Abstract
Simple Summary
Growth hormone (GH) deficiency is the most common hypothalamic-pituitary disorder due to a brain tumor during childhood, whether it is related to the tumor itself or to the treatment. Nevertheless, the use of growth hormone replacement therapy (GHRT) remains controversial due to its potential proliferative properties. Few data are available on the safety of growth hormone replacement therapy in children with low-grade gliomas (LGG). The aim of our retrospective study was to assess the impact of growth hormone replacement therapy on the risk of relapse in children treated for midline low-grade gliomas. We included 124 children treated for low-grade midline glioma. Among them, 17 were supplemented with growth hormone. There was no significant difference in terms of relapse between the group supplemented with growth hormone and the group not supplemented. These results support the safety of growth hormone in this population.
Abstract
There is little scientific evidence regarding the safety of GHRT in LGG, where GH deficiency is common. Purpose: to compare the recurrence rate in children with midline LGG, depending on whether or not they have received GHRT, in order to assess its impact on the risk of tumor recurrence. Methods: This bicentric retrospective study included 124 patients under the age of 18 who were diagnosed with a midline low-grade glial tumor between 1998 and 2016. We also reviewed literature on this subject. The main outcome measure was tumor relapse, demonstrated by brain MRI. Results: There were 17 patients in the GH-supplemented group (14%) and 107 patients in the non-supplemented group (86%). Relapse occurred in 65 patients (45.5%); 7 patients died (4.9%); no deaths occurred in patients receiving GHRT. Two patients developed a second tumor (1.4%), none of which had received GHRT. Relapse concerned 36.4% of patients without GHRT and 52.9% of patients with GHRT. The difference was not statistically significant between the two groups (p = 0.3). Conclusion: GHRT does not lead to a statistically significant increase in risk of relapse for pediatric midline low-grade pediatric glioma in our cohort. Although these results appear reassuring, future natural history or prospective studies should be done to ascertain these findings. Nevertheless, these reassuring data regarding GHRT are in agreement with the data in the current literature.
Keywords: growth hormone (GH), growth hormone replacement therapy (GHRT), growth hormone safety, low-grade glioma, tumor relapse, endocrine monitoring
1. Introduction
Long-term overall survival for children with low-grade glioma (LGG) is now 95% at 5 years [1] and 87% at 10 years [2] thanks to recent scientific advances. The major challenge is to prevent long-term sequelae in what is often a chronic, but rarely fatal, disease. Growth hormone (GH) deficiency is the most common hypothalamic-pituitary disorder due to a brain tumor during childhood, whether it is related to the tumor itself or to the treatment received [3]. Indeed, in the absence of GH replacement therapy (GHRT), 40% of pediatric brain tumor long-term survivors have a short adult height below the tenth percentile [4]. Additionally, GHRT is important to prevent short stature in adulthood, osteoporosis, or metabolic syndrome [5].
GH has a proliferative role, and it inhibits apoptosis by activating the PI3K-AKT-mTOR signaling pathway [6]. This same signaling pathway is known to be involved in the carcinogenesis of certain LGGs in children [6].
Therefore, it is important to assess the risk of tumor recurrence or progression in patients with LGG when GHRT is used, especially in the case of residual tumor. Precautions before starting GHRT are well known: the glioma should be considered inactive and all anti-tumor treatment should have been completed for at least 12 months, as recommended [5,7]. GH treatment should be discontinued if there are signs of tumor growth [8].
The safety of GHRT has already been proven by numerous studies when used in children or adults without specific risk factors for developing cancer [6,8,9]. Several studies have already shown that the risk of tumor recurrence is not increased with GHRT in patients with craniopharyngioma, medulloblastoma, or malignant germ-cell tumor [6,10,11,12,13,14,15,16,17]. Some studies have also investigated gliomas and GHRT and did not show an increased risk of relapse occurring in connection with GHRT [6,12,16]. However, these are conclusions drawn from subgroups of the population. The results are quite disparate from one study to another.
Midline location is a statistically significant independent risk factor for growth hormone deficiency due to proximity to the pituitary and hypothalamus [2,5]. Growth hormone (GH) deficiency is common in this location due to tumor growth, tumor removal, or radiation therapy in the hypothalamic-pituitary region [7]. No studies to date have focused on midline LGGs.
While overall survival is very good, progression-free survival is less than 40% at 5 years [18]. LGG therefore often becomes a chronic disease. Over the long term, overall survival is 50.4% at 18 years for gliomas of the optic pathways in particular [18]. The main cause of death is tumor progression. The neuro-cognitive sequelae are often significant in the long term [19]. Age and intracranial hypertension at diagnosis are often associated with a worse prognosis [18]. Currently, the management of LGG is primarily surgical excision, which can be curative when total excision is possible. Unlike other LGGs in which surgery is often the only treatment (e.g., posterior fossa), surgical removal of midline LGGs is often not possible due to proximity to the optic chiasm brainstem and hypothalamic pituitary axis [20]. Indeed, the risk of visual, neurological, and endocrine sequelae is major [19]. When surgery is impossible or when residual tumor persists after surgery, the risk of progression or relapse is high. In that case, chemotherapy is usually given. The three most commonly used protocols in France during this period were the BBSFOP protocol (6 cytotoxic agents given sequentially for 16 months and including carboplatin, procarbazine, etoposide, cisplatin, vincristine, and cyclophosphamide) [21], the SIOP-LGG 2004 protocol (vincristine carboplatin) [22] and the Vinblastine protocol [23]. Sometimes, radiotherapy remains the most suitable treatment, but the tendency is to use it less, due to radiation-induced later effects, especially in very young children (secondary cancer, post-radiation angiopathy, hypothalamic-pituitary dysfunction, and/or slowed cognitive development) [18].
The main objective of our study was to compare the recurrence rate in children with midline LGG, depending on whether or not they received growth hormone replacement therapy, in order to assess its impact on the risk of tumor recurrence. The secondary objective was to carry out a descriptive, retrospective analysis of the cohort of children treated for a low-grade glial tumor of the midline, between 1 January 1998 and 31 December 2016 at the university hospitals of Lille and Lyon in France.
2. Materials and Methods
The study was an observational, retrospective and descriptive study. The information was collected by a standardized and anonymized data collection sheet. In accordance with French regulations, the study protocol was approved by the Commission Nationale de l’Informatique et des Libertés (CNIL) on the basis of a declaration of conformity MR-004 (No. 2215746).
We defined the midline tumors as limited to the diencephalon, optic nerves, optic chiasm, pituitary stalk area, hypothalamus, epiphysis, thalamus, and third ventricle. We included all patients treated for low-grade midline glioma diagnosed between 1 January 1998 and 31 December 2013 in Lille (University Hospital and Center Oscar Lambret comprehensive cancer center) and between 1 January 2000 and 31 December 2016 in Lyon (IHOPe), allowing a minimum of 3 years follow-up at the time of data collection for all patients. Patients had to be between 0 and 18 years of age at the time of diagnosis. We excluded patients for whom follow-up was not carried out only in these centers, patients lost to follow-up, and those for whom there was a lack of data. Patients who died before the end of first-line oncological treatment were also excluded as well as those who had recurrence within one year of the last oncological treatment. One patient was excluded from the series because his GH replacement therapy was started before one year of remission, contrary to the recommendations [10]. We performed a comparative analysis of the tumor recurrence rate between patients substituted and unsubstituted for growth hormone.
The risk factor studied was exposure to growth hormone. For the diagnosis of GH deficiency, IGF1 was measured as well as IGFBP3 in young children. GH must be measured after stimulation in France by hypoglycemia (insulin tolerance test), by L-dopa, arginine, glucagon, propranolol, clonidine, or GHRH [24]. These stimulation tests can be used in combination. The diagnosis of GH deficiency (GHD) must be proven by two tests, a simple stimulation test and a combined stimulation test. If both tests show a result below 10 mIU/mL (3 micrograms/L), it is a severe GH deficiency. There is a partial GH deficiency if the results are between 10 and 20 mIU/L (3 to 6.7 micrograms/L). A single test with a response >6.7 micrograms/L excludes the diagnosis of GHD [24]. If there is an obvious acquired cause of GHD, a single stimulation test measuring IGF1 is necessary [24]. In our study, all patients were monitored for height and weight growth. Growth hormone deficiency was suspected in children presenting with height curve brake and confirmed on IGF1 levels and by two growth hormone stimulation tests.
