Abstract
Background:
Consumption of added sugars (AS) and sugar-sweetened beverages (SSB) may adversely affect adolescents’ weight and cardiovascular disease risk. Reliance on self-reported dietary assessment methods is a common research limitation, which could be overcome by dietary intake biomarkers.
Aim:
The investigation was a proof-of-concept study to evaluate the proposed carbon isotope ratio (δ13C) biomarker of AS intake in adolescents, using a controlled feeding design.
Methods:
Participants (n = 33, age 15.3 years, 53% female) underwent two seven-day controlled feeding periods in a randomly assigned order. Diets were matched in composition except for AS content (5% or 25% of total energy). Fasting fingerstick blood samples were collected daily during each diet period.
Results:
Fingerstick δ13C values changed from day 1 to 8 by −0.05 ± 0.071‰ on 5% AS, and +0.03 ± 0.083‰ on 25% AS (p ≤ 0.001). Reliability was demonstrated between day 7 and 8 δ13C values on the 5% (ICC = 0.996, p ≤ 0.001) and 25% (ICC = 0.997, p ≤ 0.001) AS diets.
Conclusions:
Larger scale investigations are warranted to determine if this technique could be applied to population-level research in order to help assess the effectiveness of interventions aimed at reducing the consumption of AS or SSB intake.
Keywords: Added sugar, adolescents, biomarker dietary assessment, validation
Introduction
Added sugars (AS) are defined as: “…sugars and syrups added to foods during processing or preparation (Johnson et al., 2009; US Department of Agriculture, 2015).” Adolescents consume approximately 16% of daily calories as (Ervin et al., 2012; Welsh et al., 2011). Of this, sugar-sweetened beverages (SSB) comprise about 33–60% of AS intake (Ervin et al., 2012; Guthrie and Morton, 2000; Watowicz et al., 2015; Welsh et al., 2011); adolescent consumer preference for sugary beverages has been shown to be heavily influenced by unique environmental influences (Smith et al., 2017). Consumption of AS/SSB is linked to undesirable changes in weight status (Bermudez and Gao, 2010; Lim et al., 2009; Malik et al., 2013; Wang et al., 2015) and cardiovascular disease risk factors in youth (Ambrosini et al., 2013; Gyllenhammer et al., 2014). This has prompted major health organizations to establish limits for AS intake (US Department of Agriculture, 2015; Vos et al., 2016; World Health Organization, 2015). Yet, there is debate about the health effects of AS/SSB intake, partly due to the limitations of self-reported dietary data (Davy and Jahren, 2016). Because pediatric populations tend to misreport dietary intake (Bel-Serrat et al., 2016; Lioret et al., 2011; Murakami and Livingstone, 2016; Rangan et al., 2014; Ventura et al., 2006), especially for foods containing AS (Lioret et al., 2011; Rangan et al., 2014; Ventura et al., 2006), there is a need for objective dietary intake biomarkers (Kuhnle, 2012). The δ13C value of fingerstick blood has been proposed as a biomarker for AS intake (Davy and Jahren, 2016). If validated, this technique could be applied to population-level research in order to help assess the effectiveness of interventions aimed at reducing the consumption of AS or SSB intake.
The proposed δ13C AS biomarker is based upon the differential accumulation of 13C to 12C isotopes in plant tissues (Jahren et al., 2014). Plants that perform C4 photosynthesis undergo additional chemical reactions than the C3 pathway, which leads to elevated 13C content in C4 plants (Jahren et al., 2014). The primary crops for sugar production in the United States—corn, sugarcane, and sorghum—are C4 plants (Jahren et al., 2014). Thus, the δ13C of human tissue, which is influenced by diet, can reflect AS intake. Positive correlations between AS intake and the δ13C value of plasma glucose (Cook et al., 2010), red blood cell (RBC) alanine (Choy et al., 2013), RBCs (Nash et al., 2014), and whole blood (Davy et al., 2011; Fakhouri et al., 2014; Yeung et al., 2010) have been reported. However, investigations have been limited to those which utilized self-reported dietary intake data (Chi et al., 2015; Choy et al., 2013; Davy et al., 2011; Fakhouri et al., 2014; MacDougall et al., 2018; Nash et al., 2013; Nash et al., 2014; Yeung et al., 2010) and one short-term controlled feeding study in adults (Cook et al., 2010). Of the two published investigations of the δ13C biomarker in children (Chi et al., 2015; MacDougall et al., 2018), only one assessed dietary intake (MacDougall et al., 2018). Our recent comparison of biomarker values to self-reported SSB intake in 326 children and adolescents determined that the biomarker was able discriminate between high and low SSB consumers (MacDougall et al., 2018). Controlled feeding studies are now needed to evaluate the validity of this technique for objectively assessing AS and SSB intake.
Tissue metabolic rate and δ13C turnover are positively correlated in animals (Fry and Arnold, 1982; MacAvoy et al., 2006; Tieszen et al., 1982). Since adolescents are undergoing growth and development (Rogol et al., 2000), tissue turnover times may be more rapid in adolescents than in adults, and fingerstick blood δ13C may therefore change more rapidly in response to changes in AS intake. The objective of this investigation was to evaluate the sensitivity and reliability of the proposed δ13C AS biomarker, assessed in fingerstick blood samples, in adolescents during a short-term controlled feeding trial using a crossover design. The investigation was a proof-of-concept study. It was hypothesized that fingerstick δ13C would be a sensitive and reliable indicator of actual AS intake.
