Abstract
Lung diseases continue to draw considerable attention from biomedical and public health care agencies. The lung with the largest epithelial surface area is continuously exposed to the external environment during exchanging gas. Therefore, the chances of respiratory disorders and lung infections are overgrowing. This review has covered promising and opportunistic etiologic agents responsible for lung infections. These pathogens infect the lungs either directly or indirectly. However, it is difficult to intervene in lung diseases using available oral or parenteral antimicrobial formulations. Many pieces of research have been done in the last two decades to improve inhalable antimicrobial formulations. However, very few have been approved for human use. This review article discusses the approved inhalable antimicrobial agents (AMAs) and identifies why pulmonary delivery is explored. Additionally, the basic anatomy of the respiratory system linked with barriers to AMA delivery has been discussed here. This review opens several new scopes for researchers to work on pulmonary medicines for specific diseases and bring more respiratory medication to market.
Keywords: Etiological agents, Antimicrobial agents, Infection, Lung, Pulmonary delivery, Barrier, Inhalable drug
Graphical abstract
Highlights
-
•
Targeting lungs through oral administration of AMAs is a big challenge.
-
•
Lung infection etiological agents are considerably higher than the effective pulmonary treatment available.
-
•
Many potential barriers are responsible for ineffective delivery of AMAs through pulmonary administration.
-
•
Extensive research is needed to bring more drugs to the market for pulmonary administration.
Etiological agents, Antimicrobial agents, Infection, Lung, Pulmonary delivery, Barrier, Inhalable drug.
1. Introduction
The rising incidence of lung disease has become a concern worldwide. The causative agents for these infections are bacteria, viruses, parasites, fungi, etc. Targeting antimicrobial agents (AMAs) to the lungs is essential to treat these microorganisms. However, the pulmonary route is challenging due to the nature of lung tissue. The lung has a large surface area that contains a thin epithelium layer that helps to expose AMA to lung tissue [1]. Compared to parenteral or oral administration, pulmonary delivery of AMAs poses substantial benefits for managing lower airway infections. Treating bacterial infections proves difficult due to the rapid development of drug resistance. This pulmonary delivery offers the local application of AMA to enhance drug concentrations at the infected site while minimizing systemic exposure, which leads to the development of resistance and toxicity [2]. Bypassing drug metabolism and preventing the first-pass metabolism are exciting possibilities for using the pulmonary delivery of AMA [3]. Moreover, this therapy improves patients’ compliance [4]. However, a less number of inhalable AMA formulations have reached the marketplace. This paper focuses on opportunistic etiological agents that play a vital role in lung infections and their available conventional treatments. In response to this enormous burden of respiratory diseases and causative agents, very few AMAs have been approved for pulmonary delivery, which has been discussed here. This paper explains the anatomical barriers and challenges AMAs face in the respiratory tract to bring more pulmonary medication to market.
2. Etiological agents responsible for lung infections
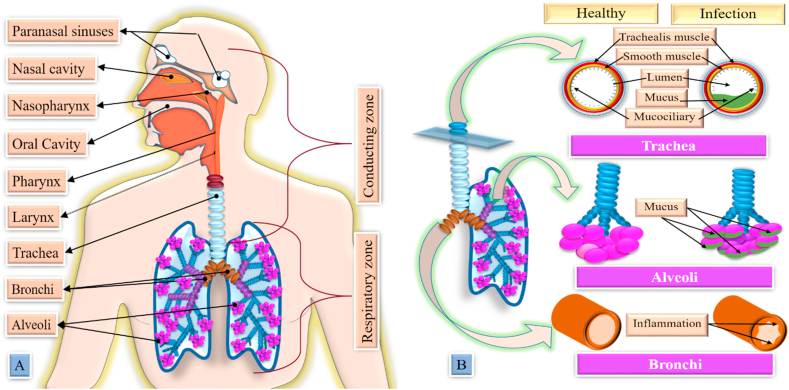
There are numerous causing agents (Figure 1) accountable for lung infections. The disease initiation involves direct or thorough travel to the lungs from the infection site.
Figure 1.
Different types of etiological agents are responsible for lung disease. Bacteria, fungi, parasites, and viruses are the main causative agents for lung infections. Four gram-positive bacteria, thirteen gram-negative bacteria, and two acid-fast staining bacteria cause lung infections. Ten fungal microorganisms and fourteen parasites have been identified in lung infection. Last but not least, twelve viruses are shorted here, which are found to be responsible for lung infections. The red ink highlighted organisms show a higher incidence of lung infections.
2.1. Gram-positive bacteria
In bacteriology, gram-positive bacteria show purple color under a microscope during Gram staining, whereas gram-negative bacteria do not. Staphylococci, Streptococci, Mycobacterium, clostridium, actinomyces, etc., are examples of gram-positive bacteria. Few bacteria cause lung infection, but the incidence is significantly high (Table 1).
Table 1.
Summary of lung infections causing bacteria and their management.
| Class | Organism | Characteristics | Recommended medicine |
|---|---|---|---|
| Gram-positive bacteria | Staphylococcus aureus | 1. A vital pathogen for CF 2. Colonizes mainly the mucous membranes of the anus, perineum, nose, and throat |
Cephalosporins (cefazolin), β-lactam antibiotics (oxacillin or nafcillin), glycopeptide antibiotics (vancomycin), cyclic lipopeptides (daptomycin), and lipoglycopeptide |
| MSSA | 1. The chances of MRSA increase with exposure to hospitals and antibiotic 2. Prevalence of this organism increase with all S. aureus |
Vancomycin, daptomycin, teicoplanin, telavancin, oxazolidinones, and tigecycline | |
| Streptococcus pneumoniae | 1. Responsible for the majority of community-acquired pneumonia 2. Significant cause of morbidity and mortality |
Amoxicillin, cephalosporins, ofloxacin and vancomycin. | |
| Bacillus anthracis | 1. Mostly used for biological warfare 2. Inhalational anthrax is denoted as the most life-threatening |
Penicillin G, linezolid and carbapenems, ciprofloxacin, Dichlorophen, oxiconazole, suloctidil, hexestrol, and bithionol | |
| Acid-fast staining bacteria | Mycobacterium tuberculosis | 1. One-third of the world population is infected with this microorganism 2. Mainly progresses in patients with impaired immunity |
Rifampicin, isoniazide, Ethambutol, pyrazinamide, Prothionamide, Ethionamide, etc. |
| Nontuberculous mycobacteria | 1. NTM are environmental microorganisms and can cause chronic lung infection 2. NTM is only dangerous to individuals with defective lung structures or immunosuppressed. |
Azithromycin or clarithromycin with rifampicin and ethambutol. Injectable amikacin or streptomycin. Rifampicin, ethambutol, isoniazid, or moxifloxacin, and injectable aminoglycoside (clarithromycin-resistant MAC) |
|
| Gram-negative bacteria | Achromobacter xylosoxidans | 1. Non-fermenting gram-negative rod that mainly causes healthcare-associated infection 2. This pathogen is widely resistant to many antibiotics and complicating treatment options |
Ceftazidime, tobramycin, and colistin, ceftazidime, carbapenems, piperacillin-tazobactam, and sulfamethoxazole-trimethoprim |
| Burkholderia cepacia complex | 1. This gram-negative bacteria comprises 18 distinct species. 2. These species are less common, and their clinical associations are less well defined. |
Ceftazidime, meropenem, trimethoprim-sulfamethoxazole, doripenem, doxycycline, minocycline, tobramycin, poly (acetyl arginyl) glucosamine, ceftazidime, and meropenem | |
| Chlamydia psittaci | 1. Transmitted to humans by any bird, and there have been rare reports of human-to-human transmission 2. Initially, replicate in respiratory epithelial cells is followed by spreading throughout the body |
Doxycycline And Tetracycline Hydrochloride | |
| Chlamydia psittaci | 1. Can infect individuals of all ages 2. It can persist in the host for months to years without showing any symptoms. |
Macrolides, tetracycline, and quinolones | |
| Francisella tularensis | 1. Inhalation of F. tularensis bacilli is responsible for the slow progression of pneumonia with a lower fatality rate. 2. This microorganism can infect many cell-like phagocytic cells, including macrophages and dendritic cells. |
Doxycycline and oral fluoroquinolone (ciprofloxacin, ofloxacin, and levofloxacin) | |
| Haemophilus influenzae | 1. This organism is mainly prevalent in children and less common in adults. 2. This organism is associated with frequent exacerbations of bronchiectasis |
Dexamethasone, ceftazidime, cefotaxime, ceftriaxone, fluoroquinolones, azithromycin, and amoxicillin/clavulanate. | |
| Klebsiella pneumoniae | 1. Accounts for a higher proportion of pneumonia acquired in hospitals. 2. This microorganism is a notable pulmonary pathogen leading to severe pneumonia and sepsis |
Third-fourth generation cephalosporin, aminoglycoside, carbapenem, polymyxin class, tigecycline, and fosfomycin | |
| Legionella pneumophila | 1. Resides in surface or drinking water and is transmitted to humans by aerosols. 2. Destructive alveolar inflammation is produced by recruiting neutrophils, monocytes, and bacterial enzymes |
β-Lactams, macrolides, rifampicin, fluoroquinolones, and omadacycline | |
| Mycoplasma pneumoniae | 1. This pathogen spreads from person to person by respiratory droplets. 2, It produces hydrogen peroxides and superoxide, damaging respiratory epithelial cells. |
macrolides, tetracycline, streptogramins, fluoroquinolones, omadacycline, lefamulin, nafithromycin, zoliflodacin, and solithromycin | |
| Moraxella catarrhalis | 1. The bacteria are transmitted exclusively between humans by direct and indirect contact. 2. It causes both upper (children) and lower (adult) respiratory tract infection |
Ampicillin, penicillin, amoxicillin-clavulanic acid, azithromycin, clarithromycin, extended-spectrum cephalosporin, tetracycline, trimethoprim-sulfa, and fluoroquinolones | |
| Pseudomonas aeruginosa | 1. This microorganism interrupts lower and upper airway homeostasis by damaging the epithelium cells. 2. No adequate remedy has been developed yet to manage this microorganism |
carbapenems (meropenem, imipenem), aminoglycosides (tobramycin, netilmicin, gentamicin, amikacin), fluoroquinolones (levofloxacin, ciprofloxacin), cephalosporins (cefepime, ceftazidime), β-lactam inhibitors (piperacillin, ticarcillin), polymyxins (polymyxin A and polymyxin B) and fosfomycin | |
| Stenotrophomonas maltophilia | 1. It is a multi-drug resistant microorganism associated with several infections 2. This microorganism is intrinsically and adaptively resistant to many antibiotics. |
Levofloxacin, ticarcillin-clavulanate, trimethoprim-sulfamethoxazole, doxycycline, fluoroquinolones, minocycline, and tigecycline | |
| Yersinia pestis | 1. The infection is usually transmitted to humans through a flea bite from a flea fed on an infected rat and then on a human. 2. The disease begins with bronchiolitis and alveolitis, progressing to a lobular and eventual lobar consolidation. |
Tetracycline, streptomycin, fluoroquinolones, chloramphenicol, gentamicin, and sulphonamides |
CF-Cystic fibrosis; MSSA-Methicillin-Susceptible S. aureus; MAC- Mycobacterium avium complex.
2.1.1. Staphylococcus aureus
Staphylococcus aureus is a gram-positive microorganism recognized as an important pathogen for cystic fibrosis (CF). The role of S. aureus in CF and the appropriate clinical response to its detection is challenging. The commonness of this microorganism among pediatric CF has become a growing concern. This microorganism colonizes mainly the mucous membranes of the anus, perineum, nose, and throat. This colonization is more frequently acquired by hospitalized patients and immediate contact persons in healthcare settings. 70–90% of the general population are the transient carriers of this microorganism. Asymptomatic carriage is a major reservoir of persistent infection spreading in the community [5]. The rising incidence of S. aureus infections leads to drug resistance. S. aureus causes lower lung function, higher airway inflammation, and mortality when detected together with P. aeruginosa [6]. S. aureus in adults with CF demonstrates a lower chance of mortality, better lung function, and lower risk of exacerbation. Several studies revealed the coinfections of S. aureus and P. aeruginosa in chronic airway diseases. During infancy, S. aureus is the key colonizing bacteria in CF lungs and declines at a later age. Additionally, the chances of P. aeruginosa increase with increasing age. The competitive interaction of these two pathogens is influenced by their survival, antibiotic susceptibility, and disease propagation [7]. Therefore, S. aureus pathogenesis is either more in children or in the absence of P. aeruginosa. The pathogenesis of S. aureus may be high for specific subtypes like methicillin-resistant S. aureus (MRSA) or small-colony variants (SCVs) of S. aureus [8]. Several antibiotics are recommended for the treatment of S. aureus infection, including cephalosporins (cefazolin), β-lactam antibiotics (oxacillin or nafcillin), glycopeptide antibiotics (vancomycin), cyclic lipopeptides (daptomycin), and lipoglycopeptide.
Methicillin-Susceptible S. aureus (MSSA) is identified either by resistance to β-lactams or by the carriage of the mecA gene [9]. Its prevalence has increased simultaneously with all S. aureus and methicillin-susceptible S. aureus (MSSA). The chances of MRSA increase with exposure to hospitals and antibiotics [10]. Staphylococcal small colony variants (SCVs) are slow-growing antibiotic-resistant variants that are difficult to detect with traditional cultures. A study found CSVs associated with lower lung function and higher rates of preceding antibiotic treatment. MRSA and SCVs cause worse outcomes in patients with pre-existing diseases and higher antibiotic treatment burdens [8]. Treatment with oxacillin, cloxacillin, flucloxacillin, and nafcillin are prolonged in MSSA. However, optimum treatment therapy is still unresolved [11]. The treatment of MRSA requires early initiation of antimicrobial therapy. Vancomycin is the first choice of treatment. Daptomycin is also equally effective, but it is a costly alternative. Ceftaroline also demonstrates promising activity in MRSA. Antibiotics like teicoplanin, telavancin, oxazolidinones, and tigecycline are also recommended in single or combination. Although a wide range of antibiotics is available for treatment often fail due to poor control of foci [12].
2.1.2. Streptococcus pneumoniae
Streptococcus pneumoniae (pneumococcus) is a gram-positive bacterium. This microorganism is responsible for the majority of community-acquired pneumonia. It is a commensal microorganism in the human respiratory tract. Acute pulmonary infection is often characterized by a higher bacterial load of S. pneumoniae in the lung. A substantial influx of polymorphonuclear cells has been observed in this infection, followed by a risk of systemic spread. It is a significant cause of morbidity and mortality globally, causing more deaths than other infectious diseases [13]. After entering the lower respiratory tract initiates pneumonia by escaping the mucous defense and gradually proceeding to the alveolus [14]. Oral amoxicillin is generally recommended as a first-line antimicrobial agent. However, the course varies from five to seven days based on the illness severity. The American Thoracic Society and Disease Society of America recommend macrolide therapy for community-acquired pneumonia without having the chance of drug-resistant S. Pneumoniae. British Infection Association recommends cephalosporins and penicillin-based agents in community-acquired pneumonia. In the case of highly resistant S. pneumoniae, ofloxacin and vancomycin are used as alternatives. β-lactam with fluoroquinolone or macrolide is recommended as the first-line in intensive care unit (ICU) patients [15].
2.1.3. Bacillus anthracis
Bacillus anthracis is the agent of anthrax, a common disease of livestock. It occasionally infects humans through direct or indirect contact with the infected animal. It is the only obligate pathogen within the genus Bacillus. This microorganism is one of the most likely agents used for biological warfare as its spores are highly resilient to degradation and easy to produce. The clinical syndromes of this infection depend on the entry route of B. anthracis. Inhalational anthrax (entering through the respiratory system) is denoted as the most life-threatening form of the disease and causes severe hemorrhagic mediastinitis (inflammation of the mediastinum) [16]. This pathogen produces exotoxin and progresses to toxemia that severely compromises pulmonary function. Pulmonary parenchymal changes, serosanguineous fluid in alveolar spaces, or mononuclear cells have been detected in a few patients. The lymph node parenchyma generally is teeming with intact and fragmented gram-positive bacilli [17]. This microorganism is susceptible to many antibiotics, but early detection and management may eradicate the bacteria. Penicillin G is generally used for naturally occurring anthrax. The WHO has recommended a combination of penicillin with fluoroquinolone or macrolide in severe cases. In gastrointestinal anthrax, a combination of penicillin with an aminoglycoside is recommended [18]. Managing this microorganism is complicated due to the lack of efficient treatment. Centers for Disease Control and Prevention has recommended a combination therapy of linezolid and carbapenems to treat late anthrax. In a study, meropenem or linezolid with or without ciprofloxacin could not protect from anthrax-meningitis mainly due to poor penetration to the blood-brain barrier [19]. One group of researchers screened 1586 clinically approved drugs to treat B. anthracis [20]. Dichlorophen, oxiconazole, suloctidil, hexestrol, and bithionol effectively inhibited this microorganism. These drugs have demonstrated broad-spectrum activity against gram-positive and -negative bacterial strains. Out of which, hexestrol exhibits superior inhibition across all strains.
2.2. Acid-fast staining bacteria
These bacteria undergo staining but resist decolorization by acids. Acid fastness is the inherent property of this class of bacteria. Acid-fast staining bacteria cause tuberculosis and certain other infections (Table 1).
2.2.1. Mycobacterium tuberculosis
Tuberculosis continues to be a significant worldwide epidemic. Approximately one-third of the world population is infected with Mycobacterium tuberculosis [21]. M. tuberculosis is the most lethal mycobacterial species. It is an indisputable pathogen that is accountable for many deaths worldwide. M. tuberculosis is an obligate-aerobic, non-motile, non-spore-forming, catalase-negative, and facultative intracellular bacteria. Primary tuberculosis occurs in patients without previous exposure or with the loss of acquired immunity [22]. It mainly progresses in patients with impaired immunity. The disease passes through progressive phases of exudation, recruitment of macrophages, T lymphocytes, and granuloma formation, followed by repair with granulation tissue, fibrosis, and mineralization. Secondary or reinfection-reactivation tuberculosis, referred to as post-primary tuberculosis, occurs in patients with previous immunity to the microorganism and accounts for most clinical cases of tuberculosis [23]. Most cases of active tuberculosis in adults with normal immunity progress into a latent infection, whereas reinfection with a new strain derived from the environment occurs in immunocompromised patients. The most common form of post-primary tuberculosis in adults involves the apices of the upper lobes, producing granulomatous lesions with greater caseation, often with cavities, variable degrees of fibrosis, and retraction of the parenchyma [8]. Several first-line [24] and second-line [25] drugs are available to treat tuberculosis. The World Health Organization (WHO) has recommended adopting a Directly Observed Treatment Short-course (DOTS) strategy to improve treatment adherence. In pulmonary and extra-pulmonary tuberculosis, rifampicin, isoniazid, pyrazinamide, and ethambutol are prescribed in the intensive phase, followed by rifampicin and isoniazid in the maintenance phase. The previously reported article explains the list of drugs in different resistance cases well [26]. All these drugs are mainly available in the market in oral form. Although the lungs are the primary site of action, a tiny fraction reaches to lungs from conventional administration [27]. Therefore, there is an emerging need to deliver drugs to the lungs directly.
2.2.2. Nontuberculous mycobacteria
The incidence and number of deaths from non-tuberculous mycobacteria (NTM) disease have steadily increased globally. NTM is only dangerous to individuals with defective lung structures or immunosuppressed. NTM are now commonly infecting seemingly immune-competent children and adults at increasing rates through pulmonary infection. It is of concern as the pathology of NTM is challenging to treat and is resistant to drugs [28]. NTM are environmental microorganisms and can easily be found in soil and water that can cause chronic lung infection. The rate of NTM infection is increasing in patients with CF. This microorganism is not transmissible from person to person or reactive to latent infection like Mycobacterium tuberculosis [29]. Out of 150 identified NTM species, only a few have been reported to cause pulmonary disease. The majority (95%) of NTM isolated from CF patients is Mycobacterium avium complex (MAC) (M. intracellulare and four M. avium subspecies) and M. abscessus complex (MABSC) (subspecies abscessus, massiliense, and bolletii) [30]. In most instances, patients with NTM infection develop chronic lung disease and other risk factors, such as AIDS, alcoholism, or diabetes. Significant heterogeneity is observed in susceptibility to standard anti-TB drugs. Thus, macrolides and injectable aminoglycosides are generally prescribed for NTM disease. Despite available guidelines, the treatment is mainly empirical. In nodular or bronchiectasis MAC lung disease, combinational therapy like azithromycin or clarithromycin with rifampicin and ethambutol is recommended as initial therapy. The addition of injectable amikacin or streptomycin is included with the standard treatment. In the clarithromycin-resistant MAC case, rifampicin, ethambutol, isoniazid, or moxifloxacin, and injectable aminoglycoside are given. M. abscessus is resistant to all front-line anti-TB drugs. Thus oral macrolides with two parenteral drugs are the primary choice. According to the American Thoracic Society (ATS) and Infectious Disease Society of America (IDSA) guidelines, amikacin, imipenem, or cefotaxime are used to treat M. abscessus infection.
2.3. Gram-negative bacteria
The high rate of respiratory infections is due to gram-negative bacteria, which can be found in all environments on Earth that support life. These pathogens are among the most significant public health problems in the world due to their high resistance to antibiotics (Table 1). The most common pneumonia causing microorganisms is Pseudomonas aeruginosa. Pneumonia causing a few more organisms are Chlamydia species, Francisella tularensis, Klebsiella pneumoniae, Legionella pneumophila, and Mycoplasma pneumoniae.
2.3.1. Achromobacter xylosoxidans
Achromobacter xylosoxidans is an aerobic, motile, oxidase-positive, non-fermenting gram-negative rod that mainly causes healthcare-associated infection [31]. As for P. aeruginosa and Burkholderia cepacia complex (BCC), A. xylosoxidans can be dominant and detected in CF patients at the end stage. The most clinically significant species of Achromobacter are xylosoxidans and denitrificans [32]. This pathogen is widely resistant to many antibiotics and complicating treatment options [33]. This species may cause frequent infection and is one of CF patients' most promising causes of post-lung transplant infections. Due to the lack of standard treatment for Achromobacter infections, antibiotics are given in systemic and/or inhalation. Inhalation therapy with ceftazidime, tobramycin, and colistin improved Achromobacter clearance compared to systemic treatment of these drugs in CF patients [34]. β-lactam antibiotics gave favorable clinical outcomes, including ceftazidime, carbapenems, piperacillin-tazobactam, and sulfamethoxazole-trimethoprim [35].
2.3.2. Burkholderia cepacia complex
Burkholderia cepacia complex (BCC) is a group of gram-negative bacteria comprising 18 distinct species. B. cenocepacia and B. multivorans are the two most common and most common CF-associated. Often, B. gladioli are discussed with BCC because of similar infections [36]. But these species are less common, and their clinical associations are less well defined. B. cenocepacia is associated with more rapid lung function than B. multivorans. Similarly, subsequent mortality has been seen with B. cenocepacia than B. multivorans. Both are transmissible between persons with CF. The essential phenotypic modifications are the variation of the lipopolysaccharide (LPS) structure at the level of the O-antigen (OAg) presence, influencing adherence, colonization, and the ability to evade the host defense mechanisms [37]. Treatment against BCC often varies case-by-case, depending on the clinical response and susceptibility data. Ceftazidime, meropenem, trimethoprim-sulfamethoxazole, and doripenem are generally prescribed in the empiric treatment for BCC. Doxycycline and minocycline are the oral alternatives to this empiric treatment [38]. BCC can resist antibiotics and develop adverse environmental conditions that make them impossible to eradicate from CF lungs. Therefore, antimicrobial therapy is limited due to broad-spectrum antimicrobial resistance. Different drugs like tobramycin, poly (acetyl arginyl) glucosamine, ceftazidime, and meropenem with distinct mechanisms of action have been tested to treat these bacteria. It has been suggested that combining these drugs may facilitate a better way of treating BCC [39].
