Abstract
In contrast to the livestock industry, sperm cryopreservation has not yet been successfully established in the poultry industry. This is because poultry sperm cells have a unique shape and membrane fluidity, differing from those of livestock sperm. The objective of this review is to discuss the cellular and molecular characteristics of rooster spermatozoa as a cause for their generally low freezability. Furthermore, here, we discuss novel developments in the field of semen extenders, cryoprotectants, and freezing processes, all with the purpose of increasing the potential of rooster sperm cryopreservation. Currently, it is very important to improve cryopreservation of rooster sperm on a global scale for the protection of gene resources due to the incidence of epidemics such as avian influenza.
Key words: cryopreservation, poultry, cryoprotectants, oxidative stress
INTRODUCTION
Methods for long-term preservation of sex cells are continuously being developed to preserve genetic diversity and to increase gene flow across populations. The success of cryopreservation is determined by many criteria and conditions of a physical and chemical nature. These conditions must guarantee the preservation of the structures of individual cell organs and membranes during the freeze–thaw procedure and allow for the maintenance of their function (Mohammad et al, 2021). The process of cryopreservation and insemination is routinely applied in cattle. For that reason, our approaches are discussed and compared with dairy- or meat-purpose cattle.
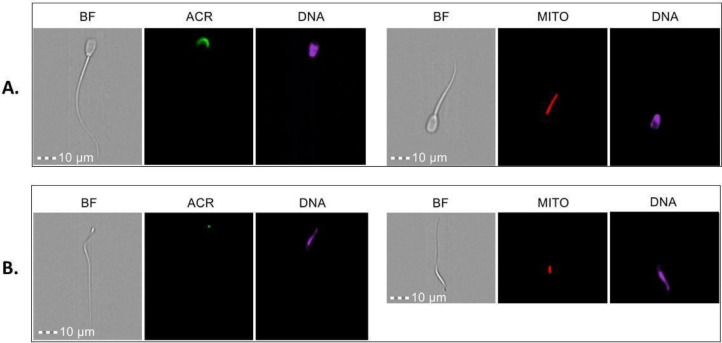
Freezing rooster spermatozoa is the only option for sex cell conservation due to the impossibility of freezing yolk-laden eggs ( Long, 2006). Rooster cryopreserved spermatozoa are associated with extremely variable fertility (Lake, 1986; Hammerstedt and Graham, 1992; Buss, 1993; Long et al., 2010; Thélie et al., 2019). Low tolerance to the cryopreservation procedure of poultry sperm can be explained by the cellular and molecular characteristics of poultry spermatozoa (Figure 1); for example, poultry sperm membranes contain higher quantities of polyunsaturated fatty acids than mammalian sperm and, therefore, may require higher antioxidant protection. Commonly used cryoprotectants for cryopreservation have a contraceptive or even toxic effect due to the peculiarity of poultry sperm cells (Çiftci and Aygün, 2018).
Figure 1.
Morphological differences between mammalian and avian spermatozoa (A - bull; B - rooster). Spermatozoa stained with PNA-lectin/FITC (acrosome, ACR), Hoechst-33342 (DNA), or Mitotracker Deep Red (mitochondria, MITO). A - frozen-thawed bull sperm. B - frozen-thawed rooster sperm. BF - bright field. Amnis ImageStream Imaging Flow Cytometer. Illustrative plots are shown.
Interspecies or even interbloodline differences in spermatozoa tolerance to cryopreservation determine the fertility of cryopreserved semen. It is essential that the development of successful freezing procedures involves more than the identification or application of novel cryoprotectants and additives (Holt, 2000). Although the first major development in poultry sperm cryopreservation was performed in the 1930s–40s (the discovery of glycerol's cryoprotective properties), its toxicity to avian cells remains an unsolved task for workers and scientists in the poultry industry (Long, 2006). Greater fertility for cryopreserved rooster spermatozoa is needed. If improved cryopreservation techniques are developed, they can be used to efficiently preserve these valuable poultry genetics. At the same time, it will be possible to share these genetics globally by sending cryopreserved sperm.
Different Reproduction Biology
Spermatozoa are highly differentiated haploid cells with highly condensed chromatin inside the cells and limited capacity for biosynthesis and cell reparation. The differences in poultry sperm cells require specific protocols that provide the unique characteristics of sperm cells in this species (Çiftci and Aygün, 2018; Santiago-Moreno et al., 2016). Rooster spermatozoa are homogametic. The cells are approximately 0.5 μm at their widest point and approximately 70 μm in length (Chenoweth and Lorton, 2014). The filiform shape of the poultry sperm head is similar in diameter to the tail (Thurston and Hess, 1987). Therefore, sperm heads have relatively low cytoplasmic volume, and thus, a relatively low volume of cryoprotectants (CPAs) can be accumulated. There are technical limitations to sperm examination by computer‑assisted sperm morphology analysis (CASA) systems, as these systems were designed to investigate the approximately spherically headed spermatozoa of mammalian species (Santiago-Moreno et al., 2016). For turkey and chicken spermatozoa, the neck region of the midpiece consists of a proximal and an elongated distal centriole. In contrast, guinea fowl spermatozoa contain only a single elongated centriole and associated pericentriolar projections (Thurston and Hess, 1987). Cross sections of the centrioles have the typical ‘pinwheel’ arrangement of nine triplet microtubules embedded in a cylindrical, dense wall (Thurston and Hess, 1987). The rooster spermatozoa midpiece contains approximately 30 mitochondria, with the mitochondria being integral in energy utilization for flagellar movement (Nguyen et al., 2014). Poultry sperm have a relatively long tail (between 90 and 100 μm; approximately 8 times longer than the sperm head) (Thurston and Hess, 1987) compared to bull spermatozoa (Bahr and Zeitler, 1964). For that reason, predisposes of poultry sperm seem to be more sensitive to freezing damage (Donoghue and Wishart, 2000).
Sperm cells mature in the testis and epididymis in most mammalian species. In roosters, as probably in other bird species, however, final maturation takes place in the vas deferens (the ducts that transport sperm from the epididymis to the ejaculatory organ) (Bernal et al. 2022).
It is known that the flagellar movement of sperm is driven by the sliding of microtubules with the motor protein dynein ATPase (Gibbons, 1988, Inaba, 2003). However, much is unresolved regarding the regulatory mechanism of movements in the axoneme. Reversible immobilization of chicken sperm motility (sperm suspended in a simple salt solution without Ca2+ were immotile at body temperature, but motility was instantly restored when the incubation temperature decreased below 30°C) has been described by Munro (1938) and Ashizawa and Nishiyama (1978). This reversible activation/inactivation of motility was also observed in drake sperm and partially in turkey sperm but not in quail sperm (Wishart and Wilson, 1999). In chicken sperm, motility inhibition at 40°C was quickly recovered when Ca2+ was included in the incubation mixture (Ashizawa et al., 1989 ). The depletion of Ca2+ from the medium by adding Ca2+ chelating reagents disturbed sperm motility even at 30°C. Therefore, it is considered that Ca2+ is essential for the maintenance of chicken sperm motility, as also reported in the sperm of many animals (Ashizawa et al., 1994b ). Later, it was found that motility inactivation at body temperature was also cancelled by an increase in the intracellular pH (pH i) of the sperm (Ashizawa et al., 1994c ). Sperm motility was activated in a 30°C temperature-controlled medium with various extracellular pH (pHe) values ranging from 7.3 to 10.1. In contrast, sperm exposed to 40°C medium, regardless of a pHe value lower than 8.1, were in a quiescent state (Ashizawa et al., 1994c ). The pHi at 40°C was approximately 0.3 units lower than the pHi at 30°C. An acidic pHi at 40°C possibly plays a role in motility immobilization at body temperature. The pH within the reproductive tract of the producing hen is generally higher than pH 8 (Fiser and Macpherson, 1974). Alternatively, the alkalization of sperm pHi is an important tool for sperm motility activation in chickens. Sperm acquire the potential for motility as they pass through the excurrent ducts (Ashizawa and Sano, 1990; Clulow, 1982; Howarth, 1983; Munro, 1938). Unfortunately, the question of how the composition of excurrent duct fluid affects sperm motility is not yet understood. In this regard, Ca2+ and HCO3− have been identified as motility agonists for in vitro assays (Ashizawa and Sano, 1990; Ashizawa and Wishart, 1987).
Sperm drastically change their flagellar movement in response to the surrounding physical and chemical environment. Testicular sperm are immotile; however, they gain the competence to initiate motility during passage through the male reproductive tract. During the process of ejaculation, the sperm is activated and promptly initiates motility. Unlike mammals, ejaculated sperm in birds are stored in specialized tubular invaginations referred to as sperm storage tubules (SSTs), located between the vagina and uterus, before fertilization. The resident sperm in the SSTs are in a quiescent state and are then reactivated after release from the SSTs. It is thought that avian sperm can undergo a motility change from a quiescent to an active state twice (Matsuzaki and Sasanami, 2022).
Sperm storage within the female reproductive tract is important for maintaining fertility. After copulation, either via natural mating or artificial insemination, in female poultry species, that is, hens, turkeys, and birds, sperm are transported through the cloaca to the junction of the uterus and the vagina (uterovaginal junction [UVJ]) of the oviduct (Bakst, 1998) and in the infundibulum, although the primary storage site for sperm is the SSTs in the UVJ (Brillard, 1993). In the UVJ, sperm enter the tubular invagination sites of the surface epithelium of the mucosa, collectively called sperm storage tubules (SSTs), where they are stored for longer periods, depending on the species, and retain their fertilizing capacity (Bréque et al., 2003; Holt and Fazeli, 2016). Uterus-vaginal protein extracts induce spermatic decapacitation in vitro (Camarillo et al., 2019). In general, the sperm storage tubules of hens are full for 24 to 48 h after insemination (Brillard, 1992; Bakst et al., 1994).
However, the molecular mechanism underlying sperm motility regulation is poorly understood (Matsuzaki and Sasanami, 2022).
In mammals, the acrosome reaction is required for sperm fertilization capacity, but thus far, there is no evidence that this process is necessary in birds (Howarth, 1970; Partyka and Niżański, 2022). Avian fertilization systems are quite different from those of mammalian species because of unique systems, such as polyspermic fertilization (Matsuzaki and Sasanami, 2022).
Another major difference between bovine and poultry reproductive biology that affects semen cryopreservation is the semen volume obtained per collection. The average ejaculate volume of a bull ranges from 5 to 8 mL, whereas semen volumes collected from poultry range from 0.1 to 1.5 mL. The rooster produces, on average, 0.6 mL of ejaculate (Taye and Esatu, 2022). Different roosters of the same species often produce different volumes of semen at different times (Getachew, 2016). The average volume ejaculated using the abdominal massage technique is approximately 0.25 mL (Gordon, 2005). The recorded semen volume was found to range from 0.37 to 0.73 mL (Peters et al., 2008). The average sperm concentration of poultry semen is much higher (6–10×109 sperm/mL) than that of bull semen (1–2×109 sperm/mL), and poultry sperm function is negatively impacted by excessive dilution, known as the dilution effect (Sexton and Fewlass, 1978; Sexton, 1981; Parker and McDaniel, 2006).
Sperm fertilizing ability is, however, drastically reduced at a dilution rate as high as 1:10 or 1:20 (Wilcox, 1958). It is possible that extensive dilution may destabilize the sperm membrane, which is detrimental to sperm motility and viability (Maxwell and Johnson, 1999). Furthermore, routinely collecting semen from donors (2–3 times a week) increases semen quality (Riaz et al., 2004).
In contrast to bulls, the pH of cockerel semen is higher, ranging from 7.2 to 7.6 (Getachew, 2016).
The biochemical characteristics and physiological roles of the various seminal plasma components in birds (carbohydrates, lipids, amino acids, hormones, and proteins) are poorly understood. Seminal plasma contains proteins expected to have an action on most cellular functions. A significant part of these proteins are common with the sperm cells, showing important exchanges between the sperm and their biological fluid. They include different metabolic functions, immunity, oxidoreduction actors and regulation, proteolysis, apoptosis, ion homeostasis, antimicrobial defences and epigenetic actors such as histones (Labas et al., 2014 ; Li et al., 2020). The variable amounts of many proteins are related to the different fertility capacities of poultry sperm. The role of seminal plasma in semen conservation (chilling and freezing) remains largely a matter of speculation, as both inhibitory and stimulating effects have been found (Santiago-Moreno and Blesbois, 2020).
The outermost membrane of the cell that surrounds the spermatozoon is called the spermatozoal plasma membrane (Chenoweth and Lorton, 2014). This membrane consists of a lipid bilayer made up of polar phospholipids, cholesterol, and proteins, a phospholipid-H2O interface on the exterior of the cell, and the glycocalyx, the outermost region of the plasma membrane (Hammerstedt and Graham, 1992). Rooster sperm plasma membranes consist of many different phospholipid species (Bongalhardo et al., 2002), which are distinct from their mammalian counterparts. These membranes have a lower protein-to-phospholipid ratio of 0.46 (w/w) than bulls (0.80) and therefore are more resistant to cold shock when reduced to temperatures of approximately 5°C than mammalian spermatozoa (Wales and White, 1959; Pickett and Komarek, 1967; Parks and Lynch, 1992). However, the rooster spermatozoal plasma membrane, while stable, is inelastic (Robertson, 1983), and when the sperm are exposed to hypotonic conditions, the membranes can easily rupture. This supports the belief that there is not enough research on the precise composition of sperm.