The primary endpoint was relapse. Relapse was defined by the reappearance of the tumor on imaging (in a patient previously in complete remission), or the progression in volume of a pre-existing remnant tumor, occurring outside of any oncological treatment, or at least one year following the diagnosis for patients who have not received any treatment apart from a possible biopsy. The tumor was considered as stable if it did not increase in volume on two consecutive MRI scans, one year apart, without any oncological treatment. If no tumor residue was seen on the MRI one year after the end of the oncological treatments, the patient was then considered to be in complete remission
In order to take into account the various confounding factors, we performed a univariate analysis. Continuous variables were investigated using Student’s and Wilcoxon’s tests. Qualitative variables were studied with Fisher’s test or Chi-square. The significance level was set arbitrarily at 5%, i.e., an expected alpha probability value such as p < 0.05, with a 95% confidence interval (95% CI). Variables from the univariate analysis with a p-value < 0.1 were then included in the multivariate analysis. We performed a logistic regression to study the different factors that may interfere with the risk of relapse. These values were expressed in an odd ratio (OR) with a 95% confidence interval. We used R statistics software version 3.5.2.
3. Results
3.1. Population Characteristics
3.1.1. Flow Chart
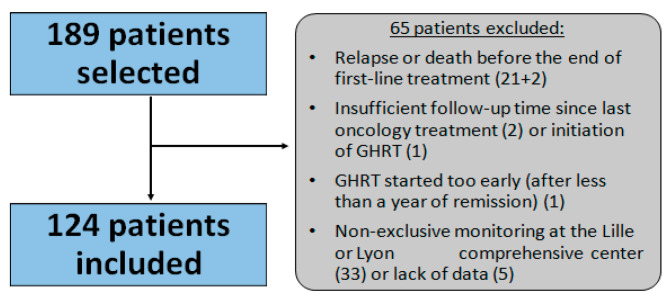
We included 124 patients (Figure 1).
Figure 1.
Flow chart depicting the selection of patients for the study. GHRT: growth hormone replacement therapy.
3.1.2. Comparison of the Population in the Two Groups
Median age at diagnosis was 4.5 years (0–17.6). Only 94 patients (76%) were referred to an endocrinologist.
Seventeen patients received GHRT (13.7%), with a median dose of 33 µg/kg/day (14–48) and with a median duration of 2.2 years (0.3–8). Population characteristics are described in Table 1.
Table 1.
Population characteristics.
| Group without GHRT | Group with GHRT | p-Value | |
|---|---|---|---|
| Total population | n (%) | n (%) | |
| 107 | 17 | ||
| Gender | 0.38 | ||
| Female | 60 (56.1%) | 7 (41.2%) | |
| Male | 47 (43.9%) | 10 (58.8%) | |
| NF1 | 39 (36.5%) | 3 (17.7%) | 0.21 |
| Tumor type | |||
| Pilocytic astrocytoma (grade I) | 95 (88.8%) | 12 (70.6%) | 0.02 |
| Astrocytoma (grade II) | 2 (1.9%) | 3 (17.6%) | |
| Oligoastrocytoma (grade II) | 3 (2.8%) | 0 (0%) | |
| Oligodendroglioma (grade II) | 3 (2.8%) | 0 (0%) | |
| Ganglioglioma (grade II) | 4 (3.7%) | 2 (11.8%) | |
| Biopsy (followed by chemo or RT or simple monitoring) | 22 (20.6%) | 6 (35.3%) | 0.21 |
| First-line treatments (can be combined) | |||
| Subtotal resection | 26 (24.3%) | 6 (35.3%) | 0.37 |
| Complete resection | 9 (8.4%) | 0 (0%) | 1 |
| Chemotherapy | 53 (49.5%) | 15 (88.2%) | 0.003 |
| Radiotherapy | 7 (6.5%) | 1 (5.9%) | 0.99 |
| First-line treatments in detail | |||
| Surgery only | 19 (17.8%) | 1 (5.9%) | 0.3 |
| Surgery + RT | 3 (2.8%) | 0 (0%) | 1 |
| Surgery + chemo | 14 (13.1%) | 5 (29.4%) | 0.14 |
| Surgery + RT + chemo | 1 (0.9%) | 0 (0%) | 1 |
| Chemo only | 37 (34.6%) | 9 (52.9%) | 0.18 |
| RT only | 2 (1.9%) | 0 (0%) | 1 |
| Chemo + RT | 1 (0.9%) | 1 (5.9%) | 0.26 |
| Simple monitoring | 30 (28%) | 1 (5.9%) | 0.07 |
NF1: Neurofibromatosis type 1; GHRT: growth hormone replacement therapy; RT: radiotherapy; Chemo: chemotherapy.
Biopsy alone was not considered as a treatment.
Twenty patients had surgery only, without radiotherapy or chemotherapy: 19 in the group without GHRT and 1 in the group with GHRT. Two patients had only received first-line radiotherapy; they were in the group without GHRT. The median total dose of radiotherapy was 50.4 Grays (25.7–56). Thirty-one patients were only observed, without any treatment (30 patients in the group without GHRT and 1 patient in the group with GHRT).
Nine patients underwent total tumor resection (8.4%). Among them, there had been no deaths, and only one relapse (p = 0.08). All were in the group without GHRT. The other patients only benefited from partial excision (26 patients in the group without GHRT [24.3%] and 6 patients in the group with GHRT [35.3%]).
Moreover, none of the patients who could benefit from total excision had received adjuvant chemotherapy. Chemotherapy was therefore administered if the tumor was not operable or when the resection was incomplete. A total of 28 patients received a biopsy, 41 patients underwent complete or incomplete resection, and 55 patients therefore did not have an anatomo-pathological diagnosis. Among them, 42 patients were carriers of NF1. In the case of NF1, the diagnosis of OPG is based on its characteristic appearance of glioma on MRI and does not require histological proof [25]. Thirteen patients did not have NF1 and did not have an anatomo-pathological confirmation because the magnetic resonance imaging (MRI) was very suggestive of an OPG, and the biopsy was too risky for their location. For one of them, the biopsy had not been done because it was a therapeutic emergency to start chemotherapy.
There was no significant difference between the two groups, apart from two characteristics: number of patients with pilocytic astrocytoma was significantly lower in the group receiving GHRT (70.9% versus 88.8%, p = 0.02); number of patients treated with chemotherapy was higher in the group receiving GHRT (88.2% versus 49.5%, p = 0.003).
The median duration of follow-up since the end of oncological treatment was 8 years (4–211 months). On average, patients substituted with GH were followed longer after the end of their treatment than non-substituted patients. The average duration of oncological follow-up was 12.6 years (±33 months) in the group with GHRT, versus 7.8 years (±53.5 months) for children without GHRT (p < 0.001). The average time between the end of oncological treatment and the start of GHRT was 67.4 months, or 5.6 years (±31.3 months). All the characteristics of patients supplemented with GH are described in Table 2, and in Table 3 for patients not supplemented.
Table 2.
