Abstract
Endothelial colony-forming cells (ECFCs) are vascular resident and circulating endothelial cell subtypes with potent angiogenic capacity, a hierarchy of single-cell clonogenic potentials, and the ability to participate in de novo blood vessel formation and endothelial repair. Existing literature regarding ECFCs in neonatal and adult pulmonary diseases is confounded by the study of ambiguously defined “endothelial progenitor cells,” which are often not true ECFCs. This review contrasts adult and fetal ECFCs, discusses the effect of prematurity on ECFCs, and examines their different pathological roles in neonatal and adult pulmonary diseases, such as bronchopulmonary dysplasia, congenital diaphragmatic hernia, pulmonary artery hypertension, pulmonary fibrosis, and chronic obstructive pulmonary disease. Therapeutic potential is also discussed in light of available preclinical data.
Keywords: ECFCs, bronchopulmonary dysplasia, congenital diaphragmatic hernia, pulmonary artery hypertension, pulmonary fibrosis
Overview of Adult and Fetal Endothelial Progenitor Cell Nomenclature and Definition of Endothelial Colony-Forming Cells
The term “endothelial progenitor cell” (EPC) lacks specificity and is now used only as an umbrella term for a wide variety of cells that have been implicated in angiogenesis and vasculogenesis (1). This ambiguity in nomenclature has created confusion in the literature as to the function of these cells and their role in health and disease. As a starting point, it is important to understand that there have been two approaches used to study EPCs: 1) in vitro cell culture isolation methodologies; and 2) flow cytometry-based assays.
Various culture methods have been used to isolate EPCs, with each technique resulting in the isolation of different cell types with inconsistent characterization (1–3). In fact, several populations originally studied as EPCs have since been shown to be neither endothelial nor progenitor cells. Rather, they are myeloid cells with potent proangiogenic functions brought about through paracrine actions. These cells include early outgrowth EPCs or myeloid angiogenic cells, circulating angiogenic cells, and colony-forming unit endothelial cells. Although distinct, myeloid angiogenic cells may function synergistically with true EPCs (4).
The cells widely accepted as true EPCs, first identified by Yoder and colleagues, have been termed endothelial colony-forming cells (ECFCs) (5). ECFCs represent a vascular resident endothelial cell type with potent intrinsic angiogenic capacity and the abilities to participate directly in endothelial repair, instigate de novo blood vessel formation, and excrete paracrine signals to promote and enrich vascular repair (5, 6). Importantly, ECFCs demonstrate a hierarchy of single-cell clonogenic potentials (7, 8). As originally described, ECFCs appear after 14–21 days in culture when mononuclear cell fractions (MNCs) are seeded onto collagen-coated tissue culture plates in endothelial growth media (EGM-2), and nonadherent cells are depleted (5). Others have isolated and cultured these cells on gelatin, fibronectin, vitronectin, and Matrigel, and while the choice of extracellular matrix does not isolate a particular type of ECFC, it may lead to shape changes, differences in proliferation, and changes in migration (M.C. Yoder, personal communication, February 28, 2022). As such, differences in ECFC isolation and culture methodologies further complicate the interpretation of the existing literature.
Using flow cytometry, many different antigens have been applied to identify EPCs, with some associated controversy and similar inclusion of hematopoietic cells. The most current flow cytometric characterization of ECFCs is listed in Table 1, in contrast to other described circulating progenitor cells. Specifically, ECFCs are a rare population within the circulating progenitor cell staining profile, but instead of CD45dim, they are CD45−, and they are contained within the AC133− fraction of cells (3, 18). The cells identified as ECFCs by the flow cytometry markers listed in Table 1 failed to form hematopoietic colonies but did yield clonogenic endothelial colonies with proliferative potential, confirming their identity in culture (15, 16). However, most studies that employ flow cytometry have studied populations of cells such as CD133− CD34+ CD144+ cells or CD45− CD34+ cells (19, 20). Although these populations may be enriched for ECFCs, they tend not to correlate with cultured ECFCs and have not been well characterized. Therefore, we limit the discussion of flow cytometric data in this manuscript to emphasize that while flow cytometry is useful in immunophenotyping, cell culture assays are the standard to define ECFCs.
Table 1.
Common Nomenclature and Markers for Flow Cytometry Identification of Endothelial Progenitor Cells
| Name | Markers | References |
|---|---|---|
| Myeloid angiogenic cells | CD45+, CD14+, CD31+, CD34−, CD146−, CD133−, and Tie2− | (1, 9, 10) |
| Circulating endothelial precursors | CD45− and/or CD34+, AC133+, and VEGFR2+ | (11, 12) |
| Proangiogenic CPC | CD45dim, CD34+, CD31+, and AC133+ | (13, 14) |
| Nonangiogenic CPC | CD45dim, CD34+, CD31+, and AC133− | (13, 14) |
| ECFC* | CD45−, CD34+, CD31+, VEGFR2+, CD105+, CD146+, CD105+, CD144+, CD73−, HLA-DR−, HLA-ABC+, CD14−, and AC133− | (1, 3, 8, 15, 16, 17) |
Definition of abbreviations: CPC = circulating progenitor cell; ECFC = endothelial colony-forming cell.
Please note that although ECFCs express these antigens, the definition of an ECFC is on the basis of cell culture methods and not immunophenotyping.
In the fetal environment, there are a variety of prenatal EPCs that are considered to be circulating embryonic cells with the potential to contribute to endothelial development (21). So-called embryonic EPCs derive from the proximal lateral mesoderm and have been described as early as embryonic day (E) 7.5 in mouse embryos. An advantage of embryonic EPCs is that they lack MHC-I (major histocompatibility complex I) expression and are resistant to killer cell-mediated cytolysis, which allows their use in nonsyngeneic animals (22). Although they are not recruited by adult blood vessels under physiologic conditions, they do home to ischemic tissues and colonize tumor metastases in mice (21). Multiple other early EPCs have also been described from the yolk sac, allantois, and fetal liver. These cells include hematovascular progenitors, G2-GATA4 endothelial progenitors, and erythro-myeloid endothelial progenitors (reviewed in Reference 21). It is unclear how these different sets of early EPCs relate to late EPC populations, but they are most likely completely different subsets. ECFCs are considered to be among the late fetal EPCs, and various groups have isolated ECFCs or related cells from the placenta and umbilical cord blood. In the following review, we will attempt to clarify the literature by focusing on cells confirmed to be adult or fetal ECFCs with any deviations from that explicitly stated. These data represent a fraction of the available EPC literature we reviewed for each disease state: 44% of bronchopulmonary dysplasia (BPD) articles (7/16), 60% of congenital diaphragmatic hernia (CDH) articles (3/5), 24% of pulmonary artery hypertension (PAH) articles (13/55), 47% of pulmonary fibrosis articles (7/15), and 21% of chronic obstructive pulmonary disease (COPD) articles (5/24).
Distinction between Adult and Fetal Endothelial Colony-Forming Cells
Direct comparisons have been made between similarly cultured fetal and adult ECFCs. Cord blood ECFCs displayed significantly enhanced clonogenic and proliferative potential in comparison to adult peripheral blood ECFCs (8); however, the two cell types did not differ in angiogenic tube formation capability or induction of VCAM-1 (vascular cell adhesion molecule-1) with inflammatory stimuli (8). Interestingly, when cord blood ECFCs were compared with placental ECFCs, both had similar quantities of high proliferative potential (HPP) clones and comparable functional capacity (16, 23), but in one study, placental ECFCs formed significantly more blood vessels in an in vivo vasculogenic assay (23). Therefore, both umbilical cord and placental ECFC types demonstrate enhanced proliferative and clonogenic potential compared with adult ECFCs isolated from peripheral blood mononuclear cells and mature endothelial cells (8, 21).
