Abstract
Background:
Puerto Rico began screening blood donations for Zika virus (ZIKV) RNA using nucleic acid amplification technology (NAT) on April 3, 2016. Follow-up of positive donors was used to assess viral and serologic marker dynamics through the early stages of ZIKV infection and estimate incidence in the Puerto Rico population.
Methods:
Individual donations from volunteer blood donors were screened for the presence of ZIKV using cobas® Zika nucleic acid testing (NAT). Positive samples were further tested to confirm infection, estimate viral load, and identify ZIKV-specific antibodies by IgM antibody capture ZIKV ELISA. Donations positive in simulated minipools were compared to those detectable only by individual donation NAT with respect to viral load and ZIKV IgM status to evaluate the infection stage. A three step analysis process was used to estimate the mean duration of NAT-reactivity of ZIKV in human plasma and to update the 2016 annual incidence estimate of ZIKV infections.
Findings:
52,942 blood donations in Puerto Rico were screened for ZIKV and 339 confirmed NAT positive (NAT+) donations were detected. IgM negative index donations had markedly higher mean viral loads (1·1 × 106 vs 8·3 × 104 IU/ml) and higher proportions (194 of 209 [93%] IgM negative/Minipool positive versus 29 of 110 [26%] IgM positive/minipool positive) of simulated minipool positive results. The rate of donations detectible only by individual donation NAT and that were IgM+ increased as the epidemic evolved. The mean duration of NAT-detectability was estimated at 11.70 (95% CI 10.06 – 14.36) days. Applying this detection period to the observed rate of NAT+ donations yielded a ZIKV seasonal incidence estimate of 768,101 infections in a population of 3,638,773 in 2016 [21.1% (95% CI 18.1% – 24.1%)].
Interpretation:
Characterization of early ZIKV infection is important for blood safety, since infectivity and utility of screening method likely correlate with viral load and serological stages of infection. Our findings also have important implications for diagnostic testing, public health surveillance and epidemiology, including estimating that ~21% of the PR population was infected during the 2016 outbreak.
Funding:
This project has been funded with Federal funds from the Biomedical Advanced Research and Development Authority, Office of the Assistant Secretary for Preparedness and Response, under Contract #HHSO100201600010C, and by NHLBI REDS-III Central Lab Contract #HHSN268201100001I.
INTRODUCTION
Zika virus (ZIKV), a mosquito-borne flavivirus predominantly transmitted through the bite of Aedes mosquitos1, has been unequivocally associated with Guillain-Barre Syndrome and fetal brain abnormalities, including severe microcephaly, if a pregnant woman is infected and congenital transmission occurs1–6. The first confirmed cases of ZIKV infection in the Americas were reported in Brazil in 2014, and ZIKV rapidly spread through the Americas and into the Caribbean in mid-2015 and 20167,8. The first autochthonous cases in the US territory of Puerto Rico were reported in late 20159. As a result of FDA guidance10, blood donations collected in Puerto Rico were screened for Zika virus (ZIKV) RNA using cobas® Zika nucleic acid testing (NAT) beginning on April 3, 201611. ZIKV RNA was immediately detected in blood donors11 and 339 confirmed infected donations (of 52,942 total) were detected through December 31, 2016 at Creative Testing Solutions (CTS).
Follow-up studies of NAT positive (NAT+) blood donors progressing through early stages of ZIKV infection enable characterization of viral and immune dynamics and estimation of the distribution of the durations of NAT-detectability in blood compartments and body fluids12 which can be employed for estimation of ZIKV infection incidence in blood donor and general populations13. Categorization of index samples into stages of infection is important for blood safety considerations, since infectivity and utility of minipool (MP) versus individual donation (ID) NAT screening likely correlate with viral load and serological stage of infection. Staging of index infections also has important implications for diagnostic testing and understanding the durations of ZIKV viral and immunological markers in blood and persistence of ZIKV in body fluids and tissues. This study: 1) describes how the viral and immunological profiles of ZIKV infection in the index NAT+ donations evolved through the course of the 2016 Puerto Rican epidemic, 2) estimates the distribution of the duration of NAT-reactivity in plasma 3), estimates the duration from NAT-reactivity to IgM seroconversion, and 4) refines a previous estimate for the seasonal incidence of ZIKV infection in Puerto Rico13 based on the updated NAT-reactivity duration estimates and NAT yield data from the complete 2016 epidemic.
METHODS
Blood donor screening for ZIKV RNA.
This study is limited to donations screened at CTS between April 3, 2016 and December 31, 2016. Appropriate protections for the use of human subjects in research were received prior to the start of NAT screening in Puerto Rico and analyses. Routine informed consent for blood donation, as well as specific information related to Zika testing and participation in the clinical study was provided to every donor prior to donation. Donations from volunteer blood donors from Puerto Rico were screened at CTS for ZIKV RNA by individual donation NAT using the Roche cobas® Zika investigational assay (subsequently licensed by FDA) which was initiated on April 3, 2016 based on FDA requirements10. Individual donation NAT positive (ID-NAT+) samples were further tested to confirm infection, estimate viral load (reported in international units [IU]/mL) by quantitative PCR, and identify ZIKV-specific antibodies by IgM antibody capture (MAC) ELISA using previously reported assays performed at Vitalant Research Institute and a confirmatory testing algorithm11,14. Simulated minipools were prepared by diluting individual donation NAT+ plasma 1:6 in negative plasma and tested by the cobas® Zika test to discriminate individual donation NAT-only positive (ID-NAT-only+) from minipool detectable (MP+) donations. Infected donors who provided informed consent were enrolled into a follow-up study that included ZIKV individual donation NAT and serological assay testing that included ZIKA reporter viral particle neutralization titration (RVP-NT) from two follow-up visits, one within 2 weeks and a second between 2 and 8 weeks after the initial cobas® Zika NAT positive donation; these data were used to further confirm infection and assess the persistence of viral and serologic markers through the early stages of ZIKV infection. For the purposes of this study, a NAT positive index donation was considered to be confirmed NAT+ based on detection of ZIKV RNA and/or ZIKV IgM antibodies on additional testing of index and/or follow-up samples11.
Staging of ZIKV RNA+ donations.
