Abstract
Introduction
In China, traditional Chinese medicine (TCM) is regarded as an effective treatment for primary liver cancer (PLC). The present study analyzed the effect of TCM on the survival period of patients with PLC by analyzing the relationship between the treatment-duration-ratio of traditional Chinese medicine (C-TDR, (traditional Chinese medicine treatment duration)/(Overall treatment duration) × 100%) and the survival time of 1002 patients with PLC.
Methods
In this study, 1002 patients with PLC admitted to TCM Oncology Department of Changhai Hospital from January, 2015 to December, 2019 were enrolled. The univariate and multivariate Cox regression equation, propensity score matching (PSM) were performed to identify independent prognostic factors for survival outcomes of PLC patients at different stages and estimate the influence of C-TDR on survival time.
Results
Cox regression analysis indicated that C-TDR was an independent prognostic factor for survival outcome (P<0.05) and a corresponding reduction of relative risk of death of 75.67% (relative risk (RR) = 0.2433; 95%Confedential Interval (CI) = 0.1747–0.3388). Similarly, it is also an independent prognostic factor for patients outcome of each stage (P<0.05). The 251 patients of BCLC-A reduced 96.09% risk of mortality (RR = 0.0391; 95%CI = 0.0151–0.1012). The 396 BCLC-B patients decreased risk of death of 81.24% (RR = 0.1876, 95%CI = 0.1112–0.3163). Moreover, 355 patients of stage C demonstrated a 51.36% lower risk of death (RR = 1.0016, 95%CI = 0.9885–1.0149). Significant differences were found in the median overall survival (OS) both higher and lower C-TDR of all patients. Even after PSM, the overall survival of two groups were significantly improved following each stage.
Conclusion
Earlier administration of traditional Chinese medicine can reduce the risk of mortality and prolong survival in patients with liver cancer.
Keywords: Treatment-duration-ratio, Traditional Chinese medicine, Risk of mortality, Primary liver cancer, Survival period
Treatment-duration-ratio, Traditional Chinese medicine, Risk of mortality, Primary liver cancer, Survival period.
1. Introduction
Primary Liver cancer (PLC)is the fourth most common malignancy and the second most common cause of death from cancer worldwide [1]. The incidence rate of hepatocellular carcinoma (HCC) varies globally, with more than 80% of the cases occurring in low-to-medium resource countries; the incidence rate is particularly high in East Asia and sub-Saharan Africa [2]. In the United States, the age-adjusted incidence rate of the disease tripled from 1992 to 2010; it has leveled off in the past few years [3, 4]. The increase in the incidence rate may be due to the high prevalence of chronic hepatitis C virus infection in the population cohort born between 1945 and 1965 as well as the greatly increased burden of the metabolic syndrome [3, 4]. Though China's population accounts for only 18.4% of the global population, 466,000 new cases of liver cancer and 422,000 liver cancer-related deaths were reported in China in 2022; these account for 55.4% and 53.9% of the corresponding global rates, respectively [5, 6]. The prognosis of PLC is generally poor, and the incidence rate-to-mortality ratio is 1:0.9. The 5-year survival rates of patients with PLC in North America and China are 15%–19% and only 12.1%, respectively. Accordingly, PLC is a serious threat to the lives and health of people [7, 8, 9, 10].
PLC can be divided into three types as follows: Hepatocellular carcinoma (HCC), intrahepatic cholangiocellular carcinoma (ICC) and a mixed HCC-ICC type. HCC accounts for about 80% of all cases [11]. A variety of treatments are recommended for PLC, with surgical resection, liver transplantation, and radiofrequency ablation being recommended for the treatment of early stage [12]. Transcatheter arterial chemoembolization (TACE) has become the standard treatment for patients with intermediate stage PLC [13]. Recently, Tyrosine kinase inhibitors (Tki) and Immune checkpoint inhibitors (ICIs) had been developed for the treatment of advanced liver cancer [14].
Because liver cirrhosis is the basis of PLC in most patients, the prognosis depends on not only the tumor burden, but also on the degree of liver dysfunction as well as the patients' performance status (PS). In most cases of solid tumors, an intraoperative pathological examination of the resected specimen is performed to determine the tumor stage, which in turn is used to determine the tumor–node–metastasis (TNM) classification. However, the TNM staging system cannot explain the degree of liver dysfunction and the patient's PS, which determine the feasibility of treatment and need to be considered during clinical decision-making for patients with HCC [1].
Several alternative staging systems have been proposed, including the Barcelona Clinical Liver Cancer (BCLC) staging system, Italian Liver Cancer Program, Japanese Comprehensive Staging, and Chinese University Prognostic Index, among others [15]. Although no staging system has been accepted universally, BCLC staging may provide the most prognostic information because it includes the assessment of the tumor burden, liver function, and patient's PS. Therefore, it is recognized by the Professional Association of Liver Diseases. The prognostic ability of BCLC staging has been verified in European, American, and Asian populations [16, 17]. The advantage of BCLC staging is that it can stratify the survival rate of patients with HCC into substrates of 0, A, B, C, and D. Therefore, it can be easily applied to patient care directly [18, 19, 20].
PLC staging is based on imaging to determine the tumor burden, i.e., the number, size, and location of the tumor and whether there is vascular invasion (even when the intrahepatic portal root and extrahepatic metastasis are involved). Following treatment (resection, ablation, and liver transplantation) of patients with very early (BCLC-0) and early (BCLC-A) HCC, the 5-year overall survival rate (OS) is 50%–75% [21]. The liver function of patients with intermediate-stage HCC (BCLC-B) is protected, without cancer-related symptoms, multinodular HCCs, vascular invasion, or extrahepatic metastasis. Transarterial chemoembolization is the standard of care for these patients and provides an OS that might reach 4 years in optimal candidates. Patients with BCLC-C HCC include those with advanced diseases who may have cancer-related symptoms; however, their liver function is relatively preserved (Child–Pugh A or B, but the liver function is not seriously damaged as described previously), and they may have vascular invasion or extrahepatic spread.