2.3.3. Chlamydia species
Chlamydia psittaci can be carried and transmitted to humans by any bird (pet or wild), not just by the Psittacidae family of birds, such as parrots, parakeets, and macaws. Although respiratory symptoms are usually the result of transmission from birds to humans, there have been rare reports of human-to-human transmission [40]. After inhalation, the microorganism establishes infection in the epithelial cells of the respiratory tract. Initial replication in respiratory epithelial cells is followed by the spread of bacteria throughout the body, affecting multiple organs (heart, liver, gastrointestinal tract) [41]. C. psittaci can cause morbidity with low mortality. Intentional aerosolization would lead to various cases of nonspecific “atypical pneumonia” with cough, fever, and headache [42]. Oral administration of doxycycline and tetracycline hydrochloride is effective against human psittacosis. In the contra-indicated case with tetracycline, azithromycin and erythromycin have been recommended as alternatives [43].
Chlamydia pneumoniae is a mysterious clinical pathogen that causes acute human respiratory disease. C. pneumoniae is an obligate intracellular bacterium causing respiratory infections such as acute pneumonia, bronchitis, sinusitis, and pharyngitis [44]. This disease is also known as community-acquired pneumonia and can infect individuals of all ages. 10% of community-acquired pneumonia cases are happened due to C. pneumoniae. It can penetrate mucosal epithelial cells and replicates gradually. It can be detected in atherosclerotic lesions, potentially linking the bacterium to atherosclerotic processes. However, it is not seen in undamaged vasculature [45]. It can persist in the host for months to years without showing any symptoms. C. pneumoniae can enter a non-replicative persistent state within host cells, forming morphologically aberrant inclusions. Nowadays, the reported cases of C. pneumonia are less than previously. This change is mainly influenced by changing epidemiological characteristics and newer diagnostic methods [46]. Antibiotics like macrolides, tetracycline, and quinolones treat this microorganism [47].
2.3.4. Francisella tularensis
Francisella tularensis is a pathogenic species of gram-negative coccobacillus, an anaerobic bacterium. It is non-spore-forming, non-motile, and the causative agent of tularemia, the pneumonic form of which is often lethal without treatment [48]. Inhalation of F. tularensis bacilli is responsible for the slow progression of pneumonia with a lower fatality rate than inhalation of anthrax or plague. Initially, hemorrhagic and ulcerative bronchiolitis occurs, followed by fibrinous lobular pneumonia with many macrophages. Necrosis then supervenes and evolves into a granulomatous reaction [8]. This microorganism can infect many cell types, but the primary targets appear to be phagocytic cells, including macrophages and dendritic cells (DCs). Targeting these cell types for replication and dissemination has multiple benefits for the bacteria. First, sequestration in the intracellular compartment allows the microorganism to escape exposure to numerous components present in the host serum, including complements and antibodies. Second, macrophages and DCs are pivotal cells for host defense. Macrophages kill microorganisms by invading through phagocytosis and present antigens to respond to T cells [49]. F. tularensis is classified as a category A biological warfare threatening agent. The most commonly prescribed antibiotics are doxycycline and oral fluoroquinolone (ciprofloxacin, ofloxacin, and levofloxacin). Often antibiotic therapy fails to treat this tularemia infection. The failure chances are more in fluoroquinolones than the doxycycline. Thus there is a need for effective novel treatments for F. tularensis [50, 51].
2.3.5. Haemophilus influenzae
Haemophilus influenzae is a small, non-motile, non-spore-forming, gram-negative pleomorphic rod that can be encapsulated or unencapsulated [52]. Haemophilus influenza is often the first microorganism detected in CF respiratory culture and is mainly prevalent in children and less common in adults. This microorganism is the most common bacteria and is associated with frequent exacerbations of bronchiectasis [53]. However, it is associated with adverse outcomes in chronic respiratory infections like non-CF bronchiectasis, COPD, etc. [54]. In susceptible cases, third-generation cephalosporin is the initial choice of antibiotics. Antibiotic resistance is a growing concern in this case. Several reports demonstrated drug resistance to ampicillin and macrolide like erythromycin. However, the culture specimens have not been found resistant to fluoroquinolones. The commonly prescribed antibiotics in Haemophilus influenzae B are ceftazidime, cefotaxime, ceftriaxone, fluoroquinolones, and azithromycin via the parenteral route. Dexamethasone is also recommended as adjunctive treatment as it reduces the cerebral edema associated with inflammation of the meninges. In non-encapsulated H. influenzae, amoxicillin high dose is the first choice. A combination of amoxicillin/clavulanate is also recommended as a choice. Surgical drainage is also employed in the complicated cases like subdural and pleural effusion [55].
2.3.6. Klebsiella pneumoniae
Klebsiella pneumoniae belongs to the Enterobacteriaceae family and is described as a gram-negative, encapsulate, and non-motile bacterium. Virulence of the bacterium is provided by many factors that can lead to infection and antibiotic resistance [56]. Klebsiella pneumoniae (Friedlander's bacillus) is a rare cause of community-acquired pneumonia but accounts for a higher proportion of pneumonia acquired in hospitals. Patients are more likely to be treated with antibiotics that permit this bacterium to dominate the pharyngeal flora [57]. This microorganism is a notable pulmonary pathogen leading to severe pneumonia and sepsis, mainly in immunocompromised patients. Klebsiella pneumoniae generally occurs in immunocompromised patients due to age, ethanol abuse, or diabetes mellitus. It is common for ventilator-associated pneumonia [58]. In the current regimens, third-fourth generation cephalosporin as monotherapy is prescribed. Additionally, respiratory quinolone is recommended as monotherapy or in conjunction with an aminoglycoside. In penicillin-allergic patients, aztreonam or a respiratory quinolone is prescribed. Carbapenem is given worldwide to treat nosocomial infection and extended-spectrum beta-lactamase cases. However, carbapenem-resistant cases are gradually increasing. In such a case, combining antibiotic options, including polymyxin class, aminoglycosides, tigecycline, and fosfomycin are available [56].
2.3.7. Legionella pneumophila
Legionella infections are caused mainly by Legionella pneumophila serogroup 1 and 13 [59]. Legionella bacterium is a small, aerobic, waterborne, gram-negative, unencapsulated bacillus non-motile, oxidase, and catalase-positive. It resides in surface or drinking water and is transmitted to humans by aerosols. This pathogen is often spread through air cooling systems that use water as a cooler [60]. The bacteria multiply intracellularly in alveolar macrophages. Destructive alveolar inflammation is produced by recruiting neutrophils, monocytes, and bacterial enzymes. Direct inoculation in surgical wounds is possible with contaminated tap water. The bacterium binds to respiratory epithelial cells and alveolar macrophages, after which it enters the cell. Once it has gained entry into the cell, it inhibits phagosome-lysosome fusion, followed by promoting its proliferation. L. pneumophila causes community-acquired and nosocomial pneumonia and should be considered a pathogen in patients with atypical pneumonia [61]. The treatment against Legionella bacterium is based on empirical decisions rather than a proper routine susceptibility test. β-Lactams are the first-line treatment for community-acquired bacterial pneumonia (CABP). Other antibiotics, like macrolides, rifampicin, and fluoroquinolones are less effective in intracellular pathogens. Both azithromycin and levofloxacin are effective against this pathogen [62]. Omadacycline in oral and intravenous form has been approved in CABP, including legionella. In a phase 3 study, omadacycline was found as safe with moxifloxacin in the treatment of CABP [63].
2.3.8. Mycoplasma pneumoniae
M. pneumoniae is a serious and common cause of community-acquired pneumonia in children and adults. M. pneumoniae lacks a rigid cell wall, allowing it to alter its size and shape to suit its surrounding conditions. It is resistant to AMA, which works by targeting the cell wall. This pathogen spreads from person to person by respiratory droplets. It is generally an extracellular pathogen that develops a specific attachment organelle for close association with host cells [64]. This close association prevents the mucociliary clearance mechanism in the host respiratory tract. M. pneumoniae produces hydrogen peroxides and superoxide. Gradually, it damages the respiratory epithelial cells at the base of the cilia. Afterward, it activates the innate immune response and produces local cytotoxic effects [65]. The selection of antibiotics is restricted in this type of infection, and choosing those antibiotics that inhibit protein synthesis of the bacterial ribosome. These antibiotics are macrolides, tetracycline, and streptogramins. Azithromycin is the first choice due to its better tolerance and longer half-life. Ketolides and macrolides serve synergistic activity of anti-inflammatory and bacteriostatic action. Fluoroquinolones are also employed to treat this infection due to their inhibitory DNA replication property. These class drugs are also equally effective as macrolides. Due to the increasing cases of macrolide-resistance, several new antimicrobial agents are developed, and they are omadacycline, lefamulin, nafithromycin, zoliflodacin, and solithromycin [66].
2.3.9. Moraxella catarrhalis
Moraxella catarrhalis is a gram-negative diplococcus that physiologically colonizes the healthy mucosal tissues of the human upper respiratory tract. The bacteria are transmitted exclusively between humans by direct and indirect contact. Risk factors include bacterial load, crowding, winter season, daycare attendance, length of stay in a medical institution, and respiratory therapy [67]. In children, M. catarrhalis causes upper respiratory tract infections (otitis media), whereas, in adults, the pathogen causes lower respiratory tract infections in previously compromised airways [68]. M catarrhalis has been shown to have increased cell adhesion and pro-inflammatory responses when cold shock (26 °C for 3 h) occurs. Physiologically, this may occur with prolonged exposure to cold air temperatures, resulting in cold-like symptoms [69]. Long-acting penicillin can clear the infection. Due to excessive production of β-lactamase, M. catarrhalis more frequently resistant to ampicillin and penicillin. In susceptibility cases, a wide range of antimicrobial agents like amoxicillin-clavulanic acid, azithromycin, clarithromycin, extended-spectrum cephalosporin, tetracycline, trimethoprim-sulfa, and fluoroquinolones are included in this treatment [70].
2.3.10. Pseudomonas aeruginosa
Pseudomonas aeruginosa is an encapsulated, rod-shaped, gram-negative bacterium responsible for acute distress syndrome and acute lung injury. This microorganism is also detected in nosocomial pneumonia or hospital-acquired pneumonia (pneumonia happens after hospitalization and is not pre-incubated at the time of hospitalization), specifically in long-term ventilated patients. No adequate remedy has been developed yet to manage this microorganism, resulting in higher morbidity in chronic obstructive pulmonary diseases (COPD) [71]. The complication in P. aeruginosa-associated lung infection happens due to biofilm formation, which is generally a structural consortium of this microorganism composed of DNA, protein, and polysaccharides. This microorganism interrupts lower and upper airway homeostasis by damaging the epithelium cells and intervening in the adaptive and innate immune response [72]. A wide range of antibiotics is generally prescribed to treat this pathogen, including carbapenems (meropenem, imipenem), aminoglycosides (tobramycin, netilmicin, gentamicin, amikacin), fluoroquinolones (levofloxacin, ciprofloxacin), cephalosporins (cefepime, ceftazidime), β-lactam inhibitors (piperacillin, ticarcillin), polymyxins (polymyxin A and polymyxin B) and fosfomycin. Often a single antibiotic may initiate drug resistance against several antibiotics using the overexpression of efflux systems. In most cases, quinolones have significantly triggered cross-resistance in different classes, including β-lactam and aminoglycoside [73]. Polymyxins are considered the only choice in drug resistance cases of P. aeruginosa. Ceftolozane-tazobactam and ceftazidime-avibactam also found to be effected in this case. In monotherapy, antipseudomonal β-lactams like ceftazidime, piperacillin-tazobactam, cefepime, and aztreonam are effective in multi/extremely-multi drug resistance cases [74].
2.3.11. Stenotrophomonas maltophilia
Stenotrophomonas maltophilia is an aerobic, gram-negative pathogen that frequently attacks, particularly common among adolescents and young adults [75]. It is a multi-drug resistant microorganism associated with several infections, including pneumonia, bacteremia, meningitis, and nosocomial infection, especially in immunocompromised patients. This microorganism is intrinsically and adaptively resistant to many antibiotics. For this reason, S. maltophilia has few pathogenic mechanisms and predominantly results in colonization rather than infection. If the infection does occur, invasive medical devices are usually the vehicles through which the microorganism bypasses typical host defense [76]. The administration of proper drugs is often delayed due to late detection. S. maltophilia is susceptible to several antibiotics like levofloxacin, ticarcillin-clavulanate, trimethoprim-sulfamethoxazole, and doxycycline [77]. Trimethoprim-sulfamethoxazole is traditionally used in the treatment of S. maltophilia. Fluoroquinolones are generally recommended as an alternative. However, the use of levofloxacin is limited due to the drug resistance, drug interaction, and safety profile. Additional antimicrobial agents like minocycline and tigecycline are high susceptibility rates [78].
2.3.12. Yersinia pestis
Yersinia pestis is a gram-negative, non-motile, rod-shaped coccobacillus bacterium with no spores. The natural host for this microorganism is a rat. The infection is usually transmitted to humans through a flea bite from a flea fed on an infected rat and then on a human. The disease begins with bronchiolitis and alveolitis, progressing to a lobular and eventual lobar consolidation. A serosanguineous intra-alveolar fluid accumulation with variable fibrin deposits, a fibrinopurulent phase, and necrotizing lesion have been detected in the histopathological study [8]. A complex set of virulence determinants play critical roles in the molecular strategies that Y. pestis employs to subvert the human immune system, allowing unrestricted bacterial replication in the lungs (pneumonic plague) [79]. There are three types of plague disease: bubonic, pneumonic, and septicemic. Pneumonic plague is more likely associated with an aerosolized release of Y. pestis [80]. FDA has approved different treatments, including tetracycline, streptomycin, and fluoroquinolones. Chloramphenicol, gentamicin, and sulphonamides are also suggested as effective therapies. CDC updates treatment and prevention for the plague from time to time [81].
2.4. Fungus
Fungi are commonly detected in respiratory samples, creating clinical uncertainty (CF patients). Fungi can travel along with dust and can survive in the environment. Thousands of conidia are inhaled by humans every day. Many of them create respiratory tract infections (Table 2).
Table 2.
Summary of lung infections causing fungus and their management.
| Organism | Characteristics | Recommended medicine |
|---|---|---|
| Histoplasma capsulatum | 1. It is associated with bird or bat droppings. 2. It can spread to various organs through blood |
Mainly with Itraconazole, fluconazole, and amphotericin B. Sometimes moxifloxacin, linezolid, azithromycin, levofloxacin, and hydroxychloroquine |
| Coccidioides immitis and Coccidioides posadasii | 1. Coccidioidal infections are caused due to airborne transmission 2. It is not contagious but spreads to other body parts like bone, joints, and skin resulting in an extrapulmonary infection. |
Fluconazole, voriconazole, itraconazole, and amphotericin B |
| Pneumocystis jirovecii | 1. Commonly affects immunocompromised patients and can be severely life-threatening in some cases. 2. This microorganism primarily resides in the lungs' alveoli and concentrates mainly in the lower respiratory tract in higher concentrations than upper respiratory tract specimens |
Co-trimoxazole (trimethoprim/sulfamethoxazole) |
| Aspergillus fumigatus | 1. This organism is almost ubiquitous in the environment and is the primary cause of the disease 2. Inhaled conidia are engulfed by alveolar macrophages and killed in a phagocyte oxidase-dependent fashion |
Voriconazole andAmphotericin B |
| Cryptococcus neoformans | 1. This microorganism can produce harmless colonies in the airways, leading to meningitis or disseminated disease. 2. Complicates reticuloendothelial malignancy, organ transplantation, corticosteroid treatment, or sarcoidosis |
Amphotericin B |
| Scedosporium prolificans and S. apiospermum | 1. S. apiospermum causes severe disease in immunocompromised patients with a higher mortality rate 2. One of the critical clinical manifestations of this infection is pneumonia. |
Amphotericin B, voriconazole |
| Blastomyces dermatitidis | 1. It attacks immunocompromised and normal immunity persons 2. It can also spread to other common sites like skin and bone and causes extrapulmonary disease |
Itraconazole, Ketoconazole and fluconazole |
| Sporothrix schenckii | 1. This organism is not a primary pathogen in the pulmonary system 2. The microorganism can produce cavitary disease in the form of a single lesion. |
Amphotericin B, caspofungin, Itraconazole, fluconazole and ketoconazole |
| Penicilliosis marneffei | 1. This fungus can infect humans in relatively rare circumstances. 2. It can produce a disseminated infection in healthy and immunocompromised hosts |
Amphotericin B and itraconazole |
| C. pneumonia | 1. It is rare and often noticed in immunocompromised patients admitted to the intensive care unit. 2. This microorganism is aspirating from a heavily colonized or infected oropharynx |
Azole antifungal agents, amphotericin B, and echinocandin |
2.4.1. Histoplasma capsulatum
Pulmonary histoplasmosis (Darling’s disease) is a fungal mycosis of the lung. The causative pathogen for this disease is Histoplasma capsulatum. Most often, it is associated with bird or bat droppings. Spores are produced from the mycelia of H. capsulatum. Upon inhalation, they are deposited in the alveoli. These spores can germinate into the yeast at normal body temperature. Gradually, it becomes parasitic, multiplies, and travels to hilar and mediastinal lymph nodes. Through blood circulation, it can spread to various organs. After 10–14 days of exposure, cellular immunity develops and is clear from macrophages. Any defects in cellular immunity result in a progressive disseminated form of an infection that can be lethal [82]. It survives as a saprophyte and frequently attacks the immunocompromised person (cancer, tuberculosis, HIV, etc.). Humans are infected with this fungus by inhaling air-containing spores. Initially, this infection is asymptomatic. Thus, the common tendency of patients is to ignore the common cold and mild flu-like symptoms. The severity of the disease and associated symptoms depend on the inoculum size. In such cases, non-productive cough, high fever, headache, and chest pain are observed. The commencement of effective antimicrobial treatment might reduce mortality and morbidity in the asymptomatic carrier [83]. Antifungal drugs like itraconazole, fluconazole, and amphotericin B have been used to treat histoplasmosis. Sometimes moxifloxacin, linezolid, azithromycin, levofloxacin, and hydroxychloroquine are administered as empirical therapy [84].
2.4.2. Coccidioides species
Coccidioidomycosis (San Joaquin Valley or Valley fever) is one type of pulmonary infection caused by inhalation of Coccidioides immitis and Coccidioides posadasii. The majority of coccidioidal infections are caused due to airborne transmission. After pulmonary inhalation, single spore deposits in bronchioles gradually transform into spherules. Within 48–72 h, they become filled with hundreds to thousands and initiate tissue inflammation. Through an autolysis process, they thin their cell walls. In the inflammation phase, macrophages engulf some endospores due to the body’s immune system. If these spores are not clear, they progress to the chronic inflammation phase [85]. Neutrophils and eosinophils are attracted to the local region when spherules rupture and release endospores. Mainly, T-helper-2-lymphocytes (Th2) work on abolishing Coccidioides species. However, Th2 deficiency of dysfunction has been detected in patients with disseminated disease [86]. In a few cases, they are not contagious. Therefore, the chances of human-to-human spreading are less. Sometimes, the spherule spreads to other body parts like bone, joints, and skin resulting in an extra-pulmonary infection. The choice of drugs, route, and duration of therapy depends on the severity and location of the infection. Initially, fluconazole prescribes due to an excellent safety record [87], followed by voriconazole and itraconazole. Lipid formulations of amphotericin B are also preferred due to their effective penetrability.
2.4.3. Pneumocystis jirovecii
Pneumocystis carinii Pneumonia (PCP) is now referred to as Pneumocystis jirovecii Pneumonia, a fungal infection most commonly affects immunocompromised patients and can be severely life-threatening in some cases. P. jirovecii is most frequently detected in AIDS-defining diseases. It is also detected in non-HIV immunocompromised patients with a deficiency in adaptive immunity or who take prolonged high-dose systemic glucocorticoids [88]. Structurally, P. jirovecii is a thick-walled cyst. This microorganism primarily resides in the lungs' alveoli and concentrates mainly in the lower respiratory tract in higher concentrations than upper respiratory tract specimens [89]. Torpid infections may occur in patients with acute or chronic bronchopulmonary disease, primarily infected infants, pregnant women, and healthcare workers in contact with infected patients. These colonized patients and patients with Pneumocystis pneumonia represent sources of infection in both the community and hospitals [90]. Co-trimoxazole (trimethoprim/sulfamethoxazole) is mainly given through the mouth or vein.
2.4.4. Aspergillus species
Aspergillus mold is the most common fungal species that can sporulate with released airborne conidia. Humans and animals continuously inhale numerous conidia of this fungus. Aspergillus fumigatus is almost ubiquitous in the environment and is the primary cause of the disease, followed by Aspergillus flavus, Aspergillus niger, Aspergillus terreus, Aspergillus nidulans, and several species of Fumigati that morphologically looks like A. fumigatus [91]. They are small enough to reach the airways and pulmonary alveoli. Inhaled conidia are engulfed by alveolar macrophages and killed in a phagocyte oxidase-dependent fashion. However, the incomplete killing of conidia results in germination and tissue invasion. One of the most well-clinical syndromes of fungal colonization in airways is allergic bronchopulmonary aspergillosis (ABPA) caused by A. fumigatus. ABPA is associated with worse lung function [92]. The most effective treatment is observed with voriconazole. Amphotericin B can be an alternative option.
2.4.5. Cryptococcus
Cryptococcosis is a disease state caused by lung exposure to Cryptococcus. Cryptococcus neoformans (Subtype of Cryptococcus) distribute widely, mainly in soil and avian habitats [93]. C. neoformans is a ubiquitous and facultative intracellular yeast. This microorganism can produce harmless colonies in the airways, leading to meningitis or disseminated disease. Cryptococcosis infection's chances mainly occur in persons with defective cell-mediated immunity, and life-threatening situations occur in HIV-infected patients. This microorganism complicates reticuloendothelial malignancy, organ transplantation, corticosteroid treatment, or sarcoidosis [94]. Both symptomatic and asymptomatic infections have been observed in this type of infection. Pulmonary injury patterns include single or multiple large nodules, segmental or diffuse infiltrates, cavitary lesions, and miliary nodules [8]. Generally, amphotericin B is recommended alone or in combination with flucytosine. Fluconazole can be an alternative option. However, amphotericin B has demonstrated better clinical improvement than intravenous or oral fluconazole due to the rapid onset of action.
2.4.6. Scedosporium species
Scedosporium species can be found abundantly in animal droppings, sewage, agricultural soil, and wastewater. Two species of this class, named S. prolificans and S. apiospermum (Pseudallescheria boydii), are rarely reported for human infections. Out of them, S. apiospermum causes severe disease in immunocompromised patients with a mortality rate of 58–100% [95]. In immunocompromised hosts, several conditions are reported, including eye, ear, central nervous system infections, etc. One of the critical clinical manifestations of this infection is pneumonia. P. boydii can grow and multiply in the lung, forming colonies without causing progressive disease [96]. Scedosporium species are generally resistant to amphotericin B. Whereas, S. prolificans stains are resistant to all available antifungal drugs. Voriconazole is a first-line treatment due to its strong in-vitro activity against Scedosporium species.
2.4.7. Blastomyces dermatitidis
Blastomycosis is a chronic granulomatous and suppurative infection produced by Blastomyces dermatitidis. This microorganism is a dimorphic fungus, growing in the environment as a mycelial form and mammalian tissue as a yeast form. This infection may occur in immunocompromised and normal immunity persons [97]. B. dermatitidis is inhaled into the lungs and causes pneumonitis. Initially, It is asymptomatic and undetectable, though severe, life-threatening complications like acute respiratory distress syndrome can occur [98, 99]. It can also spread to other common sites like skin and bone and causes extrapulmonary disease in approximately 25%–30% of patients. It shows the lung's hematogenous distribution, and the skin is the most common site of extra-pulmonary infection [100]. In the lung, pathologic manifestations include focal or diffuse infiltrates, rare lobar consolidation, miliary nodules, solitary nodules, and acute or organizing diffuse alveolar damage [8]. Pulmonary blastomycosis can occasionally persist in the chronic form where productive cough, hemoptysis, and weight loss can observe [101]. Itraconazole is the treatment of choice for all forms of the disease, except in severe, life-threatening cases. Itraconazole has relatively low toxicity and good efficacy, though it is important to remember that its absorption requires gastric acidity. Ketoconazole and fluconazole can be used as alternatives, but they possess lower efficacy. Remarkable side effects are also observed with ketoconazole administration [102].
