Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide. The mechanisms involved in NAFLD onset are complicated and multifactorial. Recent literature has indicated that altered intestinal barrier function is related to the occurrence and progression of liver disease. The intestinal barrier is important for absorbing nutrients and electrolytes and for defending against toxins and antigens in the enteric environment. Major mechanisms by which the intestinal barrier influences the development of NAFLD involve the altered epithelial layer, decreased intracellular junction integrity, and increased intestinal barrier permeability. Increased intestinal permeability leads to luminal dysbiosis and allows the translocation of pathogenic bacteria and metabolites into the liver, inducing inflammation, immune response, and hepatocyte injury in NAFLD. Although research has been directed to NAFLD in recent decades, the pathophysiological changes in NAFLD initiation and progression are still not completely understood, and the therapeutic targets remain limited. A deeper understanding on the correlation between NAFLD pathogenesis and intestinal barrier regulation must be attained. Therefore, in this review, the components of the intestinal barrier and their respective functions and disruptions during the progression of NAFLD are discussed.
Keywords: Nonalcoholic fatty liver disease, Intestinal barrier, Intestinal barrier permeability, Gut-liver axis
Graphical abstract
Introduction
Nonalcoholic fatty liver disease (NAFLD), defined as steatosis in more than 5% of hepatocytes, also known as metabolic-associated fatty liver disease,1 has become the most common liver disease in industrialized countries. NAFLD is an all-encompassing term for diseases including simple steatosis, nonalcoholic steatohepatitis (NASH), NASH-related liver fibrosis, cirrhosis, and hepatocellular carcinoma. Simple steatosis is considered a benign condition without features of NASH, a more advanced form of NAFLD. NASH is a progressive disease that can lead to cirrhosis and hepatocellular carcinoma.2–4 NAFLD commonly coexists with metabolic syndrome, which also includes signs of abdominal obesity, insulin resistance, elevated blood pressure, altered fasting glucose, and dyslipidemia. NAFLD is associated with increased risk of developing comorbidities, such as systemic vascular endothelial dysfunction, atherosclerosis, and/or reproductive system disorders.5,6 In recent decades, that supports the association between NAFLD and several extrahepatic system comorbidities such as cardiovascular disease, type 2 diabetes mellitus, chronic kidney disease, and neurological system diseases, including depressive mood disorder, anxiety, and apathy has increased.7,8
The mechanisms of NAFLD progression are complex and multifactorial. Historically, the pathogenesis of NAFLD has been described as a two-hit hypothesis. That is, at the onset of the disease, the first hit refers to the hepatic accumulation of lipids caused by a high-fat diet, obesity, and/or insulin resistance, and the second hit involves inflammatory cytokines, adipokines, mitochondrial dysfunction, and oxidative stress.9 However, the two-hit hypothesis is inadequate to explain the metabolic and molecular changes in NAFLD hepatocytes, and the pathological mechanisms of NAFLD seem to involve parallel multiple hit injuries. Therefore, the multiple hit model theory has been widely accepted to explain the pathogenesis of NAFLD.10 In that model, the first hit is increased liver fat levels, followed by the effects of multiple factors, including insulin resistance, gut microbiota, and genetic and environmental factors. Those factors affect the hepatocyte inflammatory environment and contribute to mitochondrial dysfunction in conjunction with oxidative stress and endoplasmic reticulum stress-associated processes.11,12 Fat accumulation, hepatocyte injury, and particularly, damage to any aspect of the intestinal barrier are vital factors in the pathophysiology of NAFLD. The intestinal barrier is essential for absorbing nutrients necessary for humans and preventing the invasion of micro-organisms into the lumen. Alteration to intestinal barrier function is associated with increased intestinal permeability, which plays a crucial role in the initiation and progression of hepatic and extrahepatic damage.13
Composition and function of the intestinal barrier
The physical intestinal barrier is a complex structure consisting of mechanical, chemical, immunological, and microbial barriers. In the lumen, a mixture of gastric acid, bile acid, pancreatic digestive juice, commensal bacteria, and even pathogens are in contact with the epithelial layer, which is lined by intestinal epithelial cells (IECs) that are connected by intracellular junctions. In addition, below the epithelial layer, the gut vascular barrier is critical for maintaining the water and molecules in the intestinal microcirculation to portal circulation and finally to the liver.14,15
Mechanical barrier
