Highlights
-
•
Ultrasound assisted extraction (UAE) used mild extraction strategies to extract oil.
-
•
UAE oil yield and recovery were comparable with Soxhlet extraction.
-
•
Mechanical pressing yielded < 50% of UAE.
-
•
Triacylglycerol profile and fatty acid composition were unaffected by UAE.
-
•
UAE helps in co-extracting antioxidants (carotene and tocopherol) from mahua oil.
Keywords: Advanced oil extraction, Mahua oil, Ultrasound, Underutilized, Antioxidant, Seed
Abstract
Ultrasound-assisted solvent extraction (UAE) was applied to extract underutilized Madhuca longifolia seed oil. The effect of extraction time, temperature, solvent type, solvent/sample ratio, and amplitude on the oil yield and recovery were investigated. Approximately 56.97% of oil yield and 99.54% of oil recovery were attained using mild conditions of 35 min, 35 °C, 40% amplitude, isopropanol to acetone (1:1), and solvent to sample (20 mL/g). UAE oil yield and recovery were comparable with Soxhlet extraction (SXE) whilst mechanical pressing (ME) yielded < 50% of UAE recovery. UAE does not affect the fatty acids composition (46% C18:1; 22% C16:0; 21% C18:0, 10% C18:2), and triacylglycerol profile (23% POO, 17% POS, 16% SOO, and 14% POP). Interestingly, UAE extracted oil conferred remarkably (P < 0.05) higher antioxidant capacity (IC50 of DPPH 106.60 mg/mL and ABTS 39.80 mg/mL) than SXE (IC50 of DPPH 810.40 mg/mL and ABTS 757.43 mg/mL) or ME (IC50 of DPPH 622.38 mg/mL and ABTS 392.87 mg/mL).
1. Introduction
Mahua (Madhuca longifolia), generally known as Koenig or buttercup fruit, is a wild tropical plant explicitly grown in the Indo-Pakistan sub-continent, the Central part of Sri Lanka, and Myanmar. Currently, mahua cultivation has extended to central Africa as well. This multipurpose tree (family Sapotaceae) with its seeds, flowers, leaves, and timber is economically crucial in the medicinal, nutraceutical, and renewable energy industries. Even though mahua is underutilized for oil production, seeds are valued for high oil content (50–60%). According to Indian sources, annual mahua oil production is around 60 million tonnes in India, mainly favoring industrial purposes [1]. It is prominently used in the biofuel industry, but applications in the food industry are scanty. The lack of technical information on its composition and properties may restrict its commercialization for food applications.
The mahua oil profile shows potential to be used as a specialty fat for food applications critically attributed to its fatty acid (stearic, oleic, and palmitic) and triacylglyceride compositions (POP, SOS, and POS). For example, the literature suggests that mahua butter can be used in shortening and chocolate manufacturing [2]. Another distinct feature of mahua oil is the existence of valuable phytonutrients consisting of tocopherols, phytosterols, terpenoids, and tocotrienols that results in health-promoting factors such as anticancer, anti-inflammation, and antidiabetic properties [3]. In addition, the antioxidative properties of these bioactive compounds also provide oils with excellent oxidative stability extending their shelf life. Hence, the presence of natural antioxidants co-extracted with the mahua oil is important as a potential replacer for harmful synthetic antioxidants frequently added to commercial cooking oil to delay oxidative rancidity that otherwise leads to the formation of off-odor and toxic compounds. On such an account, extraction and retention of these natural antioxidants become equally important as it aids to repurpose and valorize the underutilized mahua seeds in producing antioxidant-rich mahua seed oil, which is essential for the applications in the nutraceutical and food industries. Henceforth, finding efficient extraction strategies is crucial to co-extract the phytonutrients concentrated in mahua oil. Also, extraction technologies should be compatible with environmental sustainability.
Ultrasound-assisted extraction (UAE) is an emerging technique recently applied in seed oil extraction to replace conventional Soxhlet extraction that requires extensive solvent usage and an extended processing period. Due to its unique mechanism, UAE has distinguished itself among other extraction approaches. The UAE shows an outstanding ability to enhance oil extractability using shorter extraction times, lower temperatures, and less energy and solvents than conventional Soxhlet and mechanical extractions. Ultrasound application works on the cavitation or oscillation phenomenon generated by ultrasound probe (direct method) or bath sonicator devices (indirect method). Ultrasonic waves generate vibrations creating voids that transfer energy to solid particles immersed in the medium. In addition, cavitation bubbles grow closer to the solid surface and collapse at a higher amplitude forcing the cell wall to rupture, further accelerating the transfer of desired compounds trapped inside into the solvent medium. Simply, ultrasound vibrations generate physical forces upon acoustic streaming, cavitation shear forces, shock waves, and microjet into the cell matrix [4].
The ultrasound has given promising results in oil extraction for many oil-bearing vegetables, fruits, and flower seeds; for instance, pomegranate, kapok, and papaya seeds [5], [6], [7]. Most UAE studies demonstrated increased oil yield without modifying the oil profile; evidently, UAE resulted in 32.27% increased yield from papaya oil compared to 25.27% from Soxhlet whilst maintaining similar physicochemical properties. Concurrently, UAE can maximize antioxidant capacity, as affirmed by Böger et al., [8] who reported increased phenolic contents of 68.82 g GAE/kg, and enhanced reducing power of 44.06 MTE/kg, significantly higher than the sample without ultrasound for grape seed oil. Nevertheless, UAE has not been used to extract mahua seed oil even though some conventional methods such as Soxhlet and mechanical extractions had been reported in mahua seed oil extraction [2], [3].
Therefore, the current study aimed to investigate the extraction of mahua seed oil by UAE. The profile of the resulting oil was then compared with those extracted via conventional Soxhlet and mechanical extraction methods. The authors hypothesized that the performance of the UAE is efficient and significant enough to meet the maximum performance of Soxhlet, currently prevailing in the industry. Moreover, the mild processing conditions employed for UAE would assist in preserving bioactives eluted into the oil. As the current applications of mahua oil are confined to native Asian culinary dishes, utilization of advanced extraction technologies i.e., UAE which is regarded to be sustainable, efficient, and mild would further extend the applications of Mahua seed oil beyond the household setting to a more worthwhile application in food and nutraceutical industries.
2. Materials and methods
2.1. Materials
Mahua seeds (Madhuca longifloia) were purchased from the local market in the central part of Sri Lanka. Damaged or spoiled seeds were sorted out, eliminated, and the seed coat was removed manually. After wiping out any dust, sorted seeds were air-dried at room temperature before milling into a mesh size of ≤1.4 mm using a hammer miller and stored at −18 °C for future use. All the chemicals and solvents used were of analytical grade or HPLC grade.
2.2. Conventional mechanical extraction (ME)
Approximately 10 g of seeds was measured, put into the household oil press machine (AOSIDA, China), and kept the machine on for 5 min to preheat. Then, seeds were squeezed at a temperature above 90 °C for about 5 min, and the crude oil was collected after filtering through a 50-µm mesh size.
2.3. UAE-Extraction and Soxhlet extraction
The UAE extraction was performed using an ultrasound probe (COLE-PAPMER, USA) at 24 kHz of frequency and a maximum power of 500 W. Approximately 5 g of dried seed powder was extracted with 80 mL of n-hexane at 1:16 (w/v) of solid to solvent ratio in a closed system covered with parafilm and aluminum foil. The ultrasound probe was immersed in the solvent within 5–10 mm depth. The probe was adjusted to generate a wavelength to amplitude level of 60% for oil extraction performed at a temperature of 30 °C and duration of 30 min. A water bath was used to maintain the temperature during the ultrasound extraction. Extraction was repeated under different extraction times (5 to 45 min), temperatures (25 to 55 °C), amplitude levels (20–80%) and solvent/sample ratios (5 to 25 mL/g), and solvents (hexane, ethanol, isopropanol, acetone, and solvent mixtures of hexane and isopropanol, hexane and acetone, isopropanol and acetone in 1:1 ratio).
