Abstract
Microbial modulation of apoptosis has added a new dimension of understanding to the dynamic interaction between the human host and its microbial invaders. Persistent infection can be a by-product of inhibition of apoptosis and may significantly impact the pathogenesis of diseases caused by organisms such as Chlamydia trachomatis. We compared apoptotic responses among HeLa 229 cells acutely and persistently infected and mock infected with serovar A/HAR-13. Persistence was induced by gamma interferon at 0.2 and 2.0 ng/ml. Cells were treated with etoposide or staurosporine at 24-h intervals and assayed for apoptosis by cell count, DNA ladder formation, and cytochrome c translocation. From the 24- to 120-h time points, infected cultures were 87 and 90% viable for etoposide and staurosporine treatment, respectively, and produced no DNA ladder, and cytochrome c remained in the mitochondria. In contrast, mock-infected cells were 22 and 37% viable for etoposide (P = 0.0001) and staurosporine (P = 0.01), respectively, and displayed characteristic DNA ladders, and cytochrome c was translocated. We found that resistance to apoptotic stimuli was identical in acute and persistent infections. Since cytochrome c was not translocated from the mitochondrion, caspase-9 activity was likely not involved. The expression of chlamydial hsp60, a known stimulator of inflammation in vivo, was measured in both active and persistent infections by Western blot, with increased production in the latter with or without staurosporine treatment. Chlamydial disregulation of apoptosis and the ensuing persistence of organisms offer an alternative pathogenic mechanism for chlamydial scarring observed in trachoma and infertility populations via sustained inflammation induced by immunoreactive molecules such as hsp60.
The recent findings that some microbial pathogens modulate apoptosis to accommodate the organism's life cycle and facilitate infection (34) shed an important and exciting new light on the study of pathogenesis. Apoptosis, or programmed cell death, is the mechanism for cellular self-destruction that functions to eliminate cells during immune selection, tissue development, and tissue regeneration (37). It is a genetically programmed process in which macromolecules are broken down and released from the cell in an orderly fashion designed to avoid eliciting an inflammatory response. Apoptosis occurs in response to specific internal and environmental stimuli (17). Necrosis, in contrast, is generally the result of injury and is a rapid process wherein the cell membrane ruptures, disgorging cell contents into the system, inducing inflammation. Although apoptosis is designed to dispose of cell contents with minimal disruption to neighboring cells, it is possible that necrosis of bystander cells can occur (23).
Chlamydia has joined the expanding list of pathogens that modulate apoptosis by a diverse repertoire of methods (9, 10, 28, 30, 31), ranging from interactions with modulating proteins (22, 32) to coding for an inhibitor of inflammatory molecules (35). Chlamydia spp. cause a myriad of respiratory, ocular, and sexually transmitted diseases in humans (6). The sequelae of these infections include trachoma, the leading cause of preventable blindness in the world today (7), and pelvic inflammatory disease (PID), a major cause of infertility and ectopic pregnancy (4, 6). These sequelae involve scarring that occurs at mucosal sites of inflammation. Thus, although the pathogenesis of chlamydial diseases is not well understood, host immune factors such as recurrent inflammation from repeat infection (14) and hypersensitivity reactions to chlamydial heat shock protein 60 (hsp60) (25) are thought to be important. Pathogen-related factors most likely relate to the fact that Chlamydia has the ability to persist (1, 2).
Chlamydia organisms are obligate intracellular parasites with a distinct life cycle revolving between an inert, extracellular infectious stage, the elementary body, and an intracellular metabolic stage, the reticulate body. These stages are functionally distinct and marked by specific protein profiles (27). Another distinct protein profile is seen when a persistent state is induced in vitro by amino acid deprivation (5), antibiotic treatment (2), or gamma interferon (IFN-γ) (1). In the latter case, infected cells produce aberrant organisms that present antigen but remain noninfectious until IFN-γ is removed. Interestingly, the antigenic profile in persistent infections differs from that of both the elementary and reticulate bodies. The overall expression of all proteins decreases except for hsp60. A comparatively high concentration of this molecule is maintained for the duration of the persistent state (2). In a recent in vivo study, we found that 24% of women with recurrent Chlamydia trachomatis infections had the same ompA genotype despite appropriate treatment (8). Intervening culture-negative episodes for these women were significantly more likely to be positive for chlamydiae by ligase chain reaction than for women with recurrences due to different serovars or for women undergoing test of cure.