Complete confirmatory test results were obtained for 319 confirmed ZIKV individual donation NAT+ index donations, which included conclusive ZIKV viral load, simulated minipool NAT, and ZIKV IgM results. Individual donation NAT confirmed positive index donations were classified into stages based on index donation serology, viral load, and simulated minipool NAT results using the approach reported by Simmons et al. for chikungunya virus infected blood donations in Puerto Rico15; this classification was applied over the course of the entire study period (April 3-December 31, 2016). Staging reflects the relative timing of index donation during the infection process, with sequential stages defined by the criteria ID-NAT-only+/IgM-negative (IgM−), MP+/IgM−, MP+/IgM+, and ID-NAT-only+/IgM+.
Estimation of individual donation-NAT detection period and pre-IgM seroconversion window period.
Longitudinal results for 140 donors who were NAT+ at index donations were obtained, including viral load, IgM and IgG results on index donations, and individual donation NAT-reactivity and IgM results at 1–3 follow-up time-points. Our estimation methods for imputing time from initial plasma RNA positivity required that the donors were in the virus ramp-up phase at the time of donation to ensure that viral replication had not been suppressed by the presence of adaptive immune responses. Of the 140 donors with follow-up, 90 were IgM negative. Among these 40 donors had just one follow-up, 49 had two, and a single donor had three follow-up samples. Follow-up time-point 1 ranged from 5–70 days post-index (DPI), while follow-up time-point 2 ranged from 21–66 DPI, and the donor providing a 3rd follow-up sample at was at 46 DPI. The duration from initial NAT detectability to loss of individual donation NAT reactivity and IgM seroconversion are subject to left censoring, since donors were identified as NAT+ at enrollment. Using the time of index donation as the time of initiation of NAT detectability would bias estimates negatively for these durations, i.e., they would be underestimated. Doubling time data to estimate NAT detection pre-NAT infectious window periods has been a foundation of incidence and risk analyses in studies of other transfusion-transmitted viral infections, including HIV, HBV, HCV and WNV16–22. To estimate these distributions without this bias, an estimate of the ZIKV doubling time (ZDT) during the pre-IgM phase of replication was computed using experimental data from ZIKV infection studies in macaques, and then this ZDT estimate of 5·35 (SD 0·36) hours was used with measurements of ZIKV viral load on index donations to impute or back-calculate the time of initial NAT-reactivity for each donor detected as NAT+ prior to IgM seroconversion. The ZDT was computed using a mixed effects linear regression model for the log-viral loads as a linear function of time after infection, for 3–4 days, up to when viral replication was suppressed by immune responses and viral loads declined. Further details of this modeling component may be found in the supplemental materials. With an estimate of the ZDT, the viral load at time t after infection can be expressed as V(t) = V(0) 2 t/ZDT, with V(0) the “initial” (t = 0) positive viral load by the screening NAT assay. From this expression, for each donor i = 1, 2, …, n, the observed V(ti) yields the imputed value for time of initial NAT reactivity, given by ti = ZDT [log2 V(ti) – log2 V(0)]. In our computations, we assumed that V(0) was 7·85 IU/mL (95% CI 6·21 – 9·92) for donations that tested positive upon NAT screening, and confirmed as NAT+, but were below the limit of quantitation of the ZIKV viral load assay (7/90 [8%]); this viral load estimate is the geometric mean of the 50% LOD estimates for the Roche cobas® Zika assay (3·9 [95% CI 2·8 – 5·3] IU/mL) and the confirmatory PCR viral load assay (15·8 [95% CI 11·2 – 22·2] IU/mL) based on a Poisson analysis of half-log serial dilutions of a PF13/251013–18 culture supernatant, tested in 25 replicates per dilution (Bakkour, unpublished data). Titers of the culture supernatant were calibrated to the WHO standard. The confirmatory PCR assay reported viral load in IU/ml, wherein 1 IU/ml corresponds to 0·9 genome copies/mL, or 1.0 × 10−3 PFU/ml, based on quantification of a ZIKV culture supernatant stock by PCR using a genomic standard (Zika standard #1, strain H/PF/2013, Aix Marseille Université, European Virus Archive goes Global) and the WHO 1st International Standard (Paul-Ehrlich-Institut code 11468/16), and by viral plaque assay in Vero cells.
To estimate the distributions for the duration of individual donation NAT detectability and the duration from initial individual donation NAT reactivity to IgM seroconversion, the imputed values ti for the time of initial NAT reactivity for IgM− NAT+ donations were combined with the donors’ longitudinal follow-up NAT and IgM testing results to characterize the timing of loss of NAT reactivity and IgM seroconversion as either interval or right-censored observations. These were then used in a standard survival analysis modeling framework, where both Weibull and log-normal models were fit using maximum likelihood. Model selection was based on Akaike’s information criterion (AIC). Bootstrap resampling was used to characterize uncertainty in the estimates computed in each of the modeling steps, with bootstrap samples for appropriate estimates carried to subsequent steps in the analyses. For the survival modeling step, the model with the lower AIC between the Weibull and the lognormal over more of the bootstrap replications was selected for final inference and reporting. Estimates of model parameters for each bootstrap sample were used to compute mean and quantile values for the distributions, and corresponding 95% bootstrap CIs were computed. Explicit details of this modeling are available in the supplementary materials (appendix p. 3).
Estimation of incidence in Puerto Rico.
Estimation of the total number of ZIKV-infected individuals in Puerto Rico in 2016 was based on the methodology of Chevalier et al.13 with the following changes (appendix, p. 3–5): First, the use of bootstrapping to resample the dates of donations was incorporated to reflect a view of the present modeling effort as one modeling the underlying population transmission process, as in Biggerstaff and Petersen23 for West Nile Virus, thereby reflecting uncertainty in random presentation of donors during the course of acute infection prior to seroconversion and donation/participation in the follow-up study. Second, we use our updated estimates for the duration of NAT-reactivity computed above, rather than the previously assumed analogous value derived from a systematic review estimate of the time to viral clearance based on doubly censored data24. To account for the bootstrap uncertainty, the uncertainty in estimation of the ZDT, the uncertainty in estimation of V(0), the uncertainty in estimating the duration of NAT-reactivity, and the uncertainty in estimating the duration from NAT-detectability to IgM seroconversion, for each of the bootstrap samples of the donor results, we used one of the sampled values of the modeled mean durations to compute an estimate of the number of ZIKV infections. Because donors will self-defer due to symptoms or be deferred prior to donation due to fever, and symptom onset generally occurs following IgM seroconversion, we sorted NAT+ donations based on the IgM positivity status at index donation; this was used to weight NAT+, IgM− or IgM+ donations differentially to reflect their relative likelihood of detection and subsequent estimates for durations from initial NAT reactivity to IgM seroconversion and from IgM seroconversion to NAT non-detectability, respectively. See the supplementary materials (appendix p. 3–4) for details of these weighting steps and derivation of estimated number of ZIKV infection in the Puerto Rican population including adjusting for demographics (health region of residence, age group, and sex, as in Chevalier et al.13) of blood donors relative to the overall adult population, data for which were obtained from the US Census for 2014 to allow for direct comparison with the analysis in Chevalier et al.13.