Soxhlet extraction, which is the conventional method for oil extraction, was used as a control. Soxhlet extraction was carried out in a Soxhlet apparatus until the complete recovery of oil. Nearly 10 g of seed powder was measured into a thimble, and 200 mL of n-hexane was used for the extraction. The condenser unit was supplied with cold water continuously. Extraction was performed at a temperature above 80 °C for about 8 h.
After each extraction, mahua seed powder and solvent were separated via filtration by a Whatman No.1 under vacuum and the filtrate was further centrifuged. The solvent was removed via a rotary evaporator, and the remaining solvent was eliminated by drying in an oven at 50 °C until a constant weight was achieved. Finally, the oil was weighed and stored in amber vials at − 18 °C until further use.
2.4. Oil extraction yield and recovery
The extraction yield and recovery were calculated by the following formula;
| (1) |
| (2) |
| (3) |
2.5. Characterization of the mahua seed oil
The mahua seed oil extracted from the following UAE conditions: 35 min, 35 °C, 20 g/mL, 40%, and ISP: Acetone (1:1) which resulted in the highest oil yield and recovery of 56.97% and 99.54%, respectively was further analyzed for physicochemical properties, oil composition, and phytonutrients in comparison with the seed oil extracted from SXE and ME.
2.5.1. Physiochemical properties of oil
Iodine value, saponification value, acid value, and free fatty acid value were analyzed based on the AOAC methods 993.20, 920.160, and 940.28, respectively. The refractive index of the extracted oil was measured using an automatic digital refractometer (ATAGO PAL-3, Japan) at 25 °C. The color parameter was evaluated using a color spectrophotometer (Hunterlab-ClorFlex, USA) at 25 °C.
2.5.2. Fatty acid composition
Fatty acid composition (FAC) was determined in terms of fatty acid methyl esters (FAME) in gas chromatography (GC) (Agilent Technologies 7890A, USA) equipped with Flame Ionization Detector (FID). FAME was prepared based on AOCS Ch 1–91 with minor changes. The oil sample (200 mg) was measured into a 15 mL centrifuge tube, and 2 mL of hexane was added, followed by 1 mL of 2 M methanolic KOH. The closed tube was shaken vigorously for 30 s and centrifuged. The hexane layer containing FAME was placed in a GC vial for analysis. Then, 1 µL of the derivatized FAME was injected into the capillary column Agilent HP-INNOWax (30 m × 250 µm × 0.2 5 µm) for the analysis. The injector and detector temperatures were set at 250 °C. The oven temperature was programmed as follows: holding at 60 °C for 2 min, increasing up to 200 °C at a rate of 10 °C/min, increasing from 200 to 240 at a rate of 5 °C/min and hold for 18 min and nitrogen was used as carrier gas. The FAME mix C8-C24 (Supelco, Sigma Aldrich) standard was used for peaks identification.
2.5.3. Triacylglycerides
The triacylglycerol profile of mahua seed oil was determined by reverse-phase high performance liquid chromatography (R-HPLC) (Agilent Technologies 1200 Infinity Series, USA) equipped with a RID detector. The oil samples were prepared by dissolving the oil in an acetone and chloroform (1:1) mixture and then filtered through a 0.45 µm PTFE membrane filter before injecting 20 ul aliquots into the machine. The stationary phase was a LiChrospher C18 RP column (250 mm × 4.0 mm, 5 µm particle size), and the column temperature was set at 30 °C. The mobile phase was maintained under isocratic elution of acetone and acetonitrile in a 70:30 (w/w) ratio at a flow rate of 1.0 mL/min for 40 min. Cocoa butter (Merck, Sigma Aldrich) as the Reference TAG was used to identify peaks in the chromatogram following Equivalent Carbon Number (ECN), and quantification was based on the specific area.
| (3) |
2.5.4. Secondary metabolites
The LC-MS analysis was performed on an Agilent 1290 Infinity LC system (Thermo Finnigan, San Jose, CA, USA) coupled to Agilent 6520 Accurate-Mass Q-TOF mass spectrometer with dual ESI source. Separation of compounds was carried in an XDB-C18 column (150 mm × 2.1 mm, 3.5 µm) (Agilent Technologies, Eclipse) at a column temperature of 25 °C. Injection volume of 1.0 µL from the acetone dissolved oil samples was eluted with formic acid/water (0.1:99.9, v/v) and formic acid/acetonitrile (0.1:99.9, v/v) as the mobile phase at a flow rate of 0.5 mL/min. The total run time was 25 min including 5 min of post run. Spectra acquisition at a rate of 1.03 (spectra/s) in the Q-TOF MS detector was made in full scan (100–3200 m/z) at capillary voltage of 4000 V in positive polarity. MS parameters were set as follows: nebulizer pressure at 45 psi, skimmer 65 V, drying gas temperature at 300 °C and drying gas flow at 10 L/min. Agilent Mass Hunter Qualitative Analysis B.07.00 was used for LC-MS data analysis.
2.5.5. Thermal behavior
The crystallization and heating profiles of the mahua seed oil were analyzed using a differential scanning calorimeter (PerkinElmer DSC 4000). Mahua oil sample of 5–8 mg was placed in an aluminum pan and hermetically sealed whilst an empty pan was used as the reference. The oil samples were heated up to 80 °C and held for 10 min to destroy any crystal memory in the oil sample. Then oil pans were cooled to − 50 °C at a rate of 5 °C/min to capture the crystallization thermogram. Next, the temperature was increased from − 50 °C to 80 °C at the rate of 5 °C/min to get the heating profile.
2.5.6. Beta carotene content
The total carotenoid content of oil samples was calculated following the method mentioned by Davarnejad et al. [9] with some modifications. One percent oil solution was prepared by dissolving in chloroform, and the absorbance was measured at 450 nm using a spectrophotometer (Uviline 9400, SECOMAM). A standard curve was prepared by β-carotene, and the total carotenoid content of oil was expressed in terms of β-carotene.
2.5.7. Tocopherol content
The tocopherol content was determined by RP-HPLC (Agilent Technologies 1200 Infinity Series, USA) equipped with a DAD detector. The oil sample was dissolved in acetone: chloroform (1:1 v/v) mixture and filtered through a 0.45 µm PTFE membrane filter before injecting into the HPLC system including a RP-C18-5 column (250 × 4.0 mm), fixing the wavelength at 290 nm and 330 nm, respectively. The mobile phase was acetonitrile: methanol (1:1 v/v), and the flow rate was set at 1 mL/min. The standards of alpha, beta, and gamma tocopherols were used to prepare the calibration curves for the calculation of tocopherols. The results were expressed as mg of tocopherol per kg of oil.
2.6. Antioxidative properties
2.6.1. Sample preparation for antioxidant activity and total phenolic content
The methanolic extracts of mahua seed oil were prepared using the modified procedure described by Muangrat et al. [10] with minor changes. Briefly, 1 g of each mahua seed oil was mixed with 1 mL of n-hexane followed by vortexing with 1 mL of aqueous methanol solution (water: methanol, 1:4 v/v) and the mixture was further centrifuged at 6500 rpm for 15 min. The oil dissolved hexane layer was discarded whilst the methanolic phase was recovered into brown glass vials to protect against light and further used for total phenolic content and antioxidant capacity evaluation. The absorbance for all the analyses was measured using a spectrophotometer (Uviline 9400, SECOMAM).
2.6.2. The total phenolic content (TPC), DPPH Assay, ABTS assay and FRAP
The TPC of methanolic extracts of mahua seed oil was analyzed according to the Folin–Ciocalteu spectrophotometric method reported by Cisneros et al. [11]. Briefly, 200 µL of the extract was mixed with 250 µL of the Folin–Ciocalteu. A standard curve of gallic acid was used to determine the TPC content, and results were expressed as milligrams of Gallic Acid Equivalent per kilogram of oil (mg GAE/kg oil sample). Meanwhile, DPPH assay was followed according to the modified method of Muangrat et al. [10] for different methanolic concentrations of oil samples (50–1000 mg/mL) in the ratio of 1:4 (v/v) for UAE and ME extracted oil, and 1:1(v/v) for SXE extracted oil. ABTS assay was performed according to a standard method, as modified by Liu et al. [12] and Okoh et al. [13] Trolox was used as the reference standard for both free radical assays and the inhibition percentage against the concentration of methanolic extract or the standard was calculated following the formula.
| (5) |
where control is the absorbance of the DPPH solution and methanol (same quantity of methanolic extract), the sample is the absorbance of DPPH solution mixed with methanolic oil extract or the reference standard.
