrospectively or prospectively collected HCV retesting data are needed for key populations, including MSM living with HIV, and PWID12 HCV infection is more common among people living with HIV (PLHIV) than HIV-negative individuals, and coinfected people experience higher rates of HCV-related mortality than those with HCV monoinfection, making them a key population for HCV elimination.4,13 Given the regular engagement of PLHIV in routine HIV care, monitoring and validating HCV elimination in PLHIV in high-income countries with surveillance systems and affordable, accessible and high-quality healthcare is highly feasible.
Evidence from cohorts of PLHIV in high-income countries have shown decreases in primary HCV incidence in recent years, particularly after DAA introduction.14, 15, 16, 17, 18, 19, 20 However, in some countries, primary incidence was declining before DAA introduction, or trends in incidence have only been analysed during the DAA era. Therefore, it is unclear whether observed incidence reductions would have occurred even without DAAs. Quantifying the effect of DAA access on incidence is key for assessing how DAAs influence HCV transmission among PLHIV and whether additional interventions are needed.
The International Collaboration on Hepatitis C Elimination in HIV Cohorts (InCHEHC) was established to track progress and guide policy on elimination in PLHIV. Data are collected from 11 cohorts in six high-income countries with health systems facilitating testing, treatment, and surveillance. Using data from multiple countries, we aimed to assess 1) progress towards HCV elimination by measuring trends in primary HCV incidence by calendar year between 2010 and 2019, overall and by country, and 2) changes in primary incidence associated with limited and broad access to DAAs.
Methods
InCHEHC is a consortium including prospective cohorts among PLHIV with or without HCV co-infection from Australia, Canada, France, the Netherlands, Spain and Switzerland. InCHEHC cohorts collect socio-demographic and clinical data including longitudinal HCV antibody and RNA testing, HIV viral load, CD4 T-cell count and HIV and HCV treatment. Each cohort study team prepared and submitted data to the coordinating centre (Burnet Institute) based on the HIV Cohorts Data Exchange Protocol (HICDEP – https://hicdep.org/) for HIV collaborations. The HICDEP tool was adapted to meet InCHEHC data availability and format.
For the current analysis, we included data from six InCHEHC cohorts from five countries that have data among PLHIV with and without HCV co-infection (Supplementary Table S1). All cohorts received approval from their regulatory or national ethics committees (Supplementary material). The Alfred Hospital Ethics Committee (Melbourne, Australia) granted ethics approval for InCHEHC. No additional consent was needed from cohort participants to be included in the InCHEHC study.
Eligibility criteria and follow-up
HCV positive status was based on a positive antibody or RNA test result. Estimated HCV infection date was based on the midpoint between last HCV antibody negative test and first HCV positive test. For individuals with a negative antibody test and a simultaneous positive RNA test, estimated infection date was six weeks before the first positive RNA test date. We included individuals with at least one recorded negative antibody and a subsequent negative antibody test or a positive HCV RNA and/or antibody test. Time at risk commenced from the first HCV antibody negative test and ended at estimated infection date or the last recorded HCV antibody negative test date. We restricted analyses to data between 2010 and 2019, when all cohorts had started collecting data. Follow-up time started on January 1, 2010, for individuals who had a prior negative antibody test but no recorded HCV infection, or at the first negative antibody test date. Follow-up time ended at the last recorded HCV antibody negative test date, or December 31, 2019 for individuals with a negative antibody test after this date, or at the estimated HCV infection date. Individuals whose estimated (midpoint) infection date occurred after December 31, 2019 were censored at their last antibody negative test date during the analysis period. The Saint-Antoine Infectious Disease Clinical Cohort provided data until December 31, 2017.
Statistical analysis
Data were mostly drawn from routinely collected clinical data, so incidence could be influenced by irregular testing patterns; hence, we first assessed the distribution of testing intervals over all calendar years since January 1, 2010. We then compared the absolute number of HCV incident infections for each calendar year between the midpoint estimation assumption method and diagnosis date. We also assessed changes in the proportion of individuals with an unknown (i.e., no recorded HCV test) and/or HCV antibody negative status and at least one (subsequent) recorded HCV test result during each calendar year since 2010.
To describe trends in primary HCV incidence over calendar time, we used Poisson regression models with HCV incidence was allowed to vary smoothly over calendar years using restricted cubic splines with three knots (at the 10th, 50th and 90th percentiles of follow-up time), overall and by country. Two French cohorts were pooled to represent France in the analyses. In addition, we assessed changes in HCV incidence over calendar years among MSM and non-MSM.
To assess changes in HCV incidence following limited and broad access to DAAs, we defined three DAA access periods: pre-, limited- and broad-DAA access. The pre-DAA access period started at January 1, 2010. Start dates of limited- and broad-DAA access periods varied by country and were based on the date when DAAs became reimbursable (Supplementary Table S1).
We modelled the average incidence during each DAA access period using a piecewise exponential survival model. We included a fixed effect for each DAA period, assuming constant hazard within each period. We accounted for between-country variation at the beginning of 2010 by including a random intercept.
Then, we assessed whether the rate of change in HCV incidence (i.e., the slope) differed across DAA access periods, using a Poisson regression model with a random intercept and a random pre-DAA slope for each country. For this analysis, data were aggregated at the calendar month level. Time in months was included as a continuous variable and modelled as a linear spline with two knots at DAA access change points. In this linear spline model, incidence is assumed to change by a fixed percentage at each change point. The effect size of the change in slope at each DAA period was assumed to be common across countries but when it occurred varied, depending on the country-specific timing of policy changes. Confidence intervals at the 95% level were calculated using the empirical bootstrap method with 1000 samples. Bootstrap samples were generated by randomly sampling the study participants with replacement.
Sensitivity analyses
We assessed changes in incidence by DAA access period using an interrupted time series analysis approach. We implemented this using a segmented Poisson regression model including the same set of fixed and random effects as the main analysis, while adding a fixed change in intercept at the start of each DAA period, allowing the intercept and slope to differ.
We also performed sensitivity analysis using a restricted date of DAA introduction for Australia (January 1, 2015). Although Australia did not officially have a restricted access period, trials and compassionate use programs gave access to DAAs in Australia before the official date of broad access.
Lastly, we repeated the analysis excluding Spain, given differences in the study population characteristics and an increasing pre-DAA HCV incidence.
Analyses were performed in R (R version 4.1.2, Vienna, Austria).