Methods
Participants
Adolescents were recruited from a local campus community in Southwest Virginia between June 2015 and July 2016 through flyers, email advertisements, and word of mouth. Eligible individuals were 12–18 years of age; with a body mass index (BMI) percentile <95% per the Centers for Disease Control and Prevention’s (CDC) BMI-for-age growth charts (Centers for Disease Control and Prevention, 2015); without food allergies, intolerances, or aversions; and willing to follow a controlled diet for two separate 1-week periods. Of the 58 adolescents screened for eligibility, 33 were enrolled in the study. Based upon power analyses that assumed a minimum correlation of 0.3 within participants, plus a minimum effect size of η2p = 0.1, we estimated that a sample size of 30 would be sufficient to infer that the difference in δ13C value of fingerstick blood samples between day 1 and day 8 of each controlled feeding period would not be zero (significance level is 0.05; 90% power). Therefore, once approximately 30 participants had enrolled, recruitment was ceased. Of those enrolled, 32 participants completed the study.
Experimental design
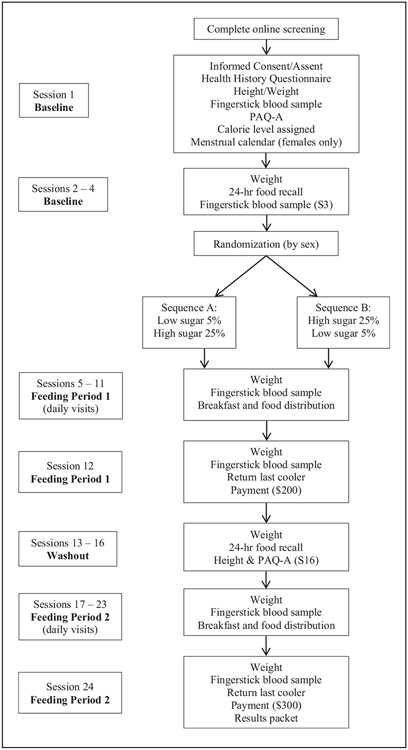
Figure 1 depicts the study design. Baseline measurements took place over four study sessions, which included assessment of demographic information, habitual dietary intake using four 24-h dietary recalls, habitual physical activity level, height, weight, and the collection of two fingerstick blood samples. Using a crossover design, participants next completed two seven-day controlled feeding periods in a randomly assigned order (5% “low” added sugars (LAS); 25% “high” added sugars (HAS)), with a four-week washout period between the two diet conditions. A study coordinator who was not involved with data collection or analysis was responsible for enrolling participants and assigning participants to their randomized sequence in which to complete the diets, LAS first (A) or HAS first (B), utilizing a computer-generated randomization scheme. Allocation of sequence was conducted to ensure that groups were approximately equal in distribution according to participants’ gender and age. The sequence was known to researchers, but not to participants, before the controlled diets were provided. Each morning during the controlled diet periods, body weight was measured to ensure weight stability, and a fasting fingerstick blood sample was obtained. During the washout period, four 24-h dietary recalls were obtained, and physical activity level, height, and weight were reassessed. Participants were compensated US$500 for completing all study sessions, and were provided with a results packet that described their baseline self-reported dietary intake.
Figure 1.
Study protocol. Sequence A: participants were assigned the 5%, LAS diet first, then the 25%, HAS diet. Sequence B: participants were assigned the 25%, HAS diet first, then the 5%, LAS diet. Unless otherwise indicated in parentheses, measurements/procedures listed in each panel took place at every visit indicated in the corresponding visit panel to the left.
PAQ-A: Physical Activity Questionnaire for Adolescents.
Experimental protocol
Controlled diet development and delivery.
Age- and sex-specific equations from the Institute of Medicine (IOM) (Food and Nutrition Board and Institute of Medicine, 2005) were used to estimate energy needs. Physical activity was self-reported using the Physical Activity Questionnaire for Adolescents (PAQ-A) (Kowalski et al., 2004), which was used to derive an activity factor for determining total daily energy requirements (Liu et al., 2016). The LAS and HAS diets (5% and 25% of calories from AS, respectively) were developed to meet dietary intake targets, which are listed in Table 1. Each seven-day diet consisted of a three-day rotating menu, which ranged from 1500 to 4500 kcal/day according to participants estimated energy requirements. Two optional 150 kcal snack modules were provided daily while on the controlled diet, to prevent energy deficits that could result from variation in daily activity level. Snack modules were designed to meet the same dietary targets as the overall diet.
Table 1.
Dietary targets for low- and high-added sugar diets.
| Nutrient | Target value |
|---|---|
| Total energy, kcal | Varies: 1500–3500 |
| Carbohydrate, % of kcal | 55% |
| Total AS, % of kcal | 5% or 25% |
| Liquid AS, % of AS | 33% |
| Solid AS, % of AS | 67% |
| Fat, % of kcal | 30% |
| Protein, % of kcal | 15% |
| Animal protein, % of protein | 42% |
| Dairy protein, % of protein | 20% |
| Vegetable/other protein, % of protein | 38% |
A list of foods popular among children was used for menu planning (American Dietetic Association, 1999) to provide foods acceptable and palatable to this population. Food items were entered in nutrition analysis software (Nutrition Data System for Research (NDS-R) 2013, University of Minnesota, Minneapolis, MN). The specific foods provided on both diets were matched closely to minimize a difference in compliance between the two feeding periods (sample menus are included as Supplemental Material).