Characteristics of patients treated with GH following a low-grade midline glial tumor between 1 January 1998 and 31 December 2016 at the Lille and Lyon CHUs.
| Patients: | Age at Diagnosis (Year) |
Sex | NF1 | Histology | Localization | Surgery | Radio Therapy |
Chemo Therapy |
GH Onset Age (y) | GH Dose (μg/kg/d) | GH Duration (Months) | Relapse | Second Cancer | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. 13 | 7.5 | M | No | Grade II astrocytoma | Bilateral thalamus | B | - | - | 15.4 | 48 | 47 | No | No | No |
| No. 14 | 4.3 | M | Yes | Pilocytic astrocytoma | OPG | - | - | Chemo | 10.8 | 36 | 33 | Yes # before GH |
No | No |
| No. 15 | 2 | F | No | Pilocytic astrocytoma | OPG | B | - | Chemo | 10.9 | 38 | 20 | Yes # before GH |
No | No |
| No. 21 | 8.1 | M | No | Grade II astrocytoma | Left thalamus | B | - | Chemo | 12.5 | 32 | 12 | No | No | No |
| No. 23 | 0.3 | F | No | Grade II astrocytoma | Chiasmatoventricular | B | - | Chemo | 9.5 | 22 | * | No | No | No |
| No. 24 | 7 | M | No | Pilocytic astrocytoma | Chiasmatoventricular | B | - | Chemo | 13.5 | 29 | 40 | Yes # before GH |
No | No |
| No. 25 | 1.1 | F | No | Pilocytic astrocytoma | Chiasmatoventricular | SR | - | Chemo | 13.2 | 33 | 39 | Yes # before GH |
No | No |
| No. 27 | 3 | M | No | Pilocytic astrocytoma | Chiasmatoventricular | SR | - | Chemo | 10.75 | 37 | 54 | Yes # before GH |
No | No |
| No. 41 | 3.2 | M | No | Pilocytic astrocytoma | OPG | - | - | Chemo | 13.7 | 37 | * | No | No | No |
| No. 43 | 6.6 | M | No | Pilocytic astrocytoma | Chiasmatoventricular and V3 | SR | - | Chemo | 14.3 | 23 | 14 | Yes # before GH |
No | No |
| No. 45 | 1.3 | F | Yes | Pilocytic astrocytoma | OPG | - | - | Chemo | 10.5 | 41 | * | No | No | No |
| No. 47 | 1.1 | M | No | Pilocytic astrocytoma | OPG | SR | - | Chemo | 13.75 | 36 | 7 | Yes under GH |
No | No |
| No. 51 | 0.7 | F | No | Pilocytic astrocytoma | Right optic strip, diencephalon | SR | - | Chemo | 10.2 | 33 | * | No | No | No |
| No. 77 | 10.1 | F | No | Pilocytic astrocytoma | OPG and V3 | B | - | Chemo | 12.8 | 35 | 6 | Yes under GH |
No | No |
| No. 81 | 4 | M | Yes | Pilocytic astrocytoma | OPG | - | RT | Chemo | 8.8 | 35 | 96 | No | No | No |
| No. 83 | 13 | M | No | Ganglioglioma | Suprasellar tumor and lateral ventricle | SR | - | - | 20 | 15 | 24 | No | No | No |
| No. 91 | 0.6 | F | No | Ganglioglioma | OPG | - | - | Chemo | 8.6 | 36 | 9 | Yes under GH |
No | No |
M: male, F: feminine, NF1: Neurofibromatosis type 1, PA: Pilocytic Astrocytoma, OPG: Optic Pathway Glioma, V3: third ventricle, CR: complete resection, SR: Subtotal resection, B: Biopsy, Chemo: Chemotherapy, RT: Radiotherapy, * GH not interrupted at the time of data collection. # first relapse, then new remission before starting GH, but no relapse on GH.
Table 3.
Characteristics of patients without GH following a low-grade midline glial tumor between 1 January 1998 and 31 December 2016 at the Lille and Lyon CHUs.
| No. | Sex | NF1 | Tumor | Localization | Surgery | RT | Chemo | Relapse | Death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | - | PA | Thalamo-peduncular | SR | RT | - | - | - |
| 2 | F | - | PA | Chiasmato-ventricular | SR | - | Chemo | Relapse | - |
| 3 | M | - | PA | OPG | B | - | Chemo | - | - |
| 4 | F | - | PA | OPG | SR | - | Chemo | Relapse | - |
| 5 | F | NF1 | PA | OPG | - | - | - | - | - |
| 6 | F | - | PA | Chiasmato-ventricular | SR | RT | Chemo | Relapse | - |
| 7 | M | NF1 | PA | OPG | - | - | - | - | - |
| 8 | F | NF1 | PA | OPG | - | - | - | - | - |
| 9 | F | - | PA | OPG | B | - | Chemo | - | - |
| 10 | F | - | PA | Thalamo-peduncular | SR | - | Chemo | Relapse | - |
| 11 | F | - | PA | Suprasellar tumor | - | - | Chemo | Relapse | - |
| 12 | M | - | PA | Chiasmato-ventricular | SR | - | Chemo | - | - |
| 16 | F | NF1 | PA | OPG | - | - | - | - | - |
| 17 | M | - | Astrocytoma grade II | Right thalamus | SR | - | - | - | - |
| 18 | M | - | PA | Thalamo-peduncular | B | - | - | Relapse | - |
| 19 | M | - | Oligoastrocytoma | Left thalamus | SR | - | Chemo | - | - |
| 20 | M | - | PA | Right thalamus | CR | - | - | - | - |
| 22 | F | NF1 | PA | OPG | - | - | - | - | - |
| 26 | M | NF1 | PA | Right optic nerve | - | - | - | - | - |
| 28 | M | - | Oligodendroglioma | Thalamus and left lateral ventricle | CR | - | - | Relapse | - |
| 29 | F | - | PA | Chiasmato-ventricular | B | RT | - | - | - |
| 30 | F | - | Oligoastrocytoma | Right optic nerve | CR | - | - | - | - |
| 31 | F | NF1 | PA | Right optic nerve | - | - | - | - | - |
| 32 | M | - | PA | Chiasmato-ventricular | SR | - | Chemo | Relapse | - |
| 33 | M | - | PA | Right temporo thalamo peduncular | SR | - | Chemo | - | Death |
| 34 | M | - | Oligodendroglioma | Right thalamus | B | RT | - | - | - |
| 35 | M | - | PA | OPG | B | - | Chemo | - | - |
| 36 | M | - | PA | Left optic nerve | CR | - | - | - | - |
| 37 | F | - | PA | Chiasmato-ventricular | SR | RT | - | Relapse | - |
| 38 | M | NF1 | PA | OPG | - | - | - | - | - |
| 39 | M | - | PA | OPG infiltrating basal ganglia | B | - | Chemo | Relapse | - |
| 40 | F | NF1 | PA | OPG | - | - | Chemo | Relapse | - |
| 42 | F | NF1 | PA | OPG | - | - | - | Relapse | - |
| 44 | M | - | PA | Chiasmato-ventricular | B | - | Chemo | Relapse | Death |
| 46 | M | NF1 | PA | Left optic nerve | CR | - | - | - | - |
| 48 | M | NF1 | PA | OPG | - | - | - | - | - |
| 49 | F | - | PA | V3 | CR | - | - | - | - |
| 50 | F | - | Oligodendroglioma | Right thalamus | B | - | - | - | - |
| 52 | F | NF1 | PA | OPG | - | - | - | - | - |
| 53 | F | NF1 | PA | OPG | - | - | Chemo | Relapse | - |
| 54 | F | NF1 | PA | OPG | - | - | Chemo | Relapse | - |
| 55 | F | NF1 | PA | OPG | - | - | Chemo | Relapse | - |
| 56 | F | - | PA | Left thalamus | CR | - | Chemo | - | - |
| 57 | M | - | Astrocytoma grade II | OPG and diencephalon | B | - | Chemo | - | - |
| 58 | F | - | PA | OPG | SR | RT | - | - | - |
| 59 | F | - | PA | OPG | - | - | Chemo | - | - |
| 60 | F | - | Oligoastrocytoma | Right V3 and thalamus | B | - | Chemo | Relapse | - |
| 61 | M | - | Ganglioglioma | Right occipital ventricular junction tumor | SR | - | - | Relapse | - |
