Abstract
Background
Adoption and outcomes for conduction system pacing (CSP), which includes His bundle pacing (HBP) or left bundle branch area pacing (LBBAP), in real-world settings are incompletely understood. We sought to describe real-world adoption of CSP lead implantation and subsequent outcomes.
Methods
We performed an online cross-sectional survey on the implantation and outcomes associated with CSP, between November 15, 2020, and February 15, 2021. We described survey responses and reported HBP and LBBAP outcomes for bradycardia pacing and cardiac resynchronization CRT indications, separately.
Results
The analysis cohort included 140 institutions, located on 5 continents, who contributed data to the worldwide survey on CSP. Of these, 127 institutions (90.7%) reported experience implanting CSP leads. CSP and overall device implantation volumes were reported by 84 institutions. In 2019, the median proportion of device implants with CSP, HBP, and/or LBBAP leads attempted were 4.4% (interquartile range [IQR], 1.9–12.5%; range, 0.4–100%), 3.3% (IQR, 1.3–7.1%; range, 0.2–87.0%), and 2.5% (IQR, 0.5–24.0%; range, 0.1–55.6%), respectively. For bradycardia pacing indications, HBP leads, as compared to LBBAP leads, had higher reported implant threshold (median [IQR]: 1.5 V [1.3–2.0 V] vs 0.8 V [0.6–1.0 V], p = 0.0008) and lower ventricular sensing (median [IQR]: 4.0 mV [3.0–5.0 mV] vs. 10.0 mV [7.0–12.0 mV], p < 0.0001).
Conclusion
In conclusion, CSP lead implantation has been broadly adopted but has yet to become the default approach at most surveyed institutions. As the indications and data for CSP continue to evolve, strategies to educate and promote CSP lead implantation at institutions without CSP lead implantation experience would be necessary.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10840-022-01417-4.
Keywords: Conduction system pacing, His bundle pacing, Left bundle branch area pacing
Introduction
His bundle pacing (HBP) and left bundle branch area pacing (LBBAP) have emerged as two approaches to pace the conduction system [1, 2], with mounting evidence that conduction system pacing (CSP) avoids the deleterious effects of right ventricular myocardial pacing [3–10]. Peer-reviewed publications on CSP have risen rapidly since 2017 [11], along with multiple national meetings on the topic, in parallel with CSP becoming a common topic of discussion on social media platforms (such as Twitter) within the electrophysiology community [12]. However, other than a marked increase in the use of the SelectSecure 3830 lead (Medtronic; Minneapolis, MN) in 2017 [11], historically the only option to perform CSP, there is little known about real-world adoption of CSP lead implantation or subsequent outcomes. Previously, worldwide surveys have been successfully utilized to study emerging techniques in electrophysiology [13, 14].
Therefore, we sought to perform a worldwide survey on CSP with HBP and LBBAP leads to determine institutional: (1) implantation experience, (2) implantation approach, and (3) outcomes for bradycardia pacing and cardiac resynchronization therapy (CRT) indications.
Methods
We performed an online cross-sectional survey on implantation of and outcomes for CSP (i.e., HBP and/or LBBAP). The survey was available for completion starting November 15, 2020, until February 15, 2021. The survey was open to all institutions regardless of location, academic affiliation, or experience with CSP. Recurring survey advertisements were disseminated to Circulation: Arrhythmia and Electrophysiology’s over 4000 Twitter followers (@CirculationEP), with tweets tagged with “#dontdistheHis” and “#EPeeps”, and its electronic mailing list, which includes over 1800 contributors. All electrophysiology fellowship program directors in the USA were also notified of the survey [15]. Up to 100 institutions that provided survey data were offered a single authorship position, with authorship determined based on completeness of provided data and on a first-come first-served basis. The data that support the findings of this study are available from the corresponding author upon reasonable request. The research protocol was reviewed by the institutional review board at Stanford University and granted an exemption from approval as it was determined to not involve human subjects.
The survey was developed by authors A. C. P., P. J. W., P. V., and P. S. S. with 73 questions across the following 5 sections: (1) institutional demographics; (2) CSP lead implantation experience; (3) CSP lead implantation procedural approach; (4) CSP outcomes for bradycardia pacing indications (short-term device outcomes, long-term device outcomes, and cardiomyopathy outcomes); and (5) CSP outcomes for CRT indications (short-term device outcomes, long-term device outcomes, and cardiomyopathy outcomes). Assignment of device indication (bradycardia pacing vs. CRT) was made at the discretion of the survey respondent, based on the primary indication for device implant. The complete survey is available in the supplemental material.
We included all survey responses with at least one answered question. Exclusion criteria included survey responses (1) with nonsensical institution name, respondent last name, and respondent email address; (2) that were duplicates from the same respondent; (3) that were duplicates from the same institution (different respondents); (4) with missing experience with CSP leads; and (5) from institutions with no device implanters. For duplicate surveys, we included the most complete survey or the most recent survey if completeness was equal.
We defined CSP experience as self-report of any attempted HBP or LBBAP lead implants at the surveyed institution. For CSP experienced institutions, we described surveyed institutions’ (1) index year CSP pacing lead implantation was attempted, (2) proportion of device (i.e., pacemaker or CRT) and pacemaker implants with CSP lead implantations attempted by year, (3) percent of proceduralists attempting and routinely implanting CSP pacing leads by device indication (i.e., bradycardia pacing vs. CRT), (4) percent of proceduralists utilizing various approaches to HBP lead implantation, (5) preprocedural exclusion criteria for CSP, and (6) selection of HBP versus LBBAP implantation sites. Results were reported for any CSP, HBP only, and LBBAP only and summarized by count and percentages when categorical and median with interquartile range (IQR) when continuous. We selected 2019 as the primary year of interest, to avoid COVID-19-related effects on overall device volume and CSP lead implantation. If the proportion of device or pacemaker implants with CSP lead attempted were > 1.0, which would be possible if CSP lead implantation was an institution’s default approach and HBP and LBBAP lead implantation were both attempted in some cases, we defined the proportion as 1.0. We reported the number of institutions that provided data for each section that could not be completed offhand (e.g., number of CSP leads attempted per year). For CSP inexperienced institutions, we described surveyed institutions rationale for not attempting CSP lead implantation.