The macronutrient composition of both diets fell within the acceptable macronutrient distribution ranges prescribed for children of 4–18 years of age (Food and Nutrition Board and Institute of Medicine, 2006). The LAS diet matched the World Health Organization’s conditional recommendation that AS should be limited to 5% of daily caloric intake (World Health Organization, 2015). The amount of AS on the HAS diet corresponded to the level of AS which has been associated with an inadequate intake of essential nutrients (Food and Nutrition Board and Institute of Medicine, 2006). The percentage of AS from solid versus liquid sources corresponded with that consumed by American adults (Ervin and Ogden, 2013), according to information available at the time of diet planning. Protein sources were consistent across diets, since meat sourced from corn-fed animals could influence δ13C biomarker values (Jahren et al., 2014); percentages matched protein intake patterns consumed by American adults (Smit et al., 1999).
On the first day of each controlled feeding period, diet instructions were reviewed with participants and their parents. Breakfast was supervised and consumed in the dining laboratory, following the measurement of weight and the collection of a fingerstick blood sample. Remaining meals and snacks for the day were provided in a portable cooler and consumed outside of the laboratory. In addition to the optional snack modules, participants were provided with three sucralose packets and three bottles of water each day, which were optional. Any uneaten food was returned the next morning, which was weighed to determine actual food consumption and to assess compliance. Participants were also asked to report consumption of non-study provided foods. If participants consumed ≥90% of food provided in the study (Hall and Most, 2005), then they were considered compliant with consuming the controlled diets.
Dietary assessment.
During the baseline and washout periods (visits 1–4 and 13–16, respectively), participants completed a total of eight, non-consecutive 24-h recalls with trained research personnel. Recalls encompassed both weekday and weekend days, in accordance with recommended dietary assessment practices (Thompson and Subar, 2013). Although participants self-reported intake, most parents accompanied their children to assist when needed (e.g. reporting details of a recipe). Researchers employed the US Department of Agriculture’s Multi-Pass Method (Raper et al., 2004) to standardize prompting. Additionally, participants used 2D and 3D food models to increase accuracy of reporting. Food recalls were analyzed using NDS-R to determine habitual macronutrient composition and AS intake.
Fingerstick blood sampling for δ13C.
The primary outcome was the δ13C values of fingerstick blood samples collected during the controlled feeding periods. Two non-fasting fingerstick samples were collected at baseline (MedLance Plus Universal Lancet, HTL-Strefa, Ozorków, Poland), to establish participant’s usual values for δ13C. Each morning of both controlled feeding periods, after a 12-h fast, a fingerstick blood sample was collected to monitor change in δ13C values in response to the high and low AS diet. Fingerstick blood samples were collected using sterilized binder-free glass microfiber filters (Whatman, type GF/D, 2.5 cm diameter, Whatman, Inc., Piscataway, NJ), and air-dried for 15–30 min. Next, the blood samples were analyzed for δ13C via natural abundance stable isotope mass spectrometry (NA-SIMS), as previously described (Davy et al., 2011). Each sample was tested in triplicate; the mean of these results was reported. There was an analytical uncertainty of ±0.046‰ with each sample measurement for the entire sample (n = 32).
Statistical methods
Data were analyzed using statistical analysis software (SPSS v. 24.0 for Windows, SPSS Inc., Chicago, IL). Descriptive statistics (mean ± SD; frequencies) were reported for participant demographics (sex, age, race/ethnicity, height, weight, BMI, and BMI percentile), self-reported dietary intake and controlled dietary intake variables (total calorie intake (kcal); carbohydrate, protein, and fat intake (g and % of kcal); AS intake (g and % of kcal); sodium (mg/day)), dietary compliance (returned food weigh-back data ((consumed/provided) × 100, expressed as %), and δ13C fingerstick values.
Sensitivity was evaluated using a repeated measures analysis of variance (RM-ANOVA) between day 1 and day 8 fingerstick δ13C values within each diet condition. A post-hoc, paired sample t-test was used if a significant diet by condition effect was found. Reliability was assessed using day 7 and day 8 fingerstick δ13C values, within each diet condition, using intra-class correlation (ICC). Paired sample t-tests were used to determine whether baseline fingerstick δ13C values were different from day 1 fingerstick δ13C value on the LAS and HAS diets. Paired sample t-tests were also used to determine if demographic and dietary intake data differed between the baseline and washout periods, and whether dietary variables differed between self-reported intake and controlled diets. All statistical tests were set with an a priori significance of α = 0.05. One participant was determined to be 88% compliant (based upon returned food) during the LAS controlled diet, and analyses were conducted with and without this participant. No differences were found; therefore, biomarker analyses with the full sample are presented.
