Summary:
Background:
Standard preoperative radiation therapy (RT) dosing of 50 Gy in 25 fractions for soft tissue sarcoma (STS) results in excellent local control with major wound complications (MWC) in approximately 35% of patients. We report primary endpoint results with 2-year follow-up of a trial of hypofractionated, three-week preoperative RT for STS.
Methods:
We conducted a prospective, single-arm controlled trial at a single tertiary cancer care center in the US. We administered preoperative 42·75 Gy in 15 fractions of 2.85 Gy to adults with non-metastatic STS of the extremity or superficial trunk and ECOG performance status 0–3. The primary endpoint was the MWC rate within 120 days of surgery defined as requiring: (1) secondary operation under either general or regional anesthesia for wound repair, (2) extensive wound management without operation, or (3) readmission to the hospital for wound care. We monitored safety for all enrolled patients per protocol using a Bayesian stopping rule One-Arm Time-To-Event Simulator comparing MWC at 120 days post-surgery among study participants to the historical rate of 35%. Recruitment is complete. Follow-up continues. This trial is registered with ClinicalTrials.gov, NCT03819985.
Findings:
From December 18, 2018 to January 6, 2021, 120 patients were enrolled and received 42·75 Gy (or CGE if protons). Median follow-up was 24 months (IQR 17–30). The MWC rate within 120 days of surgery was 31% (n=37, 95% CI, 24–40%) occurring at median 37 days (IQR 25–59). No patient experienced acute toxicity of CTCAE v4.0 ≥ grade 3, on-treatment serious adverse event, nor treatment related death. Grade ≥3 late toxic effects of RT occurred in 4 patients: 2 femur fracture, 1 lymphedema, 1 skin ulceration.
Interpretation:
Preoperative 42·75 Gy in 15 daily fractions resulted in a MWC rate that was similar to conventionally fractionated RT and was found to be safe. Long-term oncologic, late toxicity and functional outcomes are awaited.
Funding:
NIH/NCI Cancer Center Support (Core) Grant CA016672 to (PI-Pisters) The University of Texas MD Anderson Cancer Center.
INTRODUCTION:
Randomized trials have demonstrated that limb-preserving local therapy combining surgery and radiation therapy (RT) results in superior local control compared to surgery alone for patients with soft tissue sarcoma (STS), with local control rates of 85–95%. 1,2 Subsequent randomized trials showed that local control outcomes are equivalent whether the RT is delivered pre-operatively to a dose of 50 Gy in 5 weeks versus postoperatively to a dose of 60–66 Gy (depending on surgical margins) over six or more weeks.3 Pre-operative RT is associated with a higher rate of wound complications after surgery compared to delivery of RT after surgical resection, 35% vs. 17%, respectively.3 These wound complications, while non-trivial, are temporary. This contrasts with the higher rate of permanent RT-related complications -fibrosis, edema, joint stiffness and consequent functional limitations—encountered with post-operative irradiation because of the higher doses and larger treatment volumes.4,5 Due to the permanence of post-operative RT-related toxicities, most sarcoma specialists favor preoperative RT for STS of the extremity and superficial trunk.6,7 Moreover, the recent American Society for Radiation Oncology (ASTRO) Clinical Practice Guideline on radiotherapeutic management of STS in adults strongly recommended preoperative RT over postoperative RT8 as did the recently updated National Comprehensive Cancer Network STS guidelines.9 Furthermore, RT given pre-operatively can affect tumor size reduction and facilitate surgical resection in some cases.
The standard regimen for preoperative RT has been 50 Gy in 25 daily fractions delivered over 5 weeks.6,7 Given a general shift toward use of hypofractionation in the field of radiation oncology, interest has grown into whether an alternative dose-fractionation schedule for STS management, one that would be radiobiologically equivalent but shorter and more convenient for patients, might obtain similar control rates without increasing toxicity rates.7 Investigators have reported prospective data on ultrahypofractionated preoperative regimens for STS, ranging from 25–35 Gy over 5 fractions. 10–13 Broad adoption of these regimens has been limited to-date likely related to concerns about tumor control, toxicity, the use of ultrahypofractionation for such large tumors, and study participation. Thus, in the absence of randomized data to compare against standard fractionation, a more moderately hypofractionated regimen may be more appealing when shorter courses of RT are being considered. Data regarding the safety of hypofractionated pre-operative RT are needed to inform patients and providers when considering shorter, more convenient fractionation.
We conducted a prospective, single-arm clinical trial of a moderately hypofractionated course, 42·75 Gy delivered over 3 weeks for patients with STS of the extremity or superficial trunk. Based upon the linear-quadratic modeling of cell survival, the biologically equivalent dose (BED) of various radiation treatment regimens incorporates the total dose delivered along with the dose per fraction. A derivative of the formula used to calculate the BED allows one to estimate equivalent dose of a hypofractionated regimen to standard fractionation at 2 Gy / day (EQD2). For a schedule of n fractions, dose d, and assuming an α/β of 3, the EQD2 was calculated using the following formula: EQD2 = D × [(d + α/β)/(2 + α/β)]. Assuming the reported α/β of 3–5 for STS,14 this dose is radiobiologically equivalent to the 48–50 Gy in 25 fractions standard regimen and should thus not compromise local control. Our hypothesis was that this regimen would not confer a greater risk of major wound complications compared to conventional fractionation.
METHODS:
Study design and participants
This is a prospective, open-label, single-arm controlled trial conducted at a single tertiary cancer care center in the US. The study was approved by the MD Anderson Cancer Center Investigational Review Board. All eligible patients provided written informed consent prior to enrollment. Patients were eligible if they were age ≥ 18 years, had life expectancy > 6 months, and had pathologically-confirmed STS, defined as arising from non-epithelial, non-reticuloendothelial extraskeletal soft tissues15 (e.g., fibrous connective, adipose, muscle, blood and lymph vessels, peripheral nerves, serious membranous tissues) in the extremity or superficial trunk without evidence of metastasis at the time of commencement of RT. All tumor specimens were reviewed by an expert sarcoma pathologist at our institution. Multidisciplinary recommendation for combined RT and surgery was made prior to enrollment, commonly for intermediate/high grade tumors or low grade tumors with increased risk of local recurrence or morbid salvage options. Patients were excluded for ECOG performance status >3, pregnancy and previous RT to the STS anatomic site.
Procedures
Patients underwent staging with magnetic resonance imaging (MRI) of the primary tumor site and computed tomography (CT) scans of the chest. Patients with myxoid liposarcoma also underwent staging CT scans of the abdomen and pelvis and typically MRI spine. Because we are a sarcoma referral center, many patients had undergone core needle biopsy or non-oncologic excisional procedure of their tumor prior to referral. If the patient presented to our center after having undergone non-oncologic excisional procedure at an outside facility, eligibility for this trial was determined after MD Anderson surgical evaluation wherein re-resection of the site of the primary sarcoma was recommended.
The total prescribed dose was 42·75 Gy given in 15 once daily fractions of 2.85 Gy, 5 fractions per week. The RT conformed to the International Commission on Radiation Units and Measurements 50 and 62 guidelines16,17 and required standard target volume delineation.6 The gross tumor volume (GTV) was delineated using gadolinium-enhanced MRI. The clinical target volume (CTV) was constructed with an expansion of 3–4 cm longitudinally and 1·5 cm radially from the GTV. In cases where the GTV was situated in subcutaneous tissues, the CTV was a 3–4 circumferential expansion and a deep expansion of 0·5–1·0 cm into underlying non-involved muscle with inclusion of peritumoral edema and biopsy tract. If necessary, the CTV was manually edited to ensure that it encompassed any T2-weighted MRI-identified peritumoral edema. The planning target volume (PTV) was generated by expanding the CTV 0.5–1.0 cm isotropically in all dimensions. The treatment technique and modality were at the discretion of the treating radiation oncologist and could be any of the following: conventional 3D radiotherapy (3DCRT), inverse planned fixed-gantry Intensity Modulated Radiotherapy (IMRT), rotational IMRT techniques such as Volumetric Arc Radiation Therapy (VMAT), or proton therapy (3D or Intensity Modulated Proton Therapy (IMPT)). Organ-at-risk constraints included: weight-bearing bone, V35 Gy < 65%, mean ≤ 30.5 Gy; joint, V42.75 Gy ≤ 50%; femoral head and neck, V38 Gy ≤ 50%. Efforts were made that as large a corridor of limb circumference as possible receive ≤ 17 Gy and avoided 42.75 Gy to the full circumference of the femur. When femur constraints were not achievable, orthopedic oncology was alerted for consideration of fracture prophylaxis. For other normal structures established normal tissue constraints were used by estimating equivalent dose to standard fractionation at 2 Gy / day (EQD2). No dose reductions were allowed. Protocol stipulated removal from trial if constraints could not be safely met, but no patient was removed for this reason after enrollment. Acute radiotherapy toxicity was documented according to Common Terminology Criteria for Adverse Events (CTCAE),18 and assessment done weekly during RT and with telephone follow-up 1–2 weeks after RT completion
Per standard practice, the protocol stipulated that oncologic resection of the sarcoma site should generally occur 4–10 weeks after the last fraction of RT. Wound closure, including whether plastic/reconstructive surgical specialist was required for closure was at the primary surgeon’s discretion.
The use of chemotherapy was at the discretion of the multidisciplinary team and medical oncology consultant. Generally, in our center chemotherapy is considered for patients with high grade tumors that are greater than 5 cm in maximal dimension considering age, comorbidities and performance status. In cases where chemotherapy was used either neoadjuvantly or adjuvantly, the agents used and number of cycles/doses of chemotherapy delivered were at the discretion of the medical oncologist. Per protocol, chemotherapy was not used concurrently with the RT in this study.
Follow up visits with appropriate imaging (e.g., MRI of the primary and chest imaging with radiographs or CT and other imaging as indicated per standard clinical practice for STS9) were conducted every 3– 4 months in years 1 and 2 after surgery and then every 6 months in years 3–5. Every attempt was made to remedy difficulties with adherence to follow-up schedules during the SARS-CoV-2 pandemic emergency by allowing telephone and virtual follow-up visits.
Outcomes
The primary endpoint was presence of a major wound complication (MWC) within 120 days of surgery. This was defined using the criteria from the CAN-NCIC SR-2 multicenter randomized trial,3 which set the standard for understanding the rate of MWC after preoperative RT for STS. MWC was defined as requiring: (1) secondary operation under either general or regional anesthesia for wound repair, (2) extensive wound management without operation, or (3) readmission to the hospital for wound care (appendix, p 25).
Secondary outcomes were: local recurrence (identified by imaging or physical exam and required histologic confirmation); time to local relapse, patterns of local recurrence, acute toxcitiy (CTCAE), late toxicity (skin according to Radiation Therapy Oncology Group (RTOG)19, and soft tissue and joints according to CTCAE). Secondary endpoints other than local control include oncologic (overall survival, distant metastatic free survival, disease-free survival, disease specific survival) and functional outcomes, which will be reported in a later publication with longer follow-up. Recruitment was completed and follow-up is ongoing.
Statistical analyses
Because STS are relatively rare malignancies, the sample size was determined on pragmatic grounds of 120 patients. We assessed safety with respect to MWC development rate for patients treated per protocol using a Bayesian phase II one-arm time-to-event stopping rule simulator.20,21 The control MWC rate assumption was 35%.3,4 For analysis of a stopping rule of higher acute wound complications for the new dose (E) primary outcome is T = the time to a major wound complication (MWC), evaluated during a 120 day period from the date of surgery. Historical data used on the 5-week schedule (C) show that 171 of 531 patients (32·2%)3,4 who received the standard control RT schedule experienced MWC. Denoting pC = Pr(MWC | C), based on these data, one may assume, under a Bayesian model, that pC ~ beta(171, 360), which has mean 171/531 = 0·322, variance of 0·000388, and effective sample size (ESS) = 531. In this single-arm trial, the main concern that motivated the early stopping rule for safety in the statistical design was the possibility that the hypofractionated schedule may produce a higher MWC rate than C. Therefore, for statistical consideration in monitoring of outcomes for safety, at each day of follow-up between 0 and 120, each patient’s outcome data consisted of the pair (T0, d) where T0 = the time to an observed MWC or last follow-up. Denoting the time of MWC, if it occurs within 120 days, by T, we defined the event indicator d=1 if T0=T, that is, a MWC was observed at day T0, and d=0 if T0 is the patient’s most recent follow-up time without a MWC. If a patient was followed for 120 days without MWC then, for the purpose of safety monitoring, T0 = 120 and d=0 was the patient’s final outcome. The trial was required to be stopped early for lack of safety if Pr( mean(T |E) > mean (T | C) | Data ) < 0·08, with this rule applied periodically, every two months, from the start of accrual, until the last patient was accrued. The design was calibrated to have a small early stopping probability 0·10 if Pr(Time to MWC < 120 days) = Pr(T<120 days) = 0·322.
Actuarial estimations of primary endpoint, local control, and confidence intervals were conducted using the Kaplan Meier method22 and the log-rank test to evaluate for significance of differences between curves for univariable analyses of factors associated with MWC (age, sex, BMI, diabetes and smoking status, performance status, tumor size and location, prior excision procedure, recurrent presentation, RT modality, interval between RT and surgery, final resection margin, plastics reconstruction, and chemotherapy). The level of statistical significance was a 2-sided p< 0·05. All endpoints and follow-up were analyzed from the date of surgery. Statistical analyses were conducted in r version 4·1·1 and python version 3·7 (Python Software Foundation). This trial is registered with ClinicalTrials.gov, NCT03819985.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
RESULTS:
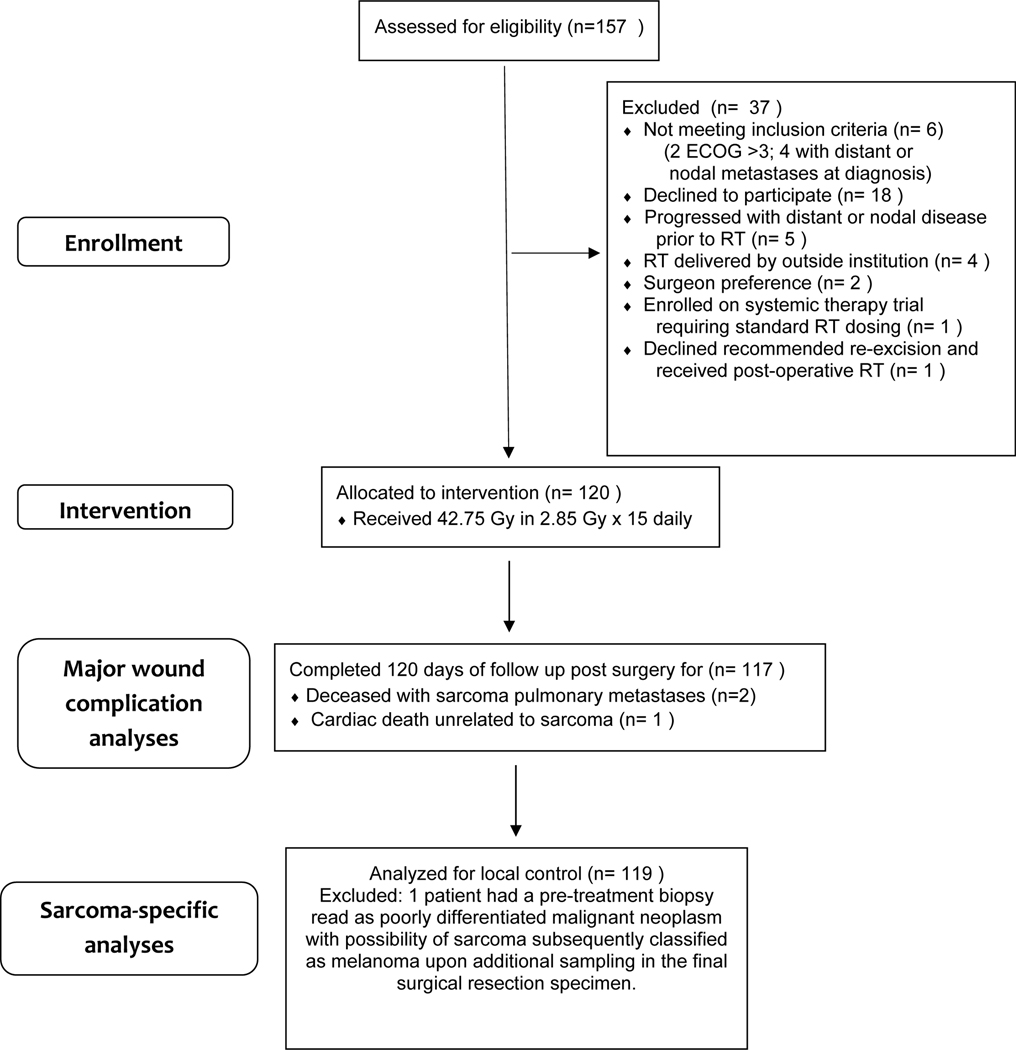
Between December 18, 2018 and January 6,2021, 120 patients were enrolled (figure 1). At no time did the stopping rule computation indicate that the trial should be stopped early for lack of safety. No on-treatment serious adverse event nor treatment related death occurred.
Figure 1. Trail profile.
ECOG=Eastern Cooperative Oncology Group. *One patient had their pretreatment biopsy read as poorly differentiated malignant neoplasm with the possibility of sarcoma, but upon additional sampling of the final surgical resection specimen, the patient’s diagnosis was revised to melanoma.
Table 1 shows the patient, tumor, and treatment characteristics. All patients received a preoperative dose of 42·75 Gy (or CGE if proton therapy) in once daily 2·85 Gy fractions over a median 20 days (IQR 18–21). Tumor was intermediate or high grade in 88 s (73%) of 120 patients. Twenty-five patients had primary wound closure by the sarcoma surgeon and 95 (79%) of 120 patients had wound closure with a vascularized tissue transfer, and/or split thickness skin grafting, or complex closure with the assistance of a plastics/reconstructive surgical specialist.
Table 1.
Patient, Tumor, and Treatment Characteristics
| Variable | All patients (n=120) Value or number (%) |
|---|---|
| Patient characteristics | |
| Age, years | |
| Median | 60 |
| IQR | 48–69 |
| Sex | |
| Female | 50 (42) |
| Male | 70 (58) |
| BMI, kg/m2 | |
| Median | 30 |
| IQR | 26–33 |
| Diabetes mellitus | |
| No | 105 (88) |
| Yes | 15 (13) |
| Tobacco smoking status | |
| Active smoker at diagnosis | 7 (6) |
| History of smoking but not active smoker | 34 (28) |
| No tobacco smoking history | 79 (66) |
| ECOG performance status | |
| 0–1 | 108 (90) |
| 2–3 | 12 (10) |
| Sarcoma presentation characteristics | |
| Tumor location | |
| Upper extremity | 20 (17) |
| Lower extremity | 78 (65) |
| Trunk | 22 (18) |
| Non-oncologic surgery prior to enrollment† | |
| No | 59 (58) |
| Yes | 51 (43) |
| Maximum Tumor Dimension, cm | |
| Median | 7·6 |
| IQR | 4·5–12·8 |
| Grade | |
| Low | 9 (8) |
| Intermediate | 27 (23) |
| High | 61 (51) |
| Unknown/ungradeable‡ | 23 (19) |
| Histopathology | |
| UPS | 26 (22) |
| Myxoid liposarcoma | 17 (14) |
| Liposarcoma (non-myxoid) | 15 (13) |
| Myxofibrosarcoma | 15 (13) |
| Unclassified | 9 (8) |
| Synovial | 7 (6) |
| Leiomyosarcoma | 6 (5) |
| Fibrosarcomatous transformation of dermatofibrosarcoma protuberans | 4 (3) |
| MPNST | 3 (3) |
| Epithelioid sarcoma | 2(2) |
| Extraskeletal myxoid chondrosarcoma | 2(2) |
| Extraskeletal osteosarcoma | 2(2) |
| Acral myxoinflammatory fibroblastic sarcoma | 2(2) |
| Low grade fibromyxoid sarcoma | 2(2) |
| Other* | 8(7) |
| Presentation | |
| Primary | 108 (90) |
| Recurrent | 12 (10) |
| Treatment characteristics | |
| RT modality/technique | |
| IMRT/VMAT | 57 (48) |
| 3DCRT | 55 (48) |
| Electrons | 5 (4) |
| Protons | 3(3) |
| Interval between RT end date and surgery, weeks | |
| Median | 5·7 |
| IQR | 4·6–6·4 |
| Final Surgical Resection Margin | |
| Positive/uncertain | 12 (10) |
| Negative | 108 (90) |
| Plastics/Reconstructive Surgical specialist participated in wound closure at time of resection | |
| Yes | 95 (79) |
| No | 25 (31) |
| Chemotherapy | |
| Neoadjuvant | 36 (30) |
| Adjuvant | 3 (3) |
| None | 81 (68) |
Abbreviations: IQR, interquartile range; UPS, unclassified pleomorphic sarcoma; MPNST, malignant peripheral nerve sheath tumor; RT, radiation therapy; Neo/Adj, neoadjuvant or adjuvant;
Defined as wide local excision with positive margins (5 patients) or excisional biopsy/unplanned excision (46 patients).
Some sarcomas are, by definition, not gradable.
Other histologies include angiosarcoma, pleomorphic rhabdomyosarcoma, sclerosing epithelioid fibrosarcoma, solitary fibrous tumor, histiocytic sarcoma, dermatofibrosarcoma protuberans (without fibrosarcomatous change) round cell sarcoma-not otherwise specified, and hemangiopericytoma. One patient’s final diagnosis was revised to melanoma upon pathological review of his complete resection specimen.
All patients underwent surgical resection after RT a median 5·7 weeks (IQR 4.6–6.4) after the last fraction of RT. Median follow-up from the date of surgery for patients alive at last follow-up was 24 months (IQR 17–30). At the time of this analysis, 12 patients had died; 9 deaths were sarcoma-related and 3 were of non-sarcoma-related causes. Three of these 12 deaths occurred prior to the end of the 120-day postoperative time period: 2 with sarcoma progression at 49 and 76 days after surgery, respectively and one from cardiac-related death 39 days after surgery. None of these 3 patients had experienced MWC at the time of death.
Among all enrolled patients, 37 (31%, 95% CI, 24–40%) of 120 patients developed a MWC a median 37 days from surgery (IQR 25–59), and 12 (32%) of 37 patients required re-operation for treatment of their MWC. Table 2 provides details provides details of MWC. The anatomic site was upper extremity in 1 patient, trunk in 8 patients, and lower extremity in 28 patients. Of the lower extremity major wound complications, 23 were proximal and 5 were distal. An additional 8 patients experienced a wound complication that was not considered major by the primary endpoint criteria. These included patients who experienced wound-edge necrosis requiring silver sulfadiazine cream for healing, those requiring prolonged dry dressing, and those necessitating oral antibiotics. Thus, 45 (38%) of 120 patients experienced any severity of wound complication.
Table 2-.
Type and frequencies of major wound complications
| Wound complication | n | % of total cohort |
|---|---|---|
| Yes | ||
| Secondary operation for wound repair | 12 | 10% |
| Invasive procedure for wound management without Secondary operation | 16 | 13% |
| Deep packing of the wound at least 2 cm with or without Prolonged dressing | 2 | 2% |
| Readmission to hospital for wound care | 7 | 6% |
| No major wound complication | 83 | 69% |
In univariable analyses (Table 3), significantly fewer patients with upper extremity tumors developed MWC compared to lower extremity and truncal tumors and those with diabetes mellitus were more likely to develop MWC. Development of MWC was not significantly associated with age, tumor size, performance status, BMI, smoking status, recurrent presentation, RT modality/technique, or receipt of chemotherapy. There was no difference in the development of MWC between those who had primary closure versus those who underwent closure with the assistance of plastics/reconstructive surgery.
Table 3.
Univariable analyses of development of major wound complication by patient, tumor, and treatment characteristics
| Variable | No. | MWC at 120 days* (Kaplan Meier curve,%) | p value |
|---|---|---|---|
| Patient characteristics | |||
| Age, years | |||
| ≤60 | 61 | 38 | 0.41 |
| > 60 | 59 | 35 | |
| Sex | |||
| Female | 50 | 26 | 0.30 |
| Male | 70 | 35 | |
| BMI, kg/m2 | |||
| < 30 | 64 | 30 | 0.77 |
| ≥30 | 56 | 32 | |
| Diabetes mellitus | |||
| No | 105 | 28 | 0.010 |
| Yes | 15 | 57 | |
| Smoking history (current or previous) | |||
| No | 79 | 31 | 0.64 |
| Yes | 41 | 32 | |
| ECOG performance status | |||
| 0–1 | 113 | 31 | 0.83 |
| 2–3 | 7 | 31 | |
| Sarcoma presentation characteristics | |||
| Tumor Location | |||
| Upper extremity | 20 | 5 | 0.029 |
| Lower extremity | 78 | 35 | |
| Trunk | 22 | 41 | |
| Non-oncologic excision prior to enrollment | |||
| No | 69 | 31 | 0.74 |
| Yes | 51 | 32 | |
| Maximum Tumor Dimension, cm | |||
| ≤10 | 80 | 28 | 0.26 |
| > 10 | 40 | 38 | |
| Presentation | |||
| Primary | 108 | 32 | 0.61 |
| Recurrent | 12 | 25 | |
| Treatment characteristics | |||
| RT modality/technique | |||
| IMRT/VMAT | 57 | 30 | 0.12 |
| 3DCRT | 55 | 31 | |
| Electrons | 5 | 20 | |
| Protons | 3 | 67 | |
| Interval between RT end date and surgery | |||
| ≤ 8 weeks | 113 | 32 | 0.36 |
| > 8 weeks | 7 | 14 | |
| Final Surgical Resection Margin | |||
| Positive/Uncertain | 12 | 43 | 0.24 |
| Negative | 108 | 30 | |
| Plastics/Reconstructive Surgical specialist participated in wound closure at time of resection | |||
| Yes | 95 | 32 | 0.75 |
| No | 25 | 29 | |
| Chemotherapy | |||
| No | 81 | 30 | 0.71 |
| Yes | 39 | 34 |
Abbreviations: MWC, major wound complication; BMI, body mass index; IMRT/VMAT, intensity modulated radiation therapy/volumetric modulated arc therapy; 3DCRT, 3D conformal radiation therapy.
Kaplan Meier curves with log-rank test for difference between curves.
No patient experienced acute toxicity grade ≥ 3 (Table 4). Grade ≥3 late toxic effects of RT were observed in 4 patients: 2 with femur fracture requiring surgical stabilization; one patient experienced severe lower extremity lymphedema necessitating lymphatic bypass procedure; and one patient who had MWC who required multiple surgeries and delayed healing with skin ulceration beyond 6 months. Detailed reporting of late toxicity and functional outcomes will be reported with longer follow-up.
Table 4:
Acute and late toxicity for RTOG and CTCAE scales
| Acute toxicity | Entire Cohort (n=120) |
|---|---|
| Skin toxicity CTCAE | |
| 0 | 34 (28%) |
| 1 | 77 (64%) |
| 2 | 9 (8%) |
| 3 | 0 |
| 4 | 0 |
| 5 | 0 |
| Fatigue CTCAE | |
| 0 | 69 (58%) |
| 1 | 49 (41%) |
| 2 | 2 (2%) |
| 3 | 0 |
| 4 | 0 |
| 5 | 0 |
| Pain CTCAE | |
| 0 | 73 (61%) |
| 1 | 34 (28%) |
| 2 | 13 (11%) |
| 3 | 0 |
| 4 | 0 |
| 5 | 0 |
| Late toxicity | Patients with ≥ 6 months of follow up (n=115) |
| Skin RTOG | |
| 0 | 71 (62%) |
| 1 | 41 (36%) |
| 2 | 2 (2%) |
| 3 | 0 |
| 4 | 1 (1%) |
| Fibrosis of superficial or deep connective tissue CTCAE | |
| 0 | 84 (73%) |
| 1 | 28 (24%) |
| 2 | 3 (3%) |
| 3 | 0 |
| 4 | 0 |
| 5 | 0 |
| Lymphedema CTCAE | |
| 0 | 98 (85%) |
| 1 | 14 (12%) |
| 2 | 2 (2%) |
| 3 | 1 (1%) |
| 4 | 0 |
| 5 | 0 |
| Joint CTCAE | |
| 0 | 91 (79%) |
| 1 | 24 (21%) |
| 2 | 0 |
| 3 | 0 |
| 4 | 0 |
| 5 | 0 |
Late toxicity at last follow-up; 5 patients missing due to death before 6 months (n=3) or duration of follow-up for toxicity assessment < 6 months (n=2).
Local control was assessed for the 119 patients. At the time of this analysis, 6 patients had experienced a local relapse at a median of 16 months (IQR 7·0–17) since surgery. Four were in the RT field, one was at the field edge, and one was wide of the radiotherapy field. One of these patients required amputation for local recurrence and was disease free 10 months at the time of this analysis. The 30-month actuarial local control rate was 93% (95% CI, 86–97%).
DISCUSSION:
To our knowledge, our single-arm trial is one of the largest prospective investigation of radiotherapeutic management for STS of the extremity and trunk in the past two decades. This study demonstrated that a hypofractionated pre-operative radiotherapy dose regimen of 42·75 Gy delivered over three weeks resulted in a MWC in 31% of patients. Using a rigorous definition of MWC to allow robust qualitative comparison to historical data, we ascertained that MWC development was not higher than rates observed with standard 50 Gy delivered over five weeks.3,4 Acute and late toxicity were acceptable, and local control was comparable to conventional fractionation at 2 years of follow-up.
Our study results compare favorably, not only with conventional fractionation, but also with those of other recent investigations of ultrahypofractionated regimens. The published literature on condensed radiotherapy regimens reflects interest in whether preoperative RT can be safely and effectively delivered using courses that are shorter than five weeks.7 In 2009, Ryan and colleagues10 reported on 8 × 3·5 Gy delivered 4 days per week sandwiched in between 3 cycles of epirubicin and ifosfomide chemotherapy. They reported a 2-year local control rate of 88% and acceptable outcomes with respect to wound complications. Hypofractionated regimens that do not incorporate concurrent chemotherapy offer more appropriate comparative data for our study. Kosela-Paterczyk and colleagues11 reported the largest prospective study of ultrahypofractionated preoperative radiotherapy for STS using 5 × 5 Gy daily. Their local control rate of 81% was lower than historically reported rates. This may have been attributable to their 25 Gy total dose computing to an equivalent dose in 2 Gy per day fractions of 40 Gy assuming α/β of 3. Kalbasi and colleagues12 have recently reported favorable outcomes for 5 × 6 Gy daily fractions in cohort of 52 patients. Similarly, Bedi and colleagues13 have reported excellent results with 5 × 7 Gy delivered every other day in a cohort of 32 patients. These ultrahypofractionated regimens are promising. However, in the absence of a randomized controlled trial comparing these to the standard schedule, practitioners may prefer a more moderately hypofractionated alternative if a shortened course of RT is considered. Our center has incorporated the results of this current study into practice by counseling patients regarding the outcomes and limitations of this study and offering this regimen as an alternative to the five-week regimen.
Our study has several limitations, chief among them, and similar to the ultrahypofractionation studies, are that it is a single-institution study and also does not offer randomized comparison to conventional fractionation. However, the rarity and heterogeneity of STS make randomized controlled trials impractical to conduct. To address this limitation, we chose a Bayesian monitoring statistical design which has been reported to facilitate high quality evidence assessment in rare diseases.23 While our data cannot offer a proven alternative to conventional fractionation, patients can be counseled regarding our findings and consider this three-week regimen in consultation with their radiation oncologist. Appropriate counseling should include that long-term toxicities are pending and there remains uncertainty as to whether this regimen may adversely impact functional outcomes compared to conventional fractionation. However, it is reassuring that an α/β of 3 is also commonly used as the best estimate for late toxicity effects. It is thus likely that our hypofractionated regimen is bioequivalent to 50 Gy in 25 fractions, not just from a standpoint of disease control for STS, but also for late toxicities. Patient outcomes are superior when patients receive treatment at a center that sees a higher volume of sarcoma cases. 24,25 Thus, this shorter RT course may be of particular relevance for resource-constrained patients or in situations wherein staying five weeks near a sarcoma referral center for RT presents a challenge. Another notable limitation is our relatively short follow-up. However, larger series of patients with longer follow-up show that median time to local recurrence for STS is 18–19 months and that two-thirds of local relapses occur by 2 years after treatment.26,27 Thus, our observed local control is consistent with conventional fractionation. Additionally, our study may have under-reported acute skin toxicity due to lack of physical examination 1–2 weeks after RT completion when acute skin reaction can be most severe. Importantly, longer follow-up will be needed to assess long-term local control as well as functional outcomes. Additionally, after our trial completed recruitment, data were published showing that patients with myxoid liposarcoma, which is particularly radiosensitive, likely is effectively treated with reduced-dose preoperative 36 Gy in 18 fractions.28 Furthermore, we look forward to results of other prospective trials of three-week hypofractionated preoperative RT regimens for STS opened at Mayo Clinic (NCT04562480)29 and the Netherlands Cancer Institute (NCT04425967)30.
In conclusion, we found that 42·75 Gy in fractions of 2·85 Gy delivered over 3-weeks offers a safe, effective, and more convenient alternative to conventional fractionation. The acute and post-surgical morbidity associated with this three-week regimen is comparable to the standard five-week regimen and early analyses suggest local control outcomes are consistent with standard fractionation. Patients could be counseled that while data are still maturing, this preoperative radiotherapy regimen offers a reasonable alternative to conventional fractionation, especially if it facilitates care at a high-volume sarcoma center or alleviates resource constraints that may interfere with accession of five-weeks of radiotherapy.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study:
Conventionally fractionated preoperative radiation therapy in 5 weeks has been the standard of care for soft tissue sarcoma management for decades. However, in recent years interest in hypofractionated approaches has increased. We searched PubMed for reports published in English between inception and manuscript submission on August 18, 2022 using the terms “sarcoma”, “soft tissue”, “preoperative”, “radiotherapy”, and/or “hypofractionation.” Prior to the first patient enrollment on December 18, 2018, three prospective studies were identified. Two studies were exploratory studies evaluating preoperative chemoradiation with pathologic endpoints, and the only other study reported an inferior local control rate of 81% compared to conventional fractionation (85–95%). All three available studies used preoperative doses that were not radiobiologically equivalent to standard conventional dosing. At the time of manuscript submission, 5 additional prospective studies investigating ultrahypofractionated preoperative radiotherapy without concurrent chemotherapy were identified (prescribing 30–40 Gy in 5 fractions). One study reported feasibility alone of 35 Gy (n=12). The remaining 4 studies reported outcomes and toxicity with cohorts of 16, 25, 32 and 52 patients, respectively. Adoption of hypofractionation or ultrahypofractionation for preoperative radiation in soft tissue sarcoma has been limited to-date, likely related to small reported cohorts with relatively short follow-up as well as concerns about ultrahypofractionation for tumors that require a large volume to be irradiated because of the potentially extensive size of sarcomas at presentation.