We next averaged the resulting estimated number of ZIKV infections to produce the final estimate, using the 2·5th and 97·5th percentiles of these values as the 95% CI for the population number of ZIKV infections. Finally, we divided the estimated number of ZIKV infections (and 95% CI) by the population size of Puerto Rico to give the estimate of the incidence of ZIKV infections for all of 2016. Other parameter specifications for the resampling estimation remained the same as in Chevalier et al.13.
Role of the Funding Source.
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Screening and Confirmatory Test Results for Puerto Rico Blood Donations.
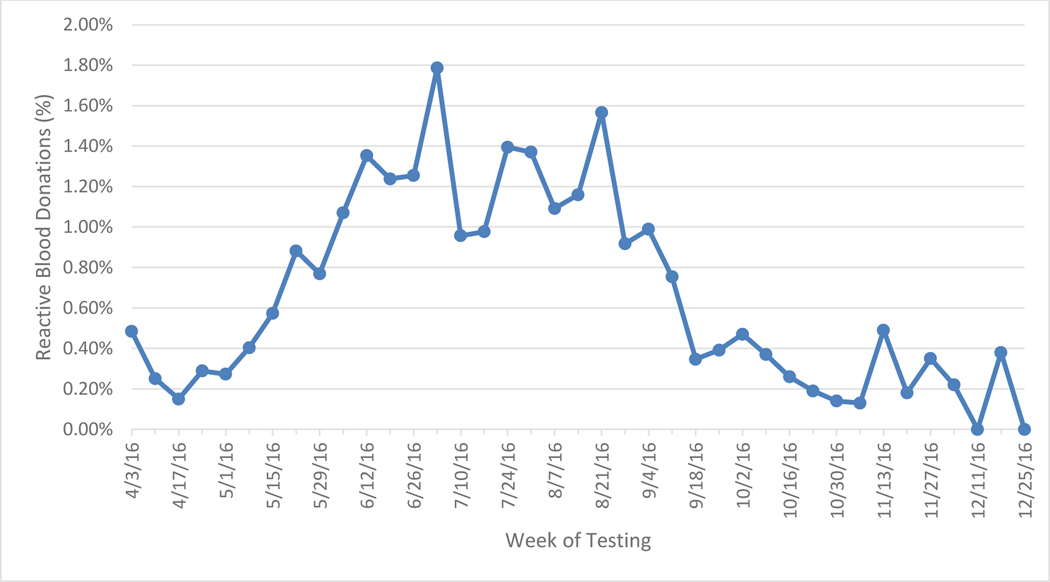
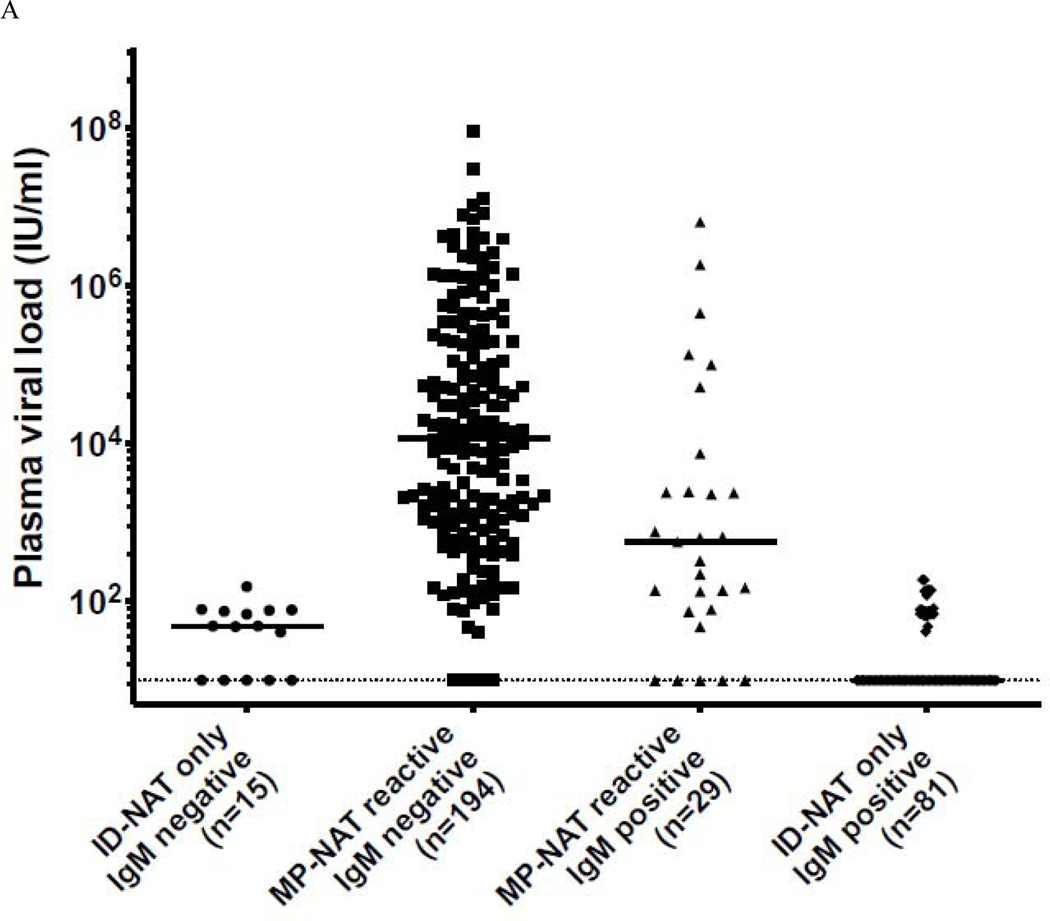
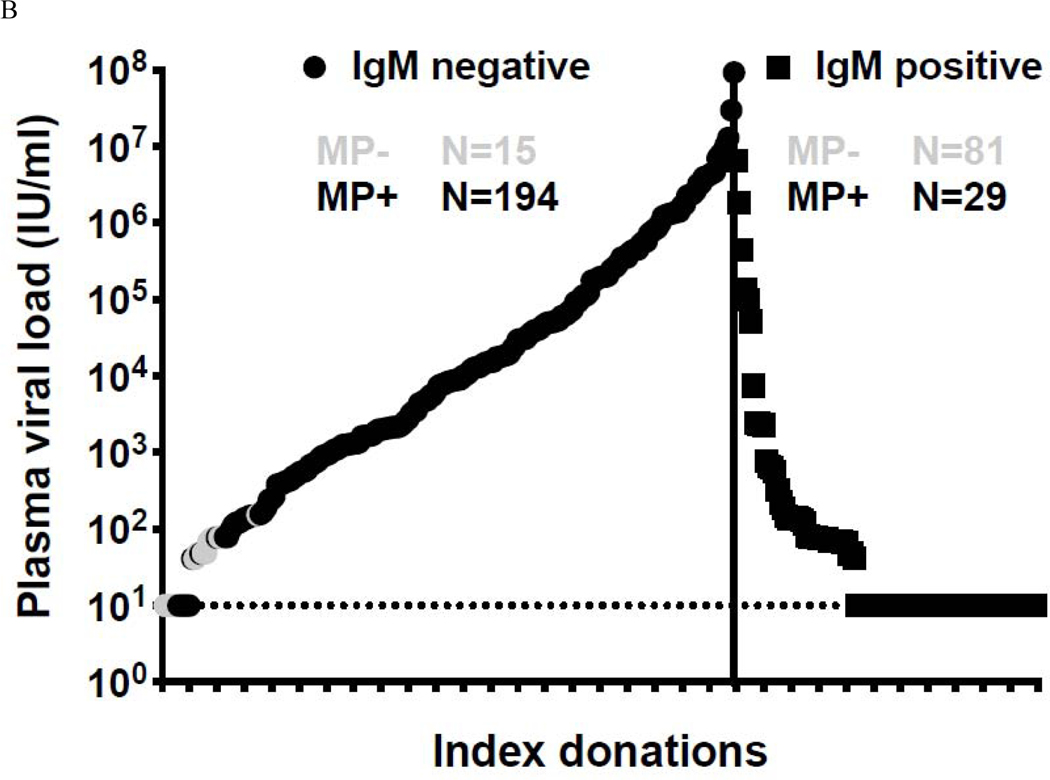
Between April 3 and December 31, 2016, 53,112 individual blood donation plasma samples from volunteer donors in Puerto Rico were screened for the presence of ZIKV RNA using the Roche cobas® Zika investigational assay at the CTS laboratory in St. Petersburg, Florida and identified 351 initially positive donations, of which 339 (96·6%) confirmed as positive. ZIKV screening with the Roche cobas® Zika NAT investigational assay was authorized under an Investigational New Drug (IND) Application. The overall frequency of confirmed ZIKV infection during the study period was 1:156, with a peak of 1·8% (19/1064) in the first week of July 2016 (Figure 1). Of the confirmed RNA-positive index donations, 319 (94·1%) demonstrated conclusive IgM results (20 serum samples were either inconclusive or no IgM data was generated due to the lack of a serum sample) and had simulated minipool data. Of these, 110 were conclusively ZIKV IgM-positive and 209 were seronegative (Figure 2 A&B). The majority of infected donations detected during the initial 6 months of screening had high viral loads and tested seronegative. As ZIKV IgM neutralizing antibodies develop, viral loads drop resulting in a characteristic distribution of viral loads over the course of the epidemic, similar to other arboviral infections 15. The majority of low viral load donations were IgM positive, indicative of recently acquired but resolving acute infections. Between October and December 2016 as the number of NAT+ waned, NAT+ donations tested IgM+ and viral loads were very low as the number of NAT+ positive donations waned, with the exception of a few outliers.
Figure 1.