Results
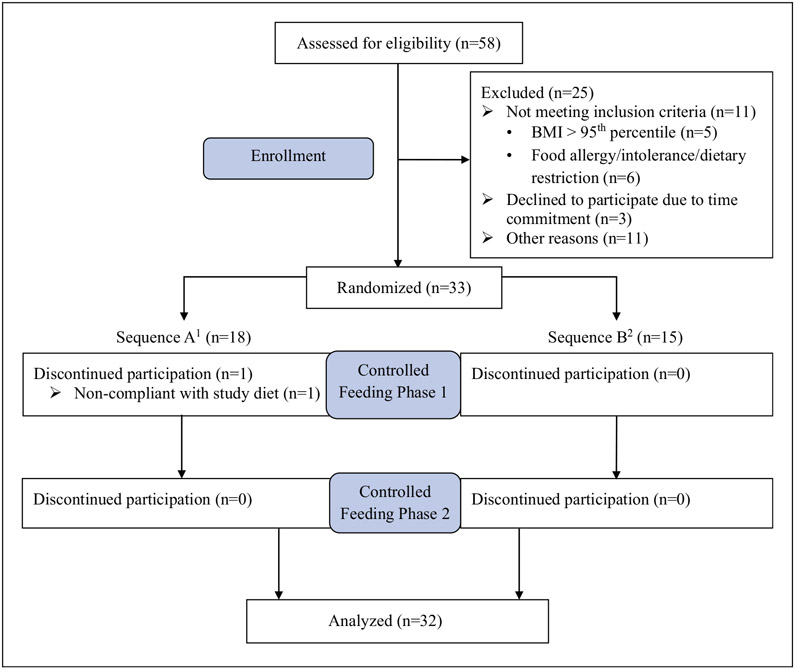
As depicted in Figure 2, 58 participants were screened for eligibility. Of these, 25 failed to meet eligibility criteria (n = 11), declined to participate (n = 3), or were excluded for other reasons, e.g. failing to keep scheduled appointments (n = 11). Additionally, one participant discontinued the study after non-compliance to the controlled diet. Thus, 32 adolescents completed the controlled feeding study (97% completion) (see Figure 2). Participant demographic characteristics, anthropometric measurements, activity level and estimated energy requirements are provided in Table 2. The sample was primarily non-Hispanic White (97%) and female (53%); mean age was 15.3 years. BMI percentile was in the normal weight range (2), and physical activity level was reported as “low active” (37). Estimated daily energy needs were 2903 kcal/day, which ranged from 1938 kcal/day to 4745 kcal/day.
Figure 2.
Consolidated Standards of Reporting Trials (CONSORT) diagram. 1Sequence A participants were assigned the 5%, LAS diet first, then the 25%, HAS diet. 2Sequence B participants were assigned the 25%, HAS diet first, then the 5%, LAS diet.
Table 2.
Baseline demographic characteristics of the study sample (n = 32).
| Mean ± SD | na (%)b | |
|---|---|---|
| Sex | ||
| Female, n | – | 17 (53) |
| Male, n | – | 15 (47) |
| Race/ethnicity | ||
| Non-Hispanic White, n | – | 31 (97) |
| Non-Hispanic Black, n | – | – |
| Hispanic, n | – | – |
| Other/unknown, n | – | 1 (3) |
| Age, years | 15.3 ± 1.6 | – |
| Education | ||
| Middle school (grades 6 – 8) | – | 10 (31) |
| High school (grades 9 – 12) | – | 22 (69) |
| Height, cm | 167.5 ± 9.6 | – |
| Weight, kg | 57.2 ± 10.2 | – |
| BMI, kg/m2 | 20.2 ± 2.1 | – |
| BMI percentile, % | 47.0 ± 25.1 | – |
| PAQ-A3 score | 2.1 ± 0.6 | – |
| EEN3, kcal/day | 2903 ± 715 | – |
SD: standard deviation; BMI: dody mass index percentile (Centers for Disease Control and Prevention, 2015); PAQ-A: Physical Activity Questionnaire for Adolescents(Kowalski et al., 2004), where scores range from 1 to 5 with 5 indicating “high physical activity”; EEN: Estimated energy needs, calculated using IOM age- and sex-specific equations(Food and Nutrition Board and Institute of Medicine, 2005) plus PAQ-A to determine physical activity level.
The number of participants, as an absolute value, in the sample that have the specified demographic characteristic.
The number of participants, as a percentage, in the sample that have the specified demographic characteristic.
Anthropometric measurements, activity level and energy requirements were re-assessed during the washout period. Weight, PAQ-A score, and estimated energy needs did not significantly differ compared with baseline values (reported in Table 2, p > 0.05 for all). However, height increased by 0.4 ± 0.7 cm, from baseline to the washout period (p ≤ 0.001). Self-reported dietary intake at baseline and washout is provided in Table 3. There were no significant differences in total energy, macronutrient (as g/day and % of kcal/day), total sugars, AS (as g/day and % of kcal/day), or sodium intake between baseline and washout periods (all p > 0.05).
Table 3.
Dietary characteristics of self-reported diet at baseline and washout, and on the LAS and HAS controlled diets (n = 32).
| Baselinea | Washouta | LAS dietb | HAS dietb | |
|---|---|---|---|---|
| Calories, kcal/day | 2519 ± 577 | 2475 ± 605 | 2763 ± 624 | 2755 ± 617 |
| Total fat, g/day | 98.5 ± 26.9 | 94.1 ± 23.9 | 92.4 ± 20.9 | 92.4 ± 20.9 |
| Fat, % | 34.4 ± 3.3 | 33.8 ± 4.4 | 30.3 ± 0.2 | 29.9 ± 0.2 |
| Total carbohydrate, g/day | 317.3 ± 69.3 | 312.8 ± 91.5 | 377.6 ± 85.8* | 378.6 ± 85.5* |
| Carbohydrate, % | 50.8 ± 3.6 | 50.3 ± 4.9 | 54.0 ± 0.2 | 54.9 ± 0.1 |
| Total protein, g/day | 91.1 ± 26.0 | 94.4 ± 28.4 | 105.3 ± 23.3* | 102.1 ± 22.0* |
| Protein, % | 14.9 ± 2.6 | 15.8 ± 3.3 | 15.7 ± 0.1 | 15.2 ± 0.2 |
| Total sugars, g/day | 127.3 ± 33.1 | 133.5 ± 48.1 | 158.2 ± 38.0* | 214.0 ± 50.4* |
| Added sugars, g/day | 76.0 ± 25.6 | 77.2 ± 35.7 | 34.5 ± 7.9* | 171.9 ± 38.7* |
| Added sugars, % | 12.2 ± 3.3 | 12.4 ± 4.4 | 5.0 ± 0.0* | 25.0 ± 0.1* |
| Sodium, mg/day | 3820 ± 1123 | 3757 ± 1310 | 3551 ± 691 | ± 724 |
HAS: high added sugar controlled diet; LAS: low added sugar controlled diet.