It is unknown if the two sets of fetal ECFCs that have been described truly reflect physiological differences in their origin, phenotype, or contribution to prenatal vascular growth. However, the integrin expression profiles of ECFCs from cord blood and placenta are the same, and microarray analysis demonstrated that only 33 genes were differentially expressed out of more than 40,000 (16). These genes were related to cell adhesion and migration, likely reflecting the fact that cord blood ECFCs are a circulating population and placental ECFCs are probably a vascular-resident population.
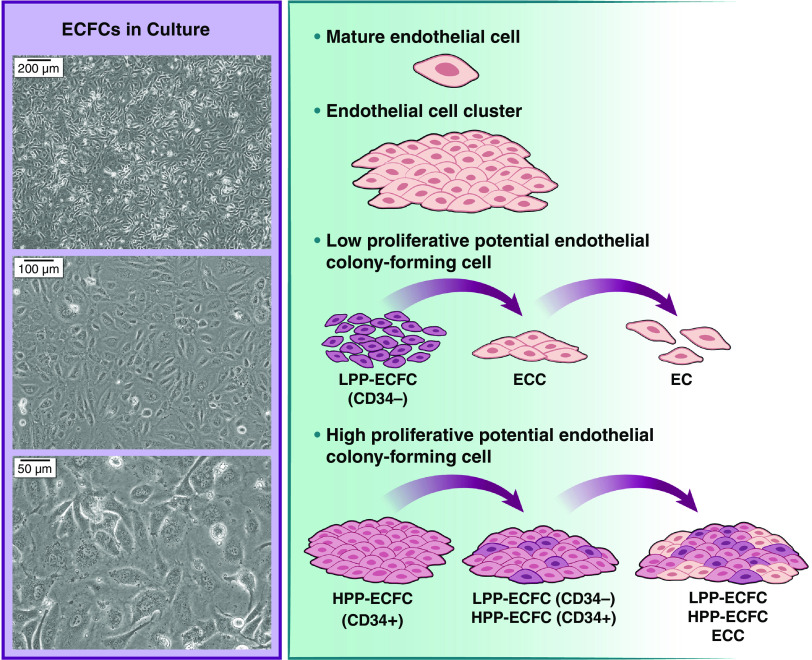
As the scientific community learns more about these different cell types, it is also becoming clear that subpopulations exist within populations of cultured ECFCs that appear homogenous with light microscopy (Figure 1). For example, well-defined hierarchies have been identified on the basis of characteristics such as proliferative potential and in vivo vasculogenic activity (8, 24). Such populations include high proliferative, low proliferative, and nonproliferating endothelial cell clusters, as well as mature, fully differentiated endothelial cells. Within cord blood, a unique population of HPP-ECFCs has been identified that can achieve at least 100 population doublings, replate into at least secondary and tertiary colonies, and retain high degrees of telomerase activity (8). Among human placental ECFCs, only CD34+ cells have the capacity to reproduce HPP-ECFCs (capable of forming colonies with greater than 2,000 cells) on replating (24). They are the only ECFCs capable of self-renewal and giving rise to both CD34+ and CD34− cells in stringent single-cell cultures, and they perform better at restoring perfusion when injected into ischemic hind limbs. Similarly, CD34+ ECFCs derived from peripheral blood exhibited higher tube-formation capacity and tip-cell gene expression than CD34− cells (25). In contrast, low proliferative potential (LPP) CD34− ECFCs form only small clusters that are lost with subsequent passaging. Interestingly, CD34+ HPP cells are relatively quiescent compared with CD34− LPP cells because of overexpression of cell cycle regulators. This is a consequence of sustained and increased Notch signaling, which permits the formation of a slowly proliferative colony (24). As colonies expand outward, contact with high degrees of Notch ligand from the CD34+ cells lessens, leading to reduced activity of cell cycle regulators and subsequent proliferation. Inhibition or loss of Notch signaling, however, also leads to loss of ability to self-renew. No other human endothelial cell has been identified with similar growth characteristics or clonogenic capacity. Improvements in single-cell genomics, flow cytometry, and other techniques are certain to facilitate and refine our evolving understanding of ECFC biology in the future.
Figure 1.
Clonogenic hierarchy of endothelial colony-forming cells (ECFCs) in culture. Despite appearing homogenous in culture, ECFCs have well-defined hierarchies on the basis of proliferative potential. CD34+ HPP ECFCs are the only cells capable of self-renewal in stringent single-cell culture. EC = endothelial cell; ECC = endothelial cell cluster; HPP = high proliferative potential; LPP = low proliferative potential.
Effect of Gestational Age (GA) on Fetal ECFCs
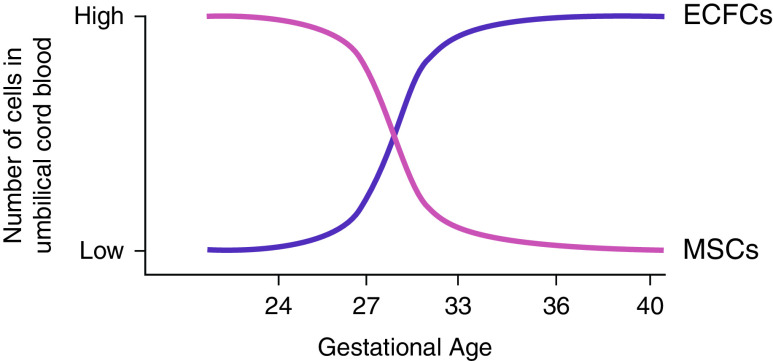
Although fetal ECFCs have vastly more proliferative potential than adult ECFCs, there is some evidence to suggest that proliferative capacity also differs on the basis of GA (26). Umbilical cord blood from infants born between 33 and 36 weeks GA yielded roughly the same number of ECFC colonies as that from term infants. However, only one-third as many ECFC colonies were enumerated from 24 to 28 weeks GA infant cord blood. Instead, this blood was enriched in mesenchymal stem cell populations, suggesting that different types of stem/progenitor cells emerge and circulate at different times during gestation (Figure 2). This is consistent with data from low birth weight preterm infants ranging from 27 to 37 weeks GA that show a decreased number of ECFC colonies; dramatically reduced capacity for tube formation, migration, and proliferation in vitro; and reduced angiogenic capability in vivo (27). The increase in the frequency of circulating ECFCs and angiogenic function as a fetus matures may be because of the necessary acceleration of capillary growth that accompanies airway surface expansion late in fetal development (28).
Figure 2.
Time course for appearance of ECFCs and mesenchymal stem cells (MSCs) in umbilical cord blood. The number of ECFC colonies enumerated from 33- to 36-week gestational age infants is comparable to that from term infants and roughly three times as many as from 24- to 28-week infants. Rather, early gestational age cord blood has been shown to be enriched in MSCs, which are not as numerous later in gestation.
However, this association has not held true in all studies (19, 20). One study looking at preeclampsia showed that ECFCs cultured on fibronectin instead of collagen were reduced in preterm infants with preeclampsia but were higher in preterm infants without preeclampsia compared with control subjects (29). In that study, preterm ECFCs from preeclamptic patients were slower to emerge in culture but ultimately maintained normal function and ability to form vascular networks. These studies again emphasize how differences in technique and clinical stresses (which are often not reported) potentially impact results.
Furthermore, there is evidence that dysfunctional preterm ECFCs remain impaired into adulthood and may be associated with risk factors for cardiovascular disease (30). ECFCs from adults who were born preterm at 29 or fewer weeks GA appeared later in culture and had reduced proliferation. In this population, reduced ECFC proliferation was associated with elevated systolic blood pressure, and ECFC impairment was more pronounced in preterm adults who had a history of BPD as neonates. It is unclear if ECFC function should be considered a biomarker for disease or a target for novel therapeutics.
