Abstract
Background
In multisystem inflammatory syndrome in children (MIS-C), diagnostic delay could be associated with severity. This study aims to measure the time to diagnosis in MIS-C, assess its impact on the occurrence of cardiogenic shock, and specify its determinants.
Methods
A single-center prospective cohort observational study was conducted between May 2020 and July 2022 at a tertiary care hospital. Children meeting the World Health Organization MIS-C criteria were included. A long time to diagnosis was defined as six days or more. Data on time to diagnosis were collected by two independent physicians. The primary outcome was the occurrence of cardiogenic shock. Logistic regression and receiver operating characteristic curve analysis were used for outcomes, and a Cox proportional hazards model was used for determinants.
Results
Totally 60 children were assessed for inclusion, and 31 were finally analyzed [52% males, median age 8.8 (5.7–10.7) years]. The median time to diagnosis was 5.3 (4.2–6.2) days. In univariable analysis, age above the median, time to diagnosis, high C-reactive protein, and high N-terminal pro-B-type natriuretic peptide (NT-proBNP) were associated with cardiogenic shock [odds ratio (OR) 6.13 (1.02–36.9), 2.79 (1.15–6.74), 2.08 (1.05–4.12), and 1.70 (1.04–2.78), respectively]. In multivariable analysis, time to diagnosis ≥ 6 days was associated with cardiogenic shock [adjusted OR (aOR) 21.2 (1.98–227)]. Time to diagnosis ≥ 6 days had a sensitivity of 89% and a specificity of 77% in predicting cardiogenic shock; the addition of age > 8 years and NT-proBNP at diagnosis ≥ 11,254 ng/L increased the specificity to 91%. Independent determinants of short time to diagnosis were age < 8.8 years [aHR 0.34 (0.13–0.88)], short distance to tertiary care hospital [aHR 0.27 (0.08–0.92)], and the late period of the COVID-19 pandemic [aHR 2.48 (1.05–5.85)].
Conclusions
Time to diagnosis ≥ 6 days was independently associated with cardiogenic shock in MIS-C. Early diagnosis and treatment are crucial to avoid the use of inotropes and limit morbidity, especially in older children.
Keywords: COVID-19, Delay in diagnosis, Heart failure, MIS-C, SARS-CoV-2
Introduction
Multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection is a rare but serious condition [1]. Its cumulative incidence was 7.2 per 100,000 children in France on July 7, 2022 [2]. Two-thirds of patients with MIS-C required critical care support. Complications included cardiogenic shock, extracorporeal membrane oxygenation (ECMO), and death [3]. In high-income countries, deaths are relatively rare, accounting for less than 2% of cases [4].
The diagnosis of MIS-C can be difficult [5]. Early diagnosis would allow rapid treatment. Anti-inflammatory treatment, including corticosteroids and intravenous immunoglobulins, resulted in rapid recovery and improved outcomes [6]. However, the delayed diagnosis could lead to delayed treatment and an unfavorable outcome. Little is known about the impact of diagnostic delay on the course of MIS-C and its main complication, cardiogenic shock [7].
Depending on the pediatric disease, the prognosis varies with the time to diagnosis. An increased time to diagnosis, defined as more than 10 days from the onset of symptoms, was associated with an increased risk of coronary artery aneurysms in Kawasaki disease [8]. However, a long time to diagnosis of new-onset heart failure was associated with increased survival in pediatric cardiomyopathies and myocarditis [9].
It is unclear whether a diagnostic delay leads to an increased risk of cardiogenic shock in MIS-C. We hypothesized that a prolonged time to diagnosis was associated with an increased occurrence of cardiogenic shock in MIS-C. The aim of the present study was to measure the time to diagnosis, to assess the consequences of a long time to diagnosis on the onset of cardiogenic shock, and to define the determinants of the diagnostic delay.
Methods
Population
A single-center cohort observational study was conducted from May 2020 to July 2022. The period of analysis was divided into two periods: before and after the implementation of the new French therapeutic guidelines on March 18, 2021 [10].
Children with an MIS-C diagnosis meeting the World Health Organization (WHO) criteria hospitalized at the tertiary hospital center were prospectively included [11]. The non-inclusion criteria were duplicates and refusal to participate. Children who had previously been vaccinated against COVID-19 were excluded to ensure that antibody positivity was not related to the vaccine but rather to an actual COVID-19 infection.
The study was registered by the French National Data Protection Commission (Commission nationale de l’informatique et des libertés, CNIL, Paris, France) under the reference identifier ID1248 and was validated by the local ethics committee in agreement with French regulations for such observational studies and with the Helsinki Declaration of 1964, as revised in 2000. Informed consent was obtained from children and legal guardians.
Time to diagnosis measurement
This study was reported in accordance with the Reporting Studies on Time to Diagnosis (REST) guidelines for time-to-diagnosis studies [12].
The period between the first MIS-C symptoms and the date of diagnosis was named the time to diagnosis, expressed in days. This period was divided into two intervals: patients and physicians. The patient interval was defined as the period between the day and hour of the first symptom and the first medical consultation. The physician interval was defined as the period between the day and hour of the first medical consultation and the diagnosis. Time to admission was defined as the period between the first symptom and the first admission to the hospital.
