Abstract
BACKGROUND:
Epidemiological study findings are inconsistent regarding associations between prenatal polycyclic aromatic hydrocarbon (PAH) exposures and childhood behavior. This study examined associations of prenatal PAH exposure with behavior at age 4–6 years in a large, diverse, multi-region prospective cohort. Secondary aims included examination of PAH mixtures and effect modification by child sex, breastfeeding, and child neighborhood opportunity.
METHODS:
The ECHO PATHWAYS Consortium pooled 1118 mother-child dyads from three prospective pregnancy cohorts in six U.S. cities. Seven PAH metabolites were measured in prenatal urine. Child behavior was assessed at age 4–6 using the Total Problems score from the Child Behavior Checklist (CBCL). Neighborhood opportunity was assessed using the socioeconomic and educational scales of the Child Opportunity Index. Multivariable linear regression was used to estimate associations per 2-fold increase in each PAH metabolite, adjusted for demographic, prenatal, and maternal factors and using interaction terms for effect modifiers. Associations with PAH mixtures were estimated using Weighted Quantile Sum Regression (WQSR).
RESULTS:
The sample was racially and sociodemographically diverse (38% Black, 49% White, 7% Other; household-adjusted income range $2,651-$221,102). In fully adjusted models, each 2-fold increase in 2-hydroxynaphthalene was associated with a lower Total Problems score, contrary to hypotheses (b= −0.80, 95% CI = −1.51, −0.08). Associations were notable in boys (b= −1.10, 95% CI = −2.11, −0.08) and among children breastfed 6+ months (b= −1.31, 95% CI = −2.25, −0.37), although there was no statistically significant evidence for interaction by child sex, breastfeeding, or neighborhood child opportunity. Associations were null for other PAH metabolites; there was no evidence of associations with PAH mixtures from WQSR.
CONCLUSION:
In this large, well-characterized, prospective study of mother-child pairs, prenatal PAH exposure was not associated with adverse child behavior scores. Future studies characterizing the magnitude of prenatal PAH exposure and studies in older childhood are needed.
Keywords: Polycyclic aromatic hydrocarbons, prenatal, behavioral development, neurodevelopment
1. INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are a class of ubiquitous chemical pollutants formed as by-products of burning fossil fuels and industrial emissions. Pathways for exposure to PAHs include inhalation of particulate matter containing PAHs, by diet through consuming barbequed or grilled meats, and from smoking (Agency for Toxic Substances and Disease Registry (ATSDR), 1995). Prenatal exposure to PAHs has been linked to adverse birth and developmental outcomes in offspring, including reduced birthweight and preterm birth, neural tube defects, and neurobehavioral deficits that continue into middle childhood (Jedrychowski et al., 2012; Perera et al., 2005; Wang et al., 2015). PAHs are readily transferred through the placenta and fetal brain barrier (Brown et al., 2007) and are predominantly metabolized into hydroxy-PAHs (Onyemauwa et al., 2009). The precise mechanisms by which PAHs affect the developing brain are poorly understood but animal studies point to disruptions to regulation of neuronal differentiation, synapse formation, and neuroplasticity (McCallister et al., 2008; Wormley et al., 2004), activation of apoptotic pathways (Nicol et al., 1995), and neuronal death (Dutta et al., 2010).
Epidemiologic studies of associations between prenatal PAH exposure and child behavior are primarily from two cohorts based in New York City and Krakow, Poland (Genkinger et al., 2015; Perera et al., 2012, 2011). In the New York cohort, prenatal PAHs were measured in both personal air and cord blood, and selected Child Behavior Checklist (CBCL) subscales of behavior were assessed at approximately aged 5 and 7 years in mother-child pairs. At both ages, higher PAH exposure was associated with higher adverse behavioral scores or greater risk for behavioral problems (Perera et al., 2012, 2011). In the Krakow cohort, prenatal PAH levels from personal air monitoring were associated with higher Internalizing and Externalizing behaviors and four syndrome subscales (Withdrawn/Depressed, Social Problems, Attention, and Aggressive behavior at age 6 to 9 years (Genkinger et al., 2015). In our previous prospective cohort study of mother-child pairs in Memphis, Tennessee, we observed inconsistent associations between urine-derived prenatal PAH exposure and toddler-age neurodevelopment that varied across PAH metabolites. For example, at two years of age, prenatal exposure to 1-hydroxypyrene was associated with a higher risk for developmental delay, while 1-hydroxynaphthalene was associated with a lower risk for behavior problems(Wallace et al., 2021). Examination of effect modification is particularly important in studies of low level neurotoxicant exposures like PAHs, as they are hypothesized to act in concert with other environmental and social determinants of child behavior (Bellinger, 2001, 2000). Prior research indicates that associations between exposures to environmental toxicants and child neurodevelopment may be sex specific (Gade et al., 2021; Lertxundi et al., 2019). Sex differences in associations between PAH exposure and behavior in schoolchildren were reported in the cross-sectional National Health and Nutrition Examination Survey (Abid et al., 2014). We also hypothesized that breastfeeding would act as a buffer to insults from PAH exposures. Breastmilk contains high concentrations of anti-inflammatory long-chain polyunsaturated fatty acids, and breastfeeding has well-established benefits on early childhood health and development (“Position of the American Dietetic Association: Promoting and Supporting Breastfeeding,” 2009). The detrimental effects of environmental exposures are also experienced unequally, with disproportional burdens on socioeconomically disadvantaged communities and families (Brooks-Gunn et al., 1996). Vulnerabilities within the community or family may also modify the effects from environmental exposures. Understanding the contribution of neighborhood-level factors to associations of prenatal PAHs and neurodevelopment is important as they are targets for policies and community-level intervention. Prior studies of prenatal PAH exposure in relation to child behavior and cognition showed differences by neighborhood quality (Lovasi et al., 2014), material hardship (Perera et al., 2018), maternal demoralization (Perera et al., 2013), and childhood early life stress (Pagliaccio et al., 2020).
We expand upon the existing literature by examining associations of prenatal PAH exposure and preschool and early school-age child behavior in a pooled sample spanning six U.S. cities in the ECHO-PATHWAYS Consortium. The study population encompassed a relatively large and diverse sample of mother-child dyads. Secondary research questions included examination of PAH mixtures and assessment of effect modification by child sex, child neighborhood opportunity, and length of breastfeeding. We hypothesized that children with higher prenatal exposures to PAHs would have more behavior problems in early childhood, and these associations would differ by child sex. We hypothesized that breastfeeding and better early childhood neighborhood opportunity would buffer adverse associations between PAHs and child behavior.
2. METHODS
2.1. Study Population
This study included mother-child dyads enrolled in three cohorts of the ECHO PATHWAYS Consortium: The Conditions Affecting Neurocognitive Development and Learning in Early childhood (CANDLE) study, the Global Alliance to Prevent Prematurity and Stillbirth (GAPPS) study and The Infant Development and the Environment Study (TIDES)(LeWinn et al., 2022). Pregnant women provided informed consent at enrollment and study protocols were approved by the respective institutions where the studies were based. The current analysis was conducted by ECHO PATHWAYS and was approved by the University of Washington IRB.
The CANDLE study is a prospective pregnancy cohort in Shelby County, Tennessee (Sontag-Padilla et al., 2015) which was designed to identify early-life determinants of neurocognitive development. The study design, recruitment experience, and data collection have been described elsewhere (LeWinn et al., 2020). Pregnant women were recruited for CANDLE between 2006–2011 and were eligible if they were between the ages of 16 and 40 years in the second trimester of a singleton low-risk pregnancy (e.g. without chronic hypertension requiring therapy, insulin-dependent diabetes, renal disease) with the intent to deliver at one of five health care settings in Shelby County. Women attended prenatal study visits that included maternal surveys, biospecimen collection, and abstraction of labor and delivery data from the medical records. Mother-child dyads were followed up at regular intervals with clinic and home visits and telephone surveys, including clinic visits at age 4–6 years and 8–9 years.
The GAPPS study was launched by Seattle Children’s Hospital in 2007 as a longitudinal biorepository, designed to focus attention and research on reducing the impact of preterm birth and stillbirth. Participants were enrolled over a period of 8 years from three hospitals in Seattle, WA and Yakima, WA at various time points in pregnancy and were eligible if they were 18 years or older, able to speak and write English, and intending to deliver in the hospital in which they were enrolled (www.gapps.org). The GAPPS study utilized both active and passive data collection, including medical records abstraction, urine and blood samples, and questionnaires. Women previously enrolled in GAPPS at these two sites were re-contacted and invited to participate in the ECHO-PATHWAYS Consortium if their child was born between 2011 and 2016 and had contributed biospecimens and prenatal questionnaire data to the repository (Senter et al., 2021). Women were recruited for postnatal follow-up visits with ECHO-PATHWAYS; this study included mother-child dyads who participated in follow-up at age 4–6 years.
TIDES is a pregnancy cohort with four sites across the US, in San Francisco, CA; Minneapolis, MN; Rochester, NY; and Seattle, WA (Barrett et al., 2014). Women were recruited in 2010–2012 during the first trimester of pregnancy from obstetrical clinics affiliated with participating academic medical centers. The initial aim of this cohort was to examine associations of prenatal phthalate and other environmental chemical exposures with early life reproductive development. Inclusion criteria included being at least 18 years old, planning to deliver at one of the study hospitals, and having a low-medical-risk pregnancy at enrollment. Prenatal study visits occurred in every trimester and included biospecimen collection and maternal surveys. Birth record reviews as well as a birth exam were performed. Mother-child pairs were followed with questionnaires and clinic visits at ages 4–5 years and 6 years.
Women from all three cohorts were included in the analytic dataset if they had urinary PAH measures during pregnancy and completed a CBCL assessment at the age 4–6 visit. We excluded one pair of twins and all children born prior to 34 weeks’ gestation, as children born very preterm are at higher risk for behavior problems (Sejer et al., 2019; Soleimani et al., 2014) and we were interested in understanding the associations of PAHs and behavior independent of the effects of preterm birth. Because one of the primary aims of this study was to assess associations of PAH exposure and behavior independent of the effects of prenatal maternal smoking, we also excluded women who reported any smoking during pregnancy or had urinary cotinine levels > 200 ng/mL at the second or third trimester study visits, a common cutoff to identify active smoking (Schick et al., 2017).
2.2. OH-PAH Analysis
Maternal prenatal PAH exposure was determined through measurement of monohydroxylated PAH metabolite concentrations (OH-PAHs) in urine samples collected in mid-pregnancy. The gestational age at time of urine collection ranged from 12.9 to 35.1 weeks, with a median of 22.1 weeks and was similar across cohorts. Samples were stored at −80 degrees C in study biorepositories until the time of analysis at the Wadsworth Laboratory, New York State Department of Health. Methods for the extraction and analysis of OH-PAHs have been published previously(Guo et al., 2013; Wallace et al., 2021). Urine samples (500 μL) were fortified with 10 ng each of an isotopically labeled internal standard mixture, and mixed with 1 mL of 0.5 M ammonium acetate buffer containing 200 units/mL of β-glucuronidase/sulfatase enzyme (MP Biomedicals, LLC, Solon, OH, USA). The samples were gently mixed and incubated overnight at 37°C, and then diluted with 2 mL of HPLC-grade water and extracted with 7 mL of 80% pentane: 20% toluene (v:v), by shaking on a reciprocating shaker for one hour, centrifuged at 3600 × g for 20 minutes, the supernatant was transferred into a new glass tube for instrumental analysis. The chromatographic separation of OH-PAHs was accomplished using a Waters Acquity I-Class UPLC system (Waters; Milford, MA, USA) connected to an Acquity UPLC BEH C18 column (50 × 2.1 mm, 1.7 μm, Waters; Milford, MA, USA). Identification and quantification of PAH metabolites was performed on an ABSCIEX 5500 triple quadrupole mass spectrometer (Applied Biosystems; Foster City, CA, USA). The laboratory participated in several external quality assurance schemes to validate OH-PAHs assay successfully (Kannan et al., 2021). The limits of detection (LOD) ranged from 0.02 to 0.12 ng/mL. Samples below the limit of detection (LOD) were calculated as: LOD/√2. For this analysis, we included each OH-PAH metabolite that was detected in greater than 80% of the study population, which yielded sufficient samples for the following OH-PAHs: 1- and 2-hydroxynaphthalene (NAP); 1-, 2-, 3-, and 9-hydroxyphenanthrene (PHEN); 2-, 3-, and 9-hydroxyfluorene (FLUO), and 1-hydroxypyrene (PYR). Measurements were unable to distinguish the isomers of hydroxyfluorenes or 1- and 9-hydroxyphenanthrene, so these measurements were quantified as summation: 2/3/9-hydroxyfluorene (2/3/9-FLUO) and 1/9-hydroxyphenanthrene (1/9-PHEN), respectively. Samples for all three cohorts were analyzed in four batches at the Wadsworth laboratory using the same method of OH-PAH determination. While raw log-transformed OH-PAH measures (in ng/mL) were used in the main analysis, we calculated and adjusted OH-PAHs for urine specific gravity in sensitivity analyses using the following formula:
Where P is the measured urinary PAH concentration, SG is the specific gravity for each participant, and SGmedian is the median batch SG(Levine and Fahy, 1945).
2.3. Child Behavior Assessment
Child behavior was assessed in all cohorts using the CBCL between the ages of 4 to 6 years (Achenbach and Rescorla, 2001, 2000). One of two versions of the CBCL was administered depending on the child’s age at assessment: the CBCL preschool form (ages 1.5–5 years) (Achenbach and Rescorla, 2000), or the CBCL school age form (ages 6–18 years)(Achenbach and Rescorla, 2001). The forms ask caregivers to rate the frequency of child behaviors over the last two (preschool form) or six (school age form) months on a scale of Not True (0), Somewhat or Sometimes True (1), to Very True or Often True (2). The preschool form includes 99 behaviors and the school age form includes 112 problem items. Additional behaviors appropriate for children up to 18 years of age are included in the school-age form but caregivers reported similar types and counts of behaviors across forms, reflecting the comparable developmental stage of the children across cohorts. The primary outcome was the Total Problems score, modeled as a raw continuous score with adjustment for child age at assessment and CBCL form version. We elected to use the raw score with adjustment for child age at assessment and CBCL form version instead of using the provided standardized t-scores due to inconsistent methods for sex adjustment in the t-score calculations between the form versions (Smucker et al., 1986). Higher scores reflect more behavior problems. Despite prior work (Genkinger et al., 2015; Perera et al., 2012, 2011) that examined associations between PAH and CBCL subscales, we elected to focus on the Total Problems both to limit to the potential of a Type 1 error and due to the significant differences in the distribution of raw subscales between the preschool and school-age forms. Higher problems across any subscale of behavior would be reflected in the Total Score.
2.4. Effect Modifiers
Infant sex was ascertained from medical record abstraction. The duration of any breastfeeding was assessed by questionnaire at approximately 4 to 6 years of age. Participants were asked “Was your child ever breast fed?” and “How long was your child breastfed?”. We categorized length of breastfeeding as none, 0–6 months, and ≥6 months and did not differentiate between mothers who exclusively breastfed from those who breastfed but also used formula. Children’s contextual opportunity at the neighborhood level was measured using the publicly-available Child Opportunity Index (COI), which was specifically designed to describe the multifaceted neighborhood-based conditions and resources that influence child development, as a tool to monitor healthy equity and to identify neighborhoods that are disadvantaged and are targets for community intervention (Acevedo-Garcia et al., 2014). The COI was geospatially-linked to maternal address at enrollment and updated at the age 4–6 visit; analyses utilized the Social and Economic domain and Education domain scores, which reflect neighborhood-level socioeconomic status (SES) and education quality, respectively. Higher scores indicate greater opportunity in the surrounding environment.
2.5. Covariates
Maternal report was used to collect characteristics at prenatal and postnatal study visits, including marital status, pre-pregnancy body mass index (BMI), household size, tobacco smoking and alcohol use during pregnancy, exposure to tobacco smoke during pregnancy and in the postnatal period, age at delivery, child’s race, and length of breastfeeding. Infant sex, gestational age and birthweight were ascertained from medical record abstraction. Maternal educational attainment and Regional Price Parity adjusted household income (Bureau of Economic Analysis, 2021) were updated at the age 4–6 visit. Children’s contextual opportunity at the neighborhood level was measured using the publicly-available Child Opportunity Index, which was geospatially-linked to maternal address at enrollment and updated at the age 4–6 visit (Acevedo-Garcia et al., 2014); analyses utilized the Social and Economic domain and Education domain scores, which reflect neighborhood-level socioeconomic status (SES) and education quality, respectively, with higher scores indicating greater opportunity in the surrounding environment. Maternal depression and maternal stress were measured at the child age 4–6 visit in GAPPS and TIDES and age 8–9 visit in CANDLE using the Patient-Reported Outcomes Measurement Information System (PROMIS) depression score (Pilkonis et al., 2011) and the Perceived Stress Scale (Cohen et al. 1983), respectively. Postnatal tobacco exposure from the child’s mother or from other family members in the home was reported by the mother at the age 4–6 visit.
2.6. Statistical Analysis
We used descriptive statistics to characterize the study population and the distributions of maternal OH-PAH exposure in the analytic sample. Multivariable linear regression with robust standard errors was used to estimate associations of individual OH-PAHs and continuous Total Problems score with corresponding 95% confidence intervals (95% CI). Separate models were fitted for each OH-PAH metabolite. For all models, OH-PAH concentrations were log transformed and analyzed in separate regression models that included specific gravity as a covariate. To ease interpretability, estimates were multiplied by ln(2) to calculate estimates per 2-fold increase in ln-OH-PAH. We took a staged approach to regression model building with adjustment for potential confounder and precision variables. In Figure 1, we display a Directed Acyclic Graph (DAG) that shows the potential confounders we determined a priori, defined as factors likely to be directly or indirectly associated with prenatal PAH exposure as well as risk factors for neurobehaviorial problems. We also identified selected precision variables with well-established associations with child behavior although not likely to be associated with PAH exposure(Lewinn et al., 2020; O’Neill et al., 2017). The minimally adjusted model includes child age at assessment (continuous year, to two decimal places), child sex (binary), CBCL form version (binary), OH-PAH analysis batch, urinary specific gravity (continuous), and study site (categorical, 7, with Seattle GAPPS and Seattle TIDES modeled separately). The fully adjusted model, considered the main analysis, adjusted for additional confounders and precision variables indicated in the DAG, including maternal demographics and health during pregnancy (maternal age at child’s birth [continuous], child birth order [binary, first born vs. not), individual-level SES variables (maternal education [categorical], income by household count [continuous, natural log transformed] (Burniaux et al., 1998), household count [categorical], interaction between adjusted income by family household size (i.e. log income X household count), marital status [married or living with partner vs. not]), neighborhood-level SES variables (Child Opportunity Index Social and Economic domain [continuous], Child Opportunity Index Education domain [continuous], and precision variables (maternal depression [PROMIS score, continuous], maternal stress [Perceived Stress scale, continuous], pre- and post-natal secondhand smoke exposure (self-reported; any vs. none), and postnatal maternal smoking (self-reported; any vs. none). The expanded adjusted model included additional precision variables associated with child behavior, but not as well-established in the literature as those included in the full model, including prenatal alcohol use (any/none), pre-pregnancy BMI (continuous), breastfeeding status (categorical; None, <6 months., ≥ 6 months). The expanded adjustment model additionally included gestational age (continuous), which we hypothesized to lie on the causal pathway, and child race (categorical; White, Black, Asian, American Indian/Alaska Native, Other, Multiple race), which we considered to be a proxy for unmeasured social conditions inequitably distributed by race that may confound associations (Brooks-Gunn et al., 1996).
Figure 1.
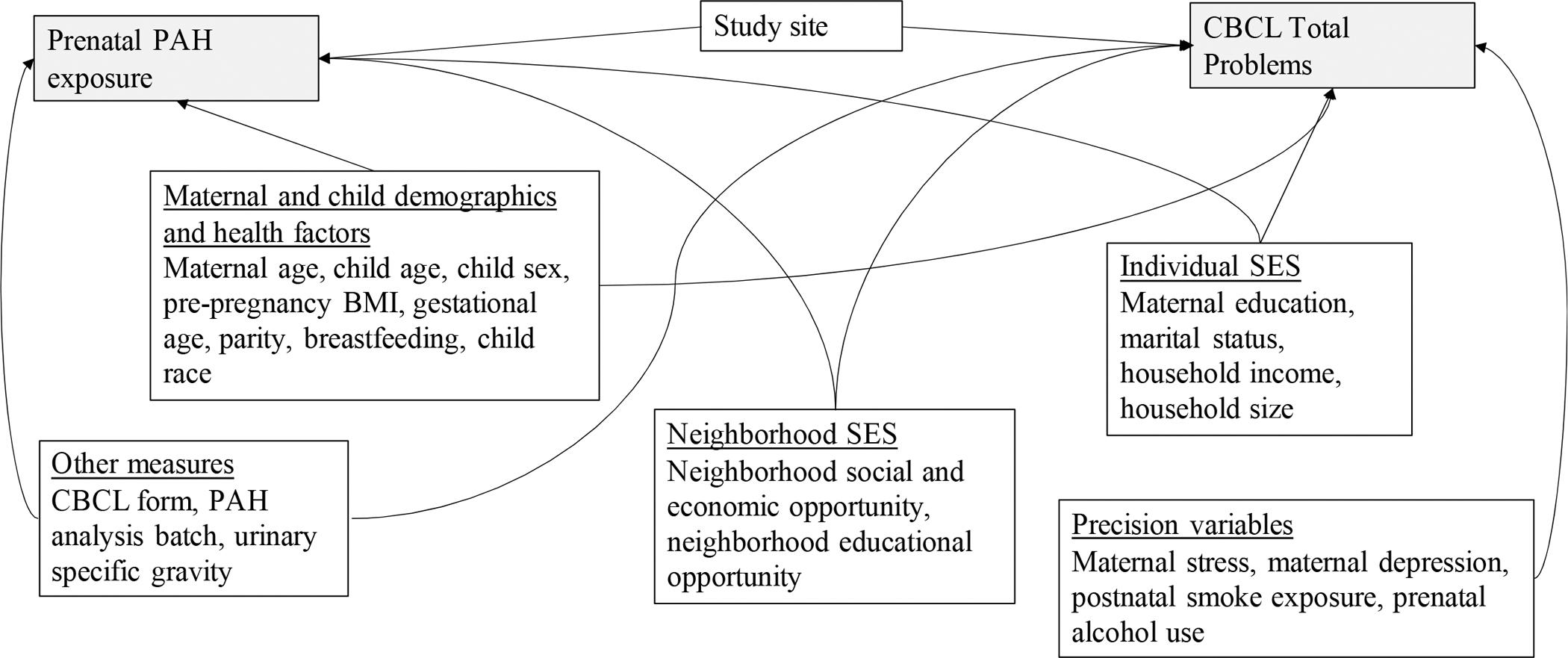
Directed acyclic graph (DAG) showing conceptual model for confounding
Abbreviations: BMI=Body mass index, CBCL=Child Behavior Checklist, PAH=Polycyclic aromatic hydrocarbon, SES=Socioeconomic status
2.6.1. Secondary Analyses
Weighted Quantile Sum Regression (WQSR) (Carrico et al., 2015; Czarnota et al., 2015) was used to characterize the association between OH-PAH mixtures and Total Problems scores while accounting for the between-component correlation of PAH metabolites. OH-PAHs were normalized by converting to quintiles, and WQSR scores composed of weighted sums of individual OH-PAHs were estimated. Weights were selected using bootstrap resampling methods (1000 bootstrap runs for each analysis) to optimize the association between the WQSR score and outcomes in multivariable linear regression models adjusted for the covariates in the fully adjusted models. Mixture effects were estimated in the positive and negative direction separately. A permutation test p-value was estimated for every full sample WQSR analysis that resulted in a 95% CI that did not include the null (Day et al., 2022; Loftus et al., 2021). Evidence for differences in all analyses was evaluated using a significance threshold of p<0.05.
Multivariable linear regression with multiplicative interaction terms and robust standard errors was used to examine whether associations of prenatal OH-PAH and Total Problems differed by child sex, breastfeeding, and Child Opportunity Index (Educational, Social and Economic domains). Models were fitted separately for each effect modifier and included all covariates in the fully adjusted model as well an interaction term between each OH-PAH metabolite and effect modifier. Breastfeeding status was modeled using 3 categories: never, 0–6 months, 6+ months; Child Educational and Social and Economic Opportunity were modeled continuously using standardized z-scores. We used a significance threshold of p<0.05 to assess evidence of effect modification, and also evaluated stratum-specific associations with 95% CIs from these models.
2.6.2. Sensitivity Analyses
We tested the robustness of our main analyses to alternative methods of specific gravity adjustment by repeating the main analyses using specific gravity-adjusted OH-PAH as the predictor variable, rather than specific gravity as a covariate. We performed a second sensitivity analysis to examine alternative methods of imputing OH-PAH concentrations below the limitation of detection by repeating the main analysis using Censored Likelihood Multiple Imputation (CLMI)(Boss et al., n.d.) rather than imputing OH-PAH concentrations with LOD/√2. Estimates from the three cohorts were combined using meta-analysis with fixed effects models. Finally, to assess the robustness of our results to site or cohort specific results, we repeated all main analyses with exclusion of one site at a time as well as stratified by cohort. All sensitivity analyses included covariates in the fully adjusted model.
All statistical analyses were conducted at the UW Pathways Data Center and analyzed using R Studio (RStudio Team, 2020). The R package “gWQS” version 2.0 package was used for the WQSR analyses (Renzetti et al., 2020).
3. RESULTS
3.1. Characteristics of the study population
The inclusion of participants from enrollment to the age 4–6 CBCL assessment and the final analytic sample sizes are provided in Figure 2. Of the 2,854 pregnant women ever enrolled in the CANDLE, GAPPS, and TIDES cohorts, 1,651 also had OH-PAH measures from pregnancy and completed a CBCL at their age 4–6 visit. Of these, we excluded 39 mother-child dyads because the child was born before 34 weeks and 144 mother-child dyads due to evidence of prenatal smoking from self-report or cotinine. After excluding participants with missing covariate data, 1,118 mother-child dyads were included in the final analytic sample (Figure 2).
Figure 2.
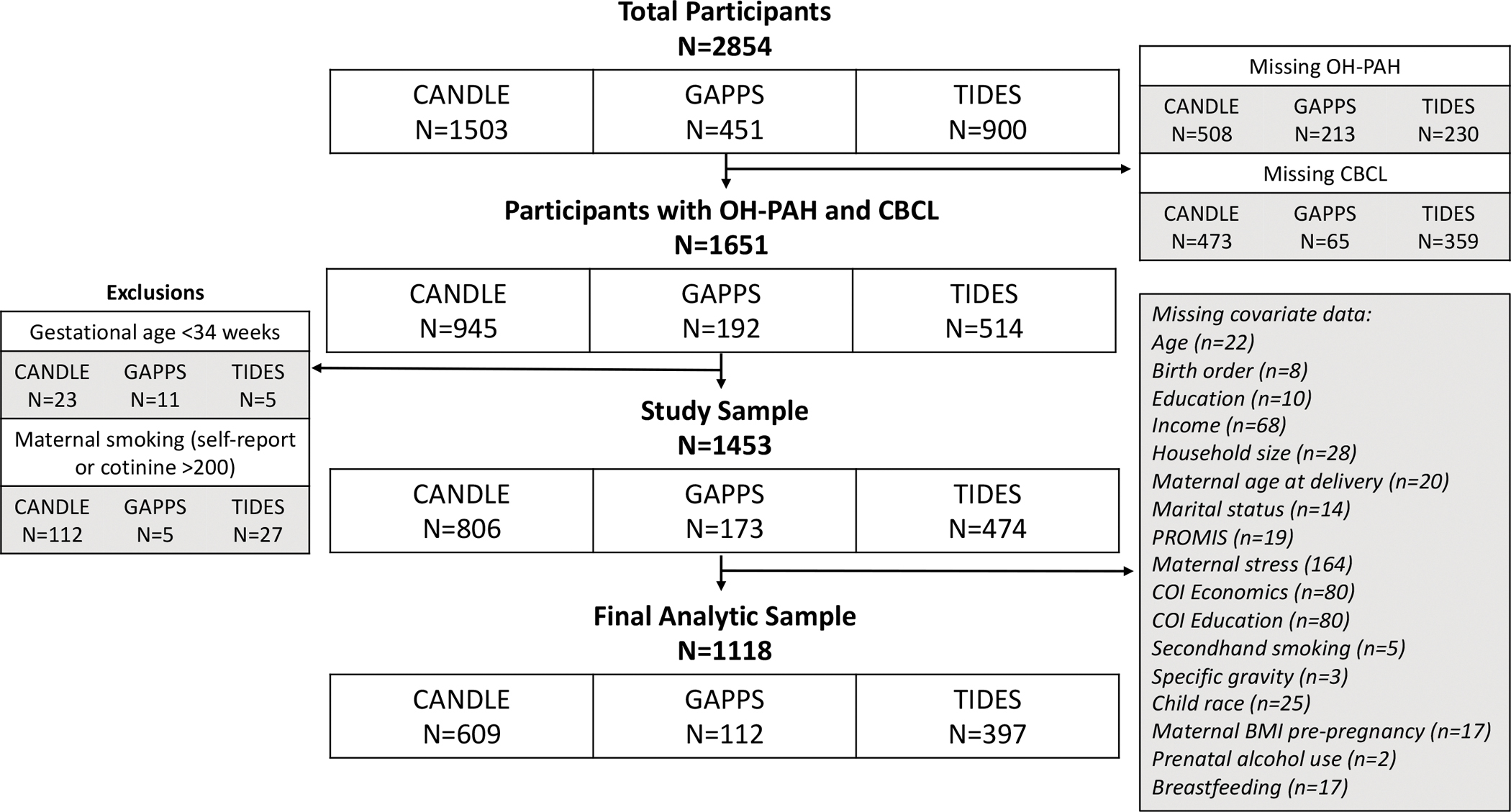
Flowchart for inclusion of study participants from enrollment to age 4–6 child visit and final analytic study sample, by ECHO-PATHWAYS cohort
Characteristics of the mothers and children in the analytic sample are summarized in Table 1. Children were, on average, 5.1 years of age (SD = 1.0) at their CBCL assessment and 52% female. Forty-nine percent identified their child’s race as White, 38% Black, 2% Asian, 0.5% American Indian or Alaska Native, 7% as Other, and 2% as Multiracial. On average, mothers were 29 years of age at their child’s birth (SD=5.8), 25% reported a high school education or less while 61% had a college education or beyond, and 73% were married or living as married. Eighteen percent of children were exposed to secondhand smoke in the pre- or post-natal period and 10% of mothers reported any prenatal alcohol use. Nineteen percent reported they never breastfed their child, 30% breastfed for 0–6 months, and 50% breastfed six months or longer. Average PROMIS maternal depression and stress scores were similar across study cohorts. A higher proportion of participants in CANDLE identified as a person of color than in GAPPS or TIDES, had secondhand smoke exposure in the home, and did not breastfeed their child. Overall, CANDLE participants had lower region- and inflation-adjusted household income compared to the other cohorts (Table 1).
Table 1.
Characteristics of the study population overall and by ECHO-PATHWAYS cohort
| Total | CANDLE | GAPPS | TIDES | |
|---|---|---|---|---|
| Characteristic | N=1118 | N=609 | N=112 | N=397 |
|
| ||||
| Child age at CBCL assessment (years), mean +/− SD | 5.12 ± 1.03 | 4.27 ± 0.34 | 5.44 ± 0.68 | 6.33 ± 0.35 |
| CBCL Study Form | ||||
| Preschool | 701 (62.7%) | 609 (100.0%) | 92 (82.1%) | 0 (0.0%) |
| School age | 417 (37.3%) | 0 (0.0%) | 20 (17.9%) | 397 (100.0%) |
| Child sex | ||||
| Male | 537 (48.0%) | 298 (48.9%) | 53 (47.3%) | 186 (46.9%) |
| Female | 581 (52.0%) | 311 (51.1%) | 59 (52.7%) | 211 (53.1%) |
| Child race | ||||
| White | 551 (49.3%) | 179 (29.4%) | 86 (76.8%) | 286 (72.0%) |
| Black | 427 (38.2%) | 386 (63.4%) | 3 (2.7%) | 38 (9.6%) |
| Asian | 26 (2.3%) | 6 (1.0%) | 1 (0.9%) | 19 (4.8%) |
| American Indian/Alaska Native | 6 (0.5%) | 2 (0.3%) | 1 (0.9%) | 3 (0.8%) |
| Other | 83 (7.4%) | 30 (4.9%) | 18 (16.1%) | 35 (8.8%) |
| Multiple | 25 (2.2%) | 6 (1.0%) | 3 (2.7%) | 16 (4.0%) |
| Maternal age at delivery (years), mean +/− SD | 29.28 ± 5.75 | 27.35 ± 5.60 | 30.84 ± 5.04 | 31.79 ± 5.04 |
| Income adjusted for household count (U.S. dollars), mean +/− SD | 73114.71 ± 54693.13 | 42700.04 ± 29515.13 | 104334.30 ± 49999.95 | 110963.40 ± 57140.16 |
| Household count, mean +/− SD | 4.32 ± 1.25 | 4.40 ± 1.34 | 4.55 ± 1.24 | 4.14 ± 1.08 |
| Household count categories | ||||
| 2–3 | 240 (21.5%) | 136 (22.3%) | 15 (13.4%) | 89 (22.4%) |
| 4 | 478 (42.8%) | 228 (37.4%) | 48 (42.9%) | 202 (50.9%) |
| 5 | 244 (21.8%) | 143 (23.5%) | 28 (25.0%) | 73 (18.4%) |
| 5+ | 156 (14.0%) | 102 (16.7%) | 21 (18.8%) | 33 (8.3%) |
| Maternal education | ||||
| Less than high school/high school/GED | 284 (25.4%) | 247 (40.6%) | 10 (8.9%) | 27 (6.8%) |
| Vocational or Technical school or Associates degree | 152 (13.6%) | 73 (12.0%) | 28 (25.0%) | 51 (12.8%) |
| College degree | 328 (29.3%) | 166 (27.3%) | 40 (35.7%) | 122 (30.7%) |
| Graduate or professional degree | 354 (31.7%) | 123 (20.2%) | 34 (30.4%) | 197 (49.6%) |
| Marital status | ||||
| Married or living with partner | 813 (72.7%) | 366 (60.1%) | 104 (92.9%) | 343 (86.4%) |
| Not married or living as single | 305 (27.3%) | 243 (39.9%) | 8 (7.1%) | 54 (13.6%) |
| Child was first born | ||||
| No | 621 (55.5%) | 369 (60.6%) | 78 (69.6%) | 174 (43.8%) |
| Yes | 497 (44.5%) | 240 (39.4%) | 34 (30.4%) | 223 (56.2%) |
| Pre-pregnancy maternal BMI, mean +/− SD | 27.00 ± 7.31 | 28.38 ± 7.82 | 26.86 ± 7.52 | 24.93 ± 5.80 |
| Child gestational age (days), mean +/− SD | 274.32 ± 9.95 | 273.57 ± 8.90 | 274.28 ± 10.42 | 275.48 ± 11.19 |
| Prenatal alcohol use | ||||
| None | 1012 (90.5%) | 562 (92.3%) | 103 (92.0%) | 347 (87.4%) |
| Any | 106 (9.5%) | 47 (7.7%) | 9 (8.0%) | 50 (12.6%) |
| Pre- and post-natal secondhand smoke exposure | ||||
| None | 920 (82.3%) | 471 (77.3%) | 99 (88.4%) | 350 (88.2%) |
| Any | 198 (17.7%) | 138 (22.7%) | 13 (11.6%) | 47 (11.8%) |
| Postnatal maternal smoking | ||||
| None | 1060 (94.8%) | 568 (93.3%) | 111 (99.1%) | 381 (96.0%) |
| Any | 58 (5.2%) | 41 (6.7%) | 1 (0.9%) | 16 (4.0%) |
| Maternal stress (Perceived Stress Scale), mean +/− SD | 2.27 ± 0.70 | 2.34 ± 0.73 | 2.24 ± 0.71 | 2.16 ± 0.64 |
| Maternal depression (PROMIS score), mean +/− SD | 48.37 ± 7.24 | 48.33 ± 6.89 | 49.09 ± 7.60 | 48.24 ± 7.66 |
| Child breastfeeding | ||||
| None | 217 (19.4%) | 186 (30.5%) | 5 (4.5%) | 26 (6.5%) |
| <6 months | 338 (30.2%) | 239 (39.2%) | 27 (24.1%) | 72 (18.1%) |
| ≥ 6 months | 563 (50.4%) | 184 (30.2%) | 80 (71.4%) | 299 (75.3%) |
| Recruitment site | ||||
| Memphis | 609 (54.5%) | 609 (100.0%) | 0 (0.0%) | 0 (0.0%) |
| San Francisco | 109 (9.7%) | 0 (0.0%) | 0 (0.0%) | 109 (27.5%) |
| Minneapolis | 127 (11.4%) | 0 (0.0%) | 0 (0.0%) | 127 (32.0%) |
| Rochester | 82 (7.3%) | 0 (0.0%) | 0 (0.0%) | 82 (20.7%) |
| Seattle - TIDES | 79 (7.1%) | 0 (0.0%) | 0 (0.0%) | 79 (19.9%) |
| Seattle - GAPPS | 50 (4.5%) | 0 (0.0%) | 50 (44.6%) | 0 (0.0%) |
| Yakima | 62 (5.5%) | 0 (0.0%) | 62 (55.4%) | 0 (0.0%) |
| Child Opportunity Index Education score (z-score), mean +/− SD | −0.01 ± 0.08 | −0.05 ± 0.06 | 0.00 ± 0.06 | 0.04 ± 0.08 |
| Child Opportunity Index Social and Economic score (z-score), mean +/− SD | −0.00 ± 0.24 | −0.09 ± 0.24 | 0.08 ± 0.12 | 0.11 ± 0.20 |
3.2. Distribution of prenatal OH-PAH concentration
Table 2 and Supplemental Table 1 show the distribution of urinary OH-PAH metabolites and the proportion below LOD overall and by study cohort. The proportion of sample below LOD varied across OH-PAH metabolites and study cohort and was lowest for 2-NAP (0.4%) and highest for 1-PYR (34.3%) (Table 2 and Supplemental Table 1). Raw geometric mean OH-PAH concentrations ranged from 0.06 ng/mL (SD=3.36) for 1-PYR to 2.94 ng/mL for 2-NAP (Table 2). OH-PAH concentrations were similar by child sex but highly variable across recruitment site and cohort (Supplemental Table 2). Across participant characteristics, women with higher OH-PAH concentrations, particularly for 1-NAP and 2-NAP, were more likely to identify their child as Black, to have a high school education or less, to report pre- or postnatal secondhand smoke exposure, and to have never breastfed their child, (Supplemental Table 2). OH-PAH metabolites demonstrated moderate to strong pairwise correlations (Supplemental Table 3).