ECFCs in BPD
BPD is a chronic lung disease of prematurity with an incidence that increases as GA and birth weight decrease (31). Approximately 80% of infants born between 22 and 24 weeks GA develop BPD, whereas only 20% of infants born at 28 weeks GA develop BPD, and 95% have a birth weight under 1,500 g. BPD is considered a developmental arrest of lung vascular and alveolar development resulting in a decreased area for gas exchange (32). The extent of chronic lung disease varies widely among preterm infants, and it is thought that impaired vascular development plays a central role in the development of BPD. Human infants that die of BPD have dysmorphic capillaries that are often on the interior of thickened alveolar septa, as well as decreased expression of vascular endothelial growth factor (VEGF) and the angiogenic receptors Fms-like tyrosine kinase 1 ([Flt-1], a.k.a. vascular endothelial growth factor receptor 1) and tyrosine kinase with immunoglobulin-like loops and epidermal growth factor homology domains-2 (TIE-2) (33). As endothelial progenitor cells are important in both angiogenesis and vasculogenesis in the developing lung, it has been hypothesized that alterations in EPCs play a role in the pathogenesis of BPD.
To date, two studies have examined ECFCs in the cord blood of premature infants and have found that ECFCs are fewer in preterm infants who subsequently develop BPD versus those who do not develop BPD (Figure 3) (34, 35). Borghesi and colleagues studied preterm infants with a GA of less than 32 weeks or a birth weight of less than 1,500 g (35) and assessed cord blood ECFCs using established clonogenic assays. They found that infants who went on to develop moderate or severe BPD had significantly lower frequencies of ECFCs than infants who developed only mild or no BPD. This held true for infants less than 28 weeks GA, even though ECFC frequencies were extremely low at that early GA. ECFC frequency was also inversely correlated with the duration of oxygen supplementation, nasal CPAP usage, and mechanical ventilation (35). A second study enrolled 62 preterm infants between 24 and 36 weeks GA, 13 of whom subsequently developed moderate or severe BPD (34). Similar to the first study, ECFCs were decreased among all patients with BPD, including mild BPD, when compared with those without BPD (1.4; interquartile range [IQR], 0–4.8 vs. 3.9; IQR, 1.3–7.4 ECFC colonies per 107 MNCs; P < 0.05). In the subset of neonates born at less than 28 weeks, all developed BPD, but none of the patients with moderate or severe BPD had detectable ECFCs, whereas infants with mild BPD had 4.9 (IQR, 2.4–9.0) ECFC colonies per 107 MNCs (P < 0.01). A univariate comparison of ECFC number and birth weight showed that the correlation between these variables was small, but the absence of ECFCs was associated with a relative risk of 8.1 (95% confidence interval, 2.5–26.2) of developing moderate to severe BPD. These data bolster the idea that the link between ECFC numbers and pulmonary outcomes is not simply because of the degree of prematurity, but there are no data regarding the function of these ECFCs.
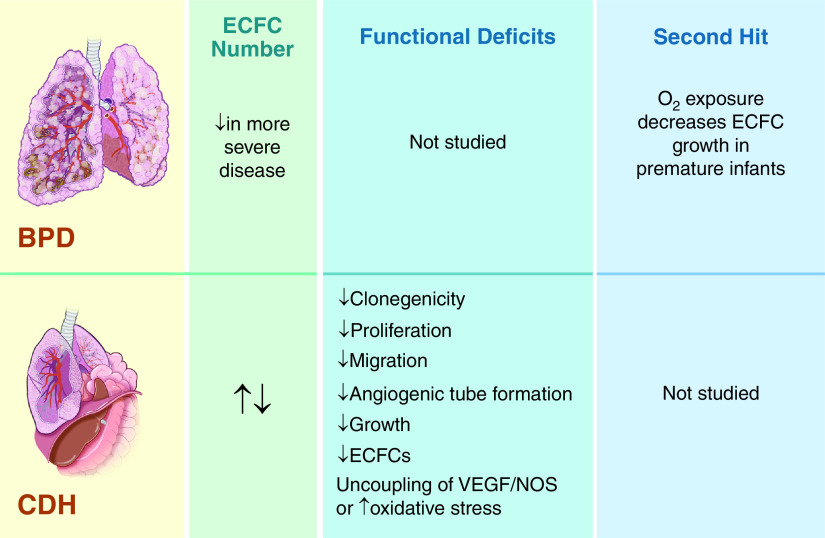
Figure 3.
Summary of available data regarding ECFCs in neonatal lung diseases, including bronchopulmonary dysplasia (BPD) and congenital diaphragmatic hernia (CDH). Top panels: BPD lungs are characterized by decreased alveolarization and vascular growth; abnormal vascular remodeling, tone, and reactivity; and emphysema, fibrosis, and increased arteriolar medial thickness. ECFC number has been shown to be reduced in more severe cases of BPD, but functional studies have not been performed with ECFCs from infants with BPD. Bottom panels: Lungs in patients with CDH are characterized by bilateral pulmonary hypoplasia (worst on the side ipsilateral to the diaphragmatic hernia), decreased airway branching and alveolarization, and vascular bed hypoplasia with decreased arborization, altered vasoreactivity, and adventitial thickening. Two human studies that looked at ECFC numbers in infants with CDH came to opposite conclusions. A fetal lamb study supports a reduction in ECFC number in CDH and is in accordance with the human study that shows reduced angiogenic functions of these cells. NOS = nitric oxide synthase; VEGF = vascular endothelial growth factor.
It may be that the normal appearance of ECFCs between 24 and 30 weeks (or more commonly after 28 wk) is key to the maturation of lung vasculature during normal development. However, there is reason to believe ECFCs are disrupted after extremely premature birth, in contrast to the physiologic increase of ECFCs seen at later GAs for fetuses that remain in utero (27). Although ECFCs have not been studied over time after delivery, in vitro data suggests that oxygen exposure after premature birth reduces the growth of ECFCs (Figure 3) (19). This second hit could further impair ECFCs and vascular development in this population of premature infants at risk for BPD.
Decreased cell proliferation has also been demonstrated in vitro for ECFCs isolated from hyperoxia-induced BPD lungs from newborn rats (36). These cells formed fewer cordlike endothelial networks in comparison to control subjects. Moreover, in single-cell clonogenic assays, 23.6 ± 2.5% of ECFCs from the control group versus only 9.3 ± 3.2% of ECFCs from the hyperoxia group were capable of forming HPP colonies with greater than 500 cells each (P < 0.05). Together, these results mirror in vitro results with human ECFCs and suggest that hyperoxia induces a functional deficiency in ECFCs that may be an appropriate therapeutic target.
ECFCs in CDH
CDH is a severe developmental anomaly that affects one out of every 2,500 newborns and is characterized by severe pulmonary hypoplasia and pulmonary hypertension. Lungs of infants with CDH have decreased vascular branching and total vascular volume, and it is thought that decreased lung vascular growth during development contributes to the etiology of CDH-associated pulmonary disease (37).
One study of six ECFC cultures from patients with CDH showed that ECFCs were increased in the cord blood of infants with CDH and that they proliferated significantly more rapidly when compared with control subjects (Figure 3) (38). Cord blood from infants with CDH was shown to yield 1.5 times more ECFC colonies compared with control cultures, and when low passage cells were cultured, the median fold increase of CDH ECFCs was 2.2× that of control ECFCs. However, one limitation of this study was that the angiogenic function of CDH ECFCs was not studied.