Traditional Chinese medicine (TCM) had been widely used as an essential treatment for liver cancer in China [22]. It has been reported that long-term of TCM is essential for prolonging survival as well as for maintaining quality of life in these patients [23]. However, there is no consensus on the duration of TCM treatment use and for how long TCM should be extended [23]. Jiedu Granule is a TCM compound that is widely used by patients with liver cancer in China. It is composed of a variety of anti-cancer TCMs. According to the TCM theory, it is used for clearing away heat and toxic substances. A number of studies conducted by our team have clearly proven the effectiveness and safety of Jiedu Granules against PLC [29, 30, 31, 32, 33]. However, we found that its treatment time may be an important factor that could affect the OS. Therefore, we designed the present study; hrein, we introduced a new concept, the treatment-duration-ratio of TCM (C-TDR), which was defined as (TCM treatment duration)/(overall treatment duration) × 100%.
The main objective of the current study was to assess the effect of TCM on the survival period of PLC by analyzing the relationship between C-TDR and the survival time.
2. Material and methods
2.1. Study design and participants
Patients with advanced PLC who received treatments from Changhai Hospital in Shanghai, China between January 2015 and December 2019 were eligible for this study. The inclusion criteria were (1) a diagnosis of PLC, confirmed histologically or cytologically, or confirmed clinically in accordance with the Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2019 Edition) [24]; (2) BCLC stages A to C categorization; (3) age ≥18 years; (4) Eastern Cooperative Oncology Group (ECOG) PS ≤ 2 [25]; (5) Child–Pugh class A or B; and (6) estimated survival ≥3 months. Exclusion criteria were: (1) patients known to be allergic to Chinese herbal medicine or TCM preparations; (2) uncontrollable heart, brain, kidney, lung, and other organ diseases; (3) previous (within 5 years) or other incurable malignant tumors at the same time; (4) history of mental illness or psychotropic substance abuse; (5) incomplete data; and (6) patients who in the judgment of the investigator, were not suitable for inclusion.
All the patients provided written informed consent before undergoing any study-specific procedures. The study has been approved by the Institutional Review Board of Chinese Clinical Trial Registry (CHiECRCT-20150073) and follows the tenants of the Declaration of Helsinki.
2.2. Procedures
This was a retrospective, real-world study. All patients entered into the liver cancer database received Traditional Chinese Medicine treatment based on Jiedu granules, twice a day, 30 min after meals, for more than 3 months. Simultaneously, all patients were administered the best supportive treatments possible; these treatments included liver protection, stomach protection, and anti-viral treatments, among others. Patients were allowed to select follow-up treatments according to their needs or preferences; these included TACE, minimally invasive therapy, radiotherapy, targeted therapy, and immunosuppressive therapy, among others.
Jiedu Granule [33](Tianjiang Pharmaceutical Factory, Jiangsu, China; Production License No. Su ZzY20010266) was administered twice a day at an individual dose of 8 g (equal to 80 g raw herbal material) 30 min after meals. The JD Granule formula is composed of Actinidia valvata root, Salvia chinensis root, Cremastra appendiculata bulb, and the gizzard membrane of Gallus gallus domesticus, in a proportion of 1:1:0.4:0.4. The herbs and gizzard membrane are extracted in hot water and lyophilized to form the compound. Treatment with JD Granule was continued until the patient died or was unable to continue taking the drug [33].
The follow-up period began immediately after the off-treatment visit and was planned to continue. Telephone and outpatient or inpatient follow-ups were performed if the patient was alive or withdrew consent, until the follow-up deadline on June 30, 2021. Patients were scheduled to be followed up for survival every 2 months, and all anticancer treatments received were reported. The primary outcome was overall survival (OS), which was the time from the onset of treatment to death.
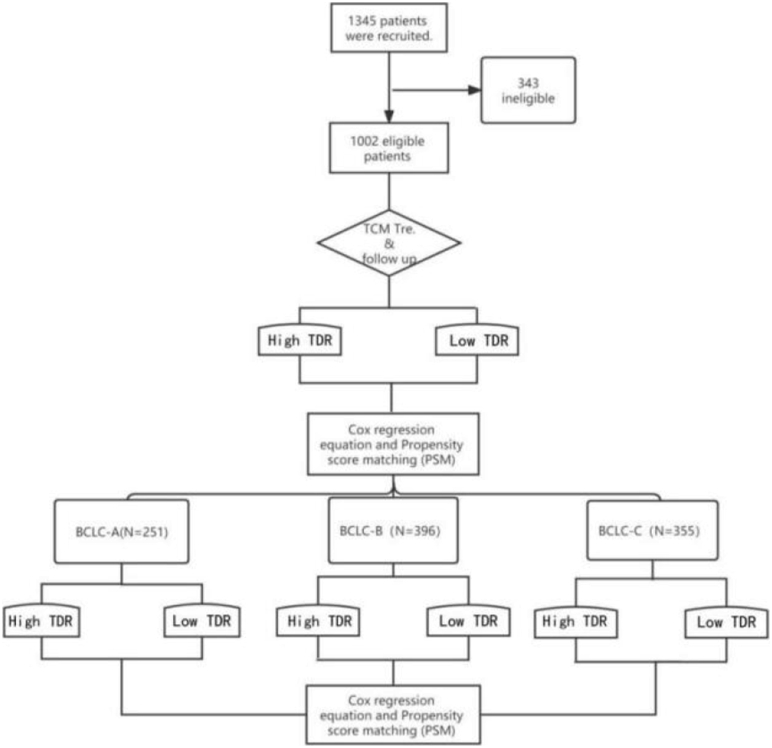
All patients were divided into three groups according to BCLC stage, including 251 in stage A, 396 in stage B, and 355 in stage C. Patients were assigned to two groups; high and low TDR groups, according to the median C-TDR at each stage (Figure 1). Those exceeding the median C-TDR are defined as high TDR. Conversely, below the median C-TDR is low TDR.
Figure 1.
Trial profile. A total of 1002 eligible patients, including 251 in stage A, 396 in stage B and 355 in stage C.
2.3. Statistical analysis
Kaplan–Meier analysis and log-rank test were used to evaluate differences in survival between the treatment groups. A subgroup analysis was also performed. Univariate and multivariate Cox regression analyses were used to assess the hazard ratio (HR) or relative risk (RR) and 95% confidence interval (CI) for prognostic variables. Two-sided P values less than 0.05 were considered statistically significant.
3. Results
3.1. Demographical and clinical characteristics
Patient baseline characteristics are shown in Table 1.
Table 1.