Table 2.
Distribution of OH-PAH metabolites (in ng/mL) in the study population
| Metabolite | N below LOD | % below LOD | Min | 1st quartile | Median | Mean | 3rd quartile | Max | Geometric meana | Geometric SD |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 1-NAP | 140 | 12.5% | 0.01 | 0.20 | 0.53 | 2.34 | 1.28 | 329.02 | 0.48 | 5.06 |
| 2-NAP | 4 | 0.4% | 0.01 | 1.39 | 3.23 | 5.71 | 6.60 | 181.50 | 2.94 | 3.36 |
| 2-PHEN | 119 | 10.6% | 0.00 | 0.03 | 0.06 | 0.18 | 0.12 | 59.00 | 0.06 | 2.84 |
| 3-PHEN | 124 | 11.1% | 0.00 | 0.03 | 0.06 | 0.19 | 0.11 | 94.00 | 0.06 | 2.83 |
| 1/9-PHEN | 146 | 13.1% | 0.00 | 0.05 | 0.17 | 0.35 | 0.41 | 44.69 | 0.14 | 4.20 |
| 2/3/9-FLUO | 308 | 27.5% | 0.01 | 0.34 | 0.50 | 1.13 | 1.13 | 88.00 | 0.56 | 2.88 |
| 1-PYR | 383 | 34.3% | 0.01 | 0.01 | 0.08 | 0.22 | 0.21 | 20.80 | 0.06 | 5.05 |
All distributions represent volumetric concentrations of raw OH-PAHs and based on detectible values
Abbreviations: 1-NAP= 1-hydroxynaphthalene, 2-NAP=2-hydroxynaphthalene, 2-PHEN=2-hydroxyphenanthrene, 3-PHEN=3-hydroxyphenanthrene, 1/9-PHEN=1/9-hydroxyphenanthrene, 2/3/9-FLUO=2/3/9-hydroxyfluorene, 1-PYR=1-hydroxypyrene, LOD = Limit of detection; SD = Standard deviation
3.3. Distribution of CBCL Total Problems
Overall, 63% of participants completed the CBCL preschool form and 37% completed the school age form. The form version varied by study cohort and child age at outcome assessment: 100% of CANDLE participants completed the preschool form while 100% of TIDES participants completed the school age form. Eighty two percent of GAPPS participants completed the preschool form and 18% completed the school age form. Average raw (i.e. unadjusted for child sex or study form) CBCL Total Problems scores were 21.9 (SD = 17.4) and were highest in CANDLE participants (mean = 22.6, SD = 18.2) followed by TIDES (mean = 21.3, SD = 16.4) and GAPPS (mean = 20.2, SD = 16.6).
3.4. Associations between individual OH-PAHs and child Total Problems score
Associations between individual OH-PAH metabolites and Total Problems scores are shown in Figure 3. There was no evidence for associations for any OH-PAH and Total Problems scores in minimally adjusted models. After full adjustment, 2-NAP was associated with a lower Total Problems score (2-fold b= −0.80, 95% CI = −1.51, −0.08) (Figure 3). This association was slightly attenuated in the expanded adjustment model (2-fold b= −0.71, 95% CI −1.44, 0.02). Associations for 1-NAP and Total Problems reached borderline significance in the fully adjusted model (2-fold b= −0.46, 95% CI −0.97, 0.04). Associations were null for all other metabolites in full and expanded adjustment models (Figure 3).
Figure 3.
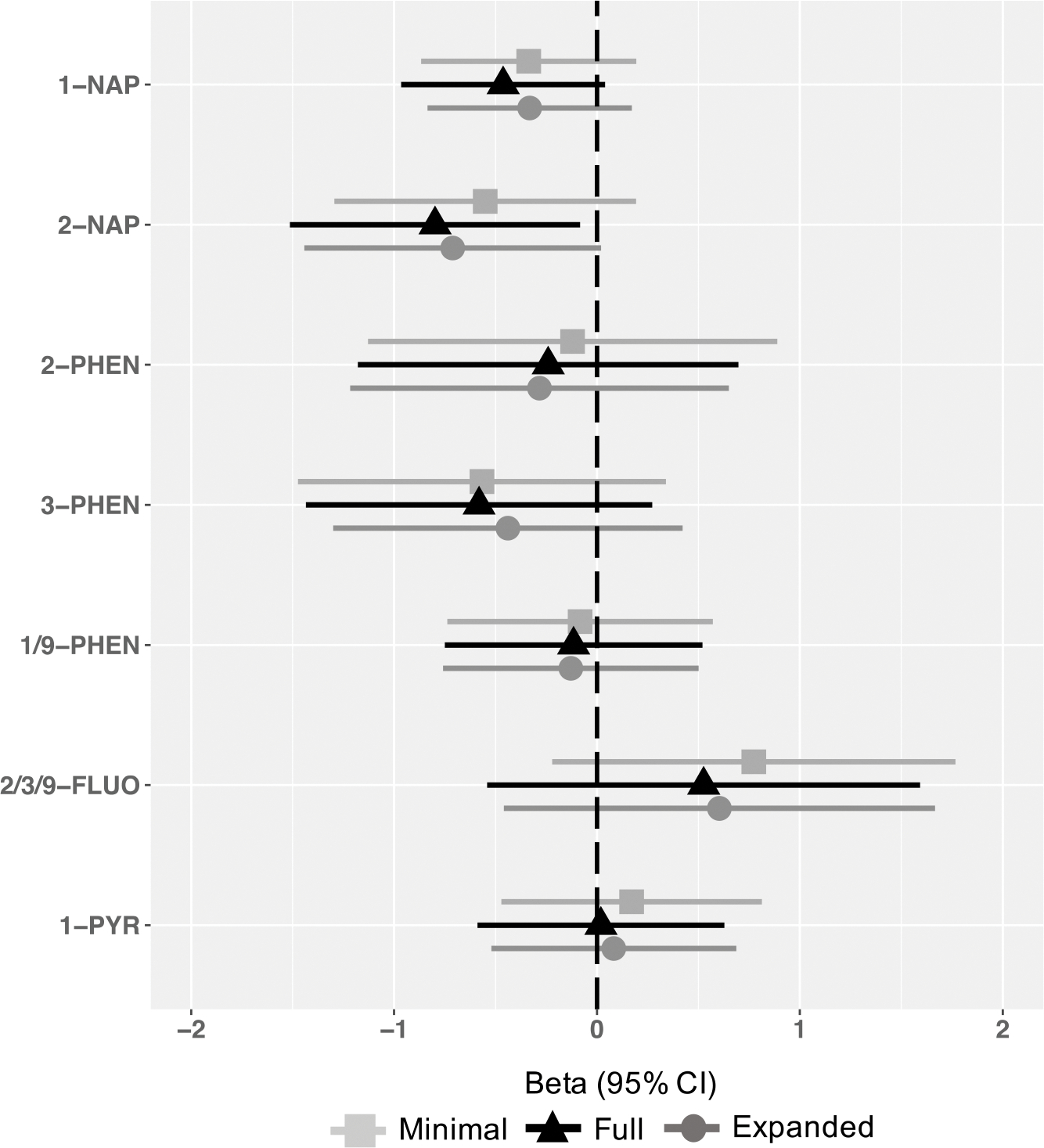
Associations between prenatal OH-PAH metabolites and age 4–6 Total Problems, by metabolite and model.
Models represent effect estimates (symbols) and 95% confidence intervals (bars) from linear regressions. The minimally adjusted model includes child age at assessment (continuous year, to two decimal places), child sex (binary), CBCL form version (binary), OH-PAH analysis batch, and urinary specific gravity (continuous). The fully adjusted model includes terms in the minimally adjusted model plus maternal education (categorical), adjusted income by household count (continuous, natural log transformed), household count (categorical), interaction between adjusted income by family household size (i.e. log income X household count), maternal age at child’s birth (continuous), marital status (married or living with partner vs. not), child birth order (binary, first born vs. not), study site (categorical, 7, with Seattle GAPPS and Seattle TIDES modeled separately), maternal depression (PROMIS score, continuous), maternal stress (Perceived Stress scale, continuous), Child Opportunity Index Social and Economic domain (continuous), Child Opportunity Index Education, pre- and post-natal secondhand smoke exposure (self-reported; any vs. none), and postnatal maternal smoking (self-reported; any vs. none). The expanded adjusted model includes terms in the fully adjusted model plus pre-pregnancy BMI (continuous), gestational age (continuous), prenatal alcohol use (continuous), breastfeeding status (categorical; None, <6 months., ≥ 6 months), as well as self-identified race (categorical; White, Black, Asian, American Indian/Alaska Native, Other, Multiple race). All estimates represent effect per 2-fold increase in log OH-PAH.
Abbreviations: 1-NAP= 1-hydroxynaphthalene, 2-NAP=2-hydroxynaphthalene, 2-PHEN=2-hydroxyphenanthrene, 3-PHEN=3-hydroxyphenanthrene, 1/9-PHEN=1/9-hydroxyphenanthrene, 2/3/9-FLUO=2/3/9-hydroxyfluorene, 1-PYR=1-hydroxypyrene, 95% CI = 95% Confidence Interval.
3.5. Analysis of OH-PAH mixtures
Table 3 shows results of analysis of OH-PAH mixtures using WQSR. There was no evidence for associations of OH-PAH mixtures and Total Problems in either the positive or negative direction (Positive b = 0.32, 95% CI −.21, 0.84; Negative b = − 0.43, 95% CI −0.92, 0.06) (Table 3). A permutation test was not performed as the 95% confidence interval did not exclude the null for either estimate.
Table 3.
Estimated effects and metabolite weights of OH-PAH matrix and age 4–6 Total Problems from Weighted Quantile Sum Regression, by direction
| Direction | Beta | 95% CI | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| Positive | 0.32 | −0.21 | 0.84 | 0.24 | |
| Negative | −0.43 | −0.92 | 0.06 | 0.08 | |
|
| |||||
| Metabolite weight | Positive | Negative | |||
|
| |||||
| 1-NAP | 0.02 | 0.18 | |||
| 2-NAP | 0.00 | 0.63 | |||
| 2-PHEN | 0.10 | 0.02 | |||
| 3-PHEN | 0.01 | 0.05 | |||
| 1/9-PHEN | 0.12 | 0.06 | |||
| 2/3/9-FLUO | 0.60 | 0.01 | |||
| 1-PYR | 0.15 | 0.06 | |||
Estimates adjusted for child age at assessment (continuous year, to two decimal places), child sex (binary), CBCL form version (binary), OH-PAH analysis batch, and urinary specific gravity (continuous). maternal education (categorical), adjusted income by household count (continuous, natural log transformed), household count (categorical), interaction between adjusted income by family household size (i.e. log income X household count), maternal age at child’s birth (continuous), marital status (married or living with partner vs. not), child birth order (binary, first born vs. not), study site (categorical, 7, with Seattle GAPPS and Seattle TIDES modeled separately), maternal depression (PROMIS score, continuous), maternal stress (Perceived Stress scale, continuous), Child Opportunity Index Social and Economic domain (continuous), Child Opportunity Index Education, pre- and post-natal secondhand smoke exposure (self-reported; any vs. none), and postnatal maternal smoking (self-reported; any vs. none).
Abbreviations: 1-NAP= 1-hydroxynaphthalene, 2-NAP=2-hydroxynaphthalene, 2-PHEN=2-hydroxyphenanthrene, 3-PHEN=3-hydroxyphenanthrene, 1/9-PHEN=1/9-hydroxyphenanthrene, 2/3/9-FLUO=2/3/9-hydroxyfluorene, 1-PYR=1-hydroxypyrene
3.6. Effect modification by child sex, breastfeeding, and neighborhood child opportunity
Observed associations of OH-PAHs and Total problems were generally more pronounced in boys than girls across metabolites, including for 2-NAP (2-fold b in boys = −1.10, 95% CI −2.11, −0.08; 2-fold b in girls = −0.53, 95% CI −1.36, 0.30), though the statistical test of interaction was null (pinteraction = 0.34) (Figure 4). There was no evidence of sex interaction or sex-specific associations for other metabolites (Figure 4). In analyses of effect modification by breastfeeding, evidence of an association between 2-NAP and Total Problems scores was strongest among in children breastfed for 6+ months (2-fold b = −1.31, 95% CI −2.25, −0.37), but the statistical test of interaction was null (pinteraction = 0.21; Figure 5). A similar pattern was observed for 1-NAP (2-fold b in children breastfed 6+ months = −0.72, 95% CI −1.43, 0.00, pinteraction = 0.78). We found no evidence of effect modification by length of breastfeeding for other OH-PAH metabolites (Figure 5) and no evidence for interaction by either domain of neighborhood child opportunity (Table 4).
Figure 4.
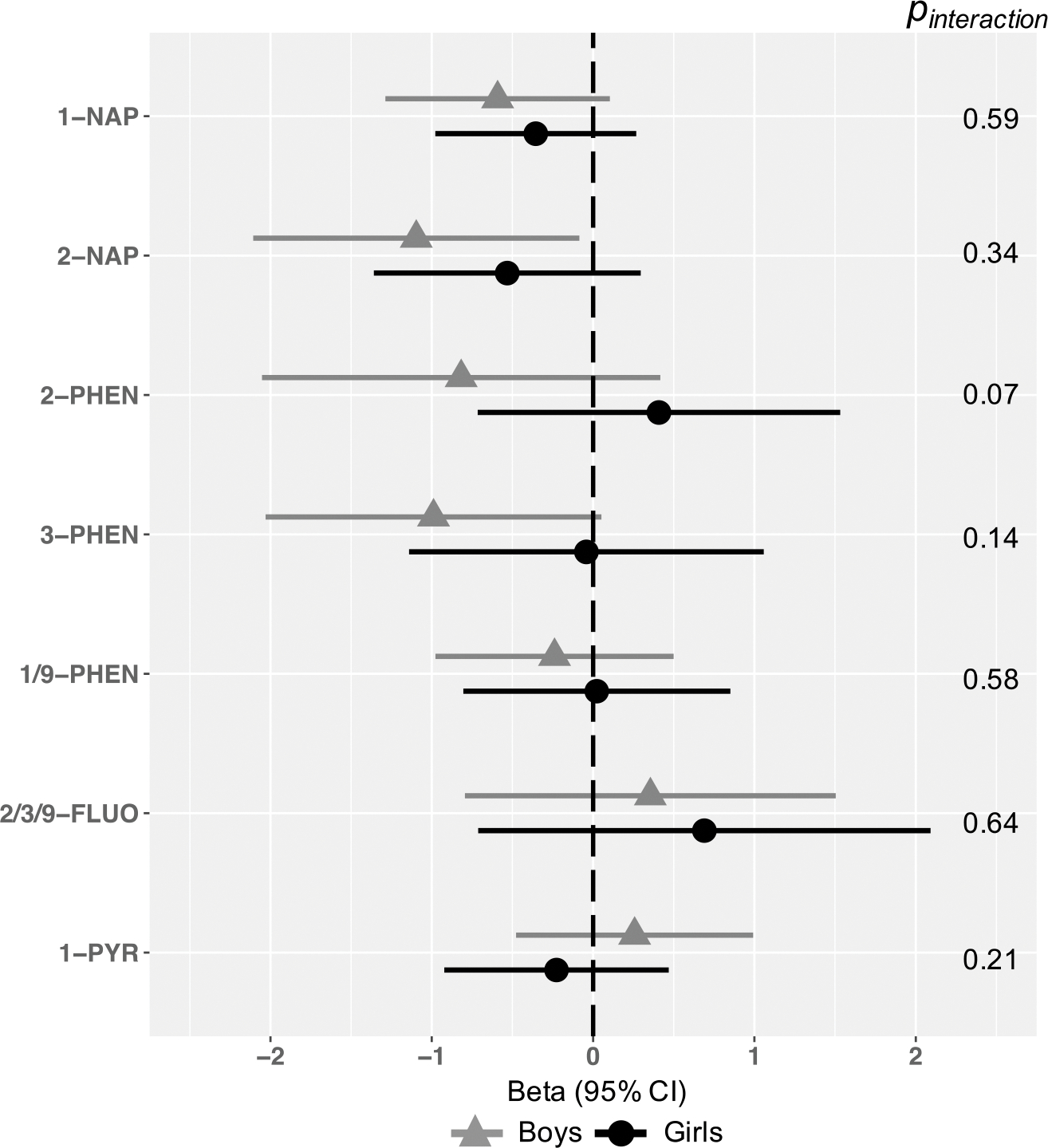
Associations between OH-PAH metabolites and age 4–6 Total Problems, by child sex.
Models represent effect estimates (symbols) and 95% confidence intervals (bars) from linear regressions. Adjusted for child age at assessment (continuous year, to two decimal places), child sex (binary), CBCL form version (binary), OH-PAH analysis batch, and urinary specific gravity (continuous). maternal education (categorical), adjusted income by household count (continuous, natural log transformed), household count (categorical), interaction between adjusted income by family household size (i.e. log income X household count), maternal age at child’s birth (continuous), marital status (married or living with partner vs. not), child birth order (binary, first born vs. not), study site (categorical, 7, with Seattle GAPPS and Seattle TIDES modeled separately), maternal depression (PROMIS score, continuous), maternal stress (Perceived Stress scale, continuous), Child Opportunity Index Social and Economic domain (continuous), Child Opportunity Index Education, pre- and post-natal secondhand smoke exposure (self-reported; any vs. none), and postnatal maternal smoking (self-reported; any vs. none). All estimates represent effect per 2-fold increase in log OH-PAH.
Abbreviations: 1-NAP= 1-hydroxynaphthalene, 2-NAP=2-hydroxynaphthalene, 2-PHEN=2-hydroxyphenanthrene, 3-PHEN=3-hydroxyphenanthrene, 1/9-PHEN=1/9-hydroxyphenanthrene, 2/3/9-FLUO=2/3/9-hydroxyfluorene, 1-PYR=1-hydroxypyrene, 95% CI = 95% Confidence Interval.
Figure 5.
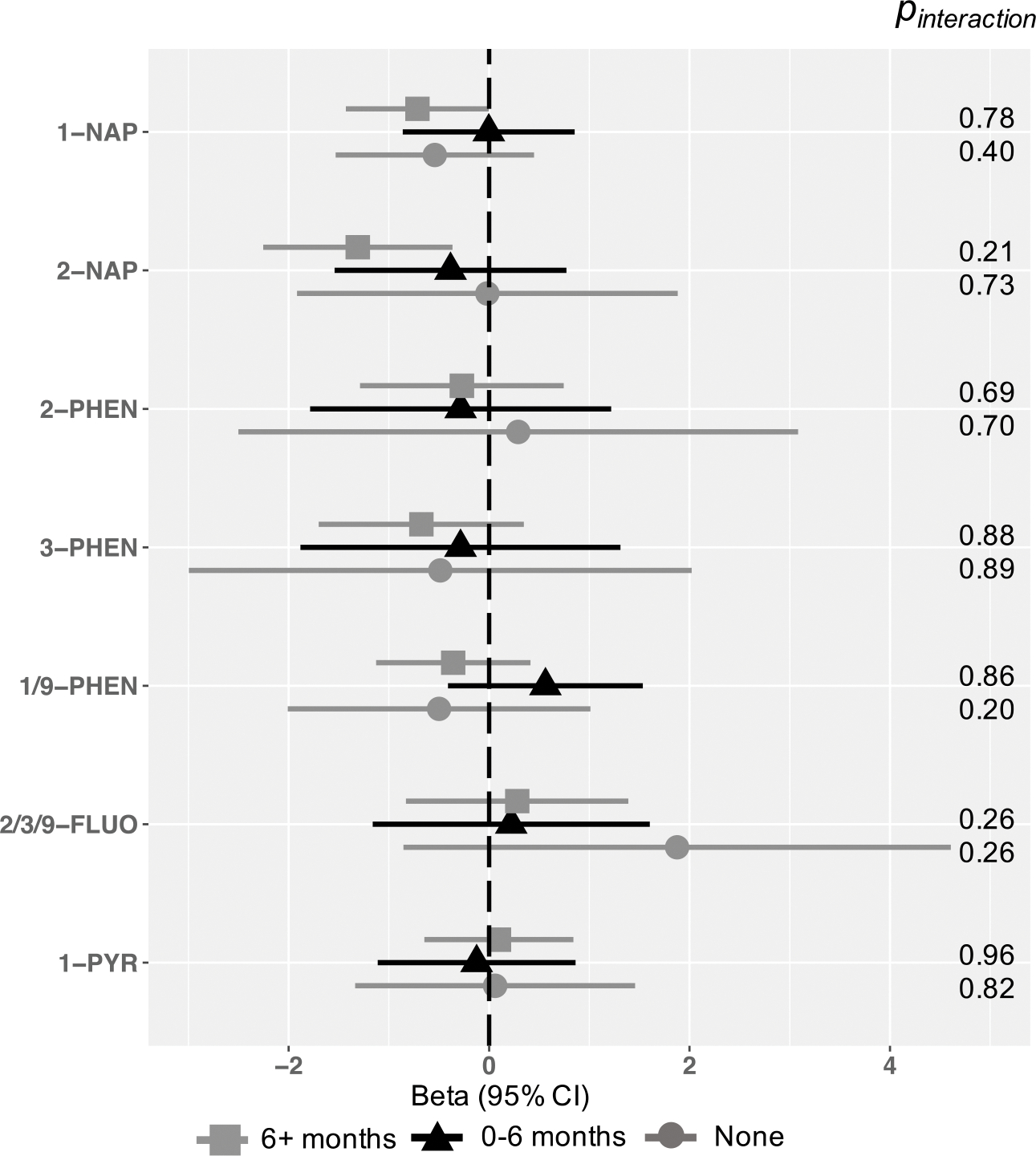
Associations between OH-PAH metabolites and age 4–6 Total Problems, by length of breastfeeding.
Models represent effect estimates (symbols) and 95% confidence intervals (bars) from linear regressions. Adjusted for child age at assessment (continuous year, to two decimal places), child sex (binary), CBCL form version (binary), OH-PAH analysis batch, and urinary specific gravity (continuous). maternal education (categorical), adjusted income by household count (continuous, natural log transformed), household count (categorical), interaction between adjusted income by family household size (i.e. log income X household count), maternal age at child’s birth (continuous), marital status (married or living with partner vs. not), child birth order (binary, first born vs. not), study site (categorical, 7, with Seattle GAPPS and Seattle TIDES modeled separately), maternal depression (PROMIS score, continuous), maternal stress (Perceived Stress scale, continuous), Child Opportunity Index Social and Economic domain (continuous), Child Opportunity Index Education, pre- and post-natal secondhand smoke exposure (self-reported; any vs. none), and postnatal maternal smoking (self-reported; any vs. none). All estimates represent effect per 2-fold increase in log OH-PAH.
Abbreviations: 1-NAP= 1-hydroxynaphthalene, 2-NAP=2-hydroxynaphthalene, 2-PHEN=2-hydroxyphenanthrene, 3-PHEN=3-hydroxyphenanthrene, 1/9-PHEN=1/9-hydroxyphenanthrene, 2/3/9-FLUO=2/3/9-hydroxyfluorene, 1-PYR=1-hydroxypyrene, 95% CI = 95% Confidence Interval.
Table 4.
Associations between OH-PAH metabolites and age 4–6 Total Problems, with interaction by continuous Child Opportunity Index domain
| Social and Economic | Education | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Metabolite | Beta (when COI=0)* | 95% | CI | p-value | Interaction p | Beta (when COI=0)* | 95% | CI | p-value | Interaction | p |
|
| |||||||||||
| 1-NAP | −0.45 | −0.96 | 0.05 | 0.08 | 0.78 | −0.39 | −0.89 | 0.11 | 0.12 | 0.15 | |
| 2-NAP | −0.80 | −1.51 | −0.09 | 0.03 | 0.67 | −0.77 | −1.47 | −0.06 | 0.03 | 0.65 | |
| 2-PHEN | −0.24 | −1.18 | 0.70 | 0.62 | 0.83 | −0.20 | −1.14 | 0.73 | 0.67 | 0.24 | |
| 3-PHEN | −0.60 | −1.45 | 0.25 | 0.17 | 0.42 | −0.57 | −1.42 | 0.27 | 0.19 | 0.28 | |
| 1/9-PHEN | −0.13 | −0.76 | 0.51 | 0.70 | 0.46 | −0.13 | −0.78 | 0.51 | 0.68 | 0.79 | |
| 2/3/9-FLUO | 0.53 | −0.54 | 1.60 | 0.33 | 0.84 | 0.63 | −0.46 | 1.72 | 0.26 | 0.27 | |
| 1-PYR | 0.02 | −0.60 | 0.63 | 0.95 | 0.96 | 0.03 | −0.58 | 0.64 | 0.93 | 0.35 | |
Models represent effect estimates and 95% confidence intervals from linear regressions. Adjusted for child age at assessment (continuous year, to two decimal places), child sex (binary), CBCL form version (binary), OH-PAH analysis batch, and urinary specific gravity (continuous). maternal education (categorical), adjusted income by household count (continuous, natural log transformed), household count (categorical), interaction between adjusted income by family household size (i.e. log income X household count), maternal age at child’s birth (continuous), marital status (married or living with partner vs. not), child birth order (binary, first born vs. not), study site (categorical, 7, with Seattle GAPPS and Seattle TIDES modeled separately), maternal depression (PROMIS score, continuous), maternal stress (Perceived Stress scale, continuous), Child Opportunity Index Social and Economic domain (continuous), Child Opportunity Index Education, pre- and post-natal secondhand smoke exposure (self-reported; any vs. none), and postnatal maternal smoking (self-reported; any vs. none). All estimates represent effect per 2-fold increase in log OH-PAH.
Abbreviations: 1-NAP= 1-hydroxynaphthalene, 2-NAP=2-hydroxynaphthalene, 2-PHEN=2-hydroxyphenanthrene, 3-PHEN=3-hydroxyphenanthrene, 1/9-PHEN=1/9-hydroxyphenanthrene, 2/3/9-FLUO=2/3/9-hydroxyfluorene, 1-PYR=1-hydroxypyrene, 95% CI = 95% Confidence Interval.
3.7. Sensitivity analyses
Modeling PAH exposure using specific gravity-adjusted OH-PAH rather than raw OH-PAH had little effect on estimates and all 95% confidence intervals included the null (Supplemental Figure 1). Sensitivity analyses using CLMI for the imputation of OH-PAH concentrations below the limitation of detection also yielded similar estimates to the main analyses (Supplemental Figure 2). Sensitivity analyses using leave-one-site-out are shown in Supplemental Figure 5. Estimates for associations of most metabolites were similar across site, including all null associations. Associations for 2-NAP and Total Problems were notable for all sites with the exception of analyses leaving out Memphis and Minneapolis, and were of the greatest magnitude when leaving out Rochester, Yakima, and Memphis (Supplemental Figure 3). Supplemental Figure 6 shows cohort-stratified associations of OH-PAHs and Total Problems. Associations for 2-NAP and Total Problems were strongest in TIDES, although confidence intervals included the null (2-fold b = −1.04, 95% CI −2.11, 0.03); there was no evidence for associations of OH-PAHs and Total Problems in CANDLE and GAPPS (Supplemental Figure 4).
4. DISCUSSION
In this large, diverse, and well-characterized multi-site prospective study of mother-child pairs participating in the ECHO-PATHWAYS consortium, we examined associations of prenatal PAH exposure measured in individual OH-PAH metabolites as well as OH-PAH mixtures and child behavior at age 4 to 6 years. We did not see evidence to support our hypothesis that prenatal OH-PAH exposure was associated with higher Total Problems scores. Most associations were null. After adjustment, 2-NAP was associated with lower Total Problems scores in our main analyses and some sensitivity and secondary analyses. The magnitudes of these effect sizes were small (e.g. most estimates reflect an approximately 1-point difference in raw behavior score), were not in the hypothesized direction, and were not robust to expanded adjustment or alternative methods of adjustment for specific gravity. There was also no evidence from WQS regression of PAH mixtures on behavior scores nor evidence for effect modification by child sex, breastfeeding, neighborhood social and economic or educational opportunity.
The predominantly null associations observed in this study stand in contrast to the findings from two prospective cohorts in New York City and Krakow. The New York City cohort was comprised of African-American and Dominican mothers and assessed associations of prenatal PAH exposure based on maternal personal sampling of airborne PAH as well as PAH-DNA adducts sampled from cord blood and selected CBCL behavior outcomes at multiple child ages, including two studies at similar age assessments as this analysis, e.g. ages 4 to 7 years (Perera et al., 2012, 2011). CBCL outcomes reported included the Anxious/Depressed and Attention Problems from the CBCL syndrome scales and DSM-oriented Anxiety problems. PAH-DNA adducts were associated with worse scores or greater odds for meeting borderline or clinical range for nearly all outcomes at age 5, and for Attention Problems at age 7. The authors conducted additional analyses of behavior outcomes at ages 6–7 utilizing personal air PAH measures coupled with cord blood PAH measurement (Perera et al., 2012). High PAHs in air were associated with greater risk for meeting the borderline or clinical range for Anxious/Depressed and Attention Problems and DSM-IV Anxiety Problems, but not DSM-IV Attention Deficit/Hyperactivity Problems. BaP-DNA adducts were associated with Attention problems but no other subscales after covariate adjustment. Later analyses at ages 9 and 11 showed behavioral deficits associated with PAHs in air and/or cord blood were more pronounced in children exposed to material hardship (Perera et al., 2018) or children’s early life stress (Pagliaccio et al., 2020), without evidence for main effects of PAHs and behavior at either age point in the absence of interaction. Effect sizes were large across time points, e.g odds ratios for notable associations ranged from 3.30–8.3 at ages 5 and 7 years and 4.0–8.9 at ages 6–7, after adjustment for sociodemographic confounding. But caution should be taken in interpreting these effect sizes, as the number of children with behavior problems in each subscale was small, e.g. 10 affected children out of less than 100 participants at age 5 and fewer than 25 out of 250 meeting borderline or clinical ranges for behavior problems at ages 6–7, may be vulnerable to residual confounding, and were not significant after Bonferroni correction.
The cohort in Krakow, Poland assessed PAHs using personal air monitoring as well as dietary PAH calculated from questionnaire data and CBCL broadband and syndrome subscales at age 6–7 years (Genkinger et al., 2015). Prenatal PAH exposure measured using personal air was associated was adverse scores for both Internalizing and Externalizing broadband scores and 4 subscales (Withdrawn/Depressed, Social Problems, Attention Problems, and Aggressive behavior. Dietary PAH was associated only with Attention problems, but no other outcome. At age 9, poorer behavior scores were associated with higher personal air PAH exposure, but only in children of mothers with maternal demoralization (Perera et al. 2013). Confounding adjustment was similar to the New York cohort, although the effect sizes observed were notably smaller than the New York cohort and were < 0.3 point difference in raw syndrome scales and <0.2 point differences in broadband scores at age 6–7, sample sizes involved fewer than 150 participants at each time point.
Notably, both the NY and Krakow cohorts primarily focused on specific syndrome and DSM subscales as outcomes. In contrast, we assessed behavior using the Total Problems score, which combines items across all domains of behavior problems captured by the CBCL. We elected not to analyze subscales of behavior in this study because of the relatively low levels of specific, subdomain clinical problems in general population samples, particularly at this young age and weak differentiation of problem types in early childhood. We also did not have a priori hypotheses about specific subscales of behavior, as the results of the Krakow study suggested adverse effects of prenatal PAHs across a spectrum of behavior scales, and we wanted to limit the potential for bias from a Type 1 error. Further, the number of items that comprise the syndrome and DSM subscale scores vary considerably between the preschool and school age CBCL instruments, which were combined in this study, presenting methodological challenges to subdomain investigation.
Our results are more consistent with other studies in the U.S. and internationally which have examined associations between PAH exposure assessed pre- and post-natally and school-aged child behavior. Two prospective cohorts in Spain (n=484) and the Netherlands (n=3120) participating in the ESCAPE consortium assessed associations of pre- and postnatal PAH using regional PM2.5 air monitoring and land use regression modeling and CBCL clinical depressive or anxiety or aggressive symptoms at age 9–10 years (Jorcano et al., 2019). Null associations with pre- and post-natal PAHs were observed in both cohorts and in the meta-analysis (Jorcano et al., 2019). Cross-sectionally, null associations have also been observed in Spain, where PAH exposure was based on air monitoring in 242 children from 35 schools and child attention and ADHD were assessed using the ADHD-DSM-IV checklist and the child Attention Network Test (Mortamais et al., 2017). Interestingly, PAHs were associated with lower caudate nucleus volume using brain MRI, but associations were null for all behavior outcomes(Mortamais et al., 2017). In the U.S., cross-sectional associations between individual urinary PAH metabolites and parent-reported behavior problems were assessed in children aged 6–15 years participating in the National Health and Nutrition Examination Survey (NHANES) (Abid et al., 2014). 2-NAP was associated with a lower risk of parent-reported ADHD diagnosis, and in analysis of effect modification by sex, the strongest inverse effects were observed in females. Inverse associations were also observed between 1-pyrene and ADHD, while 2/3-hydroxyfluorene was associated with a 2-fold greater odds of receiving special education services (Abid et al., 2014). Comparison of the prenatal PAH levels observed in these studies and other cohorts to ours is complicated by the different methods used to measure PAH exposure (e.g. air, blood, urine) and type of outcomes studied (e.g. domains and subscales of child behavior). The distribution of OH-PAH levels in this study were within the ranges reported in pregnant women participating in NHANES in 2003–2004(Woodruff et al., 2011). Notably, the air PAH levels measured in the cohorts in New York City and Krakow were high relative to those measured in other cities and studies of PAH exposure, particularly in Krakow, which experienced air PAH concentrations more than 5 times greater than those observed in New York City (Jedrychowski et al., 2005). If there is a threshold effect in the dose-response relationship, our null findings could be due to the relatively lower prenatal PAH exposures in our study population. The differences in the magnitude of the PAH exposures, coupled with heterogeneity in the patient populations and potential susceptibility to toxic effects of PAH exposures may also contribute to the variability in findings across studies and underscores the complexity in characterizing the populations most vulnerable to exposure.
Strengths of this study include the large sample size, comprising three racially and ethnically diverse prospective mother-child cohorts from six U.S. cities participating in the ECHO PATHWAYS consortium, with robust statistical power to detect small associations and effect modification. This was the largest prospective study of directly measured prenatal PAH exposure and early school-age child behavior, and used an exposure metric that captured all routes of exposure, including air and diet. Adjustment for confounding is particularly important in studies of environmental exposures and neurodevelopment (Mink et al. 2004), and it is possible that associations observed in other studies could be the result of residual confounding, if some confounders were mismeasured or not included in adjustment models. We harmonized covariates across cohorts to create a well-characterized study population with rich adjustment for maternal and sociodemographic factors within and outside of the home that influence child behavior and utilized staged models to assess the sensitivity of our findings to adjustment.
There were several limitations to this study. First, we combined data from cohorts utilizing a pooled approach and a complete case analysis to examine associations, without utilizing weights or other model-based approaches to combining data from multiple cohorts. This approach leverages the diversity of the participants across cohorts, strengthening the external generalizability, but could also induce measurement error due to heterogeneity in PAH levels and variability in methods for ascertaining covariate data. Comparison of the estimates from sensitivity analyses leaving one site out and stratified by study cohort suggest that that the predominantly null associations persist in spite of the heterogeneities across study sites and cohort. A second limitation is that OH-PAH exposure were measured using a single urine sample in pregnancy, which is known to represent only short-term exposures to PAH, having a half-life of 6–35 hours (Jongeneelen et al., 1990). PAHs exhibit weak to moderate correlation when sampled repeatedly (Cathey et al., 2018) and may not reflect the cumulative burden of exposure over pregnancy. This may have introduced nondifferential exposure misclassification, which would likely bias our findings towards the null. The proportion of samples below the limit of detection was high for some metabolites, particularly for 2/3/9-FLUO and 1-PYR, and our method of imputing <LOD values as LOD/√2 may have introduced bias in the observed associations. We excluded metabolites with %LOD above 30% to minimize the potential for bias and conducted sensitivity analyses using CLMI to impute PAH values were similar to estimates in the main analyses(Boss et al., n.d.). We observed similar findings with imputation of <LOD values.
4.1. Conclusion
The results of our study suggest that prenatal PAH exposure is not associated with adverse behavioral functioning in children at ages 4 to 6 years, which contrasts with some prior prospective studies of similarly aged children situated in dense, highly polluted urban environments. It is possible that associations of prenatal PAH exposure and child behavior are present only in the setting of very high exposure to PAHs or in concert with other stressors or vulnerabilities that extend beyond those included in the current report. Additional prospective studies in other pregnancy cohorts would help to understand the discrepancy in the published results. Future studies should also address existing gaps in the literature on the associations of PAHs on behavior, including the possibility of critical windows of exposure, the role of of postnatal PAH exposure, and wehther associations of PAHs and behavioral functioning are evident in middle childhood and adolescence.
Supplementary Material
Highlights:
This study examined associations of prenatal PAH and child behavior
This study was conducted in the ECHO PATHWAYS consortium
The study population included 3 diverse longitudinal pregnancy cohorts
We employed novel methods to examine PAH mixtures and child behavior
Adverse associations between prenatal PAH and behavior were not evident
Acknowledgments
ECHO PATHWAYS is funded by NIH (UG3/UH3OD023271, P30ES007033). The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study was funded by the Urban Child Institute. The TIDES study was funded by NIH R01ES016863 and National Institute of Environmental Health Sciences (NIEHS) Intramural Funding (ZIA10331): Reproductive outcomes and oxidative stress in TIDES (ROOST). Dr. Kannan analyzed OH-PAH metabolites in TIDES with support from the New York University ECHO Cohort Center (UG3/UH3OD023305 (PI: Leonardo Trasande)).Research including Global Alliance to Prevent Prematurity and Stillbirth (GAPPS) participants was conducted using specimens and data collected and stored on behalf of the GAPPS Repository. We are grateful for the participation of families enrolled in the CANDLE, TIDES, and GAPPS cohort, as well as the dedication of CANDLE, TIDES, and GAPPS research staff and investigators. This manuscript has been reviewed by PATHWAYS for scientific content and consistency of data interpretation with previous PATHWAYS publications.
Footnotes
Competing Financial Interests: The authors declare they have no actual or potential competing financial interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abid Z, Roy A, Herbstman JB, Ettinger AS, 2014. Urinary polycyclic aromatic hydrocarbon metabolites and attention/deficit hyperactivity disorder, learning disability, and special education in U.S. children aged 6 to 15. J Environ Public Health 2014, 628508. 10.1155/2014/628508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Garcia D, McArdle N, Hardy EF, Crisan UI, Romano B, Norris D, Baek M, Reece J, 2014. The child opportunity index: improving collaboration between community development and public health. Health Aff (Millwood) 33, 1948–1957. 10.1377/hlthaff.2014.0679 [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA, 2001. Manual for the ASEBA School-Age Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families, Burlington, VT. [Google Scholar]
- Achenbach TM, Rescorla LA, 2000. Manual for the ASEBA Preschool Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families, Burlington, VT. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR), 1995. Toxicological profile for polycyclic aromatic hydrocarbons, U.S. Department of Health and Human Services. [PubMed] [Google Scholar]
- Bar S, Milanaik R, Adesman A, 2016. Long-term neurodevelopmental benefits of breastfeeding. Curr Opin Pediatr 28, 559–566. 10.1097/MOP.0000000000000389 [DOI] [PubMed] [Google Scholar]
- Barrett ES, Sathyanarayana S, Janssen S, Redmon JB, Nguyen RHN, Kobrosly R, Swan SH, 2014. Environmental health attitudes and behaviors: Findings from a large pregnancy cohort study. European Journal of Obstetrics and Gynecology and Reproductive Biology 176, 119–125. 10.1016/j.ejogrb.2014.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC, 2001. Future directions for neurobehavioral studies of environmental neurotoxicants. Neurotoxicology 22, 645–656. 10.1016/S0161-813X(01)00036-5 [DOI] [PubMed] [Google Scholar]
- Bellinger DC, 2000. Effect modification in epidemiologic studies of low-level neurotoxicant exposures and health outcomes. Neurotoxicol Teratol 22, 133–140. 10.1016/S0892-0362(99)00053-7 [DOI] [PubMed] [Google Scholar]
- Boss J, Mukherjee B, Ferguson K, … A.A.-E., 2019, undefined, n.d. Estimating outcome-exposure associations when exposure biomarker detection limits vary across batches. ncbi.nlm.nih.gov. [DOI] [PMC free article] [PubMed]
- Brooks-Gunn J, Klebanov PK, Duncan GJ, 1996. Ethnic Differences in Children’s Intelligence Test Scores: Role of Economic Deprivation, Home Environment, and Maternal Characteristics. Child Dev 67, 396–408. 10.1111/j.1467-8624.1996.tb01741.x [DOI] [PubMed] [Google Scholar]
- Brown LA, Khousbouei H, Goodwin JS, Irvin-Wilson C. v., Ramesh A, Sheng L, McCallister MM, Jiang GCT, Aschner M, Hood DB, 2007. Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. Neurotoxicology 28, 965–978. 10.1016/J.NEURO.2007.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Economic Analysis, 2021. Real Personal Income & Regional Price Parities Methodology [WWW Document]. U.S. Department of Commerce. URL https://www.bea.gov/data/prices-inflation/regional-price-parities-state-and-metro-area (accessed 4.17.22). [Google Scholar]
- Burniaux J-M, Dang T-T, Fore D, Förster M, d'Ercole MM, Oxley H, 1998. Income Distribution and Poverty in Selected OECD Countries. 10.1787/730801800603 [DOI]