Another study examining a similar number of patients demonstrated that ECFCs were reduced in the cord blood of patients with CDH and had reduced potential for self-renewal, clonogenicity, proliferation, and migration (Figure 3) (39). The authors showed that the ECFC population in cord blood was reduced by 80% compared with control subjects when assessed by flow cytometry for CD45− CD34+ AC133− CD31+ cells, and colony counts per 107 MNCs were 72% lower. Similarly, in single-cell colony formation assays, the percentages of high proliferative potential ECFCs were reduced by 92%, and EC populations were increased by 320% in patients with CDH. The percentage of cells that migrated in a modified Boyden chamber assay was 89% fewer for CDH ECFCs. Although there were no observed differences in in vitro tube formation assays, in vivo vasculogenesis assays did demonstrate an 85% reduction in the number of chimeric vessels perfused with mouse erythrocytes in the transplanted CDH graft. The discordance between in vitro and in vivo testing of angiogenic capacity may relate to different time courses for the two studies (6–12 h in vitro vs. 14 days in vivo), or the in vitro assay may have been less comprehensive in approximating in vivo angiogenesis. It would be reasonable to conclude that given impaired ECFC counts, migration, proliferation, and reduced capacity in vivo for de novo angiogenesis that fewer and dysfunctional ECFCs are involved in vascular development in CDH, thereby contributing to the characteristic hypoplastic vasculature. Moreover, the cellular capacity for nitric oxide (NO) production was increased, but the response to VEGF was blunted, indicating disruptions in normal circulating ECFC functions (39).
The results of these two studies looking at ECFCs were essentially opposite of each other, and that may be explained by differences in GA or severity of CDH, which was not accounted for in either study, small sample size, other population-level differences, or variations in cell isolation and processing. It may be expected that the study of patients with severe CDH would reveal more significant ECFC dysfunction.
The only other study to examine HPP-ECFCs in CDH used a fetal lamb model and demonstrated that cell number, growth, and tube formation were decreased compared with control subjects (40). In contrast to the human studies, which isolated circulating ECFCs from cord blood, the cells in this model were isolated from fetal lamb pulmonary artery endothelial cells (PAECs). In this model, single-cell clonogenic assays demonstrated that the proportion of HPP-ECFCs with growth of more than 1,000 cells was markedly reduced from 29% for control subjects to 1% in CDH (P < 0.0001). Likewise, CDH ECFC growth and tube formation were reduced by 31% and 54%, respectively. The study also identified decreased intracellular NO production, decreased endothelial NO synthase (eNOS) protein concentrations, and increased VEGF and vascular endothelial growth factor receptor 2 (VEGF-R2) protein concentrations. These changes were hypothesized to represent either defects in VEGF-eNOS signaling, as cells remained responsive to VEGF despite having increased concentrations of VEGF and VEGF-R2 proteins, or oxidative stress and uncoupling of eNOS, preferentially shunting NO precursors toward superoxide production instead of NO. Importantly, the authors were unable to isolate microvascular ECFCs from CDH fetal lambs, suggesting that distal lung ECFCs may be markedly reduced. Clearly, additional studies are required, including investigations into therapeutics targeting ECFC function or cell-based interventions.
ECFCs in PAH
Adult PAH is distinct from neonatal pulmonary hypertension, which occurs in response to underdeveloped lungs. Diverse causes of this disease in older individuals may be idiopathic, heritable, drug- or toxin-induced, a consequence of left heart disease or chronic pulmonary arterial obstruction, related to a systemic illness, or because of acquired lung disease and/or hypoxemia (41). The major pathophysiology in adult disease relates to extensive vascular remodeling with angioproliferation, inflammation, and vasoconstriction of the small arteries of the lungs, which together increase pulmonary vascular resistance. As such, it is unsurprising that the role of ECFCs also differs in adults.
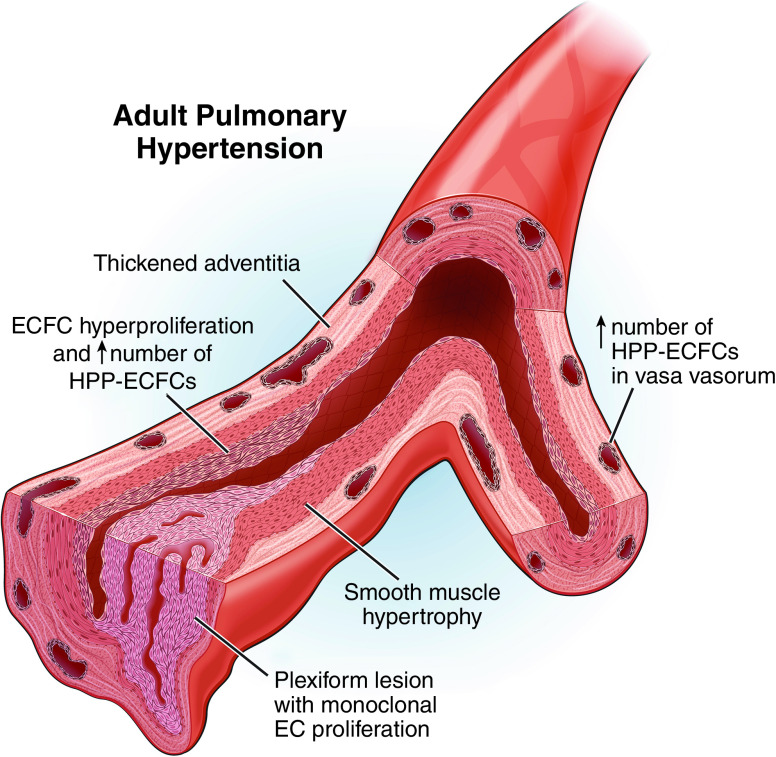
ECFCs have been identified in adult pulmonary vasculature and have been shown to be hyperproliferative in patients with PAH (Figure 4) (42). Among pulmonary artery endothelial cells, ECFCs are common, representing between 5% and 30% of sorted ECs in both healthy and PAH samples from explanted human lungs. Although the absolute number of ECFCs did not differ between these two groups, the cells had a wide range of proliferative capacities, and ECFCs from PAH lungs gave rise to larger colonies and had more HPP-ECFCs. These data are consistent with observations that idiopathic PAH PAECs were more proliferative in response to growth factors in vitro (43) and that circulating ECFCs from patients with PAH with BMPRII mutations were both more numerous and more proliferative (44). Similarly, a calf model of hypoxia-induced PAH demonstrated a significantly higher percentage of HPP-ECFCs in the vasa vasorum and a twofold increase in endoglin (CD105), which is a proliferation-associated and hypoxia-inducible protein in angiogenically active endothelial cells (45). Given that the plexiform lesions that are the hallmark of irreversible vessel disease in primary PAH are comprised of monoclonal endothelial cell proliferations (46), it is also possible that ECFCs underly these vascular anomalies. Together, these data suggest that ECFCs contribute to a proliferative angiopathic process in PAH and may give rise to lesions seen in end-stage disease.
Figure 4.
Pathogenic contribution of ECFCs to adult pulmonary artery hypertension (PAH). ECFCs in the pulmonary vasculature of patients with PAH are hyperproliferative and comprised of more HPP-ECFCs than healthy individuals. In total, ECFCs represent between 5% and 30% of endothelial cells in PAH. It is thought that ECFCs could be responsible for the development of plexiform lesions, given the fact that these lesions are monoclonal endothelial cell proliferations. HPP-ECFC numbers are also increased in the vasa vasorum. This may contribute to the hypoxia-induced angiogenic expansion of the vasa vasorum, which is an important contributor to pulmonary vascular remodeling.
There is additional evidence that angiogenic capacity may be altered in PAH ECFCs, but existing data are inconsistent. Circulating ECFCs in patients with PAH with BMPRII mutations had impaired ability to form vascular networks (44). Similarly, adult peripheral blood ECFCs responded to hypoxia in vitro with reduced tube formation and migration (47). However, tube formation was increased in HPP-ECFCs isolated from the hypoxic calf model (45), and it was unchanged in circulating ECFCs from patients with PAH (41). Further study is needed to determine the relative importance and contribution of PAH ECFC angiogenic capability and proliferative capacity to disease pathogenesis.