2.4.8. Sporothrix schenckii
Sporotrichosis is a subacute or chronic infection caused by thermally dimorphic fungi of the genus Sporothrix. For a long time, sporotrichosis was known as rosebush mycosis or gardener's mycosis. The infection usually results from inoculating the agent on the skin or mucous membrane by trauma with contaminated plant material. However, zoonotic transmissions have been reported with less frequent inhaled fungal propagules and systemic mycosis [103]. The causative agent named Sporothrix schenckii mainly attacks the skin, subcutis, and lymphatic pathways. Rarely, S. schenckii is a primary pathogen in the pulmonary system. The characteristic infection involves suppurating subcutaneous nodules progressing proximally along lymphatic channels (lymphocutaneous sporotrichosis). The microorganism can produce cavitary disease in the form of a single lesion. Infection may be bilateral, apical, progressive, destructive, or clinically identified as a solitary pulmonary nodule [8]. A rare form of sporotrichosis appears to result from inhaling the microorganism. This form is characterized by chronic cavitary pneumonia that is clinically and radiographically indistinguishable from tuberculosis and histoplasmosis [104]. In treating S. schenckii, melanin pigment may be hampered due to concurrent administration of amphotericin B and caspofungin. Due to adverse effects, azole compounds were introduced. Itraconazole is used as a first-line treatment with considerable efficacy and safety in most cases of sporotrichosis. This drug demonstrated low toxicity and well-tolerance in long-term therapy [105]. Another drug, amphotericin B, is used to treat disseminated forms, particularly in immunocompromised subjects initially. Amphotericin B may be used after 12 weeks of pregnancy in pregnant women. However, this medication has been recommended for pulmonary and disseminated forms where treatment should be initiated priority. Fluconazole is less effective than itraconazole. It is prescribed to patients who do not tolerate or have drug interactions with itraconazole. Ketoconazole showed higher toxicity and has not demonstrated a good response [106].
2.4.9. Penicilliosis marneffei
Penicilliosis is mainly caused by the Penicillium marneffei, a zoonotic parasitic and pathogenic dimorphic fungus in bamboo rats. However, this fungus can infect humans in relatively rare circumstances. The most significant transmission route is through contact with P. marneffei spores in the soil during the rainy season or at a wound site [107]. P. marneffei can produce a disseminated infection in healthy and immunocompromised hosts [108]. This infection is often detected in HIV-infected persons in South-East Asia. Sometimes, lung mass was observed in chest radiography. However, no respiratory symptoms were observed in patients [109]. Amphotericin B and itraconazole successfully controlled this pulmonary penicilliosis.
2.4.10. Candida species
Candida microorganisms are yeasts that can produce pseudohyphae and are the most common invasive fungal pathogens in humans. Secondary Candida pneumonia is commonly detected in humans, but primary C. pneumoniae is rare and often noticed in immunocompromised patients admitted to the intensive care unit. Other species of this class: C. glabrata, C. tropicalis, and C. albicans are responsible for 95% of blood-stream infections. This route is equally accountable for the acquisition of C. pneumonia. However, a non-blood-borne route is also observed for pneumonia infection, where this microorganism is aspirating from a heavily colonized or infected oropharynx. In the case of aspiration, these microorganisms may be found in the airways associated with an alveolar filling pattern of bronchopneumonia [8]. Initiation of therapy depends on the form of candidiasis and anecdotal reports. In most cases, Azole antifungal agents, amphotericin B, and echinocandin antifungal agents are recommended.
2.5. Parasites
Protozoal infections are the most prevalent intestinal infections worldwide; they rarely involve the lungs and pleura. Pulmonary infections with free-living amoebas, Toxoplasma species, Babesia species, Cryptosporidium species, Leishmania species, and Microsporidia species have been well documented (Table 3).
Table 3.
Summary of lung infections causing parasites and their management.
| Organism | Characteristics | Recommended medicine |
|---|---|---|
| B. divergens and B. microti | 1. The effect on the lung is a consequence of a systemic inflammatory response 2. Acute respiratory distress syndrome develops once the disease complicated |
Clindamycin and quinine sulfate |
| Cryptosporidium hominis and Cryptosporidium parvum | 1. Manifestations of this infection are asymptomatic shedding, acute watery diarrhea (approximately for two weeks), and persistent diarrhea 2. This microorganism targets the epithelium of the airways, gut, and biliary tract. |
Nitazoxanide, paromomycin, and indinavir |
| Dirofilaria immitis | 1. After being injected into the subcutis, this parasite travels into veins and gradually migrates to the heart 2. Parasites possibly grow in the right ventricle and are brushed into small pulmonary arteries |
Diethylcarbamazine and ivermectin |
| Entamoeba histolytica, E. dispar, and E. moshkovskii | 1. Amoebic dysentery becomes invasive in a small percentage of patients 2. The lungs may be affected by direct extension or hematogenous spread |
Metronidazole or tinidazole, paromomycin, iodoquinol, and diloxanide furoate |
| Echinococcus hatch, Echinococcus granulosus and Echinococcus multilocularis | 1. Echinococcosis is a zoonosis that occurs wherever sheep, dogs, or other canids and humans live in close contact. 2. In bronchi, cysts may rupture and cause coughs that eliminate the protoscolices (adult larva) or portions of the cyst wall |
Mebendazole and albendazole |
| Leishmania donovani | 1. transmitted to humans by several species of the Phlebotomus sandfly 2. Pulmonary leishmaniasis has been reported in HIV-infected patients and transplant recipients. |
Pentavalent antimonial derivatives, paromomycin, and liposomal amphotericin B |
| Microsporidia species | 1. microsporidiosis in humans can occur in both immune-competent and immune-compromised hosts. 2. Pulmonary microsporidiosis is often overlooked and characterized by a few non-specific symptoms like cough, fever, and dyspnoea. |
Albendazole (Albenza) and fumagillin |
| Paragonimus species | 1. target the lung and are acquired by ingesting freshwater crabs or crayfish infected with the metacercarial larvae 2. Pulmonary paragonimiasis can cause persistent hemoptysis. |
Triclabendazole, bithionol, niclofolan, praziquantel, and fenbendazole |
| P. falciparum, P. malariae, P. ovale, and P. vivax | 1. The disease is transmitted by the bite of the female Anopheles mosquito. 2. The clinical impact of malaria on the lung may range from mild to severe respiratory insufficiency. |
Antimalarial drugs |
| Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum | 1. eggs transmit to humans through snail-intermediate hosts and penetrate the skin of susceptible animals and people through the free-swimming cercaria 2. Pulmonary schistosomiasis comprises both acute and chronic forms. |
Praziquantel and artesunate |
| Strongyloides stercoralis | 1. This infection may persist as asymptomatic for years 2. It auto-infects the host body |
Ivermectin, albendazole |
| Toxoplasma gondii | 1. Infection occurs by ingesting oocysts or meat-containing live microorganisms 2. This infection is asymptomatic in some cases and is commonly detected in patients with AIDS |
Pyrimethamine, sulfadiazine/clindamycin, azithromycin, and doxycycline |
| Toxocara canis and Toxocara cati | 1. Children are more prone to infection via the fecal-oral route as they are more likely to consume Toxocara eggs. 2. The larvae cross the intestinal wall and travel to many organs, including the lungs. |
Albendazole, mebendazole |
| Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense | 1. Protozoa transmitted to man by biting flies and bedbugs. 2. Pneumonitis is the most frequent lesion in the lungs, placenta membranes, and umbilical cord. |
Nifurtimox and benznidazole |
2.5.1. Babesias species
The Babesias are intraerythrocytic protozoa, of which there exist various species, fundamentally B. divergens and B. microti. Humans acquire the infection (babesiosis) characterized fundamentally by fever and hemolysis through tick bites. The factors for systemic infection are immunosuppression, advanced age, and antecedents of splenectomy. Babesiosis is quite a rare disease in which the effect on the lung is a consequence of a systemic inflammatory response. The clinical manifestations are fever, cough, and labored breathing, with noncardiogenic pulmonary edema being the most systematic development. Acute respiratory distress syndrome developed as a disease complication requiring mechanical ventilation due to respiratory failure and hypoxemia. The treatment of choice is the joint administration of clindamycin and quinine sulfate [110].
2.5.2. Cryptosporidium species
Ten species of intracellular coccidian protozoa are currently recognized. Cryptosporidium species have been found to infect mammals, birds, reptiles, amphibians, and fish. The two species that most commonly infect humans are Cryptosporidium hominis and Cryptosporidium parvum. The former seems primarily limited to humans, and the latter has a wide range of hosts, including most major domestic livestock animal species [111]. The three main manifestations of this infection are asymptomatic shedding, acute watery diarrhea (approximately for two weeks), and persistent diarrhea for a few weeks [112]. Patients with AIDS have a more comprehensive range of disease severity and duration, including a fulminant cholera-like illness. In the lung, the microorganism targets the epithelium of the airways, just as it does the surface epithelium of the gut and biliary tract. Pulmonary cryptosporidiosis is primarily a case report event, most reports being from earlier phases of the AIDS epidemic [8]. Anti-parasitic drugs like nitazoxanide (FDA-approved treatment) can be helpful in cryptosporidiosis-associated diarrhea. However, this drug is not recommended for respiratory cryptosporidiosis. In some cases, paromomycin has been employed to treat respiratory cryptosporidiosis in AIDS patients. Indinavir (protease inhibitors) can be chosen as this drug interferes with cryptosporidium development. Supportive care and antiretroviral therapy are generally recommended without efficacious treatment of respiratory cryptosporidiosis [113].
2.5.3. Dirofilaria immitis
The zoonosis is caused by Dirofilaria immitis, a parasite of dogs. Human infection is determined by the prevalence of disease in the natural host and the extent to which humans are exposed to mosquito vectors. After being injected into the subcutis, this parasite travels into veins and gradually migrates to the heart. They generally die before they mature into adult worms. There was a misconception as Dirofilaria (heartworm) only resides in the heart. During infection, parasites possibly grow in the right ventricle and are brushed into small pulmonary arteries [114]. Gradually, they form the nidus in the arteries and cause thrombus. At this stage, Human D. immitis infection demonstrates an asymptomatic solitary pulmonary nodule in the lung periphery [8]. No specific antifilarial chemotherapy is indicated for human Dirofilaria infections yet. Thus, the various lesions (caused by worms) can be removed by surgery. No role for antiparasitic agents has been identified as effective in Dirofilaria infection during controlled trials. The antiparasitic treatment is most likely effective in arresting the worm and allowing its subsequent removal since only one infertile parasite is present [115]. Most patients are treated symptomatically with anti-inflammatory agents, including steroids. The worm has usually died long before extraction. Some authors suggested adding oral treatment with diethylcarbamazine (DEC). In some cases, oral ivermectin can be used [116]. Therapy with tetracyclines has been reported to damage D. immitis, even causing the death of adult worms [115].
2.5.4. Entamoeba species
Entamoeba is pseudopod-forming, anaerobic, protozoan parasites belongs to Entamoebidae family. Amoebic dysentery becomes invasive in a small percentage of patients [117]. After leaving the gut, the trophozoites travel to the liver. The lungs may be affected by direct extension or hematogenous spread [8]. Entamoeba histolytica causes most symptomatic diseases. Other species like E. dispar (non-pathogenic) and E. moshkovskii can cause similar infections. These microorganisms spread via the oral-fecal route. The infected cysts are often found in contaminated food and water. The chances of pleuropulmonary amebiasis (extraintestinal amebiasis) increase gradually. This is mainly occurring by inhalation of cysts of E. histolytica infected dust [118]. The first-line treatment has been initiated with metronidazole or tinidazole. Luminal agents like paromomycin, iodoquinol, and diloxanide furoate are also recommended for this infection.
2.5.5. Echinococcus species
Echinococcosis is a zoonosis that occurs wherever sheep, dogs, or other canids and humans live in close contact. Ingested eggs of the tapeworm Echinococcus hatch in the gut, releasing oncospheres, which invade the mucosa, enter the circulation, and travel to various sites, where they develop into hydatid cysts [119]. Unilocular slow-growing cysts in the lung are produced by Echinococcus granulosus. Echinococcus multilocularis proliferates by budding, producing an alveolar pattern of microvesicles. The outer fibrous layer of E. granulosus contains chronic inflammatory cells that interface with the alveolated parenchyma. In bronchi, cysts may rupture and cause coughs that eliminate the protoscolices (adult larva) or portions of the cyst wall. Abscesses (accumulation of pus) and granulomas (inflammation) may also form in the lung, pleura, and chest wall [8]. In Echinococcosis, four current treatment modalities are inadequate and controversial. Generally, treatment outcomes are improved when surgery or PAIR (puncture, aspiration, injection of proto scolicidal agent, and respiration) is combined with benzimidazole drugs given pre-and/or post-operation. Mebendazole and albendazole are the only anthelmintics effective against cystic echinococcosis. Albendazole and mebendazole are well tolerated but show different efficacy [120]. Albendazole chemotherapy was found to be the primary pharmacological treatment in CE management. Nevertheless, combined therapy with albendazole plus praziquantel resulted in higher scolicidal and anti-cyst activity [121].
2.5.6. Leishmania species
Leishmaniasis (Leishmania donovani infection) is transmitted to humans by several species of the Phlebotomus sandfly. Most Leishmania species are digenetic, i.e., they need two hosts to complete their life cycle. The parasite survives within the insect vector and proliferates in the alimentary tract extracellularly. The vertebrate host adopts an obligatory intracellular form that thrives inside phagolysosomes [122]. They are a group of vector-borne parasitic diseases with a high disease burden in the Indian sub-continent. The primary clinical forms are cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL), and visceral leishmaniasis (VL). Many of these forms affect the viscera (lungs, larynx, gastrointestinal tract, etc.) and are considered opportunistic infections in AIDS patients. The infection of the mucosa of the larynx is another complication of leishmaniasis. They are caused by different species and subspecies of Leishmania [123]. Pulmonary leishmaniasis has been reported in HIV-infected patients and transplant recipients. The microorganism (L. donovani amastigotes) can be found in the alveoli and alveolar septa. Sometimes, pleural effusion is explicitly observed in immunocompetent patients. In VL, pulmonary symptoms depend on many factors like a bacterial infection, hypoalbuminemia, and vagal nerve compression [124]. Pentavalent antimonial derivatives are the primary choice in leishmaniasis. However, other substances such as paromomycin and liposomal amphotericin B are effective alternatives.
2.5.7. Microsporidia species
Microsporidiosis is a disease caused by infection with microscopic microorganisms called microsporidia which are found worldwide in vertebrates and invertebrates and can serve as hosts for these microorganisms. Microsporidia are eukaryotic parasites that grow within other host cells to produce infective spores. At the same time, microsporidiosis in humans can occur in both immune-competent and immune-compromised hosts. It has often been seen in the immune-suppressed population [125]. The microsporidia are obligate intracellular spore-forming protozoa. More than 140 genera and 1200 species are recognized, but only seven genera and a few species have been confirmed as human pathogens [126]. Pulmonary microsporidiosis has been observed in different countries, especially in immunocompromised patients with HIV [127, 128, 129]. Pulmonary microsporidiosis is often overlooked and characterized by a few non-specific symptoms like cough, fever, and dyspnoea. In the lung, these pathogens cause bronchitis or bronchiolitis (or both), usually in patients who also have intestinal infections or disease at other sites, especially the biliary tract [8]. The most commonly used medications for microsporidiosis include albendazole (Albenza) and fumagillin. Intravenous fluid administration and electrolyte repletion may be necessary for patients with diarrhea and dehydration. Dietary and nutritional regimens may also assist with chronic diarrhea. Finally, improving immune system function with antiretroviral therapy in HIV-infected individuals may also improve symptoms [130]. However, no specific treatment for pulmonary microsporidiosis has been framed yet.
2.5.8. Paragonimus species
Paragonimiasis is an infectious disease caused by Trematodes of the genus Paragonimus. Paragonimus species target the lung and are acquired by ingesting freshwater crabs or crayfish infected with the metacercarial larvae of the Paragonimus species. The disease manifestations are related to the migratory route. In most cases, Paragonimus westermani is involved in disease propagation. However, several other species also co-exist. An eosinophil-rich inflammatory reaction occurs in the infection site that evolves to form a fibrous pseudocyst or capsule containing worms, exudate, and debris. Rupturing the cysts within bronchioles may release eggs, resulting in cough and sputum formation. These eggs may become embedded in the parenchyma, producing nodular granulomatous lesions that progress to scars [8]. Pulmonary paragonimiasis can cause persistent hemoptysis. The diagnosis is often missed due to rare occurrences and is mainly endemic in North America, Asia, and Africa [131]. Triclabendazole was approved by the FDA in 2019 for fascioliasis. The Centers for Disease Control (CDC) recommends triclabendazole off-label for treating paragonimiasis. Older therapies (e.g., bithionol, niclofolan) have unacceptable adverse effect profiles compared with praziquantel despite their effectiveness (cure rates ≥90%) [132]. Most patients with paragonimiasis are cured by standard praziquantel treatment. However, several cases have been reported with unsatisfactory responses to the standard praziquantel treatment [133]. Treatment with fenbendazole improves clinical signs and chest radiography [134].
2.5.9. Plasmodium species
Plasmodium comprises the intracellular protozoa that are responsible for malaria. It is, therefore, one of the diseases with the highest morbidity and mortality. Four species of this genus affect humans: P. falciparum, P. malariae, P. ovale, and P. vivax. The disease is transmitted by the bite of the female Anopheles mosquito. In most cases, acute respiratory distress syndrome (ARDS) is observed in the infection with P. falciparum rather than other malaria species [135]. Pulmonary edema is the principal manifestation of the effects of malaria on the lung. The increased permeability of the alveolar capillaries appears by a mechanism where the plasmatic liquid fills the alveolar spaces. The clinical impact of malaria on the lung may range from mild (fever, cough, dyspnoea, expectoration, and thoracic pain) to severe respiratory insufficiency. The selection of an antimalarial therapy depends on a series of factors, like species type, the clinical state of a patient, and the parasite's susceptibility to the drugs. One noteworthy aspect is the pulmonary toxicity produced by using mefloquine, with the development of diffuse alveolar damage [110].
2.5.10. Schistosoma species
Schistosomiasis (also known as bilharzia) is an infectious disease caused by trematode parasites of the genus Schistosoma. Disease caused by this parasite results from the immunologic reactions to egg-derived antigens produced by adult worms and the mechanical effects of eggs trapped in blood vessel walls [136]. The public health burden of schistosomiasis is enormous. Different life cycles and disease manifestations are observed in three primary Schistosoma species: Schistosoma mansoni, Schistosoma haematobium, and Schistosoma japonicum. Their eggs transmit to humans through snail-intermediate hosts and penetrate the skin of susceptible animals and people through the free-swimming cercaria [137]. After egg deposition, they develop into adult worms and live in various human venous plexuses. Pulmonary schistosomiasis comprises both acute and chronic forms. Acute diseases like Katayama syndrome manifests with fever, chills, weight loss, gastrointestinal symptoms, myalgia, and urticaria in patients with no previous exposure to the parasite [138]. Acute larval pneumonitis and Loeffler-like eosinophilic pneumonia may be seen in this setting. Chronic pulmonary disease is usually secondary to severe hepatic involvement with portal hypertension. In this setting, the eggs of S. mansoni, and rarely S. japonicum or S. haematobium may be shunted through portosystemic collateral veins to the lungs. The eggs lodge in arterioles and provoke a characteristic granulomatous endarteritis with pulmonary symptoms and radiologic infiltrates [139]. Praziquantel is the primary choice of drug in treating all species of Schistosoma. This drug increases membrane permeability resulting in vacuolation of the tegument [140]. A single dose of metrifonate reduced egg excretion but was marginally better than the placebo at achieving cure in one month. Three trials were conducted with an antimalarial drug named artesunate. Substantial anti-schistosomal effects were observed in one of the three trials, which was at unclear risk of bias due to poor reporting of the trial methods [141].
2.5.11. Strongyloides stercoralis
Strongyloides is a human parasite caused by ingesting eggs of the nematode Strongyloides stercoralis in the small intestinal mucosa. These microorganisms are distinct among the helminths family as they can replicate within the human host. Concurrently, it auto-infects the host body. This infection may persist as asymptomatic for years. When the disease occurs, filariform larvae leave the gut and travel through the pulmonary vasculature. After penetrating the alveoli, they initiate hemorrhage and inflammation. Gradually, abscesses, eosinophilic pneumonia, and Loeffler syndrome are developed. When the elimination is interrupted, these larvae may develop into adult worms, producing eggs and rhabditiform larvae [8]. 1–2 days of ivermectin administration is recommended for a chronic and asymptomatic infection. Sometimes albendazole is co-administered with ivermectin for disseminated infections and hyper-infection syndrome. The treatment duration and administration route must be individualized to eliminate the parasite [142]. However, clinicians may prefer to defer treatment for Strongyloidiasis for infected pregnant patients until after the first trimester. Empiric corticosteroid used to treat wheezing is problematic because they may cause life-threatening hyperinfection [143].
2.5.12. Toxoplasma gondii
Toxoplasma gondii, an obligate intracellular protozoon, is the agent causing toxoplasmosis, an infectious disease found worldwide. Members of the cat family (Felidae) are the hosts of this protozoan. Several mammals and birds serve as intermediate hosts. Infection occurs by ingesting oocysts or meat-containing live microorganisms [144]. Initially, this infection is asymptomatic in some cases and is commonly detected in patients with AIDS. Symptoms like fever, lymphadenopathy, muscle aches, and headache are observed in immunocompetent individuals. Brain and retina problems are generally observed in these patients. Pulmonary lesions are also detected in some disseminated disease cases. Often, they form miliary nodules with fibrinous exudates, which further develop into confluent fibrinopurulent pneumonia [8]. The most appropriate therapeutic measures are a combination of pyrimethamine and sulfadiazine administration. To prevent hematological complications, the substitution of sulfadiazine is clindamycin. Alternatives to these combinations are azithromycin and doxycycline [110].
2.5.13. Toxocara species
Toxocara species are common parasites that cause visceral larva migrants of dog tapeworm (Toxocara canis) and the less common cat tapeworm (Toxocara cati). Once ingested, embryonated eggs develop into infective larvae in the intestine of an intermediate host (especially a child with a history of a pica) [145]. Afterward, the larvae cross the intestinal wall and travel to many organs, including the lungs. T. cati is thought to more frequently cause severe human disease. Children are more prone to infection via the fecal-oral route as they are more likely to consume Toxocara eggs by ingesting soil or other contaminated substances [146]. The most common pulmonary symptoms are dyspnoea and cough. The bilateral pulmonary nodules are the most common finding in chest imaging [147]. Albendazole is widely accepted as the first choice for this infection. However, the optimal duration regimen has not been standardized yet [148]. It often requires a vitrectomy, depending on the severity and damage chances [149]. The therapy regimen in patients with visceral larva migrans (VLM) is meant to relieve symptoms and diminish the host's inflammatory response to the parasite. Corticosteroids and antihistamines are often used for this purpose. Patients with myocarditis or central nervous system (CNS) disease should always be treated with corticosteroids. Antiparasite agents, such as mebendazole, may help to reduce symptoms. However, systemic treatment with anthelmintics can result in hypersensitivity reactions [150].
2.5.14. Trypanosoma
The Trypanosoma genus comprises various species of haemoflagellated protozoa transmitted to man by biting flies and bedbugs. Trypanosoma brucei rhodesiense and T. B. gambiense are responsible for sleeping sickness. African trypanosomiasis and T. (Schizotrypanum) cruzi (South America) are responsible for Chagas' disease or trypanosomiasis Americana. The latter may affect the respiratory apparatus, organs such as the heart and esophagus, or during pregnancy through the placenta (the congenital form of the disease). In the congenital form of the disease, pneumonitis is the most frequent lesion in the lungs, placenta membranes, and umbilical cord. Nifurtimox (a nitrofuran derived) and benznidazole (a nitroimidazole) are the medicines to treat Chagas' disease [110].