Weekly Detection Rate of ZIKV RNA in Blood Donated in Puerto Rico between April 3 and December 31, 2016 and tested at CTS, n = 351.
Figure 2.
Panel A. Classification of MP+ and ID only + ZIKV NAT yield cases samples relative to IgM status between April 3 and December 31. Panel B. Index donations ordered by viral load, IgM status and mini pool status.
Black circles = IgM negative, minipool positive; Gray circles = IgM negative, ID-NAT only positive; Black Squares = IgM positive, minipool positive; Gray Squares IgM positive, ID-NAT only positive Figure only includes index donations with conclusive IgM and simulated minipool results n=319
Staging of Infected Donations through the Course of 2016 Puerto Rico Outbreak.
Analysis of viral load and IgM status in confirmed infected index donations showed a wide range of viral loads in the IgM− and IgM+ stages of acute infection (Figure 2A), demonstrating that donors present throughout acute infection. Donations without detectable anti-Zika IgM are assumed to represent donors detected in the acute ramp-up phase of viremia, whereas those with ZIKV IgM are likely in the resolution stage with declining viral load attributable to immune suppression of replication and viral clearance (Figure 2 A&B). Index donations that were IgM− had markedly higher mean viral loads (1·1 × 106 vs 8·3 × 104 IU/ml) and higher proportions of simulated MP+ results than IgM+ donations (194 of 209 [93%] IgM−/MP+ versus 29 of 110 [26%] IgM+/MP+) (categorization based on ZIKV IgG was not possible due to high levels of preexisting DENV antibody cross reactivity). The changing distribution of stages of infection over the course of the epidemic is evidenced by the shifting proportion of RNA positive donations that tested IgM seronegative to those observed that were serologically reactive. As the epidemic grew in magnitude, the rates of individual donation NAT-only detectible and of IgM+ donations increased significantly (Table 1), and a wider distribution of viral loads in the acutely infected population was observed. In the early period of testing (April-June), 75 of 110 (68·2%) of the NAT+ donations were IgM seronegative. Although the proportion of seronegative donors in July (41 of 57) did not change substantially from June (48 of 67), the number of individual donation NAT-only positive samples increased. NAT+ donations peaked in August, corresponding with the beginning of the shift in proportion of individual donation NAT-only to IgM+ donations (by September the ratio was 1:1).
Table 1.