We compared surveyed institutions’ baseline characteristics between CSP-experienced and CSP-inexperienced institutions, stratifying CSP-experienced institutions by whether they had experience with both HBP and LBBAP, only HBP, or only LBBAP. For bradycardia pacing and CRT indications, we separately compared HBP and LBBAP (1) short-term device outcomes (intraprocedural success, selective CSP, acute CSP threshold, ventricular sensing, procedure duration, acute lead revision); (2) long-term device outcomes (chronic CSP threshold, chronic lead revision, CS upgrade [CRT only]); and (3) cardiomyopathy outcomes (pacing induced cardiomyopathy [bradycardia pacing only], ejection fraction improvement [CRT only]). Difference between groups were assessed with the χ2 and 2-sample t test for categorical and continuous variables, respectively.
REDCap, version 11.1.0 (Vanderbilt University, Nashville, TN), was used (1) by investigators to build and test the survey, (2) by respondents to complete the survey, and (3) for data management and export. All analyses were performed using STATA, version 17.0 (College Station, TX).
Results
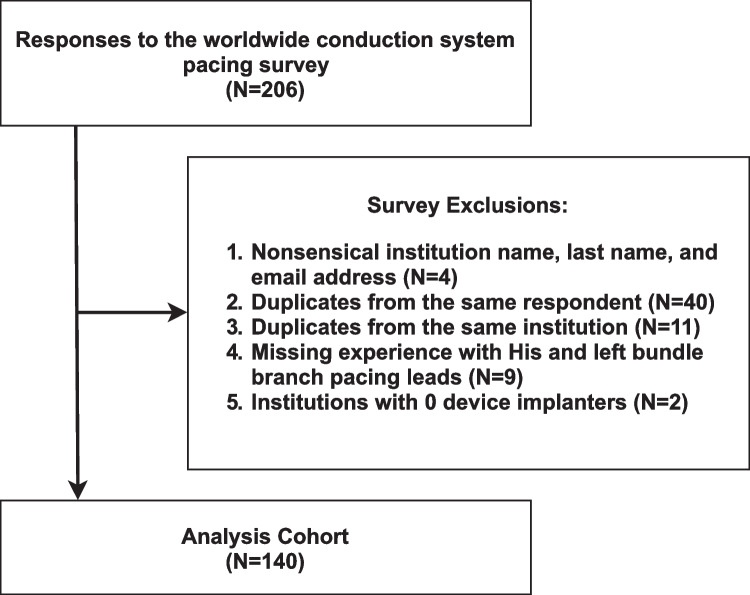
The analysis cohort included 140 institutions, located on 5 continents, who contributed data to the worldwide survey on CSP (Fig. 1). Of these, 127 institutions (90.7%) reported experience implanting CSP leads (i.e., HBP and/or LBBAP). Of the 127 institutions, experience with both HBP and LBBAP was reported by 87 institutions (68.5%), only HBP by 38 institutions (29.9%), and only LBBAP by 2 institutions (1.6%). Performance of CSP for bradycardia pacing and CRT indications was reported by 110 institutions (91.2%) and 92 institutions (79.3%), respectively. As compared to CSP experienced institutions, CSP inexperienced institutions (N = 13) were less likely to be in the USA/Canada (7.7% vs. 36.2%, p = 0.03), with fewer device implanters (3.0 ± 1.4 vs. 5.8 ± 3.4, p = 0.01) (Table 1). CSP-inexperienced institutions reported not attempting CSP primarily due to (1) increased procedural difficulty (e.g., low success rate, high implant threshold, etc.) (N = 5) and (2) lack of access to in-person proctoring for CSP (N = 4).
Fig. 1.
Cohort exclusion diagram. Inclusion and exclusion criteria used to select analysis cohort
Table 1.
Baseline Characteristics of Surveyed Institutions
| CSP Experienced | |||||||
|---|---|---|---|---|---|---|---|
| All Institutions (N = 140) |
All (N = 127) |
His and LBBAP (N = 87) |
His only (N = 38) |
LBBAP only (N = 2) |
CSP Inexperienced (N = 13) |
P Value* | |
| Region | 0.03 | ||||||
| USA/Canada | 47 (33.6%) | 46 (36.2%) | 32 (36.8%) | 13 (34.2%) | 1 (50.0%) | 1 (7.7%) | |
| Central/South America | 7 (5.0%) | 5 (3.9%) | 3 (3.4%) | 2 (5.3%) | 0 | 2 (15.4%) | |
| Western Europe | 45 (32.1%) | 42 (33.1%) | 28 (23.1%) | 14 (36.8%) | 0 | 3 (23.1%) | |
| Eastern Europe | 7 (5.0%) | 7 (5.5%) | 5 (5.7%) | 2 (5.3%) | 0 | 0 | |