Two physicians specialized in pediatric cardiology independently assessed the time to diagnosis [9]. Potential disagreements were resolved after a third review of the charts. If disagreements persisted, a third experienced physician determined the time to diagnosis. The assessors could access records from initial visits outside of the tertiary hospital when necessary. They were blinded to the primary outcome during the first diagnosis assessment. A long time to diagnosis was defined as six days or more [13].
Patient characteristics
Variables collected were demographic data, symptoms on the first day, biological and ultrasound features, and treatments. The blood biological features were: sodium, platelet, total leucocyte, neutrophil, and lymphocyte counts, blood creatinine, urea, albumin, C reactive protein (CRP), procalcitonin (PCT), D-Dimers, prothrombin time ratio, factor V activity, N-terminal pro-B-type natriuretic peptide (NT-proBNP), troponin T, lactate, and pH. Extreme values for each child during hospitalization were collected. The glomerular filtration rate (GFR) was calculated using the Schwartz formula [14]. The ultrasound features were minimal left ventricular ejection fraction (LVEF), presence of coronary dilation and dilated coronary Z score, and presence of arrhythmia or high-grade conduction block [15]. LVEF was considered depressed when below 55%: mildly depressed between 45% and 54%, moderately depressed between 35% and 44%, and severely depressed below 35% [16]. The lowest values of LVEF were used. The only potential immunomodulatory treatments used were intravenous immunoglobulins and corticosteroids. Potential antithrombotic treatments were aspirin and heparin. Data were first presented for all children; they were then compared between children with a short and a long time to diagnosis.
Outcomes
The primary outcome was the presence of cardiogenic shock. Cardiogenic shock was defined by the use of one or more inotropes in the context of signs of shock with clinical signs of heart failure (hepatomegaly, pulmonary crackles), decreased LVEF and/or increased NT-proBNP [17]. Inotropes included dobutamine, milrinone, epinephrine, and norepinephrine. Other outcomes collected were distributive shock, shock, extracorporeal membrane oxygenation (ECMO), respiratory support, encephalitis, and death. Distributive shock was defined as the need for a fluid bolus greater than 20 mL/kg and/or vasopressor (epinephrine, norepinephrine) [18]. Shock was defined as cardiogenic shock and/or distributive shock [19]. The length of stay in the pediatric intensive care unit (PICU) and/or high-dependency unit (HDU), the total length of stay, and the number of readmissions related to the MIS-C episode were collected. Patients had at least one cardiology visit within three months of discharge.
Determinants
Potential determinants and confounders included age at diagnosis analyzed as a categorical variable, sex, comorbidities, distance from patient’s home to tertiary care hospital, number of healthcare visits prior to diagnosis, specialty of the first physician encountered, period before or after the implementation of the new French therapeutic guidelines on March 18, 2021, and existence of Kawasaki clinical criteria [8, 10, 20, 21].
Statistical analysis
For the primary outcome, the linearity of the association with time to diagnosis was tested, and time to diagnosis was transformed into a polynomial and categorized in case of deviance from linearity. Associations between time to diagnosis analyzed as a categorical variable, and the primary outcome were studied by univariable analyses and multivariable analyses using a logistic regression model. The sensitivity and specificity of a long time to diagnosis in predicting cardiogenic shock were defined. Outcomes associated with cardiogenic shock were used to build a predictive score. Age was analyzed as a categorical variable; the median age was utilized to define a practical cutoff. Receiver operating characteristic (ROC) curves were used to define the cutoffs of continuous variables collected at diagnosis. The area under each ROC curve was considered acceptable above 0.8. The sensitivity, specificity, and positive and negative predictive values of this score in predicting cardiogenic shock at diagnosis were defined.
For the determinants, the associations between potential determinants and time to diagnosis, analyzed as a continuous variable, were studied with a Cox proportional hazards model since time to diagnosis is a censored variable. The hazard ratios (HRs) obtained expressed the instantaneous risk of a diagnosis: the higher the HR, the shorter the time to diagnosis. Adjusted hazard ratios (aHRs) were calculated by multivariable analysis.
Variables with a P value less than 0.05 for the primary outcome and 0.24 for determinants were retained for the multivariable analyses with a maximum of two models. The significant variables with the highest odds ratios (ORs) were chosen for the two models for outcome. A P value less than 0.05 was considered statistically significant.
For a two-tailed test with an alpha risk of 5% and a beta risk of 10%, the estimated minimum sample size was 20 patients, with at least 10 in each group [22, 23].
Statistical analyses were performed with Stata v13 (Statacorp, College Station, TX, USA). The results are presented as numbers (percentages), medians [interquartile ranges (IQR)], ORs or HRs with 95% confidence intervals (95% CIs). For categorical variables, Fisher’s exact test was used. For quantitative variables, a Shapiro-Wilk normality test was used to verify the normal distribution. With unpaired data, an unpaired t test or a Mann-Whitney test was used. With paired data, a paired t test or a Wilcoxon matched-pairs signed rank test was used.
Results
Patient characteristics
Figure 1 shows the flow chart. Totally 28 children (90%) had a positive SARS CoV-2 test: serology (24, 77%) and/or polymerase chain reaction (PCR) (8, 26%) with a previous contact at least two weeks before the onset of symptoms. All the other children (3, 10%) had contact with a patient infected with COVID-19 two to six weeks before the first symptoms.
Fig. 1.
