Abstract
Simple Summary
Malignant pleural mesothelioma (MPM) is an aggressive and still incurable cancer. There is an urgent need to identify effective and reliable tools for detecting and diagnosing the early onset of MPM. In our study, we investigated the whole miRNAs expression profile from serum extracellular vesicles to identify early changes related to MPM development. miR-11400, miR-148a-3p, and miR-409-3p levels were increased in pre-clinical MPM patients up to five years before their diagnosis. The three-miRNA pattern showed a good discrimination capacity to distinguish pre-clinical MPM from cancer-free controls. The three miRNAs also displayed high diagnostic capabilities for differentiating between MPM patients and controls. This study identified a potential EV miRNA signature in preclinical MPM up to five years before diagnosis and raises the possibility of early intervention.
Abstract
Malignant pleural mesothelioma (MPM) is an aggressive cancer with a dismal prognosis. Early therapeutic interventions could improve patient outcomes. We aimed to identify a pattern of microRNAs (miRNAs) as potential early non-invasive markers of MPM. In a case-control study nested in the European Prospective Investigation into Cancer and Nutrition cohort, we screened the whole miRNome in serum extracellular vesicles (EVs) of preclinical MPM cases. In a subgroup of 20 preclinical samples collected five years prior MPM diagnosis, we observed an upregulation of miR-11400 (fold change (FC) = 2.6, adjusted p-value = 0.01), miR-148a-3p (FC = 1.5, p-value = 0.001), and miR-409-3p (FC = 1.5, p-value = 0.04) relative to matched controls. The 3-miRNA panel showed a good classification capacity with an area under the receiver operating characteristic curve (AUC) of 0.81 (specificity = 0.75, sensitivity = 0.70). The diagnostic ability of the model was also evaluated in an independent retrospective cohort, yielding a higher predictive power (AUC = 0.86). A signature of EV miRNA can be detected up to five years before MPM; moreover, the identified miRNAs could provide functional insights into the molecular changes related to the late carcinogenic process, preceding MPM development.
Keywords: malignant pleural mesothelioma, biomarkers, microRNAs, early changes, next generation sequencing
1. Introduction
Malignant pleural mesothelioma (MPM) is the most aggressive asbestos-related disease, characterized by a dismal prognosis with a median survival of 14 months [1].
The hazardous effect of asbestos is globally well recognized and even though it has been banned in several nations, asbestos is still being used in most low- and middle-income countries. Recently, MPM accounted for 30,443 new cancer cases (0.2%) and 25,576 cancer deaths (0.3%) worldwide [2]. A peak incidence and mortality will be expected to occur before 2030, while in resource-limited countries, it will increase in the next decades. For these reasons the role of asbestos in human health still remains a concern.
Despite advances in understanding its pathogenic mechanism, MPM has remained a major clinical challenge. It is often diagnosed at advanced stages and is refractory to current treatments [3,4], but early detection could enable interventions at a potential resectable stage. Previous studies reported that patients treated with a multimodality therapy, when the tumor is limited to parietal or visceral space, have shown a better overall survival [5,6]. In 2020, phase III clinical trials positioned immunotherapy as a promising option for the first- and second-line treatment of MPM and led to a significant improvement of overall survival. However, the 5-year survival rate is still low, with a percentage of 9.6% [7].
Multiple approaches have been attempted to identify biomarkers in order to facilitate early non-invasive detection of the disease, with potential application in screening at-risk populations [8]. However, none of the identified markers achived a significant sensitivity and specificity to allow for a translation into clinical settings and the diagnosis is still challenging. Cytological samples obtained by thoracentesis or by fine-needle aspiration biopsy may be inconclusive for a definitive diagnosis [9]. Liquid biomarkers have sparked a lot of interest due to their advantages over a single tissue biopsy. Currently, three blood-based biomarkers have been extensively investigated for MPM for their diagnostic potential, such as soluble mesothelin, fibulin-3, and osteopontin. However, their clinical utility is limited by their poor sensitivity.
A growing number of reports have shown that circulating microRNAs (miRNAs) may have clinical relevance as cancer biomarkers, for their unique expression profiles, depending not only on the type of cancer but also on the disease stage. Most circulating miRNAs are released in the blood stream encapsulated in extracellular vesicles (EVs), lipid bilayer membranes involved in cell-to-cell communication with surrounding and distant cells by shaping their metabolism, and modulating gene expression [10].
EVs can be released from the originating cell either through the outward budding of the plasma membrane or through the inward budding of the endosomal membrane. The most relevant EV subtypes are exosomes (50–150 nm), microvesicles (200 nm–1 µm), and apoptotic bodies, which are all notably involved in physiological processes as well as in pathological development [11].
In addition, miRNAs in EVs are protected from Rnases degradation, making them ideal candidates for liquid biopsies and avoiding the need for invasive procedures to obtain tissue biopsies [12].
Cavalleri et al. identified miR-103a-3p and miR-30e-3p derived from plasma EVs were able to discriminate MPM from asbestos-exposed individuals [9]. However, most of the studies examined circulating cell-free miRNAs [13]. First, among all miR-126-3p was consistently down-regulated both in tissue and serum of MPM patients and could differentiate MPM from NSCLC [14].
Increased circulating expression of miR-625-3p and high levels of miR-34b/c methylation in serum-circulating DNA were associated with MPM diagnosis [15,16].
These results do not allow any conclusion regarding performance of miRNAs for the detection of MPM at early stages, deriving from the analysis of incident cases of MPM and they need to be validated in longitudinal studies.
In the present study, we performed, for the first time, a comprehensive miRNome profile using a Next Generation Sequencing (NGS) approach, of serum circulating EVs in pre-clinical MPM cases, within a case-control study nested in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort.
2. Materials and Methods
2.1. Study Cohort
EPIC is a multi-center cohort, involving 23 centers from 10 European countries (Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom). The study design has been described previously [17].
In the EPIC cohort, 148 participants developed MPM (EPIC-MESO) at the last follow-up (2016), however serum samples were available for 82 pre-clinical participants. Serum was collected at recruitment and, thus, prior to MPM diagnosis, that occurred between 17 days and 18 years after blood collection, with a median time to diagnosis of 8 years. Controls were individuals nested in the EPIC cohort without cancer diagnosis up to and including the last follow-up. One control was randomly selected for each case from the same center and matched for age at blood collection (±1.5 years), sex, and asbestos exposure.
An expert epidemiologist assessed the asbestos exposure as described in Supplementary Methods “Asbestos exposure assesment”.
All participants gave their written informed consent to participate in the EPIC study. Our study complies with the Declaration of Helsinki principles and conforms to ethical requirements.
2.2. Isolation of Serum Extracellular Vesicles and RNA Extraction
EVs were isolated from 200 µL of serum using ExoQuick precipitation solution (System Biosciences, Palo Alto, CA, USA) according to manufacturer’s instructions. Briefly, 200 µL of serum was processed with 50.4 µL ExoQuick solution and stored at 4 °C overnight. The EVs pellets were resuspended with 200 µL of nuclease free water and RNA was immediately isolated from the solution.