| Eastern Asia | 22 (15.7%) | 17 (13.4%) | 13 (14.9%) | 4 (10.5%) | 0 | 5 (38.5%) | |
| Southern Asia | 7 (5.0%) | 5 (3.9%) | 4 (4.6%) | 0 | 1 (50.0%) | 2 (15.4%) | |
| Western Asia | 3 (2.1%) | 3 (2.4%) | 1 (1.1%) | 2 (5.3%) | 0 | 0 | |
| Australia | 2 (1.4%) | 2 (1.6%) | 1 (1.1%) | 1 (2.6%) | 0 | 0 | |
| Academic-Affiliated | 127 (90.7%) | 116 (91.3%) | 79 (90.8%) | 35 (92.1%) | 2 (100%) | 11 (84.6%) | 0.43 |
| Fellowship | 111 (79.2%) | 103 (81.1%) | 75 (87.2%) | 27 (71.1%) | 1 (50.0%) | 8 (61.5%) | 0.10 |
| Number of Device Implanters | 5.5 ± 3.3 | 5.8 ± 3.4 | 5.9 ± 3.5 | 5.5 ± 3.1 | - | 3.0 ± 1.4 | 0.01 |
| CSP Indications | - | ||||||
| Bradycardia Pacing | - | 110 (91.2%) | 75 (92.6%) | 33 (89.2%) | 2 (100%) | - | - |
| CRT | - | 92 (79.3%) | 69 (85.2%) | 22 (66.7%) | 1 (50.0%) | - | - |
Values are mean ± SD or n (%). CRT: cardiac resynchronization, CSP: conduction system pacing, LBBAP: left bundle branch area pacing
*Difference between CSP experienced (all) and CSP inexperienced assessed with the χ2 and 2-sample t test for categorical and continuous variables, respectively
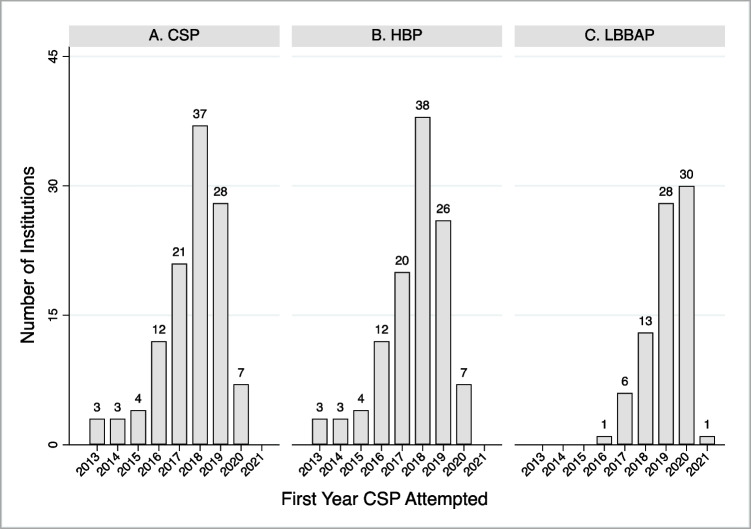
For CSP-experienced institutions, the first year HBP lead implantation was attempted was 1999 (N = 1), with 96% of HBP-experienced institutions not attempting implantation of HBP leads until during or after 2013. The largest number of institutions (N = 38) first attempted implantation of HBP leads in 2018. The first year LBBAP lead implantation was attempted was 2016, with the largest number of institutions (N = 30) first attempting implantation of LBBAP in 2020 (Fig. 2).
Fig. 2.
Index year conduction system pacing lead attempted. Institutions’ first year attempting implantation of 1) conduction system pacing (CSP) lead (panel A), 2) His bundle pacing (HBP) lead (panel B), and 3) left bundle branch area pacing (LBBAP) lead (panel C). CSP includes both HBP and LBBAP leads. Index HBP lead implant also reported in 1999 (n=1), 2003 (n=1), 2006 (n=2), and 2008 (n=1)
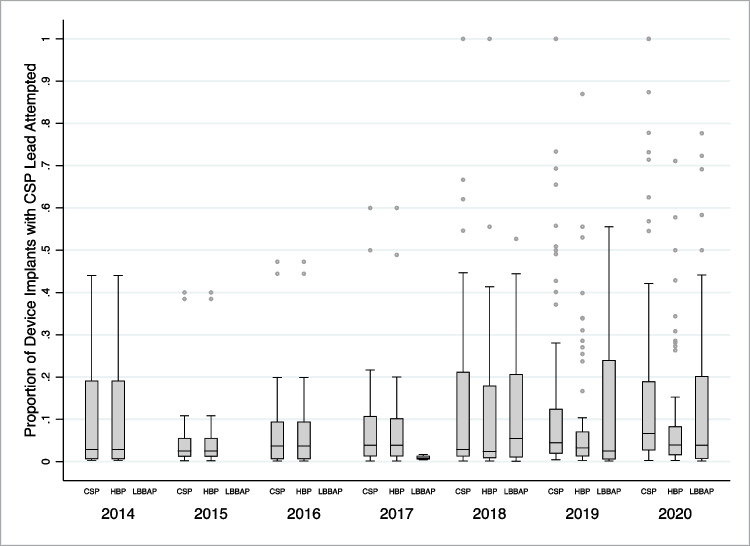
CSP and overall device implantation volumes were reported by 84 institutions, with 177,308 device implants included with a CSP lead attempted in 13,196 (7.4%). In 2019, the median proportion of device implants with CSP, HBP, and/or LBBAP leads attempted were 4.4% (interquartile range [IQR], 1.9–12.5%; range, 0.4–100%), 3.3% (IQR, 1.3–7.1%; range, 0.2–87.0%), and 2.5% (IQR, 0.5–24.0%; range, 0.1–55.6%), respectively. From 2014 to 2020, the median proportion of device implants with CSP leads attempted was numerically similar (2014, 2.9%; 2020, 6.6%). However, the number of institutions with ≥ 50% of device implants with CSP leads attempted numerically increased (2014, 0; 2020, 11) (Fig. 3). For pacemaker implants only, the median proportion with CSP leads attempted in 2019 was 5.9% (IQR, 2.6–20.0%; range, 0.6–100.0%) (Supplemental Fig. 1).
Fig. 3.