Baseline of demographic data and patient characteristics.
| BCLC-A (N = 251) | BCLC-B (N = 396) | BCLC-C (N = 355) | ||
|---|---|---|---|---|
| Gender | Male | 203 (80.8%) | 327 (82.5%) | 312 (87.8%)∗ |
| Female | 48 (19.2%) | 69 (17.5%) | 43 (12.2%)∗ | |
| Age | ≥55 | 121 (48.6%) | 187 (47.2%) | 259 (72.6%)∗ |
| <55 | 129 (51.4%) | 209 (52.8%) | 96 (27.4%)∗ | |
| Hepatitis | Absent | 30 (11.9%) | 35 (8.8%) | 22 (6.1%)∗ |
| HAV | 3 (1%) | 1 (0.2%) | 2 (0.5%)∗ | |
| HBV | 213 (84.8%) | 354 (87.1%) | 317 (89.2%)∗ | |
| HCV | 2 (0.6%) | 4 (1%) | 12 (3.3%)# | |
| Others | 3 (1%) | 2 (0.4%) | 2 (0.5%)∗ | |
| Child-Pugh | A | 245 (97.6%) | 335 (84.5%) | 270 (76.0%)∗ |
| B | 6 (2.4%) | 61 (15.5%) | 85 (24.0%)# | |
| Tumor Type | Single | 218 (86.9%) | 167 (42.1%) | 100 (28.1%)∗ |
| Multiple | 33 (13.1%) | 135 (34.0%) | 111 (31.2%)∗ | |
| Massive | 0 | 79 (19.9%) | 114 (32.1%)# | |
| Diffuse | 0 | 11 (2.7%) | 30 (8.4%)# | |
| Tumor location | Right | 142 (56.6%) | 247 (62.3%) | 227 (63.94%)∗ |
| Left | 74 (29.5) | 101 (25.5%) | 85 (23.94)∗ | |
| Left&right | 35 (13.9%) | 38 (13.2%) | 43 (12.11%)∗ | |
| Tumor size | ≤3cm | 179 (71.3%) | 59 (14.96%) | 66 (18.59%)# |
| 3–5cm | 57 (21.7%) | 98 (24.7%) | 63 (17.75%)# | |
| 5–10cm | 15 (0.06%) | 163 (41.2%) | 126 (35.49%)# | |
| ≥10cm | 0 | 76 (19.2%) | 100 (28.17%)# | |
| Tumor thrombus | Absent | 0 | 0 | 171 (48.17%)# |
| Present | 0 | 0 | 184 (51.83%)# | |
| Lymph node metastasis | Absent | 0 | 0 | 245 (69.01%)# |
| Present | 0 | 0 | 110 (30.99%)# | |
| Distant metastasis | Absent | 0 | 0 | 263 (74.08%)# |
| Present | 0 | 0 | 92 (25.92%)# | |
| Surgical history | Absent | 118 (47.01%) | 225 (56.82%) | 245 (69.01%)∗ |
| Present | 133 (52.99%) | 171 (43.18%) | 110 (30.99%)∗ | |
BCLC:Barcelona Clinic Liver Cancer.
P < 0.05 indicates statistical significance.
P > 0.05 indicates no statistical significance.
3.2. Survival analysis
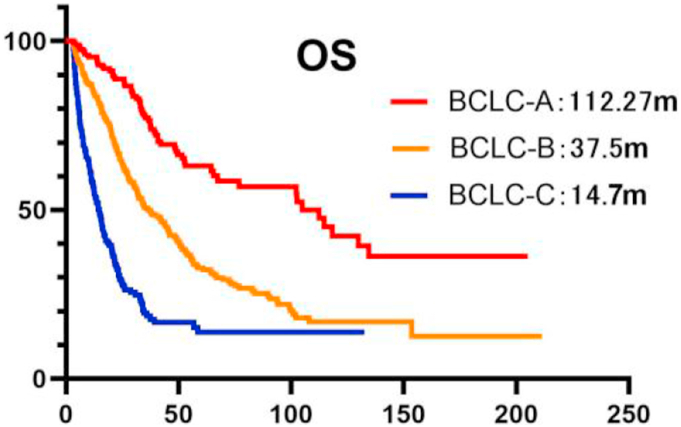
Figure 2 shows different OS outcomes for 1002 patients and the different BCLC stages. The median OS duration was 33.63 months for all patients, and 112.27 months, 37.5 months, and 14.7 months in stages A, B and C, respectively (Figure 2). The 1-, 2- and 5- year survival rates of stages A–C were 95.29% vs. 84.90% vs. 57.15%, 77.23% vs. 50.03% vs. 18.53%, and 63.05% vs. 32.24% vs. 13.72% respectively.
Figure 2.
Kaplan–Meier analysis of overall survival in 1002 and BCLC stages patients. OS:Overall Survival, mOS:Median Overall Survival, m:month, BCLC:Barcelona Clinic Liver Cancer.