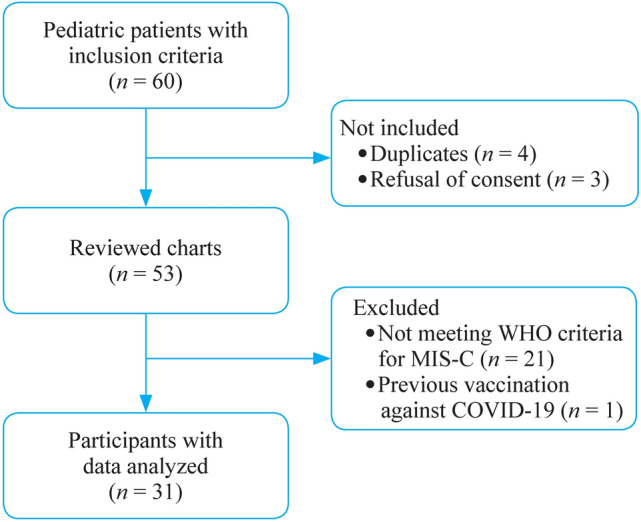
Flow chart of the study. MIS-C multisystem inflammatory syndrome in children
The characteristics of the children are presented in Table 1. All children had elevated CRP and NT-proBNP levels. Three (10%) had coronary dilation. The dilatation was moderate (+ 2.8, + 3.3 and + 3.9 standard deviations) and completely resolved in all cases before discharge. One (3%) had a high-grade atrioventricular block that resolved completely before discharge.
Table 1.
Patient characteristics
| Variables | All children | Time to diagnosis < 6 d (n = 18) | Time to diagnosis ≥ 6 d (n = 13) | P |
|---|---|---|---|---|
| Males | 16 (52) | 10 (56) | 6 (46) | 0.722 |
| Age (y) | 8.8 (5.7–10.7) | 7.8 (5.1–9.8) | 10.5 (8.5–11.8) | 0.026 |
| Weight (kg) | 29 (21–43) | 24 (19–37) | 38 (27–53) | 0.020 |
| Past medical history | 8 (26) | 4 (22) | 4 (31) | 0.689 |
| Asthma | 5 (16) | 3 (17) | 2 (15) | 1 |
| Obesitya | 4 (13) | 1 (6) | 3 (23) | 0.284 |
| CNS malformation | 1 (3) | 1 (6) | 0 (0) | 1 |
| Kidney malformation | 1 (3) | 1 (6) | 0 (0) | 1 |
| Symptoms on the first day | ||||
| Fever | 27 (87) | 17 (94) | 10 (77) | 0.284 |
| Digestive signsb | 15 (48) | 8 (44) | 7 (54) | 0.722 |
| Asthenia | 11 (35) | 4 (22) | 7 (54) | 0.128 |
| Headache | 5 (24) | 5 (28) | 0 (0) | 0.058 |
| Neck pain | 3 (10) | 1 (6) | 2 (15) | 0.558 |
| Sore throat | 3 (10) | 1 (6) | 2 (15) | 0.558 |
| Othersc | 4 (13) | 1 (6) | 3 (23) | 0.284 |
| Blood biological features | ||||
| Sodium (mmol/L) | 131 (126–133) | 132 (127–134) | 129 (126–133) | 0.410 |
| Platelets (109 /L) | 126 (101–266) | 124 (97–202) | 149 (96–290) | 0.400 |
| Leucocytes (109 /L) | 14.9 (8.3–18.6) | 10.0 (7.8–17.8) | 17.5 (12.5–26.4) | 0.012 |
| Lymphocytes (109 /L) | 0.8 (0.5–1.1) | 0.8 (0.5–1.6) | 0.8 (0.4–1.1) | 0.409 |
| Neutrophils (109 /L) | 10.9 (5.7–15.1) | 6.8 (5.5–11.2) | 14.0 (9.4–22.0) | 0.032 |
| CRP (mg/L) | 208 (125–294) | 201 (108–254) | 269 (166–414) | 0.075 |
| Procalcitonin (ng/ml)d | 17 (9.5–32) | 15 (8–19) | 24 (10–95) | 0.165 |
| Albumin (g/L)e | 25 (22–30) | 25 (23–30) | 24 (21–29) | 0.240 |
| D-Dimers (μg/L)f | 4000 (2800–6200) | 4050 (2875–5600) | 3700 (2330–7000) | 0.835 |
| Urea (g/L) | 0.43 (0.32–0.59) | 0.36 (0.29–0.45) | 0.59 (0.40–1.20) | 0.003 |
| GFR (ml/min/1.73m2) | 115 (87–138) | 128 (104–148) | 91 (35–128) | 0.012 |
| Prothrombin time ratio (%)g | 75 (66–86) | 80 (70–88) | 67 (51–79) | 0.014 |
| Lactates (mmol/L) | 2.5 (1.6–3.6) | 1.9 (1.5–3.2) | 3.2 (1.7–4.6) | 0.103 |
| Acidosish | 5 (16) | 1 (7) | 4 (31) | 0.134 |
| NT-proBNP (ng/L) | 10,012 (3527–22,304) | 5555 (2384–10,227) | 22,304 (13,859–47,889) | < 0.001 |
| Troponin T (ng/L) | 58 (29–166) | 32 (16–60) | 141 (63–316) | 0.001 |
| LVEF (%) | 45 (40–52.5) | 45 (45–56) | 40 (30–50) | 0.021 |
| Normal | 7 (23) | 5 (28) | 2 (15) | 0.667 |
| 45–54 | 12 (39) | 10 (56) | 2 (15) | 0.032 |
| 35–44 | 8 (26) | 3 (17) | 5 (38) | 0.229 |
| < 35 | 4 (13) | 0 (0) | 4 (31) | 0.023 |
Results were expressed as number (%) or median (IQR). Significant P values < 0.05 were indicated in bold
CNS central nervous system, CRP C-reactive protein, GFR Glomerular Filtration Rate, IQR interquartile range, NT-proBNP N-terminal pro-B-type natriuretic peptide, LVEF ultrasound left ventricular ejection fraction, MIS-C multisystem inflammatory syndrome in children
aObesity was defined as a body mass index above the International Obesity Task Force curve depending on age, reaching the value of 30 at 18 years old
bDigestive signs included vomiting, abdominal pain and diarrhea
cOther symptoms on the first day included myalgia and dysgeusia (same patient), arthralgia and rash (same patient), cough, and hypotonia (n = 1, 3% for each)
dn = 25 with available data
en = 29 with available data
fn = 15 with available data
gChildren with low prothrombin time had normal factor V activity (above 60%)
hAcidosis was defined as pH below 7.37 for arterial or capillary samples and below 7.33 for venous samples
Time to diagnosis and outcomes are presented in Table 2. Records from initial visits outside of the tertiary hospital were needed for one child. The mean (± standard deviation) time to diagnosis was 5.3 (± 1.6) days, and the mean difference between short and long time to diagnosis was 2.4 days. The time to diagnosis was not significantly different from the time to treatment for all children (Table 2, P = 0.753). The time to diagnosis was significantly longer than the time to admission for all children (Table 2, P < 0.001).
