Abstract
We previously showed that adoptive transfer of Borrelia burgdorferi-pulsed dendritic cells (DCs) into syngeneic mice protects animals from challenge with tick-transmitted spirochetes. Here, we demonstrate that the protective immune response is antibody (Ab) dependent and does not require the presence of major histocompatibility complex (MHC) class II molecules on DCs. Mice sensitized with B. burgdorferi-pulsed MHC class II-deficient (MHC class II−/−) DCs mounted a humoral response against protective antigens, including B. burgdorferi outer surface protein A (OspA) and OspC. B-cell help for the generation of neutralizing anti-OspC immunoglobulin G Abs could be provided by γδ T cells. In contrast, anti-OspA Ab production required the presence of αβ T cells, although this pathway could be independent of MHC class II molecules on antigen-presenting cells. Moreover, depletion of NK cells prior to transfer of antigen-pulsed MHC class II−/− DCs resulted in significant increases in the levels of neutralizing Abs induced by DCs. Altogether, these data suggest that the initial interactions between DCs and innate immune cells, such as γδ and NK cells, can influence the generation of a protective humoral response against B. burgdorferi antigens.
Borrelia burgdorferi, the causative agent of Lyme disease, is transmitted to the host during the feeding of Ixodes ticks (9). Clinical symptomatology includes a typical erythema migrans skin lesion in the early stages of infection and musculoskeletal, cardiovascular, and neurologic disorders in the tertiary stage of infection (53).
Attempts to prevent B. burgdorferi infection have led to the identification of several protective antigens. Active immunization of mice with B. burgdorferi outer surface protein A (OspA), OspB, and OspC protected against challenge with tick-transmitted spirochetes, a protective immune response mediated by the generation of neutralizing antibodies (Abs) (16, 21, 22, 25, 34, 42, 45). In addition, neutralizing anti-B. burgdorferi immunoglobulin G (IgG) Abs developed in major histocompatibility complex (MHC) class II-deficient (MHC class II−/−) as well as in CD40 ligand-deficient mice (19, 20), suggesting that effector cells other than αβ T-cell receptor-positive (TCRαβ+) CD4+ T cells could provide help to B cells for the generation of neutralizing anti-B. burgdorferi Abs. It was previously found that adoptive transfer of B. burgdorferi-pulsed dendritic cells (DCs) into syngeneic mice elicits a protective immune response against natural challenge with spirochetes (35). The goal of the present study was to elucidate the immune mechanisms underlying the protective immune response induced by DCs.
It is well established that DCs play a crucial role in the generation of Abs against T-cell-dependent protein antigens (26, 27, 52). DCs represent a family of highly specialized antigen-presenting cells (APCs) residing within lymphoid and nonlymphoid tissues (55) and are very potent in initiating a wide range of T-cell responses to foreign antigens (5, 12, 55). Both human and murine DCs are able to process and present B. burgdorferi antigens (4, 23, 35). The ability of murine DCs to present protective B. burgdorferi antigens (35) prompted us to define the immune mechanisms underlying the protective response elicited by DCs. Here, we describe a novel regulatory pathway involved in the generation of neutralizing anti-B. burgdorferi Abs induced by antigen-pulsed DCs.
MATERIALS AND METHODS
Mice.
Female 6- to 8-week-old C3H/HeN C57BL/6, B6.CB17 SCID, C56BL/6J-Igh-6 knockout (B cell−/−), C57BL/6J-Tcrd knockout (TCRγδ−/− [referred to hereafter as γδ−/−]), C57BL/6J-Tcrb knockout (TCRαβ−/−), C57BL/6J-Tcrb, and TCRd knockout (TCRβδ−/−) mice were obtained from Jackson Laboratory (Bar Harbor, Maine). C57BL/6Aβ N5 mice (MHC class II gene knockout) were purchased from Taconic Farms (Germantown, N.Y.). All mice were maintained under pathogen-free conditions in the Department of Pathology, Colorado State University.
Strain of B. burgdorferi.
Low-passage B31 spirochetes (fewer than seven passages) were cultured in Barbour-Stoenner-Kelly II medium (6) at 33°C and grown to late log phase for in vitro antigen processing. Previous studies showed that B. burgdorferi B31 expresses OspC in vitro (25, 34).
B. burgdorferi recombinant antigens.
The generation of B. burgdorferi recombinant OspC (rOspC) has been described previously (25). Recombinant OspA (rOspA) was generated as follows. The entire coding sequence minus the signal peptide of the OspA gene was amplified from B. burgdorferi B31 genomic DNA using the primers OspA-F1 (5′ CAAAATGTTAGCAGCCTT 3′) and OspA-R1 (5′ TTTTAAAGCGTTTTTAATTTC 3′), corresponding to the 5′ and 3′ ends of the gene, respectively.
The fragment was amplified by PCR as previously described (25), ligated into plasmid vector pBAD-TOPO (Invitrogen, Carlsbad, Calif.) according to the manufacturer's directions, and transformed into Escherichia coli strain TOP10 (Invitrogen). Transformants were analyzed for the presence of the insert by PCR and for the correct orientation of the insert in the vector by DNA sequence analysis. Gene expression was accomplished by growing the culture in Luria-Bertani broth until mid-log phase and subsequent induction with 0.02% arabinose after incubation for 3 to 4 h. rOspA was extracted from the cells by the B-PER extraction method (Pierce, Rockford, Ill.) according to the manufacturer's instructions. The solubilized protein was placed in a nickel cation chelating column (Novagen, Madison, Wis.) to purify six-His-tagged rOspA. The eluted protein was dialyzed in phosphate-buffered saline and stored at −20°C until use.
Infection of mice by tick bite.
B. burgdorferi B31-infected Ixodes scapularis nymphal ticks were laboratory reared and used to infect mice by natural exposure as previously described (35, 41). Infection rates in tick colonies were greater than 80% (41). In all tick challenge studies, individual mice were exposed to 10 nymphal ticks, which were allowed to feed to repletion over a 72- to 96-h period. Twenty-one days after exposure to infected ticks, B. burgdorferi infection was monitored by serologic analysis and culturing of ear biopsy specimens (35, 51) and spleen specimens.
Isolation of splenic DCs.
Low-density cells from MHC class II−/− or wild-type C57BL/6 mice were collected after density gradient centrifugation on dense bovine serum albumin columns and were further enriched by adherence on plastic and overnight incubation at 37°C as previously described (35).