3.3. Factors associated with OS
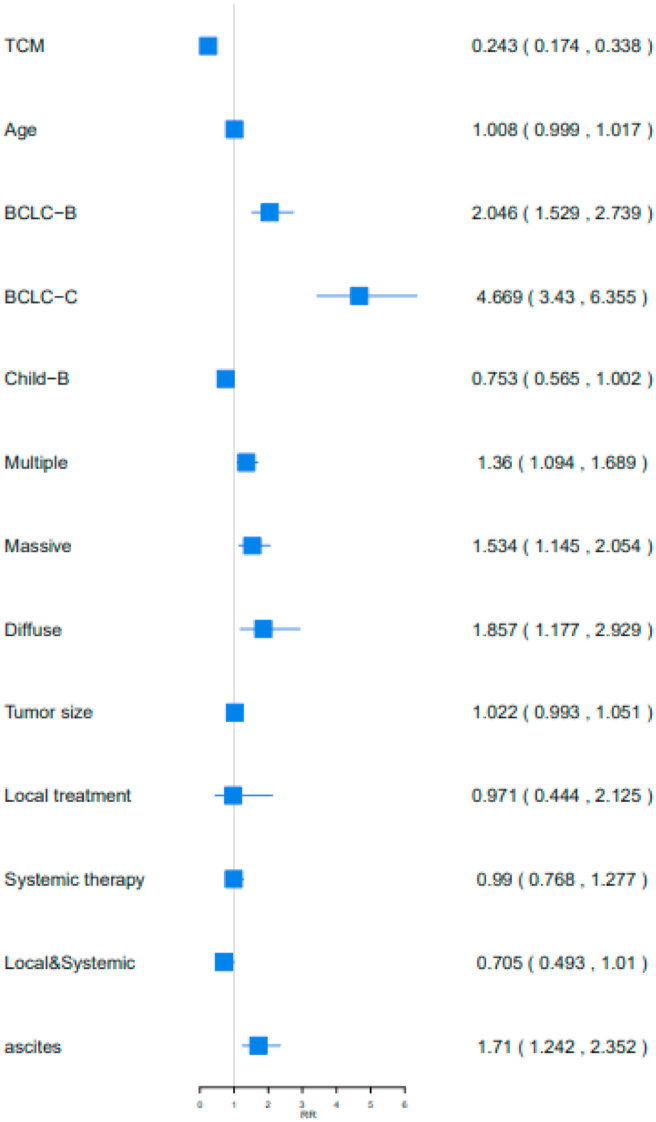
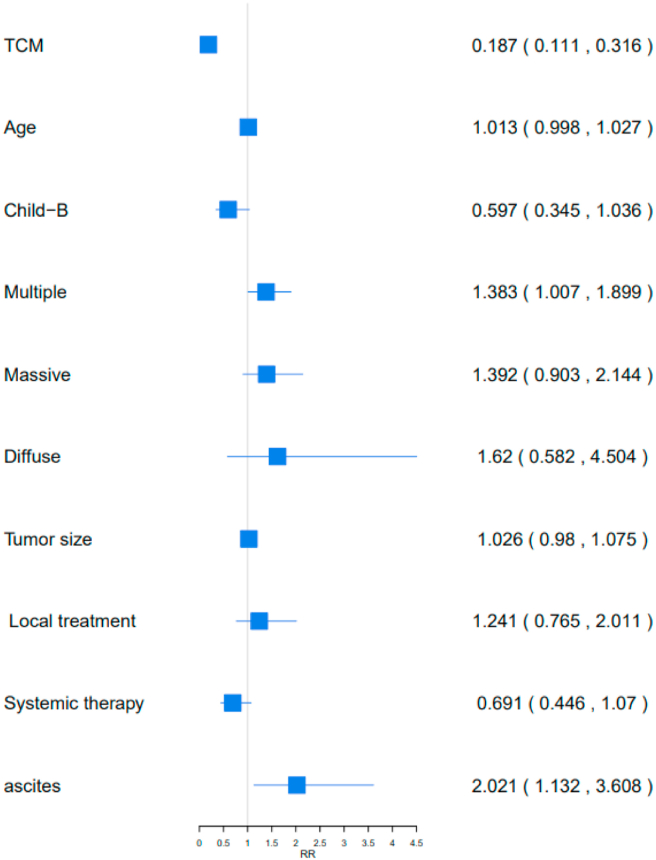
Multivariate Cox proportional hazards analysis identified prognostic factors associated with OS in the 1002 patients with PLC, including C-TDR, BCLC stages, tumor size, tumor type, and ascites (P < 0.05; Table 2). Higher C-TDR in patients with liver cancer reduced the risk of death by 75.67% (RR [95%CI] = 0.2433 [0.1747,0.3388]). Four important factors that increased the risk of death in patients with liver cancer were BCLC stage, tumor size, tumor type, and ascites (BCLC-B or BCLC-C vs. BCLC-A: 104.70% (RR [95%CI] = 2.047 [1.5296,2.7393] vs. 366.93% (RR [95%CI] = 4.6693 [3.4306,6.3554]), tumor size: 36.01% (RR [95%CI] = 1.3601 [1.0947,1.6899]), multiple vs. single: 53.46% (RR [95%CI] = 1.5346 [1.146,2.0549]), massive vs. single: 85.75% (RR [95%CI] = 1.8575 [1.1776,2.9299]), and ascites: 71.01% (RR [95%CI] = 1.7101 [1.243,2.3528]) (Figure 3).
Table 2.
The multivariate Cox proportional hazards associated with OS in 1002 patients with PLC.
| OS RR value(95%CI) | P value | Risk of death | |
|---|---|---|---|
| TDR of TCM | 0.2433 (0.1747,0.3388) | 0.000 < 0.05∗ | 75.67%↓ |
| Age | 1.0084 (0.9995,1.0175) | 0.0656 > 0.05# | - |
| BCLC-B VS BCLC-A | 2.047 (1.5296,2.7393) | 0.00 < 0.05∗ | 104.70%↑ |
| BCLC-C VS BCLC-A | 4.6693 (3.4306,6.3554) | 0.000 < 0.05∗ | 366.93%↑ |
| Child-B VS Child-A | 0.7533 (0.5658,1.0029) | 0.052 > 0.05# | - |
| Tumor size | 1.3601 (1.0947,1.6899) | 0.0055 < 0.05∗ | 36.01%↑ |
| Multiple | 1.5346 (1.146,2.0549) | 0.004 < 0.05∗ | 53.46%↑ |
| Massive | 1.8575 (1.1776,2.9299) | 0.0077 < 0.05∗ | 85.75%↑ |
| Diffuse | 1.0221 (0.9935,1.0514) | 0.1315 > 0.05# | - |
| Local treatment | 0.9719 (0.4444,2.1259) | 0.9432 > 0.05# | - |
| Systemic therapy | 0.9905 (0.7683,1.2771) | 0.9416 > 0.05# | - |
| Local&Systemic | 0.7057 (0.493,1.0102) | 0.0568 > 0.05# | - |
| Ascites | 1.7101 (1.243,2.3528) | 0.001 < 0.05∗ | 71.01%↑ |
P < 0.05 indicates statistical significance.
P > 0.05 indicates no statistical significance.
Figure 3.
Analysis of prognostic factors in 1002 patients with OS. TCM: TDR of TCM, Local&Systemic: Local treatment & Systemic therapy.