Table 2.
Time to diagnosis and outcomes
| Variables | All children | Time to diagnosis < 6 d (n = 18) | Time to diagnosis ≥ 6 d (n = 13) | P |
|---|---|---|---|---|
| First consulted physician | ||||
| General practitioner | 21 (68) | 12 (67) | 9 (69) | 1 |
| Hospital emergency | 10 (32) | 6 (33) | 4 (31) | 1 |
| Number of healthcare visits before diagnosis | 3 (2–4) | 3 (2–3.3) | 3 (3–4) | 0.221 |
| Patient interval | 2.0 (1.0–3.0) | 1.5 (1.0–3.0) | 2.2 (1.2–3.5) | 0.377 |
| Medical interval | 3.1 (2.0–4.4) | 2.7 (1.0–3.2) | 4.4 (3.6–5.6) | 0.001 |
| Time to diagnosis | 5.3 (4.2–6.2) | 4.7 (3.3–5.1) | 6.3 (6.1–7.2) | < 0.001 |
| Time to treatment | 5.3 (4.3–6.3) | 4.7 (3.6–5.1) | 6.3 (6.1–7.3) | < 0.001 |
| Time to admission | 4.0 (3.0–5.0) | 4.0 (2.8–4.2) | 5.0 (3.0–6.0) | 0.071 |
| Admission ward | ||||
| PICU | 22 (71) | 11 (61) | 11 (85) | 0.237 |
| HDU | 6 (19) | 4 (22) | 2 (15) | 1 |
| Pediatric cardiology | 3 (10) | 3 (17) | 0 | 0.245 |
| Non-invasive positive airway pressure supporta | 5 (16) | 0 (0) | 5 (38) | 0.008 |
| Shock | 11 (35) | 3 (17) | 8 (62) | 0.021 |
| Cardiogenic shock | 9 (29) | 1 (8) | 8 (62) | 0.001 |
| Distributive shock | 6 (19) | 3 (17) | 3 (23) | 0.676 |
| Inotropes | ||||
| None | 22 (71) | 17 (94) | 5 (38) | 0.001 |
| Dobutamine | 7 (23) | 0 (0) | 7 (54) | < 0.001 |
| Norepinephrine | 4 (13) | 1 (6) | 3 (23) | 0.284 |
| Epinephrine | 2 (6) | 0 (0) | 2 (15) | 0.168 |
| Milrinone | 2 (6) | 0 (0) | 2 (15) | 0.168 |
| Heparinb | 7 (23) | 1 (6) | 6 (46) | 0.012 |
| Encephalitis | 9 (29) | 5 (28) | 4 (31) | 1 |
| Death | 0 (0) |
Results were expressed as number (%) or median (IQR). Significant P values < 0.05 were indicated in bold
HDU high-dependency unit, IQR interquartile range, MIS-C multisystem inflammatory syndrome in children, PICU pediatric intensive care unit
aFour children were treated with high-flow nasal cannula and one with non-invasive ventilation
bFour children had a preventive dose and three curative.
Medical interval: time from first medical consultation to diagnosis; patient interval: time from the first symptom to first medical consultation
Among analyzed children, 21 (68%) were treated after the implementation of the new therapeutic guidelines: they were all treated with intravenous immunoglobulins and corticosteroids, apart from one who was only treated with corticosteroids. Among the 10 children (32%) treated before, they all received intravenous immunoglobulins. Eight children (80%) had corticosteroids before the new guidelines, vs. 21 (100%) after (P = 0.097).
Nine children (29%) had aspirin, including three treated at a low dose for coronary dilatation. Six children (60%) received aspirin before the new guidelines, vs. 3 (14%) after (P = 0.015).
