Abstract
Simple Summary
HER2+ metastatic breast cancer (MBC) is a highly prevalent type of breast cancer owing to its resistance to conventional anti-HER2 drugs. Therefore, novel agents that can arrest tumor progression and enhance the overall survival rates of HER2+ breast cancer patients, which would represent a major advancement toward the treatment of HER2 + MBC, need to be developed. This review provides insights into the clinical implication and utility of margetuximab, an anti-HER2 drug, in HER2 + MBC treatment, focusing on studies on the efficacy of margetuximab. Margetuximab is indeed an excellent addition to the arsenal of anti-HER2 mAb drugs that can be used for treating HER2 + MBC.
Abstract
Breast cancer (BC) is the most commonly diagnosed cancer globally, with high mortality rates. Targeted drug therapies have revolutionized cancer treatment. For example, treatment with human epidermal receptor 2 (HER2) antagonists has markedly improved the prognosis of patients with HER2-positive BC (HER2 + BC). However, HER2+ metastatic BC (MBC) remains prevalent owing to its resistance to conventional anti-HER2 drugs. Therefore, novel agents are needed to overcome the limitations of existing cancer treatments and to enhance the progression-free and overall survival rates. Progress has been made by optimizing the fragment crystallizable (Fc) domain of trastuzumab, an IgG1 monoclonal, chimeric anti-HER2 antibody, to develop margetuximab. The modified Fc domain of margetuximab enhances its binding affinity to CD16A and decreases its binding affinity to CD32B, thereby promoting its antitumor activity. This review summarizes studies on the efficacy of margetuximab, discusses its utility as an anti-HER2 monoclonal antibody drug for the treatment of HER2 + BC, and presents the latest advances in the treatment of BC. This review provides insights into the clinical implication of margetuximab in HER2 + MBC treatment.
Keywords: margetuximab, Fc engineering, clinical trials, anti-HER2 therapy, human epidermal receptor 2, positive metastatic breast cancer
1. Introduction
Breast cancer (BC), the most malignant tumor type of cancer in females, accounts for 11.7% of newly diagnosed cancer cases and 6.9% of cancer-related deaths annually [1]. Tumor heterogeneity is regarded as one of the hallmarks of cancers, including BC [2]. Owing to its molecular heterogeneity, BC is differentiated into four subtypes based on the presence or absence of the following biomarkers: hormone receptors (HRs), human epidermal receptor 2 (HER2), and Ki-67 (cell proliferation marker). The four subtypes of BC are: (I) luminal A (HR+/HER2−/low Ki-67), (II) luminal B (HR+/HER2−/high Ki-67) or (HR+/HER2+/any Ki-67), (III) non-luminal HER2+ (HR-/HER2 overexpression), and (IV) triple-negative (HR−/HER2−) along with the basal-like subtype [3,4]. These subtypes vary in etiology, risk factor profile, organ site preference for metastases, responses to therapy, and prognoses [5].
HER2 (also known as ErbB2, C-erbB2, or Her2/neu) is a tyrosine kinase protein belonging to the epidermal growth factor receptor (EGFR) family [6]. HER2 expression levels, depicting HER2 positivity (+) or negativity (−), are used as diagnostic and prognostic biomarkers for solid tumors [7]. HER2 positivity is confirmed based on the score of 3+ in ≥10% of tumor cells in the immunohistochemistry (IHC) test or a HER2/chromosome enumeration probe (CEP17) ratio of ≥2 in the in-situ hybridization (ISH) assay along with an average HER2 copy of <4 signals per cell in the pathological test [6,7]. Compared with other BC subtypes, HER2-positive cancer subtypes are characterized by decreased apoptosis and enhanced cell proliferation, mobility, adherence, invasiveness, angiogenesis, metastasis, and epithelial cell survival [7]. HER2 genomic alterations have been identified in 20–30% of invasive BC cases [8]. Moreover, HER2 + BC is a highly immunogenic and aggressive subtype with poor clinical outcomes and high recurrence rates owing to its resistance to chemotherapy [9]. Conventionally, HER2 + BC tends to grow faster and is associated with an increased risk of the progression of systemic and brain metastases. Tumor characteristics that promote metastasis in BC remain elusive [10]. Approximately 15–20% of BC cases are of the HER2 + BC subtype; the prevalence attributed to HER2 overexpression is a consequence of genomic alteration [11].
When diagnosed during the initial stages, before regional lymph node metastasis, BC is curable. However, after organ metastasis, BC cannot be cured with the current treatment strategies, resulting in high mortality rates. The extent of BC metastases is generally detected by imaging and validated by biopsy [10]. Moreover, tumor age, size, stage, histological type, and lymphovascular status are considered crucial factors for prognostication and treatment considerations [10]. The higher rates of metastasis are the result of the amplification of the ErbB2 gene. HER2 + BC metastasis is most prominently observed in the bones (65–67%), lungs (45–35%), and the brain (30–55%) [12]. It can also metastasize to distant organs such as the auxiliary lymph nodes, liver, and peritoneal cavity [10,12].
Over the last two decades, the progression-free survival (PFS) rates and prognosis of patients with HER2+ metastatic BC (MBC) have substantially improved due to the development of novel anti-HER2 targeted therapies. For example, the monoclonal antibody trastuzumab is the recommended treatment during the initial stages of HER2 + BC; it is either administered alone (monotherapy) or in combination with cytotoxic agents (taxane), followed by doxorubicin therapy [13,14]. Although this treatment shows promising outcomes in terms of survival rates and the reduction in relapse and metastasis, it shows severe cardiac toxicity, especially when used in combination with anthracycline agents, preventing its use in some patients. Moreover, resistance to trastuzumab has been reported in some patients. Consequently, to overcome toxicity, resistance, and other limitations of targeted therapy, novel HER2 inhibitors have been developed [15].
This review summarizes the current knowledge on margetixumab (Margenza®), a novel anti-HER2 drug recently approved by the United States Food and Drug Administration (US FDA), with an overview on its mechanism of action and its clinical significance. This review will be helpful for practicing clinicians.
2. Structural Biology of HER-Family Receptors
The human epidermal growth factor receptor (EGFR/ErbB) family comprises four members, namely HER1 (ErbB1), HER2 (ErbB2), HER3 (ErbB3), and HER4 (ErbB4), encoded by the ERBB1, ERBB2, ERBB3, and ERBB4, respectively [16]. These receptors are transmembrane tyrosine kinases with partial homology. Each receptor contains an extracellular domain (ECD) with four subunits (I, II, III, and IV) and a transmembrane lipophilic domain consisting of 19–25 hydrophobic amino acids. The ECD is 630 amino acids long, where the first and third subdomains (I/L1 and III/L2) are leucine-rich segments involved in ligand binding; they exhibit a β-barrel conformation. However, the second and fourth subdomains (II/CR1 and IV/CR2), which facilitate dimer formation, are cysteine-rich domains with disulfide bonds. Domain IV harbors a cleavage site for matrix metalloproteases (MMP) [17].
An Intracellular portion of about 550 amino acid residues containing (i) juxtamembrane segment, (ii) functional tyrosine kinase (TK) domain with catalytic activity, and (iii) cytoplasmic carboxyl-terminal tail with multiple phosphorylation sites, is essential for the activation of downstream signaling pathways [18]. The tyrosine kinase domain is essential for the dimerization of HER receptors. Ligand binding by the receptors is essential for the activation of the tyrosine kinase domains, which facilitates their dimerization. Dimerization can take place between molecules of the same receptor (homodimerization) or between two different HER receptors (heterodimerization) [19,20,21,22].
When inactive, i.e., in the absence of a ligand, these receptors exist as monomers in a tethered conformation due to the intramolecular interactions between domains II and IV. In this tethered conformation the “dimerization arm” (β-hairpin/loop) that exists in cysteine-rich domain II, is completely buried in the tethering arm of domain IV. This restricts the movement of receptor ECD arms and stabilizes the tethered receptor conformation, which is responsible for the auto-inhibition of ligand binding and dimerization [20,23]. However, ligand binding to domains I and III induces a conformational change that destabilizes the intramolecular tether and prevents the autoinhibitory effect. This exposes the dimerization arm and consequently allows dimerization.