It is possible that ECFC proliferation has different effects on the lungs and hearts of patients with PAH. In the lungs, abnormal ECFCs are a key component of occlusive angioproliferative vascular remodeling, but in a stressed heart, ECFCs may induce neovascularization and facilitate adaptive hypertrophic cardiac remodeling and myocardial revascularization. In one study, ECFC outgrowth from peripheral blood in patients with PAH was associated with a lower right ventricular ejection fraction, lower central venous saturation, and a shorter time to clinical worsening (5.4 vs. 36.5 months, P = 0.032) (48). Although ECFCs were more proliferative in patients with PAH in this study, the proliferative rate had no relationship to pulmonary vascular resistance; rather, it was inversely correlated with right ventricular dilation. The exact mechanisms behind these associations remain uncertain.
ECFCs in Pulmonary Fibrosis
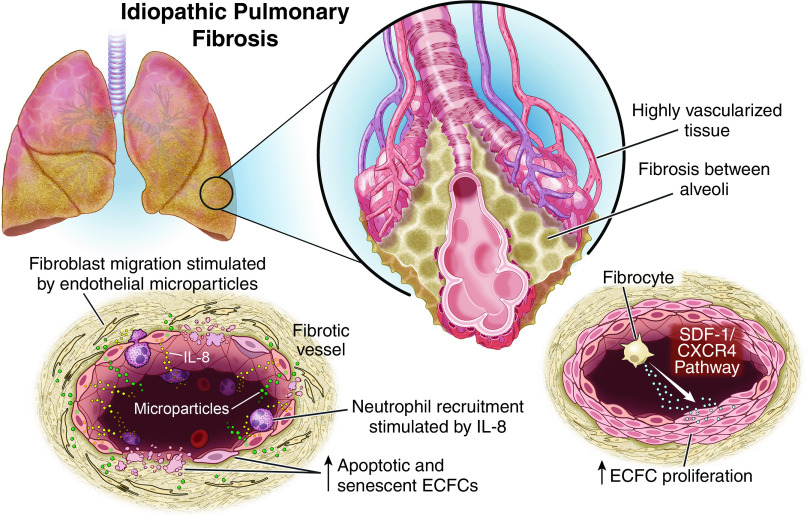
Pulmonary fibrosis is a lung disease characterized by progressive scarring of the lungs and an imbalance between pro and antiangiogenic growth factors (49). The fibrotic areas of the lung have a decreased vascular density, but adjacent nonfibrotic areas are highly vascularized (Figure 5). Among patients with idiopathic pulmonary fibrosis (IPF), ECFCs were increased in those with significantly impaired gas exchange (DlCO < 40%), and proliferation of ECFCs was increased in those with exacerbation compared with stable disease (49). It remains unclear, however, if this increased mobilization of ECFCs in severe IPF is an adaptive response to vascular ablation in fibrotic areas or a factor contributing to pathological vascular remodeling. Pulmonary hypertension, for example, is common among patients with IPF even if they do not have significant functional impairment, and these vascular derangements may be contributory. These processes may be further regulated by interactions between ECFCs and fibrocytes (Figure 5), which are a distinct population of cells that express both hematopoietic and fibroblast markers and contribute to organ fibrosis (50). Fibrocyte culture medium from patients with IPF has been shown to increase ECFC proliferation and differentiation in vitro, and fibrocytes from patients with IPF that have been coimplanted with human ECFCs formed functional microvascular beds in immunodeficient mice. These effects are achieved through the stromal-cell derived factor-1/C-X-C chemokine receptor type 4 (SDF-1/CXCR4) pathway and suggest a role for fibrocytes during disease exacerbations.
Figure 5.
Pathogenic contribution of ECFCs to idiopathic pulmonary fibrosis (IPF). Pulmonary fibrosis is a heterogeneous lung disease in which fibrotic areas have decreased vascular density and adjacent, nonfibrotic areas are highly vascularized. In humans with IPF, ECFCs were increased in those with worse gas exchange, and they were more proliferative in patients undergoing disease exacerbations. It remains unclear whether this is an adaptive response or a contributor to the pathogenesis. Two different models of ECFC function in IPF have been experimentally demonstrated. First, fibrocytes increase ECFC proliferation and differentiation in vitro through the SDF-1/CXCR4 functional pathway, perhaps implying a role for fibrocytes during disease exacerbations. In the second, ECFCs are senescent or apoptotic, leading to increased IL-8 secretion, which may contribute to lung neutrophil invasion. In addition, endothelial microparticles released from IPF ECFCs stimulate fibroblast migration and are more numerous in patients with more significantly impaired gas exchange. It is unclear if ECFCs have different roles in IPF lungs depending on their location within the remodeled lung, the severity of the disease, or the presence of a disease exacerbation. CXCR4 = C-X-C chemokine receptor type 4; IL-8 = interleuken-8; SDF-1 = stromal-cell derived factor-1.
Another study suggested that control and IPF ECFCs do not differ in the ability for adhesion, migration, or differentiation, but more IPF ECFCs are senescent or apoptotic (Figure 5) (51). These changes were associated with increased IL-8 secretion, which may contribute to lung neutrophil invasion in IPF. Furthermore, endothelial microparticles released from IPF ECFCs stimulate fibroblast migration and are more numerous in ECFCs from patients with IPF with DlCO < 40% (52). These seemingly divergent data may reflect the fact that in human IPF, the pulmonary endothelium is remodeled with highly proliferative cells in juxtaposition with apoptotic ones (53); however, further investigation is warranted. ECFCs from patients with IPF have also been shown to be more thrombogenic than those from cord or peripheral blood (54). As such, ECFCs may have several pathogenic roles in pulmonary fibrosis.
ECFCs in COPD
COPD is a progressive lung disease that results in airflow limitation from irreversible lung damage, most commonly caused by tobacco smoking. Cardiovascular disease is a common comorbidity, and there are clusters of patients that actually exhibit a “pulmonary vascular COPD phenotype,” characterized by less severe airflow limitation, hypoxemia, reduced DlCO, hypocapnia, and exercise limitation due to cardiovascular dysfunction (55, 56). Patients with COPD and smokers are, therefore, at high risk for endothelial dysfunction.
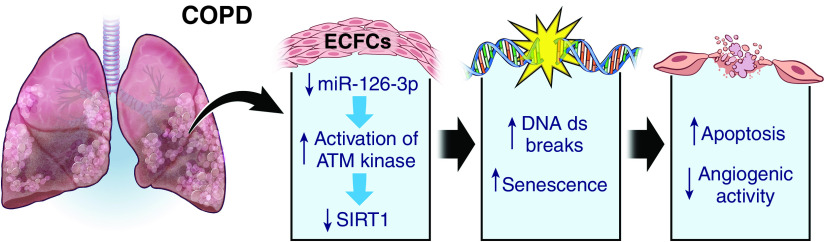
ECFCs isolated from smokers and patients with moderate COPD demonstrated increased DNA double-strand breaks and senescence compared with nonsmokers (Figure 6) (57). These epigenetic changes resulted from the activation of ataxia telangiectasia mutated (ATM) kinase, which reduces sirtuin-1 (SIRT1), a protein deacetylase that protects against DNA damage. miR-126–3p, an endothelial microRNA critical to angiogenesis and vascular homeostasis, modulates this DNA damage response through repression of ATM kinase, but it is reduced in COPD ECFCs, thereby enhancing ATM kinase and increasing DNA damage responses (58). These senescent ECFCs also display decreased in vivo angiogenic activity and increased apoptosis (57). In patients with COPD with pulmonary hypertension, peripheral blood ECFCs have also been shown to have reduced proliferation, adhesion, and migration capacities (59), which are enhanced after treatment with the Rho-kinase inhibitor Fasudil (60). These identified pathways may prove to be useful targets for therapeutic intervention, but further studies are needed.