Given the potential importance of persistence in the pathogenesis of chlamydial diseases, we investigated the apoptotic response of human epithelial cells to persistent infection with C. trachomatis serovar A. We used the in vitro immune factor model to induce persistence and tested the response to the cell death inducers staurosporine, a protein kinase C (PKC) inhibitor, and etoposide, an inhibitor of topoisomerase II. We found a similar block in apoptosis to that seen in acute infections described by Fan et al. (9). During both active and persistent infection, hsp60 was continuously expressed. In persistent infection, synthesis of most proteins was decreased, enhancing the effect of hsp60 expression. This suggests that an apoptotic block may enable colonization of the organism, while local concentrations of immunoregulatory factors may favor a chlamydial form that expresses an inflammation-inducing protein, such as hsp60. This offers a possible mechanism for the immune injury seen in scarring disease characteristic of trachoma and PID.
(This research was presented in part at the American Society of Microbiology symposium “A Cell Biology Approach to Microbial Pathogenesis,” Portland, Oreg., 25–28 April 1999.)
MATERIALS AND METHODS
Establishment of C. trachomatis infections and detection of chlamydial protein by microscopy.
Human cervical adenocarcinoma cells (HeLa 229) were seeded at 106 cells per ml onto coverslips in paired 12-well plates in minimal essential medium with 10% fetal bovine serum (MEM-10). After 24 h of incubation at 37°C in 5% CO2 with establishment of confluent monolayers, the cells were washed three times with Hanks' balanced salt solution, infected with C. trachomatis serovar A/HAR-13 at a multiplicity of infection (MOI) of 50 in 0.4 ml of 0.25 M sucrose–10 mM sodium phosphate–5 mM l-glutamic acid (pH 7.2), and incubated on a rocker for 2 h. The inoculum was removed and replaced with MEM-10 containing cycloheximide (1 μg/ml) (Sigma, St. Louis, Mo.). Coverslips were incubated at 24-h intervals, removed from the well, fixed in methanol for 30 min at −20°C, air dried, and stained with fluorescein isothiocyanate (FITC)-conjugated anti-major outer membrane protein (anti-MOMP) chlamydial antibody (Cortex Biochem, San Leandro, Calif.) for an additional 30 min at room temperature. The coverslips were washed three times with phosphate-buffered saline (PBS) and then mounted onto slides and viewed under a fluorescent microscope (Nikon Eclipse TE300, Tokyo, Japan) at 40× magnification, using a FITC filter.
Establishment of persistent C. trachomatis infections and detection of chlamydial proteins by immunoblot.
Confluent HeLa 229 cell cultures were infected with C. trachomatis as above except without cycloheximide and treated with IFN-γ at 0, 0.2, or 2.0 ng/ml (Sigma) at 2 h postinfection to induce persistence as described elsewhere (1). Uninfected HeLa cells were also propagated. These experiments were performed with and without 1 μM staurosporine at 24, 48, 72, 96, and 120 h postinfection as described below except that control HeLa cells were not treated, as this would induce apoptosis and negate the control effect of viable cells. At 24, 48, 72, 96, and 120 h postinfection, cells were harvested in 200 μl of Laemmli buffer (Bio-Rad, Richmond, Calif.), boiled for 10 min, and sonicated briefly, and 40 μl of lysate was loaded on a sodium dodecyl sulfate (SDS)–12.5% polyacrylamide gel electrophoresis (PAGE) gel. After transfer, nitrocellulose (Bio-Rad) was probed with a mouse anti-chlamydial hsp60 monoclonal antibody (Affinity Bioreagents, Golden, Colo.) and a mouse anti-chlamydial MOMP monoclonal antibody (Cortex, San Leandro, Calif.) and detected by an anti-mouse immunoglobulin G (IgG)-alkaline phosphatase conjugate (Sigma, St. Louis, Mo.). A second set of experiments were performed as described above except that at 96 h post-initial infection for the persistent infections, the medium was removed from the cells and replaced with MEM-10 without IFN-γ. The cells were allowed to grow for an additional 24 and 48 h before harvesting in Laemmli buffer. Western blot was performed as above.
Apoptosis induction and Hoechst dye assay.
HeLa 229 cells were seeded onto coverslips in 12-well plates, grown to 90% confluence, and actively, persistently, or not infected as above except for the omission of cycloheximide. Apoptosis was induced with 1 μM staurosporine (Sigma) for 4 h or 300 μM etoposide (PharMingen, San Diego, Calif.) for 6 h at 24-, 48-, 72-, 96-, and 120-h time points postinfection. Adherent cells were assayed for all apoptosis experiments. Coverslips for each time point were fixed in 4% paraformaldehyde in PBS at ambient temperature, permeabilized with 0.25% saponin (Sigma) in PBS for 30 min, and stained with 10 μM Hoechst 33258 dye (Sigma) for an additional 30 min at room temperature. The coverslips were washed three times with PBS, mounted onto slides, and viewed under a fluorescent microscope (Nikon Eclipse TE 300, Tokyo, Japan) at 40× magnification, using an FITC filter. For each type of infection, cells from five random fields were counted and divided by the total number of cells to arrive at the percent apoptotic cells.
