Abstract
Simple Summary
c-KIT has been regarded as a promising therapeutic target against gastrointestinal stromal tumor (GIST). Overcoming drug resistance of c-KIT inhibitors including imatinib is required. We designed and synthesized novel thiazolo[5,4-b]pyridine derivatives and performed structure-activity relationship (SAR) studies to overcome imatinib resistance. The SAR studies led to the identification of the derivative 6r as a potent c-KIT inhibitor. The derivative 6r is capable of strongly inhibiting a c-KIT V560G/D816V double mutant that is resistant to imatinib and remarkably attenuates proliferation of GIST-T1 and HMC1.2 cancer cells. Moreover, 6r possesses differential cytotoxicity on c-KIT D816V Ba/F3 cells relative to parental Ba/F3 cells. Kinase panel profiling revealed that 6r has reasonable kinase selectivity. Furthermore, 6r not only blocks migration and invasion, but also suppresses anchorage-independent growth of GIST-T1 cells.
Abstract
c-KIT is a promising therapeutic target against gastrointestinal stromal tumor (GIST). In order to identify novel c-KIT inhibitors capable of overcoming imatinib resistance, we synthesized 31 novel thiazolo[5,4-b]pyridine derivatives and performed SAR studies. We observed that, among these substances, 6r is capable of inhibiting significantly c-KIT and suppressing substantially proliferation of GIST-T1 cancer cells. It is of note that 6r is potent against a c-KIT V560G/D816V double mutant resistant to imatinib. Compared with sunitinib, 6r possesses higher differential cytotoxicity on c-KIT D816V Ba/F3 cells relative to parental Ba/F3 cells. In addition, kinase panel profiling reveals that 6r has reasonable kinase selectivity. It was found that 6r remarkably attenuates proliferation of cancer cells via blockade of c-KIT downstream signaling, and induction of apoptosis and cell cycle arrest. Furthermore, 6r notably suppresses migration and invasion, as well as anchorage-independent growth of GIST-T1 cells. This study provides useful SAR information for the design of novel c-KIT inhibitors overcoming imatinib-resistance.
Keywords: c-KIT; GIST; GIST-T1; HMC1.2; imatinib resistance; thiazolo[5,4-b]pyridine
1. Introduction
c-KIT, a class III receptor tyrosine kinase, consists of five immunoglobulin-like domains, one transmembrane domain, one juxta-membrane domain, and two split kinase domains [1]. Binding of a stem cell factor (SCF) ligand to c-KIT induces the c-KIT dimerization, leading to subsequent activation of its intracellular signaling cascades [2]. Dysfunctions of c-KIT, such as gain of function caused by overexpression and point mutation, result in c-KIT activation and tumorigenesis [3,4]. Activation of c-KIT is observed in various cancers including GIST, mast cell tumors, and malignant melanomas [4,5,6]. In particular, c-KIT activation is deeply associated with GISTs which are the most common gastrointestinal tumor [7,8]. More than 90% of 419 GIST cases are associated with c-KIT activation and 6−7% of the cases are implicated with PDGFRα mutations [9].
Mutations within exon 11 encoding the juxta-membrane domain of c-KIT, with a relative frequency of 67%, are most common in GIST [10]. The mutations in exon 9 (IgG-like D5 domain of c-KIT) of which the majority are duplications of A502_Y503 occur at nearly 15% in GIST [11,12]. Notably, secondary c-KIT mutations causing imatinib resistance have been known to occur most commonly in exon 13/14 (ATP-binding domain of c-KIT) and exon 17/18 (activation loop of c-KIT) [13,14,15]. The c-KIT T670I gatekeeper mutant within exon 13 corresponds to the BCR-ABL T315I gatekeeper mutant and causes drug resistance through constitutive activation and steric hindrance [16,17]. D816H/V mutants occurring in the activation loop of c-KIT are capable of accelerating auto-activation rather than wild-type c-KIT, which leads to resistant to imatinib [18]. Moreover, the double mutant of c-KIT V560G/D816V has disruption of auto-inhibitory mechanism and SCF-independent constitutive activation of c-KIT [19].