In vivo protection studies.
In vivo protection studies were performed as previously described (35). Briefly, freshly isolated DCs were pulsed with live B. burgdorferi B31 (1:5 ratio of DCs to spirochetes) for 18 to 24 h at 37°C. Approximately 104 DCs in Hanks balanced salt solution (HBSS) were injected intravenously into syngeneic mice, while control groups either received similar numbers of unpulsed DCs or were treated with HBSS alone. Mice were then challenged with 10 B. burgdorferi-infected I. scapularis nymphal ticks 10 days after DC inoculation. Infection with B. burgdorferi was monitored by culturing of ear biopsy and spleen specimens as well as serologic analysis 21 days after ticks dropped off. In all instances, mice that were protected from challenge with tick-borne B. burgdorferi did not mount an Ab response against the B. burgdorferi antigen in the 41- to 43-kDa range. In contrast, mice that were infected with B. burgdorferi, as assessed by the growth of the spirochetes in cultures, mounted an Ab response against the 41- to 43-kDa B. burgdorferi antigen, as previously shown (35). In separate studies, NK cells were depleted in vivo following intravenous injection of 200 μg of monoclonal Ab (MAb) PK136 (HB-191; American Type Culture Collection, Manassas, Va.) per mouse 7 and 3 days prior to DC transfer. Similar concentrations of normal mouse IgG2a were administered as an isotype control Ab. The efficiency of NK cell depletion was determined with poly(I-C)-treated sentinel mice using splenocytes as effector cells in a standard assay of 51Cr release by YAC-1 cells. Injection of MAb PK136 significantly decreased (up to 70%) the ability of splenocytes derived from poly(I-C)-treated mice to lyse target YAC-1 cells (data not shown).
Passive Ab transfer studies.
Sera, collected 10 days after adoptive transfer of DCs, were transferred into SCID mice (200 μl per mouse, injected intraperitoneally) 24 h prior to challenge with needle-inoculated B. burgdorferi (104 per mouse). Alternatively, passive transfer of sera was performed with C3H/HeN and C57BL/6 mice, and mice were challenged 24 h later with B. burgdorferi-infected I. scapularis ticks. Mice were monitored for B. burgdorferi infection 21 days after tick challenge as described above.
Immunoblotting and ELISA.
Immunoblotting and enzyme-linked immunosorbent assay (ELISA) were carried out as previously described (35). Briefly, 100 μg of whole-cell lysates of low-passage B. burgdorferi in Laemmli's sample buffer was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (11.5% resolving gels) and transferred to nitrocellulose membranes. For immunoblotting, all sera were diluted 1:100 in Tris-buffered saline (20 mM Tris-HCl, 150 mM NaCl)–0.05% Tween 20. Membranes were subsequently incubated with goat anti-mouse IgG (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) diluted 1:2,000 in Tris-buffered saline–0.05% Tween 20. Membranes were developed with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium (Kirkegaard & Perry Laboratories).
Anti-OspA and anti-OspC IgG Ab levels were determined by an ELISA. Polyvinyl plates were coated with rOspA or rOspC (200 ng/well each) using standard procedures (35). The recombinant antigens were used to coat different wells on the same plate to allow direct comparisons of the Ab levels in each experiment. Quantification of anti-OspA and -OspC Ab isotypes was performed by an ELISA using alkaline phosphatase-conjugated anti-mouse IgG1 and IgG2b (Pharmingen, San Diego, Calif.) diluted 1:1,000 in phosphate-buffered saline–5% fetal bovine serum. Results obtained with a 1:50 dilution of the sera are shown; similar results for the levels of anti-B. burgdorferi Abs were noted with up to 1:200 dilutions of the sera (data not shown). The color was developed with p-nitrophenylphosphate (Kirkegaard & Perry Laboratories).
Statistical analysis.
Significant differences in the mean levels of anti-B. burgdorferi Abs were determined by Student's t test. P values of less than 0.05 were considered statistically significant.
RESULTS
Adoptive transfer of B. burgdorferi-pulsed DCs can elicit a protective humoral response against T-cell-dependent, MHC class II-independent antigens.
Previous studies in this laboratory showed that adoptive transfer of B. burgdorferi-pulsed DCs into syngeneic mice mediated a protective immune response against tick-transmitted spirochetes (35). Here, we demonstrate that the protective immune response is Ab mediated, as passive transfer of sera derived from DC-immunized mice into C57BL/6 mice (Table 1) or C3H mice (data not shown) resulted in a protective immune response against challenge with tick-transmitted spirochetes. Likewise, passive transfer of sera collected from B. burgdorferi-pulsed DC-treated mice into SCID mice resulted in a protective immune response against challenge with needle-inoculated spirochetes (data not shown). Furthermore, B-cell-deficient mice failed to resist infection following immunization with antigen-pulsed DCs (Table 2). The protective humoral response did not require the presence of MHC class II molecules on DCs, as demonstrated by the ability of adoptively transferred B. burgdorferi-pulsed DCs isolated from MHC class II−/− mice to mediate protection against tick-transmitted B. burgdorferi (Table 2). TCRβδ−/− mice, lacking both αβ and γδ T cells, failed to mount a protective humoral response following adoptive transfer of B. burgdorferi-pulsed DCs (Table 2). Thus, immunization with B. burgdorferi-pulsed DCs can induce neutralizing Abs against T-cell-dependent, MHC class II-independent antigens.
TABLE 1.
Sera from B. burgdorferi-pulsed DC-immunized mice can protect against tick-borne infectiona
| Injected material | No. of infected micec | Reactivity to 41- to 43-kDa antigen after challenge |
|---|---|---|
| HBSS | 5 | + |
| Unpulsed DCs | 5 | + |
| Pulsed DCsb | 0 | − |
Passive transfer of sera collected 10 days following adoptive transfer of MHC class II+/+ DCs was performed as described in Materials and Methods. Recipient syngeneic C57BL/6 mice were challenged with B. burgdorferi-infected ticks, and infection was monitored 21 days after ticks dropped off by culturing of spleen and ear skin biopsy specimens and reactivity to a 41- to 43-kDa antigen on Western blots (+, reactive; −, nonreactive) (see Materials and Methods).
Similar results were obtained with sera collected from mice treated with MHC class II−/− DCs.
n = 5.
TABLE 2.