Ligand-induced dimerization activates intracellular tyrosine kinase and enables it to assume an asymmetric active structure to create pTyr docking sites via kinase trans-tyrosine phosphorylation. These docking sites lodge the Src homology 2 (SH2) and phosphotyrosine binding (PTB) motifs of phosphotyrosine-binding proteins (e.g., Grb2, Shc, and PLCγ) [23]. The specific interaction of the pTyr-binding motifs with signaling proteins activates downstream signaling pathways, including the Ras-mitogen activated protein kinase (MAPK), Ras/Raf/MEK/MAPK, phosphoinositide-3-kinase (PI3K)-Akt, phospholipase C-gamma (PLC-γ), Src signaling, and signal transducer and activator of transcription (STAT) pathways [20]. These signaling pathways regulate essential cellular processes, including cell proliferation, migration, motility, differentiation, and apoptosis [24]. However, the cellular effects mainly depend on the intermediate pathway that is activated, and the magnitude and duration of ligand binding, which is further diversified by various ligands and the dimer in question [21].
Except for HER2, all ErbB family receptors directly bind to ligands. This is due to HER2’s ECD lacking a known ligand-binding domain [18,19]. It is therefore thought that HER2 functions as a co-receptor with, or dimerization partner of, other ErbB receptors. Similarly, all ErbB receptors exhibit tyrosine kinase activity, except for HER3, which has no or minimal catalytic kinase activity [22]. Interestingly, heterodimerization between HER2 and HER3 exhibits the most potent pro-tumorigenic and mitogenic signaling activity [23].
3. Unique Characteristics of HER2 Promote Tumor Progression in BC
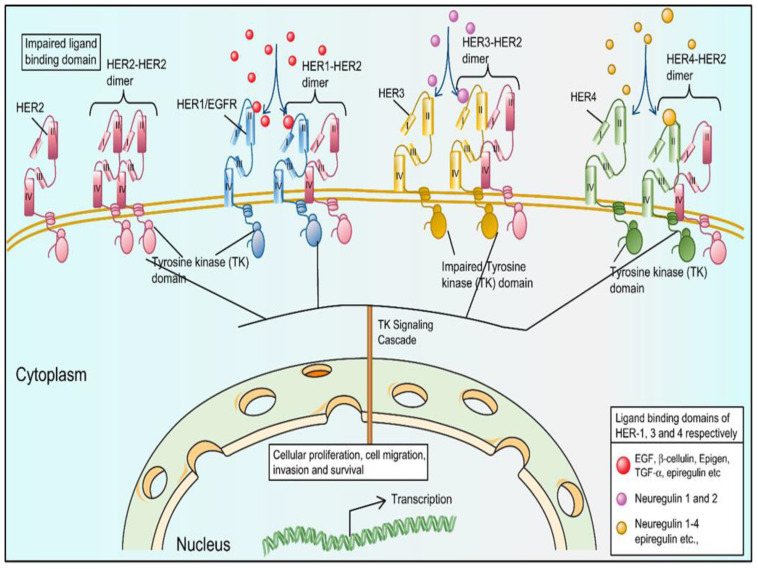
The HER2 protein has several fates (Figure 1). For example, as a monomer, as a homodimer, and as a HER2/HER3 or HER2/HER4 heterodimer [19]. The TK domain of HER2 is activated upon homo/hetero dimerization, evoking a signaling cascade that activates the receptive gene, leading to cell proliferation, migration, invasion, or cell survival, which are the hallmarks of cancer. The activation of the signaling cascades contributes to the heterogeneity of different cancer types (e.g., ovarian cancer, BC, and non-small cell lung cancer).
Figure 1.
The HER family of tyrosine kinases (TK) receptors have various ligand-binding capabilities that help orchestrate several biological processes in normal cells. The HER family of receptors comprises HER1, HER2, HER3, and HER4, depicted in blue, magenta, golden, and green, respectively. All members of the HER receptor family are structurally similar, containing an extracellular domain with a ligand-binding site (except HER2), a lipophilic transmembrane domain, and a TK intracellular domain (except for HER3). The TK domain of HER1, HER2, and HER4 is activated following homo- or heterodimerization and evokes a signaling cascade that activates a receptive gene, leading to cell proliferation, migration, invasion, or cell survival.
The overexpression/amplification of ErbB receptors shows a significant correlation with poor prognosis, cancer metastasis, decreased survival rates, and enhanced drug resistance [25]. The activation of HER2 signaling pathways is mainly responsible for cellular proliferation and cell survival phenomena, which are regarded as dominant drivers that cause tumor development and progression in nearly 85% of BC cases [26]. The amplification/overexpression of the HER-neu proto-oncogene and the HER2/HER3 heterodimer that activates oncoproteins have promiscuous roles in the pathogenesis of solid tumors [27]. Moreover, a truncated HER2 protein that lacks ECD-p95 (p95HER2) and an active C-terminal fragment, is detected in nearly 40% of HER2 + BC cases [28]. Therefore, p95HER2 is used as a predictive biomarker for cancer prognosis. Moreover, it is used to evaluate the efficacy of, or resistance to, existing treatments for BC [29,30].
4. Existing Anti-HER2 Therapies for HER2 + BC
Therapies for HER2 + BC primarily target the well-investigated HER1 and HER2 receptors and their pathways. BC cases exhibiting the overexpression of HER2 are clinically aggressive, showing moderate responses to chemotherapy. However, the development of personalized anti-HER2 treatment strategies has revolutionized the treatment of HER2 + BC, showing promising survival rates and improved patient outcomes [31,32,33,34,35]. Therapies include monoclonal antibodies (mAbs; e.g., trastuzumab and pertuzumab), antibody-drug conjugates (e.g., ado-trastuzumab emtansine, trastuzumab-deruxtecan), and tyrosine kinase inhibitors (e.g., lapatinib and neratinib) (Table 1) [13,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46].
Table 1.
Anti-HER2 targeted therapies approved by the US FDA.