2.6. Virus
Human respiratory viruses are not thought to infect the airway chronically, but they are crucial and common causes of respiratory exacerbations (Table 4). The imbalance of chronic bacterial infection and host immune response is a significant factor triggered by viral infection.
Table 4.
Summary of lung infections causing viruses and their management.
| Organism | Characteristics | Recommended medicine |
|---|---|---|
| Adenovirus | 1. It especially attacks neonates, children, and immunocompromised persons 2. This virus infection produces two arrays of lung injuries like alveolar damage and pneumonitis |
Cidofovir and ribavirin |
| Cytomegalovirus | 1. CMV infection is generally asymptomatic in older healthy children and adults 2. The chances of CMV are more in immune-compromised patients, especially AIDS and person have undergone organ transplants |
Ganciclovir, cidofovir, and phosphonoformate |
| Coronavirus | 1. Certain epidemiologic situations can cause pneumonia in children, weak elderly individuals, and immunocompromised adults 2. These viruses are responsible for most common colds |
Favipiravir |
| Epstein–Barr Virus | 1. EBV is transmitted via intimate contact with body secretions, primarily oropharyngeal secretions. 2. Most of these patients recover uneventfully, but a few develop one or more complications. |
No vaccine or specific treatment |
| Hantavirus | 1. Humans become infected by either inhaling virus-contaminated aerosols or having contact with the urine or droppings of infected animals. 2. This virus led to febrile illness with renal failure |
No vaccine or specific treatment |
| Herpes Simplex Viruses | 1. They occur primarily in patients with underlying pulmonary disease and are associated with inhalational and intubation trauma 2. They also appear in neonates and patients immunosuppressed or compromised by various chronic diseases |
Acyclovir, famciclovir, and valacyclovir |
| Human Metapneumovirus | 1. is a leading pathogen for respiratory tract infection in infants, with annual epidemics occurring during the winter and early spring months 2. The virus also causes disease in immunocompromised patients and likely explains some lower respiratory tract infections in the elderly. |
No vaccine or specific treatment |
| Influenza virus | 1. These viruses can target the ciliated epithelium of the tracheobronchial tree. 2. the virus and its attendant inflammatory response spread more distally into the respiratory bronchioles and alveoli, with hemorrhage, edema, fibrinous exudate with hyaline membranes, and patchy interstitial cellular infiltrates. |
Adamantine, rimantadine, peramivir, zanamivir, oseltamivir, baloxavir, marboxil |
| Measles Virus | 1. Measles pneumonia accounts for the vast majority of measles-related deaths. 2. The consequence of secondary pneumonia (bacterial or viral) or attributable to an aberrant immune response |
Vitamin A, ribavirin |
| Parainfluenza Virus | 1. Diffuse alveolar damage patterns of interstitial pneumonitis with giant cells are observed later to resemble measles and RSV infection 2. Observed in immunocompromised patients, especially children with congenital immunodeficiency disorders |
No specific treatment |
| Respiratory Syncytial Virus | 1. RSV has been recognized as the etiologic agent of pneumonia in community-dwelling and high-risk adults with chronic lung disease requiring hospitalization. 2. RSV targets the epithelium of the distal airway and produces bronchiolitis and epithelial cell sloughing. |
Treatment is primarily supportive care |
| Varicella-Zoster Virus | 1. This virus causes considerable morbidity in newborns, adults, and immunocompromised hosts. 2. Herpes zoster is less infectious because the only source of infection is the vesicular fluid. |
Acyclovir, penciclovir, brivudin, foscarnet, and vidarabine |
CMV- Cytomegalovirus; EBV-Epstein–Barr virus; RSV- Respiratory Syncytial Virus.
2.6.1. Adenovirus
Adenovirus comprises several genera with multiple serotypes that cause infections of the upper and lower respiratory tract, conjunctiva, and gut. These can be especially severe in neonates, children, and immunocompromised persons [151]. This virus infection produces two arrays of lung injuries, like diffuse alveolar damage with or without necrotizing bronchiolitis and pneumonitis with “dirty” or karyorrhectic necrosis. Both these patterns may coexist in some cases, and pneumonia may be accompanied by hemorrhage secondary to adenovirus-induced endothelial cell damage. Initially, an eosinophilic (Cowdry A) intra-nuclear inclusion occurs, which is surrounded by a halo with marginated chromatin, similar to HSV. Gradually, it becomes amphophilic, resulting in more basophilic obliterating in the nuclear membrane and further developing the characteristic smear cell [8]. There are no approved antiviral medicines for adenovirus infections [152]. Most adenovirus infections are mild and may require only care to help relieve symptoms, such as over-the-counter pain medicines or fever reducers. Currently, only two antiviral drugs are used as first-line adenovirus therapy. Cidofovir is an acyclic nucleoside phosphonate that shows anti-viral activity against DNA viruses. Another one is ribavirin is a broad-spectrum antiviral agent showing activity towards adenovirus infections [153].
2.6.2. Cytomegalovirus
Cytomegalovirus (CMV) is one of eight herpes viruses that infect humans. It is a β-herpes virus characterized by a restricted species range and a slow replication cycle. CMV is a double-stranded DNA virus with a large genome of the family. The virus develops an envelope consisting of several glycoproteins that helps CMV to initiate infection in a cell. Uncomplicated CMV infection causes nonspecific fever mainly due to pancytopenia. In severe cases of tissue-invasive CMV, the disease may produce a baffling array of clinical syndromes. The resolution of an active infection results in a latent state in which CMV persists indefinitely in the host tissues [154]. CMV infections can acquire throughout life. The infection is generally asymptomatic in older healthy children and adults. However, this virus can cause considerable morbidity and death in neonates [155]. The chances of CMV are more in immune-compromised patients, especially AIDS and person have undergone organ transplants. Expressions of CMV can vary and are often observed with minimal changes in scattered alveolar lining cells with typical viropathic changes [156]. Multiple eosinophilic cytoplasmic inclusions develop that progress into numerous cytomegalic cells. A typical pattern that suggests viral infection is the presence of small miliary nodules with a central hemorrhage surrounded by necrotic alveolar walls [157]. Interstitial pneumonitis is the least common pattern of CMV infection. Ulcers may be seen in the trachea and bronchi, but they occur less often than in herpetic diseases. It is advisable to look for other pathogens in CMV pneumonia, typically Pneumocystis jirovecii, but bacteria, fungi, protozoa, and other viruses are all possible co-infecting microorganisms [8]. Different strategies have been adopted to manage CMV infection. It may be prophylaxis (active case presence of a virus) or pre-emptive (laboratory evidence of active but asymptomatic). Ganciclovir, cidofovir, and phosphonoformate are frequently used [158]. Treating congenital CMV initiates with ganciclovir, an antiviral drug similar to acyclovir. After metabolism, ganciclovir triphosphate shows its antiviral effect on the CMV-infected cell. The treatment of infants for CMV can result in complications. The intravenous treatment should be instituted within the first month of life. Alternatively, valganciclovir is recommended for oral therapy. It is absorbed well after oral administration [159].
2.6.3. Coronavirus
Coronaviruses are ubiquitous RNA viruses known to cause disease in many animals. They belong to the Nidovirales order, including Coronaviridae, Arteriviridae, Mesoniviridae, and Roniviridae families [160]. Human coronaviruses belong to the family Coronaviridae, subfamily Coronavirinae, and order Nidovirales. There are generally four genera that fall into this category: α (alpha), β (beta), γ (gamma), and δ (delta). Coronaviruses infect birds and a variety of mammals. CoV groups α and β are mainly known to infect humans [161]. Along with the rhinoviruses, they are responsible for most common colds. Coinfections with other respiratory viruses occur in infants and children presenting with more severe respiratory disease. Certain epidemiologic situations can cause pneumonia in children, weak elderly individuals, and immunocompromised adults. Clinically the disease ranges from a nonhypoxemic febrile respiratory disorder (with minimal symptoms in some patients) to one of severe pulmonary dysfunction, manifesting as ARDS and eventuating in death [8]. Several newer drugs have been used to treat patients with less satisfactory effects. Nonetheless, hundreds of clinical trials are ongoing worldwide on different medications that utilize various mechanisms of action, including nucleoside inhibitors, protease inhibitors, and interleukin-6 receptor inhibitors [161]. Favipiravir is currently approved for marketing in the treatment of COVID-19.
2.6.4. Epstein–Barr virus
Epstein–Barr virus (EBV) infections are usually acquired in childhood and are generally asymptomatic. The pathologist often encounters this virus in the lung in the context of pulmonary lymphomas or in other EBV-associated lymphoproliferative disorders that can occur in transplant recipients and other immunocompromised patients [162]. EBV is transmitted via intimate contact with body secretions, primarily oropharyngeal secretions. EBV infects the B cells in the oropharyngeal epithelium. The microorganism may also be shed from the uterine cervix, implicating the role of genital transmission in some cases. On rare occasions, EBV is spread via blood transfusion [163]. However, mononucleosis is the most common symptomatic and primary form of EBV infection. Clinically, patients with active EBV infection have chronic or recurrent mononucleosis-associated symptoms, including fever, lymphadenopathy, and hepatosplenomegaly for at least three months [164]. Most of these patients recover uneventfully, but a few develop one or more complications. Pneumonitis is one of them, albeit rare and not well characterized [8]. There is no vaccine or specific treatment for EBV infection [165]. Antiviral therapy is generally ineffective for this disease. Immunomodulatory therapy has also been tried for the treatment of CAEBV. IFN-α and IFN-γ have been reported to induce remissions in some patients with CAEBV; however, long-term follow-ups have not been reported [166].
2.6.5. Hantavirus
Hantaviruses comprise the genus Hantavirus within the family Bunyaviridae. Humans become infected by either inhaling virus-contaminated aerosols or having contact with the urine or droppings of infected animals. In humans, hantaviruses cause either hemorrhagic fever with renal syndrome or hantavirus cardiopulmonary syndrome [167]. Classically this virus led to febrile illness with renal failure. Multi-organ involvement with fever, pulmonary edema, and hemorrhage occur. Liver lesions are variable and similar to those found in bacterial septicemia, albeit less severe [168]. All members of this genus are zoonotic and found in rodents worldwide. They are responsible for the cardiopulmonary syndrome, Sin Nombre, which is present in rodent feces and is acquired from the environment through inhalation. It produces florid pulmonary edema with pleural effusions, variable fibrin deposits, and focal wispy hyaline membranes [8]. There is no specific treatment, cure, or vaccine for hantavirus infection. In intensive care, patients are intubated and given oxygen therapy to help them through a period of severe respiratory distress [169]. Ribavirin was tested for efficacy in HFRS patients in China and was shown to have a statistically significant beneficial effect if initiated early in the disease course. However, ribavirin had no clinical benefit in most patients in the cardiopulmonary stage. Administration of human neutralizing antibodies during the acute phase of HPS might prove effective treatment and/or prevention of Hanta viral infections [170].
2.6.6. Herpes Simplex Viruses
Herpes Simplex Viruses (HSVs) are etiologic agents of mucocutaneous disease of the head and neck (type I) and genitalia (type II). Tracheobronchitis and pneumonia due to these viruses are rare in healthy adults with intact immune systems [171]. They occur primarily in patients with underlying pulmonary disease and are associated with inhalational and intubation trauma. They also appear in neonates and patients immunosuppressed or compromised by various chronic diseases [172]. HSV also has two types of CPE. Initially, Cowdry B (a ground-glass amphophilic intranuclear inclusion) appears with marginated chromatin. Later, Cowdry A (a single eosinophilic) inclusion surrounded by a similar halo that can be seen with adenovirus [173]. Acyclovir, famciclovir, and valacyclovir are the most effective medications for people infected with HSV. These can help to reduce the severity and frequency of symptoms but cannot cure the infection [174]. Acyclovir therapy remains an effective and often less expensive option. Famciclovir and valacyclovir offer improved oral bioavailability. However, these are more costly than acyclovir [175].
2.6.7. Human metapneumovirus
The Human Metapneumoviruses (HMPV) are enveloped, non-segmented, negative-sense, single-stranded RNA viruses. They comprise a genus of two species: Avian metapneumovirus and HMPV. The metapneumoviruses belong to Mononegavirales and the family of Pneumoviridae, including respiratory syncytial virus (RSV) [176]. HMPV is a newly recognized paramyxovirus. It is a leading pathogen for respiratory tract infection in infants, with annual epidemics occurring during the winter and early spring months [177]. The virus also causes disease in immunocompromised patients and likely explains some lower respiratory tract infections in the elderly. The clinical spectrums of croup, bronchiolitis, and pneumonia are similar to other paramyxoviruses, like RSV and parainfluenza virus. Histopathologic assessment of lung tissue in severe cases has revealed acute and organizing diffuse alveolar damage and smudge cell formation [8]. Currently, no specific antiviral therapy or vaccine is available to treat HMPV. Medical care is supportive [178]. One case study reported rapid and complete recovery with oral ribavirin and intravenous immunoglobulin [179].
2.6.8. Influenza virus
Influenza viruses are the most pathogenic respiratory viruses and predispose patients to secondary bacterial pneumonia. These viruses belong to the Orthomyxoviridae (a family of negative-sense RNA viruses that includes seven genera) family and are spherical or filamentous enveloped particles. The envelope carries a hemagglutinin attachment protein and neuraminidase [180]. These viruses can target the ciliated epithelium of the tracheobronchial tree. They produce necrotizing bronchitis-bronchiolitis and a spectrum of changes that vary depending on the stage of the disease (early vs. late), outcome (fatal vs. non-fatal), and the presence or absence of secondary bacterial pneumonia. Necrosis and desquamation of the epithelial cells to the basement membrane are associated with a relatively scant lymphocytic infiltrate [181]. However, in more severe cases, the virus and its attendant inflammatory response spread more distally into the respiratory bronchioles and alveoli, with hemorrhage, edema, fibrinous exudate with hyaline membranes, and patchy interstitial cellular infiltrates [8]. The acute symptoms persist for seven to ten days. The immune and interferon responses are responsible for the viral syndrome, including high fever, body aches, and coryza. Complications arise for chronic lung or cardiac disease patients [182].
Out of four genera of this family, only A and B be relevant for humans. These viruses are transmitted by aerosol infection through coughing, sneezing, or direct contact with the virus-contaminated surface [183]. CDC recommends four FDA-approved influenza antiviral drugs against recently circulating influenza viruses. Adamantine is the matrix proteins-2 (M2) inhibitor characterized by three condensed cyclohexane rings fused in the chair conformation. Amantadine and rimantadine (two other M2 inhibitors) were widely used in influenza. However, these drugs are inadequate for the current virus due to mutation-conferred resistance. These drugs are often replaced by nucleocapsid protein inhibitors [184]. The nucleocapsid protein inhibitors (peramivir, zanamivir, oseltamivir, baloxavir, marboxil) are also used for this disease [185]. Moreover, all influenza strains have developed high resistance against both amantadine and rimantadine.
2.6.9. Measles virus
The measles virus (MV) is a highly contagious childhood viral pathogen worldwide. Unlike varicella (chickenpox), it leads to common and severe complications. Measles pneumonia accounts for the vast majority of measles-related deaths. Most of these are the consequence of secondary pneumonia (bacterial or viral) or attributable to an aberrant immune response [186]. Despite vaccination, the measles virus is still a global pathogen and has resurfaced due to variations in vaccination rates. Primarily, viral pneumonia is uncommon and mainly observed in immunocompromised hosts [187]. Microscopically, bronchial-bronchiolar epithelial degenerate and reactive hyperplasia with squamous metaplasia. They are typically accompanied by peribronchial inflammation. Diffuse alveolar damage may occur, and quantitative immunohistochemical studies have revealed severe immune dysfunction with the loss of crucial effector cells and their cytokines [8]. There is no specific antiviral therapy for measles. Medical care is supportive and helps relieve symptoms and address complications such as bacterial infections. Severe measles cases among hospitalized children should be treated with vitamin A [188]. The long incubation period of MV before initiation of viremia serves as a wide time range for antiviral treatment. Rapid pre-emptive antiviral treatment can suppress disease development for individuals or reduce the severity before vaccination [189].
2.6.10. Parainfluenza virus
The parainfluenza virus (PIV) comprises four serotypes (I to IV) that typically target the upper respiratory tract, classically in the form of croup. Some cases involve distal airways, as in infections due to Respiratory Syncytial Virus (RSV) and influenza virus, but are milder, with less morbidity and requiring fewer hospitalizations [190]. A few documented cases have been described with diffuse alveolar damage patterns of interstitial pneumonitis with giant cells that later resemble measles and RSV infection [191]. The giant cells of parainfluenza tend to be larger and have more intracytoplasmic inclusions. Parainfluenza virus is a potential opportunist in immunocompromised patients, especially children with congenital immunodeficiency disorders [8]. There is no specific antiviral treatment for PIV illness. Most people with this illness will recover on their own [192]. Corticosteroids may be administered by mouth or given intramuscularly in dexamethasone or prednisolone. Both are superior to inhaled therapy with budesonide. The use of nebulized epinephrine is associated with short-term relief of symptoms [190]. DAS181, a novel sialidase fusion protein inhibitor, seems effective against PIV in vitro and in vivo; its use in IC children has not been evaluated [193].
2.6.11. Respiratory syncytial virus
Respiratory Syncytial Virus (RSV) causes more significant respiratory infections in early childhood than those attributable to influenza or parainfluenza viruses. The annual outbreaks of bronchiolitis and pneumonia in infants are severe during the first year of their life and for those with low birth weight or cardiopulmonary disease [194]. Considered a childhood virus, RSV has been recognized as the etiologic agent of pneumonia in community-dwelling and high-risk adults with chronic lung disease requiring hospitalization. RSV targets the epithelium of the distal airway and produces bronchiolitis with disorganization of the epithelium and epithelial cell sloughing [195]. In fatal cases, airway obstruction due to sloughed cell detritus, mucus, and fibrin is com-pounded by airway lymphoid hyperplasia. Diffuse alveolar damage may be seen in immunocompromised patients [8]. Initial signs of RSV are similar to mild cold symptoms, including congestion, runny nose, fever, cough, and sore throat. Very young infants may be irritable, fatigued, and have breathing difficulties. Usually, these symptoms will clear up on their own in a few days [196]. Treatment is primarily supportive care, and the illness resolves without complications in most children. RSV prophylaxis with palivizumab is an option for high-risk infants and children, decreasing hospitalization and length of stay. Ribavirin and palivizumab may be used for immunocompromised patients [197].
2.6.12. Varicella-Zoster virus
Varicella-zoster virus (VZV) infection produces considerable morbidity in newborns, adults, and immunocompromised hosts. This virus is equally potent in its primary form (varicella) and its reactivated form (zoster) [198]. The varicella virus can spread in many ways. Mostly, the transmission of VZV happens via respiratory droplets. In other ways, the virus can spread through direct contact with conjunctival fluid, saliva, or fluid from a vesicle of an infected individual. Herpes zoster is less infectious because the only source of infection is the vesicular fluid. Herpes zoster is less contagious because the only source of infection is the vesicular fluid. Once the vesicular lesion(s) have completely crusted over, there is no longer a risk of infection directly from the rash. Initial infection occurs when VZV gains access to regional lymph nodes from the mucosa in the upper respiratory tract [199]. The course is mild and self-limited in affected adults without underlying diseases and normal immunity. Mortality rates are significantly high if the infection progress. Microscopically, small miliary nodules of necrosis are associated with interstitial pneumonitis, edema, fibrin deposits, or patchy hyaline membranes [8]. Adults with herpes zoster can be treated with oral acyclovir. Higher doses are sometimes used for life-threatening infections, especially in immunocompromised patients. Like acyclovir, penciclovir is first mono-phosphorylated by viral TK, then further modified to the triphosphate form by cellular enzymes and also used to treat the Varicella-Zoster virus. There are some other drugs like brivudin, foscarnet, vidarabine, and interferon found to be effective against this virus [200].
3. Management of lung infections
Acute and chronic lung disease is the leading cause of morbidity and mortality worldwide. Due to the gas exchange function, the lung with the largest epithelial surface area is continuously exposed to the external environment. Therefore, the chances of respiratory disorders and lung infections are overgrowing.
3.1. Cystic fibrosis lung infections
CF lung disease describes the three most pathophysiological elements: airway obstruction, infection, and inflammation. Clinical microbiology relies on the conventional cultivation of respiratory samples, including sputum, broncho-alveolar lavage fluid, sinus samples, or oropharyngeal swabs. Using these techniques, bacteria and fungi can be identified, enumerated, isolated, and characterized. Additionally, these techniques help to analyze growth characteristics and in-vitro antibiotic susceptibilities. Therefore, diagnosing infections in individual patients, generating epidemiological information, and directing antibiotic treatment help design models of CF infection pathogenesis. Advanced laboratory techniques have been added to a growing list of CF-associated microbes. These methods are based on synthetic laboratory growth media with incubation conditions that select microbes customarily associated with lung disease pathogenesis. CF airway infections exhibit three key features: Diverse, frequently polymicrobial, and progressively evolving [201]. Aerosol therapy for CF has been explored extensively, as patients have a high risk of recurring chronic infections. Oral and parenteral antibiotics have historically been used to treat lung infections but do not directly target the lung. Increasing antibiotic concentration in the lung through this route may lead to severe side effects. The management of CF has improved with the development of the first inhaled antibiotic, TOBI containing tobramycin (1997 in the USA). Colonization of Pseudomonas aeruginosa is decreased significantly with inhaled antibiotics in CF. Inhaled tobramycin or colistimethate successfully improves lung function and reduces hospitalization rates. Inhalable antibiotics are prescribed daily (twice or thrice) for 28 days, followed by 28 days off therapy. This off therapy reduces the chances of bacterial resistance. This cycle of treatment continues for several months. Currently, two classes of antibiotics (aminoglycoside and monobactam) are approved in the USA. Polymyxin (colistimethate sodium) is also used in Europe in resistance cases with tobramycin or aztreonam. Long-term treatment using inhalable antibiotics may elevate the inflammatory response, leading to lung tissue injury and dysfunction. However, intravenous antibiotics have been used in acute pulmonary complications. Treating CF patients with inhalable antibiotics is always advisable as managing the chronic infection, and biofilm-growing mucoid is problematic. Clarithromycin acts as an immunomodulatory and bactericidal enhancer. Additionally, it can suppress the virulence factor of P. aeruginosa. Due to these activities, clarithromycin was administered with tobramycin in CF patients [202]. Lipoidal particles/vesicles have the potency to target the microbes within the biofilms and show the mucoadhesive property. A sustained-release liposomal formulation of amikacin improved patient accessibility in CF [203].
3.2. Non-CF bronchiectasis
Non-cystic fibrosis bronchiectasis (NCFB) or bronchiectasis remains a key reason for respiratory morbidity. Gradually, it becomes a leading burden worldwide. Bronchiectasis patients are frequently sick with various bacterial pathogens, resulting in persistent respiratory symptoms followed by airway damage. As a result of the experience gained with CF, inhalation therapy of antibiotics has been adopted in non-CF bronchiectasis patients suffering from P. aeruginosa infection. Prolonged antibiotic treatment is possible through an oral or inhaler route. A significant reduction of colonization has been observed after 2–4 weeks of drug administration. Inhaled antibiotics offer the advantage of delivering a higher drug dose directly to the site of bronchiectasis infection, with less potential for collateral damage and resistance; however, they are often time-consuming to administer [204]. Systemic antibiotic therapy is available, but this therapy is poorly tolerated and has shown insufficient efficacy.
Nontuberculous mycobacteria (NTM) can cause an unusual, severe infection of the musculoskeletal system. This infection necessitates intensive medical care and surgical treatment [205]. Macrophages engulf NTM. Therefore, targeting antibiotics to macrophages might improve efficiency and clinical outcomes. Oral administration of amoxicillin, co-amoxiclav, flucloxacillin, rifampicin, fucidin, and ciprofloxacin have been recommended in NCFB. Intravenous antibiotics are also employed in severe cases where oral use is limited. Benzylpenicillin, cefuroxime, ceftriaxone, and ceftazidime are administered via the parenteral route. Inhalation formulations of amikacin, tobramycin, aztreonam, fosfomycin, colistin, and ciprofloxacin have been developed for patients’ benefit. Many clinical trials were carried out on bronchiectasis patients using inhaled antibiotics, and they were found to be tolerated well with a significant reduction of bacterial load [206].