The resistance of sperm cells to cryopreservation has been verified by the membrane phospholipid content and fluidity (Holt, 2000). The fluidity of poultry sperm is closely related to the cholesterol/phospholipid ratio of the membrane (Holt, 2000) and is an indication of sperm freezability (Blesbois et al., 2005 ). It has been hypothesized that a high cholesterol content of the sperm plasma membrane is associated with a low freezing tolerance in mammals (Müller et al., 2008 ), while in poultry, the lower freezing tolerance of sperm cells is associated with lower concentrations of cholesterol and a lower fluidity of the plasma membrane (Blesbois et al., 2005 ). Membrane fluidity is dictated partially by the cholesterol:phospholipid ratio of the plasma membrane. Spermatozoon membranes with a high cholesterol:phospholipid ratio (rabbit 0.88; human 0.99) have more rigid membranes at room temperature. As these cells are cooled during cryopreservation, the high cholesterol content makes the membranes more fluid, and they incur less membrane damage than membranes from species that have low cholesterol:phospholipid ratios (rooster: 0.30; bull: 0.45) (Darin-Bennett and White, 1977; Parks and Lynch, 1992).
The plasma membranes of rooster sperm also contain a high level of unsaturated fatty acids in their phospholipid composition (Parks and Lynch, 1992). Poultry sperm cell membranes contain higher quantities of n-6 polyunsaturated fatty acids, including arachidonic (20:4n-6) and docosatetraenoic (22:4n-6) acids, than the membrane of mammalian sperm cells (Amini et al., 2015a ). This makes poultry sperm cells extremely vulnerable to lipid peroxidation (LPO) and reactive oxygen species (ROS) produced by the cellular components of semen during freezing (Nguyen et al., 2014). Experimental studies have shown that LPO causes irreversible damage to DNA and decreases sperm motility and fertility in the freezing/thawing process (Morris et al., 2011; Amini et al., 2015a; Amini et al., 2015b). The amount of maintenance of the lowest possible percentage of respiratory oxidative stress in sperm: unlike oocytes, sperm are characterized by permanent movement and high-level metabolic activity (high respiratory rate) during a very short time (Clarke et al., 1984). Seminal plasma from numerous animal species, including poultry, normally contains antioxidant properties (Bréque et al., 2003; Carolina et al., 2006; Li et al., 2010; Partyka et al., 2012; Khan et al., 2012). However, antioxidant activity is dramatically decreased by cryopreservation, resulting in higher levels of LPO (Partyka et al., 2012).
Phospholipids, specifically their fatty acids and the cholesterol concentration of a membrane, affect the fluidity and osmotic tolerance of the membrane (Parks and Lynch, 1992). Rooster sperm have a lower limit of osmotic tolerance of 17 mOsm, although its upper osmotic limit has not been characterized (Watson et al., 1992). This critical osmolality of 17 mOsm for rooster sperm is defined as the osmolality at which 50% of the cells have swollen to the point of rupture of the plasma membrane. The critical osmolality of sperm differs between species, and the ability of sperm to survive these osmotic stresses and volume changes affect their ability to survive the freezing process (Hammerstedt et al., 1990). In theory, the rooster spermatozoon's cylindrical-tapered shape should accommodate larger amounts of cell volume change to a sphere shape without rupturing (Watson et al., 1992). In contrast, it has been observed that a rooster plasma membrane swells over both the head and tail (Bakst, 1980), and when one part of the membrane does not accommodate larger amounts of cell volume, this leads to the plasma membrane bursting sooner than theorized (Watson et al., 1992). Since the upper limit of osmotic tolerance for rooster sperm is unknown, both theories could prove true until this limit is uncovered.
Morris et al. (2011) found that a combination of intracellular protein content and osmotic shrinkage causes intracellular URF of spermatozoa during cooling, leading to osmotic shock when sperm are thawed and subsequent membrane damage. The sperm membrane is the first barrier for the sperm against the outside environment, and when this membrane is altered negatively, this can lead to an alteration of the sperm's intracellular environment and cell dysfunction. However, when the sperm plasma membrane is altered positively by increasing plasma membrane fluidity at lower temperatures, this in turn increases cell cryosurvival (Partyka et al., 2016). Therefore, focusing research on plasma membrane manipulation and alternative cryoprotectants is the key to improving rooster spermatozoa cryosurvival.
Semen Contamination of Bacteria
In poultry, pathogenic bacteria have been found to be associated with sperm, but their impact on sperm quality is not yet understood (Haines et al., 2013 ). Vizzier-Thaxton et al. (2006) demonstrated that Campylobacter could attach rooster sperm, which potentially results in decreased sperm quality. Bacteria can reduce broiler sperm motility upon exposure (Haines et al., 2013 ).
The bacteria possess a relatively high tolerance to the freezing procedure: the microbial contamination of cryopreserved semen stored for 6–35 years in liquid nitrogen was reported (Bielanski et al., 2003 ). For that reason, semen diluents containing compounds antibiotics with bactericidal or bacteriostatic properties should be used. On the other hand, the use of antibiotics is a highly controversial issue today due to the environment and antimicrobial resistance (Savvulidi et al., 2018 ). In addition, some antibiotics may be toxic to spermatozoa, as Morrel and Wallgren (2014) reviewed. Therefore, there is an urgent need to find alternatives to conventional antibiotics for use in semen extenders. Therefore, new approaches for the elimination of bacterial contamination in semen were developed. Ozone treatment of semen (Gradil et al., 1995 ), using diode laser and light emitting diode on the bacterial contamination in semen (Hussein et al., 2008 ), or iron oxide (Fe3O4) nanoparticles have also been found to have antimicrobial activity against some bacteria (Guzman et al., 2012; Tsakmakidis et al., 2020; Tsakmakidis et al., 2021). Additionally, the use of antimicrobial peptides (AMPs) has become one of the most promising alternatives to the use of antibiotics in semen extender formulations to overcome the increasing bacterial resistance to antibiotics (Bussalleu et al., 2017 ).
Freezing Process
In the past 10 years, many studies have been performed to improve methods for bird sperm reproductive potential conservation after freeze‒thaw cycles (Morris et al., 2011; Amini et al., 2015a; Partyka et al., 2016). During the poultry semen cryopreservation process (including the cooling–freezing and thawing procedures), osmotic and thermic shocks may cause damage to cell structure and metabolism (Long, 2006). Epigenetic modifications, which include DNA methylation and histone acetylation in rooster sperm, were also found to be significantly reduced after cryopreservation (Salehi et al. 2020). Therefore, works aimed at the improvement of the composition of diluents for cryopreservation, the selection of cryoprotectant and freezing methods (in straws or pellets), or the freezing protocols (low/fast) are still of research interest (Abouelezz et al., 2015; Nugrahini et al., 2019; Gatti et al., 2021; Tang et al., 2021). Different freezing and thawing procedures have been developed to prevent damage or destruction of cells during cryopreservation. These procedures are summarized in Table 1, including the results of viability and other functional parameters of sperm after thawing.
Table 1.
| Author | 1 | 2 | 3 | 4 [%] | 5 | 6 | 7 | 8 [%] | 9 [%] | 10 [%] | 11 [%] | 12 [%] | 13 [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abouelezz et al. (2017) | Lake and Ravie | S | Gly | 8 | −5 to −100°C at 10°C/min, −196°C | 3min/5°C | 57.8 | 31.3 | |||||
| Lake and Ravie | P | DMA | 5 | −196°C | 60°C | 57 | 46.4 | ||||||
| Abouelezz et al. (2015) | Lake and Ravie | S | DMA | 6 | 5 to −35°C at 7°C/min; −35°C to −140°C at 60°C/min | 30sec/37°C | 21.1 | 21.3 | 10.8 | ||||
| Lake and Ravie | P | DMA | 6 | −196°C | 60°C | 18 | 19.8 | 12.8 | |||||
| Lake and Ravie | S | Gly | 11 | 5 to −35°C at 7°C/min; −35°C to −140°C at 60°C/min | 3min/5°C | 47.6 | 43.4 | 2.1 | |||||
| Lake and Ravie | P | Gly | 11 | −196°C | 60°C | 18.6 | 24 | 4.2 | |||||
| Lake and Ravie | S | Gly | 8 | 5 to −35°C at 7°C/min; −35°C to −140°C at 60°C/min | 3min/5°C | 37 | 39 | 28.8 | |||||
| Lake and Ravie | P | DMA | 3 | −196°C | 60°C | 23.6 | 24.7 | 25 | |||||
| Appiah et al. (2020) | modified | P | Gly | 13.5 | Quercetin | −196°C | 6sec/37°C | ||||||
| Appiah et al. (2019) | modified | P | Gly | 13.5 | Melatonin | −196°C | 30sec/37°C | ||||||
| Behnamifar et al. (2021) | Lake | S | DMA | 4 | 5 to −35°C at 7°C/min; −35°C to −140°C at 60°C/min | 3min30sec/5°C | 15.3 | 14.08 | |||||
| Lake | S | Gly | 8 | 5 to −35°C at 7°C/min; −35°C to −140°C at 60°C/min | 3min30sec/5°C | 40.22 | 52.17 | ||||||
| Lake + BSA | S | Gly | 8 | 5 to −35°C at 7°C/min; −35°C to −140°C at 60°C/min | 3min30sec/5°C | 35.4 | 25.06 | ||||||
| Bernal et al. (2020) | Lake and Ravie + Val | S | Gly | 8 | 5 to −35°C at 7°C/min; −35°C to −140°C at 60°C/min | 3 min/5°C | 32.6 | ||||||
| Blank et al. (2020) | Lake + FBS | S | DMF | 6 | 5°C for 15 min, −35°C for 12 min, −135°C for 5 min, −196°C | 5 min/5°C | 29.62 | ||||||
| Ehling et al. (2012) | HS1 | S | DMF + NMA 1:2 | 6,5/6,5 | −3°C/min to −35°C, −50°C/min to −130°C at 60°C/min | 4°C | 44.3 | 81.1 | |||||
| HS1 | S | DMF + NMA 1:1 | 6,5/6,5 | −3°C/min to −35°C, −50°C/min to −130°C at 60°C/min | 4°C | 47.7 | 77.3 | ||||||
| HS1 | S | MA | 6.5 | −3°C/min to −35°C, −50°C/min to −130°C at 60°C/min | 4°C | 32.7 | 39.8 | ||||||
| Fattah et al. (2017) | BPSE | S | Gly | 3 | 4 cm above the liquid N2 for 7 min | 30 sec/37°C | 52.7 | ||||||
| BPSE | S | Gly | 3 | L carnitin | 4 cm above the liquid N2 for 7 min | 30 sec/37°C | 69.1 | ||||||
| Feyzi et al. (2018) | Lake | S | Gly | 5 | 5 cm above the liquid N2 for 12 min | 30 sec/37°C | 61.2 | 48 | |||||
| Lake + NO | S | Gly | 5 | 5 cm above the liquid N2 for 12 min | 30 sec/37°C | 76.4 | 59 | ||||||
| Gatti et al. (2021) | Lake | S | DMA | 3 | 3 cm above the liquid N2 for 7 min | 20sec/37°C | 37.2 | 58.4 | 70.3 | ||||
| Lake | S | DMA | 6 | 3 cm above the liquid N2 for 7 min | 20sec/37°C | 42 | 53.7 | 74.6 | |||||
| Lake | S | DMA | 9 | 3 cm above the liquid N2 for 7 min | 20sec/37°C | 34.8 | 55.1 | 74.4 | |||||
| Lake | S | DMA | 12 | 3 cm above the liquid N2 for 7 min | 20sec/37°C | 32.4 | 50.7 | 65.8 | |||||
| Li et al. (2020) | modified | P | Gly | 13.5 | Caragenan, CLC | −196°C | 37°C | ||||||
| Long et al. (2010) | BPSE | S | Gly | 11 | −20°C for 30 min, −196°C | 2-3min/3°C | 49.6 | ||||||
| Lotfi et al. (2017) | BPSE | S | Gly | 3 | HA | 4 cm above the liquid N2 for 7 min | 46.1 | 55.3 | 65.5 | 57.5 | |||
| Madeddu et al. (2016) | Lake | S | DMA | 6 | 1cm/3cm/5cm/7cm/10 cm above the liquid nitrogen for 10 min | 30sec/38°C | 46.1 | 31.1 | |||||
| Masoudi et al. (2021) | Lake | S | Mito-tempo | 5 cm above the liquid N2 for 12 min | 30sec/37°C | 65.1 | 62.3 | 65.3 | 87.3 | ||||
| Masoudi et al. (2019) | Lake | S | Gly | 3 | Glu | 4 cm above the liquid N2 for 7 min | 30sec/37°C | 60.8 | 58.8 | 63.8 | 49.8 | 91.4 | |
| Masoudi et al. (2018) | Lake | S | Gly | 3 | Co-Q10 | 4 cm above the liquid N2 for 7 min | 30sec/37°C | 67.1 | 56.9 | 62.7 | 51.1 | 87.1 | |
| Mehaisen et al. (2020) | EK | P | DMA | 6 | 4°C for 15 min (1.13°C/min), −196°C | 6sec/60°C | 32.5 | 34.7 | 64.6 | ||||