The IC50 value shows the required concentration of the test sample to scavenge 50% of DPPH radicals. This value is calculated by the graph plotting the inhibition percentage against the different concentrations of the methanolic extract of oil and the reference standard.
According to Szydłowska-Czerniak et al. [14], FRAP method was performed for the methanolic extracts (50 µL for UAE, 500 µL for ME, and 900 µL for SXE) of mahua oil. Three standard curves were prepared from Trolox performing the same method for UAE, ME, and SXE treated methanolic extracts. Reducing power was expressed in mg of Trolox equivalent per kg of oil.
2.7. Scanning Electron microscopy
The surface morphology of mahua seed powder treated with UAE, ME, and SXE was observed through Scanning Electron Microscope (HITACHI SU8010, Japan) operating at 5 kV voltage under room temperature. Before loading into the machine, samples were gold coated (Quorum, Q150R S).
2.8. Statistical analysis
All the analysis was performed with duplicate or triplicate analysis. The result was expressed as mean ± standard deviation. One-way ANOVA and Tukey test were performed to determine the significant difference at p < 0.05.
3. Results and discussion
3.1. Effect of UAE extraction conditions on oil yield
3.1.1. Extraction time
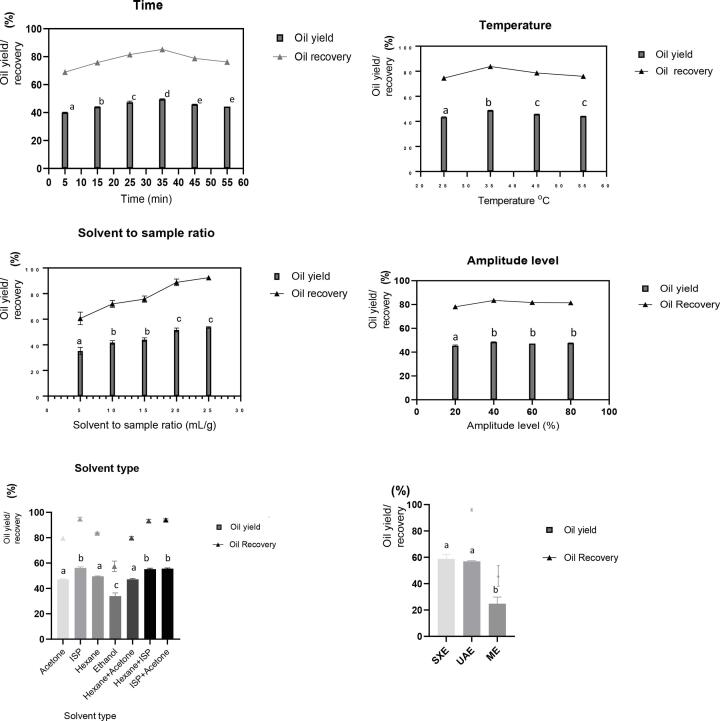
According to Fig. 1, an increase in extraction time from 25 to 35 min resulted in a significant increment of the oil yield, attaining the maximum of 49.5%, and declined slightly thereafter. At the early stage of extraction, the mass transfer rate is high due to the driving force caused by the effortless acoustic cavitation and the high concentration gradient [15], [16]. Once the equilibrium has reached, the mass transfer decelerates, resulting in lower yields. Several authors have reported a similar trend. For instance, Kapok seed oil yield increased from 5 to 10 min and then leveled off [6]. Among the optimum extraction times reported for other seed oils, which are common in the range of 5–60 min; <30 min for tea seed oil (approximately 26% yield and above 85% recovery) [17], 44 min for Isatis indigotica Fort seed oil (13.7% yield and 81% oil recovery) [18], and mahua seed oil extraction time of 35 min obtained in the present study lies in between that of literature findings. The discrepancy may be attributed to seeds' physical properties, including hardness, surface nature (smooth/rough), and the amount of oil trapped within the matrix.
Fig. 1.
UAE parameters against the yield, oil recovery, and comparison of UAE, SXE and ME; different letters over the bar shows significant difference at p < 0.05.
3.1.2. Temperature
The temperature also influences acoustic cavitation significantly. In general, temperature increment favors the mass transfer rate. The mahua oil yield peaked at 35 °C generating 48.7% of oil yield and 74.6% of oil recovery (Fig. 1). A high mass transfer can be explained by the enhanced diffusivity and penetration of solvent due to the reduction in viscosity resulting from the weakened surface tension [19]. Similarly, Thirugnanasambandham [20] and Xu et al. [21] who extracted oil from moringa seeds and rice assisting ultrasound technology, confirmed that 35 °C and 30 °C gave the best yields of 59% and 72.35%, respectively. Even though recovery of mahua oil remained above 70% throughout the temperature increment, a higher operating temperature may negatively impact oil yield. Although more bubbles generate at elevated temperatures, they collapse at a low intensity retarding oil extractability [22] which is affirmed by lower yields of mahua oil after 35 °C. Many UAE studies have exhibited a notable temperature range of 15–60 °C as favorable for oil extraction despite lower yield obtained from prolonged heating [8], [15], [23], [24].
3.1.3. Solvent to sample ratio
As compared to other UAE parameters, an apparent upward trend for oil yield can be observed with increasing solvent to sample ratio (SSR) from 5 to 25 mL/g achieving the highest of 53.7% at 25 mL/g (Fig. 1), which can be ascribed to the increasing concentration gradient between sample and solvent and the driving force which resulted from ultrasonic cavitation. Goula [25] reported a similar outcome from pomegranate seed oil, where yield increased from 35 to 42% upon the increment of SSR from 1:4 to 1:20 (w/v). More importantly, this factor exhibited the highest oil recovery (92.96%) as compared to other processing parameters: time (87.58%), temperature (84.11%), and amplitude (83.13%), suggesting that SSR is the most significant factor. Evidently, Rojo-Gutiérrez et al. [5] and Santos et al. [26] indicated that the most influencing parameter for UAE is SSR for the extraction of pomegranate and favela seed oils. Noticeably, oil recovery was minimum (57.16%) at a 1:5 (w/v) SSR ratio and maintained above 70% beyond that, which implies that the minimum requirement to activate the bubble cavitation by ultrasonic voids would be 1:10 (w/v). According to Fig. 1, SSR 1:20 (w/v) was selected as the best condition as there was no significant difference (p < 0.05) between 1:20 and 1:25 (w/v). Also, some authors suggest that the unlimited solvent volume may not increase the oil yield as less solid concentration results in lower nucleation sites for the cavitation [5], [26].
3.1.4. Amplitude level
As per Fig. 1, when the amplitude level was increased from 20% to 40%, the yield of mahua seed oil was elevated significantly, reaching 48.5%, and thereafter remained slightly lower without any significant difference (p < 0.05). The recovery of oil yield was observed between 77.3 and 83.7% when increasing the amplitude from 20 to 80%, suggesting that the efficiency of UAE is appreciable. By increasing the amplitude level, more energy is supplied into the medium. Therefore, bubbles collapse more violently, extending the collapsing radius even 10 times more, resulting in the rupture and fragmentation of the cells. At the same time, the mass transfer rate also accelerated with the increase in amplitude level. Such a phenomenon enhances the release of fat globules from plant cells. The UAE study performed by Böger et al. [8] demonstrated similar results where the recovery of grape seed oil increased from 86.2 to 90.0% when elevating the amplitude from 24 to 96 µm. However, at a certain level, the energy is dissipated among bubbles as they form in plenty resulting inefficient collapsing and less extractability. In addition, some authors suggest that amplitude must be controlled with care, as extreme conditions may force over intense cavitation, destroying molecules inside the cell. In other words, ultrasonic waves can behave in both destructive and protective manner based on the operating energy conditions [27].
3.1.5. Type of solvent
The most common solvent used in the oil industry for Soxhlet extraction is hexane due to its higher oil dissolution. However, according to the Food and Drug Administration [28], hexane is categorized in group one, defined as toxic, high risk, and with a recommended residual level of 290 ppm. In contrast, group three solvents like acetone, ethanol, and isopropanol are considered green solvents where a residual level of 5000 ppm is allowed. In comparison, the effect of acetone, hexane, ethanol, isopropanol (ISP), and their mixtures in a 1:1 ratio on the yield of mahua oil was evaluated. Compared to hexane (49.53%), acetone (47.13%) and isopropanol (56.12%) exhibited similar and better results, while ethanol yielded considerably less (33.99%). According to Freed et al. [29], the spectral polarity indexes of solvents are; hexane (2.56), isopropanol (7.87), acetone (8.01), and ethanol (8.05). Based on the polarity index, we assumed oil yield could be varied as hexane > isopropanol > acetone > ethanol. However, isopropanol attained the highest yield, as observed in the present study, which can be ascribed to the extraction of alcohol-soluble molecules such as alkaloids along with triglycerides [30]. Similar results were reported by Perrier et al. [31] in a study of rapeseed oil extraction by UAE, presenting 65% of yield from ISP and 67% from hexane whilst 41% by ethanol.
Although ISP resulted in the highest oil yield, removal of the solvent from the oil system is inefficient due to its high boiling point (84 °C). As an alternative, by mixing ISP with either hexane or acetone, the solvent boiling point can be reduced easing the solvent evaporation. Interestingly, binary mixtures of hexane + ISP (1:1), and acetone + ISP (1:1), were efficient resulting in above 50% yield and 95% recovery depicting the identical performance of a single solvent. Since acetone is more economical and ecologically feasible than hexane, the binary mixture of acetone + ISP (1:1) would be the best solvent type for a sustainable and efficient industry. Moreover, viscous compounds like waxes and resins may be extracted due to the diverse affinities of solvents. Thus, the synergetic action of cavitation and the affinity of oil towards the solvent may cause the discrepancy in the oil yields to different solvents.
3.1.6. UAE vs conventional extractions on oil recovery
UAE produced 99.5% of oil recovery (Eq 2) utilizing a shorter extraction time of 35 min and at a lower temperature of 35 °C as compared to Soxhlet, which used 8 h of extraction time and temperature above 80 °C whilst oil recovery from mechanical pressing could reach only 42.72% (Eq 3) which is not even half of the recovery obtained from UAE and SXE. Also, the energy usage of UAE (210 kJ/g) is approximately 10 times lower than SXE (1920 kJ/g) when taking into account the same solvent volume usage and two folds lower than ME (450 kJ/g). The acoustic cavitation of the ultrasound waves ruptured the cells with the subsequent oil dissolution, making the extraction process more effective than SXE and ME. Agreeable results were reported for Kapok seed oil with a 92.3% of oil recovery from UAE under a shorter extraction time of 10 min than solvent extraction by agitation (5.7 h) and SXE (8 h). More importantly, the authors highlight that the energy consumption of UAE is 80 folds lower than SXE and 50 folds lesser than solvent extraction [6]. Further, 89% of oil recovery from radish seeds was reported within 60 min compared to 8 h of SXE [15], and notably, Zhang et al. [7] reported a yield of 32.27% from UAE (20 min) comparatively higher than 25.27% from SXE (10 h). The remarkable performance of UAE in extracting oil is almost equivalent to SXE, but operated under mild processing conditions (low temperature and short time) effectively reduces the energy consumption used for oil extraction. Therefore, the effectiveness of UAE allows the use of green solvents to replace toxic solvents commonly used in conventional SXE, providing an added advantage in performing seed oil extraction in a sustainable, nature-friendly circumstance.
3.2. The physicochemical properties of mahua seed oil
The physicochemical properties of mahua oil extracted from UAE, SE, and ME are presented in Table 1. Even though the refractive index, iodine, and saponification values of the oil extracted from the three approaches are significantly different (P < 0.05), the variation is minor. The results of the physicochemical parameters of mahua oil are comparable with the data reported by Munasinghe & Wansapala [32]. However, free fatty acid and acid values of UAE oil were significantly lower than SXE and ME (p < 0.05), indicating higher hydrolytic stability. Since the hydrolysis of oil is mainly caused by moisture, when oil is exposed to air for a long time (8 h) in Soxhlet extraction, FFA may be increased, while a shorter extraction time of UAE (35 min) limits the hydrolysis. Further, as suggested by Tan et al. [33], triacylglycerols are mainly degraded by lipases, and the acoustic inactivation of such endogenous enzymes may lower the hydrolysis of oil. On the other hand, oxidation stability also improved in UAE than ME and SXE, denoted by lower peroxide and p-anisidine values. The lower temperature of 30 °C used in UAE than 80 °C (SXE and ME) may decelerate the oxidation, and according to Choe & Min [34], antioxidant activity (inherent abilities of carotenes, polyphenols etc) of the oil system crucially helps to reduce autoxidation. Supporting the fact, L*, a*(red), b*(yellow) values obtained for UAE are significantly higher than SXE and ME, and as carotenes impart yellow and red colors into the oil, UAE technique is capable of extracting and securing more carotenes in the mahua oil (Table 1).
Table 1.
The physicochemical properties of extracted mahua seed oil.
| Method | Iodine value (I2/100 g) | Saponification value (mg of KOH/g) | Acid value (mg KOH/g) | Free fatty acid (%) | Refractive index (25 °C) | Peroxide value (meq/kg) | p-anisidine value (meq/kg) | Color |
||
|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||||
| UAE | 42.75 ± 1.51a | 187.30 ± 0.26c | 33.49 ± 0.12b | 16.74 ± 0.06b | 69.17 ± 0.95a | 6.72 ± 0.48b | 0.32 ± 0.29b | 53.47a | −2.48a | 49.7a |
| SXE | 45.73 ± 0.20ab | 199.22 ± 2.58b | 44.61 ± 3.29a | 22.30 ± 1.64a | 67.60 ± 0.42 ab | 15.80 ± 1.03a | 1.05 ± 0.32ab | 4.11c | −0.32b | 1.86b |
| ME | 41.08 ± 1.42ac | 206.94 ± 3.35a | 45.22 ± 0.10a | 22.62 ± 0.05a | 70.25 ± 0.07 ac | 5.66 ± 0.32b | 1.99 ± 0.35a | 5.36b | −0.90b | 2.66b |
Note: Mean ± SD, n = 3. Different letters in the same column are significantly different between samples (p < 0.05) according to the Tukey test.
3.3. Fatty acid composition and triacylglycerol profile
As summarized in Table 2, the major FAs in mahua seed oil were oleic (46%), palmitic (22%), stearic (21%), and linoleic acids (10%). About 43% of the fatty acids in mahua oil are saturated, nearly 46% monounsaturated, and 10% polyunsaturated, which is consistent with the results reported by JMN et al. [35] and Ramadan & Moersel [2] on mahua oil. Thus, UAE increases the oil yield without modifying its FAC. In fact, most of the studies experimented on UAE for seed oils have suggested that either there is no influence of ultrasound on FAC or the impact could be minimal [6], [7], [26].
Table 2.