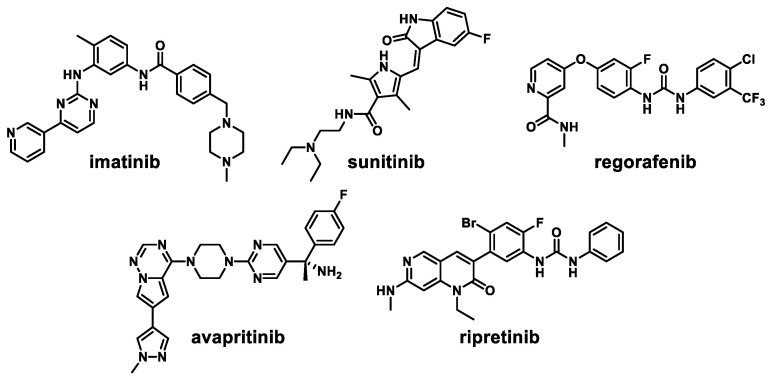
Imatinib, an innovative BCR-ABL kinase inhibitor against chronic myelogenous leukemia, has been approved as a first-line treatment for advanced GIST (Figure 1) [20]. However, 10–20% of patients taking imatinib show secondary mutations that decrease c-KIT sensitivity to imatinib [21]. Sunitinib, a second-line therapy for the treatment of GIST, possesses inhibitory activity against c-KIT V654A and gatekeeper mutant c-KIT T670I [13]. However, preclinical studies have revealed that both imatinib and sunitinib do not effectively inhibit exon 17 KIT mutations (activation loop mutants) [14]. Regorafenib was approved for the third-line treatment against imatinib-and sunitinib-resistant GISTs but it has moderate activities against the secondary mutations [22,23]. The potent c-KIT/PDGFRα inhibitor avapritinib has been approved for the GIST treatment [24]. However, secondary PDGFRα mutations (Val658Ala, Asn659Lys, Tyr676Cys, and Gly680Arg) have been observed in patients with drug resistant recurrent GIST [25]. Ripretinib, the fourth-line treatment approved for GIST, interacts reversibly to both the switch pocket and activation loop of c-KIT [26]. Although ripretinib is capable of inhibiting a wide range of c-KIT and PDGFRα mutants, it shows an overall response rate of 9.4%, suggesting additional resistance and disease progression [27].
Figure 1.
Representative c-KIT inhibitors.
In addition to clinically approved drugs, various scaffolds have been used as c-KIT inhibitors. A potent dual c-KIT/PDGFRα 1H-pyrazolo[3,4-b]pyridine inhibitor for the treatment of imatinib-resistant GISTs has been reported [28], but its anti-proliferative activity against TEL-c-KIT D816V Ba/F3 cells was not significant (GI50 > 10 μM). The derivatives having the 2-aminothiazole scaffold switch off activated c-KIT to its inactivated state, and possess activities against constitutively activated mutants in the activation loop [29]. Moreover, the thiazolo[5,4-b]pyridine scaffold has been used to discover various kinase inhibitors against PI3K [30], ITK [31], BCR-ABL [32], RAF [33], and VEGFR2 [34]. Thiazolo[5,4-b]pyridine derivatives have distinct types of binding modes depending on target kinases. The 4-nitrogen of thiazolo[5,4-b]pyridine is hinge-binding motif of PI3K kinase inhibitors while the 1-nitrogen and 2-amino group of the scaffold form H-bonding contact with the ITK kinase hinge region. In addition, the 5-position of thiazolo[5,4-b]pyridine has been functionalized to target the ATP-binding site of BCR-ABL, RAF, and VEGFR2. It is worthwhile to note that this study has, for the first time, reports functionalization on the 6-position of thiazolo[5,4-b]pyridine scaffold to identify novel c-KIT inhibitors.
In an effort to overcome drug resistance, we synthesized 31 novel thiazolo[5,4-b]pyridine derivatives and carried out a structure-activity relationship study against c-KIT enzyme and c-KIT-activated cells. The results of the SAR study for thiazolo[5,4-b]pyridine derivatives revealed that 6r possesses higher enzymatic and anti-proliferative activities than imatinib and comparable activities relative to sunitinib. Notably, 6r has 8.0-fold higher enzymatic inhibitory activity (IC50 = 4.77 μM) against c-KIT V560G/D816V double mutant and 23.6-fold higher anti-proliferative activity (GI50 = 1.15 μM) on HMC1.2 cells harboring both V560G and D816V c-KIT mutations compared with imatinib. Moreover, 6r exhibits a reasonable selectivity in the biochemical kinase panel profiling. Furthermore, 6r suppresses proliferation of cancer cells (GIST-T1 and HMC1.2) and c-KIT D816V Ba/F3 cells via blockade of c-KIT downstream signaling, and induction of apoptosis and cell cycle arrest. Notably, 6r remarkably blocks migration and invasion, as well as anchorage-independent growth of GIST-T1 cells.
2. Materials and Methods
2.1. Chemistry
All commercial reagents and solvents were purchased from chemical suppliers and directly used for synthesis without purification. Reaction monitoring was performed by thin-layer chromatography (TLC) analysis using a UV lamp, ninhydrin, or p-anisaldehyde stain for the compound detection. Reaction products were purified by column chromatography on silica gel (230−400 mesh). Purities of all compounds were analyzed by Waters LC/MS system and shown to be over 95%. 1H and 13C NMR spectra were recorded either by a Bruker 300 MHz FT-NMR (300 MHz for 1H, and 75 MHz for 13C) or a 400 MHz FT-NMR (400 MHz for 1H, and 100 MHz for 13C) spectrometer. The synthetic procedures and chemical characterizations of all compounds are described in File S1.