| Mehdipour et al. (2021) | Lake+AFP3 | S | Gly | 3.8 | 4 cm above the liquid N2 for 7 min | 30sec/37°C | 61.5 | ||||||
| Mehdipour et al. (2018) | BPSE | S | SBL | 0.5 | 4 cm above the liquid N2 for 7 min | 30sec/37°C | 43.04 | 41.79 | 41.39 | ||||
| BPSE | S | SBL | 1 | 4 cm above the liquid N2 for 7 min | 30sec/37°C | 73.56 | 68.74 | 54 | 61.65 | ||||
| BPSE | S | SBL | 1.5 | 4 cm above the liquid N2 for 7 min | 30sec/37°C | 55.7 | 50.61 | 46.85 | |||||
| BPSE | S | EY | 10 | 4 cm above the liquid N2 for 7 min | 30sec/37°C | 56.76 | 53.76 | 47.71 | |||||
| BPSE | S | EY | 15 | 4 cm above the liquid N2 for 7 min | 30sec/37°C | 64.96 | 59.5 | 55.49 | |||||
| BPSE | S | EY | 20 | 4 cm above the liquid N2 for 7 min | 30sec/37°C | 76.03 | 70.12 | 60 | 66.26 | ||||
| Mehdipour et al. (2020) | Lake | S | Gly | 2 | P188 | 4 cm above the liquid N2 for 7 min | 30sec/37°C | 66.7 | |||||
| Lake | S | Gly | 8 | P188 | 4 cm above the liquid N2 for 7 min | 59 | |||||||
| Mphaphathi et al. (2016) | Kobidil+ | S | DMSO | 8 | 5 to −20°C at −1°C/min; 5 cm above the liquid N2 for 5 min, −196°C | 5min/5°C | 46 | ||||||
| Kobidil+ | S | EG | 8 | 5 to −20°C at −1°C/min; 5 cm above the liquid N2 for 5 min, −196°C | 5min/5°C | 45 | |||||||
| Kobidil+ | S | PND | 8 | 5 to −20°C at −1°C/min; 5 cm above the liquid N2 for 5 min, −196°C | 5min/5°C | 21.8 | |||||||
| Mosca et al. (2016) | Lake | S | DMA | 6 | 3 cm above the liquid N2 for 10 min | 30sec/38°C | 46.9 | 35.8 | |||||
| Lake+T 0,1M | S | DMA | 6 | 3 cm above the liquid N2 for 10 min | 30sec/38°C | 46.4 | 38.4 | ||||||
| Lake+S 0,1M | S | DMA | 6 | 3 cm above the liquid N2 for 10 min | 30sec/38°C | 44.7 | 35.3 | ||||||
| Lake +S/T (0,1M; 0,1M) | S | DMA | 6 | 3 cm above the liquid N2 for 10 min | 30sec/38°C | 43.4 | 35.9 | ||||||
| Mosca et al. (2020) | Lake+T 0,1M | S | NMA | 6 | 3 cm above the liquid N2 for 10 min | 100sec/5°C | 50.7 | 52.3 | |||||
| Lake+T 0,1M | S | NMA | 9 | 3 cm above the liquid N2 for 10 min | 100sec/5°C | 36.6 | 35.5 | ||||||
| Lake+T 0,1M | S | NMA | 6 | 3 cm above the liquid N2 for 10 min | 30sec/38°C | 22.8 | 20 | ||||||
| Lake+T 0,1M | S | NMA | 9 | 3 cm above the liquid N2 for 10 min | 30sec/38°C | 20.5 | 18.1 | ||||||
| Miranda et al. (2018) | Kobidil+ | S | DMA | 6 | 5 cm above the liquid N2 for 15 min | 30sec/37°C | 32.3 | ||||||
| Kobidil+ | S | DMF | 7.5 | 5 cm above the liquid N2 for 15 min | 30sec/37°C | 28.6 | |||||||
| Kobidil+ | S | MA | 9 | 5 cm above the liquid N2 for 15 min | 30sec/37°C | 30.7 | |||||||
| Kobidil+ | S | EG | 8 | 5 cm above the liquid N2 for 15 min | 30sec/37°C | 27.9 | |||||||
| Kobidil+ | S | DMA | 6 | 5 cm above the liquid N2 for 15 min | 2min/5°C | 31.1 | |||||||
| Kobidil+ | S | DMF | 7.5 | 5 cm above the liquid N2 for 15 min | 2min/5°C | 39.8 | |||||||
| Kobidil+ | S | MA | 9 | 5 cm above the liquid N2 for 15 min | 2min/5°C | 31.3 | |||||||
| Kobidil+ | S | EG | 8 | 5 cm above the liquid N2 for 15 min | 2min/5°C | 46.6 | |||||||
| Nabi et al. (2016) | Nabi | S | Gly | 3 | 4 cm above the liquid N2 for 7 min | 3min/4°C | 65.4 | 73.1 | 53.5 | ||||
| BPSE | S | Gly | 3 | 4 cm above the liquid N2 for 7 min | 3min/4°C | 21.4 | 21 | ||||||
| Najafi et al. (2021) | Lake+SBL | S | DMSO | 4 | P188 | 4 cm above the liquid N2 for 7 min | 30sec/37°C | 79.5 | |||||
| Najafi et al. (2019) | BPSE | S | Gly | 3.8 | Resveratrol | 4 cm above the liquid N2 for 7 min | 30sec/37°C | 70.8 | 64.3 | 58.1 | 64.5 | ||
| BPSE | S | Gly | 3.8 | Resveratrol+NLC | 4 cm above the liquid N2 for 7 min | 30sec/37°C | 75 | 73 | 67.2 | 67.3 | |||
| Najafi et al. (2018) | BPSE | S | Gly | 8 | Lycopene | 4 cm above the liquid N2 for 7 min | 30sec/37°C | 69.7 | 64.08 | 57.8 | 59.22 | ||
| BPSE | S | Gly | 8 | Lycopene LnL | 4 cm above the liquid N2 for 7 min | 30sec/37°C | 72.91 | 68.07 | 62.2 | 64.66 | |||
| Partyka et al. (2017) | EK | P | DMA | 6 | L-carnitin 1mM | −8°C for 15 min, −196°C | 6sec/60 °c | 37.6 | 48.06 | ||||
| EK | P | DMA | 6 | L-carnitin 5mM | −8°C for 15 min, −196°C | 6sec/60 °c | 34.33 | 49.94 | |||||
| EK | P | DMA | 6 | Hypotaurin 1mM | −8°C for 15 min, −196°C | 6sec/60 °c | 36.44 | 52.18 | |||||
| EK | P | DMA | 6 | Hypotaurin 10mM | −8°C for 15 min, −196°C | 6sec/60 °c | 32.83 | 51.4 | |||||
| EK | P | DMA | 6 | Taurin 1mM | −8°C for 15 min, −196°C | 6sec/60 °c | 40.18 | 53.58 | |||||
| EK | P | DMA | 6 | Taurin 10mM | −8°C for 15 min, −196°C | 6sec/60 °c | 35.41 | 53.37 | |||||
| Rakha et al. (2018) | RFE | S | EY | 10 | 37°C to 4°C (−0.275°C/min), 4°C to −80°C (−8.4°C), −196°C | 30sec/37°C | 61.2 | 75.9 | 75.6 | ||||
| RFE | S | EY | 15 | 37°C to 4°C (−0.275°C/min), 4°C to −80°C (−8.4°C), −196°C | 30sec/37°C | 74.1 | 75 | 55.9 | 75.9 | ||||
| RFE | S | EY | 20 | 37°C to 4°C (−0.275°C/min), 4°C to −80°C (−8.4°C), −196°C | 30sec/37°C | 67.1 | 63.5 | 70.4 | |||||
| RFE | S | EY | 25 | 37°C to 4°C (−0.275°C/min), 4°C to −80°C (−8.4°C), −196°C | 30sec/37°C | 74.8 | 65.1 | 62 | |||||
| RFE | S | Gly | 20 | 37°C to 4°C (−0.275°C/min), 4°C to −80°C (−8.4°C), −196°C | 30sec/37°C | 61.9 | 51.8 | 46.5 | 65.6 | ||||
| Rakha et al. (2018) | modifided | S | Gly | 20 | 37°C to 4°C (−0.275°C/min), 4°C to −80°C (−8.4°C), −196°C | 30sec/37°C | 53.1 | ||||||
| modifided | S | DMSO | 4 | 37°C to 4°C (−0.275°C/min), 4°C to −80°C (−8.4°C), −196°C | 30sec/37°C | ||||||||
| modifided | S | DMSO | 6 | 37°C to 4°C (−0.275°C/min), 4°C to −80°C (−8.4°C), −196°C | 30sec/37°C | ||||||||
| modifided | S | DMSO | 8 | 37°C to 4°C (−0.275°C/min), 4°C to −80°C (−8.4°C), −196°C | 30sec/37°C | 73 | |||||||
| modifided | S | DMSO | 10 | 37°C to 4°C (−0.275°C/min), 4°C to −80°C (−8.4°C), −196°C | 30sec/37°C | ||||||||
| Salehi et al. (2020) | Lake | S | Gly | 3 | 4 cm above the liquid N2 for 5 min | 30sec/37°C | 60 | 77.2 | 59.3 | 32.9 | |||
| BPSE | S | Gly | 3 | 4 cm above the liquid N2 for 5 min | 30sec/37°C | 51 | 68.3 | 47.2 | 35.5 | ||||
| Murugesan and Mahapatra (2020) | Sasaki | S | DMA | 6 | 4.5 cm above the liquid N2 for 30 min | 100sec/5°C | 34.93 | 0 | 4.57 | ||||
| Sasaki | S | DMSO | 2 | 4.5 cm above the liquid N2 for 30 min | 100sec/5°C | 22.66 | 0 | 94.85 | |||||
| Lake | S | DMA | 6 | 4.5 cm above the liquid N2 for 30 min | 100sec/5°C | 34.46 | 0 | 4.14 | |||||
| Lake | S | DMSO | 2 | 4.5 cm above the liquid N2 for 30 min | 100sec/5°C | 21.23 | 0 | 91.61 | |||||
| Sasaki | S | EG | 8 | 4.5 cm above the liquid N2 for 30 min | 100sec/5°C | 23.72 | 0 | 94 | |||||
| Lake and Ravie | S | EG | 8 | 4.5 cm above the liquid N2 for 30 min | 100sec/5°C | 26.52 | 0 | 92.33 | |||||
| RFE | S | EG | 8 | 4.5 cm above the liquid N2 for 30 min | 100sec/5°C | 22.72 | 0.83 | 92.83 | |||||
| Sasaki | S | EG | 8 | 4.5 cm above the liquid N2 for 30 min | 30sec/37°C | 26 | 18.39 | 84.58 | |||||
| Lake and Ravie | S | EG | 8 | 4.5 cm above the liquid N2 for 30 min | 30sec/37°C | 24.67 | 48.12 | 69.98 | |||||
| RFE | S | EG | 8 | 4.5 cm above the liquid N2 for 30 min | 30sec/37°C | 20.33 | 38.29 | 62.73 | |||||
| Sasaki | S | DMF | 6 | 4.5 cm above the liquid N2 for 30 min | 100sec/5°C | 26 | 24.76 | 55.61 | |||||
| Lake and Ravie | S | DMF | 6 | 4.5 cm above the liquid N2 for 30 min | 100sec/5°C | 23.81 | 30.89 | 22.03 | |||||
| BPSE | S | DMF | 6 | 4.5 cm above the liquid N2 for 30 min | 100sec/5°C | 23.53 | 19.32 | 12.8 | |||||
| Shanmugam and Mahapatra (2019) | Lake and Ravie | P | DMF | 6 | −196°C | 45sec/56°C | 28.31 | 1.19 | |||||
| Lake and Ravie | P | DMF | 9 | −196°C | 45sec/56°C | 24.02 | 1.38 | ||||||
| TES/NaCl | P | DMF | 6 | −196°C | 45sec/56°C | 17.79 | 0 | ||||||
| TES/NaCl | P | DMF | 9 | −196°C | 45sec/56°C | 19.51 | 0 | ||||||
| Lake and Ravie | P | DMA | 6 | −196°C | 60°C | 40.23 | 2.75 | ||||||
| Lake and Ravie | P | DMA | 9 | −196°C | 60°C | 39.62 | 9.22 | ||||||
| Lake and Ravie+S+BSA | P | DMA | 6 | −196°C | 60°C | 39.62 | 9.58 | ||||||
| Lake and Ravie+S+BSA | P | DMA | 9 | −196°C | 60°C | 41.81 | 7.6 | ||||||
| modifide | P | DMA | 6 | −196°C | 60°C | 38.71 | 9.81 | ||||||
| modifide | P | DMA | 9 | −196°C | 60°C | 36.64 | 3.1 | ||||||
| RFE | S | NMA | 12 | 4.5 cm above the liquid N2 for 30 min | 100sec/5°C | 24.43 | 0 | ||||||
| RFE | S | DMA | 9 | 4.5 cm above the liquid N2 for 30 min | 100sec/5°C | 27.85 | 0 | ||||||
| RFE | S | DMSO | 4 | 4.5 cm above the liquid N2 for 30 min | 100sec/5°C | 27.21 | 1.9 | ||||||
| Shahverdi et al. (2015) | BPSE | S | Gly | 2 | 5 cm above the liquid N2 for 12 min | 30sec/37°C | 22.7 | ||||||
| BPSE | S | LDL | 1 | 5 cm above the liquid N2 for 12 min | 30sec/37°C | 24.1 | |||||||
| BPSE | S | LDL | 2 | 5 cm above the liquid N2 for 12 min | 30sec/37°C | 32.2 | |||||||
| BPSE | S | LDL | 4 | 5 cm above the liquid N2 for 12 min | 30sec/37°C | 43.1 | |||||||
| BPSE | S | LDL | 6 | 5 cm above the liquid N2 for 12 min | 30sec/37°C | 32.1 | |||||||
| BPSE | S | LDL | 8 | 5 cm above the liquid N2 for 12 min | 30sec/37°C | 19.8 | |||||||
| Stanishevskaya et al. (2021) | LCM | P | DMA | 6 | −196°C | 60°C | 48 | 79 | |||||
| LCM+4,8mM | P | DMA | 6 | −196°C | 60°C | 48 | 82 | ||||||
| LCM+9,5mM | P | DMA | 6 | −196°C | 60°C | 50 | 86 | ||||||
| Thélie et al. (2019) | Lake | S | Gly | 11 | −7°C/min | 3min/4°C | 83.3 | ||||||
| BHSV | S | EG | 10 | −1°C/min | 3min/4°C | 1.5 | |||||||
| BHSV | S | DMF | 6 | −15°C/min | 3min/4°C | 64.8 | |||||||
| FEB | S | DMA | 6 | −60°C/min | 5sec/40°C | 35.3 | |||||||
| Wu et al. (2020) | Lake | S | Gly | 2 | −5°C/min, −9°C/min, −13°C/min, −6.5°C/min | 3min/5°C | 34.8 | ||||||
| Lake | S | Gly | 4 | −5°C/min, −9°C/min, −13°C/min, −6.5°C/min | 3min/5°C | 75.1 | |||||||
| Lake | S | Gly | 6 | −5°C/min, −9°C/min, −13°C/min, −6.5°C/min | 3min/5°C | 77.63 | |||||||
| Lake | S | Gly | 8 | −5°C/min, −9°C/min, −13°C/min, −6.5°C/min | 3min/5°C | 70.09 | |||||||
| Lake | S | Gly | 11 | −5°C/min, −9°C/min, −13°C/min, −6.5°C/min | 3min/5°C | 57.43 |
1) extender (BSA- bovine serum albumine; Val-valine; FBS-fetal bovine serum; NO-nitric oxide; BPSE-Beltsville poultry semen extender; RFE-red fowl extender; TES-N-Tris-[hydroxymethyl]-methyl-2-aminoethansulfonic acid; LCM- Leningrad cryoprotective medium; BHSV-Blumberger Hahnen Spermna Verdünner); 2) type of packaging (P-pellets; S-straws); 3) cryoprotectant (Gly-glycerol; DMA-dimethylacetamide; DMF-dimethylformamide; MA-methylacetamide; SBL-soyabean lecithin; EY-egg yolk; DMSO-dimethylsulfoxide; EG-ethylenglycol; PND-propandiol; LDL-low-density lipoproteins); 4) concentration; 5) antioxidant (CLC-cyclodextrine; HA-hyaluronic acid; Glu-glutathion; Co-Q10-coenzyme Q10; P188-poloxamer 188; NLC-nanostructure lipid carriers); 6) freezing rate; 7) thawing temperature; 8) viability; 9) total motility; 10) fertility; 11)plasmtic membrane damage; 12) mitochondrial activity; 13) acrosomal integrity
The first studies of preserving fowl semen used a slow cooling rate (Lake and Stewart, 1978; Sexton, 1981), for example, 1 to 10°C/min to −35°C. This method of freezing permits the flow of intracellular water from the cells to the outside the cells, thereby promoting extracellular crystallization and avoiding lethal intracellular ice formation. In slow freezing, low concentrations of CPA are used, the cells dehydrate during freezing, the temperature decreases slowly, and the formation of ice crystals is controlled. Currently, fast cooling rates are preferable (Váradi et al., 2013; Long et al., 2014), for example, 20 to 100°C/min. Optimal rapid freezing avoids excessive dehydration and shrinkage of cells. One of the possible methods is ultrarapid freezing (URF). High concentrations of CPA are used in URF, the cells are dehydrated before freezing, and the rate of temperature decrease is more than 1,000°C/min. During URF, the solution solidifies at a low temperature without crystals. However, high concentrations of CPAs could be toxic to cells (Dinnyes et al., 2007).