Cytochrome c detection.
Acutely infected, persistently infected, and noninfected cells as described above were harvested with trypsin at 48, 96, and 48 h, respectively, and centrifuged at 800 rpm for 8 min at 4°C. The cytochrome c assay was performed as per Fan et al. (9). Briefly, the pellets were washed with ice-cold PBS, resuspended in up to 5 volumes of buffer A (20 mM HEPES-KOH [pH 7.5], 10 mM MgCl2, 1 mM sodium EDTA, 1 mM sodium EGTA, 1 mM dithiothreitol, 0.1 mM phenymethylsulfonyl fluoride, 250 mM sucrose), and incubated for 15 min at 4°C. Homogenization was performed using a 22 Kontes Douncer with an average of 20 strokes prior to centrifugation at 750 × g for 10 min, which was repeated twice at 4°C. The supernatants were centrifuged at 10,000 × g for 15 min at 4°C to generate the pellets containing the mitochondrial fraction. This fraction was solubilized in buffer A and stored at −70°C. The supernatants were purified of mitochondria by centrifugation in an ultracentrifuge at 100,000 × g for 1 h at 4°C. The supernatants, now representing the cytosolic fraction, were stored at −70°C. Aliquots were thawed on ice, and the proteins were quantitated by the BCA assay (Pierce, Rockford, Ill.). Twenty-five micrograms of protein from each fraction was loaded on a 15% polyacrylamide gel. After PAGE, proteins were transferred by standard procedure to nitrocellulose and probed with mouse monoclonal antibody 7H8.2C12 (IgG2b; PharMingen) specific for the denatured form of cytochrome c. An anti-mouse IgG monoclonal antibody conjugated with alkaline phosphatase (Sigma) was used for detection.
DNA ladder assay.
Confluent HeLa cells were actively, persistently, and noninfected as above but without cycloheximide and treated with 1 μM staurosporine at 48 and 96 h postinfection for 4 h. A total of 2 × 106 cells were washed with PBS after harvesting with trypsin and collected by centrifugation at 800 rpm for 10 min. The pellets were resuspended in 42°C lysis buffer containing 5 mM EDTA, 5 mM Tris (pH 8.0), and 0.5% Triton. The lysates were phenol-chloroform extracted and ethanol precipitated. The DNA was resuspended in TE (5 mM Tris, 1 mM EDTA) with RNase for 30 min at 37°C, loaded onto a 2% agarose gel, and stained with ethidium bromide.
Statistical analyses.
Cell survival was calculated as the percentage of cells surviving for each experiment. Because the percentages were not normally distributed, percentages were transformed using the arcsin of the square root of the percentage, which permitted an analysis of variance on the data. In addition, a Tukey-Kramer multiple-comparison analysis was performed to determine statistical significance at a P value of < 0.05 between two pairs.
RESULTS
Effect of persistent chlamydial infection on apoptosis induction in epithelial cells.
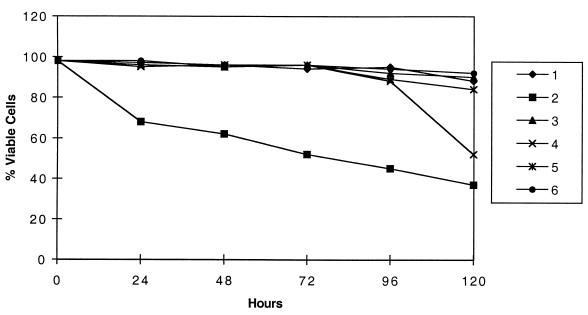
We used C. trachomatis strain A/HAR at an MOI of 50 to establish active and persistent infections in HeLa 229 cells and found that apoptotic stimulation was blocked for adherent cells treated with etoposide or straurisporine. Cell death was induced by etoposide, which inhibits topoisomerase II and is active during S phase. Cell death was also induced by staurosporine, a PKC inhibitor. Uninfected cells that were treated with low and high doses of IFN-γ showed the same sensitivity to apoptosis induction as uninfected, untreated HeLa cells. Although Chlamydia is routinely cultured in the presence of cycloheximide, it was omitted during these assays to avoid the possible confounding effect of cycloheximide on cell viability and the potential inhibitory effect on IFN-γ. An indicator of apoptosis, membrane permeability to vital dyes such as Hoechst and propidium iodide showed an average of 8% cell death for persistently infected and treated cells, while apoptosis-induced uninfected cells were almost entirely permeable by 24 h (Fig. 1). Permeability data were expressed as a percentage derived by observing slides microscopically and counting a minimum of five 40× fields per slide for three different experiments. For each 24-h interval from 24 to 120 h postinfection, infected cultures were 87 and 90% viable for etoposide (not shown) and staurosporine (shown) treatment, respectively, compared with mock-infected cells, which were 22% (P = 0.0001) and 37% (P = 0.01) viable, respectively.