Role of funding
The funders had no role in the study design, data collection, analyses or interpretation of the data.
Results
Of 104,757 InCHEHC participants, 101,045 were from cohorts with calculable primary incidence. Among participants in these cohorts who had ever tested HCV RNA positive and had follow up between 2010–2019 (Supplementary Fig. S1), the proportion who had a recorded first treatment initiation/prescription date increased during the limited and broad DAA access period compared to the pre-DAA access period in all countries.
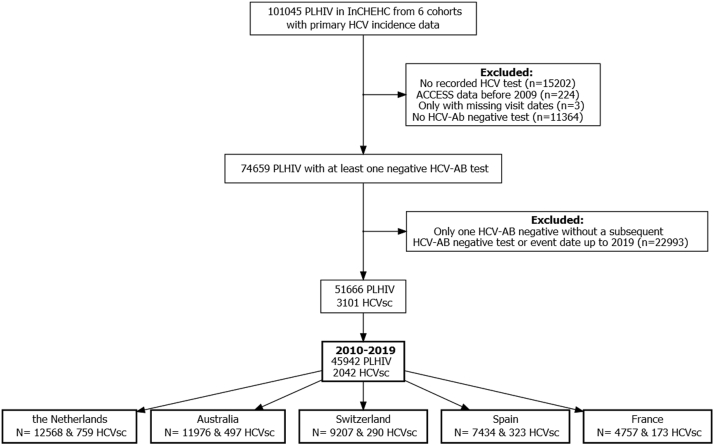
Of the 101,045 participants, 74,659 (74%) had at least one negative HCV antibody test result, of whom 69% (n = 51,666/74,659) had a subsequent test to calculate follow-up (Fig. 1). After restricting data to 2010–2019, 45,942 of 51,666 (89%) participants were included in incidence analyses.
Fig. 1.
Participant selection for the primary incidence analysis from five InCHEHC countries. InCHEHC: International Collaboration on Hepatitis C Elimination in HIV Cohorts; PLHIV: people living with HIV; HCV: hepatitis C virus; ACCESS: Australian Collaboration for Coordinated Enhanced Sentinel Surveillance; Ab: antibody; HCVsc: HCV seroconversions. We excluded ACCESS participants that only had data recorded prior to 2009 as data collection started from that year onwards (n = 244).
Cohorts from the Netherlands (ATHENA) and Switzerland (SHCS) contributed the most data, and the proportion of follow-up that each cohort contributed per calendar year was relatively stable until 2018 (Supplementary Fig. S2). From 2018 onwards, the Australian cohort (ACCESS) contributed more follow-up than previous years and more than any other cohort in 2019 (∼40% of total). Most incident infections were observed in the Netherlands (n = 759) followed by Australia (n = 497) and Spain (n = 323) (Fig. 1 and Supplementary Fig. S3).
At the start of follow-up for analyses, median age was 41 years (interquartile range [IQR] = 32, 49) and was lowest in Spain (34 years) and highest in Switzerland (43 years) (Table 1). Median CD4 T-cell count was 500 cells/mm3 (IQR = 343, 680) and 35% had a detectable HIV viral load. Median known duration since HIV diagnosis was 1.5 years (IQR = 0.02, 6.5). Participants in Spain has the shortest known duration since HIV infection (0.1 years; IQR = 0, 1.9) and participants from France the longest (5.4 years; IQR = 0.03, 6.5). Most individuals were male (87%) and MSM (69%). The proportion of males was lowest in Switzerland and highest in Australia (Table 1).
Table 1.
Baseline socio-demographic and clinical characteristics at first visit at or after the start of follow-up among 45,942 InCHEHC participants (2010–19).
| Characteristic | Australia (N = 11976) | France (N = 4757) | Spain (N = 7434) | Switzerland (N = 9207) | the Netherlands (N = 12568) |
|---|---|---|---|---|---|
| Age (years) | |||||
| Median (IQR) | 42 (33, 50) | 42 (34, 50) | 34 (28, 41) | 43 (35, 50) | 42 (33, 49) |
| Sex | |||||
| Female | 585 (4.9%) | 1085 (22.8%) | 762 (10.3%) | 2368 (25.7%) | 1184 (9.4%) |
| Male | 11328 (94.6%) | 3672 (77.2%) | 6672 (89.7%) | 6839 (74.3%) | 11384 (90.6%) |
| Unknown/Missing | 63 (0.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Year of enrolment | |||||
| Median (IQR) | 2009 (2009, 2013) | 2007 (2003, 2013) | 2012 (2009, 2015) | 2007 (1999, 2012) | 2008 (2003, 2013) |
| Key populationa | |||||
| MSM | 9121 (76.2%) | 2648 (55.7%) | 5570 (74.9%) | 4971 (54.0%) | 9514 (75.7%) |
| HIDU | 0 (0.0%) | 35 (0.7%) | 58 (0.8%) | 72 (0.8%) | 14 (0.1%) |
| Other/Unknown | 2855 (23.8%) | 2074 (43.6%) | 1806 (24.3%) | 4164 (45.2%) | 3040 (24.2%) |
| Known duration since HIV diagnosisb | |||||
| Median (IQR) | 0.6 (0.0, 2.1) | 5.4 (1.0, 12.4) | 0.1 (0.0, 1.9) | 5.2 (0.6, 12.1) | 2.9 (0.1, 8.5) |
| CD4 T-cell countc | |||||
| Median (IQR) | 560.0 (400.0, 743.0) | 508.0 (353.0, 680.0) | 458.0 (296.0, 637.8) | 491.0 (339.0, 673.0) | 480.0 (330.0, 660.0) |
| Detectable HIV viral load | |||||
| No | 1396 (11.7%) | 2408 (50.6%) | 1425 (19.2%) | 5699 (61.9%) | 5885 (46.8%) |
| Yes | 1168 (9.8%) | 1520 (32.0%) | 4878 (65.6%) | 3025 (32.9%) | 5289 (42.1%) |
| Missing | 9412 (78.6%) | 829 (17.4%) | 1131 (15.2%) | 483 (5.2%) | 1394 (11.1%) |
| Log HIV RNAc, d | |||||
| Median (IQR) | 10.4 (8.9, 11.5) | 10.7 (9.0, 11.9) | 10.8 (9.6, 12.0) | 10.6 (9.1, 11.9) | 10.7 (9.2, 12.0) |
| Follow upe | |||||
| Median (IQR) | 5.1 (2.4, 7.9) | 5.6 (3.0, 7.8) | 4.1 (1.8, 7.1) | 7.9 (4.1, 9.2) | 5.4 (2.3, 8.4) |
| Number of HCV seroconversions during follow up | |||||
| Events | 497 | 173 | 323 | 290 | 759 |
HCV: Hepatitis C virus: InCHEHC: International Collaboration on Hepatitis C Elimination in HIV Cohorts; IQR: interquartile range; MSM: men who have sex with men; PWID: people who inject drugs (lifetime or active); NA: not available; CD4 T-cell count: CD4 cluster of differentiation 4.
MSM with a history of injection drug use were included in the MSM category.
Time since first known or reported HIV positive test result until first visit at or after the start of follow-up in the analyses.
CD4 T-cell and HIV RNA represent values closest and within three months to the first visit at the start of follow-up in the analyses.
Among individuals with a detectable HIV RNA viral load.
Time between first HCV antibody negative test and last antibody negative test or event date.
HCV testing
The median number of HCV tests per person between 2010 and 2019 was five (IQR = 3, 8). Median HCV test interval was 1.0 years (IQR = 0.5, 2.0) and was relatively stable over all calendar years (Supplementary Figs. S4 and S5). Median test interval was highest in Switzerland at 1.7 years (IQR = 1.0, 2.1) and shortest in Australia at 0.7 years (IQR = 0.3, 1.2).
The proportion of individuals with an unknown or HCV antibody negative status and at least one (subsequent) HCV test during each calendar year increased slightly from 2010 and remained relatively stable after 2014 at around 48% (Supplementary Fig. S6a). Among those with recorded negative antibody status, HCV re-testing was stable over calendar time (Supplementary Fig. S6b). Trends in HCV testing varied across countries, with slight increases in testing in years when DAAs were available in Switzerland and the Netherlands, and peaking between 2014 and 2015 in France (Supplementary Fig. S7). The highest proportion of individuals tested was observed in Switzerland, above 60% in 2019.
Diagnosis and estimated infection date
Most HCV diagnoses were made in 2013–2016 (Supplementary Fig. S8). The number of infections attributed to a particular calendar year using midpoint estimation or diagnosis date were similar between 2010 and 2015. After 2015, infection dates shifted to earlier years when applying the midpoint estimation compared to the diagnosis date.
Trends in primary HCV incidence
We observed 2042 incident HCV infections over 248,189 PY of follow-up during 2010–2019. Pooled incidence was 0.82 per 100 PY (95% confidence interval [CI] = 0.78, 0.86).
Primary HCV incidence was relatively stable between 2010 and 2015 (Fig. 2). After 2015, a marked decline in annual incidence occurred, with an incidence of 0.91 per 100 PY (95%CI = 0.81, 1.03) in 2015 and 0.41 per 100 PY (95%CI = 0.30, 0.53) in 2019, a 55% decrease in pooled primary HCV incidence. A similar trend was observed when restricting data to MSM, whereas incidence among non-MSM was lower and stable over calendar years (Supplementary Fig. S9). Compared to 2015, the decrease in incidence in 2019 was highest in France (72.9%) and lowest in Spain (22.2%) (Supplementary Fig. S10).
Fig. 2.
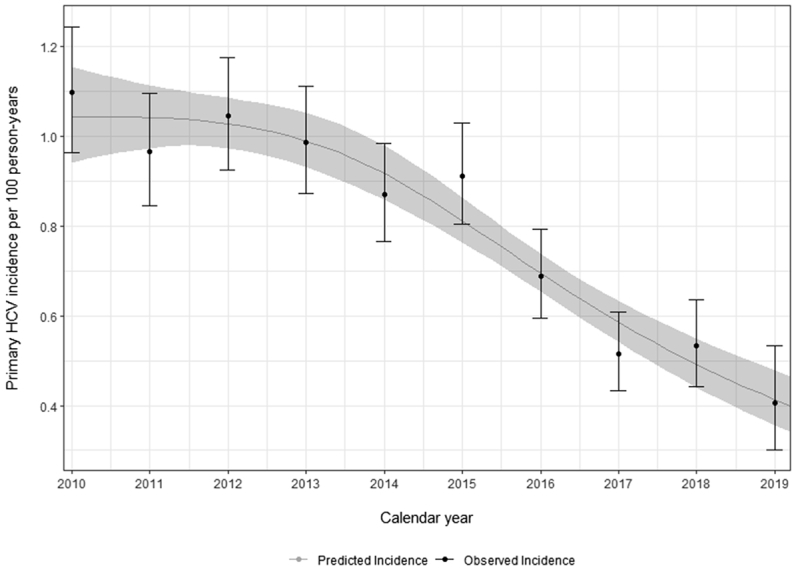
Overall HCV incidence per calendar year in the pooled dataset (2010–2019). Black dots and bars represent the observed incidence and its 95% confidence interval. Grey line and band represent the predicted incidence and its 95%CI using Poisson regression modelling with calendar year using restricted cubic splines.
Changes in primary HCV incidence after DAA introduction
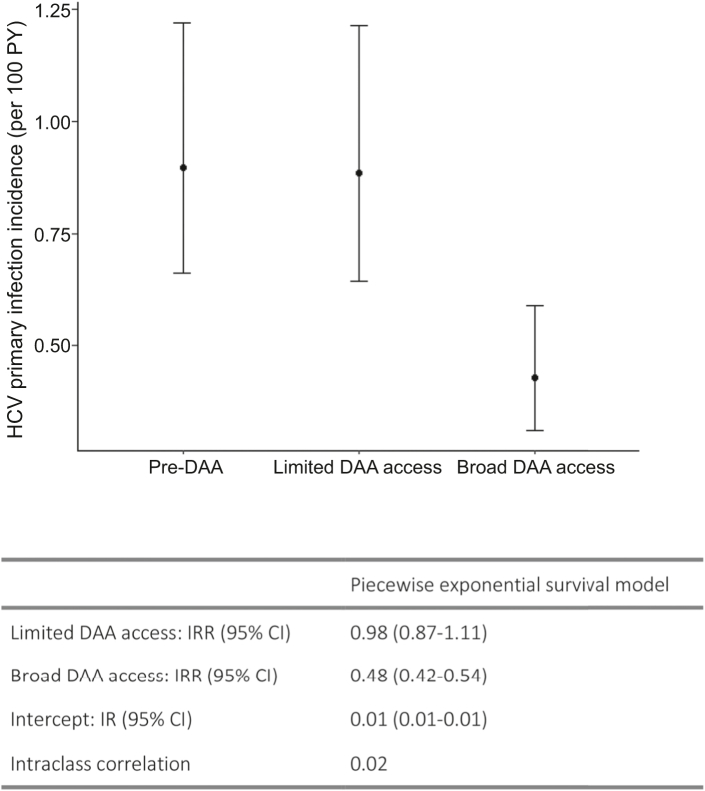
When combining data from all countries, the average predicted incidence was similar during the pre-DAA and limited-DAA access periods (predicted incidence rate pre-DAA = 0.90 per 100 PY [95%CI = 0.66, 1.22]) (Fig. 3). During the broad-DAA access period, the average predicted incidence was 52% lower than during the pre-DAA access period (incidence rate ratio [IRR] = 0.48; 95%CI = 0.42, 0.54) at 0.43 per 100 PY (95%CI = 0.31, 0.59).