The mechanical barrier is formed by a mucus layer, epithelial cells, intercellular tight junctions (TJs), and the lamina propria.16–18 As the first line of defense to resist invasion by harmful pathogens and microbes in the gastrointestinal lumen, the mucus layer overlies the epithelium and consists of proteins, glycans, and mucins with hydrophobic and surfactants that are primarily secreted by goblet cells.19,20 The gut mucosa has a large surface area exposed to the luminal environment, and it prevents harmful content from entering the systemic circulation.21 The epithelial layer is the main barrier that preserves the integrity of the intestinal barrier.22 Three intercellular junction complexes including TJs, adherens junctions, and desmosomes characterize the epithelial layer.23 TJs are composed of transmembrane proteins and related proteins involved in vesicle trafficking and intramembrane linkages. TJ transmembrane proteins include the TJ-associated proteins, claudins, and junctional adhesion molecules (JAMs). In addition, intracellular scaffold proteins such as zonula occludens (ZO)-1, ZO-2, and ZO-3, linking TJs through transmembrane proteins and to the actin cytoskeleton for exerting regulatory functions.16,24,25 The gut vascular barrier (GVB) under the epithelium is the innermost layer of multiple intestinal barriers, and it resists the entry of bacteria-derived substances moving from the lumen into the systemic circulation. The GVB is composed of vascular endothelial cells, pericytes or fibroblast cells, and enteric glial cells. These cells are united by intercellular TJs and adherens junctions.26,27
Chemical barrier
The intestinal chemical barrier protects the intestinal mucosa from invasion by micro-organisms and enzymes.28 Substances in this layer include secreted gastric acid, mucus, mucin, bile, bile acids, glycoproteins, mucopolysaccharides, digestive enzymes, lysozymes, and antimicrobial peptides. Antimicrobial peptides are microbicidal peptides that are resistant to host bacteria and pathogen element-induced barrier erosion, preventing gut barrier disruption and dysfunction.29 Gastric acid separates bacteria from the intestinal tract and prevents microbial colonization of the small intestine.30 Bile acids not only balance the metabolism of glucose, lipids, and energy but also protect the gastrointestinal environment by inactivating pathogenic bacteria.28 Furthermore, bile acids regulate intestinal epithelium cell proliferation and the gut microbiome. By modulating the functions of various species in the microbiome through their antimicrobial properties, bile acids, which are mainly deconjugated, influence the microbiome population by weakening the integrity of bacteria, particularly bacterial cell membranes.31
Immunological barrier
In addition to its barrier function that safeguards the body against pathogens, the components of the intestinal barrier, including immune cells, interact with the gut microbiome to establish an immune system that prevents micro-organisms, antigens, and pathogens in the luminal space from moving into the inner environment.20,32 The epithelium contains various IECs that trigger innate immunity. Surface goblet cells synthesize mucin on the apical barrier layer, whereas goblet cells respond to inflammatory stimuli and cooperate with IECs, mononuclear phagocytes, innate lymphoid cells, B and T lymphocytes, microbiota derivatives and metabolites to form innate and adaptive intestinal immune systems.33–36 As the first line of immune defense, antigens (i.e., self-antigens, microbiome, food molecules, and toxins) are recognized by the innate immune system. Once invading pathogens pass through the intestinal mucosa barrier, they are recognized and eliminated by immune cells such as dendritic cells, macrophages, and natural killer cells.37 These cells and pattern recognition receptors, such as toll-like receptors and nucleotide-binding oligomerization domain receptors, recognize pathogen-associated molecular patterns released from bacteria, viruses, and fungi. Additionally, a diverse population of T lymphocytes in the intestine form an essential part of the adaptive immune system.38 Goblet cells are antigen-presenting cells that deliver antigens to dendritic cells in the lamina propria, promoting the development of T regulatory cells.32,39
Microbial barrier
The human body contains a complex community of more than one hundred trillion micro-organisms, collectively referred to the microbiota.18,40 The normal microbiota includes bacteria, fungi, protozoa, archaea, and viruses.41 The gut microbiota defends against the invasion of pathogens and balances the luminal environment to maintain homeostasis.42 In addition, the host needs the microbiota to maintain the vitamin production, energy generation, cholesterol metabolism and bile deconjugation, and regulate immune functions through its interaction with mucosal immune cells and intraepithelial cells.43 The key metabolites generated from gut microbiota, including short-chain fatty acids, bile acids, trimethylamines, carotenoids, and phenolics, are regulators of intestinal metabolism and immunity.44,45 Recent research has suggested that perturbation of the gut microbiota is linked to increased intestinal permeability and host immune deficiencies, which are vital for the translocation of bacterial derivatives and contribute to the development of NAFLD.46 Studies have shown that dysbiosis or microbe-associated bacterial metabolites are harmful to the liver, contributing to the progression of liver steatosis and fibrosis. Some bacteria-derived products are pro-inflammatory stimuli that lead to increased gut permeability and are linked to systemic inflammation.47
Damaged intestinal barrier in the pathogenesis of NAFLD
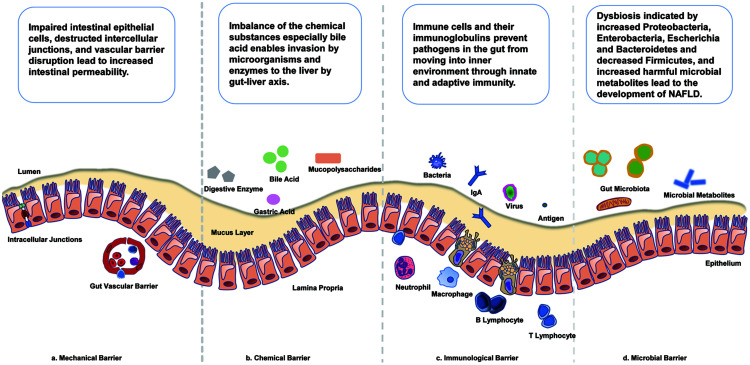
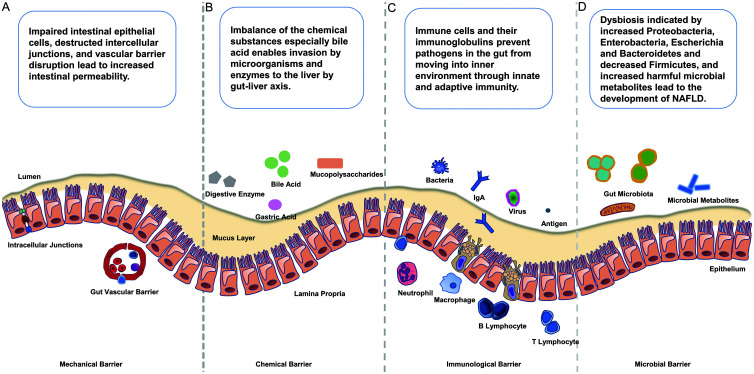
The pathogenesis of NAFLD, including disease initiation and progression, involves disruption of the intestinal barrier, alteration of intracellular junction proteins, increased intestinal permeability and disturbance of gut microbiome (Fig. 1).
Fig. 1. Intestinal barrier components and the pathophysiological changes in NAFLD.