Fatty acid and triacylglyceride compositions of mahua seed oil.
| FA | UAE | SXE | ME |
|---|---|---|---|
| C14:0 | 0.06 ± 0.00a | 0.07 ± 0.00a | 0.07 ± 0.00a |
| C16:0 | 22.07 ± 0.09a | 21.82 ± 0.05b | 21.72 ± 0.02b |
| C16:1 | 0.09 ± 0.00a | 0.09 ± 0.00a | 0.09 ± 0.00a |
| C18:0 | 21.06 ± 0.03b | 21.22 ± 0.04a | 20.87 ± 0.04c |
| C18:1 | 45.56 ± 0.01b | 45.76 ± 0.05a | 45.84 ± 0.03a |
| C18:2 | 9.79 ± 0.04a | 9.06 ± 0.01c | 9.77 ± 0.02b |
| C20:0 | 0.30 ± 0.00a | 0.30 ± 0.00a | 0.30 ± 0.00a |
| C18:3 | 0.63 ± 0.00a | 0.63 ± 0.00a | 0.63 ± 0.00a |
| C22:0 | N/D | N/D | 0.09 ± 0.00 |
| C22:1 | 0.32 ± 0.06a | 0.37 ± 0.00a | 0.33 ± 0.00a |
| C24:0 | 0.11 ± 0.02a | 0.13 ± 0.04a | 0.15 ± 0.01a |
| SFA | 43.60 | 43.54 | 43.20 |
| MUFA | 45.97 | 46.22 | 46.26 |
| PUFA | 10.42 | 9.69 | 10.40 |
| TAG | |||
| SOS | 9.21 ± 0.71a | 9.27 ± 0.07a | 8.70 ± 0.49a |
| POS | 17.41 ± 0.29a | 17.66 ± 0.11a | 17.17 ± 0.13a |
| SOO | 15.70 ± 0.64a | 15.55 ± 0.37 a | 15.00 ± 0.09a |
| POP | 13.19 ± 1.28a | 13.10 ± 0.37 a | 14.02 ± 0.09 a |
| POO | 22.93 ± 0.46a | 22.53 ± 0.46 a | 21.81 ± 0.12a |
| OOO | 8.12 ± 0.80a | 8.12 ± 0.23a | 7.83 ± 0.01a |
| PPL | 1.62 ± 0.52a | 1.81 ± 0.30 a | 2.45 ± 0.02a |
| PLO | 5.48 ± 0.46a | 5.77 ± 0.38 a | 6.55 ± 0.02 a |
| OOL | 3.09 ± 0.40a | 3.10 ± 0.05a | 3.18 ± 0.00a |
| PLL/MPL | 1.85 ± 0.0.27a | 1.82 ± 0.09a | 2.03 ± 0.00a |
| OLL/PLL | 1.39 ± 0.13a | 1.27 ± 0.01a | 1.28 ± 0.00a |
Mean ± SD; n = 3 Means represented by different letters in the same row demonstrate significant difference (p < 0.05) according to Tukey test.
The abundant TAGs species present in mahua oil were 1-palmitoyl-2,3-dioleoyl-glycerol (POO), 1-palmitoyl-2-oleoyl-3-stearoyl-glycerol (POS), 1-stearoyl-2,3-dioleoyl-glycerol (SOO), 1,3-dipalmitoyl-2-oleoyl-glycerol (POP), and an appreciable amount of 1,3-distearoyl-2-oleoyl-glycerol (SOS), and 1,2,3-trioleoyl-glycerol (OOO) contributing more than 85% of the total TAG composition (Table 2). The results are compromised with the TAG profile analyzed by JMN et al. [35] for mahua oil. Appreciably, the extraction technology has no detectable influence on changing the TAG composition. Nevertheless, it is important to determine the TAG profile as varying triglycerides present in oils and fats are fundamental to food applications. For instance, at least 70% of TAG in cocoa butter is comprised of approximately 38–45% of POS and 25–30% of SOS, and 15–19% of POP which determines the desirable properties like glossy hard texture and snap of chocolate [36]. Currently, Illipe (34% POS, 39% SOS,7% POP), Palm oil (7% POS, 0.5% SOS, 24% POP), Sal (7% POS, 26% SOS, 5% POP), Kokum (7% POS, 58% SOS, 2% POP) and Mango kernel (24–64% POP) fats are being successfully researched on developing alternatives for cocoa butter [37]. Apparently, the similar profile of mahua oil is also suitable for achieving the aforementioned TAG composition.
According to LCMS results, there are 3 monoacylglycerols (MAG), 6 diacylglycerols (DAG) and 3 triacylglycerols identified as the minor acylglycerol components of mahua oil (supplementary data). Among MAG, 1-Linoleoyl Glycerol and 1-Monopalmitin were observed in all extractions while 2-g-linolenoyl-glycerol was found in SXE and ME. Meanwhile, DAG including Hexadecyl Acetyl Glycerol, 1-pentadecanoyl-2-(9Z,12Z-octadecadienoyl)-sn-glycerol were identified from all types of extractions whilst diacylglycerol (18:4/15:0) was found from SXE and ME and, 1-Oleoyl-2-acetyl-sn-glycerol in UAE and ME extracted oil. Further, the DAG 1,2-dimyristoleoyl-sn-glycerol was solely from SXE oil and 1,2-diheptadecanoyl-sn-glycerol from ME only were identified. Additionally, TAGs 1,3-di-(6Z-octadecenoyl)-2-(9Z-octadecenoyl)-glycerol and 1-(9Z-tetradecenoyl)-2-eicosanoyl-3-(7Z,10Z,13Z,16Z,19Z-docosapentaenoyl)-sn-glycerol were prominent in UAE while Glyceryl triundecanoate was only extracted from SXE (supplementary data).
3.4. Thermal behaviour
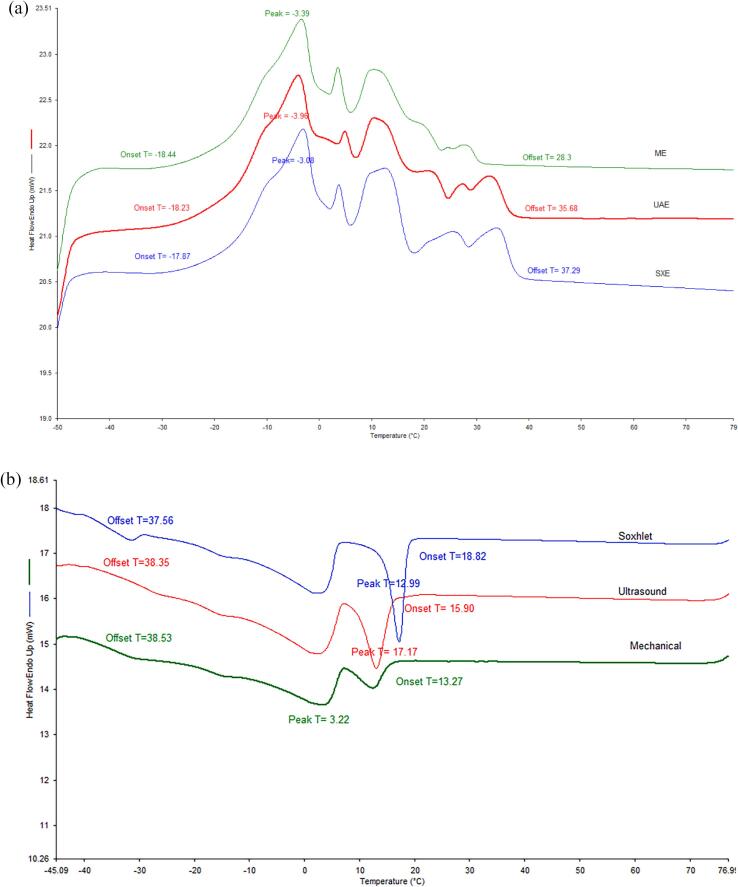
Thermal and crystallization behaviours of differently extracted mahua seed oil are depicted in Fig. 2. The onset, peak, and offset temperatures for melting and crystallization properties are also shown in Table 3. Melting curves from UAE, SXE, and ME show several peaks with melting that start at around − 18 °C and complete near 40 °C (Fig. 2a). This can be explained by the diverse triglycerides, diglycerides, and monoglycerides present in mahua oil and their different melting ranges. Although the melting curve is not the same as JMN et al. [35] reported for mahua oil, the melting temperature range is similar (-20 to 40 °C). The difference may occur as the crude mahua seed oil extracted from all extractions was used for the analysis. In this regard, the uneven nature of the curves may be caused by other compounds like waxes extracted.