Classification of ZIKV NAT yield samples relative to NAT detectability status (minipool or individual donation), Zika IgM status at index (positive or negative) and total/percent by month for the 2016.
| IgM− | IgM+ | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MP− | MP+ | MP− | MP+ | Total | ID-NAT only | % ID NAT-only | IgM reactive | % IgM reactive | |
| Apr | 0 | 10 | 1 | 4 | 15 | 1 | 6.7 | 5 | 33.3 |
| May | 1 | 16 | 6 | 5 | 28 | 7 | 25.0 | 11 | 39.3 |
| Jun | 4 | 44 | 12 | 7 | 67 | 16 | 23.9 | l9 | 28.4 |
| Jul | 4 | 40 | 16 | 1 | 61 | 20 | 32.8 | 17 | 27.9 |
| Aug | 4 | 53 | 14 | 4 | 75 | 18 | 24.0 | 18 | 24.0 |
| Sep | 0 | 16 | 15 | 3 | 34 | 15 | 44.1 | 18 | 52.9 |
| Oct | 2 | 5 | 7 | 2 | 16 | 9 | 56.3 | 9 | 56.3 |
| Nov | 0 | 9 | 6 | 0 | 15 | 6 | 40.0 | 6 | 40.0 |
| Dec | 0 | 1 | 4 | 3 | 8 | 4 | 50.0 | 7 | 87.5 |
|
| |||||||||
| Total | 15 | 194 | 81 | 29 | 319 | 96 | 110 | ||
Duration of ID-NAT-reactivity.
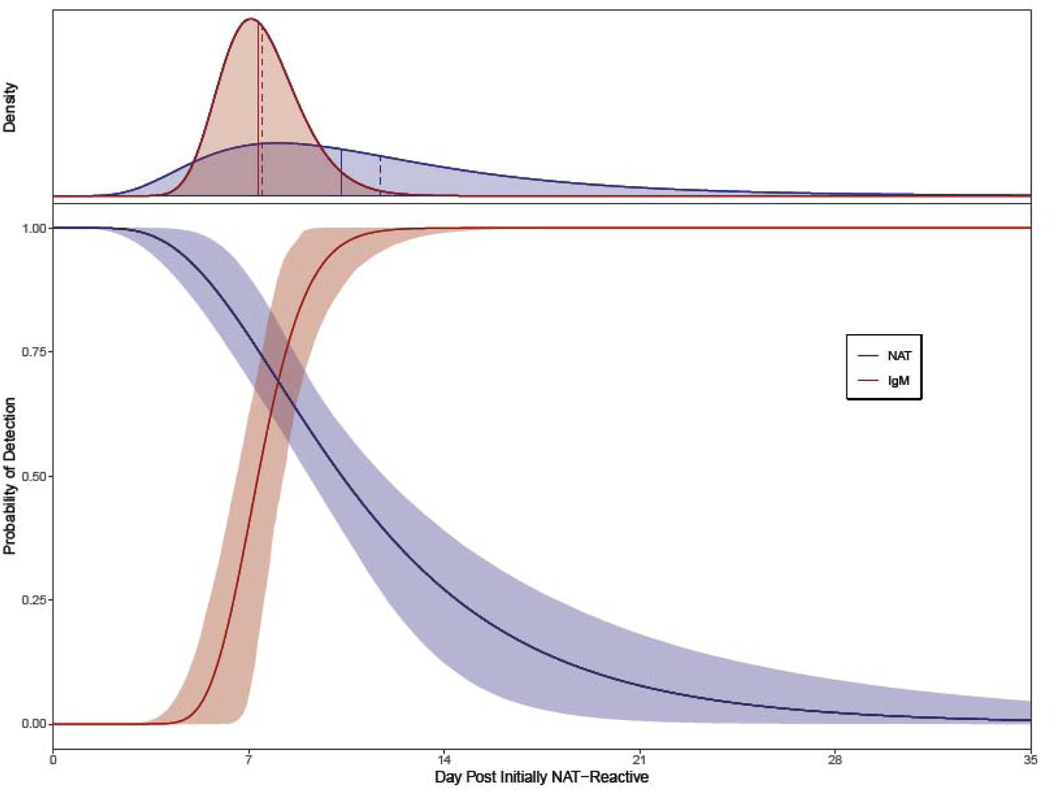
Of the 10,000 bootstrap simulations, the log-normal distribution outperformed the Weibull for the majority of the simulations, so we present only with the log-normal distribution. Estimates of means (over the simulations) of the log-normal parameters were log-scale mean = 2·34 and log-scale standard deviation = 0·50. The median duration of individual donation NAT-reactivity was estimated as 10·33 (95% CI 9·16 – 11·84) days and the 5th and 95th percentiles of the durations were 4·55 and 23·47 days, respectively. The mean duration of individual donation NAT-reactivity was 11·70 (95% CI 10·06 – 14·36) days.
Duration from initial NAT-detectability to IgM-positivity.
As with the NAT-reactivity duration analysis, in a majority of the simulations, the log-normal distribution outperformed the Weibull. Estimates of means (over the simulations) of the log-normal parameters were log-scale mean = 2·00 and log-scale standard deviation = 0·19. The estimated median duration from initial NAT-reactivity to IgM-positivity was 7·42 (95% CI 6·59 – 8·29) days, and the 5th and 95th percentiles of this duration were 5·39 and 10·21 days, respectively. The estimate of the mean duration from initial NAT-reactivity to IgM-positivity was 7·56 (95% CI 6·74 – 8·39) days.
The results of this and the previous section are summarized in Figure 3, where the estimates of the distributions of both the duration of NAT-reactivity and the duration from initial NAT-reactivity to IgM-positivity are displayed. These are plotted as probability of detection curves versus time post imputed initial NAT-reactivity (with 95% CI bands), with NAT-detectability (blue) decreasing over time and IgM-reactivity (red) increasing over time. To facilitate interpretation of the distributional shapes for these characteristics, the top panel of the figure shows these distributional estimates as densities. Marked as vertical line segments in the densities are the medians (solid) and means (dashed) for the two distributions.
Figure 3.
Fitted probabilities (lower panel) of detection curves over time for the duration of NAT-reactivity (blue) and of the duration from initial NAT-reactivity to IgM-positivity (red).
95% confidence bands shown shaded in corresponding colors. The top panel reproduces these distributional estimates as densities to facilitate interpretation of the distributional shapes for these characteristics. Marked as vertical line segments in the densities, are the medians (solid) and means (dashed) for the two distributions.
Estimation of ZIKV incidence in Puerto Rico in 2016.
NAT yield data were transformed into incidence for Puerto Rico utilizing the empirically based refined estimates of the pre-seroconversion NAT reactivity and IgM seroconversion durations and the methods of Chevalier et al.13, as described. Assuming that blood donors stratified by health region, age group, and sex are representative of the total population of Puerto Rico with respect to risk of ZIKV infection13, and applying the results detailed here, we estimated the number of individuals infected during the 2016 Zika epidemic in Puerto Rico as 768,101 (95% CI 659,298 – 878,702). This gives an estimate of the cumulative incidence for April-December in Puerto Rico of 21·1% (95% CI 18·1% - 24·1%), using a value for the population of 3,638,77325.
Discussion
This study presents the results of ZIKV screening of blood donors in Puerto Rico shortly after the initial outbreak began in 2016 and characterizes how the viral and immunological profiles of asymptomatic ZIKV RNA positive blood donors shifted through the course of the 2016 Puerto Rican epidemic. Categorization of index samples into stages of infection based on viral load (i.e., detectability by individual donation or minipool NAT) and immunologic response (IgM) is important relative for blood safety considerations, such as blood component infectivity by transfusion and utility of minipool NAT versus individual donation NAT screening26, and for understanding the durations of ZIKV viral and immunological markers in blood and persistence of ZIKV in body fluids and tissues relevant to ZIKV diagnosis and monitoring of pregnant women and travelers.
We separated the NAT-positive donors into pre- and post-IgM seroconversion groups and estimated the numbers of ZIKV infected individuals in the population for these categories using different NAT-detectability weights based on the assumption that ZIKV-infected individuals who became symptomatic had their symptom onset coincident with IgM seroconversion. The estimated mean durations from initial NAT-detectability to the loss of NAT-detectability, and from initial NAT-detectability to IgM seroconversion were used to refine the estimation approach in Chevalier et al.13, in which duration of RNA detection by NAT assays was unknown and so an estimate of viral clearance reported in a systematic literature review was used24. Using our study derived estimates for the ZIKV RNA detection period, duration to IgM seroconversion, and data on the yield of individual donation NAT-positive donations through the course of the 2016 epidemic, and then adjusting for representativeness of blood donors relative to the general population, we were able to estimate the number (768,101 [95% CI 659,298 – 878,702]) and proportion (21·1% [95% CI 18·1% - 24·1%]) of ZIKV infections in Puerto Rico in 2016. This estimate for the seasonal incidence of ZIKV during the 2016 Puerto Rican epidemic is consistent with results of a pre/post-epidemic serosurvey study from our group which demonstrated that 22·8% of the Puerto Rico donor population seroconverted to ZIKV IgG following the 2016 outbreak (manuscript in development), as well as other estimates of 2016 seasonal incidence in Puerto Rico by the CDC13,27.
Our results indicate that a sizable proportion (339/52,942[0·64%] over the 9 month period) of blood donors had detectable ZIKV RNA during the 2016 epidemic in Puerto Rico. High viral load donations which were IgM negative were derived from donors in the early stage of acute infection. These donations likely represent the greatest risk of transfusion-transmission, given absence of potentially neutralizing antibodies; further, this was the profile of donations previously implicated in transfusion-transmission of ZIKV and in transfusion transmission of other arboviruses including WNV21,28 and DENV. Based on the smooth cumulative viral load distribution curve prior to IgM detection versus the abrupt drop in viral load post IgM seroconversion (Figure 2), the infections in the pre-seroconversion stage were likely detected without a bias attributable to deferral of symptomatic potential donors. This highlights the lack of efficacy of donor questions for recent symptoms in interdicting the highest risk blood donations. Of note, no cases of transfusion transmission were documented in Puerto Rico in the context of this large outbreak following implementation of NAT screening.
Paz-Bailey, et al.29 recently estimated the length of RNA detectability in serum by the CDC Trioplex assay from symptom onset to loss of RNA detection as 15·3 days (95% CI, 13·7 – 16·9), whereas we estimated the RNA detectable window of the Roche cobas® Zika NAT assay performed on individual donations from asymptomatic blood donors to be 11·70 (95% CI 10·06 – 14·36) days. This difference could be due to differences in viral dynamics between symptomatic and asymptomatic ZIKV cases. Higher viral loads in ZIKV RNA+ blood donors who developed ZIKV symptoms relative to asymptomatic donors were documented in a study from Martinique reported by Gallian et al.30. Hence, the difference between the NAT detectability measured from symptom onset to loss of RNA detectability in Paz-Bailey et al. versus those imputed from initial NAT detectability to loss of RNA detectability in this study may result from viral load differences between symptomatic and asymptomatic ZIKA cases. Irrespective, we believe our estimate of duration of plasma RNA detection based on asymptomatic blood donors is appropriate to use for estimation of seasonal incidence based on rates of detection of NAT+ donations over time. If we had used the longer estimate by Paz-Bailey et al. we would have estimated a lower number of infections and lower seasonal incidence during the 2016 Puerto Rico outbreak, which is inconsistent with our serosurvey.
We also estimated the mean duration from initial NAT reactivity to IgM positivity, which was 7·56 days (95% CI 6·74 – 8·39). This is comparable with the estimate of IgM positivity by Lesser et al.24 of 9.1 days, as well as the incubation period (defined as time from probable date of mosquito-mediated ZIKV infection to symptom onset) of 6·4 days (95% CI 5·7 – 7·0) from Krow-Lucal et al.31, who compiled data from travelers to Zika endemic areas who developed symptomatic infections after returning to the US. This supports the inference that onset of symptoms coincides with development of detectable humoral immune responses. The issue of whether IgM is detectable concurrent with decline of RNA is important to the question of whether control of ZIKV replication precedes or is mediated by adaptive immune responses, as opposed to being partially controlled by innate or intrinsic immune responses as reported by Busch et al.21 and Tobler et al.32 for WNV, or to limited availability of target cells to support robust viral replication33. Our findings lend support to the conclusion that ZIKV IgM seroconversion occurs very soon after RNA peaks and hence adaptive immunity likely contributes substantially to suppression of viral replication and clearance of ZIKV from plasma.
Large epidemics of ZIKV struck Central and South America countries in 2015 and the Caribbean Islands in 2016. As the 2016 epidemic in Puerto Rico developed, proportions of ZIKV RNA positive blood donors peaked in early July (Figure 1). Over 36,000 symptomatic cases were reported in Puerto Rico in 2016 (https://www.cdc.gov/zika/reporting/2016-case-counts.html) demonstrating sustained levels of ZIKV infection in the general population. Our estimate that 21·1% of the Puerto Rican population experienced ZIKV infection during the 2016 epidemic supports this and is similar to other recent estimates from the CDC and Puerto Rico territorial public health authorities13,27. This is a substantially smaller outbreak when compared to outbreaks in Pacific Islands (estimated at 70% in Yap and Tahiti) and focal outbreaks in French Caribbean islands (>50% infection rates) in terms of the percentage of the population affected. Both Mitchell et al.34 and Gallian et al.30 note high numbers of donors that are asymptomatic in relation to those reporting symptoms. Given the magnitude of symptomatic cases reported during the 2016 epidemic relative to the estimated seasonal incidence of ZIKA infections in the Puerto Rico population reported in this study, only a modest proportion of infections result in reported clinical cases. Furthermore the estimate that 21% of the Puerto Rican population was infected in the 2016 indicates that many susceptible individuals remain in the population, implying a high potential for recurrent ZIKV outbreaks in future years.