2.2. Cell Culture and Reagent
GIST-T1 (Cosmobio, Tokyo, Japan, # PMC-GIST01C-COS) cells were cultured in DMEM (Welgene, # LM001-05) and HMC1.2 (Merck, Darmstadt, Germany, # SCC062) cells were cultured in IMDM (Welgene, # LM004-01). Parental Ba/F3 (DSMZ, Braunschweig, Germany, # ACC300) cells were cultured in RPMI (Welgene, # LM011-51) with 10 ng IL-3/mL (Enzo, # ALX-201-821). The culture media was supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin solution. The cells were maintained in a humidified atmosphere containing 5% CO2 at 37 °C.
2.3. In Vitro Kinase Assay
Biochemical kinase assays on c-KIT protein kinase were performed at Reaction Biology Corp. (San Diego, CA, USA). Compounds were tested with 10 μM ATP in a 10-dose IC50 mode with 3-fold serial dilution. The bioluminescent-based kinase assay was tested using an ADP-Glo assay kit. c-KIT (V560G/D816V) kinase was purchased from Promega (# VA7063). Compounds were tested with 10 μM ATP in a 10-dose IC50 mode with 3-fold serial dilution. The kinase assay followed the manufacturer’s instruction. IC50 values were calculated by GraphPad prism 8.0 software (GraphPad Software, San Diego, CA, USA).
2.4. Cell Viability Assay
Cells (GIST-T1: 5.0 × 103; HMC1.2: 1 × 104) were seeded in 96-well plates. Each compound was treated in each well at 10 dose points of 3-fold serial dilution. After treatment with each compound for 72 h, CellTiter-Glo solution (Promega, # G7572) was added to each well. Anti-proliferation activity was measured the luminescence using a 96-well plate reader (EnVision 2013).
2.5. Molecular Docking Study
The X-ray co-crystal structure of c-KIT complexed with imatinib (PDB code: 1T46) was retrieved from the Protein Data Bank and loaded into Maestro software (Schrödinger Release 2020-4, New York, NY, USA). The Protein Preparation Wizard was executed for replenishment of missing residue, addition of hydrogens, and assignment of bond orders. Restrained energy minimization was applicated in the OPLS3e force field. Ligand 6r was prepared using the LigPrep and the receptor c-KIT grid was generated considering the imatinib binding pocket. Ligand docking of 6r on c-KIT was performed using GLIDE.
2.6. Kinase Panel Profiling
Kinase panel profiling was conducted by Reaction Biology Corp. (San Diego, CA, USA). A single dose (1.0 μM) of 6r was tested against 371 recombinant human kinases in the presence of 10 μM ATP.
2.7. Western Blot Analysis
Cells were harvested and lysed using RIPA buffer containing 50 mM HEPES (pH 7.4), 1% Triton X-100, 2 mM EDTA, 150 mM NaCl, 2.5 mM NaF, 5 mM Na3VO4, and protease inhibitor cocktail tablet (Roche, # 11–878-580–001). Proteins were separated and transferred to an NC membrane. The membranes were blocked using 5% skim milk in TBS-T buffer. The p-c-KIT (Tyr703, # 3073), p-AKT (Ser473, # 9271), p-ERK (Thr202/Tyr204, # 8544), ERK (# 4695), p-PLCγ (Tyr783, # 14008), p-STAT3 (Tyr705, # 4074), and PARP (# 9542) antibodies were obtained from Cell Signaling Technologies. The β-actin (# sc-47778) and STAT3 (# sc-482) antibodies were obtained from Santa Cruz Biotechnology. AKT (A18120) antibody was purchased from ABclonal. Each primary antibody was incubated overnight at 4 °C. The secondary antibody (GenDEPOT) was incubated for 1 h at room temperature. Proteins were detected using ECL substrate.
2.8. Cell Cycle Arrest and Apoptosis Analysis
After compound treatment in GIST-T1 and HMC1.2 cells for 24 h, cells were fixed with 70% cold ethanol. Then, cells were incubated with 50 μg propidium iodide/mL solution (PI, Sigma) containing 1 mg RNase A/mL for 30 min in the dark. Cell cycle arrest was analyzed (BD Accuri), and data were processed using BD AccuriTM C6 software. For apoptosis analysis, compound treatment for 24–48 h, cells were harvested and stained with annexinV and PI solution. 1 × 105 cells were analyzed using flow cytometric analysis.
2.9. Migration Assay
GIST-T1 cells were seeded in 6-well plates at 2 × 106 cells. Next day, cells were washed with PBS and wounded in the center of each well. Then, