Abstract
Purpose:
The pH sensitivity of chemical exchange-relayed nuclear Overhauser enhancement (rNOE) signal in a saturation transfer experiment is not fully understood and needs further investigation.
Methods:
A three-pool-exchange model was simulated assuming that the magnetization transfer between an NOE pool and water is relayed by a chemical exchange (CE) pool. The saturation transfer signals from bovine serum albumin (BSA) and egg white albumin (EWA) phantoms were measured with different pH or different D2O/H2O mixture solutions.
Results:
Simulation results showed that the rNOE signal is independent of the Larmor frequency of the CE pool, indicating any CE pool can effectively relay NOE magnetization. The rNOE signal is sensitive to a change of the CE pool size and/or exchange rate only if the CE becomes a rate-limiting step in the relay process. The rNOE signal from BSA phantoms showed larger pH-dependence at −3.0 ppm than those at −1.9 and −4.0 ppm. However, rNOE signals from aliphatic protons have much weaker pH-dependence than the CEST signal, suggesting that CE is unlikely the rate-limiting step and the rNOE signals in BSA are mainly relayed by fast exchanging protons. The existence of aromatic NOE was confirmed by proton spectroscopy.
Conclusion:
The pH-sensitivity of the rNOE signal is determined by whether the CE process is a rate-limiting step in the relay. The rNOE signal has much weaker pH-sensitivity than the CEST signal in BSA proteins, which can explain the weak pH sensitivity of rNOE in vivo.
Keywords: CEST, chemical exchange, relayed, aliphatic NOE, aromatic NOE, pH
Introduction
Chemical exchange saturation transfer (CEST) MRI has emerged as a novel molecular imaging technique because it can probe many important biomolecules such as glucose1–4, glycogen5, creatine6–9, and mobile proteins10,11, and has shown great potential in the study of many diseases, including ischemic stroke, tumors, and cartilage degeneration10–17. In CEST, labile protons from biomolecules of interest are saturated by selective off-resonance irradiation and the saturated magnetization is transferred to the bulk water via chemical exchange resulting in a reduction of longitudinal water magnetization. Such indirect measurement of biomolecules through their interaction with water can often provide a sensitivity enhancement of 1–3 orders of magnitude compared to detecting the biomolecules directly by spectroscopic methods.
Besides CEST signals from labile protons, recent high-field studies have shown that significant Nuclear Overhauser enhancement (NOE) features can also be detected by such a saturation transfer process in glycosaminoglycans18, glycogen19, mobile proteins20,21, as well as in vivo data20,22. While endogenous CEST signal is usually observed in the downfield frequency range of 0.5 to 4 ppm, NOE signal has been reported in the upfield frequency range for aliphatic protons18,23–25, and has been suggested in the downfield frequency range for aromatic protons26,27. The magnitude of the upfield NOE can be much higher than that of the CEST signal and may be useful in certain disease applications28–30. The downfield aromatic NOE signal, on the other hand, may affect the quantification of some endogenous CEST signals since they overlap in their resonance frequencies.
NOE is a process of dipolar cross-relaxation between two nuclei at a close distance. There are two possible pathways for NOE, namely, direct intermolecular and exchange-relayed dipolar coupling. In exchange-relayed NOE (rNOE), the magnetization is first transferred to an exchangeable proton (such as amide, amine, or hydroxyl) on the macromolecule, which is then passed to water via chemical exchange (CE) (see Fig. 1A). Many previous studies have suggested that relayed NOE should be the dominant pathway for mobile proteins31,32, and thus, it is generally assumed that relayed NOE applies to in vivo brain CEST studies. A very recent study of glycogen has also suggested that the NOE signal may also be relayed in some carbohydrate macromolecules19,33.
Fig. 1. Diagram of the relayed NOE process.

(A) In the 3-pool relayed NOE model, the transfer of magnetization between water and an NOE pool with a pool size of fNOE and a transfer rate of kNOE (=kcb) is relayed by a CE pool with a pool size of fCE and transfer rate of kCE (=kba). (B) Two scenarios are simulated, where the NOE is relayed by amide protons with a slow chemical exchange rate or hydroxyl protons with a fast exchange rate.
The different sources of rNOE versus CEST suggests that they should have some difference in signal properties. However, compared to CEST, signal properties of rNOE have been investigated less and are not yet fully understood. Especially, their relationship with chemical exchange, such as pH sensitivity, is still controversial20,22. Although a relayed pathway suggests that rNOE signal from mobile proteins should be dependent on the CE process and pH, it is unclear whether the rNOE signal is mostly relayed by the backbone amide or the labile protons from side-chain residues, and whether the pH-sensitivity of rNOE signal would be comparable to those of CEST signals, such as the amide proton transfer effect (APT) in in vivo studies34, or is much weaker20,35–37.
In this work, we performed simulation studies to examine the signal properties of rNOE, including its dependence on saturation parameters, pool size, and exchange or magnetization transfer rate of the CE and the NOE pools. Phantom studies were performed in bovine serum albumin (BSA) and egg white albumin (EWA) at 9.4 T and 15.2 T to gain insights into the rNOE signals of upfield aliphatic and downfield aromatic protons. Part of our results has been reported in previous ISMRM meetings26,38.
Methods
To evaluate the signal properties of rNOE, we considered a three-pool exchange model, i.e., the bulk water (pool a), macromolecular labile proton (pool b, defined as CE), and macromolecular aliphatic/aromatic proton (pool c, defined as NOE) pools. We assumed that NOE is relayed by chemical exchange in this study, i.e., magnetization transfer only occurs between NOE and CE pools by spin diffusion due to dipole-dipole coupling and between CE and water pools by chemical exchange (Fig. 1A), and that direct dipole-dipole interaction between NOE and water pools is negligible.
Simulations:
The exchange-relayed NOE signals were simulated with 3-pool Bloch-McConnell Equations described in the Appendix. To evaluate the effect of saturation, relaxation, and exchange parameters on the rNOE signal, typically one specific parameter was varied with all other parameters kept at their default values. The default parameters were: B0 = 400 MHz (9.4 T), with the Larmor frequencies of the CE and NOE pools δCE = 3.5 and δNOE = −3.5 ppm10,20, respectively, and the rate of NOE (kNOE) = 5 s−1 30,39. In order to yield an rNOE signal on the order of 10% of S028,29, a fractional NOE pool size of fNOE = 0.03 was assumed. The fractional CE pool size was assumed to be an order of magnitude smaller, i.e., fCE = 0.003. For simplicity, the magnetization transfer effect of semisolid macromolecules is ignored, and the longitudinal and transverse relaxation rates R1 and R2 of protons for all three pools were assumed to be 0.5 s−1 and 1 s−1, respectively. Typically, the Z-spectrum was simulated from 6 to −6 ppm for a saturation power B1 = 1 μT and saturation pulse duration tsat = 10 s, with one parameter varied for a few selected values. To quantitatively determine the magnitude of the rNOE or CEST effect, a baseline Z-spectrum with both fCE = 0 and fNOE = 0 was calculated. The difference between Z-spectra with both fCE and fNOE of non-zero and zero values was obtained.
Endogenous labile protons which are usually detected in CEST experiments have a wide range of exchange rates10,40, including amide (<100 s−1)10,40, guanidyl (~1000 s−1)40, hydroxyl (1000 to 5000 s−1)3,40, and amine protons (>4000 s−1)3,40. To evaluate the dependence of the rNOE signal on the CE rate, two scenarios were used in the simulation of the rNOE signal, as described in Fig. 1B: a slow chemical exchange case represented by amide protons kba or kCE = 30 s−1 and a fast exchange case represented by hydroxyl or amine protons kCE = 1000 s−1. To determine the dependence of rNOE signal on fNOE·kNOE and fCE·kCE (Fig. 5B below), fNOE of 0.002 to 0.05, kNOE of 0.1 s−1 to 30 s−1, fCE of 0.0002 to 0.005, and kCE of 10 s−1 to 3000 s−1 were varied. For simplicity, the exchange rate kNOE was limited to be ≤30 s−1 so that saturation efficiency with a 1 μT pulse would always be close to 1 and not decrease significantly with an increase in kNOE.
Fig. 5. The dependence of rNOE signal on CE pool size and exchange rate in logarithms scale.
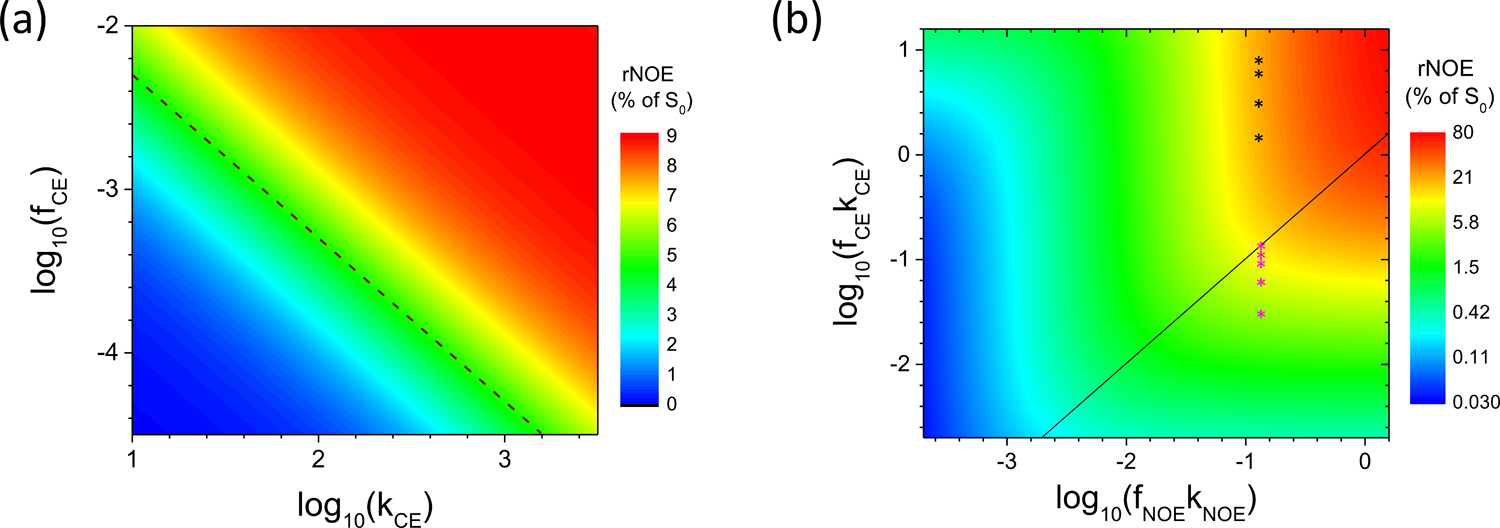
(A) The rNOE signals as a function of CE rate kCE on Y-axis and CE pool size fCE on X-axis in the log10 scale were shown for a representative fNOE of 0.01 and kNOE of 5 s−1. The dashed line indicates where fCE·kCE = fNOE·kNOE = 0.05. When fCE·kCE >> 0.05, the rNOE signal is in the red region which is insensitive to a change in either fCE or kCE. (B) The rNOE signal as functions of fNOE·kNOE and fCE·kCE in log10 scale. The line indicates where fNOE·kNOE= fCE·kCE. In regions close to or below this line, the value of fNOE·kNOE is close to or larger than fCE·kCE, while the region far above this line has fNOE·kNOE << fCE·kCE. The magenta and black stars indicate the data points simulated in Fig. 4B and 4D, respectively. The color bar indicates the magnitude of the rNOE signal in percent of S0 units.
Phantom Experiments:
All experiments were performed on a 9.4 T or a 15.2 T Bruker MRI system with a 38-mm volume coil (Rapid Biomedical or Bruker) for transmission and reception. Magnetic field homogeneity was optimized by localized shimming on a volume of interest. The dependence of the rNOE signal on pH and rNOE pool size was investigated in protein phantoms. Four sets of experiments were performed at room temperature as follows:
Saturation power-dependence of CEST vs. rNOE in BSA: 15% BSA was dissolved in phosphate-buffered saline (PBS) and titrated to pH = 5.8, 6.4, 7.0, and 7.6. Z-spectra were measured in a frequency offset range from +7 to −7 ppm with saturation power of 0.75 and 0.12 μT, and a saturation duration of 6 s.
pH dependency of CEST vs. rNOE in BSA at 15.2 T: 20% BSA was dissolved in PBS and titrated to 8 different pH values from 5.65 to 8.5. Z-spectra were acquired at a frequency offset range from +7 to −7 ppm with a 6-s and 0.5 μT saturation pulse.
CEST vs. rNOE of egg white albumin (EWA) and BSA in varied H2O/D2O mixture solution: 20% of EWA or 15% of BSA was dissolved in a mixture of distilled water (H2O) with percentages of D2O at 0, 40, 80 and 90%. The addition of D2O reduces the proton concentration in both bulk water and the exchangeable protons in the proteins, but not the concentration of non-exchangeable aliphatic and aromatic protons. Thus, the NOE pool size increases without changing the CE pool size. In both sets of phantoms, Z-spectra were measured in a frequency offset range from +8 to −8 ppm with a saturation power of 0.25 μT and a saturation duration of 14 s. Because T1 is strongly affected by the addition of D2O, the T1 of these phantoms was measured with an inversion-recovery pulse sequence.
Comparison of CEST versus MRS: 15% BSA was dissolved in 99.5% D2O to enhance the rNOE signal. Z-spectra were measured with 7-s saturation with B1 = 0.25 μT, and water-suppressed localized 1H MR spectrum was measured by a short-TE localized stimulated-echo acquisition mode (STEAM) sequence41. Parameters were TE/TR = 6/6000 ms, NEX = 100, spectral width = 8000 Hz, number of complex data points = 2048, and the voxel size = 1×1×1 mm3.
All experiments except experiment #2 were performed at 9.4 T. CEST images were measured by a single-slice spin-echo echo-planar imaging sequence with continuous-wave RF irradiation. Crushing gradients were applied immediately following the saturation pulse to suppress residual transverse magnetization. Control images were acquired at an offset of 300 ppm for signal normalization. Imaging parameters were a matrix size of 64 × 64, a field of view of 4 cm × 4 cm, and slice thickness of 5 mm. The post-acquisition recovery time, i.e. the time between the acquisition of one saturated image and the saturation pulse of the next image, was 10 s.
Data analysis
For quantitative data analysis, Z-spectra were obtained from regions of interest selected from each phantom with a small B0 inhomogeneity (with a B0 shift threshold < 5 Hz). In each phantom, a Z-spectrum with direct water saturation effect (DWS) only was fitted by excluding the data in the offset range of 0.4 to 6 ppm and −0.4 to −6 ppm. Then, the fitted Z-spectrum with DWS only was subtracted from the acquired Z-spectrum to obtain a differential Z-spectrum which removed DWS and contained only the CEST and rNOE signals.
Results:
Simulation of CEST vs. rNOE at various conditions
Since CEST and rNOE signals are both indirectly detected through water, they share many similarities. Indeed, they are both sensitive to water T1 (Fig. 2A) and are dependent on saturation duration (Fig. 2B). As expected from the 3-pool relayed-exchange model, the rNOE signal is independent of the chemical shift of the CE pool (Fig. 2C): the rNOE signal is still the same even when δCE is reduced to 0.1 ppm where the CEST signal is unrecognizable due to severe DWS. Concerning the parameters of the NOE pool, the rNOE signal increases with pool size (Fig. 3A) and NOE rate (Fig. 3B). An increase of the R2 value of the NOE proton broadens the linewidth of the rNOE signal but has little effect on its magnitude (Fig. 3C). In both Figs. 2 and 3, only one CE rate of 1000 s−1 was assumed.
Fig. 2. Simulation of the effects of 3 parameters on the rNOE signal.

The rNOE signals increase with (A) the T1 of water and (B) the saturation duration in the same way as the CEST signals. (C) The chemical shift of the CE protons does not affect the rNOE signal.
Fig. 3. Simulation of the effects of 3 parameters of the NOE pool on the rNOE signal.

The rNOE signal increases with pool size (A) and NOE rate (B). (C) An increase of the R2 of the NOE proton causes a minimal change in the magnitude of the rNOE signal but increases the linewidth.
Fig. 4 evaluates the dependence of the rNOE signal on the CE parameters assuming kNOE = 5 s−1 and fNOE = 0.03. When the relay is through a slow CE pool, the rNOE signal increases with CE pool size fCE (Fig. 4A) and kCE (Fig. 4B) similarly with the CEST signal. When the relay is through a fast CE pool, the CEST signal still increases with pool size while decreases with an increase of kCE because the saturation efficiency reduces quickly for faster exchange rates42. Nonetheless, the change in rNOE signal is very small in both cases (Fig. 4C, 4D).
Fig. 4. The dependence of rNOE on the CE pool size and exchange rate.
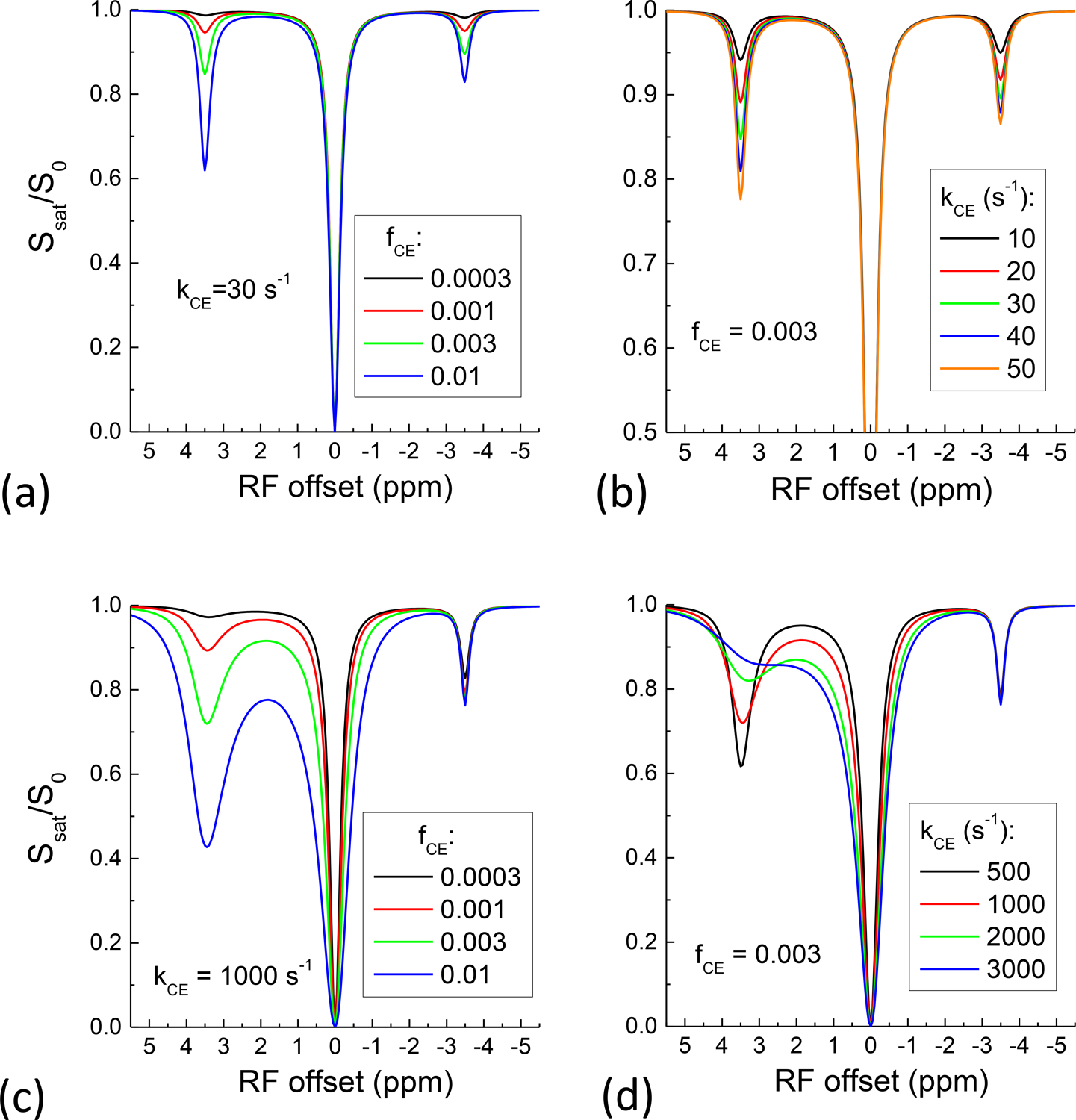
When the relay is through a slow-exchanging proton, both rNOE and CEST signal increase with CE pool size (A) and CE rate (B). When the relay is through a fast-exchanging proton, the rNOE signal is less sensitive to the CE pool size (C) and CE rate (D) than the CEST signal.