An interesting solution to sperm freezing seems to be gradual cooling by exposing the semen sample to liquid nitrogen vapor (Purdy et al., 2009; Rakha et al., 2017; Wu et al., 2020). Freezing semen packaged in straws over nitrogen vapor is a simple, quick, and inexpensive method that is widely used for cryopreservation of mammalian semen, even in commercial AI (artificial insemination) centers. This method has the great advantage of allowing the adoption of temperature gradients suitable for freezing semen in liquid nitrogen without the need for expensive dedicated equipment. In fact, the distance between the straw and the liquid nitrogen bath indirectly determines the thermal gradient during the freezing phase when the change from the liquid to the solid-state occurs. More rapid freezing rates are required to improve the survival of rooster sperm after cryopreservation, and a range of distances from 1 to 5 cm in nitrogen vapor above the surface of the liquid nitrogen is recommended for optimal sperm viability (Madeddu et al., 2016). Wu et al. (2020) as an optimal recommend exposing straws located as high as 6 cm above the liquid nitrogen surface. The methods used to control the freezing rate mainly include one-step freezing (Iaffaldano et al., 2016; Abouelezz et al., 2017; Fattah et al., 2017; Rakha et al., 2017) and 2-step freezing, that is, freezing slowly from 5°C to −35°C and then rapidly from −35°C to −150°C (Abouelezz et al., 2015; Blanch et al., 2014; Yang et al., 2016). A programmable freezing machine was also used (Santiago-Moreno et al., 2011; Blanco et al., 2012); however, it was not applied in practice, as this equipment is not always available (particularly in field conditions). Additionally, the temperature and method of thawing rooster sperm is one of the key points. During osmotic damage, the effect depends on the temperature. The formation of ice crystals is avoided during rapid warming, and extracellular thawing causes a sudden reduction in osmotic pressure, whereby the cryoprotectant is washed out before any toxic damage occurs (Wolfe and Bryant, 2001). It is difficult to compare the results of individual studies, as various papers have reported that the success of sperm cryopreservation procedures depends on the interaction among the type of cryoprotectant, semen freezing, thawing conditions, and packaging system used, each affecting sperm structure and function (Long et al., 2014; Iaffaldano et al., 2016). For example, the results of Miranda et al. (2018) indicate that the combination of EG (8%) and thawing at 5°C is the best option for rooster sperm. Compared to that, the authors Murugesan and Mahapatra (2020) , based on their research, recommend using cryoprotectant EG (8%) as well, however, with 37°C thawing temperature 37°C for 30 s. Mosca et al. (2020) investigated the effect of the CPA N-methylacetamide (MA) and of different thawing temperatures. The treatment that provided the best cryoprotective action and decreased the cellular cryodamage was the concomitant use of 6% MA and thawing at 5°C for 100 s. In contrast, Miranda et al. (2018) reported no differences comparing the effect of two thawing temperatures (5 vs. 37°C) on sperm motility of chicken semen cryopreserved with MA. Although very few studies have compared thawing temperatures in chicken semen, existing results indicate that thawing temperature may be a critical stage in the cryopreservation protocol.
Cryoprotectants
The survival of gametes after cryopreservation depends on the type and concentration of cryoprotectant (CPA) substances or solutions inserted into gametes before freezing. These are low- and high-molecular-weight organic substances readily soluble in water and are not particularly toxic even at higher concentrations. A number of these cryoprotectants are known today and are divided into freely penetrating CPAs (P-CPAs) and nonpenetrating CPAs (N-CPAs).
Thus, the important step in successful poultry semen cryopreservation is the choice of CPAs and their use during the process. Commonly used cryoprotectants are glycerol (GLY) (Wu et al., 2020), dimethylacetamide (DMA) (Abouelezz et al., 2017), dimethyl sulfoxide (DMSO) (Zhandi et al., 2017), ethylene glycol (EG) ( Murugesan and Mahapatra, 2020), and dimethylformamide (DMF) (Rakha et al., 2020). These penetrating CPAs enter the cells and avoid intracellular water crystal formation and prevent damage caused by the excessive intracellular concentration of solutes (Holt, 2000; Pereira and Marques, 2008) and have been used across various protocols and extenders. Molecularly, previous CPAs are characterized by high permeability inside the cell and low toxicity compared to other CPAs (Mohammad et al, 2021).
CPAs have a toxic effect, the severity of which varies from one type to another. This effect will lead to a decrease in the various vital and morphological characteristics of the sperm in addition to the fertility capacity. The chemical toxicity of CPAs is associated with two effects. The first is the chemical action on the cells before cryopreservation, and the second is the cause of osmotic changes in the freezing solution. Low concentrations of CPAs are usually used during slow freezing. However, the concentration of CPAs used in URF is high. Therefore, it is necessary to consider the cell permeability for each cryoprotectant to estimate CPAs toxicity. High permeability can cause osmotic stress in cells before freezing and thawing processes. The rate of penetration of CPAs is also strictly temperature dependent. Therefore, the main factors influencing the toxicity of cryoprotectants are concentration, exposure temperature, and freezing time (Benesova and Trefil, 2015). Tselutin et al. (1999) focused their work on in vitro comparison of the three CPAs glycerol, DMA, and DMSO and showed that DMSO is the most toxic CPA and glycerol the least deleterious; the highest fertility rates were obtained with DMA, but only when spermatozoa were frozen in pellets. However, for example, for gene banking, which requires high levels of safety and clear identification, glycerol and straws are more convenient. Glycerol-treated spermatozoa unfortunately have a contraceptive effect after intravaginal insemination in chickens. More than 2% glycerol in an insemination dose causes complete absence of fertility (Neville et al., 1971; Lake, 1986). Moreover, Shanmugam and Mahapatra (2021) reported that when the concentration of glycerol is above 1% during insemination, it results in contraception, and no fertile eggs are obtained. This concentration is insufficient for sperm protection during cryopreservation. The problem may be solved by dialysis or centrifugation of the insemination dose after thawing to remove glycerol (Benesova and Trefil, 2015).
In recent years, N-methylacetamide (MA) has also become popular for the cryopreservation of rooster semen. The MA compound apparently has less cytotoxicity in cryopreserved cells (Mosca et al., 2016). When Sasaki et al. (2010) used a concentration of 9% MA, the overall hatchability rate was 89.5% in Yakido chickens. NMA displayed a negative concentration-dependent effect on fertility (Kim et al., 2014 ). Consequently, the related fertilization rate remains highly variable: in chickens, fertility after artificial insemination with frozen/thawed semen cryopreserved with NMA ranged from 0 ( Shanmugam et al., 2018) to 100% (Sasaki et al., 2010), as Mosca et al. (2020) reviewed. N-Methylacetamide (NMA; H3C-C(O)-N(H)-CH3) is obtained by replacing the sulfinyl group [-S(O)-]in DMSO with an amide group [-C(O)-N(H)-] (Osuga et al., 2018). Because 6C, 7N, and 8O in the amide group have no d-orbital effects (Kauzmann, 1957) and the amide group is naturally involved in bioprocesses (Edsall et Wyman, 1958; Branden et Tooze, 1999), the cytotoxicity of NMA is lower than that of DMSO in cryopreserved human cells (Osuga et al., 2017). NMA is used in the cryopreservation of bovine spermatozoa (Jeyengran et Graham, 1980) and fowl semen (Sasaki et al., 2010; Lee et al., 2012). Polymers may be added to minimize the toxic effect of CPAs. These are nonpenetrating, remain in the space around the cells and at the same time reduce other CPAs (Partyka and Niżański, 2022). In recent years, work has also appeared on antifreeze proteins (AFPs) in the cryopreservation of poultry sperm. Unlike many other solutions, these proteins kinetically lower the temperature at which ice crystals form in a way that avoids heat shock. These proteins modify or prevent the growth of ice crystals and could stop recrystallization. This protects the cell membranes from cold-induced damage (Robles et al., 2019). Mehdipour et al. (2021) applied Type III antifreeze protein (AFP3) to the cryopreservation of spermatozoa from broiler breeder roosters, aiming to enhance post-thaw quality and fertility. The results suggested that AFP III may benefit sperm cryopreservation, considering post-thaw sperm quality and fertility parameters. Specifically, 1 mg/ml AFP III was the most appropriate concentration, with an optimal concentration potentially between 0.1 and 1 mg/ml. The high cost of AFP production can be a limiting factor for the widespread use of AFP in the cryopreservation process. However, studies have shown that AFPs can either protect or harm cells, depending on the AFP type and concentration used, other cryoprotectants present, and freeze‒thaw protocols used (Brockbank et al., 2011; Bang et al., 2013). Vitrification, in which a solution is cooled below the glass transition, is a procedure commonly used in cryopreservation to avoid problems with ice. Nevertheless, harmful ice formation is common during rewarming. Significant protective effects of AFPs after vitrification were noted, for example, in oocytes (Jo et al., 2011), sperm (Qadeer et al., 2015; Zilli et al., 2014), and embryos (Nishijima et al., 2014). The negative effects of AFPs seem to be related to the spicular shape of ice induced by fish AFPs (Rubinsky et De Vries, 1989; Ishiguro et Rubinsky, 1998), which fail to block ice growth out of the basal plane. Thus, other IBPs that bind multiple ice planes, including the basal planes, may be more promising for cryopreservation (Halwani et al., 2014).
Instead of using AFP, synthetic ice blockers (as a cost-effective alternative) might be used for sperm cryopreservation (De Leon et al., 2012; Quan et al., 2013).
The use of poloxamer 188 (P188) supplements seems promising in the cryopreservation process of poultry semen. P188 is an embryo cryopreservation supplement effective in many species and for cell lines and plant cells. Mehdipour et al. (2020) tested the suitability of P188 in the cryopreservation of rooster sperm, considering post-thawing motility, abnormalities, membrane functionality (hypo-osmotic swelling test), mitochondrial activity, viability, apoptosis status, reactive oxygen species production, and adenosine triphosphate (ATP) content after thawing and fertility and hatchability after AI. The results of this work proved that P188 could improve rooster semen cryopreservation and allow for the reduction of glycerol in extenders, with a consequent impact on the poultry industry. The positive effect of the addition of P188 extender on increased post-thaw quality and fertility of rooster sperm also proved Najafi et al. (2021).
In recent years, researchers have also focused on supplementing cryopreservation mixtures with proteins, such as fetal bovine serum (FBS), bovine serum albumin (BSA), and egg yolk (Rakha et al., 2018; Blank et al., 2020; Behnamifar et al., 2021). The outcomes of these works indicated that these proteins minimize some harmful effects of cryo-preservation, providing an alternative for chicken semen extenders.
Nonpenetrating CPAs (e.g., polyvinyl-pyrrolidone, sucrose, trehalose, raffinose, glucose, or lactose) act outside the sperm cell in the extracellular space and protect cells by dehydrating the intracellular space and limiting osmotic swelling during thawing (Rakha et al., 2020). Nonpenetrating CPAs, also known as osmoprotectants, are low-molecular-weight, hydrophilic, nontoxic molecules (Cleland et al., 2004). The addition of N-CPAs to the freezing medium serves to offset the cryodamage caused by P-CPAs. At similar concentrations, these substances are less toxic than P-CPAs, and they have multiple protective roles, such as inhibiting ice crystal growth and helping sperm stabilize internal solute concentrations under osmotic stress (Iaffaldano et al., 2016; Blank et al., 2020).