Self-reported derived from four 24-h recalls, mean ± SD.
Provided to participants during the controlled diet period, mean ± SD.
Significant difference from baseline, p ≤ 0.05.
Controlled feeding periods and dietary compliance
Estimated energy requirements were ~383 kcal/day higher than baseline self-reported energy intake (p ≤ 0.001). Weight stability and returned food were used to assess compliance to the controlled feeding periods. Weight did not significantly differ from day 1 to day 8 on either the LAS (p = 0.613) or HAS (p = 0.879) diet. Based on returned food, compliance was 98.5 ± 0.01% to the LAS diet and 97.9 ± 0.02% to the HAS diet. Three participants each reported consuming non-study foods on one day of the two seven-day controlled diet periods. Of these, one occurred during the LAS diet (one slice of cake and a sweetened sports drink), which resulted in the participant having to repeat the seven-day LAS diet period. Two occurred during the HAS diet (three breath mints (10 kcal, 0 g AS]; one s’more (126 kcal, 4 g AS)) which did not warrant repeating the seven-day controlled diet due to their minimal impact on that day’s overall AS intake.
δ13C biomarker: sensitivity and reliability
Fingerstick δ13C values from baseline and during the controlled feeding periods are reported in Table 4. There was no significant difference between group mean fingerstick δ13C values from baseline and day 1 fingerstick δ13C values on the LAS diet (p = 0.110) or the HAS diet (p = 0.330), nor was there a difference between the group mean day 1 fingerstick δ13C values of the LAS and HAS diets (p = 0.052). Additionally, no difference in results occurred according to order, or sequence, in which participants completed the controlled feeding periods.
Table 4.
Fingerstick δ13C values at baseline and on the LAS and HAS controlled diets (n = 32).
| Baseline 1, ‰ | −19.72 ± 0.50a | |
| Baseline 2, ‰ | −19.72 ± 0.50 | |
| Average baselineb, ‰ | −19.72 ± 0.50 | |
| LAS feeding period | HAS feeding period | |
|---|---|---|
| Day 1, ‰ | −19.70 ± 0.49 | −19.74 ± 0.51 |
| Day 2, ‰ | −19.70 ± 0.49 | −19.66 ± 0.56 |
| Day 3, ‰ | −19.71 ± 0.50 | −19.73 ± 0.50 |
| Day 4, ‰ | −19.71 ± 0.49 | −19.71 ± 0.50 |
| Day 5, ‰ | −19.73 ± 0.48 | −19.74 ± 0.50 |
| Day 6, ‰ | −19.72 ± 0.47 | −19.71 ± 0.50 |
| Day 7, ‰ | −19.75 ± 0.46 | −19.71 ± 0.50 |
| Day 8, ‰ | −19.75 ± 0.46* | −19.71 ± 0.49* |
HAS: high added sugar controlled diet; LAS: low added sugar controlled diet.
Values reported as mean ± standard deviation.
Mean of baseline 1 and baseline 2 fingerstick δ 13C values.
Significant difference from day 1 of the respective controlled diet, p ≤ 0.05.
A significant diet × time effect was noted between day 1 and day 8 δ13C values (p ≤ 0.001). Average fingerstick δ13C values decreased on the LAS diet and increased on the HAS diet, which were significantly different on days 1 and 8 of both feeding periods. There was a significant mean decrease between day 1 and day 8 group mean δ13C values of 0.05 ± 0.071‰ on the LAS diet (p ≤ 0.001). On the HAS diet, group mean day 8 δ13C value was 0.03 ± 0.083‰ higher compared with day 1 δ13C value (p = 0.038). Effect size was calculated as η2p = 0.39.
A high degree of reliability was found between day 7 and day 8 δ13C values on the LAS diet, with an ICC of 0.996 (95% confidence interval, 0.993 to 0.998, p ≤ 0.001). Similarly, a high degree of reliability was also found between day 7 and day 8 δ13C values on the HAS diet, with an ICC of 0.997 (95% confidence interval, 0.993 to 0.998, p ≤ 0.001).
Discussion
This investigation represents the first evaluation of the proposed δ13C added sugar biomarker in adolescents using a controlled feeding design. These results indicate that the biomarker changed in response to short-term changes in added sugar intake, using low and high added sugar intake levels. Importantly, the sugar intake levels investigated represent the recommended AS intake and the AS intake level that has been associated with nutrient inadequacy (Food and Nutrition Board and Institute of Medicine, 2006; World Health Organization, 2015). However, the group mean change in fingerstick δ13C exceeded measurement error (± 0.046‰) only for the LAS diet in the full sample (n = 32). With a longer controlled feeding period, δ13C values may continue to change until they reach a steady-state value, estimated to occur at approximately 3–4 weeks (Davy and Jahren, 2016; Jahren et al., 2014). The current study’s results differed from that obtained in the controlled feeding study conducted by Cook et al. (2010), which reported no change in fasting plasma glucose δ13C values after a seven-day controlled feeding period in adults. Consistent with previously reported findings by Nash et al. (2013), test–retest reliability was demonstrated.