Proportion of device implants with conduction system pacing lead attempted by year. Boxplots for proportion of institutions’ device implants with conduction system pacing (CSP) lead attempted, stratified by CSP lead type and year of implant. CSP includes both His bundle pacing (HBP) and left bundle branch area pacing (LBBAP) leads. Horizontal box lines (from top to bottom) represent 3rd quartile, median, and 1st quartile. Whiskers represent the largest and smallest observed value that falls within 1.5 interquartile range of the nearest quartile
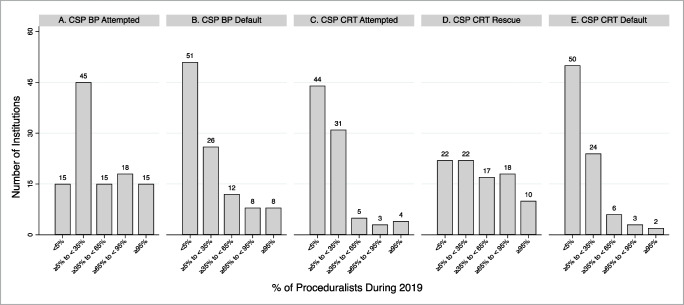
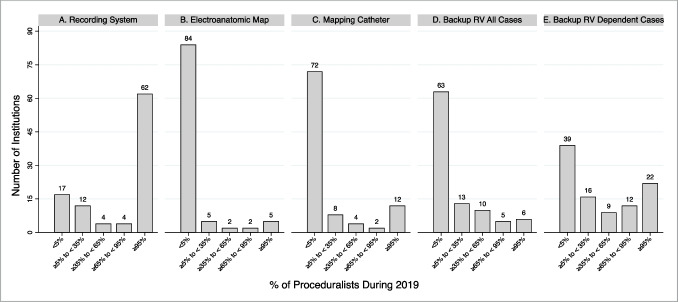
At CSP-experienced institutions in 2019, 48.6% and 58.9% of institutions had < 5% of proceduralists implanting CSP leads as a default strategy for bradycardia pacing and CRT indications, respectively. CSP lead implantation had been attempted by ≥ 95% of proceduralists for bradycardia pacing and CRT indications at 13.9% and 4.6% of institutions, respectively (Fig. 4). The most frequently reported CSP exclusion criteria for bradycardia pacing and CRT indications were AV block (14.5%) and no attempt at coronary sinus lead implantation (35.8%), respectively. No preprocedural CSP exclusion criteria for bradycardia pacing and CRT indications were reported by 63.5% and 48.1% of institutions, respectively (Supplemental Fig. 2). HBP, as compared to LBBAP, was more frequently the default CSP approach (bradycardia pacing, 55.9% vs. 18.6%; CRT, 50% vs. 17.8%) (Supplemental Fig. 3). For HBP lead implantation, the approaches with greatest variability across institutions were use of (1) an electrophysiology recording system to visualize intracardiac electrograms (SD: 1.64) and (2) backup right ventricular pacing for pacemaker-dependent patients (SD: 1.62) (Fig. 5).
Fig. 4.
Percent of proceduralist attempting and routinely implanting conduction system pacing leads by device indication. Percent of proceduralist at an institution during the 2019 calendar year who 1) attempted implantation of conduction system pacing (CSP) leads for bradycardia pacing (BP) indications (panel 1), 2) implanted CSP leads for SP indications as a default strategy (panel B), 3) attempted implantation of CSP leads for cardiac resynchronization therapy (CRT) (panel C), 4) implanted CSP leads for CRT indications after failing to place a coronary sinus lead (panel D), and 5) implanted CSP leads for CRT indications as a default strategy (panel E). CSP includes both His bundle pacing and left bundle branch area pacing leads
Fig. 5.
Percent of proceduralist utilizing various approaches to His bundle pacing lead implantation. Percent of proceduralist at an institution who implanted His Bundle Pacing (HBP) leads during the 2019 calendar year who 1) used an electrophysiology recording system to visualize intracardiac electrograms (i.e., other than the pace sense analyzer) (panel A), 2) used an electroanatomic map to identify the His bundle location (panel B), 3) used a mapping catheter to identify the His bundle location (i.e., other than the pacing lead) (panel C), 4) implanted a permanent backup right ventricle lead in addition to a HBP lead in all cases (panel D), and 5) implanted a permanent backup right ventricle lead in addition to a HBP lead in pacemaker dependent patients (panel E)
Short-term and long-term outcome data on CSP procedures were reported by 84 and 75 institutions, respectively. For bradycardia pacing indications, HBP leads, as compared to LBBAP leads, had higher implant threshold (median [IQR]: 1.5 V [1.3–2.0 V] vs 0.8 V [0.6–1.0 V], p = 0.0008) and lower ventricular sensing (median [IQR]: 4.0 mV [3.0–5.0 mV] vs. 10.0 mV [7.0–12.0 mV], p < 0.0001). Over available follow-up, HBP leads, as compared to LBBAP leads, continued to have higher threshold (median [IQR]: 1.6 V [1.3–2.0 V] vs. 0.7 V [0.6–1.0 V], p < 0.0001) and more lead revisions (median [IQR]: 5.0% [1.0–10.0%] vs. 0.0% [0.0–1.0%], p = 0.0001) (Table 2). For CRT indications, HBP leads, as compared to LBBAP, had higher implant threshold (median [IQR]: 1.7 V [1.4–2.0 V] vs 0.8 V [0.6–1.0 V], p = 0.0215) and lower ventricular sensing (median [IQR]: 3.7 mV [2.8–5.0 mV] vs. 9.0 mV [6.6–10.0 mV], p < 0.0001). Over available follow-up, HBP leads, as compared to LBBAP leads, continued to have higher threshold (median [IQR]: 1.8 V [1.5–2.0 V] vs. 0.8 V [0.6–1.0 V], p < 0.0001), more lead revisions (median [IQR]: 3.5% [0.0–8.0%] vs. 0.0% [0.0–1.0%], p = 0.0118), and more coronary sinus lead upgrades (median [IQR]: 1.0% [0.0–5.0%] vs. 0.0% [0.0–0.3%], p = 0.0268) (Table 3).
Table 2.