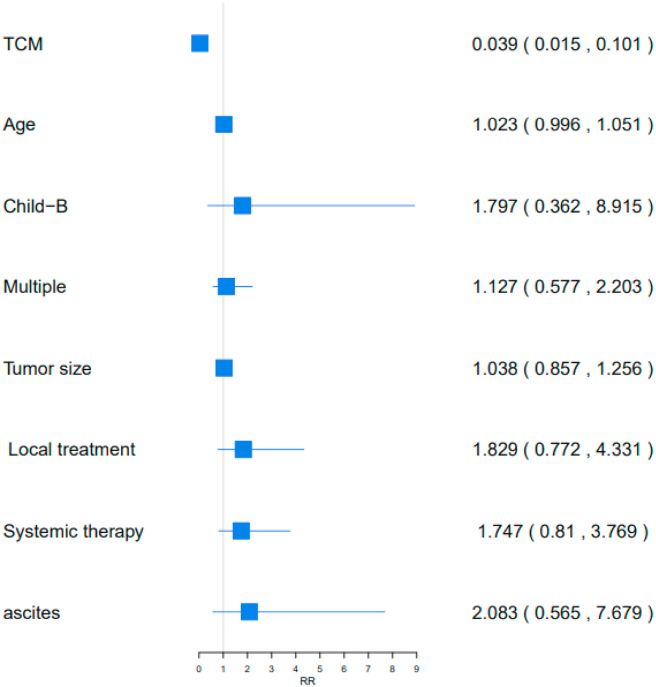
Similarly, C-TDR was an independent prognostic factor for 251 patients in stage A (P < 0.05; Table 3). A higher C-TDR in BCLC-A reduced the risk of mortality by 96.09 % (RR [95%CI] = 0.0391 [0.0151,0.1012]) (Figure 4).
Table 3.
The Cox regression analysis of 251 BCLC-A patients.
| OS RR value(95%CI) | P value | Risk of death | |
|---|---|---|---|
| TDR of TCM | 0.0391 (0.0151,0.1012) | 0.000 < 0.05∗ | 96.09%↓ |
| Age | 1.0236 (0.9964,1.0516) | 0.0895 > 0.05# | - |
| Child-B VS Child-A | 1.7974 (0.3624,8.9155) | 0.473 > 0.05# | - |
| Multiple vs single | 1.1279 (0.5772,2.204) | 0.7247 > 0.05# | - |
| Tumor size | 1.0382 (0.8575,1.2569) | 0.7011 > 0.05# | - |
| Local treatment | 1.8297 (0.7729,4.3318) | 0.1694 > 0.05# | - |
| Systemic therapy | 1.7479 (0.8105,3.7692) | 0.1544 > 0.05# | - |
| Ascites | 2.0831 (0.565,7.6795) | 0.2703 > 0.05# | - |
P < 0.05 indicates statistical significance.
P > 0.05 indicates no statistical significance.
Figure 4.
Analysis of prognostic factors in 251 BCLC-A patients with OS. TCM: TDR of TCM.
At stage B, three clinical characteristics were significantly associated with OS: C-TDR, tumor type, and ascites (P < 0.05; Table 4). A higher C-TDR of 396 in BCLC-B patients decreased the risk of death by 81.24% (RR [95%CI] = 0.1876 [0.1112,0.3163]). Tumor type and ascites elevated the risk of death for BCLC-B patients (multiple vs. single: 38.31% (RR [95%CI] = 1.3831 [1.0072,1.8992], ascites: 102.150% (RR [95%CI] = 2.0215 [1.1325,3.6082]) (Figure 5).
Table 4.
The Cox regression equation analysis of 396 BCLC-B patients.
| OS RR value(95%CI) | P value | Risk of death | |
|---|---|---|---|
| TDR of TCM | 0.1876 (0.1112,0.3163) | 0.000 < 0.05 | 81.24%↓ |
| Age | 1.0132 (0.9989,1.0276) | 0.07 > 0.05 | - |
| Child-B VS Child-A | 0.598 (0.3451,1.0361) | 0.0667 > 0.05 | - |
| Multiple | 1.3831 (1.0072,1.8992) | 0.045 < 0.05 | 38.31%↑ |
| Massive | 1.3921 (0.9035,2.1449) | 0.1337 > 0.05 | - |
| Diffuse | 1.6204 (0.5829,4.5043) | 0.3548 > 0.05 | - |
| Tumor size | 1.0267 (0.9806,1.0751) | 0.2613 > 0.05 | - |
| Local treatment | 1.241 (0.7659,2.011) | 0.3806 > 0.05 | - |
| Systemic therapy | 0.691 (0.4461,1.0703) | 0.0978 > 0.05 | - |
| Ascites | 2.0215 (1.1325,3.6082) | 0.0173 < 0.05 | 102.150%↑ |
∗P < 0.05 indicates statistical significance, #P > 0.05 indicates no statistical significance.
Figure 5.
Analysis of prognostic factors in 396 BCLC-B patients with OS
TCM: TDR of TCM.
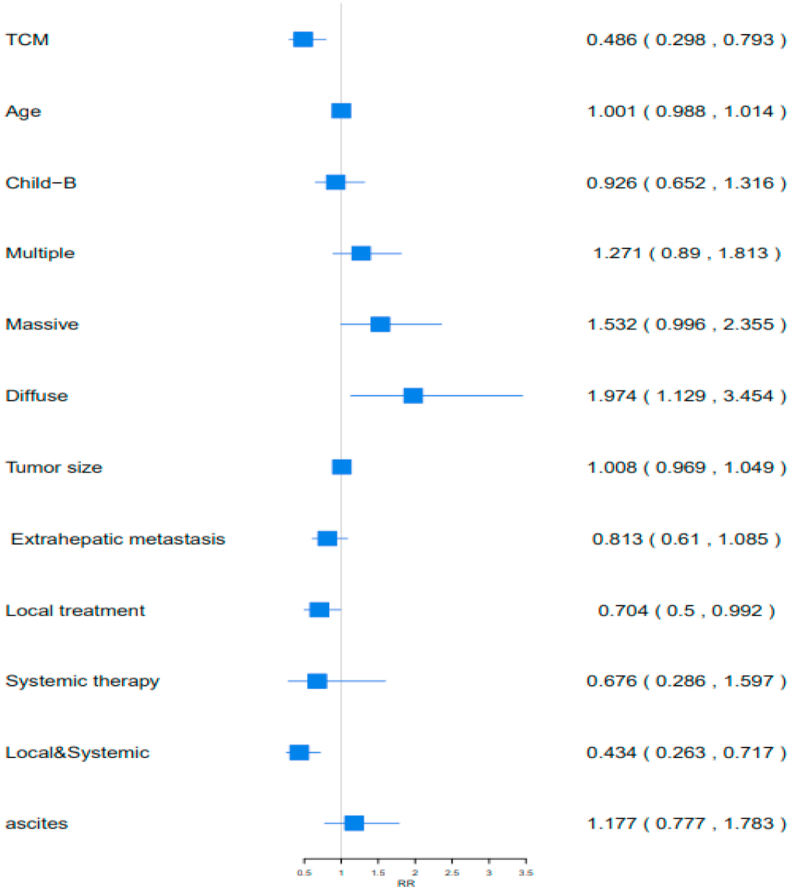
Moreover, C-TDR, Child-Pugh score, follow-up treatment, and ascites were important factors affecting survival in 355 BCLC-C patients (P < 0.05; Table 5). Higher C-TDR, accepted local treatment, and accepted combined treatment demonstrated 51.36% (RR [95%CI] = 1.0016 [0.9885,1.0149], 29.53% (RR [95%CI] = 0.7047 [0.5004,0.9924]), and 56.57% (RR [95%CI] = 0.4343 [0.263,0.7173]) lower risk of death, respectively. Diffuse exposure increased the risk of death by 97.49% (RR [95%CI] = 1.0088 [0.9696, 1.0495]) (Figure 6).