(A–D) The intestinal barrier is composed of a complex combination of mechanical (A), chemical (B), immunological (C) and microbial barriers (D). The mechanical barrier comprises the mucus layer, epithelial cells, intercellular tight junctions, and the lamina propria. The gut vascular barrier under the epithelium is composed of vascular endothelial cells, pericytes or fibroblast cells, and enteric glial cells. The chemical barrier includes gastric acid, mucus, bile and bile acids, mucopolysaccharides, digestive enzymes, lysozymes, antimicrobial peptides, and other molecules. The immunological barrier is composed of intestinal epithelial cells, mononuclear phagocytes, innate lymphoid cells, B and T lymphocytes and goblet cells. The microbial barrier includes bacteria, fungi, protozoa, archaea, and even viruses. Impaired intestinal epithelial cells, damaged intercellular junctions, and vascular barrier disruption lead to increased intestinal permeability. Imbalance of the chemical substances, especially bile acids enables invasion of the liver by micro-organisms and enzymes via the gut-liver axis. Immune cells and their immunoglobulins prevent pathogens in the gut from moving into the internal environment through innate and adaptive immunity. Dysbiosis, with increases of Proteobacteria, Enterobacteria, Escherichia, and Bacteroidetes and an decrease of Firmicutes, together with increases of harmful microbial metabolites, leads to the development of NAFLD. NAFLD, nonalcoholic fatty liver disease.
Role of the intestinal mechanical barrier in the NAFLD development
The intestinal mechanical barrier includes the intestinal mucosa and epithelial cells that are connected by junctional complexes. Destruction of junctional proteins induces intestinal inflammation and altered barrier integrity thereby inducing NAFLD.28 Xin et al.48 showed that the level of TJ protein expression in IECs was related to the occurrence and development of NAFLD. Specifically, in a group of 92 patients, ZO-1 and occludin expression was decreased in NAFLD patients and was negatively correlated with transaminase levels.48 Loss of junctional adhesion proteins led to increased intestinal permeability in mice fed a high-fat diet by affecting the expression of the F11r gene, which encodes JAM-A. Colon samples in experimental mouse models had decreased JAM-A expression and a pattern of high inflammatory protein expression, leading to the development of steatohepatitis.49 Disruption to the intestinal epithelium layer or alteration of any component in the gut barrier leads to increased intestinal permeability in patients with NAFLD.28 A meta-analysis reported that NAFLD patients exhibited increased intestinal permeability detected by an oral dual sugar test and serum zonulin levels, compared with healthy controls.50 Studies found that NAFLD patients exhibited remarkably increased intestinal permeability, which alters the population of flora and promotes the translocation of microbes into blood circulation.51,52
Vascular barrier disruption is critical for the systemic translocation of gut bacteria and bacterial products into the blood circulation.53 As a marker of gut vascular endothelial permeability, plasmalemma vesicle-associated protein-1 expression is increased in pathogenic events, such as systemic dissemination of bacteria, celiac disease, and NASH.14,27 Research has shown that disruption to the GVB is evident in the early stage of NASH. Enteric pathogens can cross over the vascular barrier by interfering with the WNT/β-catenin pathway in endothelial cells.14
Role of the intestinal chemical barrier in the NAFLD development
The liver is the primary organ to be exposed to small molecules produced by digestion, and as an excretory organ, the liver secretes materials, including antibodies, into the bile acid that enters the intestinal lumen. Bile acids then enables the reabsorption of these materials into the terminal ileum, and the molecules are trafficked back to the liver by the enterohepatic circulation.54 The bidirectional relationship between the gut and liver is termed the gut-liver axis, and it highlights the close correlation between intestine and liver functions.55 Thus, any substance that enters the gut barrier will interact with hepatocytes and participate in hepatic metabolic pathways.30
Bile acids are associated with the progression of NAFLD. Yang et al.56,57 found that patients with NAFLD had significantly elevated serum bile acids, which was correlated with steatosis severity. A study reported that removal of the gut microbiota disrupted bile acid synthesis in the liver and suppressed microbial deconjugation and dehydroxylation in the intestine, which was important to regulate obesity-induced metabolic disorders.58 The farnesoid X receptor (FXR) is a nuclear receptor, known as a regulator of bile acid synthesis, and is expressed in adipose tissue, liver, intestine, kidney and adrenal glands.59,60 Studies show that FXR is associated with metabolic regulation and nutrient absorption. Yang et al.56 reported that FXR protein and mRNA levels were reduced in NAFLD patients. In a murine model, inhibition of intestinal FXR mediated NAFLD development.61 FXR has become the target for the management of NASH and liver fibrosis. Obeticholic acid, an FXR agonist, was shown to reduce bacterial translocation from the gut to liver and improve the histological features of NASH patients.62,63
The gut-liver axis has a key role in the progression of NASH-associated portal hypertension.64 In rat models of portal hypertension, the expression of mesenteric angiogenesis factors including vascular endothelial growth factor and endothelial nitric oxide synthase and microvascular permeability was both increased.65 In addition, portal hypertension results in the disruption of the intestinal barrier integrity. Patients with portal hypertension had edema of the intestinal mucosa and dilation of intestinal intercellular spaces in the jejunum.66 A study in children with portal hypertension reported villous atrophy, capillary dilation, muscularis mucosae thickening, and increased intestinal permeability.67 In a study in Austria, patients with severe portal hypertension had increased gastroduodenal and intestinal permeability, which was correlated with the degree of the hepatic venous pressure gradient. As markers of the enteric bacteria translocation, lipopolysaccharide (LPS)-binding proteins and interleukin-6 had increased serum levels in patients with portal hypertension patients compared with controls.68
Role of the intestinal immunological barrier in NAFLD development
The immune function of the intestinal barrier includes plasma cells, lymphocytes, phagocytes, dendritic cells, and Paneth cells, gut-associated lymphoid tissue including Peyer’s patches, mesenteric lymph nodes, and mucosa-associated lymphoid tissue.69 Paneth cells secrete antimicrobial peptides into the gut lumen. Natural killer cells are activated by antimicrobial peptides released by lymphocytes. T regulatory cells recognize microbial and food antigens in the intestine and mediate the tolerance of non-pathogenic antigens.31,70 Patients with NAFLD have reduced T regulatory cells and increased Th1 and CD8+ T cells in the lamina propria.71,72 Mast cells (MCs) are immune cells in the intestinal barrier that modulate both innate and adaptive immunity. Molecules released by MCs include cytokines, histamine, and proteases that impact intestinal barrier integrity. As the main species of proteases, tryptase, and chymase lead to the cleavage of ZO-1, downregulation of JAM-A and increase of epithelial permeability.73,74 Recent evidence shows that histamine increases intestinal epithelial permeability and gut bacterial translocation in a mouse model.75 MCs release various cytokines that impact the intestinal barrier directly and also express FXR in the intestine and liver, which alters intestinal fibroblast growth factor 15 and promotes liver fibrosis.76