Figure 6.
Pathogenic contribution of ECFCs to chronic obstructive pulmonary disease (COPD). ECFCs from smokers and patients with moderate COPD display increased DNA double-strand breaks and senescence, leading to decreased in vivo angiogenic activity and increased apoptosis. ATM = ataxia telangiectasia-mutated; ds = double strand; SIRT1 = sirtuin-1.
Therapeutic Potential of ECFCs for Lung Disease
ECFCs represent a promising source for cell-based therapeutic neovascularization, which has been applied to numerous and varied diseases that are beyond the scope of this review (61, 62). They have been suggested to exert therapeutic effects through 1) direct incorporation into neovessels in target tissues; 2) a paracrine fashion; or 3) supporting the reparative ability of other stem/progenitor cells (62). ECFCs have most often been applied systemically, although evidence suggests that targeted application helps circumvent issues with low engraftment and loss of cells that are systemically distributed and/or entrapped in nontarget tissues, and it provides a greater measure of control over the cell numbers needed to achieve a therapeutic effect. Importantly, several strategies have been studied to improve the therapeutic efficacy of ECFCs, including 1) pretreatment of ECFCs with bioactive compounds or factors; 2) epigenetic activation; 3) gene manipulation; 4) coculture with other cell types, including mesenchymal stem cells; and 5) embedding in either natural or synthetic biocompatible scaffolds (62). Unfortunately, there is a paucity of data exploring the use of ECFCs in treating pulmonary disease (63).
Relatively few studies have tested the therapeutic potential of ECFCs in preclinical models of neonatal lung disease. One study achieved increased cell growth and angiogenesis in vitro using late-term fetal sheep PAECs and alveolar type 2 cells treated with human cord blood ECFC-conditioned media (ECFC-CM) from term and preterm neonates (64). Notably, ECFC-CM from preterm infants that were exposed to hyperoxia was not effective. The authors also tested ECFCs and ECFC-CM in vivo with a bleomycin rat model of BPD. ECFCs, ECFC-CM, and HUVEC-CM all improved pulmonary hypertension as evidenced by reduced right ventricular hypertrophy, but none of the treatments had any effect on lung structure, such as alveolar septation, or vessel density. Because human umbilical vein endothelial cell conditioned media (HUVEC-CM) did not have any proangiogenic effects in vitro, it may be that all these treatments provided some vasodilatory effect or a direct effect on the right heart that was unmeasured by the study. A second study using a murine bleomycin model showed that neither ECFCs from cord blood nor patients with IPF were able to prevent or treat pulmonary fibrosis (65). Unfortunately, these studies were limited by cross-species testing, uncertainty as to how many cells or what concentration of CM ended up in the lungs, the fact that daily bleomycin injections did not permit any postinsult period of recovery, which may have made the lungs more receptive to the treatments, and/or single-dose treatments with ECFCs. It should also be noted that these were postnatal treatments, whereas some neonatal lung diseases, like that associated with CDH, may be amenable to prenatal, fetal treatment.
Conversely, a third study demonstrated a positive therapeutic effect using human cord blood-derived ECFCs and the hyperoxia model of BPD (36). ECFCs administered through the jugular vein on postnatal day (P) 14 after a period of hyperoxia restored normal alveolar architecture and lung vascular growth and significantly reduced right ventricular hypertrophy by P28. These effects were persistent out to 10 months of life without adverse effects on lung structure or tumor formation, and treated mice demonstrated improved exercise capacity over the untreated hyperoxia cohort.
Subsequent experiments demonstrated that these benefits were from a clear paracrine effect (36). However, ECFCs isolated from treated mice formed more HPP colonies and formed more extensive endothelial networks than those from nontreated, hyperoxia-exposed mice. ECFC-CM preserved ECFC network formation in vitro during hyperoxia exposure, and ECFC-CM intraperitoneal injections from P4–P21 in hyperoxia-exposed rats similarly preserved alveolar growth and lung vascular growth and attenuated pulmonary hypertension. Thus, this study underscores the therapeutic benefit of promoting lung angiogenesis to repair the lung.
To our knowledge, no studies have examined the therapeutic benefit of either treating with or targeting ECFCs in models of adult pulmonary hypertension or COPD. However, in children aged approximately 2–8 years old with pulmonary hypertension, treprostinil has been shown to enhance peripheral blood ECFC numbers and cause a hyperproliferative ECFC phenotype with enhanced angiogenic potential that has been hypothesized to partly mediate the clinical benefit of prostanoids in pulmonary hypertension (66). Furthermore, others have shown that human ECFC function may be enhanced through either HIF-1α inhibition (47) or pharmacological opening of KATP channels (67) in an in vitro hypoxia model, suggesting targets for potential future therapeutics. Finally, inhaled corticosteroids have been shown to decrease senescence and reduce markers of DNA damage responses in patients with COPD. However, the protective effects of corticosteroids on the endothelium have not been directly connected to the clinical benefit of steroids.
Conclusions
The study of ECFCs in neonatal and adult lung disease has been confounded by various isolation and culture methodologies and confusing nomenclature. However, a preponderance of evidence suggests that neonatal ECFCs demonstrate more robust angiogenic function compared with adult ECFCs. Furthermore, data suggest that ECFC number and function may be reduced in neonatal lung diseases such as BPD and CDH, contrasting the angioproliferative phenotype of ECFCs in adult PAH and IPF. Different strategies to apply cell-based therapies for neonatal disease have been tested in preclinical models with some success, and potential ways to target or improve ECFC function have been preliminarily identified in adult lung diseases. ECFCs demonstrate promise in lung disease for both prognostication and treatment and provide a rich avenue for further investigation.
Acknowledgments
Acknowledgment
The authors thank Dave Schumick, a Senior Medical Illustrator at the Cleveland Clinic, for his creation of the illustrations in this manuscript.
Footnotes
Supported by the National Institutes of Health (NIH) PH R01 HL60917 (S.C.E. and K.A.).
Author Contributions: J.O.R.: conception, literature review/analysis/interpretation, drafting, critical revision, and final approval. S.C.E. and K.A.: Conception, literature interpretation, critical revision, and final approval.