Several potential limitations exist with modeling of the NAT detection period and the extrapolation to estimate infection incidence in the Puerto Rican population. We used RNA doubling times from ZIKV infection studies in macaques to estimate the NAT detectable infection dates for donors identified in the pre-IgM ramp-up phase of viral replication, which may not fully reflect the dynamics of viral replication following human autochthonous infections. Since the seronegative ramp-up stage of infection is a relatively small fraction of time from infection to clearance of the virus, any differences between doubling times in humans and macaques would minimally impact the NAT detection period estimates and associated confidence bounds. Additionally, it is unknown whether there is a difference in the ZIKV NAT detection period or the time to ZIKV IgM seroconversion between asymptomatic blood donors evaluated in our study and non-blood donors, including children and pregnant women. Such a difference could mean our estimates for general population incidence are biased, but the direction of such a bias is unknown and the likely impact on the general population incidence projection is likely small. Plaque reduction neutralization tests (PRNT) were performed on IgM negative cases, but due to IND stipulations and resource limitations, only on donors that had follow-up samples. The data showed that the majority had no ZIKV-specific PRNT reactivity at the time of an IgM negative index donation. For donations that did exhibit reactivity (12 out of 51), the ZIKV PRNT reactivity was most likely attributable to dengue cross-reactivity. From historical information, we know that >90% of the PR population has been exposed to dengue. Therefore, guidelines from the ELISA manufacturer and CDC suggest PRNT is not currently routinely recommended for confirmation of Zika MAC-ELISA results in Puerto Rico due to the difficulty in determining the specific flavivirus causing the recent infection in patients with a history of flavivirus infection.
It is important to estimate risk of collecting blood from a donor in the pre-NAT potentially infectious window period (WP) and to assess the increase in risk relative to moving from individual donation to minipool NAT35,36. Those calculations are based not only on the doubling time and sensitivity of the NAT assays in individual donation or minipool contexts, but also on the minimal infectious dose during different stages of ZIKV infection. These questions are now being addressed through escalating dose inoculation studies in macaques and mice using plasma from donors in the pre- and post-seroconversion stages of ZIKV infection.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study.
Data available on viral load dynamics during acute Zika virus infection and on the time to production IgM, IgG and neutralizing antibodies as the initial infection progresses were limited due to the rapid spread of Zika virus throughout the western hemisphere. To assess available information in the contemporary literature during manuscript preparation and prior to finalizing the submission, we searched PubMed and Medline for all publications with “Zika virus”, “infection”, “blood donors”, and “dynamics”. We found 20 documents describing the presence of viremia in blood donors (6), in bodily fluids (4), individual case reports (2), blood donor management strategies (2) and descriptions of Zika epidemics outside the US (3). Limited data (3) on Puerto Rico was available and the early nature of reporting during the epidemic limited the ability to draw significant conclusions. No publication included a detailed description of the viral and serologic progression of acute Zika infection from the estimated date of infectious exposure.
Added value of this study.
Comprehensive blood donor screening data from blood donors who were positive for the presence of Zika viral RNA at the time of donation yet were asymptomatic, and collected as the Zika epidemic spread through the Caribbean and into the US, were used to estimate the window of RNA detectability and the time to production of IgM antibodies over the course of acute infection. The imputation of time of NAT-detectability with arboviruses while differentially weighting by symptomatic and asymptomatic rates for incidence estimation are novel, providing an improvement of current estimation methods.
Implications of all the available evidence.
Our findings have important implications for diagnostic testing, public health surveillance, the safety of blood products and tissues, and public health planning. It is important to estimate risk of collecting blood from a donor in the asymptomatic and potentially infectious window period and to assess the increase in risk relative to testing strategies for ensuring safety of blood products. Furthermore, we demonstrate that although the ZIKV epidemic in 2016 was substantial, a large portion (~79%) of the Puerto Rican population remains susceptible to ZIKV, implying potential for recurrent ZIKV outbreaks in the future.
Acknowledgements:
The authors wish to give special thanks to the donors who contributed so much to this work, Dr. Matt Kuehnert and Dr. Cheryl Banez Ocfemia, Centers for Disease Control and Prevention, Dr David O’Connor from the University of Wisconsin Primate Center, for providing valuable macaque data, and the staff at Creative Testing Solutions and Vitalant Research Institute for technical assistance.
Funding:
This project has been funded in whole or in part with Federal funds from the Biomedical Advanced Research and Development Authority (BARDA), Office of the Assistant Secretary for Preparedness and Response, Office of the Secretary, Department of Health and Human Services, under Contract #HHSO100201600010C, and by the National Hearth Lung and Blood Institute (NHLBI) REDS-III Central Lab Contract # HHSN268201100001I.
Footnotes
Conflict of interest: Phillip Williamson is the Chief Scientific Officer of Creative Testing Solutions and a Principal Investigator on the Roche Molecular Systems, Inc. Zika IND; Lisa L. Pate and Susan A. Galel are employees of Roche Molecular Systems, Inc. Michael Busch is the Principal Investigator on contracts to Vitalant Research Institute from Roche Molecular Systems, Inc. to support ZIKV confirmation testing for investigational blood donor screening.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Petersen LR, Jamieson DJ, Honein MA. Zika Virus. N Engl J Med 2016; 375(3): 294–5. [DOI] [PubMed] [Google Scholar]
- 2.Sarno M, Sacramento GA, Khouri R, et al. Zika Virus Infection and Stillbirths: A Case of Hydrops Fetalis, Hydranencephaly and Fetal Demise. PLoS Negl Trop Dis 2016; 10(2): e0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa F, Sarno M, Khouri R, et al. Emergence of Congenital Zika Syndrome: Viewpoint From the Front Lines. Ann Intern Med 2016; 164(10): 689–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heymann DL, Hodgson A, Sall AA, et al. Zika virus and microcephaly: why is this situation a PHEIC? Lancet 2016; 387(10020): 719–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N Engl J Med 2016; 374(20): 1981–7. [DOI] [PubMed] [Google Scholar]
- 6.Honein MA, Dawson AL, Petersen EE, et al. Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. JAMA 2016. [DOI] [PubMed] [Google Scholar]
- 7.Musso D.Zika Virus Transmission from French Polynesia to Brazil. Emerg Infect Dis 2015; 21(10): 1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salvador FS, Fujita DM. Entry routes for Zika virus in Brazil after 2014 world cup: New possibilities. Travel Med Infect Dis 2016; 14(1): 49–51. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DL, Sharp TM, Torres J, et al. Local Transmission of Zika Virus--Puerto Rico, November 23, 2015-January 28, 2016. MMWR Morb Mortal Wkly Rep 2016; 65(6): 154–8. [DOI] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration. Recommendations for Donor Screening, Deferral, and Product Management to Reduce the Risk of Transfusion-Transmission of Zika Virus - Guidance for Industry. February 2016 ed. Silver Spring, Maryland: CBER Office of Communication, Outreach, and Developoment; 2016. [Google Scholar]
- 11.Galel SA, Williamson PC, Busch MP, et al. First Zika-positive donations in the continental United States. Transfusion 2017; 57(3pt2): 762–9. [DOI] [PubMed] [Google Scholar]
- 12.Stone M.Zika RNA and Antibody Persistence in Blood and Body Fluids and Clinical Outcomes in Infected Blood Donors. See attached manuscript file. [Google Scholar]
- 13.Chevalier MS, Biggerstaff BJ, Basavaraju SV, et al. Use of Blood Donor Screening Data to Estimate Zika Virus Incidence, Puerto Rico, April-August 2016. Emerg Infect Dis 2017; 23(5): 790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williamson PC, Linnen JM, Kessler DA, et al. First cases of Zika virus-infected US blood donors outside states with areas of active transmission. Transfusion 2017; 57(3pt2): 770–8. [DOI] [PubMed] [Google Scholar]
- 15.Simmons G, Bres V, Lu K, et al. High Incidence of Chikungunya Virus and Frequency of Viremic Blood Donations during Epidemic, Puerto Rico, USA, 2014. Emerg Infect Dis 2016; 22(7): 1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003; 17(13): 1871–9. [DOI] [PubMed] [Google Scholar]
- 17.Biswas R, Tabor E, Hsia CC, et al. Comparative sensitivity of HBV NATs and HBsAg assays for detection of acute HBV infection. Transfusion 2003; 43(6): 788–98. [DOI] [PubMed] [Google Scholar]
- 18.Glynn SA, Wright DJ, Kleinman SH, et al. Dynamics of viremia in early hepatitis C virus infection. Transfusion 2005; 45(6): 994–1002. [DOI] [PubMed] [Google Scholar]
- 19.Busch MP, Murthy KK, Kleinman SH, et al. Infectivity in chimpanzees (Pan troglodytes) of plasma collected before HCV RNA detectability by FDA-licensed assays: implications for transfusion safety and HCV infection outcomes. Blood 2012; 119(26): 6326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma ZM, Stone M, Piatak M Jr., , et al. High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection. J Virol 2009; 83(7): 3288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busch MP, Kleinman SH, Tobler LH, et al. Virus and antibody dynamics in acute west nile virus infection. J Infect Dis 2008; 198(7): 984–93. [DOI] [PubMed] [Google Scholar]
- 22.Kleinman SH, Lelie N, Busch MP. Infectivity of human immunodeficiency virus-1, hepatitis C virus, and hepatitis B virus and risk of transmission by transfusion. Transfusion 2009; 49(11): 2454–89. [DOI] [PubMed] [Google Scholar]
- 23.Biggerstaff BJ, Petersen LR. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion 2002; 42(8): 1019–26. [DOI] [PubMed] [Google Scholar]
- 24.Lessler J, Ott CT, Carcelen AC, et al. Times to key events in Zika virus infection and implications for blood donation: a systematic review. Bull World Health Organ 2016; 94(11): 841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Census Bureau. American FactFinder. 2018. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=PEP_2017_PEPANNRES&src=pt (accessed 06/13/2018 2018).
- 26.Simmons G, Van Rompay K, Coffey L, et al. Transfusion-Transmission of Zika Virus in Non-human Primate and Mouse Models. VoxSanguinis 2018; 113(Suppl. 1): 5–347. [Google Scholar]
- 27.Lozier M, Adams L, Febo MF, et al. Incidence of Zika Virus Disease by Age and Sex - Puerto Rico, November 1, 2015-October 20, 2016. MMWR Morb Mortal Wkly Rep 2016; 65(44): 1219–23. [DOI] [PubMed] [Google Scholar]
- 28.Montgomery SP, Brown JA, Kuehnert M, et al. Transfusion-associated transmission of West Nile virus, United States 2003 through 2005. Transfusion 2006; 46(12): 2038–46. [DOI] [PubMed] [Google Scholar]
- 29.Paz-Bailey G, Rosenberg ES, Doyle K, et al. Persistence of Zika Virus in Body Fluids - Final Report. N Engl J Med 2018; 379(13): 1234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallian P, Cabie A, Richard P, et al. Zika virus in asymptomatic blood donors in Martinique. Blood 2017; 129(2): 263–6. [DOI] [PubMed] [Google Scholar]
- 31.Krow-Lucal ER, Biggerstaff BJ, Staples JE. Estimated Incubation Period for Zika Virus Disease. Emerg Infect Dis 2017; 23(5): 841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobler LH, Cameron MJ, Lanteri MC, et al. Interferon and interferon-induced chemokine expression is associated with control of acute viremia in West Nile virus-infected blood donors. J Infect Dis 2008; 198(7): 979–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Best K, Perelson AS. Mathematical modeling of within-host Zika virus dynamics. Immunol Rev 2018; 285(1): 81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell PK, Mier YT- RL, Biggerstaff BJ, et al. Reassessing Serosurvey-Based Estimates of the Zika Symptomatic Proportion. Am J Epidemiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saa P, Proctor M, Foster G, et al. Investigational Testing for Zika Virus among U.S. Blood Donors. N Engl J Med 2018; 378(19): 1778–88. [DOI] [PubMed] [Google Scholar]
- 36.Food US and Administration Drug. Revised Recommendations for Reducing the Risk of Zika Virus Transmission by Blood and Blood Components - Guidance for Industry. In: CBER Office of Communication, Ooutreach, and Development, editor. July 2018 ed. Silver Spring, Maryland: CBER Office of Communication, Outreach, and Developoment; 2018. p. 1–16. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.