Abstract
Clostridium perfringens iota-toxin is a binary toxin consisting of iota a (Ia), an ADP-ribosyltransferase that modifies actin, and iota b (Ib), which binds to a cell surface protein and translocates Ia into a target cell. Fusion proteins of recombinant Ib and truncated variants were tested for binding to Vero cells and docking with Ia via fluorescence-activated cytometry and cytotoxicity experiments. C-terminal residues (656 to 665) of Ib were critical for cell surface binding, and truncated Ib variants containing ≥200 amino acids of the C terminus were effective Ib competitors and prevented iota cytotoxicity. The N-terminal domain (residues 1 to 106) of Ib was important for Ia docking, yet this region was not an effective competitor of iota cytotoxicity. Further studies showed that Ib lacking just the N-terminal 27 residues did not facilitate Ia entry into a target cell and subsequent cytotoxicity. Five monoclonal antibodies against Ib were also tested with each truncated Ib variant for epitope and structural mapping by surface plasmon resonance and an enzyme-linked immunosorbent assay. Each antibody bound to a linear epitope within the N terminus (residues 28 to 66) or the C terminus (residues 632 to 655). Antibodies that target the C terminus neutralized in vitro cytotoxicity and delayed the lethal effects of iota-toxin in mice.
Clostridium perfringens type E produces iota-toxin, a binary toxin that consists of two nonlinked proteins implicated in sporadic diarrheic outbreaks among animals (6, 28, 32). The 47-kDa enzymatic component, designated iota a (Ia), docks with and is translocated by the 80-kDa iota b (Ib) into a target cell. Iota-toxin belongs to an A-B toxin family possessing nonlinked proteins like Clostridium botulinum C2 toxin (12), Clostridium spiroforme iota-like toxin (23, 29), anthrax toxin from Bacillus anthracis (14), and the vegetative insecticidal protein (VIP) produced by Bacillus cereus (11).
The current understanding of iota-toxin is mainly centered around the Ia molecule (16, 18, 34, 35), while the structure-function relationship of Ib in iota toxicity is poorly understood. Like many clostridial toxins, Ia and Ib are activated by proteases (10). The protoxin form of Ib contains a 20-kDa amino-terminal peptide released after proteolysis. Upon binding to a protein receptor (30), activated Ib docks with Ia and facilitates entry of Ia into a cell via receptor-mediated endocytosis (23). Once inside a cell, an Ia molecule ADP-ribosylates monomeric actin and consequently disrupts formation of actin filaments necessary for the cytoskeleton, with resultant cell rounding and death (18, 35). Either Ia or Ib functionally complements the serologically related iota-like toxin proteins (Sa and Sb) produced by C. spiroforme (29). There is no biological synergy of either iota or iota-like components with other A-B proteins from anthrax (20) or C2 (22, 24, 25) toxins. However, there is a 34 and 41% sequence homology between Ib and the cell-binding proteins of anthrax toxin (protective antigen; PA) and C2 toxin (component II; C2II), respectively, which strongly suggests structural and functional relatedness among these binary toxins (13, 19, 21).
In the present study, we structurally and functionally mapped the cell-binding and Ia docking domains on Ib. This was accomplished by testing recombinantly truncated variants of Ib and monoclonal antibodies with various techniques, including fluorescence-activated cytometry, surface plasmon resonance, and cytotoxicity assays.
MATERIALS AND METHODS
Recombinant Ib and truncated Ib variants.
Recombinant Ib and truncated Ib variant genes were PCR amplified from C. perfringens type E (NCIB 10748) using the primers listed in Table 1. Amplification products were directly cloned into a pCR2.1 plasmid. After digestion with NcoI and SalI, inserts were gel purified, ligated into a pET32a expression plasmid, and then used to transform Escherichia coli BL21(DE3) for producing the corresponding recombinant proteins (1 to 2 mg/liter). Fusion proteins containing thioredoxin plus a histidine tag were purified by immobilized metal affinity chromatography (Clontech, Palo Alto, Calif.), and homogeneity was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Coomassie blue-stained gels) and Western blot analysis with monospecific rabbit antisera towards Ib. All recombinant proteins were ≥80% pure as determined by standard gels. The thioredoxin-histidine tag was removed from a fusion construct (100 μg) by 15 U of porcine enterokinase (Sigma, St. Louis, Mo.) incubated together overnight at room temperature in 20 mM phosphate buffer, pH 5.3. Additionally, wild-type Ia and Ib were purified from C. perfringens as previously described by Gibert et al. (10). All protein concentrations were determined by a bicinchoninic acid assay (Pierce, Rockford, Ill.).
TABLE 1.
Primers for recombinant Ib and truncated variants
| Ib variant | 5′ Primer | 3′ Primer |
|---|---|---|
| 1-665 | 5′-CCATGGCAGCCTGGGAAGATGAAG | 5′-GTCGACTTAACACTAAGCACTAATACCTC |
| 28-665 | 5′-CCATGGATTCAATTGCAGTAAAATGGA | 5′-GTCGACTTAACACTAAGCACTAATACCTC |
| 107-665 | 5′-CCATGGATCAAGGAAAAACAGTTTCTAGG | 5′-GTCGACTTAACACTAAGCACTAATACCTC |
| 224-665 | 5′-CCATGGCTCCAAATGAAACATATCCTAAAAAAGG | 5′-GTCGACTTAACACTAAGCACTAATACCTC |
| 358-665 | 5′-CCATGGATTCCAATTGATGAGAGC | 5′-GTCGACTTAACACTAAGCACTAATACCTC |
| 466-665 | 5′-CCATGGGATCCTTCAACTTCTAATTCAATAAC | 5′-GTCGACTTAACACTAAGCACTAATACCTC |
| 558-665 | 5′-CCATGGATTATGCAGATATAAAGCTTGACAC | 5′-GTCGACTTAACACTAAGCACTAATACCTC |
| 1-66 | 5′-CCATGGCAGCCTGGGAAGATGAAG | 5′-GTCGACTTTTTGATAATCTGTATATGG |
| 1-323 | 5′-CCATGGCAGCCTGGGAAGATGAAG | 5′-GTCGACCTCTTTAGCAGCCACACT |
| 1-466 | 5′-CCATGGCAGCCTGGGAAGATGAAG | 5′-GTCGACCTTTGAATATCCACTAAAAACATAGCG |
| 1-631 | 5′-CCATGGCAGCCTGGGAAGATGAAG | 5′-GTCGACAAAATTAATATAATTAGTTTTAGGTTGG |
| 1-655 | 5′-CCATGGCAGCCTGGGAAGATGAAG | 5′-GTCGACATCAGGTGTAACTGCATATATTC |
MAbs against Ib.