Originally Published in Press as DOI: 10.1165/rcmb.2022-0318PS on October 10, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Medina RJ, Barber CL, Sabatier F, Dignat-George F, Melero-Martin JM, Khosrotehrani K, et al. Endothelial progenitors: a consensus statement on nomenclature. Stem Cells Transl Med . 2017;6:1316–1320. doi: 10.1002/sctm.16-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dight J, Zhao J, Styke C, Khosrotehrani K, Patel J. Resident vascular endothelial progenitor definition and function: the age of reckoning. Angiogenesis . 2022;25:15–33. doi: 10.1007/s10456-021-09817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gumina DL, Su EJ. Endothelial progenitor cells of the human placenta and fetoplacental circulation: a potential link to fetal, neonatal, and long-term health. Front Pediatr . 2017;5:41. doi: 10.3389/fped.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation . 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 5. Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood . 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin RZ, Moreno-Luna R, Li D, Jaminet SC, Greene AK, Melero-Martin JM. Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc Natl Acad Sci USA . 2014;111:10137–10142. doi: 10.1073/pnas.1405388111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood . 2005;105:2783–2786. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 8. Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood . 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 9. O’Neill CL, Guduric-Fuchs J, Chambers SE, O’Doherty M, Bottazzi B, Stitt AW, et al. Endothelial cell-derived pentraxin 3 limits the vasoreparative therapeutic potential of circulating angiogenic cells. Cardiovasc Res . 2016;112:677–688. doi: 10.1093/cvr/cvw209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stitt AW, O’Neill CL, O’Doherty MT, Archer DB, Gardiner TA, Medina RJ. Vascular stem cells and ischaemic retinopathies. Prog Retin Eye Res . 2011;30:149–166. doi: 10.1016/j.preteyeres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 11. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science . 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 12. Masouleh BK, Baraniskin A, Schmiegel W, Schroers R. Quantification of circulating endothelial progenitor cells in human peripheral blood: establishing a reliable flow cytometry protocol. J Immunol Methods . 2010;357:38–42. doi: 10.1016/j.jim.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 13. Estes ML, Mund JA, Ingram DA, Case J. Identification of endothelial cells and progenitor cell subsets in human peripheral blood. Curr Protoc Cytom . 2010:1–11. doi: 10.1002/0471142956.cy0933s52. [DOI] [PubMed] [Google Scholar]
- 14. Estes ML, Mund JA, Mead LE, Prater DN, Cai S, Wang H, et al. Application of polychromatic flow cytometry to identify novel subsets of circulating cells with angiogenic potential. Cytometry A . 2010;77:831–839. doi: 10.1002/cyto.a.20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mund JA, Estes ML, Yoder MC, Ingram DA, Jr, Case J. Flow cytometric identification and functional characterization of immature and mature circulating endothelial cells. Arterioscler Thromb Vasc Biol . 2012;32:1045–1053. doi: 10.1161/ATVBAHA.111.244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patel J, Seppanen E, Chong MS, Yeo JS, Teo EY, Chan JK, et al. Prospective surface marker-based isolation and expansion of fetal endothelial colony-forming cells from human term placenta. Stem Cells Transl Med . 2013;2:839–847. doi: 10.5966/sctm.2013-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prasain N, Meador JL, Yoder MC. Phenotypic and functional characterization of endothelial colony forming cells derived from human umbilical cord blood. J Vis Exp . 2012:3872. doi: 10.3791/3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol . 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 19. Baker CD, Ryan SL, Ingram DA, Seedorf GJ, Abman SH, Balasubramaniam V. Endothelial colony-forming cells from preterm infants are increased and more susceptible to hyperoxia. Am J Respir Crit Care Med . 2009;180:454–461. doi: 10.1164/rccm.200901-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Safranow K, Kotowski M, Lewandowska J, Machalińska A, Dziedziejko V, Czajka R, et al. Circulating endothelial progenitor cells in premature infants: is there an association with premature birth complications? J Perinat Med . 2012;40:455–462. doi: 10.1515/jpm-2011-0199. [DOI] [PubMed] [Google Scholar]
- 21. Díaz Del Moral S, Barrena S, Muñoz-Chápuli R, Carmona R. Embryonic circulating endothelial progenitor cells. Angiogenesis . 2020;23:531–541. doi: 10.1007/s10456-020-09732-y. [DOI] [PubMed] [Google Scholar]
- 22. Wei J, Blum S, Unger M, Jarmy G, Lamparter M, Geishauser A, et al. Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metastases after intravenous delivery. Cancer Cell . 2004;5:477–488. doi: 10.1016/s1535-6108(04)00116-3. [DOI] [PubMed] [Google Scholar]
- 23. Rapp BM, Saadatzedeh MR, Ofstein RH, Bhavsar JR, Tempel ZS, Moreno O, et al. Resident endothelial progenitor cells from human placenta have greater vasculogenic potential than circulating endothelial progenitor cells from umbilical cord blood. Cell Med . 2011;2:85–96. doi: 10.3727/215517911X617888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel J, Wong HY, Wang W, Alexis J, Shafiee A, Stevenson AJ, et al. Self-renewal and high proliferative colony forming capacity of late-outgrowth endothelial progenitors is regulated by cyclin-dependent kinase inhibitors driven by notch signaling. Stem Cells . 2016;34:902–912. doi: 10.1002/stem.2262. [DOI] [PubMed] [Google Scholar]
- 25. Tasev D, Konijnenberg LS, Amado-Azevedo J, van Wijhe MH, Koolwijk P, van Hinsbergh VW. CD34 expression modulates tube-forming capacity and barrier properties of peripheral blood-derived endothelial colony-forming cells (ECFCs) Angiogenesis . 2016;19:325–338. doi: 10.1007/s10456-016-9506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Javed MJ, Mead LE, Prater D, Bessler WK, Foster D, Case J, et al. Endothelial colony forming cells and mesenchymal stem cells are enriched at different gestational ages in human umbilical cord blood. Pediatr Res . 2008;64:68–73. doi: 10.1203/PDR.0b013e31817445e9. [DOI] [PubMed] [Google Scholar]
- 27. Ligi I, Simoncini S, Tellier E, Vassallo PF, Sabatier F, Guillet B, et al. A switch toward angiostatic gene expression impairs the angiogenic properties of endothelial progenitor cells in low birth weight preterm infants. Blood . 2011;118:1699–1709. doi: 10.1182/blood-2010-12-325142. [DOI] [PubMed] [Google Scholar]
- 28. Schittny JC. Development of the lung. Cell Tissue Res . 2017;367:427–444. doi: 10.1007/s00441-016-2545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muñoz-Hernandez R, Miranda ML, Stiefel P, Lin RZ, Praena-Fernández JM, Dominguez-Simeon MJ, et al. Decreased level of cord blood circulating endothelial colony-forming cells in preeclampsia. Hypertension . 2014;64:165–171. doi: 10.1161/HYPERTENSIONAHA.113.03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bertagnolli M, Xie LF, Paquette K, He Y, Cloutier A, Fernandes RO, et al. Endothelial colony-forming cells in young adults born preterm: a novel link between neonatal complications and adult risks for cardiovascular disease. J Am Heart Assoc . 2018;7:e009720. doi: 10.1161/JAHA.118.009720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thébaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, Steinhorn RH, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers . 2019;5:78. doi: 10.1038/s41572-019-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hwang JS, Rehan VK. Recent advances in bronchopulmonary dysplasia: pathophysiology, prevention, and treatment. Lung . 2018;196:129–138. doi: 10.1007/s00408-018-0084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med . 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 34. Baker CD, Balasubramaniam V, Mourani PM, Sontag MK, Black CP, Ryan SL, et al. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Eur Respir J . 2012;40:1516–1522. doi: 10.1183/09031936.00017312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borghesi A, Massa M, Campanelli R, Bollani L, Tzialla C, Figar TA, et al. Circulating endothelial progenitor cells in preterm infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med . 2009;180:540–546. doi: 10.1164/rccm.200812-1949OC. [DOI] [PubMed] [Google Scholar]