| 62 | M | - | PA | Suprasellar tumor | SR | - | Chemo | Relapse | - |
| 63 | F | - | PA | Missing data | B | - | Chemo | - | - |
| 64 | F | - | PA | OPG | SR | - | Chemo | Relapse | Death |
| 65 | M | NF1 | PA | Left optic nerve | - | - | - | - | - |
| 66 | F | NF1 | PA | OPG | - | - | - | - | - |
| 67 | F | - | PA | V3 | CR | - | - | - | - |
| 68 | M | - | PA | OPG | SR | - | Chemo | Relapse | - |
| 69 | M | - | PA | OPG | SR | - | Chemo | Relapse | - |
| 70 | F | - | PA | Suprasellar tumor | SR | - | Chemo | Relapse | - |
| 71 | F | - | PA | OPG | - | - | Chemo | - | - |
| 72 | M | - | PA | Right thalamus | SR | - | - | - | - |
| 73 | M | NF1 | PA | OPG | - | - | - | - | - |
| 74 | F | - | PA | Suprasellar tumor | SR | - | - | Relapse | - |
| 75 | M | - | PA | OPG and V3 | SR | - | - | Relapse | - |
| 76 | F | NF1 | PA | OPG | - | - | - | - | - |
| 78 | F | - | PA | Quadruple blade | SR | - | - | - | - |
| 79 | M | NF1 | PA | OPG | - | - | Chemo | - | Death |
| 80 | F | - | PA | Thalamo-peduncular | B | - | Chemo | - | - |
| 82 | F | - | Ganglioglioma | V3 | SR | - | - | - | - |
| 84 | F | - | PA | Retrochiasmatic lesion | - | - | - | Relapse | - |
| 85 | M | - | Ganglioglioma | V3 | CR | - | - | - | - |
| 86 | F | - | PA | Suprasellar tumor | B | - | - | - | - |
| 87 | F | NF1 | PA | OPG | - | - | - | - | - |
| 88 | F | NF1 | PA | OPG | - | - | - | Relapse | - |
| 89 | F | NF1 | PA | OPG | - | - | - | - | - |
| 90 | F | NF1 | PA | OPG | - | - | - | - | - |
| 92 | F | - | PA | OPG | B | - | Chemo | - | - |
| 93 | F | - | PA | OPG | - | - | Chemo | Relapse | - |
| 94 | M | - | PA | OPG | SR | - | - | Relapse | - |
| 95 | F | - | PA | OPG | B | - | Chemo | - | - |
| 96 | F | NF1 | PA | OPG and V3 | - | - | - | Relapse | - |
| 97 | F | - | PA | OPG | B | - | Chemo | - | - |
| 98 | M | - | PA | OPG | - | - | Chemo | - | - |
| 99 | F | - | PA | OPG | - | - | Chemo | - | - |
| 100 | F | - | PA | OPG | - | - | Chemo | - | - |
| 101 | F | NF1 | PA | OPG | - | - | - | Relapse | - |
| 102 | M | NF1 | PA | OPG | - | - | - | - | Death |
| 103 | M | - | PA | Thalamo-peduncular | B | - | Chemo | - | - |
| 104 | M | - | PA | Right thalamus and V3 | B | - | Chemo | - | - |
| 105 | M | - | Ganglioglioma | Hypothalamus | - | - | - | Relapse | - |
| 106 | F | - | PA | Left thalamus | B | - | Chemo | Relapse | - |
| 107 | M | NF1 | PA | OPG | - | - | - | Relapse | - |
| 108 | M | NF1 | PA | OPG | - | - | - | - | - |
| 109 | M | NF1 | PA | OPG | - | - | Chemo | - | - |
| 110 | M | NF1 | PA | OPG | - | - | Chemo | - | - |
| 111 | M | - | PA | OPG | - | - | Chemo | Relapse | - |
| 112 | F | NF1 | PA | OPG | - | - | Chemo | - | - |
| 113 | M | NF1 | PA | OPG | - | - | Chemo | Relapse | - |
| 114 | F | NF1 | PA | OPG | - | - | Chemo | - | - |
| 115 | F | NF1 | PA | OPG | - | - | Chemo | Relapse | - |
| 116 | F | NF1 | PA | OPG | - | - | Chemo | - | - |
| 117 | F | NF1 | PA | OPG | - | - | Chemo | - | - |
| 118 | M | - | PA | OPG | - | RT | Chemo | - | - |
| 119 | M | - | PA | OPG | B | - | Chemo | Relapse | - |
| 120 | M | - | PA | OPG | SR | - | - | - | - |
| 121 | F | - | PA | Right capsulo-thalamic | B | - | - | - | - |
| 122 | M | - | PA | OPG | - | - | - | - | - |
| 123 | F | NF1 | PA | OPG | - | - | - | Relapse | - |
| 124 | F | - | PA | Suprasellar and V3 | SR | - | Chemo | - | - |
M: male; F: female; NF1: neurofibromatosis type 1; PA: pilocytic astrocytoma; OPG: optic pathway glioma; V3: third ventricle; CR: complete resection; SR: subtotal resection; B: biopsy; Chemo: chemotherapy; RT: radiotherapy.
3.2. Primary Endpoint: Relapse
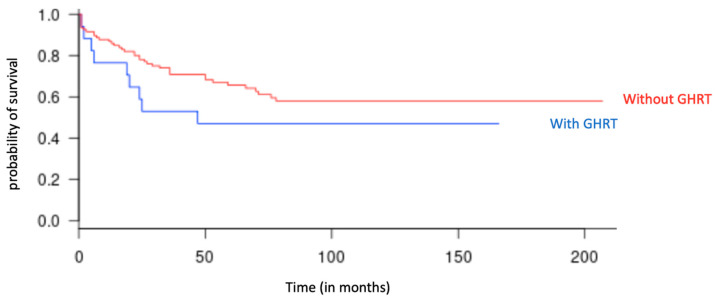
Nine relapses were observed out of the 17 patients receiving GHRT (52.9%), and 39 relapses were observed out of 107 without GHRT (36.4%) (p = 0.3). The median time to relapse in children with GHRT was 31 months (13–59) since the end of the last oncological treatment (p = 0.27), while it was 34 months (13–83) for patients without GHRT. The relapse-free survival rate over the entire duration of the study was 60.7% in the group without GHRT and 41.2% in the group with GHRT; the difference between the two groups was not significant (p = 0.13) (Figure 2).
Figure 2.
Relapse-free survival curve as a function of time (in months), in the groups with GHRT (in blue) and without GHRT (in red), over the entire duration of the study.
Relapse was not significantly associated with age at diagnosis, gender, presence of NF1, or tumor type (Table 4). All of the analyses concerning the risk factors for relapse were carried out in univariate, then the risk of relapse was studied in multivariate for the variables with a p-value < 0.1 (resection and chemotherapy).
Table 4.
The impact of different factors on relapse in patients treated for midline glioma in Lille and Lyon between 1 January 1998 and 31 December, 2016 (univariable analysis).
| Patients who Did Not Relapse | Patients who Did Relapse | ||||
|---|---|---|---|---|---|
| n = 76 | % | n = 48 | % | p-Value | |
| Age at diagnosis | 0.96 | ||||
| <12 months | 8 | 10.5 | 6 | 12.5 | |
| >12 months | 68 | 89.5 | 42 | 87.5 | |
| Gender | 0.83 | ||||
| Female | 40 | 52.6 | 27 | 56.3 | |
| Male | 36 | 47.4 | 21 | 43.7 | |
| NF1 | 0.28 | ||||
| without NF1 | 47 | 61.8 | 35 | 72.9 | |
| with NF1 | 29 | 38.2 | 13 | 27.1 | |
| Tumor type | 0.49 | ||||
| Pilocytic astrocytoma (grade I) | 64 | 84.2 | 43 | 89.6 | |
| Astrocytoma (grade II) | 5 | 6.6 | 0 | - | |
| Oligoastrocytoma (grade II) | 2 | 2.6 | 1 | 2.1 | |
| Oligodendroglioma (grade II) | 2 | 2.6 | 1 | 2.1 | |
| Ganglioglioma (grade II) | 3 | 4 | 3 | 6.2 | |
| First line chemotherapy | 0.013 | ||||
| Chemotherapy | 35 | 46 | 33 | 68.7 | |
| No chemotherapy | 41 | 54 | 15 | 31.3 | |
| First line radiotherapy | 0.47 | ||||
| Radiotherapy | 6 | 7.9 | 2 | 4.2 | |
| No radiotherapy | 70 | 92.1 | 46 | 95.8 | |
| First line type of surgery | 0.088 | ||||
| Complete resection | 8 | 10.5 | 1 | 2. 1 | |
| Subtotal resection | 13 | 17. 1 | 19 | 86.4 | |
| GHRT | 0.28 | ||||
| no GHRT | 68 | 89.5 | 39 | 81.3 | |
| with GHRT | 8 | 10.5 | 9 | 18.7 | |
NF1: neurofibromatosis type 1; GHRT: growth hormone replacement therapy.