Outcomes
Excluding children with encephalitis, children with a long time to diagnosis had a longer PICU and/or HDU length of stay [3 (2–4) vs. 2 (0.5–3) days, P = 0.025, n = 22]. They also had a longer total length of stay [7 (6.5–7.5) vs. 6 (5–6) days, P = 0.048, n = 21] before discharge from the tertiary care hospital.
Table 3 shows the factors associated with cardiogenic shock. One child (3%) received only norepinephrine. In univariable analysis, age greater than eight years, high levels of CRP and NT-proBNP, and a long time to diagnosis were associated with the use of inotropes. In multivariable analysis, a time to diagnosis equal to or greater than six days was associated with the use of inotropes, regardless of age or CRP level. Figure 2 reveals the probability of the onset of cardiogenic shock as a function of time to diagnosis.
Table 3.
Factors associated with cardiogenic shock
| Variables | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | Model 1 aOR (95% CI) |
P | Model 2 aOR (95% CI) |
P | |
| Age ≥ 8.8 y | 6.13 (1.02–36.89) | 0.048 | 3.56 (0.43–29.65) | 0.240 | ||
| CRPa | 2.08 (1.05–4.12) | 0.035 | 1.75 (0.79–3.84) | 0.165 | ||
| NT-proBNPb | 1.70 (1.04–2.78) | 0.035 | ||||
| Troponin Tc | 1.05 (0.99–1.12) | 0.120 | ||||
| Time to diagnosis (d) | 2.79 (1.15–6.74) | 0.023 | ||||
| Time to diagnosis ≥ 6 d | 27.20 (2.71–272.83) | 0.005 | 21.01 (2.00–220.81) | 0.011 | 21.21 (1.98–227.05) | 0.012 |
Data with significant P values < 0.05 were indicated in bold
aOR adjusted odds ratio, CRP C-reactive protein, MIS-C multisystem inflammatory syndrome in children, NT-proBNP N-terminal pro-B-type natriuretic peptide, OR odds ratio, 95% CI 95% confidence interval
aCRP values were analyzed as (value in mg/L)/100
bNT-proBNP values were analyzed as (value in ng/L)/10000
cTroponin T values were analyzed as (value in ng/L)/10
Fig. 2.
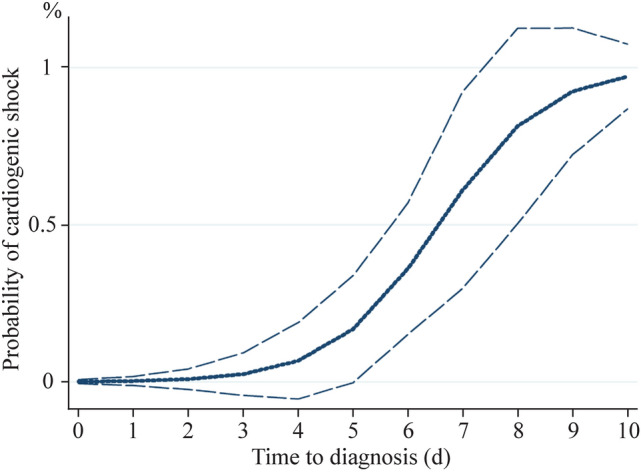
Probability of cardiogenic shock depending on time to diagnosis. The mean is indicated by the solid line and the 95% confidence interval by the dotted lines
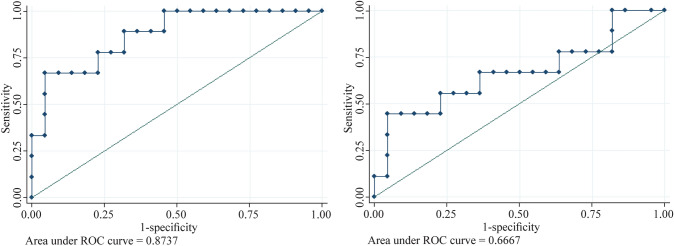
Table 4 shows the sensitivity, specificity, and positive and negative predictive values of time to diagnosis, age and NT-proBNP in predicting cardiogenic shock. Figure 3 shows the ROC curves of NT-proBNP and CRP levels at diagnosis. The area under the ROC curve was considered acceptable for NT-proBNP but not for CRP. A new score including time to diagnosis ≥ 6 days, age > 8 years, and NT-proBNP at diagnosis ≥ 11,254 ng/L (1 point for each) was built. The sensitivity, specificity, and positive and negative predictive values of a score equal to three points are described in Table 4.
Table 4.
Sensitivity, specificity, and positive and negative predictive values of time to diagnosis, age and NT-proBNP in predicting cardiogenic shock at diagnosis of MIS-C
| Variables | Se (%) | Sp (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Time to diagnosis ≥ 6 d (1 point) | 89 | 77 | 62 | 94 |
| Age > 8 y (1 point) | 89 | 50 | 42 | 92 |
| NT-proBNP at diagnosis ≥ 11,254 ng/L (1 point) | 89 | 68 | 53 | 94 |
| Predictive scorea = 3 points | 89 | 91 | 80 | 95 |
MIS-C multisystem inflammatory syndrome in children, NPV negative predictive value, NT-proBNP N-terminal pro-B-type natriuretic peptide, PPV positive predictive value, Se sensitivity, Sp specificity
aThe predictive score, indicated in bold, was defined as the addition of each of the three items
Fig. 3.