Total RNA was extracted with miRNeasy serum/plasma kit (Qiagen, Hilden, Germany) using the QIAcube extractor (Qiagen, Germany) according to manufacturer’s instructions. RNA concentration was determined for all samples with Qubit 2.0 Fluorometer with miRNA assay kit (Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Library Preparation and Next Generation Sequencing
Small non coding RNA libraries were constructed using NEBNext Multiplex Small RNA Library Prep set for Illumina (New England Biolabs, Inc., Ipswich, MA, USA) [18]. The cDNA libraries were purified with the Qiagen PCR Purification kit following the modifications indicated by NEBNext Multiplex Small RNA Library Prep instructions. Six pools of cDNA barcoded samples were prepared and each pool was finally size-selected microRNAs in a 6% PolyAcrylamide Gel (Thermo Fisher Scientific, Waltham, MA, USA). Fragments with an insert of 150 nucleotides were cut out and purified with Qiagen Gel Extraction MiniElute columns (Qiagen, Germany) following the modifications indicated in NEBNext Multiplex Small RNA Library Prep Protocol. A Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, USA) was used to check the size of each cDNA library fragment. Single-end sequencing (75 nucleotides) was performed on NextSeq550 platform (Illumina, San Diego, CA, USA).
2.4. Nanoparticle Tracking Analysis and Electron Microscopy
EV concentration was measured in a random pair of samples by Nanosight NS300 (Malvern Instruments Ltd., Malvern, UK) equipped with a 488 nm laser module that utilizes Brownian motion and refraction index. Samples were diluted 1:200 in physiologic solution filtered with 100 nm pore size. For each sample, three videos of 30 s at camera level 15 and threshold 5 were captured. All samples were characterized with Nanoparticle Tracking analysis (NTA).
The presence and integrity of EVs were assessed by Transmission electron microscopy (TEM) as described in Verta et al. [19].
2.5. miRNAs Sequencing Data Analysis
The data from miRNA sequencing (miRNA-seq) were computationally processed and analyzed as previously described [18]. Differential expression analysis was performed with the DESeq2 Bioconductor’s package (version 1.22.2).
We adjusted the analysis for age, gender, and batch effect as these parameters can affect miRNA expression [20,21]. Since the samples were collected in six different countries, we also adjusted the analysis for the country of recruitment, as this could introduce a potent confounding factor into the data. The miRNAs’ profiles could also be affected by asbestos exposure, therefore we also included this variable in the adjustment.
For each tested model, samples with missing covariates were dropped and only miRNAs with a median expression of at least ten reads were included.
MiRNAs obtained from the NGS analysis were considered significantly differentially expressed between pre-diagnostic cases and matched controls if their p-value, after adjustment for multiple testing by false discovery rate (FDR), was below the 0.05 threshold.
Logistic regression was used to create a predictive model combining the differentially expressed miRNAs into a single score which allows to detect preclinical cases. The model coefficients were re-estimated on the EPIC data using only the expression of selected miRNAs, without matched variables. Its predictive ability was investigated by the ROC (receiver operating characteristic) curve, and the area under the curve (AUC). Specificity and sensitivity were also computed for a standard cut-off value of 0.5.
2.6. Technical Validation by RT-qPCR Analysis
We validated the differentially expressed miRNAs identified in pre-clinical cases recruited up to five years before the diagnosis (20 cases and 20 controls), by quantitative reverse transcription polymerase chain reaction (RT-qPCR). To select the candidate miRNA, we used FDR ≤ 0.05 and |log2FoldChange(FC)| ≥ 0.6 as miRNAs selection criteria; however, due to the small sample study, we also included in the validation analysis de-regulated miRNAs with nominal p-value < 0.05, |log2 FC| ≥ 0.6 and read counts greater than 100. The full sequence of miRNA assayed are reported in Supplementary Table S1. Laboratory methods and statistical analysis for technical confirmation of the miRNA-seq results are detailed in Supplementary Materials “RT-qPCR analysis”.
2.7. Functional Enrichment Analysis of miRNAs Target Genes
The validated target genes of miR-409-3p and miR-148a-3p were retrieved with the MultiMiR Bioconductor’s package, from Mirecords, Mirtarbase, and Tarbase databases [22]. No available experimental evidence has been reported for miR-11400 targets, therefore potential target genes were predicted by the miRWALK2.0 tool [23]. Only those genes with a binding probability of at least 95% were considered as targets and taken into account for further analysis.
The Kyoto Encyclopedia of Genes and Genomes (KEGG) was used as reference for the pathway enrichment analyses and potential functional roles associated with the genes targeted by miR-11400, miR-409-3p, and miR-148a-3p.
The statistical significance of the enriched pathways was determined using ShinyGO v0.741 [24].
2.8. Investigation of Selected miRNAs in a Retrospective Cohort
Since a suitable prospective replication cohort could not be found, we investigated by a NGS approach the whole miRNome in 30 asbestos-exposed patients with a diagnosis of MPM and 20 asbestos-exposed cancer-free controls. Asbestos-exposed cases and controls with a well-characterized occupational and environmental asbestos exposure were recruited from two areas of northern Italy. MPM patients were diagnosed between 2018 and 2019 at the Azienda Ospedaliero-Universitaria Maggiore della Carità (Novara) as previously described in [25].
The control group belongs to the study described in Filiberti et al. [26]. The 20 retrieved controls were cancer-free at the follow-up in 2017.
The study protocols were approved by the ethics committee of the participating center and written informed consent was obtained from all individuals before enrolment.
The predictive model, previously fitted on the prospective EPIC samples, was tested for discriminating the MPM cases from the controls, using the same performance metrics previously described.
3. Results
We investigated the miRNA expression profile at the whole genome level in serum-derived EVs of 82 individuals who developed MPM in 20 years of follow-up and 82 matched cancer-free controls.
Based on the occupational history data of each participant, it was possible to perform an assessment of asbestos exposure for 58 cases, and the matched controls had the same asbestos exposure probability (Materials and Methods).
Most of the 164 paired samples were males (72%) and came from the United Kingdom (36.6%). The mean age at recruitment was 57.8 years. The characteristics of the study population are summarized in Table 1.
Table 1.