The results of Fig. 4 suggest that the rNOE signal is dependent on both fCE and kCE. To obtain more insight, the rNOE signal at −3.5 ppm was simulated as a function of fCE (0.0002 to 0.005) and kCE (10 to 103.5 s−1) with fixed fNOE = 0.01 and kNOE = 5 s−1 in Fig. 5A. The axes were shown in logarithmic scale so that a constant fCE·kCE appears in a straight line. A dashed line was plotted to show the condition that fCE·kCE = 0.05, which equals fNOE·kNOE. The same rNOE signals are observed for constant fCE·kCE values (parallel to the dashed line), indicating that the effect of a certain change in fCE is the same as an equal amount of change in kCE. When fCE·kCE is smaller than or comparable to fNOE·kNOE (regions with blue to green color), rNOE value is sensitive to a change in either kCE or fCE, or more generally, a change in the product fCE·kCE. When fCE·kCE >> fNOE·kNOE (the upper right region with red color), the rNOE value reaches a plateau and is insensitive to a change of kCE or fCE. A more general case was shown in Fig. 5B where fCE·kCE and fNOE·kNOE are both varied, and the black identity line indicates that fCE·kCE = fNOE·kNOE. The data points simulated in Fig. 4B and 4D were marked as 5 magenta and 4 black stars, respectively. The data points indicated by the magenta stars in the region below the black identity line (fCE·kCE < fNOE·kNOE) shows large variation in rNOE signals, because the CE process becomes a rate-limiting step. In contrast, the black stars in the region away from the identity line (fCE·kCE >> fNOE·kNOE) have the same rNOE signals and are insensitive to a change in the pH-sensitive kCE. Thus, further rNOE simulations were performed for the slow CE condition.
Since rNOE relies on the CE process, it is important to understand the relative magnitude of rNOE versus CEST signals as a function of NOE pool size and chemical exchange rate. For simplicity, we only considered a slow CE case so that the saturation efficiency is close to 1 (and not varied with CE rate). Fig. 6A shows simulated Z-spectra with fNOE of 0.003 to 0.1 for comparing rNOE and CEST signals. The rNOE signal increases with the NOE pool size, but is smaller than the CEST signal with a smaller CE pool size. Fig. 6B shows CEST and rNOE signals as a function of kCE for three fNOE values of 0.3%, 1%, and 3%. The rNOE change by kCE modulation was less than the CEST signal change, indicating that rNOE has a weaker pH dependence than CEST.
Fig. 6. Comparison of the magnitude and kCE-sensitivity of CEST and rNOE signals for a slow kCE = 30 s−1.
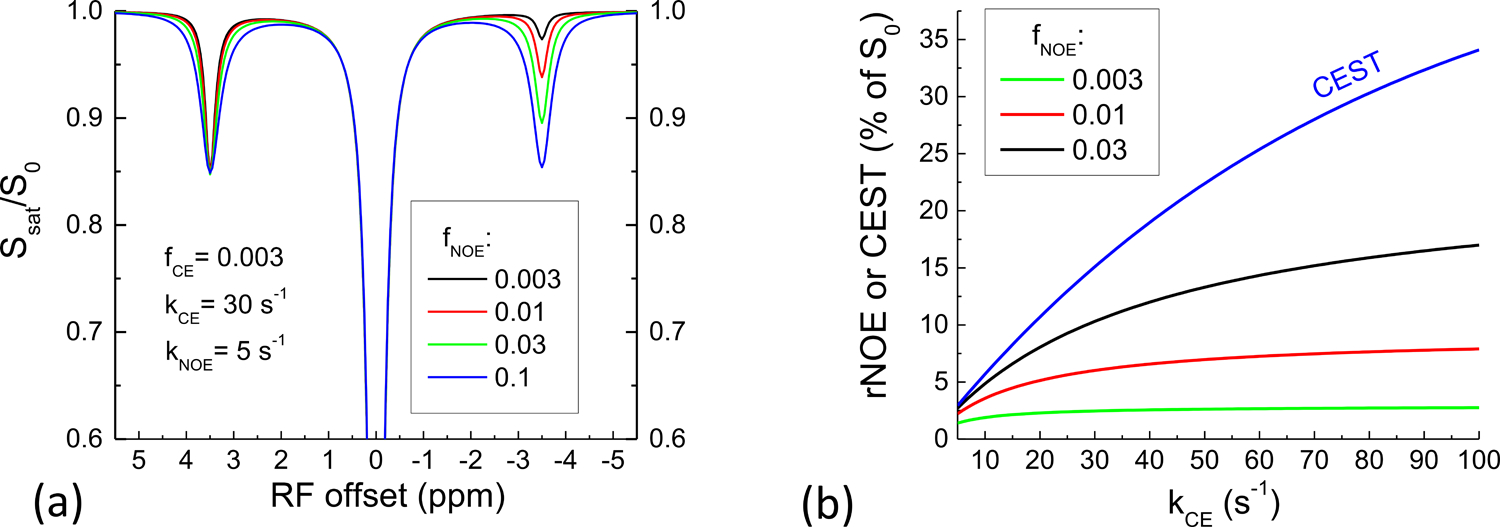
(A) the rNOE signal is smaller than the CEST signal, even when fNOE is much higher than fCE, and fNOE·kNOE is much larger than fCE·kCE. (B) Besides the difference in the magnitude, the slope of the rNOE signal versus kCE is always smaller than that of the CEST signal, suggesting a weaker pH dependence.
CEST vs. rNOE of phantoms with different pH values
The pH dependence of CEST vs. rNOE signals was investigated with phantoms with varied pH. In Z-spectra of pH-dependent BSA acquired with B1 = 0.75 μT (Fig. 7A), the downfield CEST signals at 2.8 ppm exhibited large pH-dependence due to the amine-water proton exchange from the side-chain lysine residue (kCE = ~4,000 s−1)43. In contrast, the upfield rNOE signals only slightly differed for four different pH values. The CEST signal is proportional to the saturation efficiency which increases with saturation power42,44,45. With a much smaller B1 of 0.12 μT (Fig. 7B), the CEST signals of fast-exchanging protons decreased much more significantly than that of the rNOE, because the saturation efficiency drops much faster for a faster exchange rate. To detect pH-dependence better, DWS was removed from the Z-spectra (Fig. 7C). Three aliphatic NOE peaks can be detected at about −0.8, −2, and from −3 to −4 ppm, all of which are insensitive to pH. Downfield signals contain both the pH-sensitive CEST signal and pH-insensitive NOE of aromatic protons. Since the peaks around 2.8 ppm show very weak pH-dependence, it is likely to have large contribution from aromatic NOE. When the CEST effect is minimized by a small saturation power, the differential Z-spectra of BSA (Fig. 7C) were dominated by the NOE of aromatic and aliphatic protons which have much weaker sensitivity to pH.