Immunoglobulin A (IgA) secreted by lymphocytes and plasma cells have a key role in barrier immune function. Secretory IgA is the major subtype in the gut to protect the intestinal epithelium from pathogenic micro-organisms and maintain the homeostasis in regulation of microbiota composition. There is evidence that serum IgA concentrations was significantly elevated in patients with severe NASH compared with those with early stage disease, and was associated with advanced fibrosis.77,78 However, elevated serum IgA have been observed in other chronic liver diseases, and clinical studies investigating intestinal or fecal IgA levels are limited.79 Bacteria release LPS and activate inflammatory-related cytokines in IECs. LPS is an endotoxin in the outer layer membrane of most gram-negative bacteria, and it promotes the release of signaling molecules from enterocytes, which impairs barrier function.80,81 Moreover, LPS in liver induces steatohepatitis by activating Kupffer cells.82
Role of the intestinal microbial barrier in NAFLD development
Increased intestinal mucosal inflammation and disruption of the intestinal epithelial barrier result in translocation of bacteria and their metabolites in gut.28,49 Under normal conditions, the microbiota in intestinal lumen helps the body absorb and digest nutrients and fluids. Dysbiosis, defined as an abnormal amount or imbalanced composition of intestinal bacteria, is closely associated with patients with chronic fatty liver disease or cirrhosis.83 Studies show that dysbiosis or microbe-associated bacterial metabolites are harmful to the liver and contribute to the progression of liver steatosis and fibrosis.30,52,71,84 Dysbiosis has been associated with inhibition of the expression of TJ proteins and increased intestinal permeability via toll-like receptors.85 Small intestinal bacterial overgrowth is a significant characteristic in patients with NAFLD, and it increases the occurrence of complications, especially ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, and portal hypertension.28,83 Clinical data from the past two decades has convinced scientists that NAFLD patients exhibit a reduced gut microbiome signature, as indicated by increased percentage of Proteobacteria, Enterobacteria, Escherichia, and Bacteroidetes species and decreased the percentage of Firmicutes species in the gut microbiome profile.86–88 Therefore, probiotics and prebiotics are important in maintaining a healthy intestinal microflora balance.28 Recent studies show that modulating the gut microbiota with probiotics, prebiotics, symbiotic, and agents that regulate bile acids and fecal microbiota transplantation (FMT), are practical approaches to treat NAFLD.89 FMT transfers fecal materials from healthy donors to recipients with disturbed gut microbiota. It has been widely used to treat refractory and recurrent Clostridium difficile infection.90 FMT has also become a potential therapy for the treatment of NAFLD.91 Craven et al.92 reported that endoscopic FMT to the distal duodenum in 21 patients with NAFLD resulted in improved small intestine permeability but no significant changes in insulin resistance and hepatic proton density fat fraction.92 Probiotics are live micro-organisms that improve the microbiota balance in the intestinal tract.93 They are consumed in various foods such as in yogurt and other fermented milk and food products or capsules.94 Prebiotics are fibers that are fermented by the intestinal microflora and stimulate the growth or activity of intestinal bacteria to sustain a healthy microbiome or restore balance.95 Sixty-four obese children with sonographic NAFLD were included in a randomized triple-blind clinical trial and received probiotic capsules containing Lactobacilli, Bifidobacteria, and Bifidobacterium and Lactobacillus for 12 weeks. After the bacteriotherapy, the probiotic group showed decreased liver aminotransferase and lipid levels, without changes in weight and body mass index.96 Probiotics are live micro-organisms that have benefits on intestinal gut and improvement of lipid profile. VSL#3, a mixture of probiotic medicinal food consisting of eight bacterial strains (four Lactobacillus species, namely, Lactobacillus paracasei, L. plantarum, L. acidophilus, and L. delbrueckii subspecies bulgaricus; three Bifidobacterium species, namely, Bifidobacterium. longum, B. lactis, and B. breve; and Streptococcus thermophiles), has been shown to reverse NASH in a mouse model.97,98 A randomized trial including 60 NAFLD patients reported a significant reduction in triglycerides, transaminases, and gamma-glutamyltransferase, but no change in fasting plasma glucose, total cholesterol, low-density lipoprotein-cholesterol and high-density lipoprotein-cholesterol with VSL#3 compared with controls.99 The evidence suggests probiotics and prebiotics have a possible benefit in NAFLD, high-quality studies are still limited.
Lifestyle modification as the therapeutic target for NAFLD
Research on the role of diet components in intestinal barrier integrity and function has become increasingly popular. Recent publications have demonstrated that a high-fat diet may associate with endotoxemia and lead to barrier dysfunction.100,101 Components in the diet are in close contact with epithelial cells and become the primary stimuli altering the gut barrier.102 Increased consumption of high-fat or high-fructose food may alter intestinal barrier function, thereby contributing to the progression to obesity or fatty liver disease.103 Cho et al.104 team reported that fructose induced nitrosylation of intestinal barrier junction proteins leading to a leaky gut, and finally resulting in steatohepatitis with fibrosis.104 Lifestyle modification based on a healthy diet and planned exercise is considered to be the most important management of metabolic disorders. The Mediterranean diet (MD) is characterized by high consumption of unrefined cereals, vegetables, fruit, nuts, olive oil, white meat, dairy products in moderation, limiting red meat, and moderate alcohol consumption.105,106 Recent studies have focused on the possible association between the MD and NAFLD. In a study including 46 adults with NAFLD, Gellinet et al.107 found that 6 months of a modified MD had reduced liver enzymes and lipid index at the end of treatment.107 A clinical trial of an MD in Australia reported a significant reduction in hepatic steatosis and improved insulin sensitivity compared with a low-fat high-carbohydrate diet.108 In a study by Vitaglione et al.109 coffee consumption protected against the development of NAFLD. The coffee-drinking group had more undigested lipids in luminal feces, upregulated claudin expression in the duodenum and zonulin-1 expression in both the duodenum and colon, both of which are key for maintaining intestinal barrier integrity.109
Conclusion
Disruption of any part of the intestinal barrier, including destruction of the epithelial layer, reduction in intracellular junction integrity, increased intestinal permeability, overgrowth of bacteria, and/or dysbiosis, is a key event in pathological mechanism of NAFLD. As the gut barrier function is important in maintaining homeostasis, exploring the potential mechanisms implicated in the pathogenesis and progression of NAFLD is urgently required. More studies are needed to identify and characterize the mechanisms of gut barrier dysfunction in liver damage disorders. Clinicians should be aware of the potential gut barrier dysfunction in NAFLD, which may be a therapeutic target in the near future.