Descriptive table of the samples included in the EPIC-MESO study at recruitment. ALL EPIC-MESO indicates the sequenced pre-MPM cases and matched controls. EPIC-MESO 5 years includes the subgroup of preclinical MPM collected within five years prior to MPM diagnosis and their matched controls.
| ALL EPIC-MESO (n = 164) | EPIC-MESO 5 Years (n = 48) | |||
|---|---|---|---|---|
| CA = 82 | CO = 82 | CA = 24 | CO = 24 | |
| GENDER | ||||
| M (%) | 59 (72) | 59 (72) | 20 (83.3) | 20(83.3) |
| F (%) | 23 (28) | 23 (28) | 4 (16.7) | 4(16.7) |
| AGE (mean ± sd) | 57.7 ± 8.1 | 57.8±8.1 | 60.4 ± 7.6 | 60.4 ± 7.6 |
| ASBESTOS EXPOSURE | ||||
| unexposed (%) | 18 (22) | 18 (22) | 3 (12.5) | 3 (12.5) |
| exposed (%) | 40 (48.7) | 40 (48.7) | 17 (70.8) | 17 (70.8) |
| NA | 24 (29.3) | 24 (29.3) | 4(16.7) | 4(16.7) |
| COUNTRY | ||||
| Germany (%) | 12 (14.6) | 12 (14.6) | 9 (37.5) | 9 (37.5) |
| Spain (%) | 10 (12.2) | 10 (12.2) | 1 (4.2) | 1 (4.2) |
| France (%) | 1 (1.2) | 1 (1.2) | 1 (4.2) | 1 (4.2) |
| Italy (%) | 21 (25.6) | 21 (25.6) | 4 (16.6) | 4 (16.6) |
| The Netherlands (%) | 8 (9.8) | 8 (9.8) | 1 (4.2) | 1 (4.2) |
| United Kingdom (%) | 30 (36.6) | 30 (36.6) | 8 (33.3) | 8 (33.3) |
CA: preclinical cases; CO: controls; M: male; F: female.
The presence of EVs after Exoquick precipitation, were confirmed by TEM and by NanoSight analyses. TEM analysis depicted round-shaped EVs (Supplementary Figure S1) and quantitative size analysis using NTA showed that EVs have a range of 120/150 nm (Supplementary Figure S2).
Sequencing generated per sample an average of nine million reads, ranging from 2.2–31.6 × 106 reads (Supplementary Table S2). On average, 5.7% of the reads obtained from miRNA-seq aligned on miRNA sequences (miRBase v22). Overall, 211 miRNAs were detected in 164 samples.
After read processing and filtering, four samples (two cases and two controls) were removed from the analysis as outliers.
From the analysis of miRNA-seq data derived from 80 prospectively collected MPM cases and 80 controls adjusted for sequencing run, country of recruitment, sex, and age, we did not identify any significant difference in miRNA expression.
Pre-clinical cases were further divided into two groups according to the time between blood collection and MPM diagnosis (less or more than five years before diagnosis). We did not observe any significant differentially expressed miRNAs between the two groups (Supplementary Table S3).
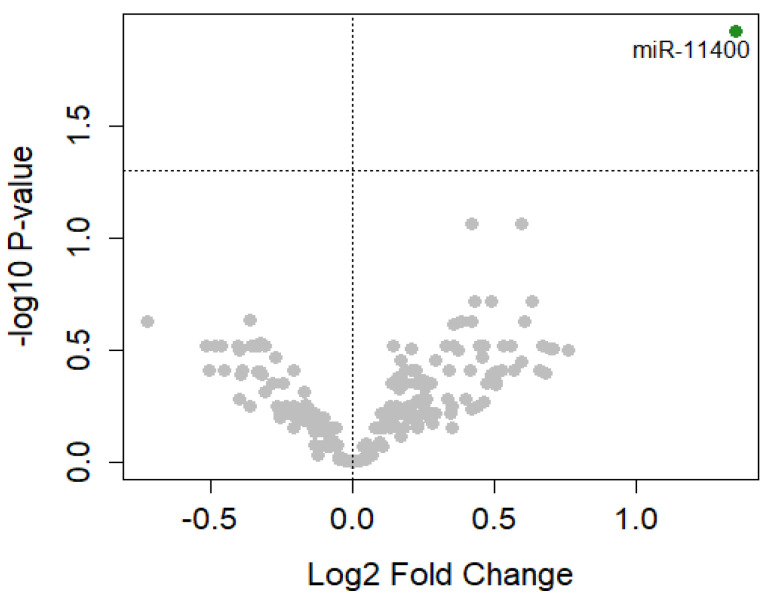
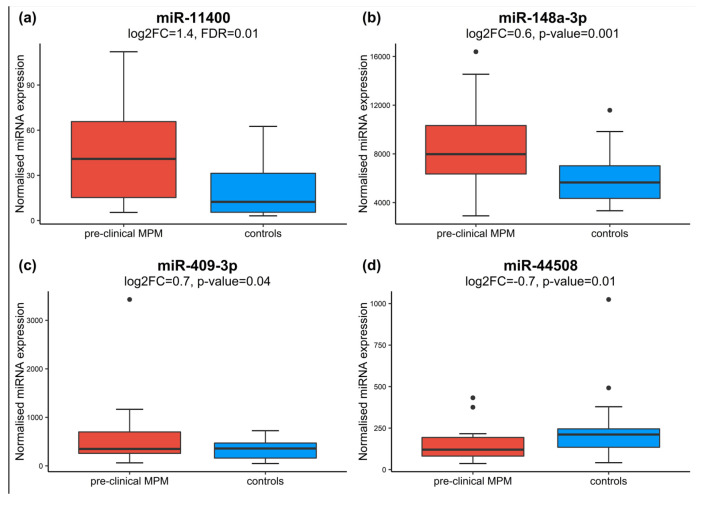
In order to observe miRNA dysregulated in relation to the cancer transformation process, we investigated the differentially expressed miRNAs in preclinical cases near to the time of diagnosis (Table 1, EPIC-MESO 5 years). In our dataset, 24 cases developed MPM within five years after the enrolment. Of them, 20 pre-clinical cases and their relative matched cancer-free controls had an assessment of asbestos exposure. We used this subgroup to perform differential expression analysis adjusted for batch effect, age, gender, country, and asbestos exposure. We identified miR-11400 (FDR = 0.01; log2FC = 1.4; 95% CI 0.69–2.0) significantly upregulated in cases compared to matched controls (Figure 1, Figure 2a, Supplementary Table S4).
Figure 1.
Volcano plot of differentially expressed miRNAs. Each dot represents a miRNA. The green dot represents miR-11400 significantly differentially expressed at FDR ≤ 0.05. The gray dots represent miRNAs that were not significantly differentially expressed at FDR.
Figure 2.
Boxplots show expression levels of miR-11400 (a), miR-148a-3p (b), miR-409-3p (c), and miR-4508 (d), in pre-clinical MPM cases collected up to five years before diagnosis and matched controls. Above each boxplot, the p-values yielded by DESeq2 after adjustment for multiple testing (FDR) or nominal p-value.
As described in Material and Methods, we also included for further analysis miR-148a-3p (log2FC = 0.6; p-value = 0.001; 95% CI 0.2, 0.82) (Figure 2b), miR-409-3p (log2FC = 0.7; p-value = 0.04; 95% CI 0.02, 1.3) (Figure 2c), and miR-4508 (log2FC = −0.7; p-value = 0.01; 95% CI −1.29, −0.16) (Figure 2d), which were significantly differentially expressed at nominal p-value.