Receiver operating characteristic (ROC) curves of NT-proBNP (left graph) and CRP (right graph) at diagnosis of MIS-C to predict cardiogenic shock. Laboratory parameters were collected on the day of diagnosis or within 12.5 h before or after diagnosis. CRP C-reactive protein, MIS-C multisystem inflammatory syndrome in children, NT-proBNP N-terminal pro-B-type natriuretic peptide
Shock was associated with age ≥ 8.8 years [OR 10.50 (1.72–63.91), P = 0.011], signs of encephalitis during hospitalization, including psychomotor slowing, behavior changes, and/or confusion [confirmed secondarily by electroencephalogram, OR 7.50 (1.14–49.26), P = 0.036], and neck pain before or at the time of diagnosis [OR 10.86 (1.03–114.58), P = 0.047]. Distributive shock was associated with neck pain before or at the time of diagnosis [OR 11.50 (1.33–99.33), P = 0.026].
None of the children required invasive ventilation, dialysis, or ECMO. All children recovered completely from clinical cardiological and neurological impairment at a median follow-up of 72 (55–412) days. One (3%) was readmitted for isolated recurrence of fever two weeks after discharge with a decrease in CRP, and corticosteroids were tapered for six weeks instead of three without recurrence.
Determinants
The determinants of short time to diagnosis are presented in Table 5. No child aged 10 or older (9 (29%)) met the clinical Kawasaki criteria.
Table 5.
Determinants of short time to diagnosis
| Variables | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age at diagnosis, y [vs. 1st half (2.6–8.8)] | ||||
| 2nd half (8.8–15.3) | 0.31 (0.13–0.76) | 0.011 | 0.34 (0.13–0.88) | 0.026 |
| Sex (female) | 0.91 (0.44–1.88) | 0.800 | ||
| Comorbiditya | 1.44 (0.64–3.28) | 0.380 | ||
| Distance from the tertiary care hospitalb | 0.49 (0.16–1.52) | 0.219 | 0.27 (0.08–0.92) | 0.036 |
| Number of healthcare visits before diagnosis | 0.77 (0.49–1.20) | 0.249 | ||
| GP as the first consulted physicianc | 1.30 (0.59–2.90) | 0.517 | ||
| Late periodd | 1.62 (0.74–3.56) | 0.232 | 2.48 (1.05–5.85) | 0.038 |
| Children with clinical Kawasaki criteria | 1.89 (0.76–4.67) | 0.171 | 1.89 (0.70–5.11) | 0.210 |
Data with significant P values < 0.05 were indicated in bold
aHR adjusted hazard ratio, GP general practitioner, HR hazard ratio, MIS-C multisystem inflammatory syndrome in children, 95% CI 95% confidence interval
aAt least one comorbidity including asthma, obesity or organ malformation
bDistance was analyzed as (value in km)/100
cVs. emergency physician
dThe late period was defined as the period after the implementation of the new French therapeutic guidelines on March 18, 2021
Discussion
This study was the first to assess the time to diagnosis in MIS-C. The median time to diagnosis of MIS-C was 5.3 days in our tertiary care hospital. More than a third had a long time to diagnosis. High age, CRP and NT-proBNP were associated with cardiogenic shock. A long time to diagnosis was independently associated with cardiogenic shock. A predictive score including time to diagnosis ≥ 6 days, age > 8 years and NT-proBNP at diagnosis ≥ 11,254 ng/L had a sensitivity of 89% and a specificity of 91% for cardiogenic shock. Independent determinants of diagnostic delay were older age, a long distance from tertiary care hospital, and the early pandemic period. Treatment within six days of symptom onset reduced morbidity, including cardiac, respiratory, and renal dysfunction, and was further associated with a reduced length of stay.
The median age, sex ratio and comorbidities of our patients with MIS-C were consistent with previous reports [19]. The rate of gastrointestinal symptoms was lower, but data collection in our study was limited to the first day of symptoms. Here, all children had an increase in NT-proBNP and CRP, as described in the literature [24]. The rates of hospitalization in the PICU, shock, and use of inotropes were similar to those of other studies [4, 25].
This study demonstrated for the first time that diagnostic delay was associated with cardiogenic shock. NT-proBNP and CRP levels were also associated with cardiogenic shock in univariable analysis. NT-proBNP has been shown to be the key cardiac marker to differentiate between serious and non-serious conditions related to SARS-CoV-2 [26]. In the studies by Whittaker et al. and Abrams et al., inflammatory markers were higher in children with shock [19, 27]. In addition, the association between troponin and severity has remained controversial [26, 27]. Troponin was not associated with cardiogenic shock in our study, although it may have been underpowered.
In the study by Bautista-Rodriguez et al., where the patients included had a relatively low mean age of seven years and a low rate of positive SARS-CoV-2 tests of 62.3%, a shorter time to admission was associated with ECMO and death in MIS-C [22]. Here, a shorter time to diagnosis was associated with a lower occurrence of cardiogenic shock in MIS-C, which seemed paradoxical. First, it was shown that the time to diagnosis was significantly different from the time to admission. Second, here, the rate of distributive shock, also called vasoplegic shock, did not differ by time to diagnosis in MIS-C [28]. In the study by Zhang et al., this has also been found in Kawasaki disease shock syndrome [29]. Additionally, in the study by Grimaud et al., vasoplegic features were frequently associated with cardiogenic shock in MIS-C, as found here [30].