Institutions’ conduction system pacing outcomes for bradycardia pacing indications
| His | LBBAP | ||||
|---|---|---|---|---|---|
| Short-Term Outcomes [Nall cases = His/LBBAP] | All Cases | 2019 Cases | All Cases | 2019 Cases | P Value* |
| Intraprocedural Success [N = 75/46]† | 75% (60–90%) | 80% (60–90%) | 81% (50–95%) | 88% (50–95%) | 0.38 |
| Selective CSP [N = 76/44]‡ | 43% (26–60%) | 45% (23–65%) | 37% (10–70%) | 45% (7–83%) | 0.57 |
| CSP Threshold (V) [N = 60/41]‡,§ | 1.5 (1.3–2.0) | 1.4 (1.3–1.5) | 0.8 (0.6–1.0) | 0.7 (0.6–0.8) | 0.0008 |
| Ventricular Sensing (mv) [N = 69/45]‡ | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | 10.0 (7.0–12.0) | 10.0 (7.0–11.0) | < 0.0001 |
| Procedure Duration (minutes) [N = 63/39]¶ | |||||
| CSP | 80 (60–100) | 80 (64–110) | 75 (60–100) | 70 (58–90) | 0.78 |
| Non-CSP | 50 (35–65) | 48 (35–65) | 60 (40–65) | 55 (40–61) | 0.39 |
| Lead Revision (acute) [N = 69/46]‡ | 2.5% (0–8.0%) | 0.3% (0–5.0%) | 0% (0–5.0%) | 0% (0–3.0%) | 0.69 |
| Long-Term Outcomes [Nall cases = His/LBBAP] | |||||
| CSP Threshold [N = 58/37]‡,# | |||||
| Threshold (V) | 1.6 (1.3–2.0) | - | 0.7 (0.6–1.0) | - | < 0.0001 |
| Follow-Up (months) | 12 (9–20) | - | 7 (6–12) | - | 0.0019 |
| Lead Revision (chronic) [N = 49/31]‡,# | |||||
| Revision | 5.0% (1.0–10.0%) | - | 0.0% (0.0–1.0%) | - | 0.0001 |
| Time To Revision (months) | 6 (3–12) | - | 3 (1–5) | - | 0.0490 |
| Pacing Induced Cardiomyopathy [N = 51/37]‡,# | |||||
| Cardiomyopathy (mean ± SD) | 1.1 ± 4.0% | - | 2.1 ± 7.4% | - | 0.40 |
| Ventricular Pacing Burden | 85% (61–95%) | - | 90% (80–100%) | - | 0.76 |
| Time To Cardiomyopathy (months) | 12 (8–24) | - | 6 (4–11) | - | 0.17 |
Values are median (interquartile range) or n (%) unless otherwise specified. CSP: conduction system pacing, LBBAP: left bundle branch area pacing, SD: standard deviation
*Difference between all His and LBBAP cases assessed with the χ2 and 2-sample t test for categorical and continuous variables, respectively
†Success defined as selective or non-selective His or left bundle branch capture with threshold less than 2.5 V @ 1.0 ms or 1.5 V @ 0.5 ms, respectively
‡For CSP cases with intraprocedural success
§CSP capture threshold defined as loss of His or left bundle branch capture at @1.0 ms or 0.5 ms, respectively. Not loss of myocardial capture
¶Procedure duration for transvenous pacemakers without cardiac resynchronization
#Last available follow-up
Table 3.
Institutions’ conduction system pacing outcomes for cardiac resynchronization therapy indications
| His | LBBAP | ||||
|---|---|---|---|---|---|
| Short-Term Outcomes [Nall cases = His/LBBAP] | All Cases | 2019 Cases | All Cases | 2019 Cases | P Value* |
| Intraprocedural Success [N = 52/37]† | 75% (52–90%) | 70% (50–90%) | 80% (63–90%) | 85% (50–91%) | 0.97 |
| Selective CSP [N = 53/35]‡ | 50% (30–65%) | 50% (25–67%) | 40 (10–70%) | 50% (18–60%) | 0.28 |
| CSP Threshold (V) [N = 44/33]‡,§ | 1.7 (1.4–2.0) | 1.6 (1.3–2.2) | 0.8 (0.6–1.0) | 0.7 (0.5–0.9) | 0.0215 |
| Ventricular Sensing (mv) [N = 50/35]‡ | 3.7 (2.8–5.0) | 3.9 (3.0–5.0) | 9.0 (6.6–10.0) | 10.0 (7.2–10.3) | < 0.0001 |
| Procedure Duration (minutes) [N = 43/29]¶ | |||||
| CSP | 110 (90–150) | 100 (90–150) | 95 (80–130) | 90 (70–130) | 0.15 |
| Non-CSP | 98 (75–120) | 95 (70–120) | 99 (70–120) | 93 (70–120) | 0.65 |
| Lead Revision (acute) [N = 50/38]‡ | 1.0 (0–7.0%) | 1.1% (0–15.0%) | 0.0 (0–5.0%) | 0.0 (0–4.0%) | 0.56 |
| Long-Term Outcomes [Nall cases = His/LBBAP] | |||||
| CSP Threshold [N = 46/33]‡,# | |||||
| Threshold (V) | 1.8 (1.5–2.0) | - | 0.8 (0.6–1.0) | - | < 0.0001 |
| Follow-Up (months) | 12 (6–19) | - | 8 (6–12) | - | 0.20 |
| Lead Revision (chronic) [N = 38/30]‡,# | |||||
| Revision | 3.5% (0.0–8.0%) | - | 0.0% (0.0–1.0%) | - | 0.0118 |
| Time To Revision (months) | 6 (3–12) | - | 4 (1–5) | - | 0.0185 |
| CS Lead Upgrade [N = 33/28]‡,# | |||||
| Upgrade | 1.0% (0.0–5.0%) | - | 0.0% (0.0–0.3%) | - | 0.0268 |
| Time to Upgrade (months) | 6 (4–12) | - | 6 (3–9) | - | 0.51 |
| EF Improvement [N = 42/28]‡,# | |||||
| EF Improvement: Binary (%) | 70% (50–80%) | - | 65% (30–80%) | - | 0.26 |
| EF Improvement: Mean (%) | 10% (10–15%) | - | 10% (7–17%) | - | 0.84 |
| Follow-Up (months) | 12 (8–20) | - | 8 (6–12) | - | 0.06 |
Values are median (interquartile range) or n (%) unless otherwise specified. CRT: cardiac resynchronization, CS: coronary sinus, CSP: conduction system pacing, LBBAP: left bundle branch area pacing
*Difference between all His and LBBAP cases assessed with the χ2 and 2-sample t test for categorical and continuous variables, respectively
†Success defined as selective or non-selective His or left bundle branch capture with threshold less than 2.5 V @ 1.0 ms or 1.5 V @ 0.5 ms, respectively
‡For CSP cases with intraprocedural success
§CSP capture threshold defined as loss of His or left bundle branch capture at @1.0 ms or 0.5 ms, respectively, not loss of myocardial capture
¶Procedure duration for CRT devices
#Last available follow-up
Discussion
In the first-ever worldwide survey of CSP, we found that CSP lead implantation has been attempted on at least 5 continents. However, overall proportion of device cases for which CSP lead implantation is attempted remains low at most CSP-experienced institutions. In fact, only 11 out of 78 institutions providing data in 2020 attempted CSP lead implantation in more than 50% of device cases. These findings highlight both the extent of experimentation with CSP lead implantation across the electrophysiology community and that a limited number of institutions and proceduralists are implanting CSP leads as a default strategy for bradycardia pacing and CRT indications.