In order to evaluate the expression levels of the three-miRNAs signature in MPM and normal tissue, we explored the database of Differentially Expressed MiRNAs in human Cancers (dbDEMC) [27,28]. We compared 25 MPM primary tumors with six normal pleural samples, identifying a significant upregulation in tumor tissue for miR-148a (log2FC = 0.4. p-value = 9.98 × 10−3) and miR-409-3p (log2FC = 1.8, p-value = 2.11 × 10−3).
3.1. Validation of miRNA-seq Data by RT-qPCR
The three-miRNA signature (miR-11400, miR-148a-3p, and miR-409-3p) was validated by RT-qPCR in the whole dataset of preclinical cases near to diagnosis and their matched controls (EPIC MESO 5 years). Although miR-4508 was eligible for validation, it was not further investigated due to the failure of TaqMan probe design. The relative expression levels of miR-11400, miR-148a-3p, and miR-409-3p obtained from RT-qPCR agreed with miRNA-seq data based on the expression trends among preclinical cases and controls, however, the differences were not statistically significant (Supplementary Figure S3).
3.2. miRNAs Target Genes and KEGG Enrichment Analysis
Functional enrichment analysis was performed to explore if the identified miRNAs were involved in relevant molecular networks related to the pathogenesis of MPM. MultiMiR Bioconductor’s package was used to retrieve miRNAs validated target genes. We identified 111 experimentally validated targets for miR-148a-3p and miR-409-3p, in particular, 110 were for miR-148a-3p and only one was for miR-409-3p (Supplementary Table S5), while no experimental evidence was available for miR-11400 target genes.
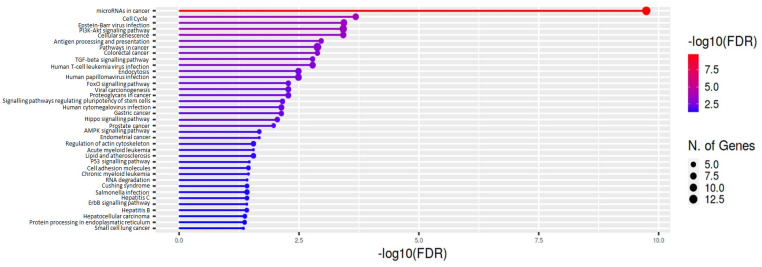
The enrichment analysis, carried out considering the validated target genes, revealed a total of 37 significantly enriched terms in KEGG (Figure 3 and Supplementary Table S6). The most significantly altered pathways in KEGG, included “TGF-beta signaling”, “PI3K-Akt signaling”, and “cell cycle pathways”, which have been defined to play essential roles in the development of MPM [29,30].
Figure 3.
Enrichment analysis of KEGG pathways relative to validated target genes of miR-409-3p and miR-148a-3p. Bar plots show the significantly enriched functional categories ordered by p-value.
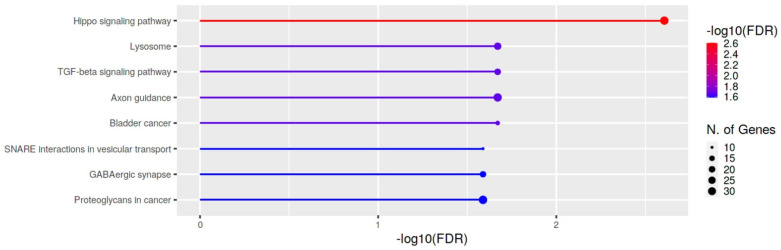
We used miRWalk 2.0 to predict target mRNAs of miR-11400, and we identified 2147 unique target genes (Supplementary Table S7). We also performed an enrichment analysis for these predicted genes and after FDR correction the potential target genes of miR-11400 resulted mainly enriched in lysosome, Hippo signaling pathway and TGF-β signaling pathway (Figure 4 and Supplementary Table S8).
Figure 4.
KEGG enrichment analysis of KEGG pathways relative to predicted target genes of miR-11400. Bar plots depict the significantly enriched terms ordered by p-value.
3.3. Prediction Model and Validation in a Retrospective Study
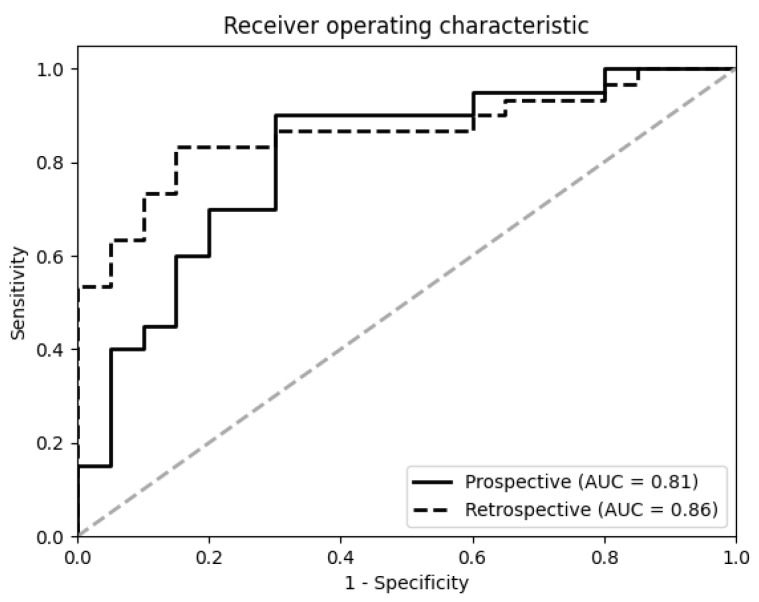
To explore the predictive power of the miRNA signature, a multivariate logistic regression model was fitted to distinguish cases diagnosed within five years after baseline and matched cancer-free controls, using the three miRNAs discovered in the previous analyses as features. The model had an AUC of 0.81 (Figure 5), a sensitivity of 75% and a specificity of 70%. The fitted model was validated in an independent retrospective cohort of 30 MPM diagnosed cases and 20 controls (Supplementary Table S9), yielding an AUC of 0.86 with a sensitivity and specificity of 53% and 95%, respectively. Table 2 showed the diagnostic statistics of the three selected miRNAs for MPM prediction.
Figure 5.
Receiver operating characteristics (ROC) curves of the three miRNAs signature (miR-11400, miR-148a-3p, and miR-409-3p) for the discrimination between preclinical MPM and matched controls (solid line), as well as for discriminating between MPM at diagnosis and healthy controls (dashed line). The area under the curve (AUC) shows the percentage of correct discrimination associating sensitivity (Y) and 1-specificity (X).
Table 2.
Diagnostic accuracy of miR-11400, miR-148-3p, and miR-409.
| Prospective | Retrospective | |
|---|---|---|
| AUC | 81% | 86% |
| Sensitivity | 75% | 53% |
| Specificity | 70% | 95% |
| PPV | 71% | 94% |
| NPV | 74 % | 57% |
| Accuracy | 73% | 70% |
AUC, area under curve; PPV, positive predictive value; NPV, negative predictive value.
4. Discussion
In the present work, we evaluated if EV-derived miRNAs could serve as reliable tumor biomarkers for early detection of MPM and to gain insight into the molecular mechanisms linked to the late carcinogenic process. By using a NGS approach, we profiled miRNAs expression enclosed in serum EVs of preclinical MPM individuals and cancer-free controls.
We observed a significant upregulation of miR-11400, miR-148a-3p, and miR-409-3p in serum EVs of 20 pre-diagnostic MPM samples collected up to five years before the clinical manifestation of the disease, compared with cancer-free individuals. In the validation phase, miR-11400 and miR-409-3p were found upregulated, while a slight increase of miR-148a-3p expression was observed in preclinical cases.
So far, numerous cell-free miRNAs have been identified as non-invasive biomarkers for MPM, but only miR-126, miR-132-3p, and miR-103a-3p were tested in plasma of preclinical MPM samples, showing that this miRNA pattern was not feasible to detect MPM at early stages [31,32,33,34,35]. Indeed, we did not find any difference in expression levels for the three described miRNAs as reported in Supplementary Table S4.
In EPIC preclinical MPM cases, a different three-miRNAs panel identified by us could predict MPM onset within five years, yielding an AUC of 0.81 with 75% sensitivity and 70% specificity. In the absence of a prospective validation cohort, we could examine the diagnostic performance of the model in a retrospective study, which confirmed the accuracy and reliability of the three-miRNA signature in distinguishing MPM cases from cancer-free controls even at the time of diagnosis (AUC = 0.86). Although the specificity was even higher than the prospective cohort at 95%, sensitivity dropped to 53% in the retrospective validation study, meaning almost half of the diagnosed cases tested as negative. This suggests that the present miRNA signature may weaken in a number of subjects over time after diagnosis and warrants further investigation. However, it is unlikely to affect undiagnosed cases at earlier stages of MPM and the specificity/sensitivity balance can be adjusted to fine tune the score threshold.
The predictive role of miR-148a-3p has been described in previous cancer studies [36,37,38]. Increasing expression levels of miR-148a-3p have been described as a biomarker for early diagnosis of laryngeal squamous cell carcinoma, and high circulating levels of this miRNA were associated with poor overall and disease-free survival of hepatocellular carcinoma patients after liver transplantation [36,37].
Josson et al. demonstrated that high expression of miR-409 delivered by EVs from prostate stromal fibroblasts promoted tumorigenesis through repression of tumor suppressor genes [39].
Limited data are available in the literature for miR-11400 [40]. The predicted target genes are highly enriched for key regulators of the lysosome, Hippo signaling, and TGF-β pathways.
The long-term toxicity of asbestos fibers is due to their bio-persistence in cells and their accumulation in lysosomes, causing autophagy as well as lysosomal dysfunctions that result in oxidative stress and inflammation [41]. As it has been demonstrated for nanoparticles, asbestos fibers could also directly affect lysosomal stability by inducing lysosomal oxidative stress, alkalization, osmotic swelling, resulting in lysosomal membrane permeabilization and the subsequent activation of NLRP3 inflammasome [41]. Thus, lysosomal dysfunction provides a mechanism for asbestos-mediated inflammation.
Significant enrichment for Hippo and TGF-β signaling has also been reported for miR-148a-3p-validated target genes. Previous studies have demonstrated a functional interaction between TGF-β and Hippo signaling pathways in MPM cell growth and proliferation [29]. In particular, the inactivation of Hippo signaling components can play a remarkable role in the development and progression of MPM [29,30]. The activated LATS kinase, in association with the tumor-suppressor MOB, predicted target of miR-11400, phosphorylates and inhibits the transcription coactivators TAZ and/or YAP [42]. Therefore, dysregulation of LATS1/2 has been shown to result in constitutive activation of the YAP1/TAZ transcriptional coactivators, conferring malignant phenotypes to mesothelial cells.
Amongst predicted miR-11400-target genes, the tumor suppressor RASSF1 and the G1-phase cell-cycle regulator CDKN2B are both frequently lost in MPM tissue and implicated in the mechanisms of carcinogenesis [43,44]. Although experimental evidence is needed to validate the functional relationship between miR-11400 and its target genes, these findings suggest its role as a mediator of inflammation and malignant transformation.
Conversely, further evidence is available for miR-148a-3p. According to pathway enrichment analysis, validated miR-148a-3p target genes were enriched in several MPM-related pathways. We obtained a significant enrichment for FOXO signaling pathway, given by downstream genes mediating the cell cycle and apoptosis. Suzuki and colleagues demonstrated that the up-regulation of miR-182 and miR-183 suppresses the expression of FOXO1, which in turn promotes cell proliferation by acting on its downstream targets CDKN1A, and CDKN1B [45]. Likewise, miR-148a-3p targeted and suppressed cell cycle and apoptotic regulators (BCL2L11, CDKN1A, CDKN1B, S1PR1) likely leading to uncontrolled proliferation [46].
It is well demonstrated that EVs represent an ideal tool used by cells of primary neoplastic lesions to shape local and distant microenvironments promoting optimal conditions for tumor growth and proliferation [47].
The levels of serum EV miRNA might reflect the profiles in cancer tissue. By using dbDEMC, we reported that miR-148a-3p and miR-409-3p were upregulated in MPM tissues in agreement with the results in serum EVs, supporting their potential mechanisms in tumor-related biological processes and potentially reflecting the MPM progression [27,28]. The expression changes in miR-11400 levels were not detected since the authors used a microarray not including miR-11400 among the probes.
Our study presents several limitations. First, the small number of prospectively collected cases limits the robustness of our results. In addition, EPIC did not include multiple sampling during follow-up; as a result, a serum sample preceding diagnosis no more than five years (i.e., presumably during the MPM pre-clinical phase) was available for only part of all EPIC cases, entailing further limitation of the study dimension. MPM is a rare malignancy and obtaining pre-clinical samples is challenging. Settings where members of high-risk groups might be invited to participate to studies of pre-clinical MPM markers are medical surveillance programs for former asbestos workers; no such program, however, is active in Italy. A validation prospective cohort was not, thus, available to us; we tested, therefore, the identified mi-RNAs panel in a case-control setting, which had the disadvantage of evaluating sensitivity and specificity at the time of MPM diagnosis, rather than during its pre-clinical phase.
More importantly, it might be questionable whether research into pre-clinical MPM markers is justified on clinical as well as public health grounds, given the current limitations of the options that might be offered to suspect pre-clinical MPM cases: the diagnostic work-up is invasive and associated with non-negligible risks, while treatment offers only limited hopes of disease eradication. However, both diagnostic approaches and treatment might evolve if a shift might be obtained towards a larger proportion of MPM cases in potentially resectable disease stages. It also has to be mentioned that the same markers may prove useful as ancillary diagnostic tools for a non-negligible proportion of cases, such as those for whom thoracoscopy is contraindicated or where microscopic samples turn out difficult to be interpreted.