A nutritional biomarker should demonstrate validity, reliability, and sensitivity to dietary intake (Hedrick et al., 2012). Previous studies suggest that blood δ13C values are a valid and reliable biomarker of AS intake when compared with self-reported intake in adults (Davy et al., 2011; Fakhouri et al., 2014; Yeung et al., 2010). Fakhouri et al. (2014) reported that for every 12 fl oz/day decrease in SSB consumption, serum δ13C values also decreased by 0.12‰ within a 18-month period, although this was based on self-reported changes in AS intake. Cook et al. (2010) reported that the δ13C value of plasma glucose was sensitive to AS intake in a previous meal but did not change in response to overall AS content of diet within a seven-day feeding period. However, these previous studies (except for Cook et al., 2010) may have underestimated the strength of associations of AS (or SSB) intake with the proposed δ13C biomarker, due to the dietary under-reporting that occurs in both adults and youth (Kuhnle, 2012; Lioret et al., 2011; MacDougall et al., 2018; Rangan et al., 2014; Thompson et al., 2010; Ventura et al., 2006).
Another proposed biomarker of sugar intake is the 24-h urinary sucrose/fructose excretion biomarker. Although urinary sugars excretion has been validated as a biomarker for total sugars intake against both self-reported dietary intake and in controlled feeding studies (Tasevska, 2015), its lack of specificity for AS is a limitation. In pre-pubertal children, urinary sugars had a higher association with natural sugar intake than with AS intake (Johner et al., 2010). The reliability of fingerstick δ13C values under controlled diet conditions in this study was higher than that of the 24-h urinary sucrose/fructose biomarker, which had an ICC of 0.67 in a controlled feeding study that included adults (Tasevska et al., 2005). Finally, although 24-h urine collections are a minimally invasive sampling method, like fingerstick blood sampling, the urinary sugars biomarker’s sensitivity to dietary changes requires multiple 24-h urine collections, which introduces challenges with participant burden and compliance (Davy and Jahren, 2016).
This investigation had several strengths. This investigation was the first controlled feeding study to evaluate the fingerstick δ13C biomarker. The minimally invasive fingerstick blood sampling method (Davy and Jahren, 2016; Jahren et al., 2014) could feasibly be utilized in field research settings, and in large epidemiological trials. The target study population is significant, in that adolescents are the highest consumers of AS and among the highest consumers of SSB (Guthrie and Morton, 2000). Finally, compliance to the controlled diet (~98%) and completion rate were high (32 of 33 participants).
We acknowledge several limitations. The sample size was small, plus lacked racial and ethnic diversity. The feeding periods were limited to two seven-day periods, and biomarker values may not have yet reached a steady state in response to the different AS intake levels. Some foods contained sweeteners that may have been derived from C3 plants (e.g. beet sugar, honey, maple syrup) that were included in AS calculations. Since the δ13C biomarker can only assess AS intake from C4 plants, AS intake from C3 sources would not have been detected (Jahren et al., 2014). However, only 8 of 71 food items used for the study diets contained C3 sources of sugar. Of those, three food items contained ≤2% of a C3 sweetener, according to ingredient lists. Also, variation of δ13C within plant matter may be as high as 5‰ (DeNiro and Epstein, 1978); it is possible that participants could have received diets with different δ13C content based on the time of the year in which they were enrolled. However, future studies could assess δ13C content of foods provided in a controlled diet. Despite these limitations, these findings may be used to justify a larger-scale feeding trial, with longer controlled diet periods.
In conclusion, group mean changes in fingerstick δ13C values changed significantly in response to known dietary AS intake within seven days, in adolescents. As with other dietary biomarkers (Brown et al., 2013), this technique may be most appropriately applied to population-level research investigating dietary intake and health outcomes. Once portable mass spectrometer technology developments are refined (Zare et al., 2009), the fingerstick δ13C biomarker could be a rapid, minimally invasive objective measure of AS intake in large-scale nutrition epidemiological studies.
Supplementary Material
Acknowledgements
William Hagopian is acknowledged for his technical support in analyzing the blood samples for δ13C via NA-SIMS technology. Several undergraduate research assistants in the Department of Human Nutrition, Foods, and Exercise are appreciated for their work in preparing daily meals for participants.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health (NIH) R21HD07863601.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Consent for publication
All co-authors consent to submit the article for publication and each has signed the Contributor Agreement.
Ethical approval
All study procedures were approved by the Virginia Tech Institutional Review Board in accordance with the Declaration of Helsinki. Participants and their parents provided written informed assent and consent before beginning the study. This study was registered at clinicaltrials.gov as NCT02455388 on 05/27/2015.
Supplemental material
Supplemental material for this article is available online.