FIG. 1.
Persistent C. trachomatis infections allow cells to resist apoptotic induction by staurosporine for up to 120 h. HeLa cells were actively, persistently, or not infected with C. trachomatis serovar A/HAR-13 as described in the text. At 24-h intervals up to and including 120 h, the cells were treated with 1 μM staurosporine for 4 h. DNA was detected using Hoechst dye, and five random fields under 40× power were counted for apoptotic cells to generate a percentage as described in the text. The graph shows the averages of three independent experiments which varied by ∼10% from each other. Series 1, uninfected, IFN-γ treated (2.0 μg) and not treated with staurosporine. Series 2, uninfected, IFN-γ (2.0 μg), and staurosporine treated. Series 3, persistently infected with serovar A/HAR-13 induced with 2.0 μg of IFN-γ (IFN-γ removed at 72 h) and not treated. Series 4, persistently infected and staurosporine treated, with IFN-γ removed at 72 h. Series 5, persistently infected and not treated. Series 6, persistently infected and staurosporine treated. Of note is that the results for persistent infections induced with 0.2 μg of IFN-γ were similar to series 3 and 4 and therefore are not shown.
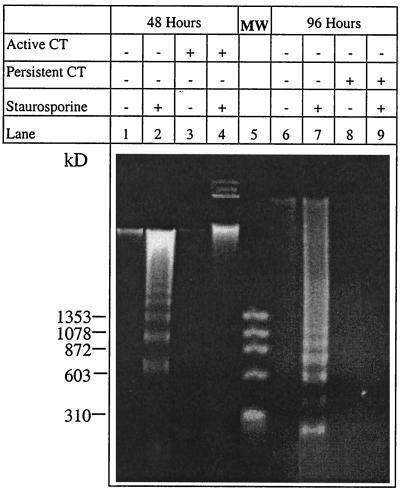
At an MOI of 50, resistance to apoptosis was demonstrated by the absence (in both acute and persistent infections) of nuclease activity, which produces a characteristic DNA ladder (Fig. 2). The ladder, however, was seen in the uninfected, staurosporine-treated (lane 2) and uninfected, IFN-γ-and staurosporine-treated (lane 7) HeLa cells, as demonstrated by SDS-PAGE (Fig. 2). Similar results were obtained for the experiments where etoposide was used to induce apoptosis (data not shown).
FIG. 2.
DNA fragmentation was pronounced in uninfected, staurosporine-treated HeLa cells but not in cells actively or persistently infected with C. trachomatis (CT) and treated. HeLa cells actively, persistently, and noninfected with C. trachomatis serovar A/HAR-13 were treated with 1 μM staurosporine at 48 and 96 h postinfection as described in the text. DNA from each time point was purified and loaded on a 2% agarose gel stained with ethidium bromide. Lane MW, size standards.
Taken together, these assays offer evidence that cell death is blocked in both acutely and persistently chlamydia-infected cells and that this death proceeds by apoptotic mechanisms in uninfected cultures given identical apoptotic stimuli.
Lack of cytochrome c relocation to the cytoplasm in response to apoptosis induction of actively and persistently infected cells.
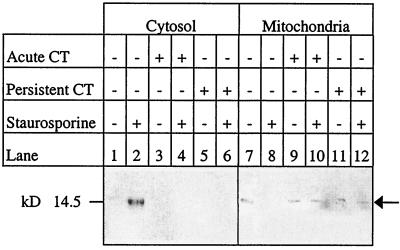
In order to compare the possible mechanisms of the apoptotic block between active and persistent infections, we determined that cytochrome c was not translocated from the mitochondrion in either type of infection (Fig. 3). In uninfected cells, cytochrome c appeared in the cytoplasm after treatment with staurosporine. Western blot analysis demonstrated that in active (up to 48 h) and persistent (up to 120 h) chlamydial infection of HeLa cells, cytochrome c remained sequestered in the mitochondrial fraction after apoptotic induction (Fig. 3). Uninfected HeLa controls that were treated with staurosporine showed translocation of cytochrome c from the mitochondria. Presumably, caspases were involved, although we did not assay for procyclic acidic repetitive protein (PARP) cleavage.
FIG. 3.