Besides the potential role of miRNAs, it would be worth identifying and validating other biomarkers of different molecular sources (e.g. proteins, DNA methylation), that in combination with small non coding RNAs might improve the diagnostic performance of MPM detection. As in other tumors, it is well known that the use of circulating biomarkers in pre-diagnostic samples may be a meaningful approach for detection at early stages [48,49]. So far, the combination of CALRETININ and MESOTHELIN has been showed to have the better diagnosis performance in plasma of preclinical asbestos exposed individuals, up to about one year before diagnosis [50]. However, several confounding factors may influence their plasma concentration and the study of additional markers that can decrease the false positive tests is warranted [51,52].
5. Conclusions
Most of the MPM group receives a diagnosis in their advanced tumor stage, which makes therapeutic approaches unfeasible, entailing a worse survival. A timely diagnosis may significantly improve the efficacy of the current therapeutic options and the overall survival.
This study identified a potential EV miRNA signature in preclinical MPM up to five years before diagnosis and raises the possibility of early intervention. If further replicated in other prospective cohorts, EV miRNAs in combination with other biomarkers may significantly impact on the management of this deadly tumor in the future.
Acknowledgments
The authors acknowledge the National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands, for their contribution and ongoing support to the EPIC Study. The authors acknowledge Giovanni Camussi (Dep of Medical Sciences, University of Turin) for his contribution in characterizing extracellular vesicles. The coordination of EPIC is financially supported by International Agency for Research on Cancer (IARC) and also by the Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London which has additional infrastructure support provided by the NIHR Imperial Biomedical Research Centre (BRC). The national cohorts are supported by: Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE), Federal Ministry of Education and Research (BMBF) (Germany); Associazione Italiana per la Ricerca sul Cancro—AIRC—Italy, Compagnia di SanPaolo and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); Health Research Fund (FIS)—Instituto de Salud Carlos III (ISCIII), Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, and the Catalan Institute of Oncology—ICO (Spain); Swedish Cancer Society, Swedish Research Council and County Councils of Skåne and Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C8221/A29017 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk; MR/M012190/1 to EPIC-Oxford) (United Kingdom).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15010125/s1. Supplementary Methods: Asbestos exposure assessment, RT-qPCR analysis. Figure S1: Representative micrograph of transmission electron microscopy of EVs isolated from 200 µL of serum; Figure S2: Representative nanoparticle tracking analysis of serum EVs showing the EVs size distribution; Figure S3: Results of RT-qPCR validation of miR-11400 (a), miR-148a-3p (b), and miR-409-3p (c) in 19 pre-clinical MPM samples and 19 matched controls. Table S1: Primary sequence of miRNAs analyzed by RT-qPCR; Table S2: MiRNA NGS pre-processing statistics; Table S3: Differentially expressed miRNAs between 80 pre-diagnostic MPM cases and 80 matched controls from NGS; Table S4: Differentially expressed miRNAs between 20 pre-diagnostic MPM cases and 20 matched controls from NGS; Table S5: Validated miRNA-target interaction results from MultiMiR Bioconductor’s package. Table S6: KEGG pathway enrichment analysis for validated target genes of miR-409-3p and miR-148a; Table S7: miRWalk 2.0 consensus target predictions for miR-11400; Table S8: KEGG pathway enrichment analysis for miR-11400 predicted target genes; Table S9: Characteristics of the retrospective study used in the validation analysis.
Author Contributions
E.C. conceptualization, methodology, writing, project administration; G.B. formal analysis, data curation, writing; B.P. methodology, investigation; A.A. (Alessandra Allione) investigation, project administration; A.R. investigation; C.C. methodology; M.M. resources; D.F. data curation; C.M. data curation; M.S. investigation; I.D. resources, data curation; F.G. resources; D.M. data curation; R.A.F. data curation; O.R. resources; C.S. (Carlotta Sacerdote) resources, data curation; M.R.-B. resources, data curation; K.S.-B. resources, data curation; S.P. resources, data curation; C.A. resources, data curation; T.J. resources, data curation; R.K. resources, data curation; R.T. resources, data curation; J.M.H. resources, data curation; E.R. resources, data curation; A.K.H. resources, data curation; C.T.-S. resources, data curation; M.B.S. resources, data curation; C.S. (Calogero Saieva) resources, data curation; P.A. resources, data curation; A.A. (Antonio Agudo) resources, data curation; E.W. resources, data curation; P.V. conceptualization; G.M. conceptualization, supervision, project administration, funding acquisition. All authors edited and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The EPIC-MESO study was reviewed and a favorable opinion given by the International Agency for Research on Cancer Ethics Committee (IEC Project No. 17-15). All participants gave their written informed consent to participate in the EPIC study. This research was conducted in accordance with the Declaration of Helsinki. The study protocol for the recruitment of individuals at the Oncology Department of the Villa Scassi Hospital, was approved by ethics committee of Regione Liguria n.605/2017 and written informed consent was obtained from all subjects prior to enrolment. All patients recruited at the Azienda Ospedaliero-Universitaria Maggiore della Carità, Novara, provided informed consent, and the protocol was approved by Comitato Etico Interaziendale AOU Maggiore della Carità, n. 169/18 (HERMES) and 205/20 (MESOMIC). All the sample collections described in the present study were carried out in accordance with the ethical principles of the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author upon request and approval by the Data Access Committee.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Italian Association for Cancer Research (AIRC, IG21390—P.I. G.M.) and from the Ministero dell’Istruzione, dell’Università e della Ricerca—c (n° D15D18000410001, to G.M.) to the Department of Medical Sciences, University of Torino.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hung Y.P., Chirieac L.R. Pathology of Malignant Pleural Mesothelioma. Thorac. Surg. Clin. 2020;30:367–382. doi: 10.1016/j.thorsurg.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Zhai Z., Ruan J., Zheng Y., Xiang D., Li N., Hu J., Shen J., Deng Y., Yao J., Zhao P., et al. Assessment of Global Trends in the Diagnosis of Mesothelioma From 1990 to 2017. JAMA Netw. Open. 2021;4:e2120360. doi: 10.1001/jamanetworkopen.2021.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mancuso M.R., Neal J.W. Novel systemic therapy against malignant pleural mesothelioma. Transl. Lung. Cancer Res. 2017;6:295–314. doi: 10.21037/tlcr.2017.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicole L., Cappellesso R., Sanavia T., Guzzardo V., Fassina A. MiR-21 over-expression and Programmed Cell Death 4 down-regulation features malignant pleural mesothelioma. Oncotarget. 2018;9:17300–17308. doi: 10.18632/oncotarget.24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wald O., Sugarbaker D.J. New Concepts in the Treatment of Malignant Pleural Mesothelioma. Annu. Rev. Med. 2018;69:365–377. doi: 10.1146/annurev-med-041316-085813. [DOI] [PubMed] [Google Scholar]