3.3. Pneumonia
Pneumonia is a respiratory infection characterized by inflammation of the bronchiole and alveolar space of the lungs. The modernization and development of industries have become the leading infectious cause of death. Primarily, it transmits via the respiration of airborne pathogens like bacteria and viruses. Based on the clinical manifestation, pneumonia classify as typical and atypical.
3.3.1. Typical pneumonia
Typical pneumonia exhibits sudden onset of illness, fever, chills, pleuritic chest pain, and productive cough. In such a case, bronchial breath sounds are perceptible. Typical pneumonia is initiated by bacterial pathogens like Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenza, Klebsiella pneumoniae, Moraxella catarrhalis, anaerobes, and aerobic gram-negative bacteria. The lower respiratory tract is frequently exposed to environmental pathogens. These bacteria attack and spread into the lung parenchyma to cause bacterial pneumonia. Host defense starts working in various modes like mechanical and chemical to inhibit the spread of microorganisms. Besides, alveolar macrophages engulf and kill these bacteria. Gradually, bacteria overcome the host’s defense capability and result in an inflammatory response [207]. Due to bacterial infection, pneumonia is treated with antibiotics. The selection of appropriate antibiotics depends on the type of bacteria and patient (inpatients or outpatients). Amoxicillin or amoxicillin with clavulanic acid or macrolides (azithromycin or clarithromycin) is generally prescribed for outpatients with no risk for resistance, no comorbidities, and no history of allergy to these drugs. In contrast, combination therapy contains β-lactam with macrolide/moxifloxacin/levofloxacin/gemifloxacin, prescribed to high-risk pneumonia patients. In the case of inpatients, combination therapy includes third-generation cephalosporin or amoxicillin with clavulanic acid in combination with a macrolide antibiotic (azithromycin or clarithromycin). Respiratory quinolone is also added to this regimen for ICU patients [208].
3.3.2. Atypical pneumonia
Atypical pneumonia presents with gradual onset of an unproductive cough, dyspnoea, and extra-pulmonary sign. These symptoms get worse over a month. This disease is never severe or needs hospitalization or bed rest. The causative microorganisms for this type of pneumonia include viruses, Legionella pneumophilia, Chlamydia psittaci, and Mycoplasma pneumoniae. Two different clinical patterns characterize legionellosis. The first is Legionnaires disease, a form of pneumonic illness with a greater than 2% fatal outcome. The second is Pontiac fever, a mild and non-pneumonic respiratory illness. Most infections are caused by Legionella pneumophila serogroup 1 and 13 [59]. Persons are infected with this microorganism when they inhale contaminated water aerosols [209]. To date, 24 different serogroups have been identified. Erythromycin and tetracycline are the primary choices of drugs due to the lower mortality rate than other antibiotics [210]. Then, alpha inhibitors, anakinra, and interleukin receptor antagonists are included in the treatment regimen. Researchers suggested early detection and treatment reduce the morbidity and mortality caused by the disease [211].
First-choice antichlamydial drugs may even induce chlamydial persistence under certain conditions. Without a functional treatment strategy, the hypothesis of a chlamydial contribution to atherogenic processes can thus neither be proved nor disproved by eradication studies. A better understanding of chlamydial pathobiology is needed before implementing clinical practice studies. Several AMA like doxycycline, tigecycline, erythromycin, azithromycin, clarithromycin, ciprofloxacin, levofloxacin, moxifloxacin, rifampin, trimethoprim, and sulfamethoxazole are active against these bacteria. Drug-loaded polymeric nanocarriers and propellant-based inhalers have the potency required to treat a chlamydial respiratory infection [212].
Macrolide antibiotics are mainly prescribed because of the low minimum inhibitory concentration (MIC). Azithromycin and clarithromycin are used extensively worldwide [213]. This mycoplasma is susceptible to tetracycline and fluoroquinolones. For children, administering these drugs increases macrolide-resistant risk [214]. C. pneumoniae and all species of Legionella are equally responsible for community-acquired bacterial pneumonia (CABP). Newly developed synthetic antibiotics are found to be active against all these microorganisms. Ketolides (semisynthetic derivatives of erythromycin) are potent against M. pneumoniae. The Food and Drug Administration (FDA) approved (2019) solithromycin for the treatment of macrolide-resistant M. pneumoniae (MRMP). Some other new AMAs are nafithromycin, acylides, and lefamulin. They are effective against many gram-positive and gram-negative bacteria [215].
Ventilator-associated pneumonia (VAP) is a lung infection that usually occurs in hospital patients who use mechanical ventilation breathing machines. The presence of an endotracheal tube or tracheostomy may result in local inflammation. Gradually, the airways are colonized with microorganisms that spread deep into the lung [216]. Generally, the insertion of an endotracheal tube increases the chances of VAP compared to patients without intubation. It is a growing concern as this microorganism affects critically ill patients admitted to the intensive care unit (ICU). Gradually, VAP becomes a leading cause of morbidity and mortality associated with multidrug-resistant (MDR) microorganisms. Inhaled antibiotic treatment was found to be more effective in VAP. Although, this therapy considers an adjunct to systemic antimicrobial therapy. Inhalable antibiotic treatment is recognized as saving patients with MDR.
Over the past decades, several aerosolized antibiotics have been studied to prevent and treat VAP. The concentration of AMA in respiratory excretions was 200 times greater than the levels found in the blood samples when administered as an aerosol. Lipophilic agents like aminoglycosides, beta-lactams, and carbapenems can penetrate extracellular lining fluid (ELF). Non-lipophilic agents like quinolones, tetracycline, clindamycin, and other newer macrolides show equal penetration. Ceftriaxone, fluoroquinolones, ampicillin, sulbactam, or ertapenem are generally used to treat VAP with no evidence of MDR. However, combination therapy recommends for MDR pathogens. Combination therapy includes antipseudomonal cephalosporins (cefepime, ceftazidime), an antipseudomonal carbapenem ((imipenem, meropenem, or doripenem), or a beta-lactam/lactamase inhibitor combination (tazobactam-piperacillin or clavulanate-ticarcillin) plus fluoroquinolone (ciprofloxacin or levofloxacin); an aminoglycoside (gentamicin, amikacin or tobramycin) plus linezolid or vancomycin [217].
Non-resolving pneumonia defines as pneumonia with a slow resolution of clinical symptoms. This non-resolving pneumonia poses a diagnostic challenge as it mimics the infectious. Many reasons are behind it, like the immune defense system, bacterial resistance, and the persistence of microorganisms [218]. Incorrect diagnosis, inadequate antibiotic therapy, impaired host defense, atypical microorganisms, resistant pathogens, non-infectious causes, tuberculosis, endobronchial lesions, etc., are the common causes of non-resolving pneumonia or slowly resolving pneumonia. Many patients respond quickly to empiric antibiotic therapy. But the complication begins if patients fail to respond to this initial therapy [219]. Treatment failure is attributed to numerous factors like antibiotic resistance, unusual microorganisms, and empyema. Patient-related risk factors like advanced age, cardia failure, hepatic disease, and alcohol abuse are equally responsible for treatment failure [220]. According to a case report, a combination therapy containing oral amoxicillin, clavulanic acid, levofloxacin, or intravenous cefoperazone-sulbactam with oral clarithromycin could not improve the patient’s conditions. However, twice daily, intravenous amphotericin B and itraconazole recovered the patient’s illness [221]. Therefore, this necessitates proper investigations and additional treatment.
3.4. Bronchitis
Bronchitis is an infection of the tube lining that leads to the lungs. Commonly, it categorizes into two different classes: acute and chronic bronchitis. Acute bronchitis is a more common symptom and persists for a few weeks without causing any serious problems. On the contrary, chronic bronchitis is another type of chronic lung disease. The details have mentioned below.
3.4.1. Acute bronchitis
Acute bronchitis occurs from acute inflammation of the bronchi caused due to various triggers, allergens, pollutants, and viral infections. Inflammation in the bronchial wall leads to mucosal thickening, denudation of the basement membrane, and epithelial-cell desquamation. Viral infection in the upper respiratory tract progress to disease in the lower respiratory tract resulting in acute bronchitis [222]. This infection characterizes by a fever that may or may not be accompanied by a cough. Viruses are mainly responsible for acute bronchitis infections (>90%), whereas the rest are due to bacteria. It is difficult to distinguish between bacterial or viral-associated bronchial infections. In such a situation, the use of antibiotics is controversial. Acute bronchitis is self-limiting, and treatment is typically symptomatic and supportive therapy. Generally, cough relief and pharmacological and non-pharmacological therapies are offered. β-agonists are routinely used in acute bronchitis patients with breathing problems. Analgesic and antipyretic agents may be used to treat associated malaise, myalgia, and fever. Seventeen randomized controlled trials (RCT) were conducted on 3969 people, including children, adolescents, and adults. Doxycycline, azithromycin, erythromycin, trimethoprim/sulfamethoxazole, amoxicillin, and cefuroxime were used in these trials. The results suggested that antibiotics reduce the duration of cough and illness [223].
3.4.2. Chronic bronchitis
Chronic bronchitis is inflammation (swelling) and irritation in the bronchial tubes. It is caused by the uncontrolled production and over-secretion of mucous from the goblet cells [224]. Epithelial cells lining the airway respond to toxic or infectious by releasing inflammatory mediators like colony-stimulating factors, interleukin 8, and other pro-inflammatory cytokines. Concurrently, an associated decrease has been observed in releasing regulatory substances like neutral endopeptidase and angiotensin-converting enzymes [225]. Finally, this overproduction obstructs the small airways and progressively reduces lung function. In this type of infection, thickened mucus and shortness of breath observes. The primary therapy aims to reduce the overproduction of mucus, suppress cough, and control inflammation. Short and long-acting beta-adrenergic receptor agonists and anticholinergic drugs help increase the airway lumen, mucous hydration, and ciliary function. Phosphodiesterase-4 inhibitor decreases inflammation and promotes airway smooth-muscle relaxation by preventing the hydrolysis of cyclic adenosine monophosphate. Glucocorticoids reduce inflammation and mucus production. Inhaled corticosteroids reduce exacerbation and improve the patient’s condition. Macrolide therapy (azithromycin and clarithromycin) is included in this treatment due to its anti-inflammatory properties [226]. Acute bronchitis usually clears up, whereas chronic bronchitis is persistent and never completely disappears. The other most commonly used antibiotic (amoxicillin) is also included in the treatment of chronic bronchitis [227].
3.4.3. Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory lung disease. It mainly affects the peripheral airways, lung parenchyma, and pulmonary vasculature. In this process, oxidative stress and protease-antiprotease imbalances involve. Emphysema describes one of the structural changes seen in COPD that obstructs airflow from the lungs. A decrease in the forced expiratory volume (FEV1) and tissue destruction leads to airflow limitation and impaired gas exchange. The progressive narrowing of the airways results in shortness of breath. Neutrophils and macrophages are recruited and release multiple inflammatory mediators. Oxidants and excess proteases lead to the destruction of the air sacs. Alpha-1 antitrypsin deficiency is a rare cause of emphysema that involves a lack of antiproteases, and the imbalance leaves the lung parenchyma at risk for protease-mediated damage. The incapability to exhale completely also causes elevations in carbon dioxide (CO2) levels. As the disease advancements, deficiency of gas exchange is often observed. Reducing ventilation or increasing physiologic dead space leads to CO2 retention [228]. Generally, long-acting bronchodilators (adrenergic receptor agonists and muscarinic receptor antagonists) are recommended for COPD patients. Inhalable corticosteroids are also administered to improve patients' conditions [229]. Antibiotics are an alternative therapeutic option in acute COPD. Selection of an appropriate AMA based on a risk stratification approach. With the help of the immune response, AMA can control the proliferation of bacterial infections. Several double-blind, randomized trials were conducted with many antibiotics like macrolide (azithromycin, clarithromycin), ketolide (telithromycin), cephalosporin (cefuroxime, cefpodoxime, or cefdinir), doxycycline, and fluoroquinolone (moxifloxacin, gemifloxacin, levofloxacin) [230]. They are found to be effective against COPD.
4. Basic anatomy-physiology and hurdle for antimicrobial agents
Many types of pneumonia are available for alveolar and tracheobronchitis infections. The gradual eventual distribution of antibiotics throughout the lungs is required for successful therapy [231]. The upper respiratory tract comprises the nasal cavity, paranasal sinuses, nasopharynx, pharynx, larynx, trachea, and bronchi (Figure 2A) [232]. Nasal and oropharyngeal are narrow angular routes with variable dimensions that prevent the entry of drugs into the lungs. Lungs are segregated into two different segments: the conducting zone (0–16 generations) and the respiratory zone (17–23 generations) [202]. The deposition of AMAs in these zones depends on interaction with the biological environments, including lung cell density, bacterial biofilm, and lung lining fluids. The biofilms are shielded with an extracellular matrix of polymeric substances, protein, DNA. These biofilms are the primary cause of chronic infection. The presence of biofilm reduces the intraluminal diameter of endotracheal tubes hinders the ability to cough up bacteria and secretions from the lower airways. In response to fluctuations in the cell-population density of bacteria, quorum sensing (QS) facilitates the production of virulence factors and promotes biofilm formation. QS coordinates the expression of various genes within the entire bacterial population and secretes many virulence factors [217]. Besides, the rapid growth of bacteria in the outer layer protects this biofilm. It is another challenging barrier for the delivery and activity of AMA. The pharynx and larynx screen foods-liquids and block them from entering the trachea. The upper respiratory tract acts on filtering and moisturizing the inhaled air. Natural breathing happens by fixing the diaphragm and escalating the thorax with the help of muscles.
Figure 2.
A. Respiratory tract anatomy: Conducting and respiratory zone: The significant parts of the respiratory tract are the paranasal sinus, nasal cavity, nasopharynx, oral cavity, pharynx, larynx, trachea, bronchi, and alveoli. The conducting zone allows the bulk flow of air to the lungs, whereas the respiratory zone helps exchange gases. B. Systematic representation of healthy and infected lungs: Common symptoms of all lung infections are hyper mucus secretion and inflammation. This mucus is responsible for shortness of breath.
The airflow into the lung occurs due to the pressure difference between intrapulmonary and mouth. Intrapulmonary pressure is decreased in the mouth, which allows inlet air. The ventilator or the respiratory muscles are the main elements of this task [233]. The variation in the pressure difference results in inertial impaction and drug deposition. The respiratory system maintains pH, hormonal balance, and host defense [234]. These activities are the primary barriers to AMAs. The mucociliary clearance helps transmit mucus toward the mouth by covering the airways. The lining fluid in the airways varies from 5-10 μm in thickness and slowly decreases towards the proceeding airway generations to the alveoli (thickness 0.5–0.8 μm) [235]. In some physiological conditions, airway mucous becomes thicker and covers most of the trachea, bronchi, and alveoli dimensions. Gradually, this hypersecretion promotes bacterial growth, obstructing drug action.
Besides, mechanical barriers like narrowing bronchoconstriction, inflammation, and mucous hypersecretion are developed in many diseases (Figure 2B). Two major cell types are found in the alveolar epithelium: type I and type II pneumocytes. Type I pneumocytes occupy about 95% of the alveolar surface, whereas type II pneumocytes are characterized by a more cuboidal morphology and cover 5% of the total alveolar surface. Type I pneumocytes cells are most likely a rate-limiting step in the uptake of compounds into the pulmonary circulation [236]. The alveolar space is covered with a complex surfactant layer (lipids-protein ratio 90:10) secreted from type II pneumocytes. This surfactant prevents the blockage of the alveoli during breathing by decreasing surface tension [237]. The airway geometry, mucociliary clearance, alveolar macrophages, and humidity act as barriers to eliciting the therapeutic response of inhaled AMAs. Likewise, the extent and place of drug deposition in the respiratory tract are crucial to maximizing the AMA's efficacy [231]. The total liquid available in the lungs (10–30 ml) promotes the dissolution of deposited AMA [235]. However, the proteolytic enzymes and surfactants can interfere with natural endopeptidase, and cathepsin H hydrolyze lung proteins and peptides to deactivate the effect of dissolved drugs. The undissolved drugs are proceeded for macrophages to engulf and act as an immunological barrier. Surfactants may prevent the adhesion of these undissolved particles to the lungs and force them to be engulfed by macrophages. This barrier removes AMA from the lungs and transfers them to the mucociliary escalator [238]. Lung inflammation causes hypersecretion of sputum that inactivates the antibiotics and reduces their bactericidal effect locally. The penetration of AMAs in the small airways is restricted if the patients suffer from bronchospasm or CF. The main reason behind this is the low airflow and viscid sputum. Similar low airflow is also seen in pneumonia patients [239].
5. Approved antimicrobial agents for lung-targeted delivery
Many anti-microbial agents are administered through different routes, like oral or parenteral, in managing lung infections effectively. Antimicrobial agents through the inhalation route are the best option to deliver higher doses directly to the infection site with minimal systemic exposure. Aerosols are a revolutionary tool to provide therapeutic agents with higher concentrations to the respiratory tract to kill microbes. Few antimicrobial agents have been approved for pulmonary delivery (Table 5).
Table 5.
List of approved anti-microbial agents for pulmonary delivery.
| Drug | Mechanism of action | Biological half-life | The volume of distribution (L/Kg) | Trade name | Disease | Marketing company/Inventor |
|---|---|---|---|---|---|---|
| Levofloxacin | Inhibition of bacterial DNA synthesis | 6–8 hr | 1.1 | MP-376 | Cystic Fibrosis | Mpex Pharmaceuticals & Horizon Pharma USA, Inc. |
| Ciprofloxacin | 4.3 hr | 1.79 | Lipoquin (ARD-3100) | Cystic Fibrosis, Bronchiectasis | Aradigm Corporation, Newark, CA | |
| ARD-1100 | Anthrax | |||||
| Aztreonam | Inhibit the synthesis of bacterial cell wall | 2 hr | 0.18 | CAYSTON® | Cystic Fibrosis | Gilead Sciences |
| Amikacin | Interfere with bacterial growth | 1.4 hr | 0.27–0.61 | (Arikace™) | Cystic Fibrosis | Transave Inc. |
| BAY41-6551 | Pneumonia | Bayer Pharma AG | ||||
| Tobramycin | Inhibiting bacterial protein synthesis | 1.8 hr | 0.363 | TOBI | Cystic fibrosis | Sun Pharmaceutical Medicare Ltd, Gujarat, India. |
| TOBI Podhaler | Cystic Fibrosis | Novartis | ||||
| Colistin | Disruption of outer cell membrane followed by bacterial death | 1.5–2 hr | 0.5 | Colymycin® | Bacterial infection | Monarch Pharmaceuticals, Bristol, US |
| Ribavirin | Inhibit viral RNA synthesis | 12 days | 9.28–15.71 | Virazole | Respiratory syncytial virus | Valeant Pharmaceuticals, Aliso Viejo, CA |
| Zanamivir | Inhibit influenza neuraminidase followed by restricting the spread of infection | 2 hr | 0.23 | RELENZA® | influenza A and B virus | GlaxoSmithKline, Brentford, UK |
5.1. Fluoroquinolones (levofloxacin, ciprofloxacin)
Ciprofloxacin is a broad-spectrum antibiotic successfully employed in COPD and cystic fibrosis. Levofloxacin is third generation fluoroquinolone that possesses broad-spectrum activity against gram-positive and gram-negative bacteria. Compared with second-generation fluoroquinolone, levofloxacin has demonstrated improved antibacterial activity Systemic intravenous administration of levofloxacin is limited due to poor penetrability in the respiratory secretion and bacterial biofilms. This problem has been overcome by developing an inhalable formulation of levofloxacin to maximize local antibiotic concentration at the pulmonary site [240]. Levofloxacin directly inhibits bacterial DNA synthesis by inhibiting DNA-gyrase in susceptible microorganisms. DNA gyrase plays a crucial role in maintaining DNA replication by resealing stand break and introducing superhelical twists in DNA that progress into an unwinding double stand [241]. The oral bioavailability of levofloxacin is almost 100%. This drug penetrates well distributed with a mean volume of distribution of 1.1. L/Kg. The plasma elimination half-life ranges from 6-8 hr with normal renal function. FDA approves levofloxacin for several treatments like community-acquired pneumonia, nosocomial pneumonia, bacterial rhinosinusitis, chronic bronchitis, acute bacterial exacerbation, etc. However, this drug is generally recommended in acute cases with no alternative treatment due to severe side effects like peripheral neuropathy, CNS effects, tendinitis, and tendon rupture).
Ciprofloxacin is another antibiotic agent of the fluoroquinolone class approved by the FDA for several bacterial infections. This drug inhibits DNA replication by interrupting the bacterial DNA topoisomerase and DNA gyrase. This antibiotic is active in a wide range of gram-negative bacteria and gram-positive bacteria. The age-dependent elimination half-life of 3.3–6.8 h for the elderly and 3–4 h for the younger persons is observed for ciprofloxacin [242]. However, a separate article also reported a short half-life of 1–1.6 hr after drug administration through oral and parenteral routes. The possible reason for this variation in half-life may be dose-dependent pharmacokinetics. Treatment efficiency in pulmonary infection caused by Francisella tularensis was improved with the liposomal formulation of ciprofloxacin [243]. Ciprofloxacin availability to the pulmonary site of infection is less after oral administration, rendering this drug inefficient in cystic fibrosis. Thus, the inhalable administration of ciprofloxacin resulted in a higher concentration of this drug in alveolar macrophages and lung epithelial fluid. Additionally, lower concentrations in plasma followed by inhalation administration decrease the systemic side effects [244]. Inhaled ciprofloxacin has gone through several clinical trials [245]. Later, the FDA granted the inhalable liposomal formulation of ciprofloxacin as an orphan drug in managing bronchiectasis and cystic fibrosis [246].
5.2. Beta-lactam antibiotics (aztreonam)
Aztreonam is a β-lactam antibiotic and clinically potent against a wide range of gram-negative bacteria. This drug possesses antimicrobial activity in different ways. It inhibits the mucopeptide synthesis in the cell wall of bacteria. Additionally, aztreonam binds to penicillin-binding protein 3 (PBP), is responsible for cell septation, and inhibits cell division, followed by cell lysis [247]. Currently, aztreonam is given in different routes like intravenous, intramuscular, intraperitoneal, and nebulizer. The oral bioavailability of this drug is <1%. This drug's average serum elimination half-life is 2 h, with the volume of distribution at a steady state of 0.18 L/kg (volume similar to extracellular fluid) [248]. Due to the short half-life, repeated administration is necessitating. Aztreonam is available in the intravenous (IV) formulation, demonstrating several potential side effects like nausea, diarrhea, rash, vomiting, and elevation of liver enzymes. Additionally, this IV formulation contains arginine salt that worsens airway inflammation or causes bronchoconstriction. This arginine salt promotes the production of nitric oxide that causes airway inflammation [249]. The inhaled formulation of aztreonam lysis was developed to make it more patient-friendly.
5.3. Aminoglycosides (amikacin, tobramycin)
Amikacin exhibits excellent antimicrobial activity against many aerobic gram-negative and resistant gram-negative bacilli. Amikacin interferes with the transcription of genetic code by binding with the 30s of bacterial ribosome subunit resulting in a disruption in bacterial protein synthesis [250]. Amikacin liposomal inhalation suspension is developed to target and localize drug delivery at the infection site of the lung with minimal systemic exposure. Generally, concentration-dependent response and post-effect of it demonstrate a broad-spectrum activity. More importantly, after parenteral administration, amikacin's associated toxicity (ototoxicity and nephrotoxicity) can be curtailed [251]. This nebulized antibiotic delivers a uniform quantity of drugs to the respiratory tract to overcome the MICs. Not only for CF and pneumonia but also this drug is approved in the USA as a part of a combination regimen for lung disease caused by Mycobacterium avium complex, where no alternative treatment is available [252].