Following previous experiments, it is recommended that media contain at least one penetrating CPA and one nonpenetrating CPA. This mixture should be dissolved in phosphate or, preferably, N-(2-hydroxyethyl)piperazine-N-(2-ethanesulfonic acid (= HEPES)), which serves as a protective medium (Pereira and Marques, 2008; Dávila et al., 2015). The use of N-CPAs that act mainly as osmoprotectants could reduce the number of P-CPAs needed in sperm cryopreservation (Neville et al., 1971). Among the disaccharides, sucrose and trehalose are N-CPAs widely studied in different mammalian species: bulls (Chen et al., 1993; Foote et al., 1993), goats (Aboagla and Terada, 2003), boars (Gutiérrez-Pérez et al., 2009), and rabbits (Dalimata and Graham, 1997). In contrast, the effect of trehalose and sucrose on the post-thaw quality of poultry sperm has been poorly studied, and few reports are available. Bird sperm show specific characteristics that need added adaptations for sperm cryopreservation. They contain the same intracellular organelles as all amniotes, including mammals. However, in contrast to most mammals, they are filiform cells with a very low cytoplasmic content and a long flagellum adapted to their long trip in the female tract before reaching the site of fertilization (Thananurak et al., 2019). They consequently contain a relatively low amount of intracytoplasmic water and very high proportions of cellular membranes that make them highly sensitive to membrane injuries (Blesbois, 2012). Avian sperm are especially intolerant to volume and osmotic changes compared to mammalian sperm. Many sugars may play a role in the protection of sperm during the freeze‒thaw process (Thananurak et al., 2019). For example, in avian species, the disaccharide sucrose showed contrasting effects on the success of sperm cryopreservation (Neville et al., 1971; Blanco et al., 2011; Blanco et al., 2012; Thananurak et al., 2019). In contrast, the disaccharide trehalose appears to be a very suitable cryoprotectant for avian sperm (Neville et al., 1971; Ehling et al., 2012; Stanishevskaya et al., 2021). Nevertheless, due to differences among the conditions used, the results reported in the literature are very variable. The addition of nonpermeating substances such as the amino acid glycine is beneficial for the cryosurvival of sperm. In rooster sperm, the inclusion of glycine into the diluent may improve sperm integrity by direct interaction with plasma membrane phospholipids (Cerolini et al., 2007; Gliozzi et al., 2011). Interesting results were obtained during the use of Ficoll (polysaccharide polymer) as a nonpermeating cryoprotectant for avian species. In a study by Miranda et al. (2018), the addition of Ficoll resulted in significantly higher post-thaw motility compared to trehalose, sucrose, or glycine. The effectiveness of Ficoll was attributed mainly to its capability of affecting solution viscosity, ensuring a greater stability of the sperm membrane and reducing mechanical stress and ice crystal formation, therefore increasing the ability to survive cryopreservation (Makarevich et al., 2008; Lagares et al., 2009).
Antioxidants
During cryopreservation, some sperm cannot take up antifreeze due to their small volume, causing the sperm to rupture and release ROS (reactive oxidizing species). In addition, cryopreservation increases ROS production in the freezing media, and ROS can attack the bis-allylic methylene group of plasma membrane phospholipids, leading to lipid peroxidation (LPO) (Hammerstedt et al., 1990; Li et al., 2010; Shahverdi et al., 2015). Therefore, avian sperm cells must be supplemented with additional antioxidants in the media to overcome the effects of lipid oxidation during freeze–thaw processes. For this reason, numerous substances with antioxidant effects, such as melatonin (Appiah et al., 2020; Mehaisen et al., 2020), k-carrageenan (k-CRG), CLC (cholesterol-loaded cyclodextrins) (Li et al., 2020), L-carnitine, hypotaurine, taurine supplementation (Partyka et al., 2017), Mito-TEMPO (Masoudi et al., 2021), Vit C, and Vit E (Amini et al., 2015a, Leao et al., 2021), and many others, have been tested. Seminal plasma from numerous animal species, including poultry, normally contains antioxidant properties (Hammerstedt et al., 1990; Watson et al., 1992; Li et al., 2010; Khan et al., 2012; Partyka et al., 2012).
The addition of antioxidants to extenders is highly desirable for both cooled and frozen rooster semen because they can reduce oxidative stress and improve fertilizing capacity. An overview of perspective antioxidants in poultry semen extenders was reviewed by Leao et al. (2021). This paper highlights glutathione, CoQ10 and L-carnitine for cooled semen, whereas for frozen semen, resveratrol, lycopene, and quercetin are most frequently used.
CONCLUSION
Currently, it is very important to improve the cryopreservation of rooster sperm for the protection of gene resources due to the occurrence of avian influenza. In cryopreservation, there are significant differences between mammalian and avian sperm cell morphology (Çiftci and Aygün, 2018). During freeze–thaw processes, sperm cells are exposed to damage such as oxidative stress, intracellular ice crystal formation during freezing and ROS accumulation (Leao et al., 2021; Masoudi et al., 2021; Partyka and Niżański, 2021). Moreover, rooster semen freezability depends on many factors, such as differences among the breeds and lines (Tselutin et al., 1999; Long, 2006; Long et al., 2010). As reported in various papers, the success of the sperm cryopreservation procedure may depend on the interaction among the type of cryoprotectant, semen freezing, thawing conditions and packaging system used, each affecting sperm structure and function (Long et al., 2014; Iaffaldano et al., 2016; Madeddu et al., 2016).
In developing the cryopreservation of poultry sperm, the main task is to improve it and simplify individual methods, including the freezing process, for a minimum period to integrate it into standard breeding practice without major demands on time and personnel.
For the widespread of cryopreservation of sperm in the poultry industry on a worldwide scale, the other very important task is to introduce novel methods and/or improve the interpretation of the results of currently used in vitro analytical methods (sperm quality tests). To this end, two aspects are essential: the ability of semen quality tests to precisely elucidate the molecular phenotype related to sperm quality and the ability of tests to predict the success of semen cryopreservation. Despite quite evident progress in the field (Blesbois et al., 2008; Labas et al., 2014), there are still a number of ambiguities with the correlation of the outputs of analytical methods and the actual fertilizing ability of rooster sperm. As an example of the essential necessity for the improvement of the results of currently used assays, the CellRox-based assay should be noted. It is broadly accepted that the CellRox dye stains only the cells with oxidative damage. Canonically, such CellRox-tagged cells are usually interpreted as damaged cells that are not of good quality. However, it was demonstrated recently that in equine sperm, the CellRox dye stains qualitatively good cells with healthy active mitochondria ( Plaza Davila et al., 2015). According to our unpublished results, for chicken sperm, the situation is similar: CellRox dye stains viable cells with active mitochondria and not cells with oxidative damage. Until the interpretation of the CellRox-based assay will not be improved, researchers should be very careful with the interpretation of CellRox-based assay results.
Regarding freezability prediction, it should be concluded that no commonly accepted test is currently available, and future research needs to fill this gap. Today, the verification of sperm quality tests by artificial inseminations is still the only tool available for researchers. Therefore, this tool must be a part of each original paper dealing with the in vitro analytical methods.
Acknowledgments
Acknowledgments
This research was supported by the Ministry of Agriculture of the Czech Republic, institutional support MZE-RO0718 (V004), by the project NAZV QK1910156, and SGS project No. 21320/1312/3134. The authors would like to thank Martin Báječný, M.Eng. (The Center for Advanced Preclinical Imaging, First Faculty of Medicine at Charles University in Prague) for his valuable help with imaging cytometry.
Disclosures
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2022.102386.
Appendix. Supplementary materials
REFERENCES
- Aboagla E.M.E., Terada T. Trehalose-enhanced fluidity of the goat sperm membrane and its protection during freezing. Biol. Reprod. 2003;69:1245–1250. doi: 10.1095/biolreprod.103.017889. [DOI] [PubMed] [Google Scholar]
- Abouelezz F., Castaño C., Toledano-Díaz A., Esteso M., Lopez-Sebastian A., Campo J., Santiago-Moreno J. Effect of the interaction between cryoprotectant concentration and cryopreservation method on frozen/thawed chicken sperm variables. Reprod. Domestic Anim. 2015;50:135–141. doi: 10.1111/rda.12464. [DOI] [PubMed] [Google Scholar]
- Abouelezz F.M.K., Sayed M.A.M., Santiago-Moreno J. Fertility disturbances of dimethylacetamide and glycerol in rooster sperm diluents: discrimination among effects produced pre and post freezing-thawing process. Anim. Reprod. Sci. 2017;184:228–234. doi: 10.1016/j.anireprosci.2017.07.021. [DOI] [PubMed] [Google Scholar]
- Amini M.R., Kohram H., Zare-Shahaneh A., Zhandi M., Sharideh H., Nabi M.M. The effects of different levels of vitamin E and vitamin C in modified Beltsville extender on rooster post-thawed sperm quality. Cell Tissue Bank. 2015;16:587–592. doi: 10.1007/s10561-015-9506-9. [DOI] [PubMed] [Google Scholar]
- Amini M.R., Kohram H., Zare-Shahaneh A., Zhandi M., Sharideh H., Nabi M.M. The effects of different levels of catalase and superoxide dismutase in modified Beltsville extender on rooster post-thawed sperm quality. Cryobiology. 2015;70:226–232. doi: 10.1016/j.cryobiol.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Appiah M.O., Li W., Zhao J., Liu H., Dong Y., Xiang J., Wang J., Lu W. Quercetin supplemented casein-based extender improves the post-thaw quality of rooster semen. Cryobiology. 2020;94:57–65. doi: 10.1016/j.cryobiol.2020.04.010. [DOI] [PubMed] [Google Scholar]
- Ashizawa K., Maeda S., Okauchi K. The mechanisms of reversible immobilization of fowl spermatozoa at body temperature. J. Reprod. Fertil. 1989;86:271–276. doi: 10.1530/jrf.0.0860271. [DOI] [PubMed] [Google Scholar]
- Ashizawa K., Nishiyama H. Effects of temperature on the vigour of motility, oxygen consumption and duration of motility of fowl spermatozoa under aerobic conditions. Japanese Poult. Sci. 1978;15:264–266. [Google Scholar]
- Ashizawa K., Sano R. Effects of temperature on the immobilization and the initiation of motility of spermatozoa in the male reproductive tract of the domestic fowl, Gallus domesticus. Comp. Biochem. Physiol. A. 1990;96:297–301. doi: 10.1016/0300-9629(90)90696-p. [DOI] [PubMed] [Google Scholar]
- Ashizawa K., Wishart G.J. Resolution of the sperm motility-stimulating principle of fowl seminal plasma into Ca2+ and an unidentified low molecular weight factor. J. Reprod. Fertil. 1987;81:495–499. doi: 10.1530/jrf.0.0810495. [DOI] [PubMed] [Google Scholar]
- Ashizawa K., Wishart G.J., Nakao H., Okino Y., Tsuzuki Y. Inhibition of temperature-dependent immobilization of fowl spermatozoa at body temperature by an increased intracellular pH. Journal of Reproduction and Fertility. 1994;101:593–598. doi: 10.1530/jrf.0.1010593. [DOI] [PubMed] [Google Scholar]
- Bahr G.F., Zeitler E. Study of bull spermatozoa – quantitative electron microscopy. J. Cell Biol. 1964;21:175–189. doi: 10.1083/jcb.21.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakst M.R. Fertilizing capacity and morphology of fowl and turkey spermatozoa in hypotonic extender. J. Reprod. Fert. 1980;60:121–127. doi: 10.1530/jrf.0.0600121. [DOI] [PubMed] [Google Scholar]
- Bakst M., Wishart R.G., Brillard J.P. Oviductal sperm selection, transport, and storage in poultry. Poult. Sci. Rev. 1994;5:117–143. [Google Scholar]
- Bakst M.R. Structure of the avian oviduct with emphasis on sperm storage in poultry. J. Exp. Zool. 1998;282:618–626. [PubMed] [Google Scholar]