Fig. 2.
Melting(a) and crystallization (b) thermograms of mahua seed oil.
Table 3.
Melting and crystallization characteristics of differently extracted mahua oil.
| Method | Melting property |
Crystallization property |
||||
|---|---|---|---|---|---|---|
| Onset T | Peak T | Offset T | Onset T | Peak T | Offset T | |
| UAE | −18.23 ± 0.01a | −3.96 ± 0.01a | 35.68 ± 0.01b | 15.90 ± 0.01b | 12.99 ± 0.01b | −38.05 ± 1.01b |
| SXE | −17.87 ± 0.01b | −3.08 ± 0.07b | 37.29 ± 0.01a | 18.82 ± 0.08a | 17.17 ± 0.01a | −37.56 ± 0.72c |
| ME | −18.44 ± 0.01a | −3.39 ± 0.03a | 28.30 ± 0.04c | 13.27 ± 0.02c | 12.46 ± 0.03c | −38.53 ± 0.31a |
Mean ± SD; n = 2 Means represented by different letters in the same column demonstrate significant difference (p < 0.05) according to Tukey test. T; temperature (°C).
The crystallization curves for UAE and SXE include dual peaks with one sharp peak attached to a broad peak (Fig. 2b). The sharp exothermic peak at 12.99 °C (UAE) and 17.17 °C (SXE) indicates the high-melting TAGs undergo crystallization first, while the second peak represents the low melting TAGs, which crystallize later. In contrast, ME shows two broad peaks. The difference in the peak temperatures may be attributed to viscous compounds like waxes i.e. extracted in UAE (fatty esters; 10E,12E,14E-Hexadecatrienyl acetate, aldehydes; 5-hydroxy caproaldehyde) and SXE (fatty esters; 8E-Tetradecenyl acetate, aldehydes; aldehydes; 5-hydroxy caproaldehyde) and the absence of such compounds in mechanical pressing, as the TAG profiles did not change significantly (supplementary data). Apparently, SXE oil crystallizes quicker than the UAE, which is correlated with the slightly higher melting temperatures of oil extracted from SXE than UAE (Fig. 2b and Table 3). Majorly, POS, SOS, and POP like tri saturated TAGs identified above make up the high melting fraction while di and tri unsaturated TAGs like SOO, POO, and OOO represent a low melting fraction. Conclusively, the extraction method could slightly affect the melting and crystallization behavior of mahua seed oil.
3.5. SEM
The SEM images of mahua seed powder treated from UAE, SXE and ME are presented in Fig. 3. The porous structure formed as an effect of the rupturing of the cell due to the cavitation effect is clearly visible in Fig. 3a. The smooth surface observed in Fig. 3c implies that the squeezing mechanism of ME is not capable enough to break the cell to release more oil, evidenced by the lowest oil yield obtained. Compared to ME the surface of SXE treated seed powder is coarse, which shows a higher yield from SXE than ME; still, random pore formation is not visible as UAE. The destructive nature of acoustic cavitation observed in Fig. 3a may be the reason for the better extraction of antioxidant compounds.
Fig. 3.
SEM images of mahua seed powder treated from UAE(a), SXE(b), and ME(c) at 2.00 k magnification.
3.6. Antioxidant activity
3.6.1. TPC, free radical assays and FRAP
Although UAE operating conditions were milder than SXE, the mechanism of acoustic cavitation as observed by SEM images was capable of providing higher yields almost equivalent to SXE. Thus, the cumulative effect of acoustic cavitation and the mild processing conditions improved the extractability of antioxidants and remained non-destructive. Additionally, the incredible color difference observed in the oil (Table 1) further prompted us to evaluate the antioxidant capacity of the extracted mahua oil. Therefore, TPC, ABTS, DPPH, and FRAP assays were conducted to determine the antioxidant capacity of mahua seed oil extracted and the results are presented in Table 4. Clearly, 301.81 mg GAE/kg of TPC obtained for UAE is more than three folds of SXE and greater than two folds of ME, implying excess polyphenols are being extracted in UAE. Further, free radical assays exhibited the same pattern of antioxidant capacity. In DPPH and ABTS assays, the lower the IC50 value, the higher the antioxidant capacity as less sample concentration is needed to scavenge the free radicals. Align to that, DPPH IC50 varied in the order of UAE; 106.60 < ME; 622.38 < SXE; 810 (mg/mL). Similarly, ABTS IC50 derived for UAE (39.80 mg/mL) was the least followed by ME and maximum in SXE (757. 43 mg/mL). On the other hand, FRAP measures the ability of antioxidants to reduce the ferric Fe(III) ion to Fe(ll), which conveys that the higher the reducing power, the more antioxidant power [38]. Results of FRAP also showed that the reducing power of the UAE oil is significantly higher than ME and two folds higher than that of SXE.
Table 4.
The results of TPC, DPPH (IC50), ABTS (IC50), and FRAP assays of mahua oil extracted from different techniques.
| Method | TPC (mg GAE/kg of oil) | DPPH(IC50) (mg/mL) |
ABTS(IC50) (mg/mL) |
FRAP (mgTrolox/kg of oil) |
|---|---|---|---|---|
| UAE | 301.81 ± 4.16a | 106.60 ± 4.29c | 39.80 ± 0.46c | 1050.84 ± 29.16a |
| SXE | 85.59 ± 0.55c | 810.40 ± 3.24a | 757.43 ± 2.23a | 584.45 ± 56.56c |
| ME | 121.20 ± 5.67b | 622.38 ± 2.57b | 392.87 ± 8.63b | 710.28 ± 35.28b |
Mean ± SD, n = 2 Means represented by different letters in the same column demonstrate significant difference (p < 0.05) according to the Tukey test.
Note. DPPH and ABTS IC50 values of Trolox solution as the reference standard were 0.0187 mg/mL, and 0.0395 mg/mL, respectively. GAE; Gallic acid equivalent.
All antioxidant assays reiterate that mahua seed oil extracted by UAE had the highest and most profound antioxidant capacity, followed by mechanical and Soxhlet, which is reasonable according to the mechanism behind each extraction method. The mechanical effect generated by ultrasonic voids and the driving force resulting from bubble cavitation may accelerate the dissolution of polyphenols into the oil. Also, polyphenols like phenolic acids that are bounded to lignin are prompted to be detached upon ultrasonication, emanating more desired biomolecules. However, in SXE, such a mechanism does not occur despite the percolation of solvent with concurrent heating for long hours, and only an external pressure is applied in ME. Evincing the fact Böger et al. [8] reported TPC of 68.82 g GAE/kg, FRAP of 44.06 MTE/kg, which were more significant than the sample without ultrasound, DPPH IC50 of 44.06 MTE/kg compared to the control of 68.82 MTE/kg for grape seed oil. Furthermore, the binary mixture of acetone and isopropanol having polar and non-polar groups was used in UAE favors the extraction of both hydrophilic and lipophilic phenolic compounds, and hexane used in SXE attracts only lipophilic molecules. Interestingly, Lee et al. [39] revealed in a UAE study of passion fruit oil a higher TPC of 46.81 and 22.62 mg GAE/g from ethanol and acetone whilst hexane attained 17.79 mg GAE/g.