Only chemotherapy was associated with a higher risk of relapse in multivariate analysis (p = 0.043).
3.3. Second Cancer, Death and Diabetes
3.3.1. Second Cancer Rate
Two patients (1.7%) developed a second cancer; both were carriers of NF1, and none of them had received GHRT. One patient had developed a mediastinal T-cell-lymphoma 4 years after treatment (the only treatment of his glioma was a complete surgical removal) and the second a cervical hamartoma 1.5 years after treatment. This patient had received only chemotherapy. The hamartoma is therefore probably linked to NF1.
3.3.2. Death Rate
Five patients died (4.7%) during the study period: two due to tumor progression and three of treatment-related complications. None of them had received GHRT.
3.3.3. Diabetes Rate
None of our patients developed type 2 diabetes on GHRT.
3.4. Particularities in GH Substituted Patients
Of all the patients substituted with GH, three patients (17,6%) presented with a relapse while undergoing treatment with GH (No. 47, 77, 91). GHRT was initiated, for two of them, after the first relapse, and after a one-year remission following this relapse (No. 47 and 108). Patient 108 had a second relapse while on GHRT. Patient 47 also relapsed on GHRT; this was his third relapse.
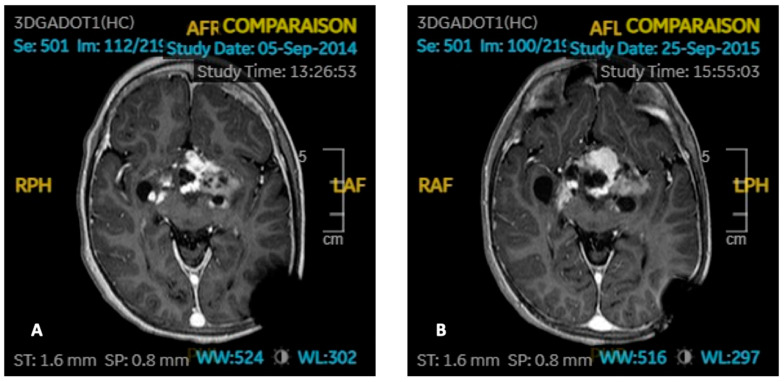
One patient (No. 91) relapsed her disease while receiving GHRT. This 7-month-old girl suffered from an optochiasmatic BRAF V600E-mutated ganglioglioma with a second localization in the 4th ventricle responsible for Russell’s syndrome and blindness at diagnosis. She was initially treated with chemotherapy (vincristine carboplatin). She then had a tumor recurrence 2.5 years later, treated with vinblastine and then with temozolomide, with partial surgery thereafter. GH replacement therapy was started 5 years after the end of any oncological treatment, since the tumor was stable. Nine months after starting GHRT, the patient again experienced tumor progression (Figure 3). The tumor then spontaneously shrank in size when GHRT was definitively stopped. She did not need tumor treatment for 5 years, then relapsed again. She still has sequelae hemiplegia, blindness, and was operated on for a fracture of the right femur due to a priori multifactorial osteoporosis (GH deficiency with contraindication to the resumption of supplementation, gonadotropic deficiency, and immobility).
Figure 3.
Imaging of Patient 91 with ganglioglioma (axial T1-weight with gadolinium). (A) Stable tumor residue before GHRT. (B) Progressive disease after 9 months of GHRT.
The second patient (No. 47) had presented with two tumor recurrences before starting GHRT. Treatment with GH started 4.7 years after the last oncological treatment. A third relapse occurred six months after initiating treatment with GH.
Finally, a last patient (No. 77) was initially treated with chemotherapy (vincristine carboplatin) for a pilocytic astrocytoma of the third ventricle. Six months after starting GHRT, she had a first relapse. She was then treated by chemotherapy (vinblastine), subtotal resection, and then by proton beam therapy. She then developed gonadotropic, corticotropic, and somatotropic insufficiency. GHRT was subsequently resumed; she then had a second tumor recurrence 24 months after the start of the substitution. GHRT has been restarted for a third time at a distance from oncological treatments; it is still in progress at the present time, and the patient is still in remission 5 years later.
4. Discussion
Our study did not show a significant difference in the risk of relapse depending on whether patients treated for midline LGG received GHRT or not. Although there appears to be a trend towards an increased risk of recurrence in the GHRT group, the difference was not significant. Of course these results may be due to a lack of study power related to the relatively small number of patients who received GHRT. The retrospective nature of the study, the small number of patients, as well as a significant difference in patients between the two groups (17 patients with GHRT versus 107 patients without GHRT) represent obvious weaknesses in our study. These results should be confirmed by further studies. However, our cohort was relatively large (124 patients), and the patients were followed over a long period (median follow-up time: 8 years.).
The span from 1998 to 2016 is a wide timeframe, and there was variation in practice over this timespan concerning the use of growth hormone (GH). Before 1985, the treatment of GH deficiency consisted of replacing the subject’s GH by injecting GH extracted from cadaveric human pituitary glands. This was reserved for severe GHD. As early as 1985, GH could be synthesized thanks to molecular biology. This is the case for all patients in our study. The treatment is still very expensive, so the indications for treatment are still limited and also depend on an economic factor [24].
During the follow-up of the treatment with GH, the clinical and biological tolerance is evaluated. The World Health Organization (WHO, Geneva, Switzerland) Expert Committee on Biological Standardization (ECBS) has recognized the need for an international standard for IGF-1 for the calibration of immunoassays and for the control of content of therapeutic products [26]. Thanks to these other standardized dosages, the treatment with GH, which consisted solely of adapting the dose to the weight of the child, was able to evolve. The initial dose still depends on the indication: in the event of GH deficiency, 25 to 35 micrograms/kg/d, then the dose is adapted to clinical tolerance, growth rate, and IGF1 level. IGF1 should be maintained within the upper half of physiological values without exceeding them (<−2SD) [5,7].
The dose of GH and the time of introduction of GHRT (beyond 12 months after the end of the anticancer treatment) were consistent with the recommendations. There were no secondary malignancies following GHRT in our study, consistent with the reassuring data in the literature [27]. The median follow-up was longer in the GHRT group (12.6 years versus 7.8 years) (p < 0.001). This is probably explained by the fact that it is recommended to follow children for a longer time in case of GHRT for fear of a potential relapse [5,7,14].
In our study, only 14% of the children were supplemented with GH. However, according to the literature, GHD concerns approximately 40% of children treated for a brain tumor, and even more so in the case of a midline tumor [4]. This low percentage of children substituted in GH can be explained by two elements. First, only 76% of the children had benefited from endocrine monitoring in our study. However, this follow-up is recommended for all children with a tumor located in the pituitary region [8,10]. It is possible that some GH deficiencies were not seen in some patients due to a lack of endocrine monitoring. In fact, all of the children in the cohort were monitored for height and weight growth, but the search for growth hormone deficiency was not carried out systematically (only in the event of loss of stature or during a consultation with an endocrinologist). Children in whom growth retardation was most evident were probably substituted more often with GH. Furthermore, a number of parents and practitioners were probably reluctant towards growth hormone substitution for fear of relapse. We do not have clear figures on this.
The higher percentage of relapse in the group substituted with GH is probably also linked to the fact that patients who received GHRT had a more serious tumor than the others and were at greater risk of relapse. Indeed, there was a higher proportion of patients with three characteristics known in the literature to confer a better prognosis in the group without GHRT: the presence of NF1, pilocytic astrocytoma, and complete resection [1,28,29]. In addition, patients in the group without GHRT had received chemotherapy less often, which is used in the tumors most at risk of relapse [28]. The two groups were therefore not completely homogeneous with regard to these risk factors. These elements could explain the lower relapse rate in the group not supplemented with GH.