Adoptive transfer of B. burgdorferi-pulsed DCs elicits a protective response in recipient mice that is T and B cell dependent and does not require MHC class II moleculesa
| Injected material | Presence (+) or absence (−) of MHC class II on DCs | Recipient mice | No. of infected mice/total no. of mice | Reactivity to 41- to 43-kDa antigen after challengeb |
|---|---|---|---|---|
| HBSS | + | WT | 10/10 | + |
| Unpulsed DCs | + | WT | 10/10 | + |
| Unpulsed DCs | + | B cell−/− | 10/10 | + |
| Pulsed DCs | + | WT | 0/10 | − |
| Pulsed DCs | + | B cell−/− | 10/10 | + |
| Pulsed DCs | + | TCRβδ−/− | 5/5 | − |
| Pulsed DCs | − | WT | 0/10 | − |
| Pulsed DCs | − | B cell−/− | 10/10 | + |
Unpulsed or B. burgdorferi-pulsed DCs isolated from wild-type (WT) or MHC class II−/− (C57BL/6) mice were adoptively transferred into naive syngeneic mice. Recipient mice were challenged 10 days later with B. burgdorferi-infected I. scapularis ticks, and infection was monitored 21 days after ticks dropped off, as described in Materials and Methods. Antigen-specific IgG Abs were not detected in TCRβδ−/− mice.
+, reactive; −, nonreactive.
Adoptive transfer of B. burgdorferi-pulsed DCs did not result in the transfer of infectious bacteria, as demonstrated by negative cultures of ear skin biopsy specimens (data not shown).
γδ−/− mice fail to mount a protective humoral response following adoptive transfer of B. burgdorferi-pulsed MHC class II−/− DCs.
Since most γδ T cells recognize MHC class II-independent antigens on APCs (8, 13, 29) and the generation of neutralizing anti-B. burgdorferi Abs can be MHC class II independent, we hypothesized that γδ T cells may be involved in providing help to B cells for the generation of a protective humoral response.
As shown in Table 3, wild-type but not γδ−/− mice resisted challenge with B. burgdorferi-infected I. scapularis ticks following adoptive transfer of MHC class II−/− DCs. Analysis of sera collected 10 days following adoptive transfer of B. burgdorferi-pulsed MHC class II−/− DCs revealed a differential recognition of B. burgdorferi antigens. In contrast to the results for wild-type mice (Fig. 1A, panel a), only minimal reactivity toward B. burgdorferi antigens was observed in γδ−/− mice (Fig. 1B, panel a). The failure of antigen-pulsed MHC class II−/− DCs to induce a protective response in γδ−/− mice correlated with low levels of anti-OspA and anti-OspC Abs (Fig. 2B). These results suggested that the presence of γδ T cells could be required for the generation of neutralizing IgG Abs against MHC class II-independent spirochete antigens. However, we subsequently found that more than just γδ T cells were involved in the generation of anti-B. burgdorferi Abs, as demonstrated by the ability of NK cell-depleted γδ−/− mice to mount a protective humoral response against challenge with B. burgdorferi-infected ticks (Table 3).
TABLE 3.
NK and γδ T cells regulate the protective immune response induced against B. burgdorferi infection by B. burgdorferi-pulsed MHC class II−/− DCsa
| Injected material | Ab treatment | Recipient mice | No. of infected mice (n = 10) |
|---|---|---|---|
| HBSS | − | WT | 10 |
| Unpulsed DCs | − | WT | 10 |
| Unpulsed DCs | − | γδ−/− | 10 |
| Pulsed DCs | − | WT | 0 |
| Pulsed DCs | − | γδ−/− | 10 |
| Pulsed DCs | − | TCRβδ−/− | 10 |
| Pulsed DCs | PK136 | γδ−/− | 0 |
| Pulsed DCs | CIg | γδ−/− | 10 |
DCs were isolated from MHC class II−/− C57BL/6 mice and adoptively transferred into syngeneic recipient mice. Mice were treated with an anti-NK1.1 MAb (PK136) or the isotype-matched control Ab (CIg) prior to DC transfer as described in Materials and Methods. −, no Ab treatment. Ten days later, recipient mice were challenged with B. burgdorferi-infected I. scapularis nymphal ticks. Infectivity with B. burgdorferi was monitored as described in Materials and Methods. Reactivity to the 41- to 43-kDa antigen was observed in infected but not in protected individuals. WT, wild type.
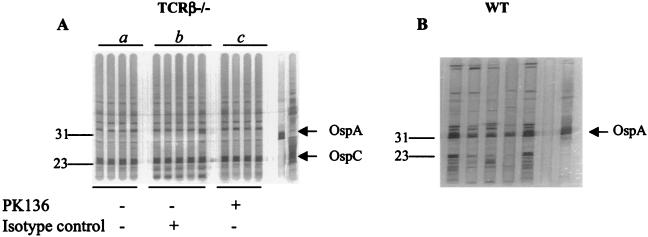
FIG. 1.
Representative Western blot (IgG) analysis of serum samples from individual mice sensitized with B. burgdorferi-pulsed MHC class II−/− DCs. (A and B) Sera were collected following adoptive transfer of B. burgdorferi-pulsed MHC class II−/− DCs into wild-type (WT) (A) and γδ−/− (B) mice. (C) TCRβ−/− mice were sensitized with the indicated B. burgdorferi-pulsed MHC class II+/+ or MHC class II−/− DCs. (D) TCRβ−/− mice were sensitized with B. burgdorferi-pulsed MHC class II−/− DCs. Mice were treated with an anti-NK1.1 MAb (PK136) or the isotype-matched control Ab prior to transfer of B. burgdorferi-pulsed DCs as described in Materials and Methods. The rightmost lanes in panels A, B, and D indicate positive control anti-OspA and anti-OspC MAbs. Numbers on the left indicate molecular masses in kilodaltons. In all experiments, adoptive transfer of unpulsed DCs did not elicit Abs against B. burgdorferi antigens, as previously described (35).
FIG. 2.
Anti-OspA and -OspC IgG Ab levels in sera of mice sensitized with MHC class II−/− DCs, as determined by an ELISA using recombinant B. burgdorferi B31 OspA or OspC. Sera were collected 10 days following adoptive transfer of antigen-pulsed DCs into recipient wild-type (WT) (A) or γδ−/− (B) mice. Treatment of mice with the anti-NK1.1 MAb PK136 was performed as described in Materials and Methods. Values are means and standard deviations. An asterisk indicates a P value of <0.05 for comparisons with the groups indicated under the horizontal bar. CIg, control Ab.
Failure of γδ−/− mice to mount a protective humoral response following adoptive transfer of B. burgdorferi-pulsed MHC class II−/− DCs can be overcome with depletion of NK cells.
Because DCs exposed to antigens could be targeted by NK cell-mediated cytotoxicity (10, 49, 58) and NK cells have been shown to play an important role in the homeostasis of Ab responses (1, 38, 57), we analyzed the humoral responses of mice depleted of NK cells prior to sensitization with B. burgdorferi-pulsed DCs. γδ−/− mice treated with B. burgdorferi-pulsed MHC class II−/− DCs, previously shown to be unable to mount a protective immune response following challenge with B. burgdorferi, now resisted infection following depletion of NK cells (Table 3). In contrast to those from wild-type mice (Fig. 1A, panel b), sera from NK cell-depleted γδ−/− mice recognized a limited set of B. burgdorferi antigens, showing strong reactivity to OspA but not other B. burgdorferi protective antigens, such as OspC (Fig. 1B, panel b), as confirmed by an ELISA (Fig. 2A and B). In addition, depletion of NK cells prior to DC transfer resulted in increases in the levels of anti-OspA IgG2b Abs in γδ−/− mice (Fig. 3B), whereas significant increases in both anti-OspA and anti-OspC IgG2b Ab levels were observed in wild-type mice (Fig. 3D). In contrast, depletion of NK cells did not alter the levels of anti-OspA and anti-OspC IgG1 Abs in mice treated with DCs (Fig. 3A and C). Anti-B. burgdorferi IgG2a levels could not be detected in B6 mice, due to the deletion of the IgG2a gene in these mice (33). Moreover, we did not find detectable levels of anti-OspA and anti-OspC IgG3 Abs (data not shown). Thus, NK cells can selectively regulate the generation of neutralizing Abs against B. burgdorferi antigens.
FIG. 3.
Anti-OspA and -OspC isotype-specific Ab levels in sera of γδ−/− and wild-type (WT) mice sensitized with MHC class II−/− DCs, as determined by an ELISA using recombinant B. burgdorferi B31 OspA or OspC. Sera were collected from γδ−/− (A and B) and WT (C and D) mice 10 days following DC transfer. Mice were treated with an anti-NK1.1 MAb (PK136) or the isotype-matched control Ab (CIg) prior to transfer of B. burgdorferi-pulsed DCs as described in Materials and Methods. Values are means and standard deviations. An asterisk indicates a P value of <0.05 for comparisons with the groups indicated under the horizontal bars.
In addition, the lack of or low levels of anti-OspC Ab production in γδ−/− mice sensitized with antigen-pulsed MHC class II−/− DCs may reflect a requirement for γδ T cells in providing B-cell help for the generation of anti-OspC Abs. In contrast, αβ T cells present in γδ−/− mice can provide the necessary signals for the generation of neutralizing anti-OspA Abs following sensitization with antigen-pulsed MHC class II−/− DCs. If this line of reasoning is correct, one should expect the generation of high levels of anti-OspC but not anti-OspA Ab production in NK cell-depleted TCRβ−/− mouse cells following sensitization with B. burgdorferi-pulsed MHC class II−/− syngeneic DCs.
The generation of a protective humoral response in TCRβ−/− mice sensitized with antigen-pulsed MHC class II−/− DCs requires the depletion of NK cells.
To test the hypothesis outlined above, we adoptively transferred B. burgdorferi-pulsed MHC class II−/− syngeneic DCs into TCRβ−/− mice, which lack all T cells except γδ T cells (36), and monitored Ab production 10 days later. Consistent with our previous findings demonstrating the down-modulatory effect of NK cells in DC-immunized mice, TCRβ−/− mice failed to elicit a protective humoral response against B. burgdorferi antigens (Fig. 1C, panel a), unless NK cells were depleted prior to the adoptive transfer of B. burgdorferi-pulsed MHC class II−/− DCs (Fig. 1D, panel b). NK cell-depleted TCRβ−/− mice sensitized with antigen-pulsed MHC class II−/− DCs resisted infection following natural challenge with B. burgdorferi-infected I. scapularis nymphal ticks (Table 4). Sera collected from NK cell-depleted TCRβ−/− mice 10 days following DC transfer reacted with a 23-kDa B. burgdorferi antigen on Western blots (Fig. 1D, panel b); this antigen was shown by an ELISA to represent OspC (Fig. 4). In addition, NK cell depletion in TCRβ−/− mice resulted in enhanced anti-OspC IgG2b but not IgG1 Ab production (Fig. 4).
TABLE 4.
Adoptive transfer of B. burgdorferi-pulsed DCs can elicit a protective immune response in the absence of αβ T cellsa
| Injected material | Presence (+) or absence (−) of MHC class II on DCs | Ab treatment | Recipient mice | No. of infected mice (n = 5) |
|---|---|---|---|---|
| HBSS | − | WT | 5 | |
| Unpulsed DCs | + | − | WT | 5 |
| Unpulsed DCs | + | − | TCRβ−/− | 5 |
| Pulsed DCs | + | − | WT | 0 |
| Pulsed DCs | + | − | TCRβ−/− | 0 |
| Pulsed DCs | − | − | WT | 0 |
| Pulsed DCs | − | − | TCRβ−/− | 5 |
| Pulsed DCs | − | PK136 | TCRβ−/− | 0 |
| Pulsed DCs | − | CIg | TCRβ−/− | 5 |
DCs isolated from MHC class II−/− or wild-type (WT) C57BL/6 mice were adoptively transferred into syngeneic recipient mice. Mice were treated with an anti-NK1.1 MAb (PK136) or the isotyped-matched control Ab (CIg) prior to DC transfer as described in Materials and Methods. −, no Ab treatment. Ten days later, recipient mice were challenged with B. burgdorferi-infected I. scapularis ticks. Infectivity with B. burgdorferi was determined as described in Materials and Methods. Reactivity to the 41- to 43-kDa antigen was observed in infected but not in protected individuals.
FIG. 4.
Anti-OspA and -OspC Ab levels in sera of TCRβ−/− mice sensitized with MHC class II−/− DCs, as determined by an ELISA using recombinant B. burgdorferi B31 OspA or OspC. Sera were collected 10 days following DC transfer. Values are means and standard deviations. An asterisk indicates a P value of <0.05 for comparisons with the groups indicated under the horizontal bars. CIg, control Ab.
Interestingly, depletion of NK cells was not required for the generation of a protective humoral response in TCRβ−/− mice sensitized with B. burgdorferi-pulsed MHC class II+/+ DCs (Table 4); this result suggested that MHC class II molecules on DCs can interfere with the down-modulatory effect of NK cells, perhaps by protecting these APCs from NK cell-mediated cytotoxicity. Ten days following the adoptive transfer of antigen-pulsed MHC class II+/+ DCs, sera from recipient TCRβ−/− mice recognized 31- and 23-kDa proteins (Fig. 1C, panel b), which represented OspA and OspC, respectively (as determined by an ELISA; data not shown).
TCRβ−/− mice fail to develop anti-OspA Abs following challenge with B. burgdorferi
Because TCRβ−/− mice naturally infected with B. burgdorferi-infected ticks may not generate anti-OspA Ab production due to the down-regulation of the expression of this protein following natural challenge (16, 24, 37, 48), mice were challenged with culture-derived B. burgdorferi in order to ensure that the injected spirochetes were expressing OspA. The injected spirochetes likely would interact with endogenous DCs expressing MHC class II molecules in recipient mice. Thus, this approach allowed us to test the hypothesis that αβ T cells are required for anti-OspA IgG Ab production in an environment where DCs expressing MHC class II molecules may be present. Twenty-one days following challenge with culture-derived B. burgdorferi, sera collected from TCRβ−/− mice consistently recognized a 23-kDa B. burgdorferi antigen (Fig. 5A) shown by an ELISA to represent OspC (Fig. 6). However, the same sera failed to recognize 31-kDa B. burgdorferi OspA (Fig. 5A, panel a, and Fig. 6) despite prior depletion of NK cells (Fig. 5A, panel c, and Fig. 6). In contrast, analysis of sera collected from wild-type mice inoculated with culture-derived spirochetes demonstrated reactivity to a 31-kDa B. burgdorferi antigen (Fig. 5B) shown by an ELISA to represent OspA (data not shown). These findings support the contention that while γδ T cells alone can support the generation of anti-OspC IgG Abs (Fig. 6), αβ T cells may be needed for the production of anti-OspA Abs (Fig. 6), regardless of the presence or absence of MHC class II molecules on APCs.
FIG. 5.
Representative Western blot (IgG) analysis of serum samples from individual TCRβ−/− (A) and wild-type (WT) (B) mice sensitized with B. burgdorferi-pulsed MHC class II−/− DCs. Sera were collected 21 days following challenge with needle-inoculated spirochetes (104 per mouse). An anti-NK1.1 MAb (PK136) or isotype control Abs were administered as described in Materials and Methods. The rightmost lanes indicate positive control anti-OspA and -OspC MAbs. Numbers on the left indicate molecular masses in kilodaltons.
FIG. 6.
Anti-OspA and -OspC IgG Ab levels in sera collected from TCRβ−/− mice 21 days following needle challenge with B. burgdorferi. Mice were treated with an anti-NK1.1 MAb (PK136) or the isotype-matched control Ab (CIg) prior to transfer of B. burgdorferi-pulsed MHC class II−/− DCs as described in Materials and Methods. Values are means and standard deviations. An asterisk indicates a P value of <0.05 for comparisons with the groups indicated under the horizontal bar.
DISCUSSION
It was previously reported that the adoptive transfer of B. burgdorferi-pulsed DCs into syngeneic mice mediated a protective immune response against tick-transmitted spirochetes (35). Here, we show that this protective response (i) is Ab dependent, (ii) can be directed against T-cell-dependent protective B. burgdorferi antigens, and (iii) does not require the presence of MHC class II molecules on DCs. A summary of these findings is presented in Table 5.
TABLE 5.
Summary of findingsa
| Mice into which B. burgdorferi-pulsed MHC class II−/− DCs were adoptively transferred | Outcome of challenge with tick-infected B. burgdorferi | Antibody response to selected B. burgdorferi antigens after challengeb |
|---|---|---|
| WT | Protection | OspA+; OspC+ |
| TCRβδ−/− | No protection | None |
| γδ−/− | No protection | OspA±; OspC± |
| γδ−/−; NK cell depleted | Protection | OspA+; OspC± |
| TCRβ−/− | No protection | OspA−; OspC− |
| TCRβ−/−; NK cell depleted | Protection | OspA−; OspC+ |
Only a summary of the findings collected for MHC class II−/− DC-immunized mice is presented. DCs were isolated from MHC class II−/− mice, pulsed with B. burgdorferi, and adoptively transferred into syngeneic mice. Protection studies were performed as described in Materials and Methods. Sera were collected 21 days after challenge, and the presence of anti-OspA and anti-OspC Abs was determined by an ELISA. WT, wild type.
+, reactive; ±, weakly reactive; −, negative.
Although the secretion of antigen-specific IgG Abs by B cells can require the appropriate help from αβ CD4+ T cells (14), we found that γδ T cells were also able to provide B-cell help for the production of neutralizing anti-B. burgdorferi Abs in mice sensitized with MHC class II−/− DCs. In addition, the generation of anti-OspC Abs in TCRβ−/− mice challenged with B. burgdorferi (Fig. 5A and 6) further supports the idea that γδ T cells alone may be sufficient for providing B-cell help for anti-OspC IgG Ab production. The ability of γδ T cells to provide help to B cells for the production of antigen-specific IgG Abs remains controversial. A recent report showed that repeated inoculation of mycobacterial antigens into TCRβ−/− mice did not result in the production of antigen-specific Abs (40). The authors demonstrated that Ab specificities were more commonly directed toward self-antigens, as opposed to the challenging pathogen. Our findings are in agreement with previous studies, which demonstrated that γδ T cells could provide the necessary signals for the generation of neutralizing IgG Abs in mice infected with vesicular stomatitis virus (32).
Interestingly, in our model, γδ T cells provided help for the elicitation of a humoral immune response against a limited set of antigens. Most noticeable was the ability of γδ T cells to support the production of anti-OspC but not anti-OspA IgG Abs in mice sensitized with antigen-pulsed MHC class II−/− DCs. A role for γδ T cells in mediating an innate immune response to common B. burgdorferi lipoproteins in Lyme disease patients has been suggested (56). Moreover, these investigators demonstrated that γδ T cells can recognize lipidated OspA and OspC antigens, independent of MHC class I or class II, CD1a, CD1b, or CD1c restriction (56). Thus, one could postulate that a rapid humoral immune response against a limited set of spirochetal antigens in infected hosts may result from a pathway that bypasses MHC class II restriction and involves γδ T cells in providing the necessary help to B cells.
The generation of neutralizing Abs against B. burgdorferi antigens in MHC class II−/− DC-immunized mice was not exclusively mediated by γδ T cells because neutralizing Abs arose in γδ−/− mice depleted of NK cells prior to DC transfer. γδ−/− mice depleted of NK cells mounted a strong anti-OspA Ab response, suggesting that αβ T cells, but not γδ T cells, provided the necessary help to B cells. The population of OspA-specific, MHC class II-independent TCRαβ+ T cells capable of providing B-cell help remains unknown. However, a population of CD4− CD8− TCRαβ+ cells capable of providing help to B cells has been described (17), and it remains to be determined if such a T-cell population can provide help to B cells for the production of anti-OspA Abs.
The mechanisms underlying the modulatory effect of NK cells in the generation of neutralizing anti-B. burgdorferi Abs remain to be determined. Because antigen-pulsed DCs may represent targets for NK cells (10, 49, 58), we cannot exclude a mechanism in which DCs presenting B. burgdorferi OspA antigens activate NK cells, which can target these APCs, resulting in down-modulation of anti-B. burgdorferi IgG Ab production. This notion would be consistent with previous findings showing transient but significant increases in anti-B. burgdorferi IgG titers in infected C3H beige mice which are defective in NK cell and granulocyte functions (7). These findings are also consistent with our observations showing increases in anti-OspA and -OspC IgG2b Ab production in NK cell-depleted wild-type mice (Fig. 3D). Given the ability of OspA to activate murine NK cells (31), it is possible that DCs exposed to B. burgdorferi outer surface proteins, such as OspA, trigger NK cell functions, resulting in the targeting of some of the injected DCs.
With Lyme disease patients, a correlation between the activation of NK cells and the humoral response to B. burgdorferi OspA has been demonstrated. Patients with severe and prolonged Lyme arthritis have suppressed NK cell activity (15), correlating with high levels of anti-OspA and -OspB IgG Abs (2, 28). In contrast, Lyme disease patients with mild and brief arthritis show no evidence of NK cell suppression (15) and have low levels of anti-OspA IgG Abs (2, 28). Thus, our findings may have important ramifications regarding the generation of high levels of anti-OspA Abs following immunization with the current recombinant OspA vaccine (50, 54).
NK cell-depleted TCRβ−/− mice sensitized with MHC class II−/− DCs were able to mount a protective humoral response against tick-transmitted spirochetes. This protective humoral response correlated with increased levels of anti-OspC Abs and low levels of anti-OspA Abs. In addition, the levels of anti-OspA but not anti-OspC Abs waned rapidly following challenge with culture-derived, OspA-expressing spirochetes (Fig. 5A and 6). These findings further support the contention that αβ T cells may be required for anti-OspA Ab production, whereas γδ T cells may be sufficient in providing help for the generation of anti-OspC Abs. We also found that, in contrast to the results for TCRβ−/− mice sensitized with antigen-pulsed MHC class II−/− DCs, depletion of NK cells was not required for the generation of a protective humoral response in TCRβ−/− mice treated with antigen-pulsed MHC class II+/+ DCs. These findings raise the interesting possibility that levels of MHC class II expression on DCs play an important role in providing protective signals against cytolysis mediated by NK cells. Indeed, a similar pathway has been shown to play a role in protecting tumor cells against NK cell-mediated cytotoxicity (30).
It is possible that antigen-pulsed DCs, targeted by NK cell cytotoxicity, release their antigenic content, which could be recycled by endogenous DCs (3, 44). Thus, injected DCs may not represent the APCs that prime T cells in situ, as demonstrated in a different model (11). However, this situation is not likely to occur in our model, for several reasons. First, adoptive transfer of B. burgdorferi-pulsed MHC class II−/− DCs into TCRβ−/− mice did not elicit any detectable antigen-specific Ab response (Fig. 1C, panel a, and Fig. 5A). In addition, differential Ab responses were observed in γδ−/− mice and in wild-type mice following sensitization with B. burgdorferi-pulsed MHC class II−/− DCs (compare Fig. 1A, panel a, to Fig. 1B, panel a). Therefore, if endogenous DCs, which express MHC class II molecules in all recipient mice, were involved in recycling the antigen content in B. burgdorferi-pulsed MHC class II−/− DCs and in cross-priming T cells in vivo, one would expect a similar Western blot profile elicited by sera derived from recipient mice. The possible absence of cross-priming in our model could be explained by the small number of adoptively transferred DCs (approximately 104), since it has been recently shown that the immunogenicity of apoptotic cells is proportional to the number of cells injected (43, 44). Future studies will be needed to clarify this issue.
Which B. burgdorferi antigen-specific Ab isotypes mediate a protective immune response in vivo has not been resolved. Recognition of a B. burgdorferi antigen by a specific Ab isotype after DC treatment showed that adoptive transfer of B. burgdorferi-pulsed DCs induced increases in the levels of anti-OspA and -OspC IgG2b but not IgG1 Abs. With an in vitro spirochete neutralization assay, it has been shown that Th1-type Abs IgG2a and IgG2b are bacteriostatic (47). Schaible et al. (46) correlated protection against challenge with syringe-inoculated B. burgdorferi with the development of B. burgdorferi antigen-specific IgG2b and IgG3 Ab isotypes after adoptive transfer of presensitized B cells into SCID mice. Likewise, Fikrig et al. (19) demonstrated that the IgG2b Ab isotype, directed against multiple antigens of B. burgdorferi, passively protects SCID mice from infection and the development of Lyme arthritis.
The specific involvement of a defined anti-B. burgdorferi Ab isotype(s) in mediating a protective humoral immune response against tick-transmitted infection after DC transfer remains undetermined. However, depletion of NK cells in γδ−/− mice resulted in significant increases in the levels of anti-OspA IgG2b Abs, correlating with the ability of the animals to resist infection with B. burgdorferi. Because the presence of different cytokines during the presentation step can greatly influence the nature of B-cell help provided by effector cells and the subsequent Ab isotype secreted (14), one could hypothesize that B. burgdorferi OspA and OspC antigens presented by DCs induce the production of gamma interferon, resulting in the secretion of the IgG2b Abs which are associated with protection in this model.