Fig. 3.
Piecewise exponential model, average primary incidence by DAA access period. DAA: direct-acting antiviral; HCV: hepatitis C virus; PY, person-years; IRR: incidence rate ratio; CI: confidence interval; IR: Incidence rate. Random effect variance for the intercept was 0.11 (standard deviation = 0.34).
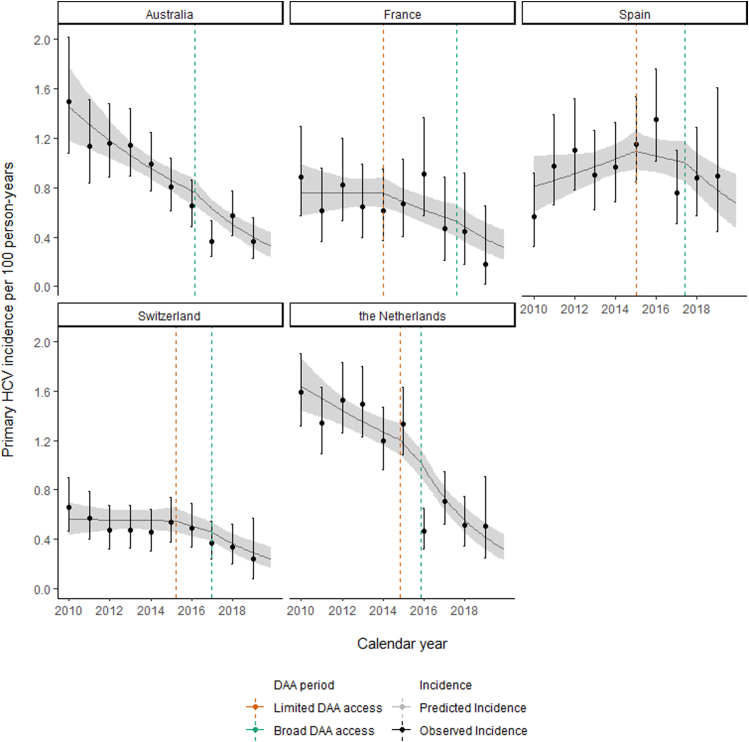
During the pre-DAA access period, the annual average decline in incidence was 2% (slope IRR = 0.98; 95%CI = 0.92, 1.04) (Fig. 4; Table 2). Incidence declined 9% more per annum during the limited-DAA access period than in the pre-DAA access period (IRR = 0.91; 95%CI = 0.82, 1.00). During the broad-DAA access period, the additional annual decline was 11% greater than in the limited-DAA access period (IRR = 0.89; 95%CI = 0.79, 1.00) and 20% greater than in the pre-DAA access period (IRR = 0.80, 95%CI = 0.73, 0.89).
Fig. 4.
Changes in primary HCV incidence trends following access to DAAs by country. HCV: hepatitis C virus; DAA: direct-acting antiviral. Predicted incidence based on the linear spline model.
Table 2.
Changes in primary HCV incidence trends following access to DAAs.
| Main analysis | Sensitivity analyses |
|||
|---|---|---|---|---|
| Model 1 AU limited date | Model 2 ITSA | Model 3 Excluding Spain |
||
| Intercept: IR (95% CI) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) | 0.01 (0.01–0.02) |
| Slope: IRR (95% CI) | 0.98 (0.92–1.04) | 0.99 (0.93–1.06) | 0.97 (0.92–1.02) | 0.93 (0.90–0.96) |
| Change in slope, limited DAA access vs pre-DAA: IRR (95% CI) |
0.91 (0.82–1.00) | 0.87 (0.78–0.98) | 1.00 (0.90–1.10) | 1.00 (0.94–1.07) |
| Change in slope, broad DAA access vs pre-DAA: IRR (95% CI) |
0.80 (0.73–0.89) | 0.84 (0.76–0.93) | 0.97 (0.85–1.10) | 0.81 (0.73–0.90) |
HCV: hepatitis C virus; DAA: direct-acting antiviral; IR: incidence rate; CI: confidence interval; IRR: incidence rate ratio.
Main analysis: Random effect variance for the intercept was 0.18 (standard deviation (SD) = 0.42) and 0.003 (SD = 0.06) for the pre-DAA access slope.
Model 1 (Australia [AU]) limited DAA access date): Random effect variance for the intercept was 0.18 (SD = 0.42) and 0.003 (SD = 0.06) for the pre-DAA access slope.
Model 2 (ITSA [interrupted time series analysis]): Random effect variance for the intercept was 0.14 (SD = 0.38) and 0.002 (SD = 0.04) for the pre-DAA access slope.
Model 3 (excluding Spain): Random effect variance for the intercept was 0.11 (SD = 0.34). A random slope was not included in this model.
Fig. 4 depicts the predicted and observed incidence over time based on the linear spline model. The model fits the data from all countries well despite variation in pre-DAA access slope and intercept, with varying pre-DAA incidence trends: declining in Australia and the Netherlands, stable in France and Switzerland and increasing in Spain.
Sensitivity analyses
When adding a limited-DAA access period for Australia and excluding Spain from the analyses, results were similar to the main analysis (Table 2; Supplementary Fig. S11; Supplementary Table S2). However, upon including a limited-DAA access period for Australia, we observed a slightly stronger change in slope between the limited-DAA access period and the pre-DAA access period than in the main analysis (Table 2).