Monoclonal antibodies (MAbs) were developed from a BALB/c mouse (National Cancer Institute, Frederick, Md.) vaccinated twice intraperitoneally with purified wild-type Ib (10 μg/dose) in aluminum hydroxide (Pierce). Spleen cells were fused with SP2/0-Ag 14 myelomas, and subsequent hybridomas were grown, screened for Ib-specific antibody, and subcloned as described previously (31). Volumes of each MAb were obtained by collecting culture fluid from hybridomas grown in vitro (Integra Biosciences, Ijamsville, Md.). MAbs were subsequently purified via protein G column chromatography (Amersham Pharmacia, Uppsala, Sweden), and homogeneity was ascertained by SDS-PAGE. Each antibody was isotyped with an enzyme-linked immunosorbent assay (ELISA) from Bio-Rad (Hercules, Calif.) and surface plasmon resonance (described below). Protein concentrations were determined by a bicinchoninic acid assay with purified bovine gamma globulin as a standard.
Cell-binding studies.
Fluorescence-activated cytometry was done with recombinant Ib and truncated Ib variants as previously described by Stiles et al. (30). Additional experiments determined if each MAb (50 μg/ml) detected cell-bound Ib (10 μg/ml) or prevented Ib binding to the Vero cell surface. A 1:100 dilution of mouse sera (normal and Ib specific) was used as controls for both sets of experiments. Detection experiments were done with Ib and truncated variants incubated with Vero cells at 37°C for 10 min. Cells were immediately washed in ice-cold Hanks balanced salts solution plus 0.2% bovine serum albumin and then incubated with MAb at 4°C for 1 h. After washing, cells were counterstained with a fluorescein isothiocyanate conjugate of goat anti-mouse immunoglobulin G (IgG) (Accurate Chemical, Westbury, N.Y.).
To determine if any MAb prevented Ib binding to the cell surface, MAb (50 μg/ml) was preincubated with Ib (10 μg/ml) for 30 min at room temperature before being added to cells (10 min at 37°C). Cells were washed with Hanks balanced salts solution plus bovine serum albumin and Ib was detected with rabbit anti-Ib sera as described previously. Geometric means of the relative fluorescence units (RFU) were considered positive if they were at least twofold greater than the mean RFU from negative-control cells not incubated with Ia or Ib but with all other reagents.
Cytotoxicity assays.
Each truncated Ib variant (10 μM) was initially mixed with Ia (10 μM) to determine the cytotoxic effects on Vero cells incubated at 37°C in 96-well plates, as described previously (30). Cytotoxic effects were recorded as a percentage of rounded cells after 18 h, and 100% cytotoxicity was evidenced by 100% cell rounding. Competition studies were also done with Ib (2 nM) mixed with increasing concentrations of a truncated Ib variant (molar ratio ranging from 1:1 to 1:2,056) and 2 nM Ia. This iota-toxin cocktail was then added to Vero cells incubated at 37°C and scored 18 h later. All cytotoxicity assays were done twice.
Epitope mapping of Ib with MAbs.
Mapping studies were initially done by surface plasmon resonance with a BIAcore 1000 (Piscataway, N.J.) and rabbit anti-mouse IgG Fc antibody (RAM Fc) (BIAcore, Uppsala, Sweden) amine coupled to a standard CM5 sensor chip. Twenty microliters of culture filtrate containing MAb was applied to the RAM Fc surface followed by 20 μl of a truncated Ib variant (70 μg/ml) or, as a control for nonspecific binding, HEPES-buffered saline containing 3.4 mM EDTA and 0.005% P20 surfactant (HBS-EP). Results were given as response units (RU) representing a direct measure of protein deposited on the sensor chip (∼1 pg/mm2 = 1 RU). All results were confirmed by an ELISA with truncated Ib variants (20 μg/ml) adsorbed onto Immulon II microtiter plates (Dynatech, Chantilly, Va.) and detected with purified MAb (10 μg/ml) according to the method of Stiles et al. (31).
To determine if identical epitopes were recognized by each MAb, 20 μl of a purified MAb (100 μg/ml) was initially applied to the RAM Fc surface. Unbound RAM Fc sites were blocked with 50 μl of naive mouse immunoglobulins. Twenty microliters of Ib (80 μg/ml) was added to the initially immobilized MAb, followed by 20 μl of a second MAb. Sensor chip surfaces were regenerated with 10 mM glycine, pH 1.5. These BIAcore results were confirmed by a competition ELISA, with MAb (10 μg/ml) in carbonate buffer initially adsorbed onto wells overnight at 4°C. After blocking, Ib (1 μg/ml) plus a competitive MAb (100 μg/ml) was added to wells for 1 h at 37°C. Wells were then washed, and rabbit anti-Ib serum was added for 1 h at 37°C, followed by goat anti-rabbit conjugate (Sigma) and finally substrate.