- 36. Alphonse RS, Vadivel A, Fung M, Shelley WC, Critser PJ, Ionescu L, et al. Existence, functional impairment, and lung repair potential of endothelial colony-forming cells in oxygen-induced arrested alveolar growth. Circulation . 2014;129:2144–2157. doi: 10.1161/CIRCULATIONAHA.114.009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mous DS, Kool HM, Wijnen R, Tibboel D, Rottier RJ. Pulmonary vascular development in congenital diaphragmatic hernia. Eur Respir Rev . 2018;27:170104. doi: 10.1183/16000617.0104-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baker CD, Black CP, Ryan SL, Balasubramaniam V, Abman SH. Cord blood endothelial colony-forming cells from newborns with congenital diaphragmatic hernia. J Pediatr . 2013;163:905–907. doi: 10.1016/j.jpeds.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fujinaga H, Fujinaga H, Watanabe N, Kato T, Tamano M, Terao M, et al. Cord blood-derived endothelial colony-forming cell function is disrupted in congenital diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol . 2016;310:L1143–L1154. doi: 10.1152/ajplung.00357.2015. [DOI] [PubMed] [Google Scholar]
- 40. Acker SN, Seedorf GJ, Abman SH, Nozik-Grayck E, Partrick DA, Gien J. Pulmonary artery endothelial cell dysfunction and decreased populations of highly proliferative endothelial cells in experimental congenital diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol . 2013;305:L943–L952. doi: 10.1152/ajplung.00226.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gelzinis TA. Pulmonary hypertension in 2021: part i-definition, classification, pathophysiology, and presentation. J Cardiothorac Vasc Anesth . 2022;36:1552–1564. doi: 10.1053/j.jvca.2021.06.036. [DOI] [PubMed] [Google Scholar]
- 42. Duong HT, Comhair SA, Aldred MA, Mavrakis L, Savasky BM, Erzurum SC, et al. Pulmonary artery endothelium resident endothelial colony-forming cells in pulmonary arterial hypertension. Pulm Circ . 2011;1:475–486. doi: 10.4103/2045-8932.93547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, et al. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol . 2007;293:L548–L554. doi: 10.1152/ajplung.00428.2006. [DOI] [PubMed] [Google Scholar]
- 44. Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LS, Marchesan D, et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med . 2009;180:780–787. doi: 10.1164/rccm.200810-1662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nijmeh H, Balasubramaniam V, Burns N, Ahmad A, Stenmark KR, Gerasimovskaya EV. High proliferative potential endothelial colony-forming cells contribute to hypoxia-induced pulmonary artery vasa vasorum neovascularization. Am J Physiol Lung Cell Mol Physiol . 2014;306:L661–L671. doi: 10.1152/ajplung.00244.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee SD, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J Clin Invest . 1998;101:927–934. doi: 10.1172/JCI1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He M, Ma S, Cai Q, Wu Y, Shao C, Kong H, et al. Hypoxia induces the dysfunction of human endothelial colony-forming cells via HIF-1α signaling. Respir Physiol Neurobiol . 2018;247:87–95. doi: 10.1016/j.resp.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 48. Smits J, Tasev D, Andersen S, Szulcek R, Botros L, Ringgaard S, et al. Blood outgrowth and proliferation of endothelial colony forming cells are related to markers of disease severity in patients with pulmonary arterial hypertension. Int J Mol Sci . 2018;19:3763. doi: 10.3390/ijms19123763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smadja DM, Mauge L, Nunes H, d’Audigier C, Juvin K, Borie R, et al. Imbalance of circulating endothelial cells and progenitors in idiopathic pulmonary fibrosis. Angiogenesis . 2013;16:147–157. doi: 10.1007/s10456-012-9306-9. [DOI] [PubMed] [Google Scholar]
- 50. Smadja DM, Dorfmüller P, Guerin CL, Bieche I, Badoual C, Boscolo E, et al. Cooperation between human fibrocytes and endothelial colony-forming cells increases angiogenesis via the CXCR4 pathway. Thromb Haemost . 2014;112:1002–1013. doi: 10.1160/TH13-08-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Blandinières A, Gendron N, Bacha N, Bièche I, Chocron R, Nunes H, et al. Interleukin-8 release by endothelial colony-forming cells isolated from idiopathic pulmonary fibrosis patients might contribute to their pathogenicity. Angiogenesis . 2019;22:325–339. doi: 10.1007/s10456-018-09659-5. [DOI] [PubMed] [Google Scholar]
- 52. Bacha NC, Blandinieres A, Rossi E, Gendron N, Nevo N, Lecourt S, et al. Endothelial microparticles are associated to pathogenesis of idiopathic pulmonary fibrosis. Stem Cell Rev Rep . 2018;14:223–235. doi: 10.1007/s12015-017-9778-5. [DOI] [PubMed] [Google Scholar]
- 53. Ebina M, Shimizukawa M, Shibata N, Kimura Y, Suzuki T, Endo M, et al. Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2004;169:1203–1208. doi: 10.1164/rccm.200308-1111OC. [DOI] [PubMed] [Google Scholar]
- 54. Billoir P, Blandinières A, Gendron N, Chocron R, Gunther S, Philippe A, et al. Endothelial colony-forming cells from idiopathic pulmonary fibrosis patients have a high procoagulant potential. Stem Cell Rev Rep . 2021;17:694–699. doi: 10.1007/s12015-020-10043-4. [DOI] [PubMed] [Google Scholar]
- 55. Kumar A, Mahajan A, Salazar EA, Pruitt K, Guzman CA, Clauss MA, et al. Impact of human immunodeficiency virus on pulmonary vascular disease. Glob Cardiol Sci Pract . 2021;2021:e202112. doi: 10.21542/gcsp.2021.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morgan AD, Zakeri R, Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Ther Adv Respir Dis . 2018;12:1753465817750524. doi: 10.1177/1753465817750524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Paschalaki KE, Starke RD, Hu Y, Mercado N, Margariti A, Gorgoulis VG, et al. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells . 2013;31:2813–2826. doi: 10.1002/stem.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Paschalaki KE, Zampetaki A, Baker JR, Birrell MA, Starke RD, Belvisi MG, et al. Downregulation of microrna-126 augments DNA damage response in cigarette smokers and patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2018;197:665–668. doi: 10.1164/rccm.201706-1304LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu P, Zhang H, Liu J, Sheng C, Zhang L, Zeng Y. Changes of number and function of late endothelial progenitor cells in peripheral blood of COPD patients combined with pulmonary hypertension. Thorac Cardiovasc Surg . 2016;64:323–329. doi: 10.1055/s-0034-1389261. [DOI] [PubMed] [Google Scholar]
- 60. Liu P, Zhang HM, Tang YJ, Sheng CF, Liu JX, Zeng YJ. Influence of Rho kinase inhibitor fasudil on late endothelial progenitor cells in peripheral blood of COPD patients with pulmonary artery hypertension. Bratisl Lek Listy . 2015;116:150–153. doi: 10.4149/bll_2015_030. [DOI] [PubMed] [Google Scholar]
- 61. Paschalaki KE, Randi AM. Recent advances in endothelial colony forming cells toward their use in clinical translation. Front Med (Lausanne) . 2018;5:295. doi: 10.3389/fmed.2018.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tasev D, Koolwijk P, van Hinsbergh VW. Therapeutic potential of human-derived endothelial colony-forming cells in animal models. Tissue Eng Part B Rev . 2016;22:371–382. doi: 10.1089/ten.TEB.2016.0050. [DOI] [PubMed] [Google Scholar]
- 63. Keighron C, Lyons CJ, Creane M, O’Brien T, Liew A. Recent advances in endothelial progenitor cells toward their use in clinical translation. Front Med (Lausanne) . 2018;5:354. doi: 10.3389/fmed.2018.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Baker CD, Seedorf GJ, Wisniewski BL, Black CP, Ryan SL, Balasubramaniam V, et al. Endothelial colony-forming cell conditioned media promote angiogenesis in vitro and prevent pulmonary hypertension in experimental bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol . 2013;305:L73–L81. doi: 10.1152/ajplung.00400.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Blandinières A, Gille T, Sadoine J, Bièche I, Slimani L, Dizier B, et al. Endothelial colony-forming cells do not participate to fibrogenesis in a bleomycin-induced pulmonary fibrosis model in nude mice. Stem Cell Rev Rep . 2018;14:812–822. doi: 10.1007/s12015-018-9846-5. [DOI] [PubMed] [Google Scholar]
- 66. Smadja DM, Mauge L, Gaussem P, d’Audigier C, Israel-Biet D, Celermajer DS, et al. Treprostinil increases the number and angiogenic potential of endothelial progenitor cells in children with pulmonary hypertension. Angiogenesis . 2011;14:17–27. doi: 10.1007/s10456-010-9192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. He M, Cui T, Cai Q, Wang H, Kong H, Xie W. Iptakalim ameliorates hypoxia-impaired human endothelial colony-forming cells proliferation, migration, and angiogenesis via Akt/eNOS pathways. Pulm Circ . 2019;9:2045894019875417. doi: 10.1177/2045894019875417. [DOI] [PMC free article] [PubMed] [Google Scholar]