Tobramycin is a broad-spectrum antibiotic that falls under the category of aminoglycosides. FDA has approved systemic administration of this drug in a wide range of susceptible microorganisms, especially gram-negative bacteria. Inhalable tobramycin formulation is approved mainly for treating cystic fibrosis caused by P. aeruginosa in six or older patients. Tobramycin inhibits the initiation step of bacterial translation by binding to 16s ribosomal RNA of 30s ribosomal unit of bacteria. After binding, this drug mistranslates the codon, resulting in misreading by transfer RNA, followed by incorrect aminoacyl units [253]. This drug also inhibits the production and expression of virulence factors of gram-negative bacteria and the associated cytotoxic effect of neutrophil-derived myeloperoxidase. This drug hardly penetrates bronchial secretions after administering it through the parenteral route. To overcome this problem, a high dose is required to achieve sufficient concentration at the site of infection, but there are chances of ototoxicity and nephrotoxicity. Due to these issues, tobramycin has been delivered through the inhalation route for better disease management [254]. The long-term clinical benefit of inhalable tobramycin has been well documented. With the success of inhaled tobramycin for treating P. aeruginosa, interest gradually rises to explore its potential application in other types of bronchiectasis. Nowadays, tobramycin inhalation is prescribed to patients with non-cystic fibrosis bronchiectasis caused by P. aeruginosa [255]. This study demonstrated that tobramycin inhalation is well-tolerated in P. aeruginosa-associated bronchiectasis. Additionally, this treatment may decrease the frequency of hospitalization rate with improved associated symptoms.
5.4. Polymyxin (colistin)
Colistin exerts its anti-microbial activity by disrupting the outer cell membrane. The cationic charged molecules interact with the anionic lipopolysaccharide (LPS) present on the outer membrane of gram-negative bacteria and initiate the displacement of calcium and magnesium ions to stabilize the LPS membrane. Gradually, cell membrane permeability increases, resulting in cell contents leakage followed by cell death. Colistin also exhibits anti-endotoxin activity. Colistin binds with lipid A portion (endotoxin of gram-negative) of LPS molecules and neutralizes. This drug is commercially available in two forms: colistin sulfate and colistimethate sodium (CMS). CMS is an inactive prodrug of colistin and is less toxic and less potent than colistin sulfate. CMS is generally administered in the form of inhalation and parenteral. Colistin has demonstrated excellent bactericidal activity against all gram-negative bacilli with MIC90 in the range of 0.5–5 mg/L (varies based on the species) [256]. After being introduced into clinical practice, colistin use was restricted due to severe toxicity. With the growing epidemic of MDR gram-negative bacteria, clinicians have started using this drug. However, the access to lung parenchyma is inadequate with the parenteral delivery of colistin. Due to this reason, inhaled colistin has been introduced to prevent and cure pulmonary infections in a patient with cystic fibrosis. This inhalation therapy of colistin is safe and well tolerable [257].
5.5. Antiviral (ribavirin, zanamivir)
Ribavirin (a guanosine analog) is a broad-spectrum antiviral agent demonstrating activity against RSV and RNA viruses. Ribavirin plays a vital role in managing chronic hepatitis C. Multiple mechanisms of action have been revealed for this drug. Mostly, antiviral activity is well documented. Ribavirin reduces the replicon of colony-forming efficiency of hepatitis C virus (HCV) in a dose-dependent manner. This drug also enhances the activity of interferon-stimulated genes that modulate cells more sensitive to exogenous interferon and concurrently increase the production of endogenous interferon. Long inter-individual variability with dose-dependent activity is observed. This drug has demonstrated a narrow therapeutic window [258]. Ribavirin in inhalation has been approved to treat hospitalized young children and infants with severe lower respiratory tract infections [259]. The suitability of this drug is also explored for COVID19. A phase 1 clinical trial has been initiated to check the safety and efficacy of VIRAZOLE® in hospitalized patients with respiratory distress due to COVID-19 [260].
Zanamivir is a sialic acid analog neuraminidase inhibitor approved for managing influenza A and B. This drug manifests tighter binding with both these types of influenza viruses. This drug's IC50 (mean inhibitory concentration by 50%) value is 0.35 nM against the H1N1 subtype and 1.1 nM for the H3N2 subtype of influenza A viruses. By inhibiting neuraminidase, this drug restricts the viral release and spread. This mechanism is mainly attributed to destroying the sialic acid-containing receptor for viral hemagglutinin. This drug is not intended to enter the intracellular as it specifically targets the extracellular enzyme function. This drug is thought to apply intranasal or oral inhalation [261].
Zanamivir is a hydrophilic compound and eliminates rapidly through the renal system, and its oral bioavailability is poor (<5%). Low oral bioavailability is another reason for delivering it through the nasal or pulmonary route [262]. Only 4–17% of oral inhalation dose is absorbed. The protein bounding of this drug is less than 10% and excreted in unchanged form through urine, whereas the unabsorbed drug is cleaned through feces. No dose adjustment is recommended for this drug in hepatic or renal clearance [263]. The amount of this drug required for inhalation depends on the inspiratory flow and delivery device discussed in a previously published article [264]. In prophylaxis family settings, this drug reduces 80% of contracting influenza if taken 48 h after the first symptom.
6. Conclusion
In this review, different types of etiological agents and the associated lung infections have been emphasized. Targeting drugs to the lungs is challenging through the oral route. Therefore, antimicrobial agents through the inhalation route are crucial for better disease management. Surprisingly, very few inhalable antimicrobial agents have been approved for marketing. To find the obstacles, we explain the basic anatomy-physiology and analyze all the possible AMA barriers in pulmonary administration. AMAs cross several obstacles before they exert their effect locally or systemically. The physicochemical characteristic of the administered antimicrobial agent may influence the inhalation process. A successful aerosol therapy reduces inertial impaction and increases the proportion of medication available for gravitational sedimentation. Respiratory disease and inhalation techniques contribute equally to the effective delivery of antimicrobials. A deep aerosol delivery in the lungs improves the fraction of the drug reaching systemic circulation. Nanocarriers can increase efficacy and decrease the parenteral toxicity of many medications. In the future, many advancements need to develop in terms of new carriers, composites, and surface modifications for pulmonary antimicrobial therapy.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Nguyen H.X. Targeted delivery of surface-modified nanoparticles: modulation of inflammation for acute lung injury. Surf. Modif. Nanoparticles Target. Drug Deliv. 2019;12:331–353. [Google Scholar]
- 2.Gao W., Chen Y., Zhang Y., Zhang Q., Zhang L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018;127:46–57. doi: 10.1016/j.addr.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Rijt S.H., Bein T., Meiners S. Medical nanoparticles for next generation drug delivery to the lungs. Eur. Respir. J. 2014;44:765–774. doi: 10.1183/09031936.00212813. [DOI] [PubMed] [Google Scholar]
- 4.Pleasants R.A., Hess D.R. Aerosol delivery devices for obstructive lung diseases. Respir. Care. 2018;63:708–733. doi: 10.4187/respcare.06290. [DOI] [PubMed] [Google Scholar]
- 5.Chmielowiec-Korzeniowska A., Tymczyna L., Wlazło Ł., Nowakowicz-Dębek B., Trawińska B. Staphylococcus aureus carriage state in healthy adult population and phenotypic and genotypic properties of isolated strains. Adv. Dermatol. Allergol. 2020;37:189. doi: 10.5114/ada.2020.94837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Limoli D.H., Hoffman L.R. Help, hinder, hide and harm: what can we learn from the interactions between Pseudomonas aeruginosa and Staphylococcus aureus during respiratory infections. Thorax. 2019;74:684–692. doi: 10.1136/thoraxjnl-2018-212616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas L., Götz F. Molecular mechanisms of Staphylococcus and Pseudomonas interactions in cystic fibrosis. Front. Cell. Infect. Microbiol. 2022;11:1383. doi: 10.3389/fcimb.2021.824042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCullough A.E., Leslie K.O. In: Pract. Pulm. Pathol. 3rd ed. Leslie K.O., Wick M.R., editors. Elsevier; 2018. Lung infections; pp. 147–225.https://reader.elsevier.com/reader/sd/pii/B9780323442848000077?token=48FE88CDBAC70D74BFE460259BE260FA50D0EFA3ED7CB50E48B291E7A277C13DF1B31C451D12815BECE0ACAAA15DABCC [Google Scholar]
- 9.Miragaia M. Factors contributing to the evolution of Meca-mediated β-lactam resistance in staphylococci: update and new insights from whole genome sequencing (WGS) Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.02723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loke H.Y., Kyaw W.M., Chen M.I.C., Lim J.W., Ang B., Chow A. Length of stay and odds of MRSA acquisition: a dose–response relationship? Epidemiol. Infect. 2019;147 doi: 10.1017/S0950268819001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karchmer A.W. Definitive treatment for methicillin-susceptible staphylococcus aureus bacteremia: data versus a definitive answer? Clin. Infect. Dis. 2017;65:107–109. doi: 10.1093/cid/cix288. [DOI] [PubMed] [Google Scholar]
- 12.Choo E.J., Chambers H.F. Treatment of methicillin-resistant Staphylococcus aureus bacteremia. Infect. Chemother. 2016;48:267–273. doi: 10.3947/ic.2016.48.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troeger C., Blacker B., Khalil I.A., Rao P.C., Cao J. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018;18:1191–1210. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henriques-Normark B., Tuomanen E.I. The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harb. Perspect. Med. 2013;3 doi: 10.1101/cshperspect.a010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown L.A., Mitchell A.M., Mitchell T.J. Streptococcus pneumoniae and lytic antibiotic therapy: are we adding insult to injury during invasive pneumococcal disease and sepsis? J. Med. Microbiol. 2017;66:1253–1256. doi: 10.1099/jmm.0.000545. [DOI] [PubMed] [Google Scholar]
- 16.Chagla Z., Sładek K., Jankowski M., Mediastinitis McMaster Textb. Intern. Med. 2019:1. https://empendium.com/mcmtextbook/chapter/B31.II.3.22 [Google Scholar]
- 17.Grinberg L.M., Abramova F.A., Yampolskaya O.V., Walker D.H., Smith J.H. Quantitative pathology of inhalational anthrax I: quantitative microscopic findings. Mod. Pathol. 2001;14:482–495. doi: 10.1038/modpathol.3880337. [DOI] [PubMed] [Google Scholar]
- 18.Savransky V., Ionin B., Reece J. Current status and trends in prophylaxis and management of anthrax disease. Pathogens. 2020;9 doi: 10.3390/pathogens9050370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sittner A., Ben-Shmuel A., Glinert I., Bar-David E., Schlomovitz J., Kobiler D., Weiss S., Levy H. Using old antibiotics to treat ancient bacterium—β-lactams for Bacillus anthracis meningitis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0228917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amakawa M., Gunawardana S., Jabbour A., Hernandez A., Pasos C., Alameh S., Martchenko Shilman M., Levitin A. Repurposing clinically approved drugs for the treatment of Bacillus cereus, a surrogate for Bacillus anthracis. ACS Omega. 2020;5:21929–21939. doi: 10.1021/acsomega.0c03207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Debnath S.K., Saisivam S., Debnath M. Validated UV-Spectrophotometric method for the ethionamide estimation in bulk, tablet and nanoparticles. Int. J. Drug Dev. Res. 2017;9:20–23. [Google Scholar]
- 22.Jilani T.N., Avula A., Zafar Gondal A., Siddiqui A.H. Active tuberculosis, StatPearls. 2020. http://www.ncbi.nlm.nih.gov/pubmed/30020618 [PubMed]
- 23.Cardona P.J. Reactivation or reinfection in adult tuberculosis: is that the question? Int. J. Mycobacteriology. 2016;5:400–407. doi: 10.1016/j.ijmyco.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Debnath S.K., Saisivam S., Debnath M., Omri A. Development and evaluation of chitosan nanoparticles based dry powder inhalation formulations of prothionamide. PLoS One. 2018;13:1–12. doi: 10.1371/journal.pone.0190976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Debnath S.K., Srinivasan S., Debnath M. Development of dry powder inhaler containing prothionamide-PLGA nanoparticles optimized through statistical design: in-vivo study. Open Nanomed. J. 2017;4:30–40. [Google Scholar]
- 26.Rabahi M.F., Da Silva Júnior J.L.R., Ferreira A.C.G., Tannus-Silva D.G.S., Conde M.B. Tuberculosis treatment. J. Bras. Pneumol. 2017;43:472–486. doi: 10.1590/S1806-37562016000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Debnath S.K., Saisivam S., Omri A. PLGA ethionamide nanoparticles for pulmonary delivery: development and in vivo evaluation of dry powder inhaler. J. Pharm. Biomed. Anal. 2017;145:854–859. doi: 10.1016/j.jpba.2017.07.051. [DOI] [PubMed] [Google Scholar]
- 28.Ratnatunga C.N., Lutzky V.P., Kupz A., Doolan D.L., Reid D.W., Field M., Bell S.C., Thomson R.M., Miles J.J. The rise of non-tuberculosis mycobacterial lung disease. Front. Immunol. 2020;11:303. doi: 10.3389/fimmu.2020.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill U.G., Floto R.A., Haworth C.S. Non-tuberculous mycobacteria in cystic fibrosis. J. R. Soc. Med. 2012;105:S18. doi: 10.1258/jrsm.2012.12s003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skolnik K., Kirkpatrick G., Quon B.S. Nontuberculous mycobacteria in cystic fibrosis. Curr. Treat. Options Infect. Dis. 2016;8:259–274. doi: 10.1007/s40506-016-0092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barragan E.P., Perez J.S., Corbella L., Orellana M.A., Fernandez-Ruiz M. Achromobacter xylosoxidans bacteremia: clinical and microbiological features in a 10-year case series. Rev. Española Quimioter. 2018;31:268–273. pmc/articles/PMC6166261/?report=abstract (accessed September 1, 2020) [PMC free article] [PubMed] [Google Scholar]
- 32.Awadh H., Mansour M., Aqtash O., Shweihat Y. Pneumonia due to a rare pathogen: Achromobacter xylosoxidans, Subspecies denitrificans. Case Rep. Infect. Dis. 2017;2017:1–4. doi: 10.1155/2017/3969682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Habib S., Fuca N., Azam M., Siddiqui A.H., Rajdev K., Chalhoub M. Achromobacter xylosoxidans/denitrificans bacteremia and subsequent fatal Escherichia coli/Streptococcus anginosus pleural empyema. Respir. Med. Case Reports. 2018;25:311–313. doi: 10.1016/j.rmcr.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taccetti G., Francalanci M., Pizzamiglio G., Messore B., Carnovale V., Cimino G., Cipolli M. Cystic fibrosis: recent insights into inhaled antibiotic treatment and future perspectives. Antibiotics. 2021;10:338. doi: 10.3390/antibiotics10030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isler B., Kidd T.J., Stewart A.G., Harris P., Paterson D.L. Achromobacter infections and treatment options, Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.01025-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinucci M., Roscetto E., Iula V.D., Votsi A., Catania M.R., De Gregorio E. Accurate identification of members of the Burkholderia cepacia complex in cystic fibrosis sputum. Lett. Appl. Microbiol. 2016;62:221–229. doi: 10.1111/lam.12537. [DOI] [PubMed] [Google Scholar]
- 37.Hassan A.A., Coutinho C.P., Sá-Correia I. Burkholderia cepacia complex species differ in the frequency of variation of the lipopolysaccharide O-antigen expression during cystic fibrosis chronic respiratory infection. Front. Cell. Infect. Microbiol. 2019;9:273. doi: 10.3389/fcimb.2019.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sfeir M.M. Burkholderia cepacia complex infections: more complex than the bacterium name suggest. J. Infect. 2018;77:166–170. doi: 10.1016/j.jinf.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Narayanaswamy V.P., Giatpaiboon S., Baker S.M., Wiesmann W.P., LiPuma J.J., Townsend S.M. Novel glycopolymer sensitizes Burkholderia cepacia complex isolates from cystic fibrosis patients to tobramycin and meropenem. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldstein E.J.C., Abrahamian F.M. In: Infect. Dis. (Auckl)., Fourth. Cohen J., Opal S.M., Powderly W.G., editors. Elsevier; Amsterdam, Netherlands: 2017. Infections from pets; pp. 656–662. e1. [Google Scholar]
- 41.Seltz L.B., Colvin M., Barton L.L. In: Kendig Chernick’s Disord. Respir. Tract Child., Eight. Wilmott R.W., Bush A., Deterding R.R., Boat T.F., Chernick V., Ratjen F., editors. Elsevier; Amsterdam, Netherlands: 2012. Atypical pneumonias in children; pp. 493–505. [Google Scholar]
- 42.Sandrock C. In: Murray Nadel’s Textb. Respir. Med. V Broaddus C., Ernst J.D., Lazarus S.C., Nadel J.A., editors. SIxth, Elsevier; Amsterdam, Netherlands: 2016. Bioterrorism; pp. 699–712. e2. [Google Scholar]
- 43.Bommana S., Polkinghorne A. Antimicrobial control of chlamydial infections in animals: current practices and issues. Front. Microbiol. 2019;10:113. doi: 10.3389/fmicb.2019.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porritt R.A., Crother T.R. Chlamydia pneumoniae infection and inflammatory diseases. Forum Immunopathol. Dis. Ther. 2016;7:237–254. doi: 10.1615/ForumImmunDisTher.2017020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honarmand H. Atherosclerosis induced by chlamydophila pneumoniae: a controversial theory. Interdiscip. Perspect. Infect. Dis. 2013;2013:11. doi: 10.1155/2013/941392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Senn L., Jaton K., Fitting J.W., Greub G. Does respiratory infection due to chlamydia pneumoniae still exist. Clin. Infect. Dis. 2011;53:847–848. doi: 10.1093/cid/cir515. accessed. [DOI] [PubMed] [Google Scholar]
- 47.Smith-Norowitz T.A., Huang Y., Loeffler J., Klein E., Norowitz Y.M., Hammerschlag M.R., Joks R., Kohlhoff S. Azithromycin decreases Chlamydia pneumoniae-mediated Interleukin-4 responses but not Immunoglobulin E responses. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mansfield K.G., Fox J.G. In: Common Marmoset Captiv. Biomed. Res. Marini R., Wachtman L., Tardif S., Mansfield K., Fox J., editors. Elsevier; Amsterdam, Netherlands: 2019. Bacterial diseases; pp. 265–287. [Google Scholar]
- 49.Warawa J., Shannon J.G., Bosio C.M. In: Encycl. Immunobiol. Ratcliffe M.J., editor. Elsevier; Amsterdam, Netherlands: 2016. Immunology of bacterial biodefense agents: Francisella tularensis, Burkholderia mallei, and Yersinia pestis; pp. 66–74. [Google Scholar]
- 50.Mechaly A., Elia U., Alcalay R., Cohen H., Epstein E., Cohen O., Mazor O. Inhibition of Francisella tularensis phagocytosis using a novel anti-LPS scFv antibody fragment. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-019-47931-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Darmon-Curti A., Darmon F., Edouard S., Hennebique A., Guimard T., Martin-Blondel G., Klopfenstein T., Talarmin J.P., Raoult D., Maurin M., Fournier P.E. Tularemia: a case series of patients diagnosed at the national reference center for rickettsioses from 2008 to 2017. Open Forum Infect. Dis. 2020;7 doi: 10.1093/ofid/ofaa440. ofaa440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gessner B.D. Berthe-marie njanpop-lafourcade, M.A. Herbert, Haemophilus influenzae, infect. Dis. Antimicrob. Agents. 2017;1 http://www.antimicrobe.org/b67.asp [Google Scholar]
- 53.Huang H.Y., Chung F.T., Lo C.Y., Lin H.C., Huang Y.T., Yeh C.H., Lin C.W., Huang Y.C., Wang C.H. Etiology and characteristics of patients with bronchiectasis in Taiwan: a cohort study from 2002 to 2016. BMC Pulm. Med. 2020;20:45. doi: 10.1186/s12890-020-1080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.King P. Haemophilus influenzae and the lung (Haemophilus and the lung) Clin. Transl. Med. 2012;1:10. doi: 10.1186/2001-1326-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khattak Z. STATPEARLS; 2021. Haemophilus Influenzae; p. 1.https://www.statpearls.com/ArticleLibrary/viewarticle/91515 [PubMed] [Google Scholar]
- 56.Ashurst J.V., Dawson A. Klebsiella pneumonia, StatPearls. 2018. http://www.ncbi.nlm.nih.gov/pubmed/30085546 [PubMed]
- 57.Corrin B., Nicholson A.G. In: Pathol. Lungs, Third. Corrin B., Nicholson A.G., editors. Elsevier; Amsterdam, Netherlands: 2011. Chapter 5- infectious diseases; pp. 155–262. [Google Scholar]
- 58.Atamni H.J.A.T., Iraqi F.A. In: Mol. Stat. Tech. Behav. Neural Res. Gerlai R.T., editor. Elsevier; Amsterdam, Netherlands: 2018. Chapter 9-Collaborative cross as the next-generation mouse genetic reference population designed for dissecting complex traits; pp. 191–224. [Google Scholar]
- 59.Kuroki T., Amemura-Maekawa J., Ohya H., Furukawa I., Suzuki M., Masaoka T., Aikawa K., Hibi K., Morit M., Lee K.I., Ohnishi M., Kura F. Outbreak of legionnaire’s disease caused by legionella pneumophila serogroups 1 and 13, Emerg. Inf. Disp. 2017;23:349–351. doi: 10.3201/eid2302.161012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brigmon R.L., Turick C.E., Knox A.S., Burckhalter C.E. The impact of storms on Legionella pneumophila in cooling tower water, implications for human health. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.543589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brady M.F., Sundareshan V. Legionnaires’ disease (Legionella infection), StatPearls. 2018. http://www.ncbi.nlm.nih.gov/pubmed/28613558
- 62.Wilson R.E., Hill R.L.R., Chalker V.J., Mentasti M., Ready D. Antibiotic susceptibility of Legionella pneumophila strains isolated in England and Wales 2007–17. J. Antimicrob. Chemother. 2018;73:2757–2761. doi: 10.1093/jac/dky253. [DOI] [PubMed] [Google Scholar]