Fig. 7. pH-sensitive vs insensitive component of Z-spectra in 15% BSA at 4 pH values.
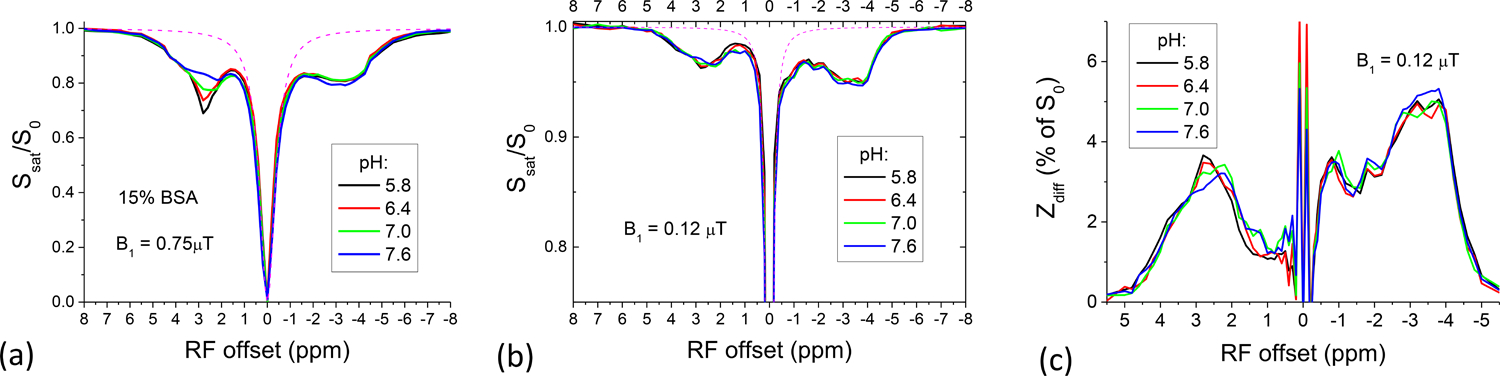
(A) The amine-water proton exchange at 2.8 ppm is dominant in the Z-spectra of 0.75 μT and is highly sensitive to pH, whereas the upfield rNOE is much less sensitive to pH. (B) At a lower power of 0.12 μT, the CEST signal is minimized due to a very low saturation efficiency, showing pH-insensitive signals at both positive and negative offsets. The magenta dashed lines indicate the fitted Z-spectra with DWS only. (C) The differential Z-spectra for 0.12 μT show very little pH-sensitivity, suggesting a large contribution from both aromatic and aliphatic rNOE.
To further investigate the pH dependency of BSA samples, we studied BSA with wider pH variation at a higher field of 15.2 T for improving spectral resolution. Clear pH-dependence can be seen for downfield and upfield frequencies in the Z-spectra and the differential Z-spectra (Fig. 8A & 8B) acquired with B1 = 0.5 μT. For quantitative comparison, CEST and rNOE signals at a few selected frequencies (Fig. 8C) were plotted as a function of pH. The CEST signal at 2.8 ppm increases with decreasing pH, as expected for fast exchanging amine protons25,46. The NOE signals show much weaker pH-dependence than the CEST signal, especially at pH <7.0. Interestingly, the NOE signal at −3 ppm shows much larger pH-dependence than that of −1.9 and −4 ppm. A linear fitted function is rNOE signal (%) = 7.1+0.19×pH for −1.9 ppm, 3.5+1.24×pH for −3ppm, and 8.9+0.43×pH for −4ppm, respectively.
Fig. 8. Comparison of the CEST and rNOE signals of BSA at 15.2 T.

(A) the Z-spectra and (B) the differential saturation transfer signal (i.e., CEST or rNOE) at 8 pH values showed varied peak intensities at different frequency offsets. (C) Although the aliphatic rNOE signals only show weak pH-dependence overall, the signal at −3 ppm has larger pH-dependence than those of −1.9 and −4 ppm.
CEST vs. rNOE of aliphatic and aromatic protons in phantoms with different H2O/D2O mixtures
To investigate the impact of the NOE pool size without changing the CE process, water proton concentration in solution was reduced by using H2O/D2O mixture. Since the CEST signal is expected to be constant, the change in saturation transfer MRI data is due to the NOE contribution. Fig. 9 shows the saturation transfer experimental results of 20% EWA and 15% BSA in varied H2O/D2O mixture. Both Z-spectra indicate that the addition of D2O enhanced the saturation transfer signal (Fig. 9A and 9B). Because the saturation transfer MR signal is proportional to T1 which increases with D2O percentage, this effect should be removed in the analysis of CEST and rNOE signal. In the T1-corrected differential Z-spectra of both proteins (Fig. 9C and 9D), the upfield rNOE signals increase with D2O percentage, which can be attributed to a relative increase of fNOE. Peak magnitude at −3.5ppm is 2.01 and 2.17 times higher in 90% D2O than that in 0% D2O for EWA and BSA, respectively, whereas the downfield peak (2 ppm for EWA and 2.2 ppm for BSA) magnitude is 1.36 and 1.48 times higher in 90% D2O than that in 0% D2O for EWA and BSA, respectively. The smaller increase of downfield signal with D2O percentage is likely due to the contribution of both D2O-insensitive CE and D2O-sensitive aromatic rNOE signals.
Fig. 9. Comparison of saturation transfer spectra of (A, C) EWA and (B, D) BSA in varied concentration of D2O.