Table 5.
The Cox regression equation analysis with 355 BCLC-C patients.
| OS RR value(95%CI) | P value | Risk of death | |
|---|---|---|---|
| TDR of TCM | 1.0016 (0.9885,1.0149) | 0.0039 < 0.05 | 51.36%↓ |
| Age | 0.9266 (0.6525,1.316) | 0.8069 > 0.05 | - |
| Child-B VS Child-A | 1.2711 (0.8907,1.8138) | 0.6703 > 0.05 | - |
| Multiple | 1.5322 (0.9967,2.3556) | 0.1861 > 0.05 | - |
| Massive | 1.9749 (1.1291,3.4541) | 0.0518 > 0.05 | - |
| Diffuse | 1.0088 (0.9696,1.0495) | 0.0171 < 0.05 | 97.49%↑ |
| Tumor size | 0.814 (0.6105,1.0852) | 0.6649 > 0.05 | - |
| Extrahepatic metastasis | 0.809 (0.6289,1.0406) | 0.1608 > 0.05 | - |
| Local treatment | 0.7047 (0.5004,0.9924) | 0.0451 < 0.05 | 29.53%↓ |
| Systemic therapy | 0.6768 (0.2867,1.5975) | 0.373 > 0.05 | - |
| Local&Systemic | 0.4343 (0.263,0.7173) | 0.0011 < 0.05 | 56.57%↓ |
| Ascites | 1.1773 (0.7771,1.7837) | 0.4412 > 0.05 | - |
∗P < 0.05 indicates statistical significance, #P > 0.05 indicates no statistical significance.
Figure 6.
Analysis of prognostic factors in 355 BCLC-C patients with OS. TCM: TDR of TCM, Local&Systemic: Local treatment & Systemic therapy.
3.4. Propensity score and survival analysis
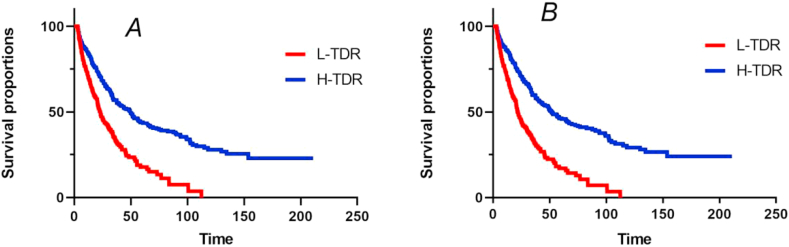
All patients were assigned to two groups; high and low TDR groups, according to the median C-TDR at every stage. Propensity score matching (PSM) and survival analysis were used to analyze the prognostic factors for 1002 patient outcomes at different stages. There was no significant difference in the baseline data between the H-TDR and L-TDR groups before and after PSM at the three stages (P > 0.05) (Appendix Table S1-S4). The H-TDR group had different outcomes for OS, compared with L-TDR, before and after PSM at every stage. Before PSM, median OS duration was 49.2 months for the 501 patients in the H-TDR group, compared with 22.77 months for the 501 patients in the L-TDR group (P = 0.00 < 0.05; HR = 0.4628 [0.3879–0.5522]) (Figure 7A). After PSM, the difference in OS was significant between the H-TDR (51.53 months) and L-TDR group (21.73 months) (P = 0.00 < 0.05, HR = 0.4217 [0.3491–0.5094]) (Figure 7B), with comparable 1-, 2-, and 5-year survival rates (86.09% vs. 70.95%, 59.79% vs. 32.25%, and 45.88% vs. 16.99%, respectively).
Figure 7.
Kaplan–Meier analysis of overall survival in H-TDR and L-TDR group before and after propensity score matching. A: Before PSM, 0: L-TDR group, 1: H-TDR group; B: After PSM, 1: L-TDR group, 2: H-TDR group.
Similarly, there were statistically significant differences in OS between the H-TDR and L-TDR groups before and after PSM at all three stages. BCLC-A stage: Before PSM, H-TDR group (125 patients) 134.47 months vs. L-TDR group (126 patients) 40.8 months (P = 0.00 < 0.05, HR = 0.3034 [0.1863–0.4941]) (Appendix Fig S1A); 1-, 2-, and 5-year survival rates (100% vs. 90.1%, 87.78% vs. 61.44%, and 75.7% vs. 38.42%). After PSM, H-TDR group (98 patients) 134.47 months vs. L-TDR group (98patients) 40.8 months (P = 0.00 < 0.05, HR = 0.3034 [0.1774–0.5190]) (Appendix Fig S1B); 1-, 2-, and 5-year survival rates (100% vs. 89.87%, 90.02% vs. 59.23%, 75.51% vs. 37.0%, respectively). BCLC-B stage: Before PSM, H-TDR group (198 patients) 49.2 months vs. L-TDR group (198patients) 26.77 months (P = 0.00 < 0.05, HR = 0.5441 (0.4151–0.7132) (Appendix Fig S2A); 1-, 2-, and 5-year survival rates (89.13% vs. 80.18%, 59.48% vs. 36.89%, and 40.78% vs. 18.5%, respectively). After PSM, H-TDR group (156 patients) 52.93 months vs. L-TDR group (156patients) 23.67 months (P = 0.00 < 0.05, HR = 0.4472 [0.3331–0.6003]) (Appendix Fig S2B); 1-, 2-, and 5-year survival rates (90.19% vs. 75.23%, 64.52% vs. 30.24%, 44.25% vs. 15.23%, respectively). BCLC-C stage: Before PSM, H-TDR groups (177 patients) 16.9 months vs. L-TDR group (178 patients) 12.27 months (P = 0.00 < 0.05, HR = 0.7260 [0.5564–0.9474]) (Appendix Fig S3A); 1- and 2-year survival rates (64.17% vs. 50.05%, 21.17% vs. 15.28%, respectively). After PSM, the H-TDR group (134 patients) 17.4 months vs. L-TDR group (134patients)10.8 months (P = 0.00 < 0.05, HR = 0.6207 [0.4613–0.8351]) (Appendix Fig S3B); 1- and 2-year survival rates (66.11% vs. 45.19% and 24.03% vs 11.87%, respectively).