| Agent |
Year of FDA
Approval |
Mechanism
of Action |
Indication | Dosage |
Major
Adverse Effects |
Box Warning |
|---|---|---|---|---|---|---|
| Trastuzumab [31,32] | 1998 | A humanized monoclonal IgG1 antibody that targets the extracellular domain (domain IV) of human HER2/neu, preventing dimerization and inducing ADCC | Treatment of HER2/neu overexpressing breast cancer/HER2 + MBC (first-line setting in therapy); used as adjuvant/neo adjuvant therapy alongside chemotherapy for ≤ 1 year. | Initial loading dose of 4 mg/kg IV followed by 2 mg/kg weekly or a loading dose of 8 mg/kg IV followed by 6 mg/kg every 3 weeks | Fever, infusion related reactions, diarrhea, headache, increased cough, rash, anemia, neutropenia, and myalgia | Cardiotoxicity, decline in left ventricular function; pulmonary toxicity (rare); infusion-related reactions |
| Lapatinib [33,34,35] | 2007 | Reversible tyrosine kinase inhibitor of HER1 and HER2 phosphorylation, resulting in an inhibition of signal transduction | Used in combination with capecitabine | 1250 mg/kg orally for 1–21 days along with 1000 mg/m2 capecitabine for 1–14 days, repeated every 3 weeks | Nausea, diarrhea, fatigue, and rash (acne, dermatitis acneiform) | Idiosyncratic hepatotoxicity |
| Pertuzumab [36,37] | 2012 | A humanized monoclonal IgG1 antibody that targets the ECD II of HER2/neu, preventing heterodimerization of HER2 with other HER family members |
Used as adjuvant/neo adjuvant therapy; given for 1 year for node positive disease; used in conjunction with trastuzumab and taxane for MBC as a first-line therapy | Initial loading dose of 840 mg/kg followed by 420 mg/kg every 3 weeks | Nausea, diarrhea, neutropenia, alopecia, rash, and peripheral neuropathy | Cardiotoxicity, decline in LVF, IRR hypersensitivity reactions/anaphylaxis, cardiomyopathy, and embryo-fetal toxicity |
| Ado-trastuzumab Emtansine (T-DM1) [38,39] |
2013 | An antibody drug conjugate comprising trastuzumab linked to a potent anti-microtubule agent DM1 (derivative of maytansine); causes cell-cycle arrest, leads to apoptosis, induces ADCC, and disrupts downstream HER2 signaling | Used to treat patients with HER2 + MBC who previously received trastuzumab and a taxane; adjuvant treatment of patients with HER2+ early BC | 3.6 mg/kg IV every 3 weeks | Fatigue, nausea, headache, thrombocytopenia, and constipation |
Hepatotoxicity, left ventricular dysfunction, pulmonary toxicity, IRR hypersensitivity reactions, thrombocytopenia, and neurotoxicity |
| Afatinib [40,41] | 2013 | Irreversible HER1 and HER2 tyrosine kinase inhibitor; induces phosphorylation leading to subsequent inhibition of signal transduction | Used as first-line therapy for advanced NSCLC patients with mutant-HER1 | Afatinib, 40 mg or 30 mg once daily | Diarrhea, paronychia, acneiform skin rash, stomatitis, and a loss of appetite | Left ventricular dysfunction, diarrhea, hepatotoxicity, hand-foot skin reaction, and interstitial lung disease |
| Neratinib [42,43,44,45] | 2017 | Irreversible HER1 and HER2 tyrosine kinase inhibitor; causes phosphorylation leading to subsequent inhibition of signal transduction |
Extended adjuvant therapy administered after trastuzumab and chemotherapy |
240 mg orally with food continued for 1 year along with prophylactic loperamide | Diarrhea, nausea, vomiting, anorexia, abdominal pain fatigue, and decreased appetite | Diarrhea (grade ≥ 3), hepatotoxicity, and embryo-fetal toxicity |
| Trastuzumab-deruxtecan [46] | 2019 | An antibody-drug conjugate comprising trastuzumab linked to topoisomerase I inhibitor (deruxtecan); induces ADCC, disrupts downstream HER2 signaling leads to apoptosis and cell-cycle arrest | Used for patients with metastatic or unresectable HER2+ breast cancer who have had one or more prior anti-HER2-based regimens | 5.4 mg/kg IV every 3 weeks | Nausea, diarrhea, vomiting, musculoskeletal pain, and myelosuppression | Interstitial lung disease, left ventricular dysfunction, neutropenia, embryo-fetal toxicity |
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; BC, breast cancer; EGFR, epidermal growth factor; HER2, human epidermal growth factor receptor 2; IRR, infusion-related reactions; LVF, left ventricular dysfunction; NSCLC, non-small cell lung cancer; MBC, metastatic breast cancer.
5. Current Insights on Margetuximab, a Novel Anti-HER2 Drug for Treatment of Positive Metastatic Breast Cancer
Research to develop anti-HER2-personalised therapeutic agents is being conducted at a rapid pace, which is evident based on the number of molecules being introduced into clinical trials. Rituximab initiated an era of immunotherapeutics, with mAbs extensively employed afterward to target tumors [47,48,49,50]. The therapeutic functionality of mAbs depends on the interactions of two regions thereof with components of the host immune system: the fragment antigen binding (Fab) region that binds to the antigen and the fragment crystallizable (Fc) region that interacts with the FcγRs and C1 complex (C1q) components of the immune system [51,52,53,54]. The mAbs exert their cytotoxic actions by promoting the interaction between Fc and FcγRs to activate the innate immune response by engaging complement-dependent cytotoxicity (CDC), antibody-dependent cellular phagocytosis (ADCP), and antibody-dependent cellular cytotoxicity (ADCC) [51,55,56,57].
Antibody-dependent cellular cytotoxicity involves a cascade of mechanisms that target the FcRIIIa (CD16A) receptor on the cell surface and the Fc domains of immunoglobulins. It acts by enhancing natural killer (NK) cells, monocytes or macrophages (CD16+ subsets), and NKT cells or γδ T cells to enhance cytolysis and exert antitumor effects [58,59,60,61,62,63]. The FcγR family in humans comprises activating receptors and inhibitory receptors. Activating receptors include FcγRI (CD64, a high-affinity receptor), FcγRIIa (CD32a), FcγRIIIa (CD16a), and GPI-linked FcγRIIIb (low affinity); inhibitory receptors include FcγRIIb. The CD16A, CD32A, and CD32B receptors are expressed on effector cells and regulate immune activation processes. Human CD16a is a transmembrane low-affinity IgG Fc receptor [64] that triggers immune NK cells for their ADCC effects via the lone FcγR present in the NK cells [65,66]. Mutations in the FcγRIIIa gene generate two FcγRIIIa polymorphs with valine (V) and phenylalanine (F) at amino acid position 158 (FcγRIIIA-V158F). This polymorphism is considered crucial as it influences the rate of tumor cell lysis via ADCC, with the high-affinity valine v/v allele being responsible for more lysis than the V/F or FF alleles [51,52].
HER2 + MBC was successfully treated using the humanized IgG1 antibody, trastuzumab [53]. This was the first anti-HER2 mAb approved by the US FDA. It hinders HER1 activity by modulating extracellular HER2-neu [54]. Specifically, trastuzumab binds to the ECD of the HER2 receptor to inhibit its homodimerization and destabilize its heterodimers. Furthermore, it inhibits the formation of p95HER2, implicated in tumor progression. Moreover, trastuzumab can mediate ADCC via the activation of NK cells, made possible by the detection of the Fc portion thereof, ultimately leading to the death of cancer cells. Trastuzumab triggers HER2 internalization followed by lysosomal degradation and activates the c-Cbl-ubiquitin ligase-mediated ubiquitination and degradation of HER2. Trastuzumab also prevents matrix metalloproteinase (MMP)-mediated HER2 shedding. All of these effects ultimately lead to the inhibition of downstream signaling cascades [55,56]. The pathways inhibited by trastuzumab include the PI3K pathway, where decreasing phosphatase and tensin Homologue (PTEN) phosphorylation and AKT dephosphorylation increases cell death. Trastuzumab also inhibits the MAPK pathway, which activates the cyclin-dependent kinase inhibitor p27 KIP1 and promotes cell-cycle arrest and apoptosis. These actions show that trastuzumab exhibits anti-proliferative and anti-angiogenic effects, ultimately leading to the death of cancer cells. However, a major setback of this drug is the occurrence of drug resistance [54].
Novel immunotherapeutic agents are being developed to overcome resistance and enhance the overall survival of HER2 + BC patients. One such drug, margetuximab (MGAH22), was authorized by the US FDA on 16 December 2020 [57,58].
6. Pharmacology of Margetuximab
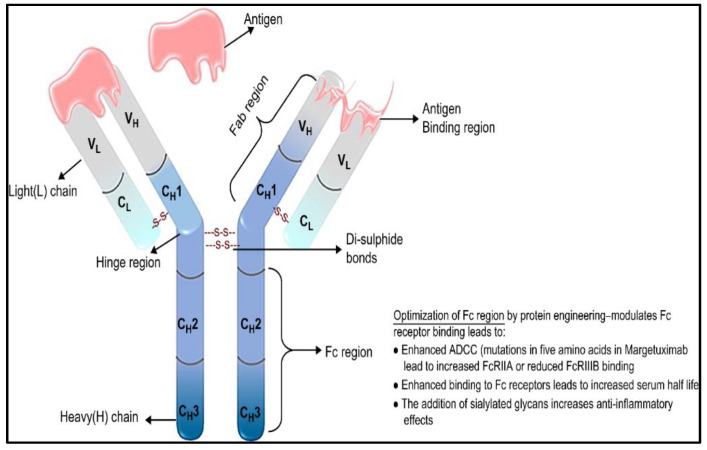
Fc-engineering strategies have been used over the years to customize mAbs to enhance their cytotoxic and antitumor potencies, margetixumab is the consequence of that effort. This drug, developed by MacroGenics, is a novel IgG1 monoclonal human/mouse chimeric antibody engineered in its Fc-domain to target HER2 (Figure 2) and is derived from 4D5, a precursor to trastuzumab [59]. The Fc-engineered domain of margetuximab exhibits mutations of five amino acid components (L235V, F243L, R292P, Y300L, and P396L) [67,68,69].
Figure 2.
Schematic representation of margetuximab. Margetuximab is a chimeric IgG1 kappa mAb with an optimized Fc region to enhance binding. The Fab and the Fc regions of IgG1 are indicated using brackets. Heavy chain domains are represented by CH1 to CH3, whereas light chain domains are denoted by CL. VH and VL represent the variable heavy chain and variable light chain, respectively.
6.1. Mechanism of Action
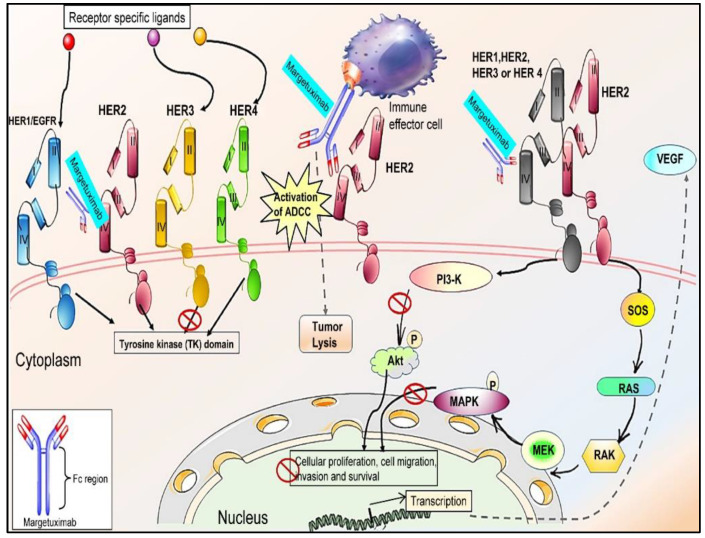
Similar to Trastuzumab, Margetixumab works by binding to the Fab epitope of the HER2 receptor with comparable specificity and affinity (Figure 3) [68] and exhibits Fc-independent antiproliferative effects. It shows elevated relative affinity towards both allelic variants of CD16A. The low-affinity allelic variant (FcγRIIIA-V158F) was found to be associated with a decreased clinical response to trastuzumab. The Fc-engineered domain of margetuximab is specifically optimized to increase its binding to all FcγRIIIA-V158F allelic variants, compared to the wild-type IgG1 [60,70]. This increased binding to FcγRIIIA is associated with the enhanced ADCC activity of human NK cells, which leads to the suppression of cell proliferation [71]. Additionally, the Fc-engineered domain of margetuximab lowers its affinity or decreases its binding to the inhibitory receptor CD32B (FcγRIIB) [53,72]. These altered binding capabilities of margetuximab, particularly in cells with lower levels of HER2, in cells resistant to trastuzumab, and in patients bearing FcγRIIIA-V158F, result in increased ADCC and enhanced anti-tumor effects [73].
Figure 3.
Schematic representation of the mechanism underlying the action of margetuximab. Margetuximab is an Fc-optimized mAb that binds to ECD IV of the HER2 receptor, thus preventing the formation of HER2–HER2 homodimers and ligand-independent HER2–HER3, HER2–HER1, and HER2–HER4 complexes/heterodimers. It enhances ADCC, leads to tumor lysis, and inhibits the TK signaling cascade, thus limiting cancer cell proliferation, migration, invasion, and survival. Figures were generated using Microsoft PowerPoint and free templates obtained from https://smart.servier.com/ (accessed on 9 June 2022).
6.2. Pharmacokinetic Properties
The molecular formula of margetuximab is C6484H10010N1726O2024S42 [74]. Its pharmacokinetics have been thoroughly investigated in Phase 1 clinical trials using pharmacokinetic two-compartmental models with parallel linear and Michaelis–Menten elimination. The approved dose used for the pharmacokinetic studies was 15 mg/kg Q3W or 6.0 mg/kg QW [73]. Bang et al. (2017) reported that the pharmacokinetic parameters of clearance (CL), central volume (V1), inter-compartment clearance (Q), and peripheral volume (V2) were 0.299, 3.73, 0.885, and 3.73 L/day, respectively. Moreover, the authors reported the distribution (t.5 dist) and half-life (t.5) at 1.12 and 15.5 days, respectively [73]. The Cmax was 466 µg/mL (20%), whereas the AUC0–21d was 4,120 µg/day/mL (21%) following administration to patients with advanced HER2 + BC and relapsed or refractory status, with a volume of distribution of 5.47 L (22%). Margetuximab is metabolized through several catabolic pathways into smaller peptides via proteases in a pattern similar to that of the other mAbs. The terminal half-life of Margetuximab was 19.2 days (28%), with a clearance rate of 0.22 L/day (24%). An approximately 3% concentration decrease in the serum levels of Margetuximab was observed four months post-discontinuation [61].
6.3. Indications/Therapeutic Use
Margetuximab, used synergistically with chemotherapy, is prescribed to adult patients with BC previously treated with two or more anti-HER2 agents, of which at least one was administered to treat metastatic diseases [61].
6.4. Tolerability and Toxicity
Treatment with margetuximab is well accepted and tolerated [61,73]. The SOPHIA clinical trial showed the safety profile of margetuximab. In this clinical trial, fatal adverse reactions were reported in 1.1% of margetuximab-receiving patients, with viral and aspiration pneumonia at 0.8% and 0.4%, respectively. Serious adverse reactions occurred in 16% of margetuximab-treated patients. The most prominent of these were left ventricular (LV) dysfunction and infusion-related reactions (IRRs) [61]. Although LV cardiac dysfunction was observed in 1.9% of margetuximab-treated patients, a lack of studies on margetuximab-treated patients with a LV ejection fraction of less than 50% or a history of the cardiac disease led to the addition to the warning and precautions section in FDA drug label [61]. The SOPHIA clinical trial showed that IRRs occurred in 13% of margetuximab-receiving patients. The majority of IRRs were reported during cycle 1 of therapy and resolved within 24 h. These infusion-related reactions were associated with symptoms such as nausea, fever, hypotension, anemia, diarrhea, headache, vomiting, fatigue, tachycardia, and certain cutaneous manifestations such as urticaria or rash. However, therapy for 9% of margetuximab-receiving patients was interrupted due to IRRS, and IRRS was discontinued in margetuximab therapy in 0.4% of treated patients. Patients experiencing severe infusion-related reactions must be carefully screened. Several toxicity parameters, including risk assessment during pregnancy and lactation, were not evaluated. Data on margetuximab-associated toxicity remains limited [61].
6.5. Dosage and Administration
A dosage of 15 mg/kg has been approved for administration via intravenous infusion. The initial dose is administered over 120 min, followed by another for approximately 30 min every three weeks until disease progression or unacceptable toxicity is observed. For therapy using a combined regimen, Margetuximab should be administered immediately following the completion of chemotherapy [61,75].
6.6. Clinical Trials of Margetuximab in BC
Clinical trials of Margetuximab began in 2010 and were conducted by MacroGenics [60,73,76]. A phase 1 trial (NCT01148849) was developed to assess the safety, efficacy, pharmacokinetic properties, pharmacodynamics, and antitumor activities of Margetuximab [73]. A total of 66 patients were included, all of whom presented with HER2-overexpressing breast and/or gastric carcinomas. Patients were divided into two groups, whereby 34 patients were treated with regimen A (intravenous infusion at a dose range of 0.1–6.0 mg/kg weekly for 3 weeks), while 32 were treated with regimen B (intravenous infusion at a dose range of 10–18 mg/kg once every 3 weeks). Margetuximab was well tolerated at the given doses without any attainable maximum tolerated dose for either regimen. The trial results indicated that, of the 60 response-evaluable patients, 12% showed partial responses, while 52% reached stable disease. Moreover, a reduction in tumor size was observed in 78% of patients. Adverse effects were mild, with constitutional symptoms such as nausea and pyrexia. The authors concluded that Margetuximab exhibited promising activity and could be used alone to treat patients with HER2+ tumors. This trial facilitated further research on the potential clinical application of Margetuximab as a single agent or in combination therapy [73,77].