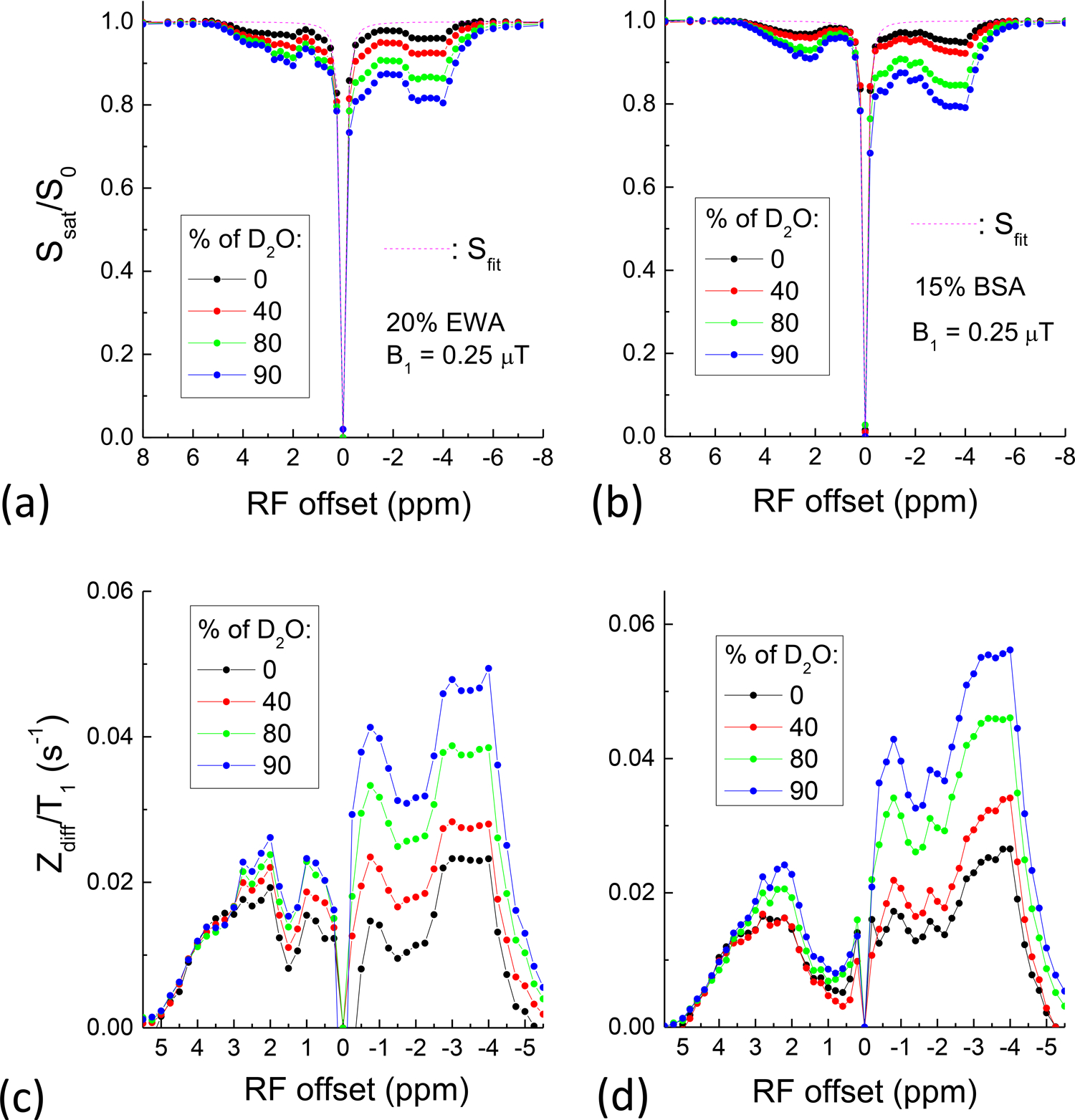
(A, B) The signal dip in the Z-spectra increases with increasing D2O percentage. (C, D) The T1-corrected differential Z-spectra signal increases in both downfield and upfield frequency ranges, with a larger increase upfield.
The existence of the aromatic NOE signal was further confirmed by saturation transfer and MRS studies of BSA in 99.5% D2O (Fig. 10). Three aliphatic NOE peaks can be seen in the differential Z-spectrum (black arrows, Fig. 10B), which were also confirmed in the proton MR spectrum (Fig. 10C). These aliphatic NOE observations match with Fig. 7C and 8B. At the downfield region, in addition to the residue 2.8 ppm peak of the amine CEST signal, two small peaks appear in the differential Z-spectrum at around 3.75 ppm and 2.2 ppm, which matches well with the frequency range of the aromatic protons in the MR spectrum, and indicates that aromatic NOE signal indeed exists.
Fig. 10. Comparison of saturation transfer and MRS spectrum for BSA in 99.5% of D2O.

With suppression of the chemical exchange effect by 99.5% D2O, the signal decay (A) in the CEST Z-spectrum is highly weighted by the rNOE signal. Differential Z spectrum (B) shows peaks with frequencies that match the aromatic and aliphatic protons on the positive (red arrows) and negative frequency (black arrows) offset side in the MRS (C) spectrum, respectively.
Discussions
Our simulation indicates that rNOE signal is closely related to the CE pool but has some unique properties which are distinct from the CEST signal. The rNOE signal is independent of the Larmor frequency of the CE pool, thus, any CE pool can effectively relay NOE magnetization. On the other hand, the rNOE signal has a complicated dependence on the CE pool size and chemical exchange rate. With our simulation parameters, when NOE is relayed by slow-exchanging amide protons, it shows a smaller magnitude and a weaker pH-dependence than the amide-CEST signal. If NOE is relayed by fast chemical exchange protons, it can be almost independent of pH.
Recently, Murase published numerical analyses of the Z-spectra in the presence of multiple exchanging pools which can be applied to exchange-relayed NOE47. Zhou et al. has reported a simplified analytical solution for relayed NOE33 and showed that the pH-sensitivity of the rNOE signal is determined by whether kCE is much faster than kNOE. This could lead to a conclusion that the rNOE signal observed in mobile protein would be independent of pH at physiological conditions, because even the slow exchanging amide (30 s−1)10 is about an order of magnitude faster than the NOE transfer rate (2–3 s−1)30,39. Our simulation showed that a more general condition for rNOE is the relationship between the product fCE·kCE and fNOE·kNOE. When fCE·kCE >> fNOE·kNOE, the capability of the relay is much higher than the magnetization transferred from the NOE pool. A change in fCE or kCE will not affect the rNOE signal as long as the condition fCE·kCE >> fNOE·kNOE is still satisfied. On the other hand, the CE process becomes a rate-limiting step for rNOE when fCE·kCE is comparable to or smaller than fNOE·kNOE. In this domain, the rNOE signal will be highly dependent on fCE and kCE.
The BSA phantom study showed that the rNOE signal has a much smaller pH-dependence than CEST signals, suggesting that the rNOE signal can be separated into two components: The majority of rNOE signals are pH-insensitive and should be relayed by fast-exchanging protons, and the pH-sensitive contribution which is relayed by the slow-exchanging amide proton is small. The high field of 15.2 T allows sufficient spectral separation within the upfield NOE signals and shows that the peak at about −3 ppm has higher pH-dependence than other frequencies. This suggests that compared to −2 ppm and −4 ppm, the rNOE signal at −3 ppm has a higher contribution from the amide-relayed process, which should come from the aliphatic protons closer to the amide groups. Note the above explanation to the pH-dependence of the rNOE signal was based on our simulation and had not been experimentally validated. Besides a change of the exchange rate of the CE pool to the pH-dependent rNOE signal, a change of the protein morphology/folding with pH may also contribute.