In a special report by Barakat et al. [11], trends in implantation of the SelectSecure 3830 lead suggested that approximately 15,096 CSP leads had been implanted in the USA from 2017 to 2018. Over this time period, worldwide CSP survey respondents from the USA, who contributed data on CSP lead implantation volume, reported implantation of 1184 CSP leads, representing 7.8% of the estimated US CSP volume. Importantly, a larger volume of CSP leads were reported to have been implanted by survey respondents not located in the USA (2472 CSP leads from 2017 to 2018) and 34% of survey respondents did not report CSP lead implantation volume. As such, the worldwide CSP survey appears to have collected data on a non-trivial proportion of all implanted CSP leads to date.
A key finding of the worldwide CSP survey is that although many institutions have attempted CSP, few implant CSP leads as a default strategy, regardless of indication. Similarly, across institutions, most proceduralists at surveyed institutions are not implanting CSP leads as a default strategy. At CSP inexperienced institutions, the primary reasons for not attempting CSP were increased procedural difficulty and lack of access to in-person proctoring for CSP, which may generalize to CSP experienced institutions who do not implant CSP leads as a default strategy. If indications for CSP lead implantations continue to expand, these findings highlight the need identify strategies to promote CSP lead implantation at institutions with and without CSP lead implantation experience.
Surveyed institutions more frequently reported that HBP lead implantation was their default CSP approach, as compared to LBBAP lead implantation. Paradoxically, institutions reported superior short- and long-term outcomes for LBBAP leads, as compared to HBP leads, with lower and more durable pacing thresholds, higher ventricular sensing, less CSP lead revisions, and fewer coronary sinus lead upgrades. These findings are consistent with what has been reported in the literature [16]. We did not inquire as to why this discrepancy is present. However, it may be due to the literature on LBBAP outcomes being relatively less mature as compared to HBP. As the evidence base for LBBAP and HBP develops, particularly clinical outcomes associated with each approach, it will be interesting to see if HBP lead implantation remains the default CSP approach world-wide.
For HBP lead implantation, the largest variability in implantation approach was seen with whether (1) an electrophysiology recording system was used to visualize intracardiac electrograms (i.e., other than the pacing system analyzer); and (2) a backup right ventricular pacing lead was implanted for pacemaker dependent patients. We did not inquire about variability in LBBAP implantation. Importantly, CSP leads and implantation tools were only available commercially from Medtronic for the majority of the surveyed time period [17]. However, other vendors have since entered the CSP lead implantation space and variability in implantation approaches would be expected to increase with unknown effects on adoption and outcomes [18, 19].
Although the worldwide CSP survey appears to have captured a relatively large proportion of the overall volume of devices implanted with CSP leads, it is plausible that results may not be applicable to CSP-experienced institutions that did not participate in the survey. For example, included institutions were predominantly academic with a relatively large number of device implanters, many with the ability to provide granular data on procedure and patient outcomes. As such, results may not generalize to dissimilar institutions. A complete list of institutions that implant devices world-wide was not available for survey distribution, making determination of the exact survey response rate impossible. Additionally, reported data was taken at face value without audit. Importantly, only a small number of CSP-inexperienced institutions participated in the survey and insight is limited into why institutions have not attempted CSP lead implantation.
Conclusions
In conclusion, CSP lead implantation has been broadly attempted but has yet to become the default approach at most surveyed institutions. If indications for CSP continue to expand, strategies to promote CSP lead implantation at institutions with and without CSP experience will be needed.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The Stanford REDCap platform (http://redcap.stanford.edu) is developed and operated by Stanford Medicine Research IT team. The REDCap platform services at Stanford are subsidized by (a) Stanford School of Medicine Research Office and (b) the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 TR001085. A complete list of institutions and survey respondents contributing data to the worldwide survey on conduction system pacing is available in the supplemental material.
Data Availability
Summary-level study data is available upon reasonable request.
Declarations
Ethical approval
The research protocol was reviewed by the institutional review board at Stanford University and granted an exemption from approval as it was determined to not involve human subjects.
Conflict of interest
Alexander C. Perino: Research support from the American Heart Association, Pfizer Inc and Bristol Myers Squibb. Consultant for Medtronic, Abbott, Biotronik, Pfizer Inc and Bristol Myers Squibb.
Paul J. Wang: None.
Michael Lloyd: Medtronic: Consulting and research; Boston Scientific: Consulting and research; Abbott: Consulting and research; Biosense Webster: Consulting.
Francesco Zanon: Speaker Fee (modest) from Biotronik, Boston Scientific, Abbott, Medtronic.