- 63.Surani S., Anzueto A., Rodriguez M., Chitra S., Gunter K. Treatment of legionella pneumophila using omadacycline vs moxifloxacin: subanalysis results from a phase 3 randomized, double-blind, multicenter study (optic) Chest. 2020;158 A307–A308. [Google Scholar]
- 64.Sastry A.S., Bhat S.K., Janagond A. In: Essentials Med. Microbiol., 1/e. Sastry A.S., Bhat S.K., Janagond A., editors. Jaypee Digital Explore Health Sciences; New Delhi, India: 2016. Mycoplasma and ureaplasma; pp. 405–408. [Google Scholar]
- 65.Rockett R. PCR Clin. Microbiol. An Aust. Int. Perspect. Springer Netherlands; 2010. Mycoplasma pneumoniae; pp. 179–182. [Google Scholar]
- 66.Bajantri B., Venkatram S., Diaz-Fuentes G. Mycoplasma pneumoniae : a potentially severe infection. J. Clin. Med. Res. 2018;10:535–544. doi: 10.14740/jocmr3421w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warnke P., Köller T., Kreikemeyer B., Barrantes I., Mach H., Podbielski A. Molecular epidemiology study of a nosocomial Moraxella catarrhalis outbreak in a neurological rehabilitation unit. J. Hosp. Infect. 2019;103:27–34. doi: 10.1016/j.jhin.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 68.Ramadan M.O., Ibrahim I.S., Shaheen A.M., Ali W.E. Significance of Moraxella catarrhalis as a causative organism of lower respiratory tract infections, Egypt. J. Chest Dis. Tuberc. 2017;66:459–464. [Google Scholar]
- 69.Constantinescu M. 2018. Moraxella Catarrhalis Infection: Background, Pathophysiology and Etiology, Epidemiology, Medscape.https://emedicine.medscape.com/article/222320-overview#a5 1. [Google Scholar]
- 70.Simmons J., Gibson S. In: Nonhum. Primates Biomed. Res. 2nd ed. Abee C.R., Tardif S., Mansfield K., Morris T., editors. Elsevier; Amsterdam, Netherlands: 2012. Bacterial and mycotic diseases of nonhuman primates; pp. 105–172. [Google Scholar]
- 71.Deshpande R., Zou C. Pseudomonas aeruginosa induced cell death in acute lung injury and acute respiratory distress syndrome. Int. J. Mol. Sci. 2020;21:1–17. doi: 10.3390/ijms21155356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoiby N., Ciofu O., Bjarnsholt T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010;5:1663–1674. doi: 10.2217/fmb.10.125. [DOI] [PubMed] [Google Scholar]
- 73.Bassetti M., Vena A., Croxatto A., Righi E., Guery B. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018:7. doi: 10.7573/dic.212527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Horcajada J.P., Montero M., Oliver A., Sorlí L., Luque S., Gómez-Zorrilla S., Benito N., Grau S. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin. Microbiol. Rev. 2019;32 doi: 10.1128/CMR.00031-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jair H.W., Lu H.F., Huang Y.W., Pan S.Y., Lin I.L., Huang H.H., Yang T.C. Roles of the two-MnSOD system of stenotrophomonas maltophilia in the alleviation of superoxide stress. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20071770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahmood S.F. Stenotrophomonas maltophilia: background, pathophysiology, epidemiology. Medscape. 2018;1 https://emedicine.medscape.com/article/237024-overview#a5 [Google Scholar]
- 77.Tillman E.M., Firmani S.E., Ackerman V.L., Slaven J.E., Cristea A.I. Evaluation of the treatment of Stenotrophomonas maltophilia in tracheostomy-dependent pediatric patients. J. Pediatr. Pharmacol. Therapeut. 2019;24:510–517. doi: 10.5863/1551-6776-24.6.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Biagi M., Tan X., Wu T., Jurkovic M., Vialichka A., Meyer K., Mendes R.E., Wenzler E. Activity of potential alternative treatment agents for stenotrophomonas maltophilia isolates nonsusceptible to levofloxacin and/or Trimethoprim-sulfamethoxazole. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.01603-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Demeure C.E., Dussurget O., Mas Fiol G., Le Guern A.S., Savin C., Pizarro-Cerdá J. Yersinia pestis and plague: an updated view on evolution, virulence determinants, immune subversion, vaccination, and diagnostics. Gene Immun. 2019;20:357–370. doi: 10.1038/s41435-019-0065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clements B.W. In: Disasters Public Heal. Clements B.W., editor. Elsevier; Amsterdam, Netherlands: 2009. Bioterrorism; pp. 27–63. [Google Scholar]
- 81.Godfred-Cato S., Cooley K.M., Fleck-Derderian S., Becksted H.A., Russell Z., Meaney-Delman D., Mead P.S., Nelson C.A. Treatment of human plague: a systematic review of published aggregate data on antimicrobial efficacy, 1939–2019. Clin. Infect. Dis. 2021;70 doi: 10.1093/cid/ciz1230. S11–S19. [DOI] [PubMed] [Google Scholar]
- 82.Kurowski R., Ostapchuk M. Overview of histoplasmosis. Am. Fam. Physician. 2002;66:2253. www.aafp.org/afp [PubMed] [Google Scholar]
- 83.Kandi V., Vaish R., Palange P., Bhoomagiri M.R. Chronic pulmonary histoplasmosis and its clinical significance: an under-reported systemic fungal disease. Cureus. 2016;8:e751. doi: 10.7759/cureus.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ling Q., Zhu W., Lu Q., Jin T., Ding S. Disseminated histoplasmosis in an immunocompetent patient from an endemic area. Medicine (Baltim.) 2018;97 doi: 10.1097/MD.0000000000011486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hospenthal D.R. 2019. Coccidioidomycosis and valley Fever, Medscape.https://emedicine.medscape.com/article/215978-overview#a3 [Google Scholar]
- 86.Akram S.M., Koirala J. StatPearls Publ. LLC; 2020. Coccidioidomycosis.https://www.ncbi.nlm.nih.gov/books/NBK448161/ [PubMed] [Google Scholar]
- 87.Lu M., Yan H., Yu C., Yuan L., Sun S. Proton pump inhibitors act synergistically with fluconazole against resistant Candida albicans. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-019-57174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Truong J., Ashurst J.V. Pneumocystis (carinii) jiroveci pneumonia, StatPearls. 2019. http://www.ncbi.nlm.nih.gov/pubmed/29493992
- 89.Hammarström H., Grankvist A., Broman I., Kondori N., Wennerås C., Gisslen M., Friman V. Serum-based diagnosis of Pneumocystis pneumonia by detection of Pneumocystis jirovecii DNA and 1,3-β-D-glucan in HIV-infected patients: a retrospective case control study. BMC Infect. Dis. 2019;19:658. doi: 10.1186/s12879-019-4289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nevez G., Hauser P.M., Le Gal S. Pneumocystis jirovecii. Trends Microbiol. 2020 doi: 10.1016/j.tim.2020.03.006. 1034-1035. [DOI] [PubMed] [Google Scholar]
- 91.Sugui J.A., Kwon-Chung K.J., Juvvadi P.R., Latgé J.P., Steinbach W.J. Aspergillus fumigatus and related species. Cold Spring Harb. Perspect. Med. 2015;5 doi: 10.1101/cshperspect.a019786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Middleton P.G., Chen S.C.A., Meyer W. Fungal infections and treatment in cystic fibrosis. Curr. Opin. Pulm. Med. 2013;19:670–675. doi: 10.1097/MCP.0b013e328365ab74. [DOI] [PubMed] [Google Scholar]
- 93.Li Z., Lu G., Meng G. Pathogenic fungal infection in the lung. Front. Immunol. 2019;10:1–20. doi: 10.3389/fimmu.2019.01524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.King J.W., Chandrasekar P.H. Vol. 1. Medscape; 2018. https://emedicine.medscape.com/article/215354-overview (Cryptococcosis: Practice Essentials, Background, Pathophysiology). [Google Scholar]
- 95.Seal P.S., Pappas P.G. In: Antibiot. Chemother. Expert Consult, Ninth. Finch R.G., Norrby S.R., Greenwood D., Whitley R.J., editors. Elsevier; Amsterdam, Netherlands: 2010. Systemic fungal infections; pp. 777–796. [Google Scholar]
- 96.Nucci M., Anaissie E.J. In: Clin. Mycol. Second. Anaissie E.J., Pfaller M.A., McGinnis M.R., editors. Elsevier; Amsterdam, Netherlands: 2009. Hyalohyphomycosis; pp. 309–327. [Google Scholar]
- 97.Johnson M.M. In: Diagnostic Pathol. Infect. Dis. Second. Kradin R.L., editor. Elsevier; Amsterdam, Netherlands: 2018. Ear, nose, and throat infections; pp. 118–142. [Google Scholar]
- 98.Drzezo. mycoses Endemic. 2019. Thorac. Key; p. 1.https://thoracickey.com/endemic-mycoses/ [Google Scholar]
- 99.Chidinma C.-M. Vol. 1. Medscape; 2019. https://emedicine.medscape.com/article/296870-overview (Blastomycosis: Practice Essentials, Background, Pathophysiology). [Google Scholar]
- 100.Hashim S., Barrios C. Disseminated blastomycosis. IDCases. 2020;21 doi: 10.1016/j.idcr.2020.e00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miller A.S., Wilmott R.W. In: Kendig’s Disord. Respir. Tract Child., Ninth. Wilmott R.W., Li A., Sly P., Bush A., Deterding R., Ratjen F., editors. Elsevier; Amsterdam, Netherlands: 2019. The Pulmonary mycoses; pp. 507–527. e3. [Google Scholar]
- 102.Miceli A., Krishnamurthy K. Blastomycosis, StatPearls. 2020. http://www.ncbi.nlm.nih.gov/pubmed/28723016 [PubMed]
- 103.Orofino-Costa R., Rodrigues A.M., de Macedo P.M., Bernardes-Engemann A.R. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An. Bras. Dermatol. 2017;92:606–620. doi: 10.1590/abd1806-4841.2017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Higuita N.I.A. Sporotrichosis: background, pathophysiology, epidemiology. Medscape. 2018;1 https://emedicine.medscape.com/article/228723-overview#a5 [Google Scholar]
- 105.Barros M.B.D.L., Paes R. de A., Schubach A.O. Sporothrix schenckii and sporotrichosis. Clin. Microbiol. Rev. 2011;24:354–633. doi: 10.1128/CMR.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kauffman C.A., Bustamante B., Chapman S.W., Pappas P.G. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the infectious diseases society of America. Clin. Infect. Dis. 2007;45:1255–1265. doi: 10.1086/522765. [DOI] [PubMed] [Google Scholar]
- 107.Chi X.-H., Xue Y.-M., Wang Q.-S., Li G.-P., Zhou H.-S., Qi Y.-S. Diagnosis and treatment of diffusible Penicillium marneffei in human immunodeficiency virus-negative patients: a challenge for the physician. Indian J. Med. Microbiol. 2017;35:617. doi: 10.4103/ijmm.IJMM_15_418. [DOI] [PubMed] [Google Scholar]
- 108.Walker D.H., McGinnis M.R. In: Pathobiol. Hum. Dis. A Dyn. Encycl. Dis. Mech. McManus L.M., Mitchell R.N., editors. Elsevier; Amsterdam, Netherlands: 2014. Diseases caused by fungi; pp. 217–221. [Google Scholar]
- 109.Nishikubo M., Doi A., Takegawa H., Yamashita D., Ohira J., Nishioka H. Asymptomatic pulmonary penicilliosis with a lung mass in an HIV-infected patient. J. Gen. Fam. Med. 2020;21:152–154. doi: 10.1002/jgf2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martinez-Giron R., Esteban J.G., Ribas A., Doganci L. Protozoa in respiratory pathology: a review. Eur. Respir. J. 2008;32:1354–1370. doi: 10.1183/09031936.00022008. [DOI] [PubMed] [Google Scholar]
- 111.Leitch G.J., He Q. Cryptosporidiosis-an overview. J. Biomed. Res. 2011;25:1–16. doi: 10.1016/S1674-8301(11)60001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thomson S., Hamilton C.A., Hope J.C., Katzer F., Mabbott N.A., Morrison L.J., Innes E.A. Bovine cryptosporidiosis: impact, host-parasite interaction and control strategies. Vet. Res. 2017;48:42. doi: 10.1186/s13567-017-0447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sponseller J.K., Griffiths J.K., Tzipori S. The evolution of respiratory cryptosporidiosis: evidence for transmission by inhalation. Clin. Microbiol. Rev. 2014;27:575–586. doi: 10.1128/CMR.00115-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Coulibaly Y.I., Klion A.D. In: Hunter’s Trop. Med. Emerg. Infect. Dis., Tenth. Ryan E.T., Solomon T., Hill D.R., Aronson N.E., editors. Elsevier; Amsterdam, Netherlands: 2020. Miscellaneous filariae; pp. 872–877. [Google Scholar]
- 115.Gunathilaka N., Siriwardana S., Wijesooriya L., Gunaratne G., Perera N. Vol. 5. SAGE Open Med. Case Reports; 2017. (Subcutaneous Dirofilariasis Caused by Dirofilaria (Nochtiella) Repens in Sri Lanka: A Potential Risk of Transmitting Human Dirofilariasis). 2050313X1770137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Albrecht H. Antimicrobe; 2002. Dirofilariasis-infectious Disease and Antimicrobial Agents; p. 1.http://www.antimicrobe.org/b11.asp [Google Scholar]
- 117.Royer T.L., Petri W.A. In: Encycl. Food Microbiol. second ed., Second. Batt C.A., Lou Tortorello M., editors. Elsevier; Amsterdam, Netherlands: 2014. Waterborne parasites: entamoeba; pp. 782–786. [Google Scholar]
- 118.Zakaria A., Al-Share B., Al Asad K. Primary pulmonary amebiasis complicated with multicystic empyema. Case Rep. Pulmonol. 2016;2016:1–4. doi: 10.1155/2016/8709347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abees Hmood Al-Khalidi K., Riyadh Al-Abodi H., Kamil Jabbar H., Hmood B.A. 2020. Echinococcus Granulosus , Overv. Echinococcosis. [Google Scholar]
- 120.Brunetti E. Echinococcosis hydatid cyst treatment & management: approach considerations, medical care, surgical care. Medscape. 2018:1. https://emedicine.medscape.com/article/216432-treatment#d7 [Google Scholar]
- 121.Velasco-Tirado V., Alonso-Sardón M., Lopez-Bernus A., Romero-Alegría Á., Burguillo F.J., Muro A., Carpio-Pérez A., Muñoz Bellido J.L., Pardo-Lledias J., Cordero M., Belhassen-García M. Medical treatment of cystic echinococcosis: systematic review and meta-analysis. BMC Infect. Dis. 2018;18:306. doi: 10.1186/s12879-018-3201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Camacho E., González-de la Fuente S., Rastrojo A., Peiró-Pastor R., Solana J.C., Tabera L., Gamarro F., Carrasco-Ramiro F., Requena J.M., Aguado B. Complete assembly of the Leishmania donovani (HU3 strain) genome and transcriptome annotation. Sci. Rep. 2019;9:1–15. doi: 10.1038/s41598-019-42511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kariyawasam U.L., Selvapandiyan A., Rai K., Wani T.H., Ahuja K., Beg M.A., Premathilake H.U., Bhattarai N.R., Siriwardena Y.D., Zhong D., Zhou G., Rijal S., Nakhasi H., Karunaweera N.D. Genetic diversity of Leishmania donovani that causes cutaneous leishmaniasis in Sri Lanka: a cross sectional study with regional comparisons. BMC Infect. Dis. 2017;17:791. doi: 10.1186/s12879-017-2883-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dasgupta S., Saha M., Chakrabarti S., Chakraborty J. Visceral leishmaniasis with pleural effusion in an immunocompetent patient. Lung India. 2014;31:56–58. doi: 10.4103/0970-2113.125913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Han B., Takvorian P.M., Weiss L.M. Invasion of host cells by microsporidia. Front. Microbiol. 2020;11:172. doi: 10.3389/fmicb.2020.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cali A., Becnel J.J., Takvorian P.M. In: Handb. Protists. second ed. Archibald J.M., Simpson A.G., Slamovits C.H., editors. Springer International Publishing; AG: 2017. Microsporidia; pp. 1559–1618. [Google Scholar]
- 127.Ozkoc S., Delibas S.B., Akisu C. Evaluation of pulmonary microsporidiosis in iatrogenically immunosuppressed patients. Tuberk. Toraks. 2016;64:9–16. doi: 10.5578/tt.10207. [DOI] [PubMed] [Google Scholar]
- 128.Scaglia M., Sacchi L., Croppo G.P., Da Silva A., Gatti S., Corona S., Orani A., Bernuzzi A.M., Pieniazek N.J., Slemenda S.B., Wallace S., Visvesvara G.S. Pulmonary microsporidiosis due to Encephalitozoon hellem in a patient with AIDS. J. Infect. 1997;34:119–126. doi: 10.1016/s0163-4453(97)92414-2. [DOI] [PubMed] [Google Scholar]
- 129.Teachey D.T., Russo P., Orenstein J.M., Didier E.S., Bowers C., Bunin N. Pulmonary infection with microsporidia after allogeneic bone marrow transplant. Bone Marrow Transplant. 2004;33:299–302. doi: 10.1038/sj.bmt.1704327. [DOI] [PubMed] [Google Scholar]
- 130.Doerr S. Vol. 1. Med. Net.; 2015. https://www.medicinenet.com/microsporidiosis/article.htm#what_is_microsporidiosis_what_causes_the_disease (Microsporidiosis Symptoms, Diagnosis & Treatment). [Google Scholar]
- 131.Chingakham D.S., Thounaojam A.S., Haobam S., Thiyam U. Pulmonary paragonimiasis, a rare cause of haemoptysis, Egypt. J. Chest Dis. Tuberc. 2016;65:361–364. [Google Scholar]
- 132.Rosenbaum S.D. Medscape; 2019. Paragonimiasis Treatment & Management: Medical Care, Surgical Care, Consultations; p. 1.https://emedicine.medscape.com/article/999188-treatment [Google Scholar]
- 133.Zheng X., Hu Y., Qian J., Yang D. Pleuropulmonary paragonimiasis with migrated lesions cured by multiple therapies. Indian J. Pathol. Microbiol. 2016 doi: 10.4103/0377-4929.174820. 56–58. [DOI] [PubMed] [Google Scholar]
- 134.Saini N., Ranjan R., Singla L.D., Anand A., Randhawa C.S. Successful treatment of pulmonary paragonimiasis in a German shepherd dog with fenbendazole. J. Parasit. Dis. 2012;36:171–174. doi: 10.1007/s12639-012-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Elzein F., Mohammed N., Ali N., Bahloul A., Albadani A., Alsherbeeni N. Pulmonary manifestation of Plasmodium falciparum malaria: case reports and review of the literature. Respir. Med. Case Reports. 2017;22:83–86. doi: 10.1016/j.rmcr.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Patel T.A., Lukawska J., Rowe J., Bailey R.L. Case report: treatment of schistosomiasis in a patient allergic to praziquantel: a desensitization and treatment protocol. Am. J. Trop. Med. Hyg. 2016;95:1041–1043. doi: 10.4269/ajtmh.16-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maguire J.H. In: Clin. Infect. Dis. second ed. Baron S., editor. Fourth, Cambridge University Press; Galveston, TX: 2015. Schistosomes and other trematodes; pp. 1268–1273. [Google Scholar]
- 138.O’Connell E.M., Nutman T.B. Eosinophilia in infectious diseases. Immunol. Allergy Clin. 2015;35:493–522. doi: 10.1016/j.iac.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Butrous G. Schistosome infection and its effect on pulmonary circulation. Glob. Cardiol. Sci. Pract. 2019;2019:1–44. doi: 10.21542/gcsp.2019.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ahmed S.H. Schistosomiasis (Bilharzia) treatment & management: approach considerations, corticosteroids, outpatient care. Medscape. 2020:1. https://emedicine.medscape.com/article/228392-treatment [Google Scholar]
- 141.Kramer C.V., Zhang F., Sinclair D., Olliaro P.L. Drugs for treating urinary schistosomiasis. Cochrane Database Syst. Rev. 2014;2014 doi: 10.1002/14651858.CD000053.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mejia R., Nutman T.B. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr. Opin. Infect. Dis. 2012;25:458–463. doi: 10.1097/QCO.0b013e3283551dbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chandrasekar P.H. Strongyloidiasis treatment & management: approach considerations, anthelmintic therapy, antibiotic therapy and supportive care. Medscape. 2019;1 https://emedicine.medscape.com/article/229312-treatment [Google Scholar]
- 144.Kochanowsky J.A., Koshy A.A. University of Texas Medical Branch at Galveston; Galveston, TX: 2018. Toxoplasma Gondii: Methods and Protocols. [Google Scholar]
- 145.Procop G.W., Neafie R.C. In: Pulm. Pathol. A Vol. Ser. Found. Diagnostic Pathol. Second. Zander D.S., Farver C.F., editors. Elsevier; Amsterdam, Netherlands: 2018. Human parasitic pulmonary infections; pp. 289–314. [Google Scholar]
- 146.Winders W.T., MenkinSmith L. StatPearls; 2019. Toxocara Canis (Visceral Larva Migrans, Toxocariasis)http://www.ncbi.nlm.nih.gov/pubmed/30860759 [Google Scholar]
- 147.Ranasuriya G., Mian A., Boujaoude Z., Tsigrelis C. Pulmonary toxocariasis: a case report and literature review. Infection. 2014;42:575–578. doi: 10.1007/s15010-014-0587-3. [DOI] [PubMed] [Google Scholar]
- 148.Hombu A., Yoshida A., Kikuchi T., Nagayasu E., Kuroki M., Maruyama H. Treatment of larva migrans syndrome with long-term administration of albendazole. J. Microbiol. Immunol. Infect. 2019;52:100–105. doi: 10.1016/j.jmii.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 149.Hare A.Q., Franco-Paredes C. Ocular larva migrans: a severe manifestation of an unseen epidemic. Curr. Trop. Med. Reports. 2014;1:69–73. [Google Scholar]
- 150.Pitetti R.D. Medscape; 2018. Visceral Larva Migrans Treatment & Management: Medical Care, Consultations, Diet; p. 1.https://emedicine.medscape.com/article/1000527-treatment [Google Scholar]
- 151.Lynch J.P., Kajon A.E. Adenovirus: epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin. Respir. Crit. Care Med. 2016;37:586–602. doi: 10.1055/s-0036-1584923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.CDC . Centers Dis. Control Prev; 2019. Adenovirus | Prevention and Treatment; p. 1.https://www.cdc.gov/adenovirus/about/prevention-treatment.html [Google Scholar]
- 153.Waye M.M.Y., Sing C.W. Anti-viral drugs for human adenoviruses. Pharmaceuticals. 2010;3:3343–3354. [Google Scholar]
- 154.Heuman D.M. What is the pathophysiology of cytomegalovirus (CMV) colitis? Medscape. 2019 https://www.medscape.com/answers/173151-174928/what-is-the-pathophysiology-of-cytomegalovirus-cmv-colitis [Google Scholar]
- 155.Dietrich M., Schieffelin J. Congenital cytomegalovirus infection. Ochsner J. 2019;19:123–130. doi: 10.31486/toj.18.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li L., Chakinala R.C. StatPearls; 2020. Cytomegalovirus Esophagitis; p. 1.http://www.ncbi.nlm.nih.gov/pubmed/32310570 [PubMed] [Google Scholar]
- 157.Azevedo L.S., Pierrotti L.C., Abdala E., Costa S.F., Strabelli T.M.V., Campos S.V., Ramos J.F., Latif A.Z.A., Litvinov N., Maluf N.Z., Filho H.H.C., Pannuti C.S., Lopes M.H., dos Santos V.A., Linardi C. da C.G., Yasuda M.A.S., Marques H.H. de S. Cytomegalovirus infection in transplant recipients. Clinics. 2015;70:515–523. doi: 10.6061/clinics/2015(07)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chakravarti A., Kashyap B., Matlani M. Cytomegalovirus infection: an Indian perspective. Indian J. Med. Microbiol. 2009;27:3–11. [PubMed] [Google Scholar]
- 159.Swanson E.C., Schleiss M.R. Congenital cytomegalovirus infection: new prospects for prevention and therapy. Pediatr. Clin. 2013;60:335–349. doi: 10.1016/j.pcl.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses Methods Protoc. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Debnath S., Srivastava R., Omri A. Emerging therapeutics for the management of COVID 19. Expet Opin. Emerg. Drugs. 2020;25:337–351. doi: 10.1080/14728214.2020.1810663. [DOI] [PubMed] [Google Scholar]
- 162.Shannon-Lowe C., Rickinson A. The global landscape of EBV-associated tumors. Front. Oncol. 2019;9:713. doi: 10.3389/fonc.2019.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cunha B.A. Vol. 1. Medscape; 2018. https://emedicine.medscape.com/article/222040-overview#a5 (Epstein-barr Virus (EBV) Infectious Mononucleosis (Mono): Background, Pathophysiology, Epidemiology). [Google Scholar]