- Bang J.K., Lee J.H., Murugan R.N., Lee S.G., Do H., et al. Antifreeze peptides and glycopeptides, and their derivatives: potential uses in biotechnology. Mar. Drugs. 2013;11:2013–2041. doi: 10.3390/md11062013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnamifar A., Bernal B., Torres O., Luis-Chincoya H., Gil M.G., Garcia-Casado P., Rahimi S., Woelders H., Santiago-Moreno J. Research Note: Evaluation of two methods for adding cryoprotectant to semen and effects of bovine serum albumin on quality characteristics of cryopreserved rooster spermatozoa. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesova B., Trefil P. Possibilities for preserving genetic resources in birds. World's Poult. Sci. J. 2015;72:629–642. [Google Scholar]
- Bernal B., Behnamifar A., Álvarez-Rodríguez C. Transit along the vas deferens results in a high percentage of filiform spermatozoa with compacted chromatin in the rooster. Reprod. Fertil. Dev. 2022;34:699–712. doi: 10.1071/RD21209. [DOI] [PubMed] [Google Scholar]
- Bernal B., Iglesias-Cabeza N., Sánchez-Rivera U., et al. Effect of supplementation of valine to chicken extender on sperm cryoresistance and post-thaw fertilization capacity. Poult. Sci. 2020;99:7133–7141. doi: 10.1016/j.psj.2020.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielanski A., Bergeron H., Lau P.C.K., Devenish J. Microbial contamination of embryos and semen during long term banking in liquid nitrogen. Cryobiology. 2003;46:146–152. doi: 10.1016/s0011-2240(03)00020-8. [DOI] [PubMed] [Google Scholar]
- Blanch E., Tomás C., Casares L., Gómez E.A., Sansano S., Giménez I., Mocé E. Development of methods for cryopreservation of rooster sperm from the endangered breed ‘Gallina Valenciana de Chulilla’ using low glycerol concentrations. Theriogenology. 2014;81:1174–1180. doi: 10.1016/j.theriogenology.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Blanco J.M., Long J.A., Gee G., Wildt D.E., Donoghue A.M. Comparative cryopreservation of avian spermatozoa: benefits of non-permeating osmoprotectants and ATP on turkey and crane sperm cryosurvival. Anim. Reprod. Sci. 2011;123:242–248. doi: 10.1016/j.anireprosci.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Blanco J.M., Long J.A., Gee G., Wildt D.E., Donoghue A.M. Comparative cryopreservation of avian spermatozoa: effects of freezing and thawing rates on Turkey and Sandhill Crane Sperm Cryosurvival. Anim. Reprod. Sci. 2012;131:1–8. doi: 10.1016/j.anireprosci.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Blank M.H., Silva V.C., Rui B.R., Novaes G.A., Castiglione V.C., Garcia Pereira R.J. Beneficial influence of fetal bovine serum on in vitro cryosurvival of chicken spermatozoa. Cryobiology. 2020;95:103–109. doi: 10.1016/j.cryobiol.2020.05.011. [DOI] [PubMed] [Google Scholar]
- Blesbois E., Grasseau I., Seigneurin F. Membrane fluidity and the ability of domestic bird spermatozoa to survive cryopreservation. Reproduction. 2005;129:371–378. doi: 10.1530/rep.1.00454. [DOI] [PubMed] [Google Scholar]
- Blesbois E., Grasseau I., Seigneurin F., Mignon-Grasteau S., Saint Jalme M., Mialon-Richard M.M. Predictors of success of semen cryopreservation in chickens. Theriogenology. 2008;69:252. doi: 10.1016/j.theriogenology.2007.09.019. -26. [DOI] [PubMed] [Google Scholar]
- Blesbois E. Biological features of the avian male gamete and their application to biotechnology of conservation. J. Poultry Sci. 2012;49:141–149. [Google Scholar]
- Bongalhardo D.C., Somnapan-Kakuda N., Buhr M.M. Isolation and unique composition of purified head plasma membrane from rooster sperm. Poult. Sci. 2002;81:1877–1883. doi: 10.1093/ps/81.12.1877. [DOI] [PubMed] [Google Scholar]
- Branden C., Tooze J. 2nd (Edn.) Garland Publishing; New York: 1999. Introduction to Protein structure; p. 16. [Google Scholar]
- Bréque C., Surai P., Brillard J.P. Roles of antioxidants on prolonged storage of avian spermatozoa in vivo and in vitro. Mol. Reprod. Dev. 2003;66:314–323. doi: 10.1002/mrd.10347. [DOI] [PubMed] [Google Scholar]
- Brillard J.P. Factors affecting oviductal sperm storage in the domestic fowl following artificial insemination. Anim. Reprod. Sci. 1992;27:247–256. [Google Scholar]
- Brillard J.P. Sperm storage and transport following natural mating and artificial insemination. Poult. Sci. 1993;72:923–928. doi: 10.3382/ps.0720923. [DOI] [PubMed] [Google Scholar]
- Brockbank K.G.M., Campbell L.H., Greene E.D., Brockbank M.C.G., Duman J.G. Lessons from nature for preservation of mammalian cells, tissues, and organs. In Vitro Cell. Dev. Biol. 2011;47:210–217. doi: 10.1007/s11626-010-9383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss E.G. Cryopreservation of Rooster Sperm. Poult. Sci. 1993;72:944–954. doi: 10.3382/ps.0720944. [DOI] [PubMed] [Google Scholar]
- Bussalleu E., Sancho S., Briz M.D., Yeste M., Bonet S. Do antimicrobial peptides PR-39, PMAP-36 and PMAP-37 have any effect on bacterial growth and quality of liquid-stored boar semen? Theriogenology. 2017;89:235–243. doi: 10.1016/j.theriogenology.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Camarillo R., Jimenez I., Guzman A., Rosales A.M., Rodriguez F., Perez-Rivero J.J., Herrera J.A. Oviductal proteins effect in rooster spermatic cryopreservation. Cryo Lett. 2019;40:352–356. [PubMed] [Google Scholar]
- Cerolini S., Zaniboni L., Maldjian A., Gliozzi T. Effect of docosahexaenoic acid and α-tocopherol enrichment in chicken sperm on semen quality, sperm lipid composition and susceptibility to peroxidation. Theriogenology. 2006;66:877–886. doi: 10.1016/j.theriogenology.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Cerolini S., Zaniboni L., Mangiagalli M.G., Gliozzi T.M. Effect of glycine on cryopreservation of chicken spermatozoa. Avian Poult. Biol. Rev. 2007;18:65. [Google Scholar]
- Chen Y., Foote R.H., Brockett C.C. Effect of sucrose, trehalose, hypotaurine, taurine, and blood serum on survival of frozen bull sperm. Cryobiology. 1993;30:423–431. doi: 10.1006/cryo.1993.1042. [DOI] [PubMed] [Google Scholar]
- Chenoweth P.J., Lorton S.P. CABI; Wallingford, UK: 2014. Animal andrology: theories and applications. [Google Scholar]
- Çiftci H.B., Aygün A. Poultry semen cryopreservation technologies. World's Poult. Sci. J. 2018;74:699–710. [Google Scholar]
- Clarke R.N., Bakst M.R., Ottinger M.A. Morphological changes in chicken and turkey spermatozoa incubated under various conditions. Poult. Sci. 1984;63:801–805. doi: 10.3382/ps.0630801. [DOI] [PubMed] [Google Scholar]
- Cleland D., Krader P., Mccree C., Tang J., Emerson D. Glycine betaine as a cryoprotectant for prokaryotes. J. Microbiol. Methods. 2004;58:31–38. doi: 10.1016/j.mimet.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Clulow J. Production, transport, maturation, storage and survival of spermatozoa in the male Japanese quail, Coturnix coturnix. J. Reprod. Fertil. 1982;64:259–266. doi: 10.1530/jrf.0.0640259. [DOI] [PubMed] [Google Scholar]
- Dalimata A.M., Graham J.K. Cryopreservation of rabbit spermatozoa using acetamide in combination with trehalose and methylcellulose. Theriogenology. 1997;48:831–841. doi: 10.1016/s0093-691x(97)00305-1. [DOI] [PubMed] [Google Scholar]
- Darin-Bennett A., White I.G. Influence of the cholesterol content of mammalian spermatozoa on susceptibility to cold-shock. Cryobiology. 1977;14:466–470. doi: 10.1016/0011-2240(77)90008-6. [DOI] [PubMed] [Google Scholar]
- Dávila S.G., Campo J.L., Gil M.G., Castano C., Santiago-Moreno J. Effect of the presence of hens on roosters sperm variables. Poult. Sci. 2015;94:1645–1649. doi: 10.3382/ps/pev125. [DOI] [PubMed] [Google Scholar]
- De Leon P.M.M., Campos V.F., Corcini C.D., Santos E.C.S., Rambo G., Lucia T., Deschamps J.C., Collares T. Cryopreservation of immature equine oocytes, comparing a solid surface vitrification process with open pulled straws and the use of a synthetic ice blocker. Theriogenology. 2012;77:21–27. doi: 10.1016/j.theriogenology.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Dinnyes A., Liu J., Nedambale T.L. Novel gamete storage. Reprod. Fertility Dev. 2007;19:719–731. doi: 10.1071/rd07035. [DOI] [PubMed] [Google Scholar]
- Donoghue A.M., Wishart G.J. Storage of poultry semen. Anim. Reprod. Sci. 2000;62:213–232. doi: 10.1016/s0378-4320(00)00160-3. [DOI] [PubMed] [Google Scholar]
- Edsall J.T., Wyman J. Academic Press; New York Sec 3: 1958. Biophysical chemistry. [Google Scholar]
- Ehling C.h., Taylor U., Baulain U., Weigend S., Henning M., Rath R. Cryopreservation of semen from genetic resource chicken lines. Agricult. Forestry Res. 2012;62:151–158. [Google Scholar]
- Fattah A., Sharafi M., Masoudi R., Shahverdi A., Esmaeili V. L-carnitine is a survival factor for chilled storage of rooster semen for a long time. Cryobiology. 2017;74:13–18. doi: 10.1016/j.cryobiol.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Feyzi S., Sharafi M., Rahimi S. Stress preconditioning of rooster semen before cryopreservation improves fertility potential of thawed sperm. Poult. Sci. 2018;97:2582–2590. doi: 10.3382/ps/pey067. [DOI] [PubMed] [Google Scholar]
- Fiser P.S., Macpherson J.W. Development of embryonic structures from dispersed chick blastoderm cells. Poult. Sci. 1974;53:565–571. doi: 10.3382/ps.0530565. [DOI] [PubMed] [Google Scholar]
- Foote R.H., Chen Y., Brockett C.C., Kaproth M.T. Fertility of bull spermatozoa frozen in whole milk extender with trehalose, taurine, or blood serum. J. Dairy Sci. 1993;76:1908–1913. doi: 10.3168/jds.S0022-0302(93)77524-4. [DOI] [PubMed] [Google Scholar]
- Gatti N.L.S., Corcini C.D., Filho J.S., Soares S.L., Anciuti S.N., Barbosa R.M., Knabah N.W., Tavares A.T., Bongalhardo D.C., Varela Junior A.S. Dimethylacetamide in rooster semen cryopreservation. Cryo Lett. 2021;42:39–43. [PubMed] [Google Scholar]
- Getachew T.A. Review article of artificial insemination in poultry. World. Vet. J. 2016;6:25–33. [Google Scholar]
- Gibbons I.R. Dynein ATPases as microtubule motors. J. Biol. Chem. 1988;263:15837–15840. [PubMed] [Google Scholar]
- Gliozzi T.M., Zaniboni L., Cerolini S. DNA fragmentation in chicken spermatozoa during cryopreservation. Theriogenology. 2011;75:1613–1622. doi: 10.1016/j.theriogenology.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Gordon I. In: Reproductive technologies in farm animals. Gordon I, editor. CABI Publishing UK; Oxfordshire: 2005. Artificial Insemination; pp. 49–81. [Google Scholar]
- Gradil C., Eaglesome M.D., Stewart B., Garcia M.M., Quimby F. Bactericidal effects of ozone at nonspermicidal concentrations. Can. J .Vet. Res. 1995;59:183–186. [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Pérez O., Uribe C.S., Juárez M.M.L., Trujillo O M.E. Cryoprotective effects with low glycerol freezing extender for boar spermatozoa added to different trehalose concentrations. Theriogenology. 2009;70:1398. [Google Scholar]
- Guzman M., Dille J., Godet S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomedicine. 2012;8:37–45. doi: 10.1016/j.nano.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Haines M.D., Parker H.M., McDaniel C.D., Kiess A.S. Impact of 6 different intestinal bacteria on broiler breeder sperm motility in vitro. Poult. Sci. 2013;92:2174–2181. doi: 10.3382/ps.2013-03109. [DOI] [PubMed] [Google Scholar]
- Halwani D.O., Brockbank K.G.M., Duman J.G., Campbell L.H. Recombinant Dendroides canadensis antifreeze proteins as potential ingredients in cryopreservation solutions. Cryobiology. 2014;68:411–418. doi: 10.1016/j.cryobiol.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerstedt R.H., Graham J.K., Nolan J.P. Cryopreservation of mammalian sperm: what we ask them to survive. J. Androl. 1990;11:73–88. [PubMed] [Google Scholar]
- Hammerstedt R.H., Graham J.K. Cryopreservation of poultry sperm: the enigma of glycerol. Cryobiology. 1992;29:26–38. doi: 10.1016/0011-2240(92)90004-l. [DOI] [PubMed] [Google Scholar]
- Holt W.V. Basic aspects of frozen storage of semen. Anim. Reprod. Sci. 2000;62:3–22. doi: 10.1016/s0378-4320(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Holt W.V., Fazeli A. Sperm storage in the female reproductive. Annu. Rev. Anim. Biosci. 2016;4:291–310. doi: 10.1146/annurev-animal-021815-111350. [DOI] [PubMed] [Google Scholar]
- Howarth B. An examination for sperm capacitation in the fowl. Biol. Reprod. 1970;3:338–341. doi: 10.1093/biolreprod/3.3.338. [DOI] [PubMed] [Google Scholar]
- Howarth B. Fertilizing ability of cock spermatozoa from the testis epididymis and vas deferens following lntramagnal insemination. Biol. Reprod. 1983;28:586–590. doi: 10.1095/biolreprod28.3.586. [DOI] [PubMed] [Google Scholar]
- Hussein Z.M., El-Tayeb T.A., El-Keraby F., Harith M.A. The effect of diode laser and light emitting diode on the bacterial contamination of semen medium for artificial insemination. Biologicals. 2008;36:303–307. doi: 10.1016/j.biologicals.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Iaffaldano N., Di Iorio M., Miranda M., Zaniboni L., Manchisi A., Cerolini S. Cryopreserving turkey semen in straws and nitrogen vapour using DMSO or DMA: effects of cryoprotectant concentration, freezing rate and thawing rate on post-thaw semen quality. Br. Poult. Sci. 2016;57:264–270. doi: 10.1080/00071668.2016.1148261. [DOI] [PubMed] [Google Scholar]