In addition to the epitope-mapping experiments, each MAb was also isotyped by BIAcore with hybridoma culture fluid and a mouse IgG subclass kit (BIAcore), as recommended by the manufacturer.
In vitro and in vivo neutralization of iota-toxin.
Dilutions of each MAb (1.50 μM to 1.0 nM) were incubated with 2 nM Ia and wild-type Ib (1 h at 37°C) and then added to Vero cells. Viability was scored as a percentage of unrounded cells after 18 h.
In vivo studies were done by premixing 12.5 pmol of Ia and Ib with 1 nmol of MAb for 1 h at 37°C. BALB/c mice were then injected intraperitoneally (n = 3 per tested antibody) with this mixture, and time to death was recorded over 26 h.
RESULTS
Cloning, production, and characterization of truncated Ib variants.
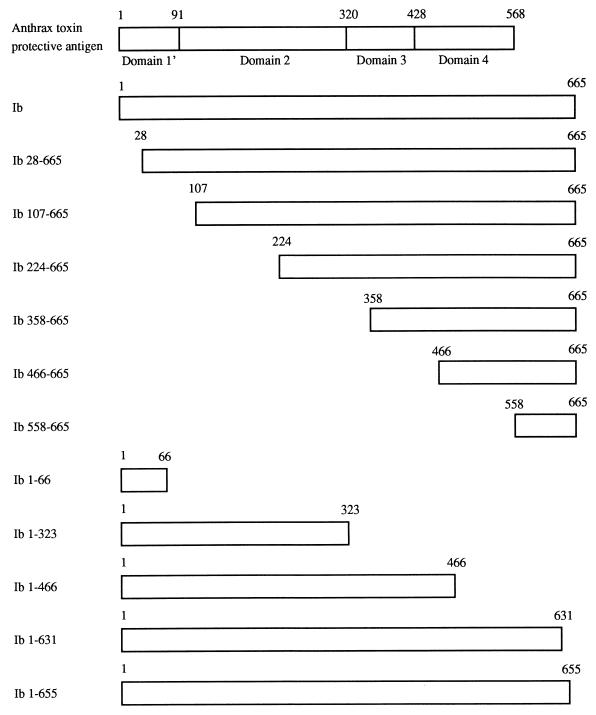
To analyze Ib domains that interact with the cell surface and Ia, we recombinantly generated fusion proteins of Ib and 11 truncated Ib variants linked to a thioredoxin-histidine tag via the N terminus (Fig. 1). Recombinant Ib (full length, residues 1 to 665) fusion protein was only four- to eightfold less active than wild-type Ib when combined with Ia, and biological activity of the recombinant was indistinguishable from wild-type Ib following enterokinase removal of the thioredoxin-histidine tag. None of the truncated Ib variants, when used at a very high concentration (10 μM) with equimolar Ia, were cytotoxic to Vero cells. Further studies with Ib 28-665 treated with and without enterokinase did not reveal any difference in cytotoxicity when combined with Ia. Each truncated Ib variant (except Ib 1-66) reacted in an ELISA with Ib antiserum at levels equivalent to whole-molecule Ib (data not shown), thus enabling us to develop fluorescence-activated cytometry experiments for binding of each Ib fusion protein to Vero cells. Fusion proteins were used for all subsequent studies because enterokinase removal of the thioredoxin-histidine tag from various constructs was highly variable.
FIG. 1.
Schematic presentation of proteolytically activated forms of anthrax toxin PA (21) showing the four putative domains, recombinant Ib, and truncated Ib variants.
Binding of truncated Ib variants to Vero cells.
Fluorescence-activated cytometry was used to characterize the binding of recombinant Ib and each truncated variant to the surface of Vero cells. Ib 28-665, Ib 107-665, Ib 224-665, Ib 358-665, and Ib 466-665 effectively bound to Vero cells (Table 2). However, Ib lacking residues 632 to 665 or 656 to 665 or truncated variant Ib 558-665 was much less efficient at binding to the cell surface, indicating that the receptor binding domain of Ib is localized within the C-terminal residues 466 to 665. Moreover, residues 466 to 558 and 656 to 665 of Ib were apparently critical for cell receptor recognition and/or conformational integrity of the Ib molecule.
TABLE 2.
Binding of recombinant Ib, truncated Ib variants, and Ia to Vero cells, as determined by fluorescence-activated cytometry
| Recombinant Ib or truncated variant | Binding to cell surface receptor (RFU)a | Docking of Ia to cell-bound Ib or truncated variant (RFU) |
|---|---|---|
| Ib | 17.6 | 23.1 |
| Ib 28-665 | 13.6 | NDb |
| Ib 107-665 | 14.6 | 8 |
| Ib 224-665 | 11.6 | 9.3 |
| Ib 358-665 | 12.1 | 11.1 |
| Ib 466-665 | 12.2 | 10.6 |
| Ib 558-665 | 7.6 | ND |
| Ib 1-66 | 6 | ND |
| Ib 1-323 | 7.2 | ND |
| Ib 1-466 | 8 | ND |
| Ib 1-631 | 7.2 | ND |
| Ib 1-655 | 5.2 | ND |
| None | 4.6 | 7.7 |
Geometric mean of the RFU. Results were considered positive if the RFU were at least twofold higher than the negative control (None) consisting of no Ib or truncated Ib variant but all other reagents.
ND, not determined.
Ia docking domain on Ib.