- 6.Nowak A.K., Chansky K., Rice D.C., Pass H.I., . Kindler H.L., Shemanski L., Bille A., Rintoul R.C., Batirel H.F., Thomas C.F., et al. The IASLC Mesothelioma Staging Project: Proposals for Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Pleural Mesothelioma. J. Thorac. Oncol. 2016;11:2089–2099. doi: 10.1016/j.jtho.2016.08.147. [DOI] [PubMed] [Google Scholar]
- 7.Mielgo-Rubio X., Cardena Gutierrez A., Sotelo Pena V., Sanchez Becerra M.V., Gonzalez Lopez A.M., Rosero A., Trujillo-Reyes J.C., Counago F. Tsunami of immunotherapy reaches mesothelioma. World J. Clin. Oncol. 2022;13:267–275. doi: 10.5306/wjco.v13.i4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J., Guan X., Fan Z., Ching L.M., Li Y., Wang X., Cao W.M., Liu D.X. Non-Invasive Biomarkers for Early Detection of Breast Cancer. Cancers. 2020;12:2767. doi: 10.3390/cancers12102767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Zandwijk N., Clarke C., Henderson D., Musk A.W., Fong K., Nowak A., Loneragan R., McCaughan B., Boyer M., Feigen M., et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J. Thorac. Dis. 2013;5:E254–E307. doi: 10.3978/j.issn.2072-1439.2013.11.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu R., Rai A., Chen M., Suwakulsiri W., Greening D.W., Simpson R.J. Extracellular vesicles in cancer—implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018;15:617–638. doi: 10.1038/s41571-018-0036-9. [DOI] [PubMed] [Google Scholar]
- 11.Doyle L.M., Wang M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui M., Wang H., Yao X., Zhang D., Xie Y., Cui R., Zhang X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019;10:626. doi: 10.3389/fgene.2019.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari L., Carugno M., Mensi C., Pesatori A.C. Circulating Epigenetic Biomarkers in Malignant Pleural Mesothelioma: State of the Art and critical Evaluation. Front. Oncol. 2020;10:445. doi: 10.3389/fonc.2020.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Rivera V., Negrete-Garcia M.C., Avila-Moreno F., Ortiz-Quintero B. Secreted and Tissue miRNAs as Diagnosis Biomarkers of Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2018;19:595. doi: 10.3390/ijms19020595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirschner M.B., Cheng Y.Y., Badrian B., Kao S.C., Creaney J., Edelman J.J., Armstrong N.J., Vallely M.P., Musk A.W., Robinson B.W., et al. Increased circulating miR-625-3p: A potential biomarker for patients with malignant pleural mesothelioma. J. Thorac. Oncol. 2012;7:1184–1191. doi: 10.1097/JTO.0b013e3182572e83. [DOI] [PubMed] [Google Scholar]
- 16.Muraoka T., Soh J., Toyooka S., Aoe K., Fujimoto N., Hashida S., Maki Y., Tanaka N., Shien K., Furukawa M., et al. The degree of microRNA-34b/c methylation in serum-circulating DNA is associated with malignant pleural mesothelioma. Lung Cancer. 2013;82:485–490. doi: 10.1016/j.lungcan.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez C.A., Riboli E. Diet and cancer prevention: Contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Cancer. 2010;46:2555–2562. doi: 10.1016/j.ejca.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 18.Ferrero G., Cordero F., Tarallo S., Arigoni M., Riccardo F., Gallo G., Ronco G., Allasia M., Kulkarni N., Matullo G., et al. Small non-coding RNA profiling in human biofluids and surrogate tissues from healthy individuals: Description of the diverse and most represented species. Oncotarget. 2018;9:3097–3111. doi: 10.18632/oncotarget.23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verta R., Grange C., Skovronova R., Tanzi A., Peruzzi L., Deregibus M.C., Camussi G., Bussolati B. Generation of Spike-Extracellular Vesicles (S-EVs) as a Tool to Mimic SARS-CoV-2 Interaction with Host Cells. Cells. 2022;11:146. doi: 10.3390/cells11010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rani A., Barter J., Kumar A., Stortz J.A., Hollen M., Nacionales D., Moldawer L.L., Efron P.A., Foster T.C. Influence of age and sex on microRNA response and recovery in the hippocampus following sepsis. Aging. 2022;14:728–746. doi: 10.18632/aging.203868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francavilla A., Gagliardi A., Piaggeschi G., Tarallo S., Cordero F., Pensa R.G., Impeduglia A., Caviglia G.P., Ribaldone D.G., Gallo G., et al. Faecal miRNA profiles associated with age, sex, BMI, and lifestyle habits in healthy individuals. Sci. Rep. 2021;11:20645. doi: 10.1038/s41598-021-00014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ru Y., Kechris K.J., Tabakoff B., Hoffman P., Radcliffe R.A., Bowler R., Mahaffey S., Rossi S., Calin G.A., Bemis L., et al. The multiMiR R package and database: Integration of microRNA-target interactions along with their disease and drug associations. Nucleic Acids Res. 2014;42:e133. doi: 10.1093/nar/gku631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sticht C., De La Torre C., Parveen A., Gretz N. miRWalk: An online resource for prediction of microRNA binding sites. PLoS ONE. 2018;13:e0206239. doi: 10.1371/journal.pone.0206239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge S.X., Jung D., Yao R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics. 2020;36:2628–2629. doi: 10.1093/bioinformatics/btz931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sculco M., La Vecchia M., Aspesi A., Pinton G., Clavenna M.G., Casalone E., Allione A., Grosso F., Libener R., Muzio A., et al. Malignant pleural mesothelioma: Germline variants in DNA repair genes may steer tailored treatment. Eur. J. Cancer. 2022;163:44–54. doi: 10.1016/j.ejca.2021.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Filiberti R., Marroni P., Spigno F., Merlo D.F., Mortara V., Caruso P., Cioe A., Michelazzi L., Bruzzone A., Bobbio B., et al. Is soluble mesothelin-related protein an upfront predictive marker of pleural mesothelioma? A prospective study on Italian workers exposed to asbestos. Oncology. 2014;86:33–43. doi: 10.1159/000355687. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y., Zheng M., Merritt R.E., Shrager J.B., Wakelee H.A., Kratzke R.A., Hoang C.D. miR-1 induces growth arrest and apoptosis in malignant mesothelioma. Chest. 2013;144:1632–1643. doi: 10.1378/chest.12-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z., Wu L., Wang A., Tang W., Zhao Y., Zhao H., Teschendorff A.E. dbDEMC 2.0: Updated database of differentially expressed miRNAs in human cancers. Nucleic Acids Res. 2017;45:D812–D818. doi: 10.1093/nar/gkw1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sekido Y. Targeting the Hippo Pathway Is a New Potential Therapeutic Modality for Malignant Mesothelioma. Cancers. 