References
- Ambrosini G, Oddy W, Huang R, et al. (2013) Prospective associations between sugar-sweetened beverage intakes and cardiometabolic risk factors in adolescents. American Journal of Clinical Nutrition 98(2): 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Dietetic Association (1999) Well-Controlled Diet Studies in Humans: A Practical Guide to Design and Management. Madison, WI: University of Wisconsin. [Google Scholar]
- Bel-Serrat S, Julián-Almárcegui C, González-Gross M, et al. (2016) Correlates of dietary energy misreporting among European adolescents: the Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study. British Journal of Nutrition 115(8): 1439–1452. [DOI] [PubMed] [Google Scholar]
- Bermudez O and Gao X (2010) Greater consumption of sweetened beverages and added sugars is associated with obesity among US young adults. Annals of Nutrition & Metabolism 57(3–4): 211–218. [DOI] [PubMed] [Google Scholar]
- Brown I, Dyer A, Chan Q, et al. (2013) Estimating 24-hour urinary sodium excretion from casual urinary sodium concentrations in Western populations: The INTERSALT study. American Journal of Epidemiology 177(11): 1180–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2015) About child & teen BMI. Available at: https://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html (accessed xx month xxxx). [Google Scholar]
- Chi D, Hopkins S, O’Brien D, et al. (2015) Association between added sugar intake and dental caries in Yup’ik children using a novel hair biomarker. BMC Oral Health 15: 1–8. Available at: https://bmcoralhealth.biomedcentral.com/articles/10.1186/s12903-015-0101-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy K, Nash S, Kristal A, et al. (2013) The carbon isotope ratio of alanine in red blood cells is a new candidate biomarker of sugar-sweetened beverage intake. The Journal of Nutrition 143(6): 878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C, Alvig A, Liu Y, et al. (2010) The natural 13C abundance of plasma glucose is a useful biomarker of recent dietary caloric sweetener intake. The Journal of Nutrition 140(2): 333–337. [DOI] [PubMed] [Google Scholar]
- Davy B and Jahren A. (2016) New markers of dietary added sugar intake. Current Opinion in Clinical Nutrition and Metabolic Care 19(4): 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy B, Jahren A, Hedrick V, et al. (2011) Association of d13C in fingerstick blood with added sugars and sugar-sweetened beverage intake. Journal of the American Dietetic Association 111(6): 874–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNiro M and Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochimica et Cosmochimica Acta 42(5): 495–506. [Google Scholar]
- Ervin R, Kit B, Carroll M, et al. (2012) Consumption of added sugar among US children and adolescents, 2005–2008. Report for the National Center for Health Statistics, US Department of Health and Human Services. Report no. 87, March. Hyattsville, MD. Available at: https://www.cdc.gov/nchs/data/databriefs/db87.pdf [Google Scholar]
- Ervin R and Ogden C (2013) Consumption of added sugars among US adults, 2005-2010. Report for the National Center for Health Statistics, US Department of Health and Human Services. Report no. 122, May. Hyattsville, MD. [Google Scholar]
- Fakhouri T, Jahren A, Appel L, et al. (2014) Serum carbon isotope values change in adults in response to changes in sugar-sweetened beverage intake. The Journal of Nutrition 144(6): 902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Nutrition Board and Institute of Medicine (2005) Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington, DC: National Academies Press, pp.177–182. [Google Scholar]
- Food and Nutrition Board and Institute of Medicine (2006) Dietary reference intakes: The essential guide to nutrient requirements. Washington, DC: National Academies Press, 70, pp.103–104. [Google Scholar]
- Fry B and Arnold C (1982) Rapid 13C/12C turnover during growth of brown shrimp (Penaeus aztecus). Oecologia 54(2): 200–204. [DOI] [PubMed] [Google Scholar]
- Guthrie J and Morton J (2000) Food sources of added sweeteners in the diets of Americans. Journal of the American Dietetic Association 100(1): 43–51. [DOI] [PubMed] [Google Scholar]
- Gyllenhammer L, Weigensberg M, Spruijt-Metz D, et al. (2014) Modifying influence of dietary sugar in the relationship between cortisol and visceral adipose tissue in minority youth. Obesity 22(2): 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D and Most M (2005) Dietary adherence in well-controlled feeding studies. Journal of the American Dietetic Association 105(8): 1285–1288. [DOI] [PubMed] [Google Scholar]
- Hedrick VE, Dietrich AM, Estabrooks PA, et al. (2012) Dietary biomarkers: advances, limitations and future directions. Nutrition Journal 11(1): 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahren A, Bostic J and Davy B (2014) The potential for a carbon stable isotope biomarker of dietary sugar intake. Journal of Analytical Atomic Spectrometry 29(5): 795–816. [Google Scholar]
- Johner S, Libuda L, Shi L, et al. (2010) Urinary fructose: a potential biomarker for dietary fructose intake in children. European Journal of Clinical Nutrition 64(11): 1365–1370. [DOI] [PubMed] [Google Scholar]
- Johnson R, Appel L, Brands M, et al. (2009) Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 120(11): 1011–1020. [DOI] [PubMed] [Google Scholar]
- Kowalski K, Crocker P and Donen R (2004) The Physical Activity Questionnaire for Older Children (PAQ-C) and Adolescents (PAQ-A) Manual. Saskatoon, Canada: University of Saskatchewan. [Google Scholar]
- Kuhnle G (2012) Nutritional biomarkers for objective dietary assessment. Journal of the Science of Food and Agriculture 92(6): 1145–1149. [DOI] [PubMed] [Google Scholar]
- Lim S, Zoellner J, Lee J, et al. (2009) Obesity and sugar-sweetened beverages in African-American preschool children: a longitudinal study. Obesity 17(6): 1262–1268. [DOI] [PubMed] [Google Scholar]