Like Ib, the docking of Ia to Ib is also detected by fluorescence-activated cytometry after mixing both components with Vero cells (30). In contrast to whole-molecule Ib, Ia did not dock with any truncated Ib variant (Table 2). The Ib 107-665 variant readily bound to the cell surface but did not support docking of Ia, suggesting that the N-terminal 106 residues of Ib were important for interactions with Ia. As stated earlier, studies with a larger N-terminal truncated variant, Ib 28-665, before and after enterokinase treatment revealed that this molecule did not effectively interact with Ia and thus failed to facilitate cytotoxic effects on Vero cells. In agreement with findings for recombinant Ib (Ib 1-665) containing the thioredoxin and histidine tag, the inability of N-terminal truncated variants of Ib to dock with Ia was not due to steric hindrance from the thioredoxin-histidine tag.
Inhibition of iota cytotoxicity by truncated Ib variants.
Competitive cytotoxicity assays between wild-type Ib and various concentrations of each truncated Ib variant in the presence of Ia were done with Vero cells (Table 3). Each truncated Ib variant that bound to Vero cells during fluorescence-activated cytometry experiments afforded varying levels of protection against iota cytotoxicity, thus suggesting that binding was specific for a functional receptor. Ib 28-665 and Ib 107-665 were the best inhibitors of iota cytotoxicity, while Ib 224-665, Ib 358-665, and Ib 466-665 were less effective. As evidenced by Ib 1-66, Ib 1-323, Ib 1-466, Ib 1-631, and Ib 1-655, deletions within the C terminus of Ib yielded ineffective competitors of iota cytotoxicity. Truncated Ib variants that did not bind to the cell surface did not inhibit iota cytotoxicity, indicating that the Ia docking and translocation domains of Ib were not competitors of iota cytotoxicity.
TABLE 3.
Competition between wild-type Ib and truncated Ib variants as determined by Vero cytotoxicity
| Truncated Ib variant | Truncated Ib variant/Ib molar ratio yielding 50% protectiona |
|---|---|
| Ib 28-665 | 125:1 |
| Ib 107-665 | 125:1 |
| Ib 224-665 | 1,028:1 |
| Ib 358-665 | 1,028:1 |
| Ib 466-665 | 1,028:1 |
| Ib 558-665 | >2,056:1 |
| Ib 1-66 | >2,056:1 |
| Ib 1-323 | >2,056:1 |
| Ib 1-466 | >2,056:1 |
| Ib 1-631 | >2,056:1 |
| Ib 1-655 | >2,056:1 |
Wild-type Ib (2 nM) and a serial dilution of each truncated Ib variant were added to Vero cells with Ia (2 nM). Results represent the molar ratio of truncated Ib variant to wild-type Ib that afforded 50% protection against iota cytotoxicity.
Epitope and functional mapping of Ib with MAbs.
Five MAbs developed against Ib to further define functional domains on Ib were designated (and isotyped) 1D11, 1E12, 4H7, and 10A6 (all IgG1) and 4F6 (IgG2b). Truncated Ib variants were used to map epitopes for each MAb by BIAcore technology. MAbs 10A6, 4H7, and 1E12 bound within the N terminus (amino acids 1 to 66), while 1D11 and 4F6 recognized the C-terminal residues 632 to 655 (Table 4). All BIAcore results were subsequently confirmed by an ELISA (data not shown).
TABLE 4.
Binding of Ib MAbs to truncated Ib variants by BIAcore
| Protein | MAb binding (RU)a
|
|||||
|---|---|---|---|---|---|---|
| 10A6 | 1D11 | 4H7 | 1E12 | 4F6 | Control MAbb | |
| Ib | 342 | 639 | 285 | 234 | 299 | 87 |
| Ib 107-665 | 0 | 664 | −27 | −29 | 532 | 10 |
| Ib 224-665 | −8 | 794 | −37 | −27 | 351 | 10 |
| Ib 358-665 | −3 | 563 | −6 | −2 | 632 | 5 |
| Ib 466-665 | −12 | 687 | −34 | −29 | 454 | 16 |
| Ib 1-66 | 244 | 18 | 206 | 232 | −43 | 36 |
| Ib 1-323 | 242 | −27 | 202 | 281 | 21 | −13 |
| Ib 1-466 | 274 | −1 | 191 | 157 | 4 | 22 |
| Ib 1-631 | 142 | −8 | 117 | 121 | 5 | 3 |
| Ib 1-655 | 181 | 144 | 133 | 147 | 131 | 7 |
| HBS-EPc | −46 | −36 | −47 | −36 | 22 | 6 |
MAbs captured by the RAM Fc surface varied between 1,130 and 1,730 RU. Values represent the RU due to MAb binding of the designated recombinant Ib or truncated variant. Results were considered positive if the RU were 2.5-fold or greater than that for the control MAb.
Control MAb recognized C. botulinum neurotoxin A, but not Ib, as determined by an ELISA.
HBS-EP, negative control containing only buffer and surfactant.
To determine whether the MAbs shared common epitopes on Ib, additional competitive binding studies were done with BIAcore (Table 5). MAbs 10A6, 4H7, and 1E12 blocked each other bidirectionally and evidently bound to the same epitope in the N terminus, with similar avidities. MAbs 4F6 and 1D11 recognized unique epitopes within the C terminus. In fact, binding of either 4F6 or 1D11 to Ib enhanced binding of the other C-terminal targeting antibody, suggesting conformational changes and more optimal presentation of epitopes within this region of Ib. These results were also confirmed by a competitive ELISA (data not shown).
TABLE 5.