It should be noted that no patient in our study had benefited from a targeted therapy (MEK inhibitor or BRAF inhibitor), because these drugs were not available in France for the older patients, or not indicated. BRAF alterations (V600E mutations or BRAF-fusions) are frequently observed in LGGs. To date, targeted therapy is a promising option for pediatric gliomas harboring BRAF alterations [30]. Since these targeted therapies are very recent, we do not know yet precisely the duration of treatment (often 18 to 24 months, depending on the studies) [31,32]. Changing practices related to the prescription of MEK and BRAF inhibitors may lead to a review of the time required before GH is prescribed. This will need to be assessed by further studies.
As described before, one child particularly caught our attention: Patient 91 had relapsed only 9 months after initiation of GHRT, while the tumor had remained stable for 5 years before. This was his second relapse. When GHRT was stopped, the tumor spontaneously shrank in size, without oncological treatment. This patient then relapsed 5 years later, without any new GHRT. The association of these two events, namely the relapse under GHRT and the spontaneous tumor reduction on stopping GHRT, may turn out to be quite accidental, but they may legitimately raise the question of the safety of the GHRT. In addition, she suffered a fracture of the femur due to a priori multifactorial osteoporosis, which could possibly have been avoided if the resumption of the GHRT had been authorized.
Patient 47 also relapsed under GHRT, but this was then his third relapse. However, we know that patients who have already had a relapse are more likely to relapse in the future, independently of GHRT [1].
A minority of our patients (6%) received first-line radiotherapy (alone or combined with another treatment), and two of them had even received only radiotherapy. These are patients diagnosed with LGG in the 2000s, when the use of radiotherapy in LGG was common. Patients with a more recent diagnosis are treated with chemotherapy and/or surgery as the first line. In the study by Journy et al. studying the risk factors for relapse of all brain tumors after radiotherapy, relapse was not more frequent in the case of GHRT. However, the number of patients with glioma treated with GH was too small to analyze in this subgroup [33].
To our knowledge, to date, no study has specifically studied the risk of tumor recurrence in the event of treatment with GH in children treated for a low-grade glial tumor, which remains the primary etiology of brain tumors in children. Most of the studies looked at all types of brain tumors [2,7,10,11,15].
Despite the theoretical arguments regarding the pathophysiology of GH on carcinogenesis [34], there is no evidence of an increased risk of tumor recurrence after GH substitution.
A recent review encompassing safety data from the GH registries of various pharmaceutical companies between 1988 and 2016 showed no evidence of an increased risk of new malignancy, leukemia, brain tumor, or recurrence of malignant brain tumor in children treated with GH without any other risk factors [11]. In contrast, an increased risk of secondary cancer has been shown in patients irradiated for tumor of the central nervous system [11]. There may be an increased risk of type 2 diabetes in patients treated with GH, but this seems mostly confined to patients who have pre-existing risk factors for diabetes [13,35]; none of our patients were affected. The authors of this study conclude that patients with risk factors for cancer or type 2 diabetes, if they need to be treated, should be followed closely [12]. The published data are therefore rather reassuring in terms of the long-term safety profile of GH treatment.
The safety of GHRT after treatment of craniopharyngioma or even medulloblastoma has already been demonstrated. According to some studies, GHRT even reduces the risk of tumor recurrence [36,37]. A 2017 meta-analysis of 3487 patients with craniopharyngioma showed that the recurrence rate of the latter was significantly lower in children supplemented with growth hormone (10.9%; 95% CI: 9.8–12.1%) compared to children without supplementation (35.2%; 95% CI: 23.1–49.6%) [38].
Two major meta-analyses found a reduced risk of relapse in the event of GHRT [12,13]. In that of Shen L et al., progression or recurrence of intracranial tumors was not associated with GHRT (RR 0.48, 95% CI 0.39–0.56). In the subgroup analysis, the risk of recurrence and progression was decreased when patients received GHRT for craniopharyngioma, medulloblastoma, astrocytoma, or glioma but not for pituitary adenomas and ependymomas [12]. The meta-analysis by Zhi-Feng Wang et al., covering all brain tumors, found a tumor recurrence rate of 21.0% in children supplemented with GH and 44.3% in the GH-untreated group [13]. The pooled RR for recidivism was 0.470 (95% CI 0.372–0.593; p = 0.000). In the astrocytoma subgroup, the RR was 0.515 (95% CI 0.285–0.929, p = 0.028).
No studies have focused specifically on low-grade midline gliomas in GHRT. Only a few cohorts have been able to perform subgroup analyzes for astrocytomas based on their location [10,16,39]. Three cohorts of children supplemented with GH and suffering from various brain tumors did not find an increased risk of tumor recurrence in the subgroup of astrocytomas. Among the large cohort of Darendeliler et al., 400 patients were treated for a glial tumor. Among them, 39 presented a tumor recurrence (9.7%) [39]. The disease-free survival rate in these patients was 69% over 9.1 years of follow-up and was similar to literature data for children not supplemented with GHRT. Patients who relapsed were 1.5 years younger at diagnosis (p = 0.021) and were less than 2.2 years old at the start of GH treatment (p < 0.001) [39]. The author concludes, however, that prolonged follow-up for the detection of recurrences and secondary cancers remains essential. In the study by Swerdlow et al. [16], the risk of recurrence was lower for patients on GHRT, particularly for astrocytomas, with a relative risk of 0.5 (95% CI: 0.3–0.9). In the cohort of Sklar et al. [10], there was no increased risk of tumor recurrence in the event of treatment with GH (RR 0.98, CI 0.35–2.75, p = 0.96) in the 68 patients who had an astrocytoma.
We have summarized the GHRT in pediatric gliomas in Table 5. Our results are therefore in line with the various studies carried out in the past.
Table 5.
Main articles in the literature concerning growth hormone supplementation in children with brain tumors.
| References | Study Groups | Recurrence | Authors Conclusions |
|---|---|---|---|
| Karavitaki et al., 2006 [40] | Craniopharyngioma: 32 patients with GHRT (but 11 started during adult life), 53 without GHRT | 4 patients treated with GH and 22 non-GH treated ones developed tumour recurrence (p = 0.06; RR = 0.309) | GH replacement does not increase the risk of recurrence in patients with craniopharyngioma |
| Rohrer et al., 2010 [41] | 108 craniopharyngioma, medulloblastoma, and ependymoma patients | 13/44 GH-treated and 28/59 non-GH-treated children relapsed | No increased risk of recurrence under GHRT |
| Alotaibi et al., 2017 [38] | Craniopharyngioma: 3436 pediatric patients were treated with GHRT after surgery (GHRT duration ranged between 1.9 and 6.4 years), and 51 were not. | The recurrence rate of the latter was significantly lower in children supplemented with GH (10.9%; 95% CI: 9.80–12.1%) compared to children without supplementation (35.2%; 95% CI: 23.1–49.6%) | This meta-analysis demonstrated a lower recurrence rate of craniopharyngioma among children treated with GHRT than those who were not. |
| Shen et al., 2015 [12] | All brain tumors, meta-analysis of 15 studies, 2232 patients with GHRT and 3606 without GHRT | RR of recurrence: 0.44, (95% CI = 0.34 to 0.54; p = 0.680) In the subgroup analysis, the risks of recurrence were decreased for craniopharyngioma, medulloblastoma, astrocytoma, glioma, but not for pituitary adenomas and ependymomas |
Recurrence of intracranial tumors was not associated with GHRT |
| Zhi-Feng Wang et al., 2014 [13] | All brain tumors, meta-analysis of 10 studies | Tumor recurrence rate of 21.0% in children with GHRT and 44.3% without GHRT. RR for recidivism: 0.470 (95% CI 0.372–0.593; p = 0.000). In the astrocytomas subgroup, the RR was 0.515 (95% CI 0.285–0.929, p = 0.028) | No increased risk of recurrence under GHRT |
| Darendeliler et al., 2006 [39] | 400 patients were treated for a glial tumor with GHRT | 39 presented a tumor recurrence (9.7%) The disease-free survival rate in these patients was 69% over 9.1 years of follow-up, similar to literature data for children not supplemented with GHRT. |
Recurrence of glial tumors was not associated with GHRT. Prolonged follow-up for the detection of recurrences and secondary cancers remains essential. |
| Swerdlow et al., 2000 [16] | All brain tumors after radiotherapy, 180 children with GHRT and 891 children without GHRT. | Thirty-five first recurrences occurred in the GH-treated children and 434 in the untreated children. RR of first recurrence for all brain tumors: 0.6; 95% CI 0.4–0.9). RR for astrocytomas: 0.5 (95% CI: 0.3–0.9). | Risk of recurrence was lower for patients on GHRT, particularly for astrocytomas |
5. Conclusions
Our study did not find a significant difference in the rate of LGG tumor recurrence between children supplemented with GH and patients without supplementation. There were no deaths and no second cancers directly related to growth hormone supplementation.