3.5. Discussion
In recent years, the combination of Chinese and western medicine has been a relatively acceptable treatment method [22, 23]. TCM has unique advantages for the prevention and treatment of PLC [22, 23]. An increasing number of studies indicate that TCM has a unique advantage in relieving and curing PLC because of its good curative effect and fewer adverse reactions. Previous study showed that Huaier granule, a traditional Chinese medicine, can significantly prolong recurrence-free surviva and reduced extrahepatic recurrence for HCC after curative liver resection [26]. Another study explored the benefits of TCM therapy in the long-term survival of 3483 patients with PLC and found that using TCM as adjuvant therapy can probably prolong median survival time and improve the OS among patients with HCC [27]. However, there is no consensus in the world on the length of TCM treatment and for how long this therapy should be extended [22, 23].
This is the first and largest real-world study in China to evaluate the significance of TCM taking duration in the treatment of PLC. There were significant differences in the Child–Pugh class; tumor type, location, size, and thrombus; and prevalence of lymph node and distant metastases among the BCLC stage A,B and C (Table 1). Patients with different stages of PLC have different conditions and OS. Generally, compared to BCLC-A and BCLC-B, BCLC-C is more serious and is associated with a shorter OS (Figure 2). Here we introduced a new concept, treatment-duration-ratio of Traditional Chinese Medicine (C-TDR) to investigate the association of C-TDR and survival outcomes. The treatment-duration-ratio of Traditional Chinese Medicine (C-TDR) refers to the percentage of time receiving TCM treatment in overall treatment time. The higher percentage means that the time of starting traditional Chinese medicine treatment is close to the time of onset and adheres to traditional Chinese medicine treatment for a long time. The results showed high C-TDR was an independent prognostic factors for OS of TCM intervention. In other words, the earlier TCM, the longer OS. Similar results were also obtained using PSM analysis and multivariable Cox regression analysis (Appendix Fig S1-S3). The propensity score is a balanced score that can be used to explain the systematic differences between the exposure and control groups in an observational study. The score is constructed by estimating the exposure probability of the patients in each study cohort; this is achieved by limiting the exposure probability to the available observed variables. Propensity scores can be estimated by regressing the treatment allocation of the observed baseline characteristics using a logistic regression model. At the individual level, this is a way to measure a person's likelihood of receiving treatment after considering their baseline characteristics [28]. Variables used to construct the propensity scores in our study were stages; age; sex; Child–Pugh class; tumor type, location, size, and thrombus; lymph node metastasis, ascites, and distant metastasis. The choice of these covariates was based on literature on the associated or predisposing factors for PLC prognosis. The adequacy of balance was assessed using standardized differences. These methods were conducted to balance the baseline and control for confounding factors.
This study started in 2015 to assess OS in patients with PLC who were undergoing TCM treatment with Low C-TDR and High C-TDR. Our previous studies showed that Chinese Herbal Medicine effectively prolongs OS of PLC Patients for every stage [27, 28, 29, 30, 31, 32, 33]. A multicenter, open-label, randomized, controlled study [29]which compare the efficacy of a TCM regimen and TACE in preventing recurrence in post-resection patients with early HCC found that the THM regimen had prolonged the recurrence-free survival and overall survival. The retrospective study [32]which the effect of combined therapy with TACE and Jiedu granule preparation in the treatment of patients with Metaphase Liver Cancer on survival showed TACE combined with Jiedu granule can prolong OS of patients. Another prospective cohort study [33] have revealed that sorafenib and Jiedu Granule had the same effect for the treatment of Chinese patients with advanced HCC. Based on studies aforesaid, the present study took C-TDR as the research object for the first time. The outcome showed that earlier traditional Chinese medicine treatment obtain a longer OS for patients with liver cancer. Therefore, TCM treatment could be useful as a effective treatment. The longer the TCM treatment duration, the better prognosis of PLC.
There were several limitations in our study. First, this was a retrospective analysis study that inevitably had a selective bias in patient selection. Second, radiology reports and images were not evaluated by an independent reviewer and inaccurate recording of tumor lesions by the patient's treating provider may have occurred. Finally, other treatment duration or doses of other concurrent medications were not considered in our analysis, such as checkpoint inhibitor (anti-PD1) and targeted (antibody or small molecule) therapies. In addition, more data and more sites are warranted to assess the impact of patients' characteristics on the selection of TCM treatments.
4. Conclusions
In conclusion, high C-TDR was an independent prognostic factors for OS of intervention. Patients with PLC receiving earlier Traditional Chinese Medicine can reduces risk of mortality and prolong survival time.
Declarations
Author contribution statement
Simo Cheng; Changquan Ling: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Hetong Zhao; Yongbin Meng: Performed the experiments; Analyzed and interpreted the data.
Yuyu Guo; Man Yao; Xiaowan Xu: Contributed reagents, materials, analysis tools or data.
Xiaofeng Zhai: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
ChangQuan Ling was supported by National Natural Science Foundation of China [82030117].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
Acknowledgements
Special thanks to Professor Ye Xiaofei for his statistical professional support of this study.
Contributor Information
Xiaofeng Zhai, Email: zhaixfch@163.com.