- Inaba K. Molecular architecture of the sperm flagella: molecules for motility and signaling. Zool. Sci. 2003;20:1043–1056. doi: 10.2108/zsj.20.1043. [DOI] [PubMed] [Google Scholar]
- Ishiguro H., Rubinsky B. Influence of fish antifreeze proteins on the freezing of cell suspensions with cryoprotectant penetrating cells. Int. J. Heat Mass Transf. 1998;41:1907–1915. [Google Scholar]
- Jeyendran R.S., Graham E.F. An evaluation of cryoprotective compounds on bovine spermatozoa. Cryobiology. 1980;17:458–464. doi: 10.1016/0011-2240(80)90055-3. [DOI] [PubMed] [Google Scholar]
- Jo J.W., Jee B.C., Lee J.R., Suh C.S. Effect of antifreeze protein supplementation in vitrification medium on mouse oocyte developmental competence. Fertil. Steril. 2011;96:1239–1245. doi: 10.1016/j.fertnstert.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Kauzmann W. Academic Press; New York: 1957. Quantum Chemistry : An Introduction. [Google Scholar]
- Khan R., Laudario V., Tufarelli V. Semen traits and seminal plasma biochemical parameters in white leghorn layer breeders. Reprod. Domest. Anim. 2012;47:190–195. doi: 10.1111/j.1439-0531.2011.01821.x. [DOI] [PubMed] [Google Scholar]
- Kim S.W., Choi J.S., Ko Y.G., Do Y.J., Byun M., Park S.B., Seong H.H., Kim C.D. Effect of N-Methylacetamide Concentration on the Fertility and Hatchability of Cryopreserved Ogye Rooster Semen. Korean J. Poult. Sci. 2014;41:21–27. [Google Scholar]
- Labas V., Grasseau I., Cahier K., Gargaros A., Harichaux G., Teixeira-Gomes A.P., Alves S., Bourin M., Gérard N., Blesbois E. Data for chicken semen proteome and label free quantitative analyses displaying sperm quality biomarkers. Data Brief. 2014;1:37–41. doi: 10.1016/j.dib.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagares M.A., Castanheira P.N., Amaral D.C.G., Vasconcelos A.B., Veado J.C.C., Arantes R.M.E., Stahlberg R. Addition of ficoll and disaccharides to URF solutions improve in vitro viability of vitrified equine embryos. Cryo Lett. 2009;30:408–413. [PubMed] [Google Scholar]
- Lake P.E., Stewart J.M. Preservation of fowl semen in liquid nitrogen-an improved method. Br. Poult. Sci. 1978;19:187–194. doi: 10.1080/00071667808416462. [DOI] [PubMed] [Google Scholar]
- Lake P.E. The history and future of the cryopreservation of avian germ plasm. Poult. Sci. 1986;65:1–15. doi: 10.3382/ps.0650001. [DOI] [PubMed] [Google Scholar]
- Lee H.J., Kim S.K., Jang H.J., Kang K.S., Kim J.H., Choi S.B., Han J.Y. Cryopreservation of Korean Oge chicken semen using N-methylacetamide. Cryo Lett. 2012;33:427–434. PMID: 23250402. [PubMed] [Google Scholar]
- Leao A.P.A., de Souza A.V., Mesquita N.F., Pereira L.J., Zangeronimo M.G. Antioxidant enrichment of rooster semen extenders-A systematic review. Res. Vet. Sci. 2021;136:111–118. doi: 10.1016/j.rvsc.2021.02.005. [DOI] [PubMed] [Google Scholar]
- Li Z., Lin Q., Liu R., Xiao W., Liu W. Protective effects of ascorbate and catalase on human spermatozoa during cryopreservation. J. Androl. 2010;31:437–444. doi: 10.2164/jandrol.109.007849. [DOI] [PubMed] [Google Scholar]
- Long J.A. Avian semen cryopreservation: what are the biological challenges? Poult. Sci. 2006;85:232–236. doi: 10.1093/ps/85.2.232. [DOI] [PubMed] [Google Scholar]
- Long J.A., Bongalhardo D.C., Pelaéz J., Saxena S., Settar P., O'Sullivan N.P., Fulton J.E. Rooster semen cryopreservation: Effect of pedigree line and male age on post thaw sperm function. Poult. Sci. 2010;89:966–973. doi: 10.3382/ps.2009-00227. [DOI] [PubMed] [Google Scholar]
- Long J.A., Purdy P.H., Zuidberg K., Hiemstra S.J., Velleman S.G., Woelders H. Cryopreservation of turkey semen: effect of breeding line and freezing method on post-thaw sperm quality, fertilization, and hatching. Cryobiology. 2014;68:371–378. doi: 10.1016/j.cryobiol.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Li W., Apiah M.O., Zhao J., Liu H., Wang J., Lu W. Effects of k-carrageenan supplementation or in combination with cholesterol-loaded cyclodextrin following freezing-thawing process of rooster spermatozoa. Cryobiology. 2020;95:36–43. doi: 10.1016/j.cryobiol.2020.06.009. [DOI] [PubMed] [Google Scholar]
- Lotfi S., Mehri M., Sharafi M., Masoudi R. Hyaluronic acid improves frozen-thawed sperm quality and fertility potential in rooster. Anim. Reprod. Sci. 2017;184:204–210. doi: 10.1016/j.anireprosci.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Madeddu M., Mosca F., Abdel Sayed A., Zaniboni L., Mangiagalli M.G., Colombo E., Cerolini S. Effect of cooling rate on the survival of cryopreserved rooster sperm: comparison of different distances in the vapor above the surface of the liquid nitrogen. Anim. Reprod. Sci. 2016;171:58–64. doi: 10.1016/j.anireprosci.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Makarevich A.V., Parkanyi V., Ondruska Ľ., Kubovicova E., Flak P., Slezakova M., Pivko J., Rafay J. Evaluation of fertilizing capacity of rabbit sperm on the basis of annexin-V-labelled membrane changes. Slovak. J. Anim. Sci. 2008;41:1–5. [Google Scholar]
- Masoudi R., Asadzadeh N., Sharafi M. Effects of freezing extender supplementation with mitochondria-targeted antioxidant Mito-TEMPO on frozen-thawed rooster semen quality and reproductive performance. Anim. Reprod. Sci. 2021;225 doi: 10.1016/j.anireprosci.2020.106671. [DOI] [PubMed] [Google Scholar]
- Masoudi R., Sharafi M., Shahneh A.Z., Kohram H., Nejati-Amiri E., Karimi H., Khodaei-Motlagh M., Shahverdi A. Supplementation of extender with coenzyme Q10 improves the function and fertility potential of rooster spermatozoa after cryopreservation. Anim. Reprod. Sci. 2018;198:193–201. doi: 10.1016/j.anireprosci.2018.09.019. [DOI] [PubMed] [Google Scholar]
- Masoudi R., Sharafi M., ZareShahneh A., Khodaei-Motlagh M. Effects of reduced glutathione on the quality of rooster sperm during cryopreservation. Theriogenology. 2019;128:149–155. doi: 10.1016/j.theriogenology.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M., Sasanami T. Sperm motility regulation in male and female bird genital tracts. Poult. Sci. 2022;59:1–7. doi: 10.2141/jpsa.0200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell W.M.C., Johnson L.A. Physiology of spermatozoa at high dilution rates: the influence of seminal plasma. Theriogenology. 1999;52:1353–1362. doi: 10.1016/s0093-691x(99)00222-8. [DOI] [PubMed] [Google Scholar]
- Mehaisen G.M.K., Partyka A., Ligocka Z., Nizanski W. Cryoprotective effect of melatonin supplementation on post-thawed rooster sperm quality. Anim. Reprod. Sci. 2020;212 doi: 10.1016/j.anireprosci.2019.106238. [DOI] [PubMed] [Google Scholar]
- Mehdipour M., Daghigh-Kia H., Martínez-Pastor F. Poloxamer 188 exerts a cryoprotective effect on rooster sperm and allows decreasing glycerol concentration in the freezing extender. Poult. Sci. 2020;99:6212–6220. doi: 10.1016/j.psj.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdipour M., Daghigh-Kia H., Najafi A., Martínez-Pastor F. Type III antifreeze protein (AFP) improves the post-thaw quality and in vivo fertility of rooster spermatozoa. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M., Kulíková B., Vašíček J., Olexiková L., Iaffaldano N., Chrenek P. Effect of cryoprotectants and thawing temperatures on chicken sperm quality. Reprod. Domest. Anim. 2018;53:93–100. doi: 10.1111/rda.13070. [DOI] [PubMed] [Google Scholar]
- Mohammad M.S., Mardenli O., Amin Al-Tawash A.S. Evaluation of the cryopreservation technology of poultry sperm: a review study. IOP Conf. Ser. 2021;735 [Google Scholar]
- Morrel J., Wallgren M. Alternatives to antibiotics in semen extenders: a review. Pathogens. 2014;3:934–946. doi: 10.3390/pathogens3040934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G.J., Acton E., Murray B.J., Fonseca F. Freezing injury: The special case of the sperm cell. Cryobiology. 2011;64:71–80. doi: 10.1016/j.cryobiol.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Mosca F., Madeddu M., Sayed A.A., Zaniboni L., Iaffaldano N., Cerolini S. Combined effect of permeant and non-permeant cryoprotectants on the quality of frozen/thawed chicken sperm. Cryobiology. 2016;73:343–347. doi: 10.1016/j.cryobiol.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Mosca F., Zaniboni L., Abdel Sayed A., Iaffaldano N., Soglia D., Schiavone A., Cerolini S. Effect of n-methylacetamide concentration and thawing rate on chicken sperm quality after cryopreservation. Animals. 2020;10:824. doi: 10.3390/ani10050824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mphaphathi M.L., Seshoka M.M., Luseba D., Sutherland B., Nedambale T.L. The characterisation and cryopreservation of Venda chicken semen. Asian Pac. J. Reprod. 2016;5:132–139. [Google Scholar]
- Müller K., Müller P., Pincemy G., Kurz A., Labbe C. Characterization of sperm plasma membrane properties after cholesterol modification: consequences for cryopreservation of rainbow trout spermatozoa. Biol. Reprod. 2008;78:390–399. doi: 10.1095/biolreprod.107.064253. [DOI] [PubMed] [Google Scholar]
- Murugesan S., Mahapatra R. Cryopreservation of Ghagus chicken semen: effect of cryoprotectants, diluents and thawing temperature. Reprod. Domest. Anim. 2020;55:951–957. doi: 10.1111/rda.13734. [DOI] [PubMed] [Google Scholar]
- Nabi M.M., Kohram H., Zhandi M., Mehrabani-Yeganeh H., Sharideh H., Zare-Shahaneh A., Esmaili V. Comparative evaluation of Nabi and Beltsville extenders for cryopreservation of rooster semen. Cryobiology. 2016;72:47–52. doi: 10.1016/j.cryobiol.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Najafi A., AliTaheri R., Mehdipour M., Farnoosh G., Martínez-Pastor F. Lycopene-loaded nanoliposomes improve the performance of a modified Beltsville extender broiler breeder roosters. Animal Reproduction Science. 2018;195:168–175. doi: 10.1016/j.anireprosci.2018.05.021. [DOI] [PubMed] [Google Scholar]
- Najafi A., Daghigh-Kia H., Martínez-Pastor F. Poloxamer 188 and hydroxyethyl starch have a cryoprotective synergic effect improving post-thawing quality and fertility of rooster spermatozoa. Anim. Reprod. Sci. 2021;228 doi: 10.1016/j.anireprosci.2021.106738. [DOI] [PubMed] [Google Scholar]
- Najafi A., Kia H.D., Hamishehkar H., Moghaddam G., Alijani S. Effect of resveratrol-loaded nanostructured lipid carriers supplementation in cryopreservation medium on post-thawed sperm quality and fertility of roosters. Anim. Reprod. Sci. 2019;201:32–40. doi: 10.1016/j.anireprosci.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Neville W.J., Macpherson J.W., Reinhart B. Contraceptive action of glycerol in chickens. Poult. Sci. 1971;90:2047–2053. doi: 10.3382/ps.0501411. [DOI] [PubMed] [Google Scholar]
- Nguyen T.M., Alves S., Grasseau I., Metayer-Custard S., Praud C., Froment P., Blesbois E. Central role of 5′ -AMP-activated protein kinase in chicken sperm functions. Biol. Reprod. 2014;91:121. doi: 10.1095/biolreprod.114.121855. [DOI] [PubMed] [Google Scholar]
- Nishijima K., Tanaka M., Sakai Y., Koshimoto C., Morimoto M., et al. Effects of type III antifreeze protein on sperm and embryo cryopreservation in rabbit. Cryobiology. 2014;69:22–25. doi: 10.1016/j.cryobiol.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Nugrahini Y., Ismaya I., Harimurti S. The effects of different DMA level on sperm quality of Bangkok Chicken offspring. J. Livestock Sci. Prod. 2019;3:131–135. [Google Scholar]
- Osuga T., Shimizu N., Takanashi N., Tamura Y., Makino S., Asai T. N-Methylacetamide for cryopreservation - an alternative to Dimethyl Sulfoxide. Nanomed. Nanotechnol. 2017;2 [Google Scholar]
- Osuga T., Hasegawa T., Aoki M., Fujie M., Nakane T., Asai T. Efficiencies of the cryoprotectants N-Methylacetamide,N-Methylformamide and Dimethyl Sulfoxide, and the cell protectants trehalose and hydroxyethyl starch in cryopreservation of swine sperm. Nanomed. Nanotechnol. 2018;3 [Google Scholar]
- Parker H.M., McDaniel C.D. The immediate impact of semen diluent and rate of dilution on the sperm quality index, ATP utilization, gas exchange, and ionic balance of broiler breeder sperm. Poult. Sci. 2006;85:106–116. doi: 10.1093/ps/85.1.106. [DOI] [PubMed] [Google Scholar]
- Parks J.E., Lynch D.V. Lipid composition and thermotropic phase behavior of boar, bull, stallion, and rooster sperm membranes. Cryobiology. 1992;29:255–266. doi: 10.1016/0011-2240(92)90024-v. [DOI] [PubMed] [Google Scholar]