Growth hormone supplementation therefore seems to be safe in these patients. It is nevertheless necessary to ensure the follow-up of the supplemented patients.
These results are in line with the data from recent literature and constitute an additional argument to reassure practitioners and parents about the safety of this treatment.
Acknowledgments
The authors would like to sincerely thank Lucie Joswiak who collected data from Lille patients and planned this study. They also thank Matthieu Vinchon and Alexandre Vasiljevic who provided the list of patients to be included in the study.
Author Contributions
Conceptualization, P.L.; methodology, P.L.; validation, C.V., P.L., A.H. and L.K.; formal analysis, A.H.; investigation, C.P.; data curation, C.P.; writing—original draft preparation, C.P. and P.A.B.; writing—review and editing, P.L., P.A.B., L.K., I.G., M.-A.K. and C.V.; visualization, C.P.; supervision, P.L.; project administration, C.P.; funding acquisition: none. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the French Ethics Committee (Commission Nationale de l’Informatique et des Libertés, CNIL). We have produced a declaration of conformity, MR-004, as part of research work not involving human beings, with studies and evaluations in the field of health (No. 2215746) on 11/11/2019.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article (Table 2 and Table 3).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sturm D., Pfister S., Jones D.T.W. Pediatric Gliomas: Current Concepts on Diagnosis, Biology, and Clinical Management. J. Clin. Oncol. 2017;35:2370–2377. doi: 10.1200/JCO.2017.73.0242. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong G.T., Conklin H.M., Huang S., Srivastava D., Sanford R., Ellison D.W., Merchant T.E., Hudson M.M., Hoehn M.E., Robison L.L., et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011;13:223–234. doi: 10.1093/neuonc/noq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Iersel L., Li Z., Srivastava D.K., Brinkman T.M., Bjornard K.L., Wilson C.L., Green D.M.E., Merchant T., Pui C.-H., Howell R.M., et al. Hypothalamic-Pituitary Disorders in Childhood Cancer Survivors: Prevalence, Risk Factors and Long-Term Health Outcomes. J. Clin. Endocrinol. Metab. 2019;104:6101–6115. doi: 10.1210/jc.2019-00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas-Teinturier C., Salenave S. Endocrine sequelae after treatment of pediatric cancer: From childhood to adulthood. Bull. Cancer. 2015;102:612–621. doi: 10.1016/j.bulcan.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Tamhane S., Sfeir J., Kittah M.N.E.N., Jasim M.S., Chemaitilly M.W.E., Cohen M.L., Murad M.H. GH Therapy in Childhood Cancer Survivors: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2018;103:2794–2801. doi: 10.1210/jc.2018-01205. [DOI] [PubMed] [Google Scholar]
- 6.Boguszewski C.L., Boguszewski M.C.D.S. Growth Hormone’s Links to Cancer. Endocr. Rev. 2019;40:558–574. doi: 10.1210/er.2018-00166. [DOI] [PubMed] [Google Scholar]
- 7.Boguszewski M.C.S., Cardoso-Demartini A.A., Boguszewski C.L., Chemaitilly W., Higham C.E., Johannsson G., Yuen K.C.J. Safety of growth hormone (GH) treatment in GH deficient children and adults treated for cancer and non-malignant intracranial tumors—A review of research and clinical practice. Pituitary. 2021;24:810–827. doi: 10.1007/s11102-021-01173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richmond E., Rogol A.D. Treatment of growth hormone deficiency in children, adolescents and at the transitional age. Best Pr. Res. Clin. Endocrinol. Metab. 2016;30:749–755. doi: 10.1016/j.beem.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Raman S., Grimberg A., Waguespack S.G., Miller B., Sklar C.A., Meacham L.R., Patterson B. Risk of Neoplasia in Pediatric Patients Receiving Growth Hormone Therapy—A Report From the Pediatric Endocrine Society Drug and Therapeutics Committee. J. Clin. Endocrinol. Metab. 2015;100:2192–2203. doi: 10.1210/jc.2015-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sklar C.A., Antal Z., Chemaitilly W., Cohen L.E., Follin C., Meacham L.R., Murad M.H. Hypothalamic–Pituitary and Growth Disorders in Survivors of Childhood Cancer: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018;103:2761–2784. doi: 10.1210/jc.2018-01175. [DOI] [PubMed] [Google Scholar]
- 11.Stochholm K., Kiess W. Long-term safety of growth hormone—A combined registry analysis. Clin. Endocrinol. 2017;88:515–528. doi: 10.1111/cen.13502. [DOI] [PubMed] [Google Scholar]
- 12.Shen L., Sun C.M., Li X.T., Liu C.J., Zhou Y.X. Growth hormone therapy and risk of recurrence/progression in intracranial tumors: A meta-analysis. Neurol. Sci. 2015;36:1859–1867. doi: 10.1007/s10072-015-2269-z. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z.-F., Chen H.-L. Growth hormone treatment and risk of recurrence or development of secondary neoplasms in survivors of pediatric brain tumors. J. Clin. Neurosci. 2014;21:2155–2159. doi: 10.1016/j.jocn.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Allen D.B., Backeljauw P., Bidlingmaier M., Biller B.M.K., Boguszewski M., Burman P., Butler G., Chihara K., Christiansen J., Cianfarani S., et al. GH safety workshop position paper: A critical appraisal of recombinant human GH therapy in children and adults. Eur. J. Endocrinol. 2015;174:P1–P9. doi: 10.1530/EJE-15-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deodati A., Ferroli B.B., Cianfarani S. Association between growth hormone therapy and mortality, cancer and cardiovascular risk: Systematic review and meta-analysis. Growth Horm. IGF Res. 2014;24:105–111. doi: 10.1016/j.ghir.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Swerdlow A.J., Reddingius R.E., Higgins C.D., Spoudeas H.A., Phipps K., Qiao Z., Ryder W.D.J., Brada M., Hayward R.D., Brook C.G.D., et al. Growth Hormone Treatment of Children with Brain Tumors and Risk of Tumor Recurrence. J. Clin. Endocrinol. Metab. 2000;85:4444–4449. doi: 10.1210/jc.85.12.4444. [DOI] [PubMed] [Google Scholar]
- 17.Swerdlow A.J., Cooke R., Beckers D., Borgström B., Butler G., Carel J.-C., Cianfarani S., Clayton P., Coste J., Deodati A., et al. Cancer Risks in Patients Treated with Growth Hormone in Childhood: The SAGhE European Cohort Study. J. Clin. Endocrinol. Metab. 2017;102:1661–1672. doi: 10.1210/jc.2016-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rakotonjanahary J., De Carli E., DeLion M., Kalifa C., Grill J., Doz F., Leblond P., Bertozzi A.-I., Rialland X., Brain Tumor Committee of SFCE Mortality in Children with Optic Pathway Glioma Treated with Up-Front BB-SFOP Chemotherapy. PLoS ONE. 2015;10:e0127676. doi: 10.1371/journal.pone.0127676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traunwieser T., Kandels D., Pauls F., Pietsch T., Warmuth-Metz M., Bison B., Krauss J., Kortmann R.-D., Timmermann B., Thomale U.-W., et al. Long-term cognitive deficits in pediatric low-grade glioma (LGG) survivors reflect pretreatment conditions—Report from the German LGG studies. Neuro-Oncol. Adv. 2020;2:vdaa094. doi: 10.1093/noajnl/vdaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomale U.-W., Gnekow A.K., Kandels D., Bison B., Driever P.H., Witt O., Pietsch T., Koch A., Capper D., Kortmann R.-D., et al. Long-term follow-up of surgical intervention pattern in pediatric low-grade gliomas: Report from the German SIOP-LGG 2004 cohort. J. Neurosurgery: Pediatr. 2022;30:316–329. doi: 10.3171/2022.6.PEDS22108. [DOI] [PubMed] [Google Scholar]
- 21.Laithier V., Grill J., Le Deley M.-C., Ruchoux M.-M., Couanet D., Doz F., Pichon F., Rubie H., Frappaz D., Vannier J.-P., et al. Progression-Free Survival in Children with Optic Pathway Tumors: Dependence on Age and the Quality of the Response to Chemotherapy—Results of the First French Prospective Study for the French Society of Pediatric Oncology. J. Clin. Oncol. 2003;21:4572–4578. doi: 10.1200/JCO.2003.03.043. [DOI] [PubMed] [Google Scholar]
- 22.Gnekow A.K., Walker D.A., Kandels D., Picton S., Perilongo G., Grill J., Stokland T., Sandstrom P.E., Warmuth-Metz M., Pietsch T., et al. A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (≤16 years) low grade glioma–A final report. Eur. J. Cancer. 2017;81:206–225. doi: 10.1016/j.ejca.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lassaletta A., Scheinemann K., Zelcer S.M., Hukin J., Wilson B.A., Jabado N., Carret A.S., Lafay-Cousin L., Larouche V., Hawkins C.E., et al. Phase II Weekly Vinblastine for Chemotherapy-Naïve Children With Progressive Low-Grade Glioma: A Canadian Pediatric Brain Tumor Consortium Study. J. Clin. Oncol. 2016;34:3537–3543. doi: 10.1200/JCO.2016.68.1585. [DOI] [PubMed] [Google Scholar]
- 24.Journal Officiel Arrêté Du 24 Février 2003 Modifiant La Liste Des Spécialités Pharmaceutiques Remboursables Aux Assurés Sociaux. 2003. [(accessed on 24 February 2022)]. Available online: https://solidarites-sante.gouv.fr/fichiers/bo/2003/03-11/a0110796.htm?TSPD_101_R0=087dc22938ab200033c5694e4b4eac43c6a2ec98888f293ebd9a639ba2ce8dc3457111f2e798da6c08137ddd63143000e0264336f85a6335ede74c6ce2b8015d11f6a839a31873fcd3dd1ab835a6f8ee2c12094ba7dc878ba949ffb266d1c61a.