The results of the BSA and EWA phantoms also confirmed the previous findings of our group and a few other studies finding that aromatic rNOE signal can be detected in the downfield frequency range26,27. The overlap of aromatic rNOE with endogenous CEST signals could make the interpretation of CEST contrast in in vivo studies more difficult, thus, it is important to adjust the acquisition or post-processing method to minimize aromatic rNOE signals. For example, a saturation pulse with higher power and shorter duration will be more weighted to the faster exchanging CE pool than the rNOE pool with a slower transfer rate48. Alternatively, an aliphatic rNOE based correction may be performed assuming a certain ratio between aromatic and aliphatic NOE signals which does not vary significantly by saturation parameters, tissue types, or pathology30.
APT and rNOE signals can both be detected in the Z-spectrum with relatively small power, thus, they are often measured together and have been postulated to provide complementary information. Recent in vivo studies at high magnetic fields have reported that the magnitude of aliphatic rNOE signal is 8–15% in the white matter28,29, much larger than the value of ~3% usually observed for APT10,20,49. Our simulation shows that the rNOE signal, if relayed by the slow exchanging amide pool, should always be smaller than that of the APT CEST signal (e.g., Fig. 6B). This suggests that in addition to the amide group, a large contribution of the rNOE signal should be relayed through faster-exchanging protons such as hydroxyl and amine groups. Thus, the rNOE signal would only have weak pH-sensitivity. Indeed, the change of the aliphatic rNOE signal in ischemic tissue, where the local tissue acidosis can cause a pH drop of more than 0.5 unit50–52, was reported to be much smaller than that of APT in several animal studies20,36,37.
Conclusions
Our three-pool simulation shows that the rNOE signal is dependent on the CE pool size and exchange rate and more specifically, the relationship between fCE·kCE and fNOE·kNOE. The rNOE signal is sensitive to a change of the CE pool size and/or exchange rate only if the CE becomes a rate-limiting step in the relay, and rNOE signal should have a weaker pH-dependence than CEST. Our phantom results strongly suggest that both aliphatic and aromatic NOE signals can be detected in mobile proteins with weak pH-dependence.
Acknowledgments
This work is supported by NIH grant NS100703, and the Institute for Basic Science in Korea (IBS-R015-D1). We thank Dr. Julius Chung for acquiring 15.2 T data and proofreading the manuscript.
Appendix
The spin diffusion between the NOE and CE pools follows the same mathematical model as the chemical exchange between the CE and water pools. Hence, the magnetization of each pool can be described by the Bloch-McConnell Equations which incorporate the magnetization transfer process (i.e., CE and NOE). For a three-pool exchange model, we assume the NOE pool is relayed by the CE pool, and time-dependent magnetization changes are
| [1] |
| [2] |
| [3] |
| [4] |
| [5] |
| [6] |
| [7] |
| [8] |
| [9] |
where
R1i and R2i are the longitudinal and transverse relaxation rate of pool i, respectively; δi is the Larmor frequency of pool i; Ω is the frequency of the saturation pulse; ω1 is the Rabi frequency of the saturation pulse (=γB1 where γ is the gyromagnetic ratio and B1 is the saturation power); is the j-th component of the magnetization of pool i; and is the initial magnetization of pool i.
References:
- 1.Jin T, Autio J, Obata T, Kim SG. Spin-locking versus chemical exchange saturation transfer MRI for investigating chemical exchange process between water and labile metabolite protons. Magn Reson Med. 2011;65(5):1448–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan KWY, McMahon MT, Kato Y, et al. Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn Reson Med. 2012;68(6):1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin T, Mehrens H, Hendrich K, Kim SG. Mapping brain glucose uptake with chemical exchange-sensitive spin-lock magnetic resonance imaging. J Cereb Blood Flow Metab. 2014;34(8):1402–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker-Samuel S, Ramasawmy R, Torrealdea F, et al. In vivo imaging of glucose uptake and metabolism in tumors. Nat Med. 2013;19(8):1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Zijl PCM, Jones CK, Ren J, Malloy CR, Sherry AD. MRI detection of glycogen in vivo by using chemical exchange saturation transfer imaging (glycoCEST). Proc Natl Acad Sci U S A. 2007;104(11):4359–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haris M, Nanga RPR, Singh A, et al. Exchange rates of creatine kinase metabolites: feasibility of imaging creatine by chemical exchange saturation transfer MRI. NMR Biomed. 2012;25(11):1305–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kogan F, Haris M, Singh A, et al. Method for High-Resolution Imaging of Creatine In Vivo Using Chemical Exchange Saturation Transfer. Magn Reson Med. 2014;71(1):164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung JJ, Jin T, Lee JH, Kim SG. Chemical Exchange Saturation Transfer (CEST) Imaging of Phosphocreatine in the muscle. Magn Reson Med. 2018;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Barker PB, Weiss RG, van Zijl PCM, Xu JD. Creatine and phosphocreatine mapping of mouse skeletal muscle by a polynomial and Lorentzian line-shape fitting CEST method. Magn Reson Med. 2019;81(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou JY, Payen JF, Wilson DA, Traystman RJ, van Zijl PCM. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9(8):1085–1090. [DOI] [PubMed] [Google Scholar]
- 11.Zhou JY, van Zijl PCM. Chemical exchange saturation transfer imaging and spectroscopy. Progress in Nuclear Magnetic Resonance Spectroscopy. 2006;48(2–3):109–136. [Google Scholar]
- 12.Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson. 2000;143(1):79–87. [DOI] [PubMed] [Google Scholar]
- 13.Sun PZ, Zhou JY, Sun WY, Huang J, van Zijl PCM. Detection of the ischemic penumbra using pH-weighted MRI. J Cereb Blood Flow Metab. 2007;27(6):1129–1136. [DOI] [PubMed] [Google Scholar]
- 14.Zhou JY, Blakeley JO, Hua J, et al. Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn Reson Med. 2008;60(4):842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia GA, Abaza R, Williams JD, et al. Amide Proton Transfer MR Imaging of Prostate Cancer: A Preliminary Study. J Magn Reson Imaging. 2011;33(3):647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou JY, Tryggestad E, Wen ZB, et al. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med. 2011;17(1):130–U308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Zijl PCM, Yadav NN. Chemical Exchange Saturation Transfer (CEST): what is in a name and what isn’t? Mag Reson Med. 2011;65(4):927–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST). Proc Natl Acad Sci U S A. 2008;105(7):2266–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y, van Zijl PCM, Xu X, et al. Magnetic resonance imaging of glycogen using its magnetic coupling with water. Proc Natl Acad Sci U S A. 2020;117(6):3144–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin T, Wang P, Zong X, Kim SG. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magn Reson Med. 2013;69(3):760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones CK, Polders D, Huang W, et al. in vivo three-dimensional whole-brain pulsed steady-state chemical exchange saturation transfer at 7 T. Magn Reson Med. 2012;67(6):1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones CK, Huang A, Xu JD, et al. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7 T. Neuroimage. 2013;77:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mougin OE, Coxon RC, Pitiot A, Gowland PA. Magnetization transfer phenomenon in the human brain at 7 T. Neuroimage. 2010;49(1):272–281. [DOI] [PubMed] [Google Scholar]