When assessing the changes in HCV incidence using an interrupted time series approach, we observed a strong immediate decrease in incidence in the broad DAA access period (Supplementary Fig. S12 & Supplementary Table S3), but the rate of change remained similar to the main analysis during the broad DAA access periods (Table 2).
Discussion
This is the first formal evaluation of how access to DAAs changed HCV incidence among PLHIV in care across countries with different pre-DAA incidence trends. Using data from six cohorts in five countries, we show that access to DAAs was associated with a ∼50% reduction in HCV incidence in the broad-DAA access period compared to the pre-DAA access era, indicative of a TasP effect. This decline in incidence, based on empirical data, places these countries on track to eliminate HCV among PLHIV—a micro-elimination approach21—based on the WHO's 30% incidence reduction 2020 progress target.
We observed an additional 9% annual decrease in incidence during the limited DAA access period compared to the pre-DAA period, although there was no evidence of a significant change in slope. Moreover, we did not observe a significant change in the average HCV incidence during the limited-DAA compared to the pre-DAA access period. This may be because treatment was less available during this period to those engaging in ongoing high-risk practices, mainly due to restrictions for reimbursement (e.g., for those with severe liver fibrosis only). Moreover, a lagged effect of DAA on incidence might be expected, as decreases in incidence are unlikely to be observed immediately following DAA access.
While trends in incidence varied across countries during the pre-DAA access era, and particularly in Spain, it was encouraging to observe a decline in HCV incidence in all countries after DAAs became broadly accessible. These findings suggest that DAA access can change incidence trends irrespective of the underlying HCV epidemic. Another study using InCHEHC data showed that HCV reinfection remained stable or declined in most evaluated countries following broad access to DAAs, although declines were less pronounced than in primary incidence.21,22
Despite high DAA treatment uptake in Spain,23 broad access to DAAs led to a smaller absolute decline in HCV incidence than in other countries, possibly due to cohort characteristics: Spaniards were younger and had more recently diagnosed HIV infections, so potentially engaged in more high-risk behaviours. Sustained broad access to DAAs and higher uptake of other (non-)biomedical preventive interventions are needed in countries with expanding HCV epidemics prior to DAA access to achieve declines similar to those in countries with stable or declining trends.
We observed a strong decline in HCV incidence in the years immediately following broad-DAA access, slowing over time. It is unclear whether HCV incidence will continue to fall in coming years, or stabilise at a lower rate, making HCV elimination by 2030 less likely in PLHIV. It is unknown whether these trends continued after the study period, but evidence from InCHEHC countries shows decreases in HCV testing and diagnosis since the COVID-19 pandemic began in 2020.24, 25, 26, 27 This may have increased time to diagnosis, providing more opportunities for transmission. Conversely, there is evidence of decreases in behaviours associated with HCV infection,28,29 particularly early in the pandemic, which could have offset lower testing rates. International travel contracted during the pandemic, reducing the likelihood of external HCV introductions across countries.30 The expansion of international travel in 2022 could increase new infections, fuelled by undiagnosed infections from previously restricted access to testing and an increase in behaviours associated with HCV infection.
Studies conducted within InCHEHC countries have reported high initial DAA uptake rates,6,19,23,31,32 and we observed an increase in the proportion treated during the limited and broad DAA access period compared to the pre-DAA period in all countries. However, some countries have reported that HCV treatment uptake has declined since DAAs were first introduced, and those remaining to be treated are less engaged in care, reducing opportunities for HCV care.33,34 Decreasing the time between infection and treatment and reducing loss to care are key to achieving HCV elimination. For example, in the Swiss TasP trial offering PCR-based screening to all MSM, one in three patients with a new HCV infection lacked HCV antibodies despite testing RNA positive. Authors estimated a median delay of 197 days in diagnosing a new HCV infection if annual antibody testing had been implemented.19 Moreover, an Australian study of individuals with well-estimated dates of HCV seroconversion accessing clinics specialized in PWID care reported that in 2018, only 33% and 43% of RNA-positive individuals had evidence of DAA prescription within 90 and 365 days of their RNA-positive results, respectively.35 This indicates that even in countries with strong HCV elimination programs, transition times through the care cascade can be shortened.35 Decentralization of health care, peer-based or nurse-led models of care, increased testing frequency or home-based testing, incentives and simplifying the HCV cascade of care all facilitate diagnosis and treatment.36, 37, 38
Crucially, we show that HCV incidence remains 10 times higher in 2019, at 50 per 100,000 PY, than the absolute incidence target of ≤5 per 100,000 PY that WHO defines as “elimination” in the general population.4 Given that PLHIV have higher rates of HCV infection than the general population, we suggest that a PLHIV-specific absolute incidence elimination target should be considered, similar to separate targets established for PWID. This is particularly important because general population estimates are contingent on the size of the at-risk populations, which are difficult to measure within and to compare across countries.
Our analysis has limitations. First, we did not include data on risk behaviours or adjust analyses for other factors which may have contributed to changes in HCV incidence following access to DAAs. There is evidence of increases in condomless anal sex among MSM and sexually transmitted infections among MSM living with HIV in high-income countries.39,40 Nevertheless, we observed a significant reduction in HCV incidence following DAA access, even if the population continued engaging in risky behaviours, in line with previous behavioural trends. We are unaware of simultaneous major changes that would have affected the HCV epidemic other than DAA access, so argue that unmeasured or unadjusted factors cannot explain the observed changes in incidence, particularly because DAA access dates varied across countries but incidence changes were consistent. Second, we observed slight increases in HCV testing after DAAs became available, possibly due to health professionals offering it more often, and higher acceptability of testing given simplified and better treatments. This could have increased case finding and lengthened negative–positive testing intervals, affecting the accuracy of the estimated trends. However, the time between tests was relatively stable over calendar years and differences in testing rates over time were small. Third, most participants were MSM from high-income counties in HIV care, hence caution must be taken when generalizing our findings to PLHIV in other local epidemics where injection drug use drives HCV incidence or to countries where DAAs are available but not reimbursed. Nonetheless, in most European countries HCV transmission among PLHIV is concentrated in MSM, and in Australia, HCV/HIV co-infection among PWID is rare. Moreover, cohorts included in this analysis cover a representative sample of the PLHIV population in care41 or comprise a large proportion of the PLHIV population in care in their respective jurisdictions, while only a minority of PLHIV remain undiagnosed in these countries.42, 43, 44 Nevertheless, although only a small minority of PLHIV in these countries is estimated to remain undiagnosed for HIV, HCV incidence may be higher among them. Moreover, loss to care rates may be higher in those at high risk of HCV leading to missed HCV diagnoses and thus underestimating HCV incidence trends.