There is increasing evidence that DCs play a critical role in linking the innate and adaptive arms of the immune system (18, 39). Our present findings further support this contention by depicting a regulatory pathway involving DCs, γδ cells, and NK cells in controlling the generation of neutralizing IgG Abs against B. burgdorferi antigens.
ACKNOWLEDGMENTS
We are grateful to Gregory K. DeKrey and Joseph D. Smith for critical reading of the manuscript.
REFERENCES
- 1.Abruzzo L V, Rowley D A. Homeostasis of the antibody response: immunoregulation by NK cells. Science. 1983;222:581–585. doi: 10.1126/science.6685343. [DOI] [PubMed] [Google Scholar]
- 2.Akin E, McHugh G L, Flavell R A, Fikrig E, Steere A C. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect Immun. 1999;67:173–181. doi: 10.1128/iai.67.1.173-181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert L M, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 4.Altenschmidt U, Ricciardi-Castagnoli P, Modolell M, Otto H, Wiesmuller K H, Jung G, Simon M M. Bone marrow-derived macrophage lines and immortalized cloned macrophage and dendritic cells support priming of Borrelia burgdorferi-specific T cell responses in vitro and/or in vivo. Immunol Lett. 1996;50:41–49. doi: 10.1016/0165-2478(96)02517-5. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 6.Barbour A G. Isolation and cultivation of the Lyme disease spirochete. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 7.Barthold S W, de Souza M. Exacerbation of Lyme arthritis in beige mice. J Infect Dis. 1995;172:778–784. doi: 10.1093/infdis/172.3.778. [DOI] [PubMed] [Google Scholar]
- 8.Boismenu R, Havran W L. An innate view of γδ T cells. Curr Opin Immunol. 1997;9:57–63. doi: 10.1016/s0952-7915(97)80159-8. [DOI] [PubMed] [Google Scholar]
- 9.Burgdorfer W A, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 10.Carbone E, Terrazzano G, Ruggiero G, Zanzi D, Ottaino A, Manzo C, Karre K, Zappacosta S. Recognition of autologous dendritic cells by human NK cells. Eur J Immunol. 1999;29:4022–4029. doi: 10.1002/(SICI)1521-4141(199912)29:12<4022::AID-IMMU4022>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 11.Cayeux S, Richter G, Becker C, Pezzuto A, Dorken B, Blankenstein T. Direct and indirect T cell priming by dendritic cell vaccines. Eur J Immunol. 1999;29:225–234. doi: 10.1002/(SICI)1521-4141(199901)29:01<225::AID-IMMU225>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 13.Chien Y H, Jores R, Crowley M P. Recognition by γ/δ T cells. Annu Rev Immunol. 1996;14:511–532. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 14.Croft M, Swain S L. B cell response to T helper subsets. II. Both the stage of T cell differentiation and the cytokine secreted determine the extent and nature of helper activity. J Immunol. 1991;147:3679–3689. [PubMed] [Google Scholar]
- 15.Dattwyler R J, Thomas J A, Benach J L, Golightly M G. Cellular immune response in Lyme disease: the response to mitogens, live Borrelia burgdorferi, NK cell function and lymphocyte subsets. Zentbl Bakteriol Mikrobiol Hyg Ser A. 1986;263:151–159. doi: 10.1016/s0176-6724(86)80118-3. [DOI] [PubMed] [Google Scholar]
- 16.De Silva A M, Telford III S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erard F, Wild M T, Garcia-Sanz J A, Le Gros G. Switch of CD8+ T cells to noncytolytic CD4−CD8− cells that make TH2 cytokines and help B cells. Science. 1993;260:1802–1805. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez N C, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 19.Fikrig E, Barthold S W, Chen M, Chang C E, Flavell R A. Protective antibodies develop, and murine Lyme arthritis regresses, in the absence of MHC class II and CD4+ T cells. J Immunol. 1997;159:5682–5686. [PubMed] [Google Scholar]
- 20.Fikrig E, Barthold S W, Chen M, Grewal I S, Craft J, Flavell R A. Protective antibodies in Lyme disease arise independently of CD40 ligand. J Immunol. 1996;157:1–3. [PubMed] [Google Scholar]
- 21.Fikrig E, Barthold S W, Kantor F S, Flavell R A. Protection of mice against Lyme disease agent by immunizing against recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- 22.Fikrig E, Barthold S W, Marcantonio N, Deponte K, Kantor F S, Flavell R A. Roles of OspA, OspB, and flagellin in protective immunity to Lyme borreliosis in laboratory mice. Infect Immun. 1992;60:657–661. doi: 10.1128/iai.60.2.657-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filgueira L, Nestle F O, Rittig M, Joller H J, Groscurth P. Human dendritic cells phagocytose and process Borrelia burgdorferi. J Immunol. 1996;157:2998–3005. [PubMed] [Google Scholar]
- 24.Gern L, Schaible U E, Simon M M. Mode of inoculation of the Lyme disease agent Borrelia burgdorferi influences infection and immune responses in inbred strain of mice. J Infect Dis. 1993;167:971–975. doi: 10.1093/infdis/167.4.971. [DOI] [PubMed] [Google Scholar]
- 25.Gilmore R D, Kappel K J, Dolan M C, Burkot T R, Johnson B J B. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64:2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inaba K, Steinman R M. Protein-specific helper T-lymphocyte formation initiated by dendritic cells. Science. 1985;229:475–479. doi: 10.1126/science.3160115. [DOI] [PubMed] [Google Scholar]
- 27.Inaba K, Steinman R M, Van Voorhis W C, Muramatsu S. Dendritic cells are critical accessory cells for thymus-dependent antibody responses in mouse and in man. Proc Natl Acad Sci USA. 1983;80:6041–6045. doi: 10.1073/pnas.80.19.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalish R A, Leong J M, Steere A C. Early and late antibody responses to full-length and truncated constructs of outer surface protein A of Borrelia burgdorferi. Infect Immun. 1995;63:2228–2235. doi: 10.1128/iai.63.6.2228-2235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufmann S H. γ/δ and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci USA. 1996;93:2272–2279. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lobo P I, Patel H C. A novel role for MHC class II antigens: evidence implicating a protective effect on tumor cells against cytotoxicity by NK and LAK cells. Immunology. 1994;83:240–244. [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Y, Seiler K P, Tai K F, Yang L, Woods M, Weis J J. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect Immun. 1994;62:3663–3671. doi: 10.1128/iai.62.9.3663-3671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maloy K J, Odermatt B, Hengartner H, Zinkernagel R M. Interferon gamma-producing gammadelta T cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection. Proc Natl Acad Sci USA. 1998;95:1160–1165. doi: 10.1073/pnas.95.3.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin R M, Liew A M. Is IgG2a a good Th1 marker in mice? Immunol Today. 1998;19:49. doi: 10.1016/s0167-5699(97)87499-3. [DOI] [PubMed] [Google Scholar]