Abbreviations
- FMT
fecal microbiota transplantation
- FXR
farnesoid X receptor
- GVB
gut vascular barrier
- IEC
intestinal epithelial cell
- IgA
Immunoglobulin A
- JAM
junctional adhesion molecule
- LPS
lipopolysaccharide
- MC
mast cell
- MD
Mediterranean diet
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- TJ
tight junction
- ZO
zonula occluden
References
- 1.Fouad Y, Waked I, Bollipo S, Gomaa A, Ajlouni Y, Attia D. What’s in a name? Renaming ‘NAFLD’ to ‘MAFLD’. Liver Int. 2020;40(6):1254–1261. doi: 10.1111/liv.14478. [DOI] [PubMed] [Google Scholar]
- 2.Gariani K, Jornayvaz FR. Pathophysiology of NASH in endocrine diseases. Endocr Connect. 2021;10(2):R52–R65. doi: 10.1530/ec-20-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown GT, Kleiner DE. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism. 2016;65(8):1080–1086. doi: 10.1016/j.metabol.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nalbantoglu IL, Brunt EM. Role of liver biopsy in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(27):9026–9037. doi: 10.3748/wjg.v20.i27.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin S, Huang J, Wang M, Kumar R, Liu Y, Liu S, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int. 2020;40(9):2082–2089. doi: 10.1111/liv.14548. [DOI] [PubMed] [Google Scholar]
- 6.Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The metabolic syndrome. Endocr Rev. 2008;29(7):777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Moretti R, Caruso P, Gazzin S. Non-alcoholic fatty liver disease and neurological defects. Ann Hepatol. 2019;18(4):563–570. doi: 10.1016/j.aohep.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Peverill W, Powell LW, Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int J Mol Sci. 2014;15(5):8591–8638. doi: 10.3390/ijms15058591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bessone F, Razori MV, Roma MG. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell Mol Life Sci. 2019;76(1):99–128. doi: 10.1007/s00018-018-2947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemente MG, Mandato C, Poeta M, Vajro P. Pediatric non-alcoholic fatty liver disease: Recent solutions, unresolved issues, and future research directions. World J Gastroenterol. 2016;22(36):8078–8093. doi: 10.3748/wjg.v22.i36.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65(8):1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Farré R, Vicario M. Abnormal Barrier Function in Gastrointestinal Disorders. Handb Exp Pharmacol. 2017;239:193–217. doi: 10.1007/164_2016_107. [DOI] [PubMed] [Google Scholar]
- 14.Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 2019;71(6):1216–1228. doi: 10.1016/j.jhep.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu P, Bian Y, Fan Y, Zhong J, Liu Z. Protective Effect of Naringin on In Vitro Gut-Vascular Barrier Disruption of Intestinal Microvascular Endothelial Cells Induced by TNF-α. J Agric Food Chem. 2020;68(1):168–175. doi: 10.1021/acs.jafc.9b06347. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim Sci J. 2020;91(1):e13357. doi: 10.1111/asj.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chopyk DM, Grakoui A. Contribution of the Intestinal Microbiome and Gut Barrier to Hepatic Disorders. Gastroenterology. 2020;159(3):849–863. doi: 10.1053/j.gastro.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardoso-Silva D, Delbue D, Itzlinger A, Moerkens R, Withoff S, Branchi F, et al. Intestinal Barrier Function in Gluten-Related Disorders. Nutrients. 2019;11(10):E2325. doi: 10.3390/nu11102325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10(6):352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salvo Romero E, Alonso Cotoner C, Pardo Camacho C, Casado Bedmar M, Vicario M. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig. 2015;107(11):686–696. doi: 10.17235/reed.2015.3846/2015. [DOI] [PubMed] [Google Scholar]
- 21.Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17(1):362–381. doi: 10.1002/ibd.21403. [DOI] [PubMed] [Google Scholar]
- 22.Bermudez-Brito M, Plaza-Díaz J, Muñoz-Quezada S, Gómez-Llorente C, Gil A. Probiotic mechanisms of action. Ann Nutr Metab. 2012;61(2):160–174. doi: 10.1159/000342079. [DOI] [PubMed] [Google Scholar]
- 23.FARQUHAR MG, PALADE GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plaza-Díaz J, Solís-Urra P, Rodríguez-Rodríguez F, Olivares-Arancibia J, Navarro-Oliveros M, Abadía-Molina F, et al. The Gut Barrier, Intestinal Microbiota, and Liver Disease: Molecular Mechanisms and Strategies to Manage. Int J Mol Sci. 2020;21(21):E8351. doi: 10.3390/ijms21218351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slifer ZM, Blikslager AT. The Integral Role of Tight Junction Proteins in the Repair of Injured Intestinal Epithelium. Int J Mol Sci. 2020;21(3):E972. doi: 10.3390/ijms21030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Zhou J, Qiu J, Huang Z, Wang W, Wu P, et al. Berberine reduces gut-vascular barrier permeability via modulation of ApoM/S1P pathway in a model of polymicrobial sepsis. Life Sci. 2020;261:118460. doi: 10.1016/j.lfs.2020.118460. [DOI] [PubMed] [Google Scholar]
- 27.Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350(6262):830–834. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- 28.Cui Y, Wang Q, Chang R, Zhou X, Xu C. Intestinal Barrier Function-Non-alcoholic Fatty Liver Disease Interactions and Possible Role of Gut Microbiota. J Agric Food Chem. 2019;67(10):2754–2762. doi: 10.1021/acs.jafc.9b00080. [DOI] [PubMed] [Google Scholar]
- 29.Mohammed I, Abedin A, Tsintzas K, Abedin SA, Otri AM, Hopkinson A, et al. Increased expression of hepcidin and toll-like receptors 8 and 10 in viral keratitis. Cornea. 2011;30(8):899–904. doi: 10.1097/ICO.0b013e31820126e5. [DOI] [PubMed] [Google Scholar]