- Lioret S, Touvier M, Balin M, et al. (2011) Characteristics of energy under-reporting in children and adolescents. British Journal of Nutrition 105(11): 1671–1680. [DOI] [PubMed] [Google Scholar]
- Liu S, Moore L, Halliday T, et al. (2016) Validation of a method to predict total daily energy needs in non-obese adolescents. Journal of the Academy of Nutrition and Dietetics 116(Suppl 9): A62. [Google Scholar]
- MacAvoy S, Arneson L and Bassett E (2006) Correlation of metabolism with tissue carbon and nitrogen turnover rate in small mammals. Oecologia 150(2): 190–201. [DOI] [PubMed] [Google Scholar]
- MacDougall C, Hill C, Jahren A, et al. (2018) The δ13C value of fingerstick blood is a valid, reliable, and sensitive biomarker of sugar-sweetened beverage intake in children and adolescents. The Journal of Nutrition 148(1): 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik V, Pan A, Willett W, et al. (2013) Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. American Journal of Clinical Nutrition 98(4): 1084–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K and Livingstone M (2016) Prevalence and characteristics of misreporting of energy intake in US children and adolescents: National Health and Nutrition Examination Survey (NHANES) 2003–2012. British Journal of Nutrition 115(2): 294–304. [DOI] [PubMed] [Google Scholar]
- Nash S, Kristal A, Bersamin A, et al. (2013) Carbon and nitrogen stable isotope ratios predict intake of sweeteners in a Yup’ik study population. The Journal of Nutrition 143(2): 161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash S, Kristal A, Hopkins S, et al. (2014) Stable isotope models of sugar intake using hair, red blood cells, and plasma, but not fasting plasma glucose, predict sugar intake in a Yup’ik study population. The Journal of Nutrition 144(1): 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangan A, Allman-Farinelli M, Donohoe E, et al. (2014) Misreporting of energy intake in the 2007 Australian Children’s Survey: differences in the reporting of food types between plausible, under- and over-reporters of energy intake. Journal of Human Nutrition and Dietetics 27(5): 450–458. [DOI] [PubMed] [Google Scholar]
- Raper N, Perloff B, Ingwersen L, et al. (2004) An overview of USDA’s dietary intake data system. Journal of Food Composition and Analysis 17(3–4): 545–555. [Google Scholar]
- Rogol A, Clark P and Roemmich J (2000) Growth and pubertal development in children and adolescents: effects of diet and physical activity. American Journal of Clinical Nutrition 72(2): 521S–528S. [DOI] [PubMed] [Google Scholar]
- Smit E, Nieto F, Crespo C, et al. (1999) Estimates of animal and plant protein intake in US adults: results from the third National Health and Nutrition Examination Survey, 1988-1991. Journal of the American Dietetic Association 99(7): 813–820. [DOI] [PubMed] [Google Scholar]
- Smith A, Fildes A, Forwood S, et al. (2017) The individual environment, not the family is the most important influence on preferences for common non-alcoholic beverages in adolescence. Scientific Reports 7(1): 16822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasevska N (2015) Urinary sugars—a biomarker of total sugars intake. Nutrients 7(7): 5816–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasevska N, Runswick S, McTaggart A, et al. (2005) Urinary sucrose and fructose as biomarkers for sugar consumption. Cancer Epidemiology, Biomarkers & Prevention 14(5): 1287–1294. [DOI] [PubMed] [Google Scholar]
- Thompson F and Subar A (2013) Dietary assessment methodology. In: Coulston A, Boushey C and Ferruzzi M (eds) Nutrition in the Prevention and Treatment of Disease. 3rd ed. London: Elsevier, pp.5–46. [Google Scholar]
- Thompson F, Subar A, Loria C, et al. (2010) Need for technological innovation in dietary assessment. Journal of the American Dietetic Association 110(1): 48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieszen L, Boutton T, Tesdahl K, et al. (1982) Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia 57(1–2): 32–37. [DOI] [PubMed] [Google Scholar]
- US Department of Agriculture (2015) 2015–2020 dietary guidelines for Americans, 8th ed. Hyattsville, MD: US Department of Health and Human Services. [Google Scholar]
- Ventura A, Loken E, Mitchell D, et al. (2006) Understanding reporting bias in the dietary recall data of 11-year-old girls. Obesity 14(6): 1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos M, Kaar J, Welsh J, et al. (2016) Added sugars and cardiovascular disease risk in children: a scientific statement from the American Heart Association. Circulation 134(23): 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shang L, Light K, et al. (2015) Associations between added sugar (solid vs. liquid) intakes, diet quality, and adiposity indicators in Canadian children. Applied Physiology, Nutrition, and Metabolism 40(8): 835–841. [DOI] [PubMed] [Google Scholar]
- Watowicz R, Anderson S, Kaye G, et al. (2015) Energy contribution of beverages in US children by age, weight, and consumer status. Childhood Obesity 11(4): 475–483. [DOI] [PubMed] [Google Scholar]
- Welsh J, Sharma A, Grellinger L, et al. (2011) Consumption of added sugars is decreasing in the United States. American Journal of Clinical Nutrition 94(3): 726–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2015) Sugars intake for adults and children. Available at: http://www.who.int/nutrition/publications/guidelines/sugars_intake/en/ (accessed 25 April 2017).
- Yeung E, Saudek C, Jahren A, et al. (2010) Evaluation of a novel isotope biomarker for dietary consumption of sweets. American Journal of Epidemiology 172(9): 1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare R, Kuramoto D, Haase C, et al. (2009) High-precision optical measurements of 13C/12C isotope ratios in organic compounds at natural abundance. Proceedings of the National Academy of Sciences of the United States of America 106(27): 10928–10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.