Nordstrom et al. (2011) reported that margetuximab exhibited a promising safety profile, with 0.1 mg/kg as a minimum human equivalent dose in trials on cynomolgus monkeys receiving 150 mg/kg [60]. ‘No observed adverse effect level’ (NOAEL) was observed; the dose was derived from the minimum effective dose of 1 mg/kg used in transgenic mice bearing human CD16A-V158F (xenograft models). Furthermore, margetuximab treatment was considered acceptable at a dose range of 15–150 mg/kg [60]. This study was followed by a phase II clinical trial (NCT01828021) that employed a Simon 2-stage design in 41 patients to investigate the efficacy and activity of margetuximab in patients with advanced BC, in either relapsed or refractory status, and low HER2 expression, as evidenced using a fluorescence ISH test [78]. A series of trials involving Chinese patients has also been conducted (NCT04398108 and NCT04262804). The most prominent clinical trial was a phase III trial (SOPHIA, NCT02492711). This randomized, parallel assignment, open-label trial design comprised 536 patients aged ≥ 18 years with confirmed HER2 + MBC or unresectable BC previously treated with at least two HER2-directed therapies in the metastatic setting [76]. A 1:1 randomization was performed based on the chemotherapy treatment (capecitabine, gemcitabine, eribulin, or vinorelbine) administered along with margetuximab (15 mg/kg) or trastuzumab (6 mg/kg; loading dose, 8 mg/kg) over three-week cycles. The authors reported that PFS was enhanced in patients receiving margetuximab compared to those receiving trastuzumab, with a median of 5.8 vs. 4.9 months (95% CI, 4.2–5.6; hazard ratio [HR], 0.76; p = 0.033; 95% CI, 0.59–0.98 at 24% relative risk reduction). The median overall survival (OS) was 21.6 months with margetuximab vs. 19.8 months with trastuzumab (HR, 0.89; 95% CI, 0.69–1.13; p = 0.33). The final results of the SOPHIA trial reported superior overall survival (OS) in HER2 + MBC with CD16A-158F low-affinity allele patients in comparison to the trastuzumab-treated group where the median OS was 23.6 vs. 19.2 months, respectively (HR 0.72; 95% CI 0.52-1.00; nominal p = 0.05). Although this trial exhibited a comparable safety profile between margetuximab and trastuzumab-treated groups, IRRs were commonly seen in the margetuximab-treated group (13.6%) compared to the trastuzumab group [79]. Margetuximab was approved following a series of clinical trials (Table 2) to treat HER2 + MBC in December 2020. Margetuximab is currently undergoing several other clinical trials to further investigate its usage, safety, and efficacy in other cancer types, such as gastric and gastroesophageal junction cancer [80,81]. The trials currently registered with Clinicaltrials.gov are listed in Table 3.
Table 2.
Clinical trials involving Margetuximab in breast cancer patients.
| NCT Identifier | Year of Clinical Study | Study Title | Phase and Study Design |
Study Participant | Study Type | Subject Number | Status | Study Arm |
|---|---|---|---|---|---|---|---|---|
| NCT01148849 [73] | 2010 | Safety study of Margetuximab in HER2+ carcinomas | I-Single Group Assignment, open label, treatment purpose | ≥18 years (adults, older adults), with confirmed HER2 + MBC | IV | 66 | Completed | Margetuximab |
| NCT01828021 [60] | 2013 | Phase 2 study of Margetuximab in patients with relapsed or refractory advanced BC | II-Single Group Assignment, open label, treatment purpose | Age ≥ 18 years (adults, older adults), with confirmed invasive BC | IV | 25 | Completed | Margetuximab |
| NCT02492711 [76] | 2015 | Margetuximab plus chemotherapy vs. Trastuzumab plus chemotherapy in the treatment of HER2 + MBC (SOPHIA) |
III-Randomized, parallel assignment, open label, treatment purpose | Age ≥ 18 years (adults, older adults), with confirmed HER2 + MBC | IV | 624 | Completed | Margetuximab and the chosen chemotherapy (Capecitabine/Vinorelbine/Eribulin/Gemcitabine) vs. Trastuzumab and the chosen chemotherapy |
| NCT03133988 | 2017 | Margetuximab Expanded Access Program | not available | Children, adults, older adults | EA | Case -by-case basis | Approved for marketing | Margetuximab |
| NCT04262804 | 2020 | A study to evaluate the efficacy and safety of Margetuximab plus chemotherapy in the treatment of Chinese patients with HER2 + MBC | II-Randomized, parallel assignment, open label, treatment purpose | Male or female, age ≥ 18 years, with confirmed HER2 + MBC; have received at least 2 prior lines of anti-HER2 directed therapy in the metastatic setting | IV | 120 | Recruiting | Margetuximab and the chosen chemotherapy (Capecitabine/Vinorelbine/Gemcitabine) vs. Trastuzumab and the chosen chemotherapy |
| NCT04398108 | 2020 | A study to evaluate the pharmacokinetics of Margetuximab in Chinese patients with HER2 + MBC | I-Single group assignment, open label, treatment purpose | Male or female, age ≥ 18 years, with confirmed HER2 + MBC; have received at least 2 prior lines of anti-HER2 directed therapy in the metastatic setting | IV | 16 | Completed | Margetuximab and the chosen chemotherapy (Capecitabine/Vinorelbine/Gemcitabine) |
| NCT04425018 | 2020 | MARGetuximab or trastuzumab (MARGOT) (MARGOT) | II-Randomized, parallel assignment, open label, treatment purpose | Male or female, age ≥ 18 years, with confirmed Stage II or III invasive BC | IV | 171 | Recruiting | Arm (a): Paclitaxel, Pertzumab, and Margetuximab; arm (b): Paclitaxel, Pertzumab, and Trastuzumab |
Note: ClinicalTrials.gov entries as of September 2021 are listed. Abbreviations: IV, Interventional; EA, Expanded Access; BC, breast cancer; MBC, metastatic breast cancer.
Table 3.
Clinical trials involving margetuximab in other types of cancer patients.
| NCT Identifier | Study Title | Phase and Study Design |
Study Participant | Study Type | Subjects, n | Status | Study Arm | Indications |
|---|---|---|---|---|---|---|---|---|
| NCT02689284 [80] | Combination of margetuximab and pembrolizumab for advanced, metastatic HER2+ gastric or gastroesophageal junction cancer | Ib/2 Single group assignment, open label, treatment purpose | Age ≥ 18 years’ old; with confirmed HER2 + MGEJ or gastric cancer; Have received trastuzumab or at least 1/>lines of cytotoxic CT in the metastatic setting | Interventional | 95 | Completed | Margetuximab plus pembrolizumab | Gastric, stomach, and esophageal cancer |
| NCT04082364 [81] | Combination of margetuximab, INCMGA00012, MGD013, and chemotherapy Phase 2/3 trial in HER2+ gastric/GEJ cancer (MAHOGANY) | II/III Randomized, parallel assignment, open label, treatment purpose | Age ≥ 18 years (Adult, Older Adult), with confirmed HER2 + M (GEJ) or gastric cancer | Interventional | 860 | Recruiting | Cohort A: single-arm cohort. (safety efficacy evaluation); Cohort B Part 1: randomized, 4-arm segment; Cohort B Part 2: randomized, 2-arm segment |
Gastric cancer, gastroesophageal junction cancer, and HER2+ gastric cancer |
| NCT03219268 [76] | A study of MGD013 in patients with unresectable or metastatic neoplasms | I Non-randomized, single group assignment, open label, treatment purpose | Age ≥18 years (Adult, Older Adult), with confirmed advanced unresectable or metastatic solid tumors; cohort expansion only | Interventional | 353 | Active, not recruiting | Dose escalation followed by Cohort Expansion Phase at the MTD. MGD013; MGD013 + margetuximab |
Advanced solid tumors, hematologic neoplasms, ovarian cancer, HER2 + BC, NSCLC, cervical cancer, TNBC etc. |
Note: ClinicalTrials.gov entries as of September 2021 are listed. Abbreviations: BC, breast cancer; CT, chemotherapy; NSCLC, non-small cell lung cancer; HER2 + MGEJ, HER2+ metastatic gastroesophageal junctional cancer; TNBC, triple-negative breast cancer.