3.6.2. Tocopherol and carotene contents
The antioxidant assays exhibit the overall effect of antioxidants such as phenolic acids, tocopherols, tocotrienols, and carotenoids, however, the effect of each extraction mechanism upon specific antioxidants could be diverse. Of the minor compounds available in mahua oil, tocopherols and carotene are significant. The impact of the extraction technique correlates with the content of tocols and carotenoids extracted into the oil. Total carotene in oil is expressed in terms of beta carotene equivalent (BCE) and 3726.32 mg BCE/kg of UAE oil is more than twice that of SXE and significantly higher than ME treated oil (Table 5). Regarding the tocopherol contents, α tocopherol seems to be slightly higher for UAE (97 mg/kg) than SXE (91 mg/kg) and β, and γ were significantly higher (p < 0.05) in UAE (247.11 and 124.79 mg/kg) oil than SXE (124.79 and 123.34 mg/kg). Generally, carotenoid degradation initiates around 50 °C and accelerates on prolonged heat treatments. The acoustic cavitation of UAE enhances releasing of carotenoids and the mild thermal conditions (35 °C and 35 min) preserve carotenoids resulting in the highest antioxidant content. The frictions generate in ME elevate the internal temperature instantly while degrading the carotenoids. However, the short extraction duration (10 min) of ME resulted in a significantly higher carotenoid yield than SXE operated for 6 h. In contrast, tocopherols are comparatively heat-stable, and the intensity of temperature is more influential than the exposure duration. The temperature increment in ME is uncontrollable and may rapidly increase beyond 100 °C resulting in the lowest tocopherol yield. On the other hand, the temperature maintained in SXE was comparatively less around 80 °C which resulted in higher tocols than ME while the lowest temperature maintained in UAE attained the maximum tocol yield. Also, by ultrasound, Xu et al. [40] reported an increment of total tocopherols from 20.32 to 34.22 mg per 100 g of Favel seed oil. Therefore, the ability of ultrasonication to rupture the cell structure and release biomolecules without increasing the medium temperature in a short period of time is significantly important for the oil extraction industry to embrace sustainability.
Table 5.
Tocopherol and carotene content in mahua oil.
| Extraction technique | Carotene (mg BCE/kg of oil) | Tocopherols (mg/kg of oil) |
||
|---|---|---|---|---|
| Alpha | Gama | Beta | ||
| UAE | 3726.32 ± 93.04a | 104.20 ± 6.84a | 165.15 ± 19.28a | 245.20 ± 1.50a |
| SXE | 1826.97 ± 99.55c | 92.51 ± 0.84b | 145.42 ± 22.07b | 73.62.20 ± 28.32b |
| ME | 2732.89 ± 102.34b | 91.07 ± 1.42c | 132.20 ± 5.58c | 47.16 ± 0.71c |
Mean ± SD, n = 3 Means represented by different letters in the same column demonstrate significant difference (p < 0.05) according to the Tukey test. BCE; beta carotene equivalent.
3.6.3. Other minor constituents
Apart from the tocopherols and carotenes, the antioxidant activity of mahua oil could be attributed to sterol and flavonoid compounds as identified from the LCMS analysis (supplementary data). All types of extractions showed isoprenoids of 5-(1-hydroxypropan-2-yl) isolongifol-4-ene and Emmotin A which has a supportive function for antioxidants. UAE oil resulted 22α-Hydroxy-5α-campestan-3-one which is a sterol lipid. On the other hand, 24-ethyl-5alpha-cholest-25-en-3alpha,12alpha,16alpha-triol which is a stigmasterol and 4,5-Di-O-methyl-8-prenylafzelechin-4beta-ol which is a flavonoid were identified from SXE and ME. The extraction mechanism and the operative conditions including solvent types used for the extraction of mahua oil may have resulted in different minor compounds in the oil.
4. Conclusion
In the present study, ultrasound was successfully applied to extract mahua seed oil for the first time. Results revealed that UAE produced more than 99% of oil recovery and oil yield of 56.97% under minimum energy usage, shorter extraction time (35 min), and lower temperature (35 °C) with a binary mixture of acetone and isopropanol (1:1 v/v). Even though the oil profile mainly comprised of fatty acids (46% oleic; 22% palmitic; 21% stearic, 10% linoleic), triacylglycerides (23% POO, 17% POS, 16% SOO, 14% POP and 9% SOS) and melting (complete 38 °C) seemed unchanged by UAE mechanism, antioxidant capacity and bioactive compounds elution were remarkably enhanced by UAE. Enhanced oil yield and antioxidants co-extracted from UAE outperform conventional methods like Soxhlet and mechanical extractions. Since present and future consumer expectations are continually leveling up to a more ecological and healthy perception, advanced techniques like UAE are more worth scaling up to an industrial level. Thus, the ultrasonic cavitation-assisted extraction technique is a promising energy and environmental-friendly approach for extracting antioxidant-rich mahua seed oil. This research finding is valuable for the oil industry concerning sustainability where efficient extraction strategies are essential to increase oil production and bioactive components extraction utilizing mild processing conditions and nature-friendly solvents.
Funding source
The research study was funded by School of Science ECR grant and School of Science Graduate Research Funding, Monash University Malaysia.
CRediT authorship contribution statement
R.C.N. Thilakarathna: Conceptualization, Data curation, Formal analysis, Writing – original draft. Lee Fong Siow: Supervision, Investigation, Resources, Writing – review & editing. Teck-Kim Tang: Supervision, Writing – review & editing. Eng-Seng Chan: Supervision, Writing – review & editing. Yee-Ying Lee: Conceptualization, Funding acquisition, Resources, Supervision, Investigation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge Mohd Syamil Abdul-Razak, Mayathevann Asokan, Afiq Bin Anwar and Nasihah Musa for HPLC, GC, SEM and LCMS technical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2022.106280.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Baskar G., Gurugulladevi A., Nishanthini T., Aiswarya R., Tamilarasan K. Optimization and kinetics of biodiesel production from Mahua oil using manganese doped zinc oxide nanocatalyst. Renew. Energy. 2017;103:641. doi: 10.1016/j.renene.2016.10.077. [DOI] [Google Scholar]
- 2.M.F. Ramadan J.T. Moersel Mowrah butter: Nature's novel fat INFORM – International News on Fats, Oils and Related Materials. 17 2 2006 pp. 124–6.https://www.scopus.com/inward/record.uri?eid=2-s2.0.
- 3.Ramadan MF, Wagdi Abdel-Hamed EM. Health-promoting Potential and Nutritional Value of Madhuca longifolia Seeds. Nuts and Seeds in Health and Disease Prevention2020. p. 229-37. https://doi.org/10.1016/b978-0-12-818553-7.00017-6.