- 164.Oliver S., James S.H. In: Int. Encycl. Public Heal. Second. Quah S.R., editor. Elsevier; Amsterdam, Netherlands: 2016. Herpesviruses; pp. 621–627. [Google Scholar]
- 165.CDC . Centers Dis. Control Prev.; 2018. Epstein-barr Virus and Infectious Mononucleosis; p. 1.https://www.cdc.gov/epstein-barr/about-ebv.html [Google Scholar]
- 166.Cohen J.I. Optimal treatment for chronic active Epstein-Barr virus disease: Editorial. Pediatr. Transplant. 2009;13:393–396. doi: 10.1111/j.1399-3046.2008.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Muyangwa M., Martynova E.V., Khaiboullina S.F., Morzunov S.P., Rizvanov A.A. Hantaviral proteins: structure, functions, and role in Hantavirus infection. Front. Microbiol. 2015;6:1326. doi: 10.3389/fmicb.2015.01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ferreira A.V.A. In: Pract. Hepatic Pathol. A Diagnostic Approach. second ed., Second. Saxena R., editor. Elsevier; Amsterdam, Netherlands: 2018. Acute viral hepatitis; pp. 191–209. [Google Scholar]
- 169.CDC . Vol. 1. Centers Dis. Control Prev; 2012. https://www.cdc.gov/hantavirus/hps/diagnosis.html (Diagnosis & Treatment | Hantavirus). [Google Scholar]
- 170.Jonsson C.B., Hooper J., Mertz G. Treatment of hantavirus pulmonary syndrome. Antivir. Res. 2008;78:162–169. doi: 10.1016/j.antiviral.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Whitley R., Kimberlin D.W., Prober C.G. In: Hum. Herpesviruses Biol. Ther. Immunoprophyl. Arvin A., Campadelli-Fiume G., Mocarski E., editors. Cambridge University Press; Cambridge: 2007. Pathogenesis and disease.http://www.ncbi.nlm.nih.gov/pubmed/21348130 [Google Scholar]
- 172.Zaki S.R., Keating M.K. Viral diseases. Pulm. Pathol. A Vol. Ser. Found. Diagnostic Pathol. 2018:244–288. 2018. [Google Scholar]
- 173.Caruso J.L., Childs J.M. In: Lennette’s Lab. Diagnosis Viral Infect. Fourth Ed. Infect. Dis. Ther. Ser., Fourth. Jerome K.R., editor. Informa Healthcare; New York: 2010. Surgical pathology and diagnostic cytology of viral infections; pp. 151–172. [Google Scholar]
- 174.WHO . World Heal. Organ.; 2020. Herpes Simplex Virus; p. 1.https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus [Google Scholar]
- 175.Emmert D.H. Treatment of common cutaneous herpes simplex virus infections. Am. Fam. Physician. 2000;61:1697–1704. [PubMed] [Google Scholar]
- 176.Williams J., Shafagati N. Human metapneumovirus - what we know now. F1000Research. 2018;7 doi: 10.12688/f1000research.12625.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Crowe J.E., Williams J.V., Paramyxoviruses . In: Viral Infect. Humans. Kaslow R., Stanberry L., Le D.J., editors. Springer US; Boston, MA: 2014. Respiratory syncytial virus and human metapneumovirus; pp. 601–627. [Google Scholar]
- 178.CDC . Centers Dis. Control Prev; 2019. NREVSS | Human Metapneumovirus Clinical Features; p. 1.https://www.cdc.gov/surveillance/nrevss/hmpv/clinical.html [Google Scholar]
- 179.Kitanovski L., Kopriva S., Pokorn M., Dolničar M.B., Rajić V., Stefanović M., Jazbec J. Treatment of severe Human Metapneumovirus (hMPV) pneumonia in an immunocompromised child with oral ribavirin and IVIG. J. Pediatr. Hematol. Oncol. 2013;35 doi: 10.1097/MPH.0b013e3182915d2d. e311–e313. [DOI] [PubMed] [Google Scholar]
- 180.Couch R.B. In: Med. Microbiol. 4th ed. Baron S., editor. Galveston (TX): University of Texas Medical Branch; Galveston: 1996. Orthomyxoviruses.https://www.ncbi.nlm.nih.gov/books/NBK8611/ [PubMed] [Google Scholar]
- 181.Sell S., McKinstry K., Strutt T. Mouse models reveal role of T-cytotoxic and T-reg cells in immune response to influenza: implications for vaccine design. Viruses. 2019;11:52. doi: 10.3390/v11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Boktor S., Hafner J. StatPearls Publ.; 2020. Influenza, Treasure Isl.https://www.ncbi.nlm.nih.gov/books/NBK459363/ [Google Scholar]
- 183.Kutter J.S., Spronken M.I., Fraaij P.L., Fouchier R.A., Herfst S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018;28:142–151. doi: 10.1016/j.coviro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Barik S. New treatments for influenza. BMC Med. 2012;10:104. doi: 10.1186/1741-7015-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.FDA, Influenza (Flu) Antiviral Drugs and Related Information. U. S. Food Drug Adm.; 2019. p. 1.https://www.fda.gov/drugs/information-drug-class/influenza-flu-antiviral-drugs-and-related-information [Google Scholar]
- 186.Misin A., Antonello R.M., Di Bella S., Campisciano G., Zanotta N., Giacobbe D.R., Comar M., Luzzati R. Measles: an overview of a re-emerging disease in children and immunocompromised patients. Microorganisms. 2020;8:276. doi: 10.3390/microorganisms8020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Paules C.I., Marston H.D., Fauci A.S. Measles in 2019- going backward. N. Engl. J. Med. 2019;380:2185–2187. doi: 10.1056/NEJMp1905099. [DOI] [PubMed] [Google Scholar]
- 188.CDC . Centers Dis. Control Prev.; 2018. For Healthcare Professionals- Diagnosing and Treating Measles; p. 1.https://www.cdc.gov/measles/hcp/index.html [Google Scholar]
- 189.Plemper R.K., Snyder J.P. Measles control- Can measles virus inhibitors make a difference? Curr. Opin. Invest. Drugs. 2009;10:811–820. pmc/articles/PMC2728049/?report=abstract. accessed. [PMC free article] [PubMed] [Google Scholar]
- 190.Branche A.R., Falsey A.R. Parainfluenza virus infection. Semin. Respir. Crit. Care Med. 2016;37:538–554. doi: 10.1055/s-0036-1584798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mosenifar Z. Medscape; 2020. Viral Pneumonia: Practice Essentials, Background, Pathophysiology; p. 1.https://emedicine.medscape.com/article/300455-overview [Google Scholar]
- 192.CDC . Centers Dis. Control Prev; 2019. Human Parainfluenza Viruses | HPIV Prevention and Treatment.https://www.cdc.gov/parainfluenza/about/prevention-treatment.html [Google Scholar]
- 193.Waghmare A., Wagner T., Andrews R., Smith S., Kuypers J., Boeckh M., Moss R., Englund J.A. Successful treatment of parainfluenza virus respiratory tract infection with DAS181 in 4 immunocompromised children. J. Pediatric Infect. Dis. Soc. 2015;4:114–118. doi: 10.1093/jpids/piu039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tregoning J.S., Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pickles R.J., DeVincenzo J.P. Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis. J. Pathol. 2015;235:266–276. doi: 10.1002/path.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.ALA . Am. Lung Assoc.; 2020. RSV Symptoms and Diagnosis; p. 1.https://www.lung.org/lung-health-diseases/lung-disease-lookup/rsv/symptoms-diagnosis [Google Scholar]
- 197.Eiland L.S. Respiratory syncytial virus: diagnosis, treatment and prevention. J. Pediatr. Pharmacol. Therapeut. 2009;14:75–85. doi: 10.5863/1551-6776-14.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gershon A.A., Breuer J., Cohen J.I., Cohrs R.J., Gershon M.D., Gilden D., Grose C., Hambleton S., Kennedy P.G.E., Oxman M.N., Seward J.F., Yamanishi K. Varicella zoster virus infection. Nat. Rev. Dis. Prim. 2015;1 doi: 10.1038/nrdp.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Denny J.T., Rocke Z.M., McRae V.A., Denny J.E., Fratzola C.H., Ibrar S., Bonitz J., Tse J.T., Cohen S., Mellender S.J., Kiss G.K. Varicella pneumonia: case report and review of a potentially lethal complication of a common disease. J. Investig. Med. High Impact Case Reports. 2018;6 doi: 10.1177/2324709618770230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gnann J., John W. In: Hum. Herpesviruses Biol. Ther. Immunoprophyl. Arvin A., Campadelli-Fiume G., Mocarski E., editors. Cambridge University Press; Cambridge, England: 2007. Antiviral therapy of varicella-zoster virus infections; pp. 1–43.http://www.ncbi.nlm.nih.gov/pubmed/21348091 [PubMed] [Google Scholar]
- 201.Zemanick E.T., Hoffman L.R. Cystic fibrosis: microbiology and host response. Pediatr. Clin. 2016;63:617–636. doi: 10.1016/j.pcl.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wenzler E., Fraidenburg D.R., Scardina T., Danziger L.H. Inhaled antibiotics for gram-negative respiratory infections. Clin. Microbiol. Rev. 2016;29:581–632. doi: 10.1128/CMR.00101-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cipolla D., Chan H.K. Inhaled antibiotics to treat lung infection. Pharm. Pat. Anal. 2013;2:647–663. doi: 10.4155/ppa.13.47. [DOI] [PubMed] [Google Scholar]
- 204.Kelly C., Chalmers J.D., Crossingham I., Relph N., Felix L.M., Evans D.J., Milan S.J., Spencer S. Macrolide antibiotics for bronchiectasis. Cochrane Database Syst. Rev. 2018;2018:CD012406. doi: 10.1002/14651858.CD012406.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Goldstein N., St Clair J.B., Kasperbauer S.H., Daley C.L., Lindeque B. Nontuberculous mycobacterial musculoskeletal infection cases from a tertiary referral center, Colorado, USA. Emerg. Infect. Dis. 2019;25:1075–1083. doi: 10.3201/eid2506.181041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Laska I.F., Crichton M.L., Shoemark A., Chalmers J.D. The efficacy and safety of inhaled antibiotics for the treatment of bronchiectasis in adults: a systematic review and meta-analysis. Lancet Respir. Med. 2019;7:855–869. doi: 10.1016/S2213-2600(19)30185-7. [DOI] [PubMed] [Google Scholar]
- 207.Ramya C.M. Bacterial pneumonia. Res. J. Pharm. Technol. 2014;7:942–945. [Google Scholar]
- 208.Correa R. de A., Costa A.N., Lundgren F., Michelin L., Figueiredo M.R., Holanda M., Gomes M., Teixeira P.J.Z., Martins R., Silva R., Athanazio R.A., da Silva R.M., Pereira M.C. Recommendations for the management of community acquired pneumonia. J. Bras. Pneumol. 2018;44:405–423. doi: 10.1590/S1806-37562018000000130. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Waak M.B., LaPara T.M., Halle C., Hozalski R.M. Occurrence of Legionella spp. in water-main biofilms from two drinking water distribution systems. Environ. Sci. Technol. 2018;52:7630–7639. doi: 10.1021/acs.est.8b01170. [DOI] [PubMed] [Google Scholar]
- 210.Gibson D.H., Fitzgeorge R.B., Baskerville A. Antibiotic therapy of experimental airborne Legionnaires disease. J. Infect. 1983;7:210–217. doi: 10.1016/s0163-4453(83)97034-2. [DOI] [PubMed] [Google Scholar]
- 211.Bodro M., Carratala J., Paterson D.L. Legionellosis and biologic therapies. Respir. Med. 2014;108:1223–1228. doi: 10.1016/j.rmed.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 212.Bharatwaj B., Wu L., Whittum-Hudson J.A., da Rocha S.R.P. The potential for the noninvasive delivery of polymeric nanocarriers using propellant-based inhalers in the treatment of Chlamydial respiratory infections. Biomaterials. 2010;31:7376–7385. doi: 10.1016/j.biomaterials.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 213.Pereyre S., Goret J., Bebear C. Mycoplasma pneumoniae: current knowledge on macrolide resistance and treatment. Front. Microbiol. 2016;7:974. doi: 10.3389/fmicb.2016.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lee H., Yun K.W., Lee H.J., Choi E.H. Antimicrobial therapy of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Expert Rev. Anti Infect. Ther. 2018;16:23–34. doi: 10.1080/14787210.2018.1414599. [DOI] [PubMed] [Google Scholar]
- 215.Waites K.B., Xiao L., Liu Y., Balish M.F., Atkinson T.P. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin. Microbiol. Rev. 2017;30:747–809. doi: 10.1128/CMR.00114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Smaldone G.C., Palmer L.B. Aerosolized antibiotics: current and future. Respir. Care. 2000;45:667–675. [PubMed] [Google Scholar]
- 217.Abu-Salah T., Dhand R. Inhaled antibiotic therapy for ventilator-associated tracheobronchitis and ventilator-associated pneumonia: an update. Adv. Ther. 2011;28:728–747. doi: 10.1007/s12325-011-0051-z. [DOI] [PubMed] [Google Scholar]
- 218.Jayaprakash B., Varkey V., Anithakumari K. Etiology and clinical outcome of non-resolving pneumonia in a tertiary care centre. J. Assoc. Phys. India. 2012;60:98–101. [PubMed] [Google Scholar]
- 219.Ramesh P.M., Saravanan M. A clinical study on non-resolving pneumonia in tertiary care centre. Int. J. Adv. Med. 2018;5:604. [Google Scholar]
- 220.Lynch J.P., Sitrin R.G. Noninfectious mimics of community-acquired pneumonia. Semin. Respir. Infect. 1993;8:14–45. [PubMed] [Google Scholar]
- 221.Sarkar S., Saha K., Maji A., Kundu A. Non-resolving pneumonia: a rare presentation of progressive disseminated histoplasmosis. Lung India. 2014;31:73–75. doi: 10.4103/0970-2113.125993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kinkade S., Long N.A. Acute bronchitis. Am. Fam. Physician. 2016;94:560–565. [PubMed] [Google Scholar]
- 223.Smith S.M., Fahey T., Smucny J., Becker L.A. Antibiotics for acute bronchitis. Cochrane Database Syst. Rev. 2014;2014:CD000245. doi: 10.1002/14651858.CD000245.pub3. [DOI] [PubMed] [Google Scholar]
- 224.Shen Y., Huang S., Kang J., Lin J., Lai K., Sun Y., Xiao W., Yang L., Yao W., Cai S., K H., Shen Y., Huang S., Kang J., Lin J., Lai K., Sun Y., Xiao W., Yang L., Yao W., Cai S., Huang K., Wen F. Management of airway mucus hypersecretion in chronic airway inflammatory disease: Chinese expert consensus (English edition) Int. J. COPD. 2018;13:399–407. doi: 10.2147/COPD.S144312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hanson C.W., Mathew G. Vol. 53. Essence Anesth. Pract; 2011. (Chronic Bronchitis). [Google Scholar]
- 226.Widysanto A., Mathew G. StatPearls Publ; 2020. Chronic Bronchitis; p. 1.https://www.ncbi.nlm.nih.gov/books/NBK482437/ [PubMed] [Google Scholar]
- 227.Auwaerter P.G., Bartlett J.G. Johns Hopkins ABX Guid; 2020. Chronic Bronchitis, Acute Exacerbations.https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_ABX_Guide/540124/all/Chronic_Bronchitis__Acute_Exacerbations accessed June 1, 2020) [Google Scholar]
- 228.Agarwal A.K., Raja A., Brown B.D. StatPearls. StatPearls Publishing; 2020. Chronic obstructive pulmonary disease (COPD)http://www.ncbi.nlm.nih.gov/pubmed/32644707 [PubMed] [Google Scholar]
- 229.Ali M.E., McConville J.T., Lamprecht A. Pulmonary delivery of anti-inflammatory agents. Expet Opin. Drug Deliv. 2015;12:929–945. doi: 10.1517/17425247.2015.993968. [DOI] [PubMed] [Google Scholar]
- 230.Siddiqi A., Sethi S. Optimizing antibiotic selection in treating COPD exacerbations. Int. J. COPD. 2008;3:31–44. doi: 10.2147/copd.s1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Labiris N.R., Dolovich M.B. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003;56:588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Lewis A.D., Prongay K. In: Nonhum. Primate Nonclinical Drug Dev. Saf. Assess. Bluemel J., Schenck E., Korte S., Weinbauer G.F., editors. Elsevier; Amsterdam, Netherlands: 2015. Basic physiology of Macaca mulatta; pp. 87–113. [Google Scholar]
- 233.Null D.M., Suresh G.K. In: Assist. Vent. Neonate an Evidence-Based Approach to Newborn Respir. Care. sixth ed. Goldsmith J.P., Karotkin E., Keszler M., editors. Elsevier; 2016. Pulmonary function and graphics; pp. 108–117. e1. [Google Scholar]
- 234.Hsia C.C.W., Hyde D.M., Weibel E.R. Lung structure and the intrinsic challenges of gas exchange. Compr. Physiol. 2016;6:827–895. doi: 10.1002/cphy.c150028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Frohlich E., Mercuri A., Wu S., Salar-Behzadi S. Measurements of deposition, lung surface area and lung fluid for simulation of inhaled compounds. Front. Pharmacol. 2016;7:181. doi: 10.3389/fphar.2016.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Groneberg D.A., Witt C., Wagner U., Chung K.F., Fischer A. Fundamentals of pulmonary drug delivery. Respir. Med. 2003;97:382–387. doi: 10.1053/rmed.2002.1457. [DOI] [PubMed] [Google Scholar]
- 237.Cerrada A., Haller T., Cruz A., Pérez-Gil J. Pneumocytes Assemble Lung Surfactant as highly packed/dehydrated states with optimal surface activity. Biophys. J. 2015;109:2295–2306. doi: 10.1016/j.bpj.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Newman S.P. Drug delivery to the lungs: challenges and opportunities. Ther. Deliv. 2017;8:647–661. doi: 10.4155/tde-2017-0037. [DOI] [PubMed] [Google Scholar]
- 239.Antoniu S.A., Cojocaru I. Inhaled colistin for lower respiratory tract infections. Expet Opin. Drug Deliv. 2012;9:333–342. doi: 10.1517/17425247.2012.660480. [DOI] [PubMed] [Google Scholar]
- 240.Stockmann C., Sherwin C.M.t., Ampofo K., Spigarelli M.G. Development of levofloxacin inhalation solution to treat Pseudomonas aeruginosa in patients with cystic fibrosis. Ther. Adv. Respir. Dis. 2014;8:13–21. doi: 10.1177/1753465813508445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Podder V., Sadiq N.M. In: Kucers Use Antibiot. A Clin. Rev. Antibacterial, Antifung. Antiparasit. Antivir. Drugs. seventh ed. Hooper D.C., editor. CRC Press; 2017. Levofloxacin; pp. 2055–2084. [Google Scholar]
- 242.Thai T., Salisbury B.H., Zito P.M. StatPearls Publishing; Treasure Island: 2021. Ciprofloxacin.http://www.ncbi.nlm.nih.gov/pubmed/30571075 [PubMed] [Google Scholar]
- 243.Conley J., Yang H., Wilson T., Blasetti K., Di Ninno V., Schnell G., Wong J.P. Aerosol delivery of liposome-encapsulated ciprofloxacin: aerosol characterization and efficacy against Francisella tularensis infection in mice. Antimicrob. Agents Chemother. 1997;41:1288–1292. doi: 10.1128/aac.41.6.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.El-Gendy N., Desai V., Berkland C. Agglomerates of ciprofloxacin nanoparticles yield fine dry powder aerosols. J. Pharm. Innov. 2010;5:79–87. [Google Scholar]
- 245.Chorepsima S., Kechagias K.S., Kalimeris G., Triarides N.A., Falagas M.E. Spotlight on inhaled ciprofloxacin and its potential in the treatment of non-cystic fibrosis bronchiectasis. Drug Des. Dev. Ther. 2018;12:4059–4066. doi: 10.2147/DDDT.S168014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.The Boomer Esiason Foundation . Vol. 1. Esiason.Org.; 2019. https://www.esiason.org/aradigm-gets-fda-orphan-drug-designation-for-ciprofloxacin-for-inhalation-in-bronchiectasis/ (Aradigm Gets FDA Orphan Drug Designation for Ciprofloxacin for Inhalation in Bronchiectasis). [Google Scholar]
- 247.Gallagher J.J., Williams-Bouyer N., Villarreal C., Heggers J.P., Herndon D.N. In: Total Burn Care. Third. Herndon D.N., editor. Elsevier; Amsterdam, Netherlands: 2007. Treatment of infection in burns; pp. 136–176. [Google Scholar]
- 248.Ramsey C., MacGowan A.P. A review of the pharmacokinetics and pharmacodynamics of aztreonam. J. Antimicrob. Chemother. 2016;71:2704–2712. doi: 10.1093/jac/dkw231. [DOI] [PubMed] [Google Scholar]
- 249.Kirkby S., Novak K., McCoy K. Aztreonam (for inhalation solution) for the treatment of chronic lung infections in patients with cystic fibrosis: an evidence-based review. Core Evid. 2011;6:59–66. doi: 10.2147/CE.S11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sizar O., Rahman S., Sundareshan V. In: Kucers Use Antibiot. A Clin. Rev. Antibacterial, Antifung. Antiparasit. Antivir. Drugs. seventh ed. Nesbitt W.J., Aronoff D., editors. CRC Press; 2017. Amikacin; pp. 1009–1027. [Google Scholar]
- 251.Torres A., Motos A., Battaglini D., Li Bassi G. Inhaled amikacin for severe gram-negative pulmonary infections in the intensive care unit: current status and future prospects. Crit. Care. 2018;22 doi: 10.1186/s13054-018-1958-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Shirley M. Amikacin liposome inhalation suspension: a review in Mycobacterium avium complex lung disease. Drugs. 2019;79:555–562. doi: 10.1007/s40265-019-01095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Reyhanoglu G. 2020. Tobramycin, STATPEARLS.https://www.statpearls.com/ArticleLibrary/viewarticle/35928 1. [PubMed] [Google Scholar]
- 254.Vendrell M., Muñoz G., de Gracia J. Evidence of inhaled tobramycin in non-cystic fibrosis Bronchiectasis. Open Respir. Med. J. 2015;9:30–36. doi: 10.2174/1874306401509010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tanriverdi E., Yildirim B.Z., Gul S., Ugur Chousein E.G., Turan D., Çınarka H., Özgül M.A., Cetinkaya E. Results of tobramycin inhalation therapy in patients with noncystic fibrosis bronchiectasis with Pseudomonas aeruginosa colonization: real life management. J. Aerosol Med. Pulm. Drug Deliv. 2020 doi: 10.1089/jamp.2020.1606. [DOI] [PubMed] [Google Scholar]
- 256.Gupta S., Govil D., Kakar P., Prakash O., Arora D., Das S., Govil P., Malhotra A. Colistin and polymyxin B: a re-emergence. Indian J. Crit. Care Med. 2009;13:49–53. doi: 10.4103/0972-5229.56048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Choi H.K., Kim Y.K., Kim H.Y., Uh Y. Inhaled colistin for treatment of pneumonia due to colistin-only-susceptible Acinetobacter baumannii. Yonsei Med. J. 2014;55:118–125. doi: 10.3349/ymj.2014.55.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Loustaud-Ratti V., Debette-Gratien M., Jacques J., Alain S., Marquet P., Sautereau D., Rousseau A., Carrier P. Ribavirin: past, present and future. World J. Hepatol. 2016;8:123–130. doi: 10.4254/wjh.v8.i2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Luo Y.H., Huang C.Y., Yang K.Y., Lee Y.C. Inhaled ribavirin therapy in adult respiratory syncytial virus-induced acute respiratory distress syndrome. Arch. Bronconeumol. 2011;47:315–317. doi: 10.1016/j.arbres.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 260.Bausch Health Americas Inc . ClinicalTrials.Gos; 2020. Study to evaluate the safety and efficacy of VIRAZOLE® in hospitalized adult participants with respiratory distress due to COVID-19; p. 1.https://clinicaltrials.gov/ct2/show/NCT04356677 [Google Scholar]
- 261.Colman P.M. Zanamivir: an influenza virus neuraminidase inhibitor. Expert Rev. Anti Infect. Ther. 2005;3:191–199. doi: 10.1586/14787210.3.2.191. [DOI] [PubMed] [Google Scholar]
- 262.Shie J.J., Fang J.M. Development of effective anti-influenza drugs: congeners and conjugates. J. Biomed. Sci. 2019;26:1–20. doi: 10.1186/s12929-019-0567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ostrosky-Zeichner L., Rex J.H. In: Crit. Care Med. Princ. Diagnosis Manag. Adult, Third. Parrillo J.E., Dellinger R.P., editors. Elsevier; Amsterdam, Netherlands: 2008. Antifungal and antiviral therapy; pp. 1089–1109. [Google Scholar]
- 264.Debnath S.K., Srivastava R., Debnath M., Omri A. Status of inhalable antimicrobial agents for lung infection: progress and prospects. Expet Rev. Respir. Med. 2021;44:1–20. doi: 10.1080/17476348.2021.1919514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.