7. Conclusions
ErbB2 receptors are capable of enhancing the malignancy of solid tumors, including breast and gastric cancers, and sustaining cancer types resistant to conventional therapies. The scientific community deemed HER2 an effective target for cancer treatment, prevention, and diagnosis. The development of novel cancer-targeted therapies is a major advancement toward the treatment of HER2 + MBC. A step towards this was achieved by optimizing the Fc domain of trastuzumab to create the novel mAb margetuximab. Clinical trials with margetuximab have demonstrated its efficacy in treating HER2 + MBC. Importantly, compared with the standard trastuzumab combined with chemotherapy, the combination of margetuximab with chemotherapy showed favorable results in terms of overall response rate (ORR) and PFS. However, further evidence is required to determine its optimal use in a variety of clinical settings. Studies on treatment resistance to this drug and toxicity profiling are also warranted.
Data Availability Statement
All data are contained within the article.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Turashvili G., Brogi E. Tumor heterogeneity in breast cancer. Front. Med. 2017;4:227. doi: 10.3389/fmed.2017.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang J., Xu B. Targeted therapeutic options and future perspectives for HER2-positive breast cancer. Signal Transduct. Target. Ther. 2019;4:34. doi: 10.1038/s41392-019-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong C.W.S., Wu M., Cho W.C.S., To K.K.W. Recent Advances in the Treatment of Breast Cancer. Front. Oncol. 2018;8:227. doi: 10.3389/fonc.2018.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pashayan N., Antoniou A.C., Ivanus U., Esserman L.J., Easton D.F., French D., Sroczynski G., Hall P., Cuzick J., Evans D.G., et al. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat. Rev. Clin. Oncol. 2020;17:687–705. doi: 10.1038/s41571-020-0388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schettini F., Pascual T., Conte B., Chic N., Brasó-Maristany F., Galván P., Martínez O., Adamo B., Vidal M., Muñoz M., et al. HER2-enriched subtype and pathological complete response in HER2-positive breast cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2020;84:101965. doi: 10.1016/j.ctrv.2020.101965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.English D.P., Roque D.M., Santin A.D. HER2 Expression Beyond Breast Cancer: Therapeutic Implications for Gynecologic Malignancies. Mol. Diagn. Ther. 2013;17:85–99. doi: 10.1007/s40291-013-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wahdan-Alaswad R., Liu B., Thor A.D. Targeted lapatinib anti-HER2/ErbB2 therapy resistance in breast cancer: Opportunities to overcome a difficult problem. Cancer Drug Resist. 2020;3:179–198. doi: 10.20517/cdr.2019.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zielińska M., Zarankiewicz N., Kosz K., Kuchnicka A., Ciseł B. HER2-positive breast cancer—Available anti-HER2 therapies and new agents under investigation. J. Pre-Clin. Clin. Res. 2020;14:44–48. doi: 10.26444/jpccr/122068. [DOI] [Google Scholar]
- 10.Peyvandi S., Lan Q., Lorusso G. Chemotherapy-induced immunological breast cancer dormancy: A new function for old drugs? J. Cancer Metastasis Treat. 2019;5:44. doi: 10.20517/2394-4722.2019.16. [DOI] [Google Scholar]
- 11.Griguolo G., Pascual T., Dieci M.V., Guarneri V., Prat A. Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. J. Immunother. Cancer. 2019;7:90. doi: 10.1186/s40425-019-0548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gote V., Nookala A., Bolla P., Pal D. Drug Resistance in Metastatic Breast Cancer: Tumor Targeted Nanomedicine to the Rescue. Int. J. Mol. Sci. 2021;22:4673. doi: 10.3390/ijms22094673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mezni E., Vicier C., Guerin M., Sabatier R., Bertucci F., Gonçalves A. New Therapeutics in HER2-Positive Advanced Breast Cancer: Towards a Change in Clinical Practices? Cancers. 2020;12:1573. doi: 10.3390/cancers12061573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schramm A., De Gregorio N., Widschwendter P., Fink V., Huober J. Targeted Therapies in HER2-Positive Breast Cancer—A Systematic Review. Breast Care. 2015;10:173–178. doi: 10.1159/000431029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li S.G., Li L. Targeted therapy in HER2-positive breast cancer. Biomed. Rep. 2013;1:499–505. doi: 10.3892/br.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iqbal N., Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: Overexpression and therapeutic implications. Mol. Biol. Int. 2014;2014:852748. doi: 10.1155/2014/852748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchini C., Lucia P., Cristina K., Chiara G., Federico G., Elena Q., Manuela I., Serenella M.P., Elda T., Augusto A. Oncogene and Cancer-From Bench to Clinic. IntechOpen; London, UK: 2013. Her2-driven carcinogenesis: New mouse models for novel immunotherapies. [Google Scholar]
- 18.Ferreira P.M.P., Pessoa C. Molecular biology of human epidermal receptors, signaling pathways and targeted therapy against cancers: New evidences and old challenges. Braz. J. Pharm. Sci. 2017;53:e16076. doi: 10.1590/s2175-97902017000216076. [DOI] [Google Scholar]
- 19.Kiyatkin A., Rosenburgh I.K.V.A.V., Klein D.E., Lemmon M.A. Kinetics of receptor tyrosine kinase activation define ERK signaling dynamics. Sci. Signal. 2020;13:1. doi: 10.1126/scisignal.aaz5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maruyama I.N. Mechanisms of Activation of Receptor Tyrosine Kinases: Monomers or Dimers. Cells. 2014;3:304–330. doi: 10.3390/cells3020304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross J.S., Fletcher J.A., Linette G.P., Stec J., Clark E., Ayers M., Bloom K.J. The Her-2/neu gene and protein in breast cancer 2003: Biomarker and target of therapy. Oncologist. 2003;8:307–325. doi: 10.1634/theoncologist.8-4-307. [DOI] [PubMed] [Google Scholar]
- 22.Fichter C.D., Przypadlo C.M., Buck A., Herbener N., Riedel B., Schäfer L., Nakagawa H., Walch A., Reinheckel T., Werner M., et al. A new model system identifies epidermal growth factor receptor-human epidermal growth factor receptor 2 (HER2) and HER2-human epidermal growth factor receptor 3 heterodimers as potent inducers of oesophageal epithelial cell invasion. J. Pathol. 2017;243:481–495. doi: 10.1002/path.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wee P., Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers. 2017;9:52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roskoski R., Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res. 2014;79:34–74. doi: 10.1016/j.phrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z. ErbB Receptor Signaling. Volume 1652. Humana Press; New York, NY, USA: 2017. ErbB Receptors and Cancer; pp. 3–35. [DOI] [PubMed] [Google Scholar]
- 26.Brandão M., Aftimos P., Azim H.A., Sotiriou C. Molecular biology of breast cancer. In: Tsongalis G.J., editor. Essential Concepts in Molecular Pathology. 2nd ed. Academic Press; Cambridge, MA, USA: 2020. pp. 449–461. [Google Scholar]
- 27.Inoue K., Fry E.A. Aberrant Splicing of Estrogen Receptor, HER2, and CD44 Genes in Breast Cancer. Genet. Epigenetics. 2015;7:19–32. doi: 10.4137/GEG.S35500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz I.R., Vicario R., Morancho B., Morales C.B., Arenas E.J., Herter S., Freimoser-Grundschober A., Somandin J., Sam J., Ast O., et al. p95HER2–T cell bispecific antibody for breast cancer treatment. Sci. Transl. Med. 2018;10:eaat1445. doi: 10.1126/scitranslmed.aat1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arribas J., Baselga J., Pedersen K., Parra-Palau J.L. p95HER2 and Breast Cancer: Figure 1. Cancer Res. 2011;71:1515–1519. doi: 10.1158/0008-5472.CAN-10-3795. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez C., Schiff R. HER2: Biology, Detection, and Clinical Implications. Arch. Pathol. Lab. Med. 2011;135:55–62. doi: 10.5858/2010-0454-RAR.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudis C.A. Trastuzumab–Mechanism of Action and Use in Clinical Practice. N. Engl. J. Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 32.Zardavas D., Pugliano L., Ades F., Bozovic-Spasojevic I., Capelan M., de Azambuja E. Targeted treatments of HER2-positive metastatic breast cancer: Trastuzumab and beyond. Breast Cancer Manag. 2012;1:217–233. doi: 10.2217/bmt.12.35. [DOI] [Google Scholar]