Katsuhito Fujiu: Consultant and speaker frees from Medtronic, Abbott, Boston Scientific and Biotronik.
Faizel Osman: Research grants: Medtronic, Abbott Ltd.
Sem Briongos-Figuero: Consultant fees from Medtronic and Biotronik.
Toshiaki Sato: Endowed division by the Biotronik Japan.
Tolga Aksu: None.
Marek Jastrzebski: Consultant for Medtronic.
Skevos Sideris: None.
Praveen Rao: None.
Krzysztof Boczar: None.
Xu Yuan-ning: None.
Michael Wu: None.
Narayanan Namboodiri: None.
Rodrigue Garcia: Consultant for Abbott, Boston Scientific, Zoll; Research grant from Medtronic, Abbott.
Vikas Kataria: None.
Jan De Pooter: Speakerfee and travelgrants of Biotronik, Boston and Medtronic.
Oliver Przibille: Consultant: Medtronic, Abbott, Biotronik.
Anil K. Gehi: Research funding: Bristol Myers Squib Foundation; Honoraria / consultation: Abbott Medical, Zoll Medical.
Oscar Cano: Consultant fees from Medtronic, Boston Scientific and Biotronik.
Grigorios Katsouras: Consultant fees for Medtronic, Abbott.
Binni Cai: None.
Klaus Astheimer: None.
Tanyanan Tanawuttiwat: None.
Tomas Datino: Consultant fees from Medtronic.
Jacques Rizkallah: Educator: Medtronic.
Mohammad Alasti: None.
Gregory Feld: None.
Maria Teresa Barrio-Lopez: None.
Mark Gilmore MB BCh: None.
Sergio Conti: Consultant fees: Abbott Medical. Speaker honoraria: Biosense Webster.
Satoshi Yanagisawa: Endowed department sponsored by Medtronic Japan.
Julia H. Indik: None.
Jiangang Zou: None.
Sandeep A. Saha: Speakers Bureau, Medtronic Inc.
Daniel Rodriguez-Munoz: Consultant: Medtronic, Boston Scientific.
Kuan-Cheng Chang: None.
Dmitry S. Lebedev: Honoraria: Medtronic, Abbott, Biotronik, Boston Scientific.
Miguel A. Leal: None.
Andreas Haeberlin: Travel/educational grants from Medtronic and Philips/Spectranetics. Co-founder and head of Act-Inno, a cardiovascular device testing company. Consultant/advisor for DiNAQOR and Biotronik.
Alexander R. J. Dal Forno: Consultant/honoraria Medtronic, Abbott, Biotronik.
Michael Orlov: Consultant: Abbott, grants support BSCi.
Manuel Frutos: None.
Pilar Cabanas-Grandio: None.
Jonathan Lyne: Consultant Fees from Medtronic, Abbott.
Francisco Leyva: Consultant and has received research funding from Medtronic, Boston Scientific, Abbott and Microport.
Jose Maria Tolosana: Consultant: Medtronic, Boston, Abbott.
Pierre Ollitrault: Consultant: Medtronic, Abbott, Boston Scientific, Abbott.
Pasquale Vergara: None.
Cristina Balla: None.
Subodh R. Devabhaktuni: None.
Giovanni Forleo: None.
Konstantinos P. Letsas: None.
Atul Verma: Grant from Medtronic.
Jeffrey P. Moak: None.
Abhijeet B. Shelke: None.
Karol Curila: Consultant fees from Medtronic, Boston Scientific and Biotronik.
Edmond M. Cronin: None.
Piotr Futyma: Patent applications related to bipolar and high-voltage ablation. Equity in CorSystem.
Elaine Y. Wan: NIH R01 HL15223, Consulting fees from Medtronic, Abbott and Boston Scientific.
Pietro Enea Lazzerini: None.
Felipe Bisbal MD, PhD: None.
Michela Casella MD, PhD: Speaker honoraria from Abbott and Biosense Webster.
Gioia Turitto MD: None.
Lawrence Rosenthal PhD, MD: None.
T. Jared Bunch MD: Research grants: Altathera, Boehringer Ingelheim, Boston Scientific.
Artur Baszko MD, PhD: Research grants Boehringer Ingelheim and Abbott. Consulting Fees Johnson & Johnson. Speaker honoraria Biotronik, Boston Scientific, Abbott, Bayer, Johnson & Johnson.
Nicolas Clementy: None.
Yong-Mei Cha: None.
Huang-Chung Chen: None.
Vincent Galand: Consultant for Abbott, Medtronic.
Robert Schaller: Consultant: Medtronic.
Julian W.E. Jarman: None.
Masahide Harada: None.
Yong Wei: None.
Kengo Kusano: Speaker honoraria from DAIICHI SANKYO COMPANY, Ltd., Japan, Bristol-Myers Squibb, Biotronik Japan, and Medtronic Japan, and research grants from Medtronic Japan.
Constanze Schmidt: No disclosure.
Marco Antonio Arguello Hurtado: None.
Niyada Naksuk: None.
Tadashi Hoshiyama: None.
Krishna Kancharla: Consultant for Varian medical system, Boston Scientific.
Yoji Iida: None.
Mashiro Mizobuchi: None.
Daniel P. Morin: Consultant: Abbott. Speaker: Boston Scientific, Zoll.
Serkan Cay: Travel grants and speaker’s honoraria from Medtronic, Biosense Webster and Abbott, and proctor of Medtronic.
Gabriele Paglino: None.
Tillman Dahme: Consultant fees from Medtronic, Boehringer Ingelheim; speakers honoraria from Daichii-Sankyo, Bayer, Boehringer Ingelheim.
Sharad Agarwal: None.
Pugazhendhi Vijayaraman: Honoraria, research, fellowship support, Medtronic; consultant, Abbott, Biotronik, Boston Scientific, patent for HBP delivery tool.
Parikshit S. Sharma: Honoraria, Medtronic; consultant, Medtronic, Abbott, Biotronik, Boston Scientific.