Cytochrome c was not translocated from the mitochondria in cells actively or persistently infected with C. trachomatis (CT) after staurosporine challenge. HeLa cells actively and persistently infected with C. trachomatis serovar A/HAR-13 were treated with 1 μM staurosporine. The cells were sampled at 24-h intervals up to 120 h and fractionated into cytosolic and mitochondrial fractions. The actively infected HeLa cells represent the 48-h time point, while the persistently infected cells represent the 120-h time point. Western blots were probed with antibody specific for denatured cytochrome c and reacted with a secondary antibody conjugated with alkaline phosphatase. Arrow points to cytochrome c antibody.
Cultures actively infected with C. trachomatis become sensitive to apoptotic stimuli, whereas persistently infected cells continue to resist apoptotic induction.
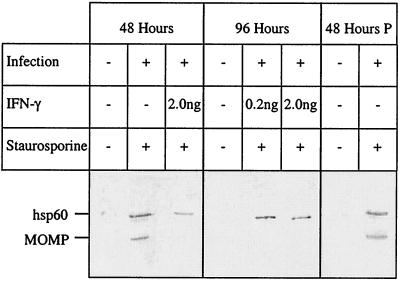
During the time course of an active or persistent infection, permeability remained intact and DNA was not fragmented in response to apoptotic induction. The characteristic DNA ladder produced by nuclease activity was not seen during the course of the infection. However, starting at 48 h and at longer time points, actively infected cells underwent apoptosis with loss of protection followed by permeability (Fig. 1) and DNA fragmentation (data not shown). Persistent infections that were maintained up to 120 h continued to resist DNA fragmentation upon staurosporine treatment. However, within 24 to 48 h of washing cells and removing IFN-γ, an active infection resumed, as evidenced by resumption of expression of both hsp60 and MOMP (Fig. 4). These infections ultimately lysed the host cells, which died by apoptotic mechanisms, as evidenced by cell count (Fig. 1) and DNA ladder formation (data not shown).
FIG. 4.
hsp60 was preferentially expressed by persistently C. trachomatis-infected HeLa 229 cells compared with active infection. HeLa cells were actively and persistently infected with C. trachomatis serovar A/HAR-13 or mock infected as described in the text. For each set of experiments, cells were either treated or not treated with 1 μM staurosporine at 24, 48, 72, 96, and 120 h postinfection, with the exception that the control HeLa cells were not treated. The cells were harvested at 24-h intervals up to 120 h. Protein from the active and persistent infections at 48 h and from the persistent infections at 96 h was run on an SDS–12.5% polyacrylamide gel, transferred to nitrocellulose, and probed with anti-chlamydial hsp60 monoclonal antibody and anti-MOMP monoclonal antibody. In addition, IFN-γ was withdrawn from persistent infections at 96 h, and these rescued infections were assayed similarly 48 h later (lanes 48 Hours P).
Expression of C. trachomatis proteins during active and persistent infection.
Both MOMP and hsp60 were expressed during active infection, but almost undetectable amounts of MOMP were expressed during persistent infections at the same time point of 48 h (Fig. 4). At 96 h postinfection, hsp60 continued to be expressed for persistent infections induced by either 0.2 or 2.0 ng of IFN-γ. Treatment with staurosporine had no effect on protein expression. Uninfected control HeLa cells were not treated with staurosporine, as this would have instituted cell death, with no cells for remaining time points. Similar results were obtained in the same experiments when staurosporine was omitted (data not shown). Forty-eight hours after removal of IFN-γ, there was resumption of near-normal MOMP expression for the persistent infections that had been treated with 0.2 ng but not 2.0 ng of IFN-γ.
DISCUSSION
Chlamydia successfully evades host defense mechanisms, including apoptosis, by growing within an inaccessible cellular compartment, the inclusion body, derived from components in the exocytic pathway (15). The organism appears to confer resistance to apoptosis upon actively infected cells, which prolongs the life of the infected cell, allowing the organism to complete its complex intracellular life cycle within 48 to 72 h. In vitro data suggest that host cytokines cooperatively induce and prolong apoptotic resistance by allowing Chlamydia to persist in tissue. In this study, a persistent infection was established with concentrations as low as 0.2 ng of IFN-γ, which is in the physiologic range seen in tissue. Thus, although the initial host response of inflammatory molecules, including interferons, occurs to eradicate infection, it may also contribute to initiation of a persistent chlamydial infection. The duration of persistence in vivo is not known, but recent evidence suggests that organisms may persist for as long as 5 years in the genital tract (8); in vitro persistence has been documented for up to 1 year (2).