Broad access to DAAs was associated with a marked reduction in primary HCV incidence, suggesting a TasP effect among PLHIV in the first years since DAA availability in countries with high DAA uptake. We showed that InCHEHC countries are on track to meet the WHO's 80% incidence relative reduction target among PLHIV, but that incidence remains 10 times higher than the absolute elimination target in the general population. PLHIV have a higher prevalence of HCV infection than HIV-negative individuals, so a PLHIV-specific absolute elimination target should be considered. Whether DAAs will continue to reduce HCV incidence after 2019 and how the COVID-19 pandemic affected incidence is largely unknown, hence continued monitoring is warranted.
Contributors
DK van Santen: Writing - Original Draft; DK van Santen and R Sacks-Davis: Conceptualization, Visualization, Data Curation, Project administration, Methodology, Software, Formal analysis, Writing - Review & Editing. A Boyd, J Young and T Spelman: Conceptualization, Methodology, Writing - Review & Editing. M Requena, J Asselin, C Smit and A Stewart: Resources, Data Curation, Writing - Review & Editing. M van der Valk, A Rauch, DL Braun, I Jarrin, J Berenguer, K Lacombe, L Wittkop, O Leleux, D Salmon, F Bonnet, G Matthews, JS Doyle, MB Klein, M Prins, MA Stoové: Conceptualization, Resources, Writing - Review & Editing. JV Lazarus: Writing - Review & Editing. M Hellard: Conceptualization, Resources, Supervision, Funding acquisition, Writing - Review & Editing.
Data sharing statement
The data dictionary based on the HIV Cohorts Data Exchange Protocol available at https://hicdep.org/ is available upon request. Data (analyses) requests are welcome subject to approval by the study steering committee. Requests and enquiries should be directed to the data coordinator, Rachel Sacks-Davis (rachel.sacks-davis@burnet.edu.au).
Declaration of interests
Juan Berenguer reports honoraria for advice or public speaking from AbbVie, Gilead, MSD, JANSSEN, and ViiV Healthcare; and grants from AbbVie, Gilead, MSD, and ViiV Healthcare. Dominique L Braun reports honoraria for advice or public speaking from, Gilead, MSD and ViiV Healthcare. Marina Klein reports grants for investigator-initiated studies from ViiV Healthcare, AbbVie, Merck, and Gilead, and consulting fees from ViiV Healthcare, AbbVie, and Gilead, all outside the submitted work. Marina Klein is supported by a Tier I Canada Research Chair. Jeffrey V Lazarus acknowledges grants and speaker fees from AbbVie, Gilead Sciences and MSD and speaker fees from Genfit, Intercept, Janssen, Novo Nordisk and ViiV, outside of the submitted work. Andri Rauch reports support to his institution for advisory boards and/or travel grants from Abbvie, MSD, Gilead Sciences, and Pfizer, and an investigator initiated trial (IIT) grant from Gilead Sciences. All remuneration to Andri Rauch went to his home institution and not to Andri Rauch personally, and all remuneration was provided outside the submitted work. Karine Lacombe reports honoraria for advice or public speaking from Abbvie, Gilead, MSD, Janssen and ViiV Healthcare. Fabrice Bonnet reports grants from Gilead and honoraria from Gilead, ViiV healthcare and MSD. Maria Prins reports unrestricted research grants and speaker/advisor fees from Gilead Sciences and MSD; all of which were paid to her institution and unrelated to the current work. Marc van der Valk reports unrestricted research grants from Gilead and MSD and fees for participation in advisory boards from Gilead, MSD and ViiV (all paid to his institution). Joseph S Doyle reports funding to his institution for investigator-initiated research from Gilead Sciences and AbbVIe, and honoraria to his institution for educational events from AbbVie. Linda Wittkop reports grants/financial support for the work under consideration from the French Agency ANRS Emerging Infectious Diseases (ANRS—MIE) paid to her institution. Gail Matthews reports grants from Gilead, AbbVie and ViiV, all paid to her institution and financial support for participating in advisory board from Gilead and ViiV. The CEASE study is supported by Gilead. Inma Jarrin reports grants from MSD and ViiV healthcare, all paid to her institution, honoraria for lectures/presentations from Gilead and ViiV healthcare, support from Gilead for attending meetings/travel, and support from Gilead to participate in an advisory board. Margaret Hellard reports investigator-initiated research grants from Gilead and Abbvie. Dominique Salmon reports support for attending meetings/travel from Gilead and AbbVie. Mark Stoové reports funding from Gilead and AbbVie for investigator-initiated research unrelated to this work and consultant fees from Gilead Sciences for activities unrelated to this work.
Acknowledgments
The authors thank the study participants for their contribution to the research. The authors acknowledge the contribution of the ACCESS team members and ACCESS advisory committee members who are not co-authors of this article. The authors also acknowledge all clinical services participating in ACCESS. The list of ACCESS team members, ACCESS advisory committee members and participating ACCESS services can be found on the ACCESS website (accessproject.org.au). ACCESS is a partnership between the Burnet Institute, Kirby Institute and National Reference Laboratory. The ATHENA database is maintained by Stichting HIV monitoring and supported by a grant from the Dutch Ministry of Health, Welfare and Sport through the Centre for Infectious Disease Control of the National Institute for Public Health and the Environment (https://www.hiv-monitoring.nl/en/research-using-our-data/submit-research-proposal/rules-acknowledgement). The authors acknowledge the contribution of the Swiss HIV Cohort Study participants and team members (https://shcs.ch/184-for-shcs-publications). This study has been financed within the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (grant #201369) and by the SHCS research foundation. Data from the SHCS are gathered by the Five Swiss University Hospitals, two Cantonal Hospitals, 15 affiliated hospitals and 36 private physicians (listed in http://www.shcs.ch/180-health-care-providers). The authors would like to thank all clinicians and clinical research technicians participating to the SAIDCC database of the Infectious Diseases Unit of St Antoine Hospital, AP-HP, Paris, France. The authors acknowledge the contribution of the AQUITAINE (ANRS CO3 AQUITAINE/AquiVIH-NA) members their participants (website: https://aquivih-na.fr/). The authors acknowledge the contribution of the CoRIS steering committee members their participants. We wish to thank Campbell Aitken for editing the manuscript. This study was funded by the Australian Government National Health and Medical Research Council (Grant number GNT1132902). The funders had no role in the study design, data collection, analyses or interpretation of the data.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2022.101810.