Informed consent
Not applicable.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deshmukh P, Casavant DA, Romanyshyn M, Anderson K. Permanent, Direct His-Bundle Pacing. Circulation. 2000;101(8):869–877. doi: 10.1161/01.cir.101.8.869. [DOI] [PubMed] [Google Scholar]
- 2.Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, Ellenbogen KA. A Novel Pacing Strategy With Low and Stable Output: Pacing the Left Bundle Branch Immediately Beyond the Conduction Block. Can J Cardiol. 2017;33(12):1736.e1–1736.e3. doi: 10.1016/j.cjca.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Abdelrahman M, Subzposh FA, Beer D, Durr B, Naperkowski A, Sun H, … Vijayaraman P. Clinical Outcomes of His Bundle Pacing Compared to Right Ventricular Pacing. J Am Coll Cardiol, 2018;71(20):2319–2330. 10.1016/j.jacc.2018.02.048. [DOI] [PubMed]
- 4.Sharma PS, Dandamudi G, Herweg B, Wilson D, Singh R, Naperkowski A, … Vijayaraman P. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: A multicenter experience. Heart Rhythm, 2018;15(3):413–420. 10.1016/j.hrthm.2017.10.014. [DOI] [PubMed]
- 5.Sharma PS, Dandamudi G, Naperkowski A, Oren JW, Storm RH, Ellenbogen KA, Vijayaraman P. Permanent His-bundle pacing is feasible, safe, and superior to right ventricular pacing in routine clinical practice. Heart Rhythm. 2015;12(2):305–312. doi: 10.1016/j.hrthm.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Arnold AD, Shun-Shin MJ, Keene D, Howard JP, Sohaib SMA, Wright IJ, … Whinnett ZI. His Resynchronization Versus Biventricular Pacing in Patients With Heart Failure and Left Bundle Branch Block. J Am College Cardiol, 2018;72(24):3112–3122. 10.1016/j.jacc.2018.09.073. [DOI] [PMC free article] [PubMed]
- 7.Upadhyay GA, Vijayaraman P, Nayak HM, Verma N, Dandamudi G, Sharma PS, … Investigators H-S. His Corrective Pacing or Biventricular Pacing for Cardiac Resynchronization in Heart Failure. J Am Coll Cardiol, 2019;74(1):157–159. 10.1016/j.jacc.2019.04.026. [DOI] [PubMed]
- 8.Salden FCWM, Luermans JGLM, Westra SW, Weijs B, Engels EB, Heckman LIB, … Vernooy K. Short-Term Hemodynamic and Electrophysiological Effects of Cardiac Resynchronization by Left Ventricular Septal Pacing. J Am Coll Cardiol, 2020;75(4):347–359. 10.1016/j.jacc.2019.11.040. [DOI] [PubMed]
- 9.Huang W, Wu S, Vijayaraman P, Su L, Chen X, Cai B, … Tung R. Cardiac Resynchronization Therapy in Patients With Nonischemic Cardiomyopathy Using Left Bundle Branch Pacing. JACC: Clin Electrophysiol, 2020;6(7):849–858. 10.1016/j.jacep.2020.04.011. [DOI] [PubMed]
- 10.Vijayaraman P, Ponnusamy S, Cano Ó, Sharma PS, Naperkowski A, Subsposh FA, … Jastrzebski M. Left Bundle Branch Area Pacing for Cardiac Resynchronization Therapy Results From the International LBBAP Collaborative Study Group. JACC: Clin Electrophysiol, 2020. 10.1016/j.jacep.2020.08.015. [DOI] [PubMed]
- 11.Barakat AF, Inashvili A, Alkukhun L, Shalaby AA, Wang NC, Bhonsale A, … Kancharla K. Use Trends and Adverse Reports of SelectSecure 3830 Lead Implantations in the United States: Implications for His Bundle Pacing. Circ: Arrhythm Electrophysiol, 2020;13(7). 10.1161/circep.120.008577. [DOI] [PubMed]
- 12.Beer D, Dandamudi G, Mandrola JM, Friedman PA, Vijayaraman P. His-bundle pacing: impact of social media. EP Europace. 2019;21(10):1445–1450. doi: 10.1093/europace/euz169. [DOI] [PubMed] [Google Scholar]
- 13.Cappato R, Calkins H, Chen S-A, Davies W, Iesaka Y, Kalman J, … Skanes A. Worldwide Survey on the Methods, Efficacy, and Safety of Catheter Ablation for Human Atrial Fibrillation. Circulation, 2005;111(9):1100–1105. 10.1161/01.cir.0000157153.30978.67. [DOI] [PubMed]
- 14.Cappato R, Calkins H, Chen S-A, Davies W, Iesaka Y, Kalman J, … Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol, 2009;3(1):32–8. 10.1161/circep.109.859116. [DOI] [PubMed]
- 15.List of Programs by Specialty. Accreditation Council for Graduate Medical Education (ACGME), n.d.. Retrieved November 1, 2020, from https://apps.acgme-i.org/ads/Public/Reports/Report/1.
- 16.Padala SK, Ellenbogen KA. Left bundle branch pacing is the best approach to physiological pacing. Heart Rhythm O2. 2020;1(1):59–67. doi: 10.1016/j.hroo.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medtronic SelectSecure Lead Model 3830. U.S. Department of Health & Human Services. U.S. Food & Drug Administration. Premarket Approval (PMA), n.d.. Retrieved July 20, 2021, from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?ID=422107.
- 18.Abbott Agilis HisPro Steerable Catheter With Electrodes. U.S. Department of Health & Human Services. U.S. Food & Drug Administration. 510(k) Premarket Notification, n.d.. Retrieved July 20, 2021, from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K200721.
- 19.Ravi V, Baba ME, Sharma PS. His bundle pacing: Tips and tricks. Pacing Clin Electrophysiol. 2021;44(1):26–34. doi: 10.1111/pace.14108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Summary-level study data is available upon reasonable request.