- 34.Mbow M L, Gilmore R D, Jr, Titus R G. An OspC-specific monoclonal antibody passively protects mice from tick-transmitted infection of Borrelia burgdorferi strain B31. Infect Immun. 1999;67:5470–5472. doi: 10.1128/iai.67.10.5470-5472.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mbow M L, Zeidner N, Panella N, Titus R G, Piesman J. Borrelia burgdorferi-pulsed dendritic cells induce a protective immune response against tick-transmitted spirochetes. Infect Immun. 1997;65:3386–3390. doi: 10.1128/iai.65.8.3386-3390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann S H. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 37.Montgomery R R, Malawista S E, Feen K J M, Bockenstedt L. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nabel G, Allard W J, Cantor H. A cloned cell line mediating natural killer cell function inhibits immunoglobulin secretion. J Exp Med. 1982;156:658–663. doi: 10.1084/jem.156.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palucka K, Banchereau J. Linking innate and adaptive immunity. Nat Med. 1999;5:868–870. doi: 10.1038/11303. [DOI] [PubMed] [Google Scholar]
- 40.Pao W, Wen L, Smith A L, Gulbranson-Judge A, Zheng B, Kelsoe G, McLennan I C M, Owen M J, Hayday A C. γδ T cell help of B cells is induced by repeated parasitic infection, in the absence of other T cells. Curr Biol. 1996;6:1317–1325. doi: 10.1016/s0960-9822(02)70718-5. [DOI] [PubMed] [Google Scholar]
- 41.Piesman J. Standard system for infecting ticks (Acari: Ixodidae) with the Lyme disease spirochete. J Med Entomol. 1993;30:199–203. doi: 10.1093/jmedent/30.1.199. [DOI] [PubMed] [Google Scholar]
- 42.Probert W S, LeFebvre R B. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB, or OspC, but not with OspD or the 83-kilodalton antigen. Infect Immun. 1994;62:1920–1926. doi: 10.1128/iai.62.5.1920-1926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ronchetti A, Rovere P, Iezzi G, Galati G, Heltai S, Protti M P, Garancini M P, Manfredi A A, Rugarli C, Bellone M. Immunogenicity of apoptotic cells in vivo: role of antigen load, antigen-presenting cells, and cytokines. J Immunol. 1999;163:130–136. [PubMed] [Google Scholar]
- 44.Rovere P, Vallinoto C, Bondanza A, Crosti M C, Rescigno M, Ricciardi-Castagnoli P, Rugarli C, Manfredi A A. Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J Immunol. 1998;161:4467–4471. [PubMed] [Google Scholar]
- 45.Schaible U E, Kramer M D, Eichmann K, Modolell M, Museteanu C, Simon M M. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci USA. 1990;87:3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaible U E, Wallich R, Kramer M D, Nerz G, Stehle T, Museteanu C, Simon M M. Protection against Borrelia burgdorferi infection in SCID mice is conferred by presensitized spleen cells and partially by B but not T cells alone. Int Immunol. 1994;6:671–681. doi: 10.1093/intimm/6.5.671. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz J, Schell R F, Callister S M, Lovrich S D, Day S P, Coe J E. Immunoglobulin G2 confers protection against Borrelia burgdorferi in LSH hamsters. Infect Immun. 1992;60:2677–2682. doi: 10.1128/iai.60.7.2677-2682.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah P D, Gilbertson S M, Rowley D A. Dendritic cells that have interacted with antigen are targets for natural killer cells. J Exp Med. 1985;162:625–636. doi: 10.1084/jem.162.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sigal L H, Zahradnik J M, Lavin P, Patella S J, Bryant G, Haselby R, Hilton E, Kunkel M, Adler-Klein D, Doherty T, Evans J, Molloy P J, Seidner A L, Sabetta J R, Simon H J, Klempner M S, Mays J, Marks D, Malawista S E. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. Recombinant Outer-Surface Protein A Lyme Disease Vaccine Study Consortium. N Engl J Med. 1998;339:216–222. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 51.Sinsky R J, Piesman J. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J Clin Microbiol. 1989;27:1723–1727. doi: 10.1128/jcm.27.8.1723-1727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sornasse T, Flamand V, de Becker G, Bazin H, Thielemans F, Thielemans K, Urbain J, Leo O, Moser M. Antigen-pulsed dendritic cells can efficiently induce an antibody response in vivo. J Exp Med. 1992;175:15–21. doi: 10.1084/jem.175.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–592. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 54.Steere A C, Sikand V K, Meurice F, Parenti D L, Fikrig E, Schoen R T, Nowakowski J, Schmid C H, Laukamp S, Buscarino C, Krause D S. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 55.Steinman R M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 56.Vincent S M, Roessner K, Sellati T, Hutson C D, Sigal L H, Behar S M, Radolf J D, Budd R C. Lyme arthritis synovial γδ T cells respond to Borrelia burgdorferi lipoproteins and lipidated hexapeptides. J Immunol. 1998;161:5762–5771. [PubMed] [Google Scholar]
- 57.Wilder J A, Koh C Y, Yuan D. The role of NK cells during in vivo antigen-specific antibody responses. J Immunol. 1996;156:146–152. [PubMed] [Google Scholar]
- 58.Wilson J L, Heffler L C, Charo J, Scheynius A, Bejarano M T, Ljunggren H G. Targeting of human dendritic cells by autologous NK cells. J Immunol. 1999;163:6365–6370. [PubMed] [Google Scholar]