- 25.Protocole National de Diagnostic et de Soins (PNDS) Neurofibromatose Published online August 2021. [(accessed on 1 September 2021)]. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2022-07/pndsnf1final.pdf.
- 26.Burns C., Rigsby P., Moore M., Rafferty B. The First International Standard for Insulin-like Growth Factor-1 (IGF-1) for immunoassay: Preparation and calibration in an international collaborative study. Growth Horm. IGF Res. 2009;19:457–462. doi: 10.1016/j.ghir.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Thomas-Teinturier C., Oliver-Petit I., Pacquement H., Fresneau B., Allodji R.S., Veres C., Bolle S., Berchery D., Demoor-Goldschmidt C., Haddy N., et al. Influence of growth hormone therapy on the occurrence of a second neoplasm in survivors of childhood cancer. Eur. J. Endocrinol. 2020;183:471–480. doi: 10.1530/EJE-20-0369. [DOI] [PubMed] [Google Scholar]
- 28.Stokland T., Liu J.-F., Ironside J.W., Ellison D.W., Taylor R., Robinson K.J., Picton S.V., Walker D.A. A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: A population-based cohort study (CCLG CNS9702) Neuro-oncology. 2010;12:1257–1268. doi: 10.1093/neuonc/noq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aihara Y., Chiba K., Eguchi S., Amano K., Kawamata T. Pediatric Optic Pathway/Hypothalamic Glioma. Neurol. Medico-chirurgica. 2018;58:1–9. doi: 10.2176/nmc.ra.2017-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan J.Y., Wijesinghe I.V.S., Alfarizal Kamarudin M.N., Parhar I. Paediatric Gliomas: BRAF and Histone H3 as Biomarkers, Therapy and Perspective of Liquid Biopsies. Cancers. 2021;13:607. doi: 10.3390/cancers13040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novartis Pharmaceuticals. Phase II Pediatric Study with Dabrafenib in Combination with Trametinib in Patients with HGG and LGG. Published online 2021. [(accessed on 29 November 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02684058.
- 32.Entz-Werle N. Pediatric Low Grade Glioma-MEKinhibitor TRIal vs. Chemotherapy (PLGG-MEK-TRIC) [(accessed on 8 July 2022)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT05180825.
- 33.Journy N.M.Y., Zrafi W.S., Bolle S., Fresneau B., Alapetite C., Allodji R.S., Berchery D., Haddy N., Kobayashi I., Labbé M., et al. Risk Factors of Subsequent Central Nervous System Tumors after Childhood and Adolescent Cancers: Findings from the French Childhood Cancer Survivor Study. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2021;30:133–141. doi: 10.1158/1055-9965.EPI-20-0735. [DOI] [PubMed] [Google Scholar]
- 34.Samani A.A., Yakar S., Leroith D., Brodt P. The Role of the IGF System in Cancer Growth and Metastasis: Overview and Recent Insights. Endocr. Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 35.Abs R., Mattsson A.F., Thunander M., Verhelst J.I., Góth M., Wilton P., Kołtowska-Häggström M., Luger A. Prevalence of diabetes mellitus in 6050 hypopituitary patients with adult-onset GH deficiency before GH replacement: A KIMS analysis. Eur. J. Endocrinol. 2013;168:297–305. doi: 10.1530/EJE-12-0807. [DOI] [PubMed] [Google Scholar]
- 36.Alotaibi N.M., Zaidi H., Noormohamed N., Cote D.J., Crocker E., Doucette J., Bi W.L., Alharthy S., Quevedo P.V., Mekary R.A., et al. Comparison of Physiologic Growth Hormone Replacement Therapy to No Replacement on Craniopharyngioma Recurrence in Pediatric Patients. J. Neurol. Surg. Part B: Skull Base. 2017;78:S1–S156. doi: 10.1055/s-0037-1600744. [DOI] [Google Scholar]
- 37.Losa M., Castellino L., Pagnano A., Rossini A., Mortini P., Lanzi R. Growth Hormone Therapy Does Not Increase the Risk of Craniopharyngioma and Nonfunctioning Pituitary Adenoma Recurrence. J. Clin. Endocrinol. Metab. 2020;105:1573–1580. doi: 10.1210/clinem/dgaa089. [DOI] [PubMed] [Google Scholar]
- 38.Alotaibi N.M., Noormohamed N., Cote D.J., Alharthi S., Doucette J., Zaidi H.A., Mekary R.A., Smith T.R. Physiologic Growth Hormone–Replacement Therapy and Craniopharyngioma Recurrence in Pediatric Patients: A Meta-Analysis. World Neurosurg. 2017;109:487–496.e1. doi: 10.1016/j.wneu.2017.09.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darendeliler F., Karagiannis G., Wilton P., Ranke M.B., Albertsson-Wikland K., Price D.A. Recurrence of brain tumours in patients treated with growth hormone: Analysis of KIGS (Pfizer International Growth Database) Acta Paediatr. 2006;95:1284–1290. doi: 10.1080/08035250600577889. [DOI] [PubMed] [Google Scholar]
- 40.Karavitaki N., Warner J.T., Marland A., Shine B., Ryan F., Arnold J., Turner H.E., Wass J.A.H. GH replacement does not increase the risk of recurrence in patients with craniopharyngioma. Clin. Endocrinol. 2006;64:556–560. doi: 10.1111/j.1365-2265.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- 41.Rohrer T.R., Langer T., Grabenbauer G.G., Buchfelder M., Glowatzki M., Dörr H.G. Growth Hormone Therapy and the Risk of Tumor Recurrence after Brain Tumor Treatment in Children. J. Pediatr. Endocrinol. Metab. 2010;23:935–942. doi: 10.1515/jpem.2010.150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this article (Table 2 and Table 3).