Added value of this study:
To our knowledge, this is one of the largest prospective trials completed in the modern radiation era for patients with soft tissue sarcomas of the extremity and superficial trunk. Our regimen investigates hypofractionation for soft tissue sarcoma using total dose and fractionation selected to have radiobiologic equivalency for tumor control to conventional fractionation and offers a more moderately hypofractionated alternative to the ultrahypofractionation regimens recently reported. This single-arm, controlled clinical trial provides high-quality evidence that a three-week shortened course of hypofractionated preoperative radiation therapy is safe and well tolerated with respect to acute surgical and radiation-related morbidity when compared to the five-week standard regimen.
Implications of all the available evidence:
Our findings support a three-week hypofractionated preoperative radiotherapy regimen as an alternative to the historical standard of five weeks of conventional fractionation. MWC complications occurred in 31% of study participants and local control was 93% at 2 years. Acute and late morbidity is comparable to conventional fractionation as is local control at 2 years. Given the importance of specialized care for the management of soft tissue sarcomas, this shorter radiation regimen may facilitate ease of access for patients seeking care at a sarcoma specialty center. Long-term toxicity, oncologic and functional outcomes associated with this regimen are awaited.
Acknowledgments:
Funding Source: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health Cancer Center Support (Core) Grant under award number CA016672 to (PI-Pisters) The University of Texas MD Anderson Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors would like to thank the patients who participated and acknowledge Albert C. Koong, MD, PhD, FACR, FASTRO, Division Head, Radiation Oncology at MD Anderson Cancer Center, for facilitating departmental research resource support for this study.
Declarations of interests:
BAG reports grant support for research to her institution from the Cancer Prevention and Research Institute of Texas (CPRIT) (Grant RP1606074, Guadagnolo co-PI) that did not relate to this work.
CR reports research funding to her institution from Bristol Myers Squibb and received honoraria to her personally from Lumanity.
KT reports research funding to her institution from the US National Science Foundation and Department of Defense as well as consulting fees to her personally from the US Department of Defense.
KH reports research funding to the institution from Cairn Surgical, Eli Lilly & Co, and Lumicell and is Medical Advisory Board member for Armada Health and AstraZeneca.
JB received personal consulting fees from GT Technologies.
RS has grant funding to the institution from the US Department of Defense for research unrelated to this report.
RR reports research funding to the institution from Springworks and C4 Theratpeutics and consulting fees to the institution form Ayala pharmaceuticals as well as personal consulting fees an honoraria from Bayer and Epizyme.
Footnotes
The rest of the authors do not have any financial disclosures nor any type of conflict related to this research.
Data sharing:
Data collected for the study are not currently available to those outside MD Anderson Cancer center. Data sharing of MD Anderson patient data for research purposes requires a signed internal-investigator-initiated data sharing and transfer agreement vetted and signed by MD Anderson’s legal counsel prior to any sharing of any data. The full protocol can be accessed by contacting the corresponding author via email.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
B. Ashleigh Guadagnolo, Department of Radiation Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Roland L Bassett, Department of Biostatistics, MD Anderson Cancer Center, Houston, TX, USA.
Devarati Mitra, Department of Radiation Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Ahsan Farooqi, Department of Radiation Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Caroline Hempel, Department of Radiation Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Courtney Dorber, Department of Radiation Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Tiara Willis, Department of Radiation Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Wei-Lien Wang, Department of Pathology, MD Anderson Cancer Center, Houston, TX, USA.
Ravin Ratan, Department of Sarcoma Medical Oncology, MD Anderson Cancer Center, Houston, TX USA.
Neeta Somaiah, Department of Sarcoma Medical Oncology, MD Anderson Cancer Center, Houston, TX USA.
Robert S Benjamin, Department of Sarcoma Medical Oncology, MD Anderson Cancer Center, Houston, TX USA.
Keila E Torres, Department of Surgical Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Kelly K Hunt, Department of Surgical Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Christopher P Scally, Department of Surgical Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Emily Z Keung, Department of Surgical Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Robert L Satcher, Department of Orthopaedic Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Justin E Bird, Department of Orthopaedic Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Patrick P Lin, Department of Orthopaedic Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Bryan S Moon, Department of Orthopaedic Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Valerae O Lewis, Department of Orthopaedic Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Christina L Roland, Department of Surgical Oncology, MD Anderson Cancer Center, Houston, TX, USA.
Andrew J Bishop, Department of Radiation Oncology, MD Anderson Cancer Center, Houston, TX, USA.