Epitope mapping of MAbs against Ib by BIAcore
| MAb 1a | Binding of MAb 1 to RAM Fc | Binding of Ib to MAb 1 | MAb 2b
|
||||
|---|---|---|---|---|---|---|---|
| 10A6 | 4H7 | 1E12 | 4F6 | 1D11 | |||
| 10A6 | 1,053 | 175 | 28 | 35 | 36 | 85 | 177 |
| 4H7 | 1,025 | 140 | 37 | 42 | 45 | 84 | 178 |
| 1E12 | 1,042 | 136 | 38 | 43 | 44 | 84 | 171 |
| 4F6 | 1,077 | 305 | 226 | 237 | 239 | −35 | 469 |
| 1D11 | 1,255 | 395 | 217 | 227 | 217 | 420 | 15 |
MAb 1 (100 μg/ml) was added to a RAM Fc-coated sensor chip, followed by surface blocking, Ib (80 μg/ml), and MAb 2 (100 μg/ml). All values are presented in RU.
The RU after addition of MAb 2 indicate binding to the Ib-MAb 1 complex. MAb 1 and MAb 2 recognized the same epitope if binding of MAb 2 to Ib was ±10 RU of the value obtained when MAb 1 and MAb 2 were identical (boldface).
Additionally, each MAb reacted with Ib in a Western blot after SDS-PAGE as well as with the protoxin form of wild-type Ib in an ELISA (data not shown). These results suggest that the C- and N-terminal epitopes were linear and not masked or conformationally altered by the 20-kDa protoxin peptide at the N terminus. Furthermore, ELISA and Western blot data with Ib 28-665 showed that each MAb bound to this construct, and the epitope recognized by the N-terminal MAbs was more precisely mapped within residues 28 to 66.
None of the MAbs recognized Ib bound to the cell surface, and only preincubation of Ib with MAb 4F6 effectively prevented Ib binding to the cell, as determined by fluorescence-activated cytometry (data not shown). Cross-reactivity studies (ELISA and Western blot) revealed that each MAb reacted strongly with C. spiroforme Sb, but only MAb 4F6 recognized C. botulinum C2II (data not shown). The PA molecule from B. anthracis was not detected by any Ib MAb.
In vitro and in vivo neutralization of iota-toxin by Ib MAbs.
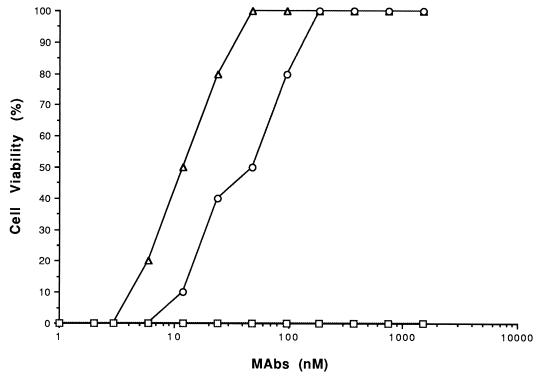
Various concentrations of each MAb were preincubated with 2 nM iota-toxin, and this mixture was then added to Vero cells for in vitro neutralization studies (Fig. 2). MAbs binding to the N terminus of Ib (1E12, 10A6, and 4H7) did not neutralize iota cytotoxicity. However, C-terminal binding MAbs 4F6 and 1D11 at 12 and 39 nM, respectively, neutralized 50% of the iota cytotoxicity. MAb 4F6, although cross-reactive with C2II, did not neutralize C2 cytotoxicity (data not shown).
FIG. 2.
Neutralization of wild-type iota-toxin (2 nM Ia plus 2 nM Ib) by various concentrations of each MAb in a Vero cell protection assay. MAbs 1D11 (○) and 4F6 (▵) afforded protection against iota-toxin versus MAbs lE12, 10A6, and 4H7, or a negative-control MAb, 1G6 (each designated by a square), developed against staphylococcal enterotoxin A.
In vivo neutralization of iota-toxin in mice yielded results similar to in vitro experiments with Vero cells. Although none of the MAbs prevented lethality, only the C-terminal binding antibodies prolonged the time to death approximately two- to threefold versus controls that were given iota-toxin without antibody. Based on in vitro and in vivo results, neutralizing antibodies for Ib evidently targeted the receptor binding, not Ia docking, domain.
DISCUSSION
Although the enzymatic properties of C. perfringens iota-toxin attributed to Ia have been well studied, there has been little understanding of how Ib targets a cell and facilitates Ia entry into the cytosol. Ib shares common epitopes and high sequence homology with the cell-binding proteins of anthrax (PA) and C2 (C2II) toxins (13, 19, 20), thus suggesting similar structure-function properties amongst these molecules. Most insight into the structure and function of these proteins, involving the binding to a target cell and translocation of another protein into the cytosol, has been derived from studies with PA. The crystal structure of PA reveals four domains, as described by Petosa et al. (21). The N and C termini, respectively designated as domains I and IV, represent the docking site for lethal or edema factor and the cell surface binding site. The central regions of PA, containing domains II and III, evidently play a role in translocating lethal or edema factor into the cytosol. No functional complementarity, as evidenced by in vitro cytotoxicity, occurs between iota, C2, and anthrax toxin components, suggesting that domain I is toxin specific (20, 22). However, there may be docking of heterologous toxin components to Ib, C2II, or PA on the cell surface without efficient translocation into the cytosol and a resultant biological effect. This possibility could be further investigated by fluorescence-activated cytometry.