Three clinical patterns of MIS-C have already been defined in the study by Whittaker et al., namely, children in shock, children with Kawasaki criteria, and children without shock or Kawasaki criteria. Children in shock were older, had signs of heart failure, high NT-proBNP, and reduced LVEF [19, 22]. In the study by Theocharis et al., their LVEF course has been described in agreement with our definition of a long time to diagnosis: children were frequently admitted on day 5 from the onset of symptoms, and the lowest LVEF was on day 7 [13]. Cardiogenic shock often appeared after six days in children in shock, while in others, cardiogenic shock never occurred. In the study by Bautista-Rodriguez et al., signs of severity were associated with a shorter time to admission [22]. On the one hand, in a population where the time to admission was long, as in the literature, children in shock were admitted on day 7 because of signs of severity, whereas children without shock were admitted later. On the other hand, in a population where the time to admission was short, as in the present study, most children were treated before the onset of signs of severity [median time to treatment (IQR) 5.3 (4.3–6.3) days]. In the current study, the time to admission was indeed short (mean ± standard deviation 3.8 ± 1.7 days) compared to the literature (5 ± 3 days) [22, 23].
To clarify the factors that could explain the diagnostic delay, we showed that children over 8.8 years of age were three times more likely to have a delayed diagnosis, as in heart failure [9]. Older age was then an independent determinant of diagnostic delay but also a predictor of severe MIS-C, as in the study by Karunakar et al. [25]. Moreover, in MIS-C, the specialty of the first consulted physician was not a determinant of the diagnostic delay, unlike congenital heart diseases [9]. In the current study, in MIS-C, the number of healthcare visits before diagnosis was not a determinant either, unlike Kawasaki disease [8]. However, in MIS-C, children living far from our tertiary center were diagnosed significantly later, as previously described in Kawasaki disease, possibly as a result of a delay in physician recognition of MIS-C [8]. Medical knowledge about this disease needs to be deepened, although it has probably improved since the start of the COVID-19 pandemic because the time to diagnosis was shorter in the second half of our study period.
MIS-C diagnosis is challenging, and a differential diagnosis is often evoked. Time is often needed to rule out diagnoses such as bacterial infection or hematological cancer. We suggest prompt treatment when the time to diagnosis is equal to or greater than six days, with all the WHO criteria. When the time to diagnosis is less than six days, the probability of cardiogenic shock seems low, and time may be granted to collect the results of the differential diagnostic tests. High inflammation and increased NT-proBNP have proven their diagnostic value in increasing the likelihood of MIS-C [31]. Echocardiography within 24 hours of admission is advised as LVEF and other more sensitive parameters, including global longitudinal strain, could help to guide the diagnosis [32]. Echocardiography should then initially be repeated daily until a probable diagnosis is found [13].
At the time of diagnosis, predicting shock could improve triage and impact monitoring and care. We described for the first time a three-point score to predict cardiogenic shock, including a cutoff for NT-proBNP. In the study by Ganguly et al., NT-proBNP > 935 ng/L had a sensitivity of 70% and a specificity of 77.5% in diagnosing MIS-C [31]. In the present study, the association of time to diagnosis ≥ 6 days, age > 8 years and NT-proBNP at diagnosis ≥ 11,254 ng/L had a sensitivity of 89% and a specificity of 91% in predicting cardiogenic shock in MIS-C. Treatment could then be intensified.
Our study has several limitations. First, although 21 children (35%) were excluded because they were misclassified, a small proportion of children were not included for refusal of consent. However, the databases used were comprehensive for our tertiary center, as children with SARS-CoV-2-related conditions were prospectively included. Second, although two physicians assessed the time points independently to limit measurement bias, these data were collected retrospectively. Third, our study only included children admitted to our tertiary care hospital. Milder cases admitted to secondary care centers could then have been omitted. Indeed, the rate of cardiac systolic dysfunction (77%) was high in our study, although the same proportions as in the literature of mildly, moderately and severely depressed LVEF were found [16]. Finally, this study was monocentric with a limited number of patients and hence of analyzable variables on multivariable logistic regression, and the predictive score needs external validation.
In conclusion, for MIS-C, children with a time to diagnosis of at least six days had a higher risk of developing cardiogenic shock, especially children over eight years of age, with an NT-proBNP at diagnosis ≥ 11,254 ng/L. Although the time to diagnosis has improved during the last period of the COVID-19 pandemic, a long distance from the tertiary care center was a risk factor for the diagnostic delay. High NT-proBNP and low LVEF could help to diagnose MIS-C in cases of prolonged fever with signs of severity to initiate urgent treatment and limit morbidity. Distributive shock was not associated with time to diagnosis. Larger multicentric studies are needed to better describe this condition.
Acknowledgements
The authors thank Dr Florence Flamein for her help in study design.