In order to compare the possible mechanisms of the apoptotic block between active and persistent infections, we determined that cytochrome c was not translocated from the mitochondrion in either type of infection. This is a critical upstream event in caspase activation, which occurs early in apoptosis. In uninfected cells, cytochrome c has been shown to appear in the cytoplasm as early as 1 h following treatment with staurosporine (38). Release into the cytoplasm allows cytochrome c to bind Apaf-1 and, in the presense of ATP, cleaves procaspase-9 to the active caspase-9. Caspase-9 in turn activates caspase-3 and continues the cascade toward dismantling the cell (21). We found that in active and persistent chlamydial infections, cytochrome c remained sequestered in the mitochondrial fraction after apoptotic induction. However, noninfected, treated cells showed translocation of cytochrome c from the mitochondria. Persistently infected cells also resisted two different modes of apoptotic induction (etoposide and staurosporine), suggesting that the block occurred at a common point upstream of varied mechanisms for implementing programmed cell death. Presumably caspases are involved, although we did not assay for PARP cleavage.
It has been suggested that cytochrome c depletion from the mitochondrion may also be an indicator of necrotic cell death, since it results in a dismantling of the electron transport chain (ETC) (36). However, this did not appear to be a factor in the control cells, since necrosis derived from ETC degradation occurs in a very delayed manner, as long as 24 h post-apoptotic induction (23). For both the permeability (Hoechst dye) and DNA fragmentation experiments, samples were treated for 4 h with staurosporine at 24-h intervals and then immediately assayed. Thus, these data do not support necrosis as a mechanism for the demise of uninfected control cells.
Our findings are similar to those of others who have investigated antiapoptotic events in vitro in C. trachomatis-infected human cell lines (9, 30). Inhibition of apoptosis has also been described for Chlamydia pneumoniae, whereby one of the mechanisms appeared to involve induction of interleukin-10 (10). In contrast (9, 30), infection with the mouse pneumonitis strain (MoPn) of C. trachomatis induced apoptosis. Furthermore, induction of tumor necrosis factor alpha appeared to precipitate additional programmed death of surrounding cells. Chlamydia psittaci has been reported to induce cell death in infected and bystander macrophages and epithelial cells by a mechanism that is independent of known caspases (11, 28). These conflicting data may reflect the fact that human C. trachomatis strains, MoPn, and C. psittaci represent vastly different subtypes and species, with unique host and tissue tropisms. Alternatively, it is possible that C. trachomatis both induces apoptosis and protects infected cells from programmed cell death. These dual properties have been reported for other microorganisms, such as adenovirus (34). Additional research will be required to fully understand the properties that can be attributed to human C. trachomatis strains.
The ability to establish a persistent infection is an important step in the pathogenesis of scarring disease seen in both trachoma and chlamydial sexually transmitted diseases. A recent in vitro model showed that macrophages infected with C. trachomatis serovar K induced apoptosis of uninfected T cells, which supports the notion that chlamydia-infected macrophages may persist by avoiding T-cell surveillance via reduction of their overall numbers (20). Resistance to cell death induced by these infections may also allow a continuous antigenic pool that may stimulate ongoing inflammation and subsequent fibrosis. Persistent chlamydial organisms are noninfectious and exhibit a protein profile that is completely different from that seen in active infection, where the predominant antigen expressed is the MOMP (26), the most abundant and immunogenic protein of the organism. We found that in persistent infections, hsp60 was expressed in greater abundance than MOMP, which is consistent with the findings of others (1). Treatment with staurosporine did not affect expression of either hsp60 or MOMP. After withdrawal of IFN-γ from persistently infected cells, active infection resumed, with expression of MOMP and hsp60. However, we were not able to recover active infection when cells had been treated with 2.0 ng of IFN-γ.
Hypersensitivity reactions are known to be elicited by chlamydial hsp60 (24, 25, 29), which is produced throughout the life cycle of the organism (1). Since it has long been known that host factors are important in disease progression, the protein profile shift offers an explanation for how this response is induced. Persistently infected cells, by blocking apoptosis, ensure indefinite survival of the bacteria while expressing their most inflammatory antigen. Reversion to active infection would result in a fully infectious organism that may spread through tissue and precipitate disease both during active infection and on resumption of a persistent state. Since hsp60 is the most abundant protein expressed during both types of infections and parallels the resistance to apoptotic stimuli, it is possible that hsp60 may play a role in the inability of infected cells to undergo apoptosis. Chlamydiae are known to have a type III secretory apparatus (19, 33), which may facilitate transport of molecules such as hsp60 to the host cytoplasm. This would provide the opportunity for hsp60 to interact with cellular molecules such as procaspase-9 to prevent activation and thereby interrupt apoptosis. This mechanism has been described for mammalian heat shock proteins (3). If this is the case, it could account for one mechanism for how chlamydiae establish a persistent infection.