- Carrico C, Gennings C, Wheeler DC, Factor-Litvak P, 2015. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat 20, 100–120. 10.1007/s13253-014-0180-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathey A, Ferguson KK, McElrath TF, Cantonwine DE, Pace G, Alshawabkeh A, Cordero JF, Meeker JD, 2018. Distribution and predictors of urinary polycyclic aromatic hydrocarbon metabolites in two pregnancy cohort studies. Environ Pollut 232, 556–562. 10.1016/j.envpol.2017.09.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J Health Soc Behav 24, 385–396. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- Czarnota J, Gennings C, Wheeler DC, 2015. Assessment of weighted quantile sum regression for modeling chemical mixtures and cancer risk. Cancer Inform 14, 159–171. 10.4137/CIN.S17295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D, Sathyanarayana S, LeWinn KZ, Karr CJ, Mason A, Szpiro AA, 2022. A permutation test-based approach to strengthening inference on the effects of environmental mixtures: comparison between single index analytic methods. Environ Health Perspect in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta K, Ghosh D, Nazmi A, Kumawat KL, Basu A, 2010. A common carcinogen benzo[a]pyrene causes neuronal death in mouse via microglial activation. PLoS One 5, e9984. 10.1371/journal.pone.0009984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gade M, Comfort N, Re DB, 2021. Sex-specific neurotoxic effects of heavy metal pollutants: Epidemiological, experimental evidence and candidate mechanisms. Environ Res 201. 10.1016/j.envres.2021.111558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genkinger JM, Stigter L, Jedrychowski W, Huang T-J, Wang S, Roen EL, Majewska R, Kieltyka A, Mroz E, Perera FP, 2015. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure, antioxidant levels and behavioral development of children ages 6–9. Environ Res 140, 136–144. 10.1016/j.envres.2015.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Rubenstein J, Popoli R, Capulong R, Till C, 2020. Sex-specific neurotoxic effects of early-life exposure to fluoride: A review of the epidemiologic and animal literature. Curr Epidemiol Rep 7, 263–273. 10.1007/s40471-020-00246-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Senthilkumar K, Alomirah H, Moon H-B, Minh TB, Mohd MA, Nakata H, Kannan K, 2013. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environ Sci Technol 47, 2932–2938. 10.1021/es3052262 [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Tang D, Zhu D, Qu L, Sjödin A, Li Z, Camann D, Perera FP, 2012. Prenatal exposure to polycyclic aromatic hydrocarbons, benzo[a]pyrene-DNA adducts, and genomic DNA methylation in cord blood. Environ Health Perspect 120, 733–8. 10.1289/ehp.1104056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski W, Galas A, Pac A, Flak E, Camman D, Rauh V, Perera F, 2005. Prenatal ambient air exposure to polycyclic aromatic hydrocarbons and the occurrence of respiratory symptoms over the first year of life. Eur J Epidemiol 20, 775–782. 10.1007/S10654-005-1048-1 [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Perera FP, Tang D, Stigter L, Mroz E, Flak E, Spengler J, Budzyn-Mrozek D, Kaim I, Jacek R, 2012. Impact of barbecued meat consumed in pregnancy on birth outcomes accounting for personal prenatal exposure to airborne polycyclic aromatic hydrocarbons: Birth cohort study in Poland. Nutrition 28, 372–377. 10.1016/j.nut.2011.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski WA, Perera FP, Camann D, Spengler J, Butscher M, Mroz E, Majewska R, Flak E, Jacek R, Sowa A, 2015. Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children. Environ Sci Pollut Res Int 22, 3631–3639. 10.1007/s11356-014-3627-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorcano A, Lubczyńska MJ, Pierotti L, Altug H, Ballester F, Cesaroni G, el Marroun H, Fernández-Somoano A, Freire C, Hanke W, Hoek G, Ibarluzea J, Iñiguez C, Jansen PW, Lepeule J, Markevych I, Polańska K, Porta D, Schikowski T, Slama R, Standl M, Tardon A, Vrijkotte TGM, von Berg A, Tiemeier H, Sunyer J, Guxens M, 2019. Prenatal and postnatal exposure to air pollution and emotional and aggressive symptoms in children from 8 European birth cohorts. Environ Int 131, 104927. 10.1016/j.envint.2019.104927 [DOI] [PubMed] [Google Scholar]
- Kannan K, Stathis A, Mazzella MJ, Andra SS, Barr DB, Hecht SS, Merrill LS, Galusha AL, Parsons PJ, 2021. Quality assurance and harmonization for targeted biomonitoring measurements of environmental organic chemicals across the Children’s Health Exposure Analysis Resource laboratory network. Int J Hyg Environ Health 234. 10.1016/J.IJHEH.2021.113741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertxundi A, Andiarena A, Martínez MD, Ayerdi M, Murcia M, Estarlich M, Guxens M, Sunyer J, Julvez J, Ibarluzea J, 2019. Prenatal exposure to PM2.5 and NO2 and sex-dependent infant cognitive and motor development. Environ Res 174, 114–121. 10.1016/j.envres.2019.04.001 [DOI] [PubMed] [Google Scholar]
- Levine L, Fahy J, 1945. Evaluation of urinary lead concentrations. I. The significance of the specific gravity. J Ind Hyg Toxicol 27, 217–223. [Google Scholar]
- LeWinn KZ, Bush NR, Batra A, Tylavsky F, Rehkopf D, 2020. Identification of Modifiable Social and Behavioral Factors Associated With Childhood Cognitive Performance. JAMA Pediatr 174, 1063–1072. 10.1001/jamapediatrics.2020.2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeWinn KZ, Karr CJ, Hazlehurst M, Carroll K, Loftus C, Nguyen R, Barrett E, Swan SH, Szpiro AA, Paquette A, Moore P, Spalt E, Younglove L, Sullivan A, Colburn T, Byington N, Sims Taylor L, Moe S, Wang S, Cordeiro A, Mattias A, Powell J, Johnson T, Norona-Zhou A, Mason A, Bush NR, Sathyanarayana S, 2022. Cohort profile: the ECHO prenatal and early childhood pathways to health consortium (ECHO-PATHWAYS). BMJ Open 12, e064288. 10.1136/BMJOPEN-2022-064288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus CT, Bush NR, Day DB, Ni Y, Tylavsky FA, Karr CJ, Kannan K, Barrett ES, Szpiro AA, Sathyanarayana S, LeWinn KZ, 2021. Exposure to prenatal phthalate mixtures and neurodevelopment in the Conditions Affecting Neurocognitive Development and Learning in Early childhood (CANDLE) study. Environ Int 150, 106409. 10.1016/j.envint.2021.106409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovasi GS, Eldred-Skemp N, Quinn JW, Chang H-W, Rauh VA, Rundle A, Orjuela MA, Perera FP, 2014. Neighborhood Social Context and Individual Polycyclic Aromatic Hydrocarbon Exposures Associated with Child Cognitive Test Scores. J Child Fam Stud 23, 785–799. 10.1007/s10826-013-9731-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallister MM, Maguire M, Ramesh A, Aimin Q, Liu S, Khoshbouei H, Aschner M, Ebner FF, Hood DB, 2008. Prenatal exposure to benzo(a)pyrene impairs later-life cortical neuronal function. Neurotoxicology 29, 846–854. 10.1016/j.neuro.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink PJ, Goodman M, Barraj LM, Imrey H, Kelsh MA, Yager J, 2004. Evaluation of uncontrolled confounding in studies of environmental exposures and neurobehavioral testing in children. Epidemiology 15, 385–393. 10.1097/01.ede.0000128402.86336.7e [DOI] [PubMed] [Google Scholar]
- Mortamais M, Pujol J, van Drooge BL, Macià D, Martínez-Vilavella G, Reynes C, Sabatier R, Rivas I, Grimalt J, Forns J, Alvarez-Pedrerol M, Querol X, Sunyer J, 2017. Effect of exposure to polycyclic aromatic hydrocarbons on basal ganglia and attention-deficit hyperactivity disorder symptoms in primary school children. Environ Int 105, 12–19. 10.1016/j.envint.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Nicol CJ, Harrison ML, Laposa RR, Gimelshtein IL, Wells PG, 1995. A teratologic suppressor role for p53 in benzo(a)pyrene–treated transgenic p53-deficient mice. Nat Genet 10, 181–187. 10.1038/NG0695-181 [DOI] [PubMed] [Google Scholar]
- O’Neill S, Rajendran K, Mahbubani SM, Halperin JM, 2017. Preschool Predictors of ADHD Symptoms and Impairment During Childhood and Adolescence. Curr Psychiatry Rep 19. 10.1007/S11920-017-0853-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyemauwa F, Rappaport SM, Sobus JR, Gajdosová D, Wu R, Waidyanatha S, 2009. Using liquid chromatography-tandem mass spectrometry to quantify monohydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J Chromatogr B Analyt Technol Biomed Life Sci 877, 1117–1125. 10.1016/j.jchromb.2009.02.067 [DOI] [PubMed] [Google Scholar]
- Pagliaccio D, Herbstman JB, Perera F, Tang D, Goldsmith J, Peterson BS, Rauh V, Margolis AE, 2020. Prenatal exposure to polycyclic aromatic hydrocarbons modifies the effects of early life stress on attention and Thought Problems in late childhood. J Child Psychol Psychiatry. 10.1111/jcpp.13189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera F, Herbstman J, 2011. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol 31, 363–373. 10.1016/J.REPROTOX.2010.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, Tu YH, Andrews H, Barr DB, Camann DE, Diaz D, Dietrich J, Reyes A, Kinney PL, 2005. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology 26, 573–587. 10.1016/j.neuro.2004.07.007 [DOI] [PubMed] [Google Scholar]
- Perera FP, Tang D, Wang S, Vishnevetsky J, Zhang B, Diaz D, Camann D, Rauh V, 2012. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6–7 years. Environ Health Perspect 120, 921–926. 10.1289/ehp.1104315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Wang S, Rauh V, Zhou H, Stigter L, Camann D, Jedrychowski W, Mroz E, Majewska R, 2013. Prenatal exposure to air pollution, maternal psychological distress, and child behavior. Pediatrics 132, e1284–94. 10.1542/peds.2012-3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Wang S, Vishnevetsky J, Zhang B, Cole KJ, Tang D, Rauh V, Phillips DH, 2011. Polycyclic aromatic hydrocarbons-aromatic DNA adducts in cord blood and behavior scores in New York city children. Environ Health Perspect 119, 1176–1181. 10.1289/ehp.1002705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Wheelock K, Wang Y, Tang D, Margolis AE, Badia G, Cowell W, Miller RL, Rauh V, Wang S, Herbstman JB, 2018. Combined effects of prenatal exposure to polycyclic aromatic hydrocarbons and material hardship on child ADHD behavior problems. Environ Res 160, 506–513. 10.1016/j.envres.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D, 2011. Item banks for measuring emotional distress from the patient-reported outcomes measurement information system (PROMIS®): Depression, anxiety, and anger. Assessment 18, 263–283. 10.1177/1073191111411667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Position of the American Dietetic Association: Promoting and Supporting Breastfeeding, 2009. . J Am Diet Assoc 109, 1926–1942. 10.1016/J.JADA.2009.09.018 [DOI] [PubMed] [Google Scholar]
- Quigley MA, Hockley C, Carson C, Kelly Y, Renfrew MJ, Sacker A, 2012. Breastfeeding is associated with improved child cognitive development: a population-based cohort study. J Pediatr 160, 25–32. 10.1016/j.jpeds.2011.06.035 [DOI] [PubMed] [Google Scholar]
- Renzetti S, Curtin P, Just AC, Bello G, Gennings C, 2020. gWQS: Generalized Weighted Quantile Sum Regression.
- RStudio Team, 2020. RStudio: Integrated Development Environment for R.
- Schick SF, Blount BC, Jacob PR, Saliba NA, Bernert JT, el Hellani A, Jatlow P, Pappas RS, Wang L, Foulds J, Ghosh A, Hecht SS, Gomez JC, Martin JR, Mesaros C, Srivastava S, St Helen G, Tarran R, Lorkiewicz PK, Blair IA, Kimmel HL, Doerschuk CM, Benowitz NL, Bhatnagar A, 2017. Biomarkers of exposure to new and emerging tobacco delivery products. Am J Physiol Lung Cell Mol Physiol 313, L425–L452. 10.1152/ajplung.00343.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejer EPF, Bruun FJ, Slavensky JA, Mortensen EL, Schiøler Kesmodel U, 2019. Impact of gestational age on child intelligence, attention and executive function at age 5: A cohort study. BMJ Open 9. 10.1136/bmjopen-2019-028982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senter CC, Bush NR, Loftus CT, Szpiro AA, Fitzpatrick AL, Carroll KN, Lewinn KZ, Alex Mason W, Sathyanarayana S, Akingbade OA, Karr CJ, 2021. Maternal stressful life events during pregnancy and atopic dermatitis in children aged approximately 4–6 years. Int J Environ Res Public Health 18. 10.3390/ijerph18189696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucker MR, Craighead WE, Craighead LW, Green BJ, 1986. Normative and reliability data for the children’s depression inventory. J Abnorm Child Psychol 14, 25–39. 10.1007/BF00917219 [DOI] [PubMed] [Google Scholar]
- Soleimani F, Zaheri F, Abdi F, 2014. Long-term neurodevelopmental outcomes after preterm birth. Iran Red Crescent Med J 16, e17965–e17965. 10.5812/ircmj.17965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag-Padilla L, Burns RM, Shih RA, Griffin BA, Martin LT, Chandra A, Tylavsky F, 2015. The Urban Child Institute CANDLE Study: Methodological Overview and Baseline Sample Description. RAND Corporation, Santa Monica, CA. 10.7249/RR1336 [DOI] [Google Scholar]
- Wallace ER, Ni Y, Loftus CT, Sullivan A, Masterson E, Szpiro AA, Day DB, Robinson M, Kannan K, Tylavsky FA, Sathyanarayana S, Bush NR, LeWinn KZ, Karr CJ, 2021. Prenatal urinary metabolites of polycyclic aromatic hydrocarbons and toddler cognition, language, and behavior. Environ Int 159, 107039. 10.1016/j.envint.2021.107039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Jin L, Ren A, Yuan Y, Liu J, Li Z, Zhang L, Yi D, Wang LL, Zhang Y, Wang X, Tao S, Finnell RH, 2015. Levels of polycyclic aromatic hydrocarbons in maternal serum and risk of neural tube defects in offspring. Environ Sci Technol 49, 588–596. 10.1021/ES503990V [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM, 2011. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect 119, 878–885. 10.1289/EHP.1002727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormley DD, Ramesh A, Hood DB, 2004. Environmental contaminant-mixture effects on CNS development, plasticity, and behavior. Toxicol Appl Pharmacol 197, 49–65. 10.1016/j.taap.2004.01.016 [DOI] [PubMed] [Google Scholar]
- Yu Y, Wang Xilong, Wang B, Tao S, Liu W, Wang Xuejun, Cao J, Li B, Lu X, Wong MH, 2011. Polycyclic aromatic hydrocarbon residues in human milk, placenta, and umbilical cord blood in Beijing, China. Environ Sci Technol 45, 10235–10242. 10.1021/es202827g [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