- 24.Jones CK, Polders D, Huang W, et al. in vivo three-dimensional whole-brain pulsed steady-state chemical exchange saturation transfer at 7 T. Magn Reson Med. 2012(67):1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin T, Wang P, Zong X, Kim SG. Magnetic resonance imaging of the Amine-Proton EXchange (APEX) dependent contrast. Neuroimage. 2012;59(2):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin T, Kim SG. In vivo saturation transfer imaging of nuclear overhauser effect from aromatic and aliphatic protons: implication to APT quantification. Paper presented at: Proceedings of 21st Annual Meeting of ISMRM2013; Salt Lake City, Utah, USA. [Google Scholar]
- 27.Goerke S, Zaiss M, Kunz P, et al. Signature of protein unfolding in chemical exchange saturation transfer imaging. NMR Biomed. 2015;28(7):906–913. [DOI] [PubMed] [Google Scholar]
- 28.Zhang XY, Wang F, Jin T, et al. MR imaging of a novel NOE-mediated magnetization transfer with water in rat brain at 9.4T. Magn Reson Med. 2017;78(2):588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaiss M, Schuppert M, Deshmane A, et al. Chemical exchange saturation transfer MRI contrast in the human brain at 9.4 T. Neuroimage. 2018;179:144–155. [DOI] [PubMed] [Google Scholar]
- 30.Zaiss M, Windschuh J, Goerke S, et al. Downfield-NOE-suppressed amide-CEST-MRI at 7 Tesla provides a unique contrast in human glioblastoma. Magn Reson Med. 2017;77(1):196–208. [DOI] [PubMed] [Google Scholar]
- 31.Vandeven FJM, Janssen H, Graslund A, Hilbers CW. Chemically Relayed Nuclear Overhauser Effects - Connectivities between Resonances of Nonexchangeable Protons and Water. J Magn Reson. 1988;79(2):221–235. [Google Scholar]
- 32.van Zijl PCM, Zhou JY, Mori N, Payen JF, Wilson D, Mori S. Mechanism of magnetization transfer during on-resonance water saturation. A new approach to detect mobile proteins, peptides, and lipids. Magn Reson Med. 2003;49:440–449. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Y, van Zijl PCM, Xu JD, Yadav NN. Mechanism and quantitative assessment of saturation transfer for water-based detection of the aliphatic protons in carbohydrate polymers. Magn Reson Med. 2021;85(3):1643–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heo HY, Zhang Y, Burton TM, et al. Improving the detection sensitivity of pH-weighted amide proton transfer MRI in acute stroke patients using extrapolated semisolid magnetization transfer reference signals. Magn Reson Med. 2017;78(3):871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Zu ZL, Zaiss M, et al. Imaging of amide proton transfer and nuclear Overhauser enhancement in ischemic stroke with corrections for competing effects. NMR Biomed. 2015;28(2):200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y, Zhou IY, Lu DS, et al. pH-sensitive amide proton transfer effect dominates the magnetization transfer asymmetry contrast during acute ischemiaquantification of multipool contribution to in vivo CEST MRI. Magn Reson Med. 2018;79(3):1602–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu LM, Jiang L, Sun PZ. Investigating the origin of pH-sensitive magnetization transfer ratio asymmetry MRI contrast during the acute stroke: Correction of T-1 change reveals the dominant amide proton transfer MRI signal. Magn Reson Med. 2020;84(5):2702–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin T, Chung J, Kim S-G. Saturation transfer of relayed Nuclear Overhauser Enhancement (rNOE): its relationship with chemical exchange. Paper presented at: Proceesings of ISMRM 27th Annual Meeting2019; Montreal, Canada. [Google Scholar]
- 39.Xu J, Yadav NN, Bar-Shir A, et al. Variable delay multi-pulse train for fast chemical exchange saturation transfer and relayed-nuclear overhauser enhancement MRI. Magn Reson Med. 2014;71(5):1798–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liepinsh E, Otting G. Proton exchange rates from amino acid side chains - Implications for image contrast. Magn Reson Med. 1996;35(1):30–42. [DOI] [PubMed] [Google Scholar]
- 41.Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41(4):649–656. [DOI] [PubMed] [Google Scholar]
- 42.Jin T, Kim SG. Approximated analytical characterization of the steady-state chemical exchange saturation transfer (CEST) signals. Magn Reson Med. 2019;82(5):1876–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zong X, Wang P, Kim S-G, Jin T. Sensitivity and Source of Amine-Proton Exchange and Amide-Proton Transfer Magnetic Resonance Imaging in Cerebral Ischemia. Magn Reson Med. 2014;71(1):118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou JY, Wilson DA, Sun PZ, Klaus JA, van Zijl PCM. Quantitative description of proton exchange processes between water and endogenous and exogenous agents for WEX, CEST, and APT experiments. Magn Reson Med. 2004;51(5):945–952. [DOI] [PubMed] [Google Scholar]
- 45.Sun PZ, van Zijl PCM, Zhou JY. Optimization of the irradiation power in chemical exchange dependent saturation transfer experiments. J Magn Reson. 2005;175(2):193–200. [DOI] [PubMed] [Google Scholar]
- 46.Cai K, Haris M, Singh A, et al. Magnetic resonance imaging of glutamate. Nat Med. 2012;18(2):302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murase K. Numerical Analysis of the Magnetization Behavior in Magnetic Resonance Imaging in the Presence of Multiple Chemical Exchange Pools. Open Journal of Applied Sciences. 2017(7):1–14. [Google Scholar]
- 48.Jin T, Kim SG. Advantages of chemical exchange-sensitive spin-lock (CESL) over chemical exchange saturation transfer (CEST) for hydroxyl–and amine-water proton exchange studies. NMR Biomed. 2014;27:1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun PZ, Zhou JY, Huang J, van Zijl P. Simplified quantitative description of amide proton transfer (APT) imaging during acute ischemia. Magn Reson Med. 2007;57(2):405–410. [DOI] [PubMed] [Google Scholar]
- 50.Rehncrona S. Brain Acidosis. Ann Emerg Med. 1985;14(8):770–776. [DOI] [PubMed] [Google Scholar]
- 51.Back T, Hoehnberlage M, Kohno K, Hossmann KA. Diffusion nuclear magnetic resonance imaging in experimental stroke. Correlation with cerebral metabolites. Stroke. 1994;25(2):494–500. [DOI] [PubMed] [Google Scholar]
- 52.Bereczki D, Csiba L. Spatial and temporal changes in tissue pH and ATP distribution in a new model of reversible focal forebrain ischemia in the rat. Metab Brain Dis. 1993;8(3):125–135. [DOI] [PubMed] [Google Scholar]