- 4.Chemat F., Rombaut N., Sicaire A.-G., Meullemiestre A., Fabiano-Tixier A.-S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017;34:540. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 5.Rojo-Gutiérrez E., Carrasco-Molinar O., Tirado-Gallegos J.M., Levario-Gómez A., Chávez-González M.L., Baeza-Jiménez R., et al. Evaluation of green extraction processes, lipid composition and antioxidant activity of pomegranate seed oil. J. Food Measur. Character. 2021;15(2):2098–2107. doi: 10.1007/s11694-020-00804-7. [DOI] [Google Scholar]
- 6.Senrayan J., Venkatachalam S. Ultrasonic acoustic-cavitation as a novel and emerging energy efficient technique for oil extraction from kapok seeds. Innov. Food Sci. Emerg. Technol. 2020 doi: 10.1016/j.ifset.2020.102347. [DOI] [Google Scholar]
- 7.Zhang W., Pan Y.G., Huang W., Chen H., Yang H. Optimized ultrasonic-assisted extraction of papaya seed oil from Hainan/Eksotika variety. Food Sci Nutr. 2019;7(8):2692. doi: 10.1002/fsn3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Böger B.R., Salviato A., Valezi D.F., Di Mauro E., Georgetti S.R., Kurozawa L.E. Optimization of ultrasound-assisted extraction of grape-seed oil to enhance process yield and minimize free radical formation. J. Sci. Food Agric. 2018;98(13):5019. doi: 10.1002/jsfa.9036. [DOI] [PubMed] [Google Scholar]
- 9.Davarnejad R., Kassim K.M., Zainal A., Sata S.A. Supercritical fluid extraction of β-carotene from crude palm oil using CO2. J. Food Eng. 2008;89(4):472. doi: 10.1016/j.jfoodeng.2008.05.032. [DOI] [Google Scholar]
- 10.Muangrat R., Veeraphong P., Chantee N. Screw press extraction of Sacha inchi seeds: Oil yield and its chemical composition and antioxidant properties. J. Food Process. Preserv. 2018;42(6) doi: 10.1111/jfpp.13635. [DOI] [Google Scholar]
- 11.Cisneros F.H., Paredes D., Arana A., Cisneros-Zevallos L. Chemical composition, oxidative stability and antioxidant capacity of oil extracted from roasted seeds of Sacha-inchi (Plukenetia volubilis L.) J. Agric. Food Chem. 2014;62(22):5191–5197. doi: 10.1021/jf500936j. %@ 0021–8561. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q., Xu Y., Zhang P., Na Z., Tang T., Shi Y. Chemical composition and oxidative evolution of Sacha Inchi (Plukentia volubilis L.) oil from Xishuangbanna (China) Grasas Aceites. 2014;65(1):e012. [Google Scholar]
- 13.Okoh S.O., Asekun O.T., Familoni O.B., Afolayan A.J. Antioxidant and free radical scavenging capacity of seed and shell essential oils extracted from Abrus precatorius (L) Antioxidants. 2014;3(2):278. doi: 10.3390/antiox3020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szydlowska-Czerniak A., Dianoczki C., Recseg K., Karlovits G., Szlyk E. Determination of antioxidant capacities of vegetable oils by ferric-ion spectrophotometric methods. Talanta. 2008;76(4):899. doi: 10.1016/j.talanta.2008.04.055. [DOI] [PubMed] [Google Scholar]
- 15.Stevanato N., da Silva C. Radish seed oil: Ultrasound-assisted extraction using ethanol as solvent and assessment of its potential for ester production. Ind. Crop. Prod. 2019;132:283. doi: 10.1016/j.indcrop.2019.02.032. [DOI] [Google Scholar]
- 16.Zhang L., Zhou C., Wang B., Yagoub A.-E.-G.-A., Ma H., Zhang X., et al. Study of ultrasonic cavitation during extraction of the peanut oil at varying frequencies. Ultrason. Sonochem. 2017;37:106. doi: 10.1016/j.ultsonch.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 17.Shalmashi A. Ultrasound-assisted extraction of oil from tea seeds. J. Food Lipids. 2009;16(4):465–474. [Google Scholar]
- 18.Li T., Qu X.-y., Zhang Q.-a., Wang Z.-Z. Ultrasound-assisted extraction and profile characteristics of seed oil from Isatis indigotica Fort. Ind. Crop. Prod. 2012;35(1):98. doi: 10.1016/j.indcrop.2011.06.013. [DOI] [Google Scholar]
- 19.Santos S.B., Martins M.A., Caneschi A.L., Aguilar P.R.M., Coimbra J.S.R. Kinetics and thermodynamics of oil extraction from Jatropha curcas L. using ethanol as a solvent. Int. J. Chem. Eng. 2015 doi: 10.1155/2015/871236. [DOI] [Google Scholar]
- 20.Thirugnanasambandham K. Ultrasound-assisted extraction of oil from Moringa oleifera Lam. seed using various solvents. Energy Sources Part A. 2018;40(3):343. doi: 10.1080/15567036.2017.1416708. [DOI] [Google Scholar]
- 21.Xu G., Liang C., Huang P., Liu Q., Xu Y., Ding C., et al. Optimization of rice lipid production from ultrasound-assisted extraction by response surface methodology. J. Cereal Sci. 2016;70:23–28. doi: 10.1016/j.jcs.2016.05.007. [DOI] [Google Scholar]
- 22.Mohammadpour H., Sadrameli S.M., Eslami F., Asoodeh A. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Ind. Crop. Prod. 2019;131:106–116. doi: 10.1016/j.indcrop.2019.01.030. [DOI] [Google Scholar]
- 23.Massa T.B., Stevanato N., Cardozo-Filho L., da Silva C. Pumpkin (Cucurbita maxima) by-products: obtaining seed oil enriched with active compounds from the peel by ultrasonic-assisted extraction. J. Food Process. Eng. 2019;42(5):e13125. doi: 10.1111/jfpe.13125. [DOI] [Google Scholar]
- 24.Sicaire A.-G., Vian M.A., Fine F., Carré P., Tostain S., Chemat F. Ultrasound induced green solvent extraction of oil from oleaginous seeds. Ultrason. Sonochem. 2016;31:319. doi: 10.1016/j.ultsonch.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Goula A.M. Ultrasound-assisted extraction of pomegranate seed oil–Kinetic modeling. J. Food Eng. 2013;117(4):492. doi: 10.1016/j.jfoodeng.2012.10.009. [DOI] [Google Scholar]
- 26.Santos K.A., da Silva E.A., da Silva C. Ultrasound-assisted extraction of favela (Cnidoscolus quercifolius) seed oil using ethanol as a solvent. J. Food Process. Preserv. 2021;45(6):e15497. doi: 10.1111/jfpp.15497. [DOI] [Google Scholar]
- 27.Hosseini S., Gharachorloo M., Tarzi B.G., Ghavami M., Bakhoda H. Effects of ultrasound amplitude on the physicochemical properties of some edible oils. J. Am. Oil Chem. Soc. 2015;92(11–12):1717–1724. doi: 10.1007/s11746-015-2733-1. [DOI] [Google Scholar]
- 28.FDA F. Q3C—Tables and list guidance for industry. ICH (Revision 3). 2017.
- 29.Freed B.K., Biesecker J., Middleton W.J. Spectral polarity index: a new method for determining the relative polarity of solvents [1] J. Fluor. Chem. 1990;48(1):63. doi: 10.1016/S0022-1139(00)82602-0. [DOI] [Google Scholar]
- 30.Panadare D.C., Gondaliya A., Rathod V.K. Comparative study of ultrasonic pretreatment and ultrasound assisted three phase partitioning for extraction of custard apple seed oil. Ultrason Sonochem. 2020;61 doi: 10.1016/j.ultsonch.2019.104821. [DOI] [PubMed] [Google Scholar]
- 31.Perrier A., Delsart C., Boussetta N., Grimi N., Citeau M., Vorobiev E. Effect of ultrasound and green solvents addition on the oil extraction efficiency from rapeseed flakes. Ultrason. Sonochem. 2017;39:58–65. doi: 10.1016/j.ultsonch.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Munasinghe M., Wansapala J. Oil extraction from Madhuca longifolia (J. Konig) JF Macbr seeds and evaluation of physico-chemical properties, fatty acid profile, antioxidant potential and sensory characteristics. Trop. Agric. 2016;93(1):29–35. doi: 10.1111/jfpp.13635. [DOI] [Google Scholar]
- 33.Tan C.-H., Ghazali H.M., Kuntom A., Tan C.-P., Ariffin A.A. Extraction and physicochemical properties of low free fatty acid crude palm oil. Food Chem. 2009;113(2):645. doi: 10.1016/j.foodchem.2008.07.052. [DOI] [Google Scholar]
- 34.Choe E., Min D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Safety. 2006;5(4):169–186. doi: 10.1111/j.1541-4337.2006.00009.x. [DOI] [Google Scholar]
- 35.Jmn M., Hm G., Long K. Composition and thermal characteristics of Madhuca longifolia seed fat and its solid and liquid fractions. J. Oleo Sci. 2010;59(1):7. doi: 10.5650/jos.59.7. [DOI] [PubMed] [Google Scholar]
- 36.Lonchampt P., Hartel R.W. Fat bloom in chocolate and compound coatings. Eur. J. Lipid Sci. Technol. 2004;106(4):241. doi: 10.1002/ejlt.200400938. [DOI] [Google Scholar]
- 37.Talbot G. Properties of cocoa butter and vegetable fats. Beckett's Industrial Chocolate Manufacture and Use. 2017:153-84.
- 38.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 39.Lee S.Y., Fu S.Y., Chong G.H. Ultrasound-assisted extraction kinetics, fatty acid profile, total phenolic content and antioxidant activity of green solvents' extracted passion fruit oil. Int. J. Food Sci. Technol. 2015;50(8):1831. doi: 10.1111/ijfs.12844. [DOI] [Google Scholar]
- 40.Xu D.P., Zheng J., Zhou Y., Li Y., Li S., Li H.B. Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: Optimization and comparison with conventional methods. Food Chem. 2017;217:552. doi: 10.1016/j.foodchem.2016.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.