- 30.Nicoletti A, Ponziani FR, Biolato M, Valenza V, Marrone G, Sganga G, et al. Intestinal permeability in the pathogenesis of liver damage: From non-alcoholic fatty liver disease to liver transplantation. World J Gastroenterol. 2019;25(33):4814–4834. doi: 10.3748/wjg.v25.i33.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Ciaula A, Baj J, Garruti G, Celano G, De Angelis M, Wang HH, et al. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk. J Clin Med. 2020;9(8):E2648. doi: 10.3390/jcm9082648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers. 2017;5(4):e1373208. doi: 10.1080/21688370.2017.1373208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang S, Yu M. Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J Inflamm Res. 2021;14:3171–3183. doi: 10.2147/jir.S318327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birchenough GM, Johansson ME, Gustafsson JK, Bergström JH, Hansson GC. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015;8(4):712–719. doi: 10.1038/mi.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabat AM, Srinivasan N, Maloy KJ. Modulation of immune development and function by intestinal microbiota. Trends Immunol. 2014;35(11):507–517. doi: 10.1016/j.it.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Zhu L, Qin S. Gut Microbiota Modulation on Intestinal Mucosal Adaptive Immunity. J Immunol Res. 2019;2019:4735040. doi: 10.1155/2019/4735040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 38.Wang C, Li Q, Ren J. Microbiota-Immune Interaction in the Pathogenesis of Gut-Derived Infection. Front Immunol. 2019;10:1873. doi: 10.3389/fimmu.2019.01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483(7389):345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62(1):159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, et al. The NIH Human Microbiome Project. Genome Res. 2009;19(12):2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leser TD, Mølbak L. Better living through microbial action: the benefits of the mammalian gastrointestinal microbiota on the host. Environ Microbiol. 2009;11(9):2194–2206. doi: 10.1111/j.1462-2920.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- 43.Walter J, Ley R. The human gut microbiome: ecology and recent evolutionary changes. Annu Rev Microbiol. 2011;65:411–429. doi: 10.1146/annurev-micro-090110-102830. [DOI] [PubMed] [Google Scholar]
- 44.Ji Y, Yin Y, Li Z, Zhang W. Gut Microbiota-Derived Components and Metabolites in the Progression of Non-Alcoholic Fatty Liver Disease (NAFLD) Nutrients. 2019;11(8):E1712. doi: 10.3390/nu11081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plaza-Díaz J, Solis-Urra P, Aragón-Vela J, Rodríguez-Rodríguez F, Olivares-Arancibia J, Álvarez-Mercado AI. Insights into the Impact of Microbiota in the Treatment of NAFLD/NASH and Its Potential as a Biomarker for Prognosis and Diagnosis. Biomedicines. 2021;9(2):145. doi: 10.3390/biomedicines9020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beisner J, Filipe Rosa L, Kaden-Volynets V, Stolzer I, Günther C, Bischoff SC. Prebiotic Inulin and Sodium Butyrate Attenuate Obesity-Induced Intestinal Barrier Dysfunction by Induction of Antimicrobial Peptides. Front Immunol. 2021;12:678360. doi: 10.3389/fimmu.2021.678360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chakaroun RM, Massier L, Kovacs P. Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients. 2020;12(4):E1082. doi: 10.3390/nu12041082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xin D, Zong-Shun L, Bang-Mao W, Lu Z. Expression of intestinal tight junction proteins in patients with non-alcoholic fatty liver disease. Hepatogastroenterology. 2014;61(129):136–140. [PubMed] [Google Scholar]
- 49.Rahman K, Desai C, Iyer SS, Thorn NE, Kumar P, Liu Y, et al. Loss of Junctional Adhesion Molecule A Promotes Severe Steatohepatitis in Mice on a Diet High in Saturated Fat, Fructose, and Cholesterol. Gastroenterology. 2016;151(4):733–746.e12. doi: 10.1053/j.gastro.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Munck TJI, Xu P, Verwijs HJA, Masclee AAM, Jonkers D, Verbeek J, et al. Intestinal permeability in human nonalcoholic fatty liver disease: A systematic review and meta-analysis. Liver Int. 2020;40(12):2906–2916. doi: 10.1111/liv.14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allam-Ndoul B, Castonguay-Paradis S, Veilleux A. Gut Microbiota and Intestinal Trans-Epithelial Permeability. Int J Mol Sci. 2020;21(17):E6402. doi: 10.3390/ijms21176402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu H, Lin A, Kong M, Yao X, Yin M, Xia H, et al. Intestinal microbiome and NAFLD: molecular insights and therapeutic perspectives. J Gastroenterol. 2020;55(2):142–158. doi: 10.1007/s00535-019-01649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brescia P, Rescigno M. The gut vascular barrier: a new player in the gut-liver-brain axis. Trends Mol Med. 2021;27(9):844–855. doi: 10.1016/j.molmed.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 55.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148(1):30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang ZX, Shen W, Sun H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol Int. 2010;4(4):741–748. doi: 10.1007/s12072-010-9202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bechmann LP, Kocabayoglu P, Sowa JP, Sydor S, Best J, Schlattjan M, et al. Free fatty acids repress small heterodimer partner (SHP) activation and adiponectin counteracts bile acid-induced liver injury in superobese patients with nonalcoholic steatohepatitis. Hepatology. 2013;57(4):1394–1406. doi: 10.1002/hep.26225. [DOI] [PubMed] [Google Scholar]