- Partyka A., Łukaszewicz E., Niżański W. Effect of cryopreservation on sperm parameters, lipid peroxidation and antioxidant enzymes activity in fowl semen. Theriogenology. 2012;77:1497–1504. doi: 10.1016/j.theriogenology.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Partyka A., Bonarska-Kujawa D., Sporniak M., Strojecki M., Niżański W. Modification of membrane cholesterol and its impact on frozen-thawed chicken sperm characteristics. Zygote. 2016;24:714–723. doi: 10.1017/S0967199416000022. [DOI] [PubMed] [Google Scholar]
- Partyka A., Rodak O., Bajzert J., Kochan J., Niżański W. The effect of l-carnitine, hypotaurine, and taurine supplementation on the quality of cryopreserved chicken semen. Biomed. Res. Int. 2017;2017:1–8. doi: 10.1155/2017/7279341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partyka A., Niżański W. Supplementation of avian semen extenders with antioxidants to improve semen quality—is it an effective strategy? Antioxidants. 2021;10:1927. doi: 10.3390/antiox10121927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partyka A., Niżański W. Advances in storage of poultry semen. Anim. Reprod. Sci. 2022;246 doi: 10.1016/j.anireprosci.2021.106921. [DOI] [PubMed] [Google Scholar]
- Pereira R.M., Marques C.C. Animal oocyte and embryo cryopreservation. Cell Tissue Bank. 2008;9:267–277. doi: 10.1007/s10561-008-9075-2. [DOI] [PubMed] [Google Scholar]
- Peters S.O., Shoyebo O.D., Ilori B.M., Ozoje M.O., Ikeobi C.O.N., Adebambo O.A. Semen quality traits of seven strain of chickens raised in humid tropics. Int. J. Poult. Sci. 2008;7:949–953. [Google Scholar]
- Pickett B.W., Komarek R.J. Effect of cold show and freezing on loss of lipid from spermatozoa. J. Dairy Sci. 1967;50:753–757. doi: 10.3168/jds.S0022-0302(67)87506-4. [DOI] [PubMed] [Google Scholar]
- Plaza Davila M., Martin Munoz P., Tapia J.A., Ortega Ferrusola C., Balao Da Silva C., Pena F.J. and Drevet J.R., Inhibition of mitochondrial complex I leads to decreased motility and membrane integrity related to increased hydrogen peroxide and reduced ATP production, while the inhibition of glycolysis gas less impact on sperm motility [e-pub ahead of print]. PLoS One,10, 2015, doi: 10.1371/journal.pone.0138777, accessed December 28, 2022. [DOI] [PMC free article] [PubMed]
- Purdy P.H., Song Y., Silversides F.G., Blackburn H.D. Evaluation of glycerol removal techniques, cryoprotectants and insemination methods for cryopreserving rooster sperm with implications of regeneration of breed or line or both. Poult. Sci. 2009;88:2184–2191. doi: 10.3382/ps.2008-00402. [DOI] [PubMed] [Google Scholar]
- Qadeer S., Khan M.A., Ansari M.S., Rakha B.A., Ejaz R., et al. Efficiency of antifreeze glycoproteins for cryopreservation of Nili-Ravi (Bubalus bubalis) buffalo bull sperm. Anim. Reprod. Sci. 2015;157:56–62. doi: 10.1016/j.anireprosci.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Quan G.B., Wu S.S., Lan Z.G., Yang H.Y., Shao Q.Y., Hong Q.H. The effects of 1,4-cyclohexanediol on frozen ram spermatozoa. Cryo Lett. 2013;34:217–227. [PubMed] [Google Scholar]
- Rakha B.A., Ansari M.S., Akhter S., Zafar Z., Hussain I., Santiago-Moreno J., Blesbois E. Cryopreservation of Indian red jungle fowl (Gallus gallus murghi) semen with polyvinylpyrrolidone. Cryobiology. 2017;78:27–33. doi: 10.1016/j.cryobiol.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Rakha B.A., Ansari M.S., Akhter M.S., Santiago-Moreno J., Blesbois E. Cryoprotectant effects of egg yolk on Indian red jungle fowl (Gallus gallus murghi) sperm. Theriogenology. 2018;119:150–155. doi: 10.1016/j.theriogenology.2018.06.015. [DOI] [PubMed] [Google Scholar]
- Rakha B.A., Ansari M.S., Akhter S., Akhter A., Blesbois E., Santiago-Moreno J. Effect of dimethylformamide on sperm quality and fertilizing ability of Indian red jungle fowl (Gallus gallus murghi) Theriogenology. 2020;149:55–61. doi: 10.1016/j.theriogenology.2020.03.023. [DOI] [PubMed] [Google Scholar]
- Riaz A., Aleem M., Ijaz A., Saeed M.A., Latif A. Effect of collection frequency on the semen quality of broiler breeder. Br. Poult. Sci. 2004;45:823–827. doi: 10.1080/00071660400012691. [DOI] [PubMed] [Google Scholar]
- Robertson R.N. Cambridge University Press; Cambridge, UK: 1983. The lively membranes. [Google Scholar]
- Robles V., Valcarce D.G., Riesco M.F. The use of antifreeze proteins in the cryopreservation of gametes and embryos. Biomolecules. 2019;9:181. doi: 10.3390/biom9050181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsky B., DeVries A.L. Effect of ice crystal habit on the viability of glycerol-protected red blood cells. Cryobiology. 1989;26:580. [Google Scholar]
- Salehi M., Mahdavi A.H., Sharafi M., Shahverdi A. Cryopreservation of rooster semen: evidence for the epigenetic modifications of thawed sperm. Theriogenology. 2020;142:15–25. doi: 10.1016/j.theriogenology.2019.09.030. [DOI] [PubMed] [Google Scholar]
- Santiago-Moreno J., Blesbois E. Functional aspects of seminal plasma in bird reproduction. Int. J. Mol. Sci. 2020;21:102130. doi: 10.3390/ijms21165664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Moreno J., Castaño C., Toledano-Díaz A., Coloma M.A., López-Sebastián A., Prieto M.T., Campo J.L. Semen cryopreservation for the creation of a spanish poultry breeds cryobank: optimization of freezing rate and equilibration time. Poult. Sci. 2011;90:2047–2053. doi: 10.3382/ps.2011-01355. [DOI] [PubMed] [Google Scholar]
- Santiago-Moreno J., Esteso M.C., Villaverde-Morcillo S., Toledano-Deaz A., Castaño C., Velázquez R., López-Sebastián A., Goya A.L., Martínez J.G. Recent advances in bird sperm morphometric analysis and its role in male gamete characterization and reproduction technologies. Asian J. Androl. 2016;18:882–888. doi: 10.4103/1008-682X.188660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Tatsumi T., Tsutsui M., Niinomi T., Imai T., Naito M., Tajima A., Nishi Y. A method for cryopreserving semen from Yakido roosters using N-methylacetamide as a cryoprotective agent. J. Poult. Sci. 2010;47:297–301. [Google Scholar]
- Sexton T.J., Fewlass T.A. A new poultry semen extender. Poult. Sci. 1978;57:277–284. doi: 10.3382/ps.0570277. [DOI] [PubMed] [Google Scholar]
- Savvulidi F.G., Ptáček M., Stádník L. Pathogens in processed ram semen and approaches for their elimination. Acta Univ. Agric. Silvic. Mendelianae Brun. 2018;66:1065–1072. [Google Scholar]
- Sexton T.J. Effects of prefreeze treatments on the fertilizing capacity of unfrozen and frozen chicken semen: Extender characteristics and dilution method. Poult. Sci. 1981;60:1552–1557. doi: 10.3382/ps.0622255. [DOI] [PubMed] [Google Scholar]
- Shahverdi A., Sharafi M., Gourabi H., AmiriYekta A., Esmaeili V., Sharbatoghli M., Janzamin E., Hajnasrollahi M., Mostafayi F. Fertility and flow cytometric evaluations of frozen-thawed rooster semen in cryopreservation medium containing low-density lipoprotein. Theriogenology. 2015;83:78–85. doi: 10.1016/j.theriogenology.2014.07.044. [DOI] [PubMed] [Google Scholar]
- Shanmugam M., Kumar K.P., Mahapatra R.K., Laxmi N.A. Effect of different cryoprotectants on post-thaw semen parameters and fertility in Nicobari chicken. Indian J. Poult. Sci. 2018;53:208–211. [Google Scholar]
- Shanmugam M., Mahapatra R. Pellet method of semen cryopreservation: effect of cryoprotectants, semen diluents and chicken lines. Braz. Arch. Biol. Technol. 2019;62 [Google Scholar]
- Shanmugam M., Mahapatra R.K. ICAR-Directorate of Poultry Research; Hyderabad: 2021. Chicken semen cryopreservation. [Google Scholar]
- Stanishevskaya O., Silyukova Y., Pleshanov N., Kurochkin A., Fedorova E., Fedorova Z., Perinek O., Prituzhalova A., Meftakh I. Effects of saccharides supplementation in the extender of cryopreserved rooster (Gallus domesticus) semen on the fertility of frozen/thawed spermatozoa. Animals. 2021;11:89. doi: 10.3390/ani11010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M., Cao J., Yu Z., Liu H., Yang F., Huang S., He J., Yan H. New semen freezing method for chicken and drake using dimethylacetamide as the cryoprotectant. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taye S., Esatu W. Potential and possibility of artificial insemination in poultry: a review article. Int. J. Adv. Res. Biol. Sci. 2022;9:90–97. [Google Scholar]
- Thananurak P., Chuaychu-Noo N., Thélie A., Phasuk Y., Vongpralub T., Blesbois E. Sucrose increases the quality and fertilizing ability of cryopreserved chicken sperms in contrast to raffinose. Poult. Sci. 2019;98:4161–4171. doi: 10.3382/ps/pez196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thélie A., Bailliard A., Seigneurin F., Zerjal T., Bichard M., Blesbois E. Chicken semen cryopreservation and use for the restoration of rare genetic resources. Poult. Sci. 2019;98:447–455. doi: 10.3382/ps/pey360. [DOI] [PubMed] [Google Scholar]
- Thurston R.J., Hess R.A. Ultrastructure of spermatozoa from domesticated birds: comparative study of turkey, chicken and guinea fowl. Scanning Microsc. 1987;1:1829–1838. [PubMed] [Google Scholar]
- Tsakmakidis I.A., Samaras T., Anastasiadou S., Basioura A., Ntemka A., Michos I. Iron oxide nanoparticlesas an alternative to antibiotics additiveon extended boar semen. Nanomaterials. 2020;10:1568–1583. doi: 10.3390/nano10081568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakmakidis I.A., Samaras T., Anastasiadou S., Basioura A., Ntemka A., Michos I. Toxic and microbiologicaleffects of iron oxide and silvernanoparticles as additives on extendedram semen. Animals. 2021;11:1011–1026. doi: 10.3390/ani11041011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tselutin K., Seigneurin F., Blesbois E. Comparison of cryoprotectants and methods of cryopreservation of fowl spermatozoa. Poult. Sci. 1999;78:586–590. doi: 10.1093/ps/78.4.586. [DOI] [PubMed] [Google Scholar]
- Váradi É., Végi B., Liptói K., Barna J., Drevet J.R. Methods for cryopreservation of Guinea Fowl Sperm. PLoS One. 2013;8:e62759. doi: 10.1371/journal.pone.0062759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizzier-Thaxton Y., Cox N.A, Richardson L.J., Buhr R., McDaniel C.D., Cosby D.E., Wilson J.L., Bourassa D., Ard M. Apparent attachment of Campylobacter and Salmonella to broiler breeder rooster spermatozoa. Poult. Sci. 2006;85:619–624. doi: 10.1093/ps/85.4.619. [DOI] [PubMed] [Google Scholar]
- Wales R.G., White I.G. The susceptibility of spermatozoa to temperature shock. J. Endocrinol. 1959;19:211–220. [Google Scholar]
- Watson P.F., Kunze E., Cramer P., Hammerstedt R.H. A comparison of critical osmolality and hydraulic conductivity and its activation energy in fowl and bull spermatozoa. J. Androl. 1992;13:131–138. [PubMed] [Google Scholar]
- Wilcox F.H. The effect of dilution and concentration of chicken semen on fertility. Poult. Sci. 1958;37:1357–1362. [Google Scholar]
- Wishart G.J., Wilson Y.I. Temperature-dependent inhibition of motility in spermatozoa from different avian species. Anim. Reprod. Sci. 1999;57:229–235. doi: 10.1016/s0378-4320(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Wolfe J., Bryant G. Cellular cryobiology: thermodynamic and mechanical effects. Int. J. Refrig. 2001;24:438–450. [Google Scholar]
- Wu B.X., Yang X.H., Yan H.F. Improving the quality of rooster semen frozen in straws by screening the glycerol concentration and freezing rate. Br. Poultry Sci. 2020;61:173–179. doi: 10.1080/00071668.2019.1686126. [DOI] [PubMed] [Google Scholar]
- Yang X.H., Zhang B.Z., Sun A., Zhu L.J., Jia X.L., Yan H.F. Study on the technology of glycerol removal in frozen straw semen. China Poultry. 2016;3:15–19. [Google Scholar]
- Zhandi M., Ansari M., Roknabadi P., Zare Shahneh A., Sharafi M. Orally administered chrysin improves post-thawed sperm quality and fertility of rooster. Reprod. Domest. Anim. 2017;52:1004–1010. doi: 10.1111/rda.13014. [DOI] [PubMed] [Google Scholar]
- Zilli L., Beirao J., Schiavone R., Herraez M.P., Gnoni A., Vilella S. Comparative proteome analysis of cryopreserved flagella and head plasma membrane proteins from sea bream spermatozoa: effect of antifreeze proteins. PLoS One. 2014;9:e99992. doi: 10.1371/journal.pone.0099992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S.S. Fowl sperm immobilization by a temperature-media interaction and its biological significance. Q. J. Exp. Physiol. 1938;27:281–291. [Google Scholar]
- Ashizawa K., Tomonaga H. and Tsuzuki Y. Regulation of flagellar motility of fowl spermatozoa: evidence for the involvement of intracellular free Ca2+ and calmodulin, Journal of Reproduction and Fertility, 101, 1994b, 265-272. [DOI] [PubMed]
- Mehdipour M., Daghigh Kia H., Moghaddam G. and Hamishehkar H. Effect of egg yolk plasma and soybean lecithin on rooster frozen-thawed sperm quality and fertility, Theriogenology, 116, 2018, 89-94. [DOI] [PubMed]
- Appiah M.O., Beibei H.E., Wenfa L.U. and Wang J. Antioxidative effect of melatonin on cryopreserved chicken semen, Cryobiology, 89, 2019, 90-95 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.