Another mechanism is suggested from apoptotic regulation, which occurs at the protein level by the formation of hetero- and homodimers among members of the Bcl-2 family of proteins. This family consists of antagonistic members that inhibit or induce apoptosis, depending upon the ratio of proapoptotic to antiapoptotic proteins within the cell following a stimulus. These proteins have regions of homology that are conserved in mammals from humans to nematodes, and homologues have been identified in viral pathogens (17, 18). With the addition of homologous viral proteins, the cellular protein balance can be shifted. An interesting example is provided by adenovirus, in which the E1A and E1B loci code for proteins that are pro- and antiapoptotic, respectively, serving the purpose of prolonging cell survival to allow replication and then inducing apoptosis to facilitate virion release and spread to other cells (34). The E1B 19-kDa protein blocks apoptosis by binding to the cellular apoptosis-inducing protein Bax and titrating out its effect (16). However, overexpression of E1B enhances survival of adenovirus-infected human but not mouse cells (12, 13). These data suggest that it is the overall protein environment of the cell that finally determines whether a protein or organism will provide an apoptotic stimulus or not.
Although C. trachomatis has been shown to modulate apoptosis, no proteins with homology to Bcl-2 have been identified, and this mechanism has not been explored. However, it is intriguing that overexpression of Bcl-2 has been shown to block the translocation of cytochrome c from the mitochondrion after apoptotic induction, whereas other apoptotic inhibitors such as caspase had no effect (38). This suggests that a Bcl-2 homologue or a nonhomologous protein that can interact with the mitochondrial membrane in a similar fashion may be involved. Bcl-2 is thought to stabilize the mitochondrial membrane, where it is located in the outer membrane, preventing the release of cytochrome c from the intermembrane space, where it is localized. It is interesting that exogenous cytochrome c added to the cytosol can mitigate the effect of Bcl-2 apoptotic inhibition and induce caspase activation (21). Bcl-2 may play additional roles in mitochondrial function as well. Further research will be required to determine whether C. trachomatis produces a homologue or nonhomologue protein with similar activity and whether hsp60 can be functionally described as an antiapoptotic protein.
ACKNOWLEDGMENTS
We thank Amy Helmer and Shanya Becha for excellent technical assistance.
This research was supported by Public Health Service grant R01 AI39499 (to D.D.) from the National Institutes of Health.
REFERENCES
- 1.Beatty W L, Byrne G I, Morrison R P. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA. 1993;90:3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beatty W L, Morrison R P, Byrne G I. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beere H M, Wolf B B, Cain K, Mosser D D, Mahboubi A, Kuwana T, Tailor P, Morimoto R I, Cohen G M, Green D R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 4.Brunham R C, Peeling R W. Chlamydia trachomatis antigens: role in immunity and pathogenesis. Infect Agents Dis. 1994;3:218–233. [PubMed] [Google Scholar]
- 5.Coles A M, Reynolds D J, Harper A, Devitt A, Pearce J H. Low-nutrient induction of abnormal chlamydial development: a novel component of chlamydial pathogenesis? FEMS Microbiol Lett. 1993;106:193–200. doi: 10.1111/j.1574-6968.1993.tb05958.x. [DOI] [PubMed] [Google Scholar]
- 6.Dean D. Chlamydia trachomatis sexually transmitted diseases. In: Connor D H, Schwartz D A, Chandler F W, editors. Diagnostic pathology of infectious diseases. Stamford, Conn: Appleton and Lange Publishers; 1997. pp. 473–490. [Google Scholar]
- 7.Dean D. Trachoma. In: Connor D H, Schwartz D A, Chandler F W, editors. Diagnostic pathology of infectious diseases. Stamford, Conn: Appleton and Lange Publishers; 1997. pp. 498–507. [Google Scholar]
- 8.Dean D, Suchland R, Stamm W. Evidence for long-term cervical persistence of Chlamydia trachomatis by omp1 genotyping. J Infect Dis. 2000;182:909–916. doi: 10.1086/315778. [DOI] [PubMed] [Google Scholar]
- 9.Fan T, Lu H, Hu H, Shi L, McClarty G A, Nance D M, Greenberg A H, Zhong G. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med. 1998;187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng Y, Shane R B, Berencsi K, Gonczol E, Zaki M H, Margolis D J, Trinchieri G, Rook A H. Chlamydia pneumoniae inhibits apoptosis in human peripheral blood mononuclear cells through induction of IL-10. J Immunol. 2000;164:5522–5529. doi: 10.4049/jimmunol.164.10.5522. [DOI] [PubMed] [Google Scholar]
- 11.Golstein P, Ojcius D M, Young J D. Cell death mechanisms and the immune system. Immunol Rev. 1991;121:29–65. doi: 10.1111/j.1600-065x.1991.tb00822.x. [DOI] [PubMed] [Google Scholar]