- 58.Sun L, Pang Y, Wang X, Wu Q, Liu H, Liu B, et al. Ablation of gut microbiota alleviates obesity-induced hepatic steatosis and glucose intolerance by modulating bile acid metabolism in hamsters. Acta Pharm Sin B. 2019;9(4):702–710. doi: 10.1016/j.apsb.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sumida Y, Yoneda M, Ogawa Y, Yoneda M, Okanoue T, Nakajima A. Current and new pharmacotherapy options for non-alcoholic steatohepatitis. Expert Opin Pharmacother. 2020;21(8):953–967. doi: 10.1080/14656566.2020.1744564. [DOI] [PubMed] [Google Scholar]
- 60.Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89(1):147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 61.Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125(1):386–402. doi: 10.1172/jci76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sorribas M, Jakob MO, Yilmaz B, Li H, Stutz D, Noser Y, et al. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J Hepatol. 2019;71(6):1126–1140. doi: 10.1016/j.jhep.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 64.Simbrunner B, Mandorfer M, Trauner M, Reiberger T. Gut-liver axis signaling in portal hypertension. World J Gastroenterol. 2019;25(39):5897–5917. doi: 10.3748/wjg.v25.i39.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geerts AM, De Vriese AS, Vanheule E, Van Vlierberghe H, Mortier S, Cheung KJ, et al. Increased angiogenesis and permeability in the mesenteric microvasculature of rats with cirrhosis and portal hypertension: an in vivo study. Liver Int. 2006;26(7):889–898. doi: 10.1111/j.1478-3231.2006.01308.x. [DOI] [PubMed] [Google Scholar]
- 66.Norman DA, Atkins JM, Seelig LL, Jr, Gomez-Sanchez C, Krejs GJ. Water and electrolyte movement and mucosal morphology in the jejunum of patients with portal hypertension. Gastroenterology. 1980;79(4):707–715. [PubMed] [Google Scholar]
- 67.Borkar VV, Poddar U, Kumari N, Singh S, Roy R, Yachha SK. Duodenal morphometry and small bowel permeability in children with portal hypertension. J Pediatr Gastroenterol Nutr. 2015;60(2):171–176. doi: 10.1097/mpg.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 68.Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, et al. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58(5):911–921. doi: 10.1016/j.jhep.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 69.Portincasa P, Bonfrate L, Khalil M, Angelis M, Calabrese FM, D’Amato M, et al. Intestinal Barrier and Permeability in Health, Obesity and NAFLD. Biomedicines. 2021;10(1):83. doi: 10.3390/biomedicines10010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sharma A, Rudra D. Emerging Functions of Regulatory T Cells in Tissue Homeostasis. Front Immunol. 2018;9:883. doi: 10.3389/fimmu.2018.00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72(3):558–577. doi: 10.1016/J.JHEP.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 72.Luck H, Tsai S, Chung J, Clemente-Casares X, Ghazarian M, Revelo XS, et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21(4):527–542. doi: 10.1016/j.cmet.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 73.Wilcz-Villega EM, McClean S, O’Sullivan MA. Mast cell tryptase reduces junctional adhesion molecule-A (JAM-A) expression in intestinal epithelial cells: implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am J Gastroenterol. 2013;108(7):1140–1151. doi: 10.1038/ajg.2013.92. [DOI] [PubMed] [Google Scholar]
- 74.Scudamore CL, Jepson MA, Hirst BH, Miller HR. The rat mucosal mast cell chymase, RMCP-II, alters epithelial cell monolayer permeability in association with altered distribution of the tight junction proteins ZO-1 and occludin. Eur J Cell Biol. 1998;75(4):321–330. doi: 10.1016/s0171-9335(98)80065-4. [DOI] [PubMed] [Google Scholar]
- 75.Potts RA, Tiffany CM, Pakpour N, Lokken KL, Tiffany CR, Cheung K, et al. Mast cells and histamine alter intestinal permeability during malaria parasite infection. Immunobiology. 2016;221(3):468–474. doi: 10.1016/j.imbio.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meadows V, Kennedy L, Ekser B, Kyritsi K, Kundu D, Zhou T, et al. Mast Cells Regulate Ductular Reaction and Intestinal Inflammation in Cholestasis Through Farnesoid X Receptor Signaling. Hepatology. 2021;74(5):2684–2698. doi: 10.1002/hep.32028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tomita K, Teratani T, Yokoyama H, Suzuki T, Irie R, Ebinuma H, et al. Serum immunoglobulin a concentration is an independent predictor of liver fibrosis in nonalcoholic steatohepatitis before the cirrhotic stage. Dig Dis Sci. 2011;56(12):3648–3654. doi: 10.1007/s10620-011-1771-2. [DOI] [PubMed] [Google Scholar]
- 78.McPherson S, Henderson E, Burt AD, Day CP, Anstee QM. Serum immunoglobulin levels predict fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;60(5):1055–1062. doi: 10.1016/j.jhep.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 79.Inamine T, Schnabl B. Immunoglobulin A and liver diseases. J Gastroenterol. 2018;53(6):691–700. doi: 10.1007/s00535-017-1400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stephens M, von der Weid PY. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes. 2020;11(3):421–432. doi: 10.1080/19490976.2019.1629235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu XX, Huang XL, Chen RR, Li T, Ye HJ, Xie W, et al. Paeoniflorin Prevents Intestinal Barrier Disruption and Inhibits Lipopolysaccharide (LPS)-Induced Inflammation in Caco-2 Cell Monolayers. Inflammation. 2019;42(6):2215–2225. doi: 10.1007/s10753-019-01085-z. [DOI] [PubMed] [Google Scholar]
- 82.Stams AJ, Plugge CM. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat Rev Microbiol. 2009;7(8):568–577. doi: 10.1038/nrmicro2166. [DOI] [PubMed] [Google Scholar]