REFERENCES:
- 1.Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 1982; 196: 305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol Off J Am Soc Clin Oncol 1998; 16: 197–203. [DOI] [PubMed] [Google Scholar]
- 3.O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet Lond Engl 2002; 359: 2235–41. [DOI] [PubMed] [Google Scholar]
- 4.Cannon CP, Ballo MT, Zagars GK, et al. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer 2006; 107: 2455–61. [DOI] [PubMed] [Google Scholar]
- 5.Davis AM, Sennik S, Griffin AM, et al. Predictors of functional outcomes following limb salvage surgery for lower-extremity soft tissue sarcoma. J Surg Oncol 2000; 73: 206–11. [DOI] [PubMed] [Google Scholar]
- 6.Haas RLM, Delaney TF, O’Sullivan B, et al. Radiotherapy for management of extremity soft tissue sarcomas: why, when, and where? Int J Radiat Oncol Biol Phys 2012; 84: 572–80. [DOI] [PubMed] [Google Scholar]
- 7.Haas RLM, Miah AB, LePechoux C, et al. Preoperative radiotherapy for extremity soft tissue sarcoma; past, present and future perspectives on dose fractionation regimens and combined modality strategies. Radiother Oncol J Eur Soc Ther Radiol Oncol 2016; 119: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salerno KE, Alektiar KM, Baldini EH, et al. Radiation Therapy for Treatment of Soft Tissue Sarcoma in Adults: Executive Summary of an ASTRO Clinical Practice Guideline. Pract Radiat Oncol 2021; 11: 339–51. [DOI] [PubMed] [Google Scholar]
- 9.Soft Tissue Sarcoma (Version 2.2022). National Comprehensive Cancer Network, 2022. https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf (accessed Sept 8, 2022). [Google Scholar]
- 10.Ryan CW, Montag AG, Hosenpud JR, et al. Histologic response of dose-intense chemotherapy with preoperative hypofractionated radiotherapy for patients with high-risk soft tissue sarcomas. Cancer 2008; 112: 2432–9. [DOI] [PubMed] [Google Scholar]
- 11.Koseła-Paterczyk H, Szacht M, Morysiński T, et al. Preoperative hypofractionated radiotherapy in the treatment of localized soft tissue sarcomas. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2014; 40: 1641–7. [DOI] [PubMed] [Google Scholar]
- 12.Kalbasi A, Kamrava M, Chu F-I, et al. A Phase II Trial of 5-Day Neoadjuvant Radiotherapy for Patients with High-Risk Primary Soft Tissue Sarcoma. Clin Cancer Res Off J Am Assoc Cancer Res 2020; 26: 1829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bedi M, Singh R, Charlson JA, et al. Is 5 the New 25? Long-Term Oncologic Outcomes From a Phase II, Prospective, 5-Fraction Preoperative Radiation Therapy Trial in Patients With Localized Soft Tissue Sarcoma. Adv Radiat Oncol 2022; 7: 100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas RL, Floot BGJ, Scholten AN, et al. Cellular Radiosensitivity of Soft Tissue Sarcoma. Radiat Res 2021; 196: 23–30. [DOI] [PubMed] [Google Scholar]
- 15.Weiss SW, Goldblum JR. General Considerations. In: Enzinginger and Weiss’s Soft Tissue Tumors, 5th edn. Mosby: Elsevier, 2008: 1. [Google Scholar]
- 16.Landberg T, Chavaudra J, Dobbs J, et al. Report 50. J ICRU 1993; os-26.
- 17.Landberg T, Chavaudra J, Dobbs J, et al. Report 62. J ICRU 1999; os-31.
- 18.Common Terminology Criteria for Adverse Events (CTCAE). 2018; published online March 1. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed May 3, 2018).
- 19.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31: 1341–6. [DOI] [PubMed] [Google Scholar]
- 20.Thall PF, Wooten LH, Tannir NM. Monitoring event times in early phase clinical trials: some practical issues. Clin Trials Lond Engl 2005; 2: 467–78. [DOI] [PubMed] [Google Scholar]
- 21.Thall PF, Simon R. Practical Bayesian guidelines for phase IIB clinical trials. Biometrics 1994; 50: 337–49. [PubMed] [Google Scholar]
- 22.Kaplan E, Meier P. Nonparametric estimator from incomplete observations. J Am Stat Assoc 1958; 53: 457–81. [Google Scholar]
- 23.Zohar S, Teramukai S, Zhou Y. Bayesian design and conduct of phase II single-arm clinical trials with binary outcomes: a tutorial. Contemp Clin Trials 2008; 29: 608–16. [DOI] [PubMed] [Google Scholar]
- 24.Blay J-Y, Soibinet P, Penel N, et al. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann Oncol Off J Eur Soc Med Oncol 2017; 28: 2852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venigalla S, Nead KT, Sebro R, et al. Association Between Treatment at High-Volume Facilities and Improved Overall Survival in Soft Tissue Sarcomas. Int J Radiat Oncol Biol Phys 2018; 100: 1004–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zagars GK, Ballo MT, Pisters PWT, Pollock RE, Patel SR, Benjamin RS. Prognostic factors for disease-specific survival after first relapse of soft-tissue sarcoma: analysis of 402 patients with disease relapse after initial conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys 2003; 57: 739–47. [DOI] [PubMed] [Google Scholar]
- 27.Maretty-Nielsen K, Aggerholm-Pedersen N, Safwat A, et al. Prognostic factors for local recurrence and mortality in adult soft tissue sarcoma of the extremities and trunk wall: a cohort study of 922 consecutive patients. Acta Orthop 2014; 85: 323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lansu J, Bovée JVMG, Braam P, et al. Dose Reduction of Preoperative Radiotherapy in Myxoid Liposarcoma: A Nonrandomized Controlled Trial. JAMA Oncol 2021; 7: e205865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clinic Mayo. A Phase II Study of Hypofractionated Pre-Operative Radiation Therapy for Localized, Resectable Soft Tissue Sarcoma of the Extremity and Superficial Trunk. clinicaltrials.gov, 2022. https://clinicaltrials.gov/ct2/show/NCT04562480 (accessed July 6, 2022).
- 30.The Netherlands Cancer Institute. Short Course Of Preoperative Radiotherapy in Head and Neck-, Trunk- and Extremity Soft Tissue Sarcomas; a Randomized Phase II Clinical Trial. clinicaltrials.gov, 2021. https://clinicaltrials.gov/ct2/show/NCT04425967 (accessed July 6, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.