- 12.Gooding L R. Virus proteins that counteract host immune defenses. Cell. 1992;71:5–7. doi: 10.1016/0092-8674(92)90259-f. [DOI] [PubMed] [Google Scholar]
- 13.Gooding L R, Aquino L, Duerksen-Hughes P J, Day D, Horton T M, Yei S P, Wold W S. The E1B 19,000-molecular-weight protein of group C adenoviruses prevents tumor necrosis factor cytolysis of human cells but not of mouse cells. J Virol. 1991;65:3083–3094. doi: 10.1128/jvi.65.6.3083-3094.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grayston J T, Wang S P, Yeh L J, Kuo C C. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985;7:717–725. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- 15.Hackstadt T, Scidmore M A, Rockey D D. Lipid metabolism in Chlamydia trachomatis-infected cells: directed trafficking of Golgi-derived sphingolipids to the chlamydial inclusion. Proc Natl Acad Sci USA. 1995;92:4877–4881. doi: 10.1073/pnas.92.11.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han J, Modha D, White E. Interaction of E1B 19K with Bax is required to block Bax-induced loss of mitochondrial membrane potential and apoptosis. Oncogene. 1998;17:2993–3005. doi: 10.1038/sj.onc.1202215. [DOI] [PubMed] [Google Scholar]
- 17.Hockenbery D M. The bcl-2 oncogene and apoptosis. Semin Immunol. 1992;4:413–420. [PubMed] [Google Scholar]
- 18.Hockenbery D M, Oltvai Z N, Yin X M, Milliman C L, Korsmeyer S J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 19.Hsia R C, Pannekoek Y, Ingerowski E, Bavoil P M. Type III secretion genes identify a putative virulence locus of Chlamydia. Mol Microbiol. 1997;25:351–359. doi: 10.1046/j.1365-2958.1997.4701834.x. [DOI] [PubMed] [Google Scholar]
- 20.Jendro M C, Deutsch T, Korber B, Kohler L, Kuipers J G, Krausse-Opatz B, Westermann J, Raum E, Zeidler H. Infection of human monocyte-derived macrophages with Chlamydia trachomatis induces apoptosis of T cells: a potential mechanism for persistent infection. Infect Immun. 2000;68:6704–6711. doi: 10.1128/iai.68.12.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 22.Lowe S W, Ruley H E. Stabilization of the p53 tumor suppressor is induced by adenovirus 5 E1A and accompanies apoptosis. Genes Dev. 1993;7:535–545. doi: 10.1101/gad.7.4.535. [DOI] [PubMed] [Google Scholar]
- 23.Majno G, Joris I. Apoptosis, oncosis, and necrosis: an overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison R P. Chlamydial hsp60 and the immunopathogenesis of chlamydial disease. Semin Immunol. 1991;3:25–33. [PubMed] [Google Scholar]
- 25.Morrison R P, Belland R J, Lyng K, Caldwell H D. Chlamydial disease pathogenesis: the 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989;170:1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison R P, Manning D S, Caldwell H D. Immunology of Chlamydia trachomatis infections: immunoprotective and immunopathogenic responses. In: Quinn T C, editor. Sexually transmitted diseases. Vol. 8. New York, N.Y: Raven Press; 1992. pp. 57–84. [Google Scholar]
- 27.Moulder J W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985;49:298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ojcius D M, Souque P, Perfettini J L, Dautry-Varsat A. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J Immunol. 1998;161:4220–4226. [PubMed] [Google Scholar]
- 29.Patton D L, Sweeney Y T C, Kuo C C. Demonstration of delayed hypersensitivity in Chlamydia trachomatis salpingitis in monkeys: a pathogenic mechanism of tubal damage. J Infect Dis. 1994;169:680–683. doi: 10.1093/infdis/169.3.680. [DOI] [PubMed] [Google Scholar]
- 30.Perfettini J L, Darville T, Gachelin G, Souque P, Huerre M, Dautry-Varsat A, Ojcius D M. Effect of Chlamydia trachomatis infection and subsequent tumor necrosis factor alpha secretion on apoptosis in the murine genital tract. Infect Immun. 2000;68:2237–2244. doi: 10.1128/iai.68.4.2237-2244.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry L L, Feilzer K, Hughes S, Caldwell H D. Clearance of Chlamydia trachomatis from the murine genital mucosa does not require perforin-mediated cytolysis or Fas-mediated apoptosis. Infect Immun. 1999;67:1379–1385. doi: 10.1128/iai.67.3.1379-1385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao L, Debbas M, Sabbatini P, Hockenbery D, Korsmeyer S, White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci USA. 1992;89:7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephens R S, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 34.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tewari M, Dixit V M. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 36.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 37.Wyllie A H, Kerr J F, Currie A R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T I, Jones D P, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]