- 83.Augustyn M, Grys I, Kukla M. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease. Clin Exp Hepatol. 2019;5(1):1–10. doi: 10.5114/ceh.2019.83151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang R, Tang R, Li B, Ma X, Schnabl B, Tilg H. Gut microbiome, liver immunology, and liver diseases. Cell Mol Immunol. 2021;18(1):4–17. doi: 10.1038/s41423-020-00592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joshi-Barve S, Kirpich I, Cave MC, Marsano LS, McClain CJ. Alcoholic, Nonalcoholic, and Toxicant-Associated Steatohepatitis: Mechanistic Similarities and Differences. Cell Mol Gastroenterol Hepatol. 2015;1(4):356–367. doi: 10.1016/j.jcmgh.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murphy GP, Brede HD, Weber HW, Groenewald JH, Schoonees R, Van Zyl JJ, et al. Treatment of baboon renal allografts with subcellular kidney cell fractions. Surg Forum. 1968;19:215–217. [PubMed] [Google Scholar]
- 87.Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11(7):868–75.e1-3. doi: 10.1016/j.cgh.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 88.Duarte SMB, Stefano JT, Oliveira CP. Microbiota and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH) Ann Hepatol. 2019;18(3):416–421. doi: 10.1016/j.aohep.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Suk KT, Kim DJ. Gut microbiota: novel therapeutic target for nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2019;13(3):193–204. doi: 10.1080/17474124.2019.1569513. [DOI] [PubMed] [Google Scholar]
- 90.Gupta M, Krishan P, Kaur A, Arora S, Trehanpati N, Singh TG, et al. Mechanistic and physiological approaches of fecal microbiota transplantation in the management of NAFLD. Inflamm Res. 2021;70(7):765–776. doi: 10.1007/s00011-021-01480-z. [DOI] [PubMed] [Google Scholar]
- 91.Gu X, Lu Q, Zhang C, Tang Z, Chu L. Clinical Application and Progress of Fecal Microbiota Transplantation in Liver Diseases: A Review. Semin Liver Dis. 2021;41(4):495–506. doi: 10.1055/s-0041-1732319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Craven L, Rahman A, Nair Parvathy S, Beaton M, Silverman J, Qumosani K, et al. Allogenic Fecal Microbiota Transplantation in Patients With Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. Am J Gastroenterol. 2020;115(7):1055–1065. doi: 10.14309/ajg.0000000000000661. [DOI] [PubMed] [Google Scholar]
- 93.Williams NT. Probiotics. Am J Health Syst Pharm. 2010;67(6):449–458. doi: 10.2146/ajhp090168. [DOI] [PubMed] [Google Scholar]
- 94.Sáez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda FJ, Plaza-Diaz J, Gil A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int J Mol Sci. 2016;17(6):E928. doi: 10.3390/ijms17060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5(4):1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Famouri F, Shariat Z, Hashemipour M, Keikha M, Kelishadi R. Effects of Probiotics on Nonalcoholic Fatty Liver Disease in Obese Children and Adolescents. J Pediatr Gastroenterol Nutr. 2017;64(3):413–417. doi: 10.1097/mpg.0000000000001422. [DOI] [PubMed] [Google Scholar]
- 97.Cheng FS, Pan D, Chang B, Jiang M, Sang LX. Probiotic mixture VSL#3: An overview of basic and clinical studies in chronic diseases. World J Clin Cases. 2020;8(8):1361–1384. doi: 10.12998/wjcc.v8.i8.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jena PK, Sheng L, Li Y, Wan YY. Probiotics VSL#3 are effective in reversing non-alcoholic steatohepatitis in a mouse model. Hepatobiliary Surg Nutr. 2020;9(2):170–182. doi: 10.21037/hbsn.2019.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Derosa G, Guasti L, D’Angelo A, Martinotti C, Valentino MC, Di Matteo S, et al. Probiotic Therapy With VSL#3® in Patients With NAFLD: A Randomized Clinical Trial. Front Nutr. 2022;9:846873. doi: 10.3389/fnut.2022.846873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stenman LK, Holma R, Gylling H, Korpela R. Genetically obese mice do not show increased gut permeability or faecal bile acid hydrophobicity. Br J Nutr. 2013;110(6):1157–1164. doi: 10.1017/S000711451300024X. [DOI] [PubMed] [Google Scholar]
- 101.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, et al. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87(5):1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 102.Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11(9):821–834. doi: 10.1080/17474124.2017.1343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kirpich IA, Marsano LS, McClain CJ. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin Biochem. 2015;48(13-14):923–930. doi: 10.1016/j.clinbiochem.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cho YE, Kim DK, Seo W, Gao B, Yoo SH, Song BJ. Fructose Promotes Leaky Gut, Endotoxemia, and Liver Fibrosis Through Ethanol-Inducible Cytochrome P450-2E1-Mediated Oxidative and Nitrative Stress. Hepatology. 2021;73(6):2180–2195. doi: 10.1002/hep.30652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol. 2018;24(19):2083–2094. doi: 10.3748/wjg.v24.i19.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abenavoli L, Boccuto L, Federico A, Dallio M, Loguercio C, Di Renzo L, et al. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int J Environ Res Public Health. 2019;16(17):E3011. doi: 10.3390/ijerph16173011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gelli C, Tarocchi M, Abenavoli L, Di Renzo L, Galli A, De Lorenzo A. Effect of a counseling-supported treatment with the Mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J Gastroenterol. 2017;23(17):3150–3162. doi: 10.3748/wjg.v23.i17.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ryan MC, Itsiopoulos C, Thodis T, Ward G, Trost N, Hofferberth S, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59(1):138–143. doi: 10.1016/j.jhep.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 109.Vitaglione P, Mazzone G, Lembo V, D’Argenio G, Rossi A, Guido M, et al. Coffee prevents fatty liver disease induced by a high-fat diet by modulating pathways of the gut-liver axis. J Nutr Sci. 2019;8:e15. doi: 10.1017/jns.2019.10. [DOI] [PMC free article] [PubMed] [Google Scholar]