Appendix ASupplementary data
References
- 1.Polaris Observatory HCV Collaborators Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7(5):396–415. doi: 10.1016/S2468-1253(21)00472-6. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Hepatitis C. 2022. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
- 3.Sogni P., Gilbert C., Lacombe K., et al. All-oral direct-acting antiviral regimens in HIV/hepatitis C virus-coinfected patients with cirrhosis are efficient and safe: real-life results from the prospective ANRS CO13-HEPAVIH cohort. Clin Infect Dis. 2016;63(6):763–770. doi: 10.1093/cid/ciw379. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . 2021. Interim guidance for country validation of viral hepatitis elimination. [DOI] [PubMed] [Google Scholar]
- 5.Busschots D., Ho E., Blach S., et al. Ten years countdown to hepatitis C elimination in Belgium: a mathematical modeling approach. BMC Infect Dis. 2022;22(1):397. doi: 10.1186/s12879-022-07378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon J.A., Dore G.J., Grebely J., et al. Australia on track to achieve WHO HCV elimination targets following rapid initial DAA treatment uptake: a modelling study. J Viral Hepat. 2019;26(1):83–92. doi: 10.1111/jvh.13013. [DOI] [PubMed] [Google Scholar]
- 7.van Dijk M., Brakenhoff S.M., Isfordink C.J., et al. The Netherlands is on track to meet the World Health Organization hepatitis C elimination targets by 2030. J Clin Med. 2021;10(19) doi: 10.3390/jcm10194562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salazar-Vizcaya L., Kouyos R.D., Zahnd C., et al. Hepatitis C virus transmission among human immunodeficiency virus-infected men who have sex with men: modeling the effect of behavioral and treatment interventions. Hepatology. 2016;64(6):1856–1869. doi: 10.1002/hep.28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser H., Martin N.K., Brummer-Korvenkontio H., et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol. 2018;68(3):402–411. doi: 10.1016/j.jhep.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall A.D., Cunningham E.B., Nielsen S., et al. Restrictions for reimbursement of interferon-free direct-acting antiviral drugs for HCV infection in Europe. Lancet Gastroenterol Hepatol. 2018;3(2):125–133. doi: 10.1016/S2468-1253(17)30284-4. [DOI] [PubMed] [Google Scholar]
- 11.hepColalition . 2021. Have a heart, save my liver? Which countries restrict treatment? [Google Scholar]
- 12.Artenie A., Luhmann N., Lim A.G., et al. Methods and indicators to validate country reductions in incidence of hepatitis C virus infection to elimination levels set by WHO. Lancet Gastroenterol Hepatol. 2022;7(4):353–366. doi: 10.1016/S2468-1253(21)00311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thein H.-H., Yi Q., Dore G.J., Krahn M.D. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22(15):1979–1991. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 14.Castry M., Cousien A., Bellet J., et al. Hepatitis C virus (HCV) incidence among men who have sex with men (MSM) living with HIV: results from the French Hospital Database on HIV (ANRS CO4-FHDH) cohort study, 2014 to 2017. Euro Surveill. 2021;26(38) doi: 10.2807/1560-7917.ES.2021.26.38.2001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyle J.S., van Santen D.K., Iser D., et al. Microelimination of hepatitis C among people with human immunodeficiency virus coinfection: declining incidence and prevalence accompanying a multicenter treatment scale-up trial. Clin Infect Dis. 2021;73(7):e2164–e2172. doi: 10.1093/cid/ciaa1500. [DOI] [PubMed] [Google Scholar]
- 16.Harney B.L., Sacks-Davis R., van Santen D.K., et al. The incidence of hepatitis C among gay, bisexual, and other men who have sex with men in Australia, 2009-2019. Clin Infect Dis. 2021;74(10):1804–1811. doi: 10.1093/cid/ciab720. [DOI] [PubMed] [Google Scholar]
- 17.Smit C., Boyd A., Rijnders B.J.A., et al. HCV micro-elimination in individuals with HIV in The Netherlands 4 years after universal access to direct-acting antivirals: a retrospective cohort study. Lancet HIV. 2021;8(2):e96–e105. doi: 10.1016/S2352-3018(20)30301-5. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson A.L., van Santen D.K., Traeger M.W., et al. Hepatitis C incidence among patients attending primary care health services that specialise in the care of people who inject drugs, Victoria, Australia, 2009 to 2020. Int J Drug Policy. 2022;103 doi: 10.1016/j.drugpo.2022.103655. [DOI] [PubMed] [Google Scholar]
- 19.Braun D.L., Hampel B., Ledergerber B., et al. A treatment-as-prevention trial to eliminate hepatitis C among men who have sex with men living with human immunodeficiency Virus (HIV) in the Swiss HIV cohort study. Clin Infect Dis. 2021;73(7):e2194–e2202. doi: 10.1093/cid/ciaa1124. [DOI] [PubMed] [Google Scholar]
- 20.Kusejko K., Salazar-Vizcaya L., Shah C., et al. Sustained effect on hepatitis C elimination among men who have sex with men in the Swiss HIV Cohort Study: a systematic re-screening for hepatitis C RNA two years following a nation-wide elimination program. Clin Infect Dis. 2022;75(10):1723–1731. doi: 10.1093/cid/ciac273. [DOI] [PubMed] [Google Scholar]
- 21.Lazarus J.V., Safreed-Harmon K., Thursz M.R., et al. The micro-elimination approach to eliminating hepatitis C: strategic and operational considerations. Semin Liver Dis. 2018;38(3):181–192. doi: 10.1055/s-0038-1666841. [DOI] [PubMed] [Google Scholar]
- 22.Sacks-Davis RvS., Boyd D.K., Young A., Spelman J., Stewart T.A. 2022. Incidence of HCV reinfection among people with HIV prior to and during periods of limited and broad access to direct-acting antiviral therapies for HCV in six countries AIDS 2022. Montreal, Canada. [Google Scholar]