- 33.Burris H.A., Hurwitz H.I., Dees E.C., Dowlati A., Blackwell K.L., O’Neil B., Marcom P.K., Ellis M.J., Overmoyer B., Jones S.F., et al. Phase I Safety, Pharmacokinetics, and Clinical Activity Study of Lapatinib (GW572016), a Reversible Dual Inhibitor of Epidermal Growth Factor Receptor Tyrosine Kinases, in Heavily Pretreated Patients with Metastatic Carcinomas. J. Clin. Oncol. 2005;23:5305–5313. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 34.Dai C., Ma S., Wang F., Zhao H., Wu X., Huang Z., Chen Z.-S., To K., Fu L. Lapatinib promotes the incidence of hepatotoxicity by increasing chemotherapeutic agent accumulation in hepatocytes. Oncotarget. 2015;6:17738–17752. doi: 10.18632/oncotarget.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon J.Y., Han J.M., Seo I., Gwak H.S. Risk factors associated with the incidence and time to onset of lapatinib-induced hepatotoxicity. Breast Cancer Res. Treat. 2019;178:239–244. doi: 10.1007/s10549-019-05382-x. [DOI] [PubMed] [Google Scholar]
- 36.Patel R., Bates J.S. Pertuzumab in Metastatic Breast Cancer. J. Adv. Pract. Oncol. 2012;3:391. doi: 10.6004/jadpro.2012.3.6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Royce M., Herold K. New Agents for the Management of Advanced HER2-Positive Breast Cancer. J. Adv. Pract. Oncol. 2016;7:295–298. [PMC free article] [PubMed] [Google Scholar]
- 38.Krop I., Winer E.P. Trastuzumab Emtansine: A Novel Antibody–Drug Conjugate for HER2-Positive Breast Cancer. Clin. Cancer Res. 2014;20:15–20. doi: 10.1158/1078-0432.CCR-13-0541. [DOI] [PubMed] [Google Scholar]
- 39.Dhillon S. Trastuzumab Emtansine: A Review of Its Use in Patients with HER2-Positive Advanced Breast Cancer Previously Treated with Trastuzumab-Based Therapy. Drugs. 2014;74:675–686. doi: 10.1007/s40265-014-0201-0. [DOI] [PubMed] [Google Scholar]
- 40.Gharwan H., Groninger H. Kinase inhibitors and monoclonal antibodies in oncology: Clinical implications. Nat. Rev. Clin. Oncol. 2015;13:209–227. doi: 10.1038/nrclinonc.2015.213. [DOI] [PubMed] [Google Scholar]
- 41.Ho G.-F., Chai C.-S., Alip A., Wahid M.I.A., Abdullah M.M., Foo Y.-C., How S.-H., Zaatar A., Lam K.-S., Leong K.-W., et al. Real-world experience of first-line afatinib in patients with EGFR-mutant advanced NSCLC: A multicenter observational study. BMC Cancer. 2019;19:1–11. doi: 10.1186/s12885-019-6107-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burstein H.J., Sun Y., Dirix L.Y., Jiang Z., Paridaens R., Tan A.R., Awada A., Ranade A., Jiao S., Schwartz G., et al. Neratinib, an Irreversible ErbB Receptor Tyrosine Kinase Inhibitor, in Patients with Advanced ErbB2-Positive Breast Cancer. J. Clin. Oncol. 2010;28:1301–1307. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 43.Martin M., Holmes F.A., Ejlertsen B., Delaloge S., Moy B., Iwata H., von Minckwitz G., Chia S.K.L., Mansi J., Barrios C.H., et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1688–1700. doi: 10.1016/S1470-2045(17)30717-9. [DOI] [PubMed] [Google Scholar]
- 44.Paranjpe R., Basatneh D., Tao G., De Angelis C., Noormohammed S., Ekinci E., Abughosh S., Ghose R., Trivedi M.V. Neratinib in HER2-Positive Breast Cancer Patients. Ann. Pharmacother. 2019;53:612–620. doi: 10.1177/1060028018824088. [DOI] [PubMed] [Google Scholar]
- 45.Puma Biotechnology, Inc. NERLYNX (Neratinib) [Package Insert]. U.S. Food and Drug Administration. [(accessed on 4 September 2021)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208051s000lbl.pdf.
- 46.Daiichi Sankyo, Inc. ENHERTU (Fam-Trastuzumab Deruxtecan-Nxki) [Package Insert]. U.S. Food and Drug Administration. Revised July 2017. [(accessed on 20 October 2022)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761139s011lbl.pdf.
- 47.Bešlija S., Gojković Z., Cerić T., Abazović A.M., Marijanović I., Vranić S., Mustedanagić–Mujanović J., Skenderi F., Rakita I., Guzijan A., et al. 2020 consensus guideline for optimal approach to the diagnosis and treatment of HER2-positive breast cancer in Bosnia and Herzegovina. Bosn. J. Basic Med Sci. 2020;21:120–135. doi: 10.17305/bjbms.2020.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mestres J.A., Imolins A.B., Martínez L.C., López-Muñiz J.I.C., Gil E.C., Ferré A.D.J., Berrón S.D.B., Pérez Y.F., Mata J.G., Palomo A.G., et al. Defining the optimal sequence for the systemic treatment of metastatic breast cancer. Clin. Transl. Oncol. 2017;19:149–161. doi: 10.1007/s12094-016-1520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Der Horst H.J., Nijhof I.S., Mutis T., Chamuleau M.E.D. Fc-Engineered Antibodies with Enhanced Fc-Effector Function for the Treatment of B-Cell Malignancies. Cancers. 2020;12:3041. doi: 10.3390/cancers12103041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu H., Saxena A., Sidhu S.S., Wu D. Fc Engineering for Developing Therapeutic Bispecific Antibodies and Novel Scaffolds. Front. Immunol. 2017;8:38. doi: 10.3389/fimmu.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang T.H., Jung S.T. Boosting therapeutic potency of antibodies by taming Fc domain functions. Exp. Mol. Med. 2019;51:1–9. doi: 10.1038/s12276-019-0345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ilieva K.M., Fazekas-Singer J., Achkova D.Y., Dodev T.S., Mele S., Crescioli S., Bax H.J., Cheung A., Karagiannis P., Correa I., et al. Functionally Active Fc Mutant Antibodies Recognizing Cancer Antigens Generated Rapidly at High Yields. Front. Immunol. 2017;8:1112. doi: 10.3389/fimmu.2017.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Costa R.L.B., Czerniecki B.J. Clinical development of immunotherapies for HER2+ breast cancer: A review of HER2-directed monoclonal antibodies and beyond. npj Breast Cancer. 2020;6:10. doi: 10.1038/s41523-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pento J.T. Monoclonal Antibodies for the Treatment of Cancer. Anticancer Res. 2017;37:5935–5939. doi: 10.21873/anticanres.12040. [DOI] [PubMed] [Google Scholar]
- 55.Church D.N., Price C.G. A Review of Trastuzumab-Based Therapy in Patients with HER2-positive Metastatic Breast Cancer. Clin. Med. Ther. 2009;1:CMT-S35. doi: 10.4137/CMT.S35. [DOI] [Google Scholar]
- 56.Fan X., Brezski R., Deng H., Dhupkar P.M., Shi Y., Gonzalez A., Zhang S., Rycyzyn M., Strohl W., Jordan R.E., et al. A Novel Therapeutic Strategy to Rescue the Immune Effector Function of Proteolytically Inactivated Cancer Therapeutic Antibodies. Mol. Cancer Ther. 2015;14:681–691. doi: 10.1158/1535-7163.MCT-14-0715. [DOI] [PubMed] [Google Scholar]
- 57.Ge J.Y., Overmoyer B. Prolonged Survival in Patients with Metastatic HER2-Positive Inflammatory Breast Cancer: A Case Report and Review of the Literature. Case Rep. Oncol. 2021;14:1071–1079. doi: 10.1159/000516760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cai H.H. Therapeutic Monoclonal Antibodies Approved by FDA in 2020. Clin. Res. Immunol. 2021;4:1–2. [Google Scholar]
- 59.Brezski R.J. Novel Approaches and Strategies for Biologics, Vaccines and Cancer Therapies. Elsevier; Amsterdam, The Netherlands: 2015. Novel Generation of Antibody-Based Therapeutics; pp. 125–146. [Google Scholar]
- 60.Nordstrom J.L., Gorlatov S., Zhang W., Yang Y., Huang L., Burke S., Li H., Ciccarone V., Zhang T., Stavenhagen J., et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res. 2011;13:R123. doi: 10.1186/bcr3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacroGenics MARGENZA (Margetuximab-Cmkb): Highlights of Prescribing Information. [(accessed on 27 August 2021)];2020 Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761150s000lbl.pd.