To delineate the cell-binding and Ia docking domains of Ib, fluorescence-activated cytometry and competitive cytotoxicity methods were used with each truncated Ib variant. For these studies, homogenous populations of Ib fusion proteins were used, since enterokinase removal of the thioredoxin-histidine tag was variably incomplete amongst the different constructs. As with PA and C2II, the cell-binding domain of Ib is located within the C terminus, but the cell surface receptor(s) recognized by PA, C2II, and Ib is unique (5, 8, 9, 27, 30). Domain IV of full-length, nonproteolytically cleaved PA encompasses the C-terminal residues 596 to 735, and a PA fragment representing amino acids 663 to 735 effectively competes with PA for binding to the cell surface (17). Domain IV of full-length C2II (residues 592 to 721) has also been defined recently as the receptor binding site, yet a truncated variant lacking this domain still formed oligomers in solution (5). The C terminus of proteolytically processed Ib (residues 466 to 665) bound the Vero cell surface and effectively prevented iota cytotoxicity, while a peptide encompassing amino acids 558 to 665 did not. Although the binding domain of Ib was localized to the C-terminal 200 residues, a truncated Ib variant lacking only the C-terminal 10 residues did not bind Vero cells. These data suggest that the distal C terminus is important for cell recognition and/or preserves conformational integrity of the Ib binding domain, possibly like the recent findings for PA (36) and C2II (5). The binding domain on Ib could be further resolved by mutagenesis studies, as demonstrated with domain IV of PA (36). Mutations within a 15-residue span (679 to 693) yielded a biologically inactive PA molecule, yet modifications in a region encompassing amino acids 704 to 722 did not decrease anthrax cytotoxicity.
The Ia docking domain on Ib was more difficult to delineate, as none of the truncated Ib variants bound Ia or elicited any signs of iota cytotoxicity. However, variants lacking the N-terminal 27 or 106 residues (Ib 28-665 and Ib 107-665, respectively) readily bound the cell surface and effectively inhibited iota cytotoxicity, thus revealing that these molecules recognize cell surface receptor, like wild-type Ib. Like PA of anthrax toxin, we propose that the Ia docking domain on Ib lies within the N terminus. Interestingly, the protoxin form of Ib that has not been proteolytically activated also binds to the cell surface but does not effectively interact with Ia (30). An inability of Ia to dock with the protoxin form of Ib may be due to steric hindrance from a 20-kDa N-terminal peptide of Ib removed after proteolysis. However, we found that a 16-kDa polypeptide (thioredoxin plus histidine tag) fused to the N terminus of Ib resulted in minimal loss of iota cytotoxicity when combined with Ia. Additional cytotoxicity studies using Ib 28-665, with and without the N-terminal thioredoxin-histidine tag and in the presence of Ia, revealed no cytotoxicity. Although Ib 28-665 binds to the cell surface, the data strongly suggest that the N terminus of Ib is important for docking with Ia.
The PA molecule of anthrax toxin, which docks to lethal factor or edema factor, also requires proteolytic activation (14). However, proteolysis of PA can occur in solution or after binding to a cell, generating a 20-kDa peptide (167 residues) from the N terminus and activated PA, known as PA63. Both PA63 and C2II form heptamers on the surface of a target cell (3, 26), although no such structures have yet been described for Ib. Truncated variants of Ib that were competitive and lacking the Ia docking domain, as determined by cytotoxicity assays, could be explained if a particular conformation or oligomerization of Ib is required for docking with Ia. Further studies are now in progress to determine whether Ib forms oligomers on the cell surface, although Ib is not significantly processed or released from Vero cells after 2 h at 37°C (30).
In addition to the structure-function experiments with truncated Ib variants, MAbs developed against Ib were also useful for further defining biologically functional and neutralizing sites on this molecule. MAbs that recognized the cell-binding C-terminal region (residues 632 to 655) of Ib neutralized iota-toxin in vitro and delayed the time to death in mice. MAb 4F6 clearly prevented Ib binding to the cell surface, but MAb 1D11, the other C-terminal-targeting antibody which neutralized iota cytotoxicity, did not inhibit binding of Ib to the cell surface. MAb 1D11 may alter the conformation of Ib or perhaps Ib oligomerization on the cell surface, leading to inefficient docking with Ia. None of the N-terminal-binding MAbs prevented Ib binding to the cell surface or neutralized in vitro or in vivo effects of iota-toxin. Several hypotheses could explain these findings for the N-terminal-targeting antibodies and include the following: (i) MAb avidity for Ib may be less than Ia avidity for cell-bound Ib, thus resulting in Ia displacement of an N-terminal-binding antibody; and (ii) binding of Ib to the cell receptor produces a conformational change, and possibly oligomers, that displaces N-terminally targeted antibodies.
Similar to our MAb results with Ib, three different MAbs against PA also recognize the cell-binding domain within residues 671 to 721 of domain IV (15). However, in contrast to our murine experiments with either C-terminal-binding MAb and iota-toxin, the MAbs for PA provide absolute protection against the lethal effects of anthrax toxin in rats. A recent study with recombinant PA devoid of the cell-binding domain revealed that this molecule is a weak immunogen versus wild-type PA (7). Additionally, polyclonal antibodies against an area of domain IV within C2II neutralize C2 cytotoxicity in vitro (5). Based on these cumulative results from different laboratories, the receptor binding domain of Ib, PA, and C2II represents an immunodominant site for toxin-neutralizing antibodies.
As of this time, the crystal structure for Ib remains undetermined, but highly resolved coordinates would obviously provide additional clues towards understanding the intoxication process of iota-toxin and the development of more effective mutagenesis studies for identifying biologically critical residues on Ib. Ia has been recently crystallized, and preliminary structural data have been presented by Tsuge et al. (33). A more-highly-resolved crystal structure for an insecticidal ADP-ribosyltransferase, VIP2, produced by B. cereus and sharing 32% sequence identify with Ia, reveals two structurally homologous domains (11). The C terminus of VIP2 contains the NAD binding cleft, while the N terminus putatively docks with the complementary cell-binding protein, VIP1. Additionally, the N terminus of C. botulinum C2I or B. anthracis LF, respectively, docks with C2II or PA bound to the cell surface (1, 4). It is possible, although it has not been proven experimentally, that the N terminus of Ia contacts the N terminus of Ib once it has bound to a target cell.