- 23.Fanciulli C., Berenguer J., Busca C., et al. Epidemiological trends of HIV/HCV coinfection in Spain, 2015-2019. HIV Med. 2022;23(7):705–716. doi: 10.1111/hiv.13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonneveld M.J., Veldhuijzen I.K., van de Laar T.J.W., Op de Coul E.L.M., van der Meer A.J. Decrease in viral hepatitis diagnoses during the COVID-19 pandemic in The Netherlands. J Hepatol. 2021;77(3):896–897. doi: 10.1016/j.jhep.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binka M., Bartlett S., Velasquez Garcia H.A., et al. Impact of COVID-19-related public health measures on HCV testing in British Columbia, Canada: an interrupted time series analysis. Liver Int. 2021;41(12):2849–2856. doi: 10.1111/liv.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shakeri A., Konstantelos N., Chu C., et al. Global Utilization trends of Direct Acting Antivirals (DAAs) during the COVID-19 pandemic: a time series analysis. Viruses. 2021;13(7) doi: 10.3390/v13071314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traeger M.W., van Santen D.K., Sacks-Davis R., et al. Impact of COVID-19 lockdown restrictions on hepatitis C testing in Australian primary care services providing care for people who inject drugs. J Viral Hepat. 2022;29(10):908–918. doi: 10.1111/jvh.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Bilsen W.P.H., Zimmermann H.M.L., Boyd A., et al. Sexual behavior and its determinants during COVID-19 restrictions among men who have sex with men in amsterdam. J Acquir Immune Defic Syndr. 2021;86(3):288–296. doi: 10.1097/QAI.0000000000002581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reyniers T., Rotsaert A., Thunissen E., et al. Reduced sexual contacts with non-steady partners and less PrEP use among MSM in Belgium during the first weeks of the COVID-19 lockdown: results of an online survey. Sex Transm Infect. 2021;97(6):414–419. doi: 10.1136/sextrans-2020-054756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koopsen J., Parker E., Han A.X., et al. Hepatitis C virus transmission among men who have sex with men in amsterdam: external introductions may complicate microelimination efforts. Clin Infect Dis. 2021;72(12):e1056–e1063. doi: 10.1093/cid/ciaa1830. [DOI] [PubMed] [Google Scholar]
- 31.Boerekamps A., Newsum A.M., Smit C., et al. High treatment uptake in HIV/HCV-coinfected patients after unrestricted access to direct-acting antivirals in The Netherlands. Clin Infect Dis. 2017;66(9):1352–1359. doi: 10.1093/cid/cix1004. [DOI] [PubMed] [Google Scholar]
- 32.Pol S., Fouad F., Lemaitre M., et al. Impact of extending Direct Antiviral Agents (DAA) availability in France: an observational cohort study (2015-2019) of data from French administrative healthcare databases (SNDS) Lancet Reg Health Eur. 2022;13 doi: 10.1016/j.lanepe.2021.100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isfordink C.J., Smit C., Boyd A., et al. Low hepatitis C virus-viremia prevalence yet continued barriers to direct-acting antiviral treatment in people living with HIV in The Netherlands. AIDS. 2022;36(6):773–783. doi: 10.1097/QAD.0000000000003159. [DOI] [PubMed] [Google Scholar]
- 34.Lee Wilkinson A., Pedrana A., Traeger M.W., et al. Real-world monitoring progress towards the elimination of hepatitis C virus in Australia using sentinel surveillance of primary care clinics; an ecological study of hepatitis C virus antibody tests from 2009 to 2019 - CORRIGENDUM. Epidemiol Infect. 2022;150:e44. doi: 10.1017/S0950268822000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Traeger M.W., van Santen D.K., Sacks-Davis R., et al. Virtual; 2021. Trends in time to treatment initiation among people diagnosed with hepatitis c in a network of Australian clinical services between 2015-2019. The International Network on Health and Hepatitis in Substance Users. [Google Scholar]
- 36.Aleman S., Soderholm J., Busch K., Kovamees J., Duberg A.S. Frequent loss to follow-up after diagnosis of hepatitis C virus infection: a barrier towards the elimination of hepatitis C virus. Liver Int. 2020;40(8):1832–1840. doi: 10.1111/liv.14469. [DOI] [PubMed] [Google Scholar]
- 37.Harney B.L., Whitton B., Paige E., et al. A multi-site, nurse-coordinated hepatitis C model of care in primary care and community services in Melbourne, Australia. Liver Int. 2022;42(3):522–531. doi: 10.1111/liv.15107. [DOI] [PubMed] [Google Scholar]
- 38.Prinsenberg T., Schinkel J., Zantkuijl P., Davidovich U., Prins M., van der Valk M. Internet-guided HCV-RNA testing: a promising tool to achieve hepatitis C micro-elimination among men who have sex with men. J Viral Hepat. 2022;29(8):677–684. doi: 10.1111/jvh.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durham M.D., Armon C., Novak R.M., et al. Longitudinal changes in, and factors associated with, the frequency of condomless sex among people in care for HIV infection, HIV Outpatient Study USA, 2007-2019. AIDS Behav. 2022;26(10):3199–3209. doi: 10.1007/s10461-022-03655-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacGregor L., Speare N., Nicholls J., et al. Evidence of changing sexual behaviours and clinical attendance patterns, alongside increasing diagnoses of STIs in MSM and TPSM. Sex Transm Infect. 2021;97(7):507–513. doi: 10.1136/sextrans-2020-054588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serrano-Villar S., Martinez-Sanz J., Ron R., et al. Effects of first-line antiretroviral therapy on the CD4/CD8 ratio and CD8 cell counts in CoRIS: a prospective multicentre cohort study. Lancet HIV. 2020;7(8):e565–e573. doi: 10.1016/S2352-3018(20)30202-2. [DOI] [PubMed] [Google Scholar]
- 42.Boender T.S., Smit C., Sighem A.V., et al. AIDS Therapy Evaluation in The Netherlands (ATHENA) national observational HIV cohort: cohort profile. BMJ Open. 2018;8(9) doi: 10.1136/bmjopen-2018-022516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMahon J.H., Moore R., Eu B., et al. Clinic network collaboration and patient tracing to maximize retention in HIV care. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0127726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scherrer A.U., Traytel A., Braun D.L., et al. Cohort profile update: the Swiss HIV Cohort Study (SHCS) Int J Epidemiol. 2022;51(1) doi: 10.1093/ije/dyab141. 33-4j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.