2018;10:90. doi: 10.3390/cancers10040090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Y., Pan D. The Hippo Signaling Pathway in Development and Disease. Dev. Cell. 2019;50:264–282. doi: 10.1016/j.devcel.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavalleri T., Angelici L., Favero C., Dioni L., Mensi C., Bareggi C., Palleschi A., Rimessi A., Consonni D., Bordini L., et al. Plasmatic extracellular vesicle microRNAs in malignant pleural mesothelioma and asbestos-exposed subjects suggest a 2-miRNA signature as potential biomarker of disease. PLoS ONE. 2017;12:e0176680. doi: 10.1371/journal.pone.0176680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goricar K., Holcar M., Mavec N., Kovac V., Lenassi M., Dolzan V. Extracellular Vesicle Enriched miR-625-3p Is Associated with Survival of Malignant Mesothelioma Patients. J. Pers. Med. 2021;11:1014. doi: 10.3390/jpm11101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber D.G., Gawrych K., Casjens S., Brik A., Lehnert M., Taeger D., Pesch B., Kollmeier J., Bauer T.T., Johnen G., et al. Circulating miR-132-3p as a Candidate Diagnostic Biomarker for Malignant Mesothelioma. Dis. Markers. 2017;2017:9280170. doi: 10.1155/2017/9280170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L., Zhou Q., Han Y.Q., Li P., Ouyang P.H., Wang M.Y., Hu Z.D. Diagnostic accuracy of circulating miR-126 for malignant pleural mesothelioma: A systematic review and meta-analysis. Transl. Cancer Res. 2021;10:1856–1862. doi: 10.21037/tcr-21-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber D.G., Brik A., Casjens S., Burek K., Lehnert M., Pesch B., Taeger D., Bruning T., Johnen G. Are circulating microRNAs suitable for the early detection of malignant mesothelioma? Results from a nested case-control study. BMC Res. Notes. 2019;12:77. doi: 10.1186/s13104-019-4113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng K.T., Lo C.M., Wong N., Li C.X., Qi X., Liu X.B., Geng W., Yeung O.W., Ma Y.Y., Chan S.C., et al. Early-phase circulating miRNAs predict tumor recurrence and survival of hepatocellular carcinoma patients after liver transplantation. Oncotarget. 2016;7:19824–19839. doi: 10.18632/oncotarget.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Y., Yu J., Ma Y., Wang F., Liu H. miR-148a and miR-375 may serve as predictive biomarkers for early diagnosis of laryngeal carcinoma. Oncol. Lett. 2016;12:871–878. doi: 10.3892/ol.2016.4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X., Zou W., Wang Y., Liao Z., Li L., Zhai Y., Zhang L., Gu S., Zhao X. Plasma-based microRNA signatures in early diagnosis of breast cancer. Mol. Genet. Genomic Med. 2020;8:e1092. doi: 10.1002/mgg3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Josson S., Gururajan M., Sung S.Y., Hu P., Shao C., Zhau H.E., Liu C., Lichterman J., Duan P., Li Q., et al. Stromal fibroblast-derived miR-409 promotes epithelial-to-mesenchymal transition and prostate tumorigenesis. Oncogene. 2015;34:2690–2699. doi: 10.1038/onc.2014.212. [DOI] [PubMed] [Google Scholar]
- 40.Sun Q., Jia X., Gao J., Zhang P., Mou W., Yang C., Tong H., Wen X., Tian Y. Identification and characterization of novel serum microRNAs in unstable angina pectoris and subclinical atherosclerotic patients. Exp. Cell Res. 2015;333:220–227. doi: 10.1016/j.yexcr.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Zolondick A.A., Gaudino G., Xue J., Pass H.I., Carbone M., Yang H. Asbestos-induced chronic inflammation in malignant pleural mesothelioma and related therapeutic approaches-a narrative review. Precis Cancer Med. 2021;4:27. doi: 10.21037/pcm-21-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu F.X., Zhang Y., Park H.W., Jewell J.L., Chen Q., Deng Y., Pan D., Taylor S.S., Lai Z.C., Guan K.L. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dubois F., Bazille C., Levallet J., Maille E., Brosseau S., Madelaine J., Bergot E., Zalcman G., Levallet G. Molecular Alterations in Malignant Pleural Mesothelioma: A Hope for Effective Treatment by Targeting YAP. Target. Oncol. 2022;17:407–431. doi: 10.1007/s11523-022-00900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang H., Xu D., Schmid R.A., Peng R.W. Biomarker-guided targeted and immunotherapies in malignant pleural mesothelioma. Ther. Adv. Med. Oncol. 2020;12:1758835920971421. doi: 10.1177/1758835920971421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki R., Amatya V.J., Kushitani K., Kai Y., Kambara T., Takeshima Y. miR-182 and miR-183 Promote Cell Proliferation and Invasion by Targeting FOXO1 in Mesothelioma. Front. Oncol. 2018;8:446. doi: 10.3389/fonc.2018.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou H., Jia X., Yang F., Shi P. miR-148a-3p suppresses the progression of acute myeloid leukemia via targeting cyclin-dependent kinase 6 (CDK6) Bioengineered. 2021;12:4508–4519. doi: 10.1080/21655979.2021.1956400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mills J., Capece M., Cocucci E., Tessari A., Palmieri D. Cancer-Derived Extracellular Vesicle-Associated MicroRNAs in Intercellular Communication: One Cell’s Trash Is Another Cell’s Treasure. Int. J. Mol. Sci. 2019;20:6109. doi: 10.3390/ijms20246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang Y.A., Weng S.L., Yang S.F., Chou C.H., Huang W.C., Tu S.J., Chang T.H., Huang C.N., Jong Y.J., Huang H.D. A Three-MicroRNA Signature as a Potential Biomarker for the Early Detection of Oral Cancer. Int. J. Mol. Sci. 2018;19:758. doi: 10.3390/ijms19030758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yee N.S., Zhang S., He H.Z., Zheng S.Y. Extracellular Vesicles as Potential Biomarkers for Early Detection and Diagnosis of Pancreatic Cancer. Biomedicines. 2020;8:581. doi: 10.3390/biomedicines8120581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnen G., Burek K., Raiko I., Wichert K., Pesch B., Weber D.G., Lehnert M., Casjens S., Hagemeyer O., Taeger D., et al. Prediagnostic detection of mesothelioma by circulating calretinin and mesothelin—A case-control comparison nested into a prospective cohort of asbestos-exposed workers. Sci. Rep. 2018;8:14321. doi: 10.1038/s41598-018-32315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pass H.I., Alimi M., Carbone M., Yang H., Goparaju C.M. Mesothelioma Biomarkers: A Review Highlighting Contributions from the Early Detection Research Network. Cancer Epidemiol. Biomarkers Prev. 2020;29:2524–2540. doi: 10.1158/1055-9965.EPI-20-0083. [DOI] [PubMed] [Google Scholar]
- 52.Casjens S., Johnen G., Raiko I., Pesch B., Taeger D., Topfer C., Schonefeld S., Moebus S., Jockel K.H., Bruning T., et al. Re-evaluation of potential predictors of calretinin and mesothelin in a population-based cohort study using assays for the routine application in clinical medicine. BMJ Open. 2021;11:e039079. doi: 10.1136/bmjopen-2020-039079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author upon request and approval by the Data Access Committee.