- 62.Ochoa M.C., Minute L., Rodriguez I., Garasa S., Ruiz E.P., Inogés S., Melero I., Berraondo P. Antibody-dependent cell cytotoxicity: Immunotherapy strategies enhancing effector NK cells. Immunol. Cell Biol. 2017;95:347–355. doi: 10.1038/icb.2017.6. [DOI] [PubMed] [Google Scholar]
- 63.Kohrt H., Rajasekaran N., Chester C., Yonezawa A., Zhao X. Enhancement of antibody-dependent cell mediated cytotoxicity: A new era in cancer treatment. ImmunoTargets Ther. 2015;4:91–100. doi: 10.2147/ITT.S61292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blázquez-Moreno A., Park S., Im W., Call M.J., Call M.E., Reyburn H.T. Transmembrane features governing Fc receptor CD16A assembly with CD16A signaling adaptor molecules. Proc. Natl. Acad. Sci. USA. 2017;114:E5645–E5654. doi: 10.1073/pnas.1706483114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patel K.R., Benavente M.C.R., Lorenz W.W., Mace E.M., Barb A.W. Fc γ receptor IIIa/CD16a processing correlates with the expression of glycan-related genes in human natural killer cells. J. Biol. Chem. 2021;296:100183. doi: 10.1074/jbc.RA120.015516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jing Y., Ni Z., Wu J., Higgins L., Markowski T.W., Kaufman D., Walcheck B. Identification of an ADAM17 Cleavage Region in Human CD16 (FcγRIII) and the Engineering of a Non-Cleavable Version of the Receptor in NK Cells. PLoS ONE. 2015;10:e0121788. doi: 10.1371/journal.pone.0121788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mahaweni N.M., Olieslagers T.I., Rivas I.O., Molenbroeck S.J.J., Groeneweg M., Bos G.M.J., Tilanus M.G.J., Voorter C.E.M., Wieten L. A comprehensive overview of FCGR3A gene variability by full-length gene sequencing including the identification of V158F polymorphism. Sci. Rep. 2018;8:15983. doi: 10.1038/s41598-018-34258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larionov A.A. Current Therapies for Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer Patients. Front. Oncol. 2018;8:89. doi: 10.3389/fonc.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kellner C., Otte A., Cappuzzello E., Klausz K., Peipp M. Modulating Cytotoxic Effector Functions by Fc Engineering to Improve Cancer Therapy. Transfus. Med. Hemotherapy. 2017;44:327–336. doi: 10.1159/000479980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X., Mathieu M., Brezski R.J. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018;9:63–73. doi: 10.1007/s13238-017-0473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaplon H., Muralidharan M., Schneider Z., Reichert J.M. Antibodies to watch in 2020. mAbs. 2020;12:1703531. doi: 10.1080/19420862.2019.1703531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen D., Zhao Y., Li M., Shang H., Li N., Li F., Wang W., Wang Y., Jin R., Liu S., et al. A general Fc engineering platform for the next generation of antibody therapeutics. Theranostics. 2021;11:1901–1917. doi: 10.7150/thno.51299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bang Y.J., Giaccone G., Im S.A., Oh D.Y., Bauer T.M., Nordstrom J.L., Li H., Chichili G.R., Moore P.A., Hong S., et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann. Oncol. 2017;28:855–861. doi: 10.1093/annonc/mdx002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.National Center for Biotechnology Information PubChem Substance Record for SID 172232539, Margetuximab (USAN), Source: KEGG. [(accessed on 2 September 2021)];2021 Available online: https://pubchem.ncbi.nlm.nih.gov/substance/172232539.
- 75.Margetuximab. [(accessed on 4 September 2021)]. Available online: https://go.drugbank.com/drugs/DB14967.
- 76.Tarantino P., Morganti S., Uliano J., Giugliano F., Crimini E., Curigliano G. Margetuximab for the treatment of HER2-positive metastatic breast cancer. Expert Opin. Biol. Ther. 2020;21:127–133. doi: 10.1080/14712598.2021.1856812. [DOI] [PubMed] [Google Scholar]
- 77.Pegram M.D., Tan-Chiu E., Miller K., Rugo H.S., Yardley D.A., Liv S., Stewart S.J., Erban J.K. A single-arm, open-label, phase 2 study of MGAH22 (margetuximab) [fc-optimized chimeric anti-HER2 monoclonal antibody (mAb)] in patients with relapsed or refractory advanced breast cancer whose tumors express HER2 at the 2+ level by immunohistochemistry and lack evidence of HER2 gene amplification by FISH. J. Clin. Oncol. 2014;32:TPS671. doi: 10.1200/jco.2014.32.15_suppl.tps671. [DOI] [Google Scholar]
- 78.Rugo H.S., Im S.-A., Cardoso F., Cortés J., Curigliano G., Musolino A., Pegram M.D., Wright G.S., Saura C., Escrivá-De-Romaní S., et al. Efficacy of Margetuximab vs Trastuzumab in Patients with Pretreated ERBB2-Positive Advanced Breast Cancer. A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021;7:573–584. doi: 10.1001/jamaoncol.2020.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rugo H.S., Im S.A., Cardoso F., Cortes J., Curigliano G., Musolino A., Pegram M.D., Bachelot T., Wright G.S., Saura C., et al. Margetuximab Versus Trastuzumab in Patients with Previously Treated HER2-Positive Advanced Breast Cancer (SOPHIA): Final Overall Survival Results From a Randomized Phase 3 Trial. J. Clin. Oncol. 2022 doi: 10.1200/JCO.21.02937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Catenacci D.V.T., Kang Y.-K., Park H., Uronis H.E., Lee K.-W., Ng M.C.H., Enzinger P.C., Park S.H., Gold P.J., Lacy J., et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22–05): A single-arm, phase 1b–2 trial. Lancet Oncol. 2020;21:1066–1076. doi: 10.1016/S1470-2045(20)30326-0. [DOI] [PubMed] [Google Scholar]
- 81.Catenacci D.V., Rosales M., Chung H.C., Yoon H.H., Shen L., Moehler M., Kang Y.-K. MAHOGANY: Margetuximab combination in HER2+ unresectable/metastatic gastric/gastroesophageal junction adenocarcinoma. Future Oncol. 2021;17:1155–1164. doi: 10.2217/fon-2020-1007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within the article.