Author contributions
SB: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization, writing–original draft, writing– review & editing. MB: data curation, investigation, resources, and writing–review & editing. MR, JS, FD, SL, AH, FG: resources, validation, and writing–review & editing. M-EL, J-BB, HR, OD, TR, AD: resources, and writing–review & editing. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
No funding was secured for this study.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethical approval
This study was validated by the local ethics committee (Univ. Lille, CHU Lille) with the reference identifier ID1248, in agreement with French regulations for such observational studies. Informed consent to participate in the study has been obtained from participants and their parent or legal guardian.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet Lond Engl. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SPF. Situation épidémiologique liée à la COVID-19 chez les 0–17 ans. Point au 7 juillet 2022. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/enquetes-etudes/situation-epidemiologique-liee-a-la-covid-19-chez-les-0-17-ans.-point-au-7-juillet-2022. Accessed 20 Aug 2022
- 3.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet Lond Engl. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radia T, Williams N, Agrawal P, Harman K, Weale J, Cook J, et al. Multi-system inflammatory syndrome in children & adolescents (MIS-C): a systematic review of clinical features and presentation. Paediatr Respir Rev. 2021;38:51–57. doi: 10.1016/j.prrv.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouldali N, Toubiana J, Antona D, Javouhey E, Madhi F, Lorrot M, et al. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA. 2021;325:855–864. doi: 10.1001/jama.2021.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global sars-cov-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 8.Wilder MS, Palinkas LA, Kao AS, Bastian JF, Turner CL, Burns JC. Delayed diagnosis by physicians contributes to the development of coronary artery aneurysms in children with Kawasaki syndrome. Pediatr Infect Dis J. 2007;26:256–260. doi: 10.1097/01.inf.0000256783.57041.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bichali S, Malorey D, Benbrik N, Le Gloan L, Gras-Le Guen C, Baruteau AE, et al. Measurement, consequences and determinants of time to diagnosis in children with new-onset heart failure: a population-based retrospective study (DIACARD study) Int J Cardiol. 2020;318:87–93. doi: 10.1016/j.ijcard.2020.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Société Française de Pédiatrie. Mise à jour du protocole PIMS par le groupe Inflammation pédiatrique COVID. https://www.sfpediatrie.com/actualites/mise-jour-du-protocole-pims-groupe-inflammation-pediatrique-covid. Accessed 19 Aug 2022.
- 11.WHO. Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Accessed 19 Aug 2022.
- 12.Launay E, Cohen JF, Bossuyt PM, Buekens P, Deeks J, Dye T, et al. Reporting studies on time to diagnosis: proposal of a guideline by an international panel (REST) BMC Med. 2016;14:146. doi: 10.1186/s12916-016-0690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theocharis P, Wong J, Pushparajah K, Mathur SK, Simpson JM, Pascall E, et al. Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J Cardiovasc Imaging. 2021;22:896–903. doi: 10.1093/ehjci/jeaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol JASN. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dallaire F, Dahdah N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2011;24:60–74. doi: 10.1016/j.echo.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, et al. Characteristics and outcomes of us children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vahdatpour C, Collins D, Goldberg S. Cardiogenic shock. J Am Heart Assoc. 2019;8:e011991. doi: 10.1161/JAHA.119.011991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith N, Lopez RA, Silberman M. StatPearls. Treasure Island, FL: StatPearls; 2022. Distributive shock. [PubMed] [Google Scholar]
- 19.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 21.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bautista-Rodriguez C, Sanchez-de-Toledo J, Clark BC, Herberg J, Bajolle F, Randanne PC, et al. Multisystem inflammatory syndrome in children: an international survey. Pediatrics. 2021;147:e2020024554. doi: 10.1542/peds.2020-024554. [DOI] [PubMed] [Google Scholar]
- 23.Alali A, O’Neil E, Anders M, Abella J, Shekerdemian LS, Vogel TP, et al. Vasoplegic shock represents a dominant hemodynamic profile of multisystem inflammatory syndrome following covid-19 in children and adolescents. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2022;23:e295–e299. doi: 10.1097/PCC.0000000000002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaushik S, Aydin SI, Derespina KR, Bansal PB, Kowalsky S, Trachtman R, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): a multi-institutional study from New York City. J Pediatr. 2020;224:24–29. doi: 10.1016/j.jpeds.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karunakar P, Ramamoorthy JG, Anantharaj A, Parameswaran N, Biswal N, Dhodapkar R, et al. Clinical profile and outcomes of multisystem inflammatory syndrome in children (MIS-C): hospital-based prospective observational study from a tertiary care hospital in South India. J Paediatr Child Health. 2022;58:1964–1971. doi: 10.1111/jpc.16129. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Patel J, Huang Y, Yin L, Tang L. Cardiac markers of multisystem inflammatory syndrome in children (MIS-C) in COVID-19 patients: a meta-analysis. Am J Emerg Med. 2021;49:62–70. doi: 10.1016/j.ajem.2021.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abrams JY, Oster ME, Godfred-Cato SE, Bryant B, Datta SD, Campbell AP, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5:323–331. doi: 10.1016/S2352-4642(21)00050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambden S, Creagh-Brown BC, Hunt J, Summers C, Forni LG. Definitions and pathophysiology of vasoplegic shock. Crit Care Lond Engl. 2018;22:174. doi: 10.1186/s13054-018-2102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang MM, Shi L, Li XH, Lin Y, Liu Y. Clinical analysis of Kawasaki disease shock syndrome. Chin Med J (Engl) 2017;130:2891–2892. doi: 10.4103/0366-6999.219151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10:69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganguly M, Nandi A, Banerjee P, Gupta P, Sarkar SD, Basu S, et al. A comparative study of IL-6, CRP and NT-proBNP levels in post-COVID multisystem inflammatory syndrome in children (MISC) and Kawasaki disease patients. Int J Rheum Dis. 2022;25:27–31. doi: 10.1111/1756-185X.14236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsubara D, Kauffman HL, Wang Y, Calderon-Anyosa R, Nadaraj S, Elias MD, et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol. 2020;76:1947–1961. doi: 10.1016/j.jacc.2020.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.