Finally, identification of the protein receptor for Ib (30), which differs from the asparagine-linked carbohydrate recognized by C2II (8), would provide useful information regarding iota intoxication. This structure-function study of Ib yielded some important clues towards the potential use of this binary toxin as a targeted delivery system, similar to that described for anthrax (1, 2) and C2 toxins (4). Clearly though, additional experiments are needed to more fully understand iota-toxin, its mechanism of action, and how it may be used as an effective biological tool.
ACKNOWLEDGMENTS
We thank Yvette Campbell for MAb purification and processing of Vero cells for fluorescence-activated cytometry and Erin Fleming for protein purification. As always, we appreciate the diligence of the cell culture group at USAMRIID, and the efforts of Shawn Guest were again invaluable for generating and maintaining hybridomas.
This work was performed while Jean-Christophe Marvaud held a National Research Council-USAMRIID Research Associateship.
REFERENCES
- 1.Arora N, Leppla S H. Residues 1–254 of anthrax toxin lethal factor are sufficient to cause cellular uptake of fused polypeptides. J Biol Chem. 1993;268:3334–3341. [PubMed] [Google Scholar]
- 2.Ballard J D, Doling A M, Beauregard K, Collier R J, Starnbach M N. Anthrax toxin-mediated delivery in vivo and in vitro of a cytotoxic T-lymphocyte epitope from ovalbumin. Infect Immun. 1998;66:615–619. doi: 10.1128/iai.66.2.615-619.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barth H, Blocker D, Behlke J, Bergsma-Schutter W, Brisson A, Benz R, Aktories K. Cellular uptake of Clostridium botulinum C2 toxin requires oligomerization and acidification. J Biol Chem. 2000;275:18704–18711. doi: 10.1074/jbc.M000596200. [DOI] [PubMed] [Google Scholar]
- 4.Barth H, Hofmann F, Olenik C, Just I, Aktories K. The N-terminal part of the enzyme component (C2I) of the binary Clostridium botulinum C2 toxin interacts with the binding component C2II and functions as a carrier system for a Rho ADP-ribosylating C3-like fusion toxin. Infect Immun. 1998;66:1364–1369. doi: 10.1128/iai.66.4.1364-1369.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blocker D, Barth H, Maier E, Benz R, Barbieri J T, Aktories K. The C terminus of component C2II of Clostridium botulinum C2 toxin is essential for receptor binding. Infect Immun. 2000;68:4566–4573. doi: 10.1128/iai.68.8.4566-4573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosworth T J. On a new toxin produced by Clostridium welchii. J Comp Pathol. 1943;53:245–255. [Google Scholar]
- 7.Brossier F, Weber-Levy M, Mock M, Sirard J C. Role of toxin functional domains in anthrax pathogenesis. Infect Immun. 2000;68:1781–1786. doi: 10.1128/iai.68.4.1781-1786.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckhardt M, Barth H, Blocker D, Aktories K. Binding of Clostridium botulinum C2 toxin to asparagine-linked complex and hybrid carbohydrates. J Biol Chem. 2000;275:2328–2334. doi: 10.1074/jbc.275.4.2328. [DOI] [PubMed] [Google Scholar]
- 9.Escuyer V, Collier R J. Anthrax protective antigen interacts with a specific receptor on the surface of CHO-K1 cells. Infect Immun. 1991;59:3381–3386. doi: 10.1128/iai.59.10.3381-3386.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibert M, Petit L, Raffestin S, Okabe A, Popoff M R. Clostridium perfringens iota-toxin requires activation of both binding and enzymatic components for cytopathic activity. Infect Immun. 2000;68:3848–3853. doi: 10.1128/iai.68.7.3848-3853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han S, Craig J A, Putnam C D, Carozzi N B, Tainer J A. Evolution and mechanism from structures of an ADP-ribosylating toxin and NAD complex. Nat Struct Biol. 1999;6:932–936. doi: 10.1038/13300. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki M, Ohishi I, Sakaguchi G. Evidence that botulinum C2 toxin has two dissimilar components. Infect Immun. 1980;29:390–394. doi: 10.1128/iai.29.2.390-394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura K, Kubota T, Ohishi I, Isogai E, Fujii N. The gene for component-II of botulinum C2 toxin. Vet Microbiol. 1998;62:27–34. doi: 10.1016/s0378-1135(98)00195-3. [DOI] [PubMed] [Google Scholar]
- 14.Leppla S H. The bifactorial Bacillus anthracis lethal and oedema toxins. In: Alouf J E, Freer J H, editors. The comprehensive sourcebook of bacterial protein toxins. 2nd ed. London, England: Academic Press; 1999. pp. 243–263. [Google Scholar]
- 15.Little S F, Novak J M, Lowe J R, Leppla S H, Singh Y, Klimpel K R, Lidgerding B C, Friedlander A M. Characterization of lethal factor binding and cell receptor binding domains of protective antigen of Bacillus anthracis using monoclonal antibodies. Microbiology. 1996;142:707–715. doi: 10.1099/13500872-142-3-707. [DOI] [PubMed] [Google Scholar]