Changquan Ling, Email: changquanling@smmu.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Ferlay J., Colombet M., Soerjomataram I., et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144(8):1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16(10):589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rich N.E., Yopp A.C., Singal A.G., Murphy C.C. Hepatocellular carcinoma incidence is decreasing among younger adults in the United States. Clin. Gastroenterol. Hepatol. 2020;18(1):242–248. doi: 10.1016/j.cgh.2019.04.043. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiels M.S., O'Brien T.R. Recent decline in hepatocellular carcinoma rates in the United States. Gastroenterology. 2020;158(5):1503–1505. doi: 10.1053/j.gastro.2019.12.030. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Serag H.B. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273. doi: 10.1053/j.gastro.2011.12.061. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajarizadeh B., Grebely J., Dore G.J. Epidemiology and natural history of HCV infection. Nat. Rev. Gastroenterol. Hepatol. 2013;10(9):553–562. doi: 10.1038/nrgastro.2013.107. [DOI] [PubMed] [Google Scholar]
- 7.Zhou M., Wang H., Zeng X., et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;394(10204):1145–1158. doi: 10.1016/S0140-6736(19)30427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allemani C., Weir H.K., Carreira H., et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385(9972):977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 10.Chen W., Zheng R., Baade P.D., et al. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 11.Galle P.R., Forner A., Llovet J.M., et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Tsukamoto M., Imai K., Yamashita Y.I., et al. Endoscopic hepatic resection and endoscopic radiofrequency ablation as initial treatments for hepatocellular carcinoma within the Milan criteria. Surg. Today. 2020;50(4):402–412. doi: 10.1007/s00595-019-01903-9. [DOI] [PubMed] [Google Scholar]
- 13.Kudo M., Matsui O., Izumi N., et al. Transarterial chemoembolization failure/refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(Suppl 1):22–31. doi: 10.1159/000368142. [DOI] [PubMed] [Google Scholar]
- 14.Sonbol M.B., Riaz I.B., Naqvi S.A.A., et al. Systemic therapy and sequencing options in advanced hepatocellular carcinoma: a systematic review and network meta-analysis. JAMA Oncol. 2020;6(12) doi: 10.1001/jamaoncol.2020.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruix J., Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marrero J.A., Kudo M., Bronowicki J.P. The challenge of prognosis and staging for hepatocellular carcinoma. Oncol. 2010;15(Suppl 4):23–33. doi: 10.1634/theoncologist.2010-S4-23. [DOI] [PubMed] [Google Scholar]
- 17.Llovet J.M., Ducreux M., Lencioni R., Di Bisceglie A., Galle P., Dufour J. European association for the study of the liver European organisation for research and treatment of cancer: EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J. Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Marrero J.A., Fontana R.J., Barrat A., et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41(4):707–716. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- 19.Cillo U., Vitale A., Grigoletto F., et al. Prospective validation of the Barcelona clinic liver cancer staging system. J. Hepatol. 2006;44(4):723–731. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Chen C.H., Hu F.C., Huang G.T., et al. Applicability of staging systems for patients with hepatocellular carcinoma is dependent on treatment method--analysis of 2010 Taiwanese patients. Eur. J. Cancer. 2009;45(9):1630–1639. doi: 10.1016/j.ejca.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Bruix J., Qin S., Merle P., et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 22.Hu B., Wang S.S., Du Q. Traditional Chinese medicine for prevention and treatment of hepatocarcinoma: from bench to bedside. World J. Hepatol. 2015;7(9):1209–1232. doi: 10.4254/wjh.v7.i9.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xi S.Y., Minuk G.Y. Role of traditional Chinese medicine in the management of patients with hepatocellular carcinoma. World J. Hepatol. 2018;10(11):799–806. doi: 10.4254/wjh.v10.i11.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Standard for diagnosis and treatment of primary liver cancer (2019 Edition) Infect. Dis. Inform. 2020;33(6):481–500. [Google Scholar]
- 25.Oken M.M., Creech R.H., Tormey D.C., et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am. J. Clin. Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 26.Chen Q., Shu C., Laurence A.D., et al. Effect of Huaier granule on recurrence after curative resection of HCC: a multicentre, randomised clinical trial. Gut. 2018;67(11):2006–2016. doi: 10.1136/gutjnl-2018-315983. [DOI] [PubMed] [Google Scholar]
- 27.Liu X., Li M., Wang X., et al. Effects of adjuvant traditional Chinese medicine therapy on long-term survival in patients with hepatocellular carcinoma. Phytomedicine. 2019;62 doi: 10.1016/j.phymed.2019.152930. [DOI] [PubMed] [Google Scholar]
- 28.Johnson S.R., Tomlinson G.A., Hawker G.A., Granton J.T., Feldman B.M. Propensity score methods for bias reduction in observational studies of treatment effect. Rheum. Dis. Clin. N. Am. 2018;44(2):203–213. doi: 10.1016/j.rdc.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Zhai X.F., Liu X.L., Shen F., Fan J., Ling C.Q. Traditional herbal medicine prevents postoperative recurrence of small hepatocellular carcinoma: a randomized controlled study. Cancer. 2018;124(10):2161–2168. doi: 10.1002/cncr.30915. [DOI] [PubMed] [Google Scholar]
- 30.Zhai X.F., Chen Z., Li B., et al. Traditional herbal medicine in preventing recurrence after resection of small hepatocellular carcinoma: a multicenter randomized controlled trial. J. Integr. Med. 2013;11(2):90–100. doi: 10.3736/jintegrmed2013021. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z., Chen H.Y., Lang Q.B., et al. Preventive effects of jiedu granules combined with cinobufacini injection versus transcatheter arterial chemoembolization in post-surgical patients with hepatocellular carcinoma: a case-control trial. Chin. J. Integr. Med. 2012;18(5):339–344. doi: 10.1007/s11655-012-1083-1. [DOI] [PubMed] [Google Scholar]
- 32.Chen L.Y., Zhai X.F., Chen Z., et al. Jie-du granule preparation for the treatment of advanced hepatocellular carcinoma: a retrospective cohort study of 177 patients. Oncotarget. 2017;8(18):30471–30476. doi: 10.18632/oncotarget.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao H.T., Meng Y.B., Zhai X.F., et al. Comparable effects of Jiedu Granule, a compound Chinese herbal medicine, and sorafenib for advanced hepatocellular carcinoma: a prospective multicenter cohort study. J Integr Med. 2020;18(4):319–325. doi: 10.1016/j.joim.2020.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.