- 16.Nagahama M, Sakaguchi Y, Kobayashi K, Ochi S, Sakurai J. Characterization of the enzymatic component of Clostridium perfringens iota toxin. J Bacteriol. 2000;182:2096–2103. doi: 10.1128/jb.182.8.2096-2103.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noskov A N, Kravchenko T B, Noskova V P. Detection of the functionally active domains in the molecule of protective antigen of the anthrax exotoxin. Mol Genet Mikrobiol Virusol. 1996;3:16–20. [PubMed] [Google Scholar]
- 18.Perelle S, Domenighini M, Popoff M R. Evidence that Arg-295, Glu-378, and Glu-380 are active-site residues of the ADP-ribosyltransferase activity of iota toxin. FEBS Lett. 1996;395:191–194. doi: 10.1016/0014-5793(96)01035-6. [DOI] [PubMed] [Google Scholar]
- 19.Perelle S, Gibert M, Boquet P, Popoff M R. Characterization of Clostridium perfringens iota-toxin genes and expression in Escherichia coli. Infect Immun. 1993;61:5147–5156. doi: 10.1128/iai.61.12.5147-5156.1993. . (Author's correction, 63:4967, 1995.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perelle S, Scalzo S, Kochi S, Mock M, Popoff M R. Immunological and functional comparison between Clostridium perfringens iota toxin, C. spiroforme toxin, and anthrax toxins. FEMS Microbiol Lett. 1997;146:117–121. doi: 10.1111/j.1574-6968.1997.tb10180.x. [DOI] [PubMed] [Google Scholar]
- 21.Petosa C, Collier R J, Klimpel K R, Leppla S H, Liddington R C. Crystal structure of the anthrax toxin protective antigen. Nature. 1997;385:833–838. doi: 10.1038/385833a0. [DOI] [PubMed] [Google Scholar]
- 22.Popoff M R, Boquet P. Clostridium spiroforme toxin is a binary toxin which ADP-ribosylates cellular actin. Biochem Biophys Res Commun. 1988;152:1361–1368. doi: 10.1016/s0006-291x(88)80435-2. [DOI] [PubMed] [Google Scholar]
- 23.Popoff M R, Milward F W, Bancillon B, Boquet P. Purification of the Clostridium spiroforme binary toxin and activity of the toxin on HEp-2 cells. Infect Immun. 1989;57:2462–2469. doi: 10.1128/iai.57.8.2462-2469.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson L L, Stiles B G, Zepeda H H, Wilkins T D. Molecular basis for the pathological actions of Clostridium perfringens iota-toxin. Infect Immun. 1987;55:118–122. doi: 10.1128/iai.55.1.118-122.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson L L, Stiles B G, Zepeda H, Wilkins T D. Production by Clostridium spiroforme of an iota-like toxin that possesses mono(ADP-ribosyl)transferase activity: identification of a novel class of ADP-ribosyltransferases. Infect Immun. 1989;57:255–261. doi: 10.1128/iai.57.1.255-261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh Y, Klimpel K R, Goel S, Swain P K, Leppla S H. Oligomerization of anthrax toxin protective antigen and binding of lethal factor during endocytic uptake into mammalian cells. Infect Immun. 1999;67:1853–1859. doi: 10.1128/iai.67.4.1853-1859.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh Y, Klimpel K R, Quinn C P, Chaudhary V K, Leppla S H. The carboxyl-terminal end of protective antigen is required for receptor binding and anthrax toxin activity. J Biol Chem. 1991;266:15493–15497. [PubMed] [Google Scholar]
- 28.Songer J G. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stiles B G. Purification and characterization of Clostridium perfringens iota toxin. Ph.D. thesis. Blacksburg: Virginia Polytechnic Institute and State University; 1987. [Google Scholar]
- 30.Stiles B G, Hale M L, Marvaud J-C, Popoff M R. Clostridium perfringens iota-toxin: binding studies and characterization of cell surface receptor by fluorescence-activated cytometry. Infect Immun. 2000;68:3475–3484. doi: 10.1128/iai.68.6.3475-3484.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stiles B G, Lidgerding B C, Sexton F W, Guest S B. Production and characterization of monoclonal antibodies against Naja naja atra cobrotoxin. Toxicon. 1991;29:1195–1204. doi: 10.1016/0041-0101(91)90192-t. [DOI] [PubMed] [Google Scholar]
- 32.Stiles B G, Wilkins T D. Purification and characterization of Clostridium perfringens iota-toxin: dependence on two nonlinked proteins for biological activity. Infect Immun. 1986;54:683–688. doi: 10.1128/iai.54.3.683-688.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsuge H, Nagahama M, Nishimura T, Sakaguchi Y, Katunuma N, Sakurai J. Crystallization and preliminary X-ray studies of the Ia component of Clostridium perfringens iota toxin complexed with NADPH. J Struct Biol. 1999;126:175–177. doi: 10.1006/jsbi.1999.4124. [DOI] [PubMed] [Google Scholar]
- 34.van Damme J, Jung M, Hofmann F, Just I, Vandekerckhove J, Aktories K. Analysis of the catalytic site of the actin ADP-ribosylating Clostridium perfringens iota toxin. FEBS Lett. 1996;380:291–295. doi: 10.1016/0014-5793(96)00052-x. [DOI] [PubMed] [Google Scholar]
- 35.Vandekerckhove J, Schering B, Barmann M, Aktories K. Clostridium perfringens iota toxin ADP-ribosylates skeletal muscle actin in Arg-177. FEBS Lett. 1987;225:48–52. doi: 10.1016/0014-5793(87)81129-8. [DOI] [PubMed] [Google Scholar]
- 36.Varughese M, Teixeira A V, Liu S, Leppla S H. Identification of a receptor-binding region within domain 4 of the protective antigen component of anthrax toxin. Infect Immun. 1999;67:1860–1865. doi: 10.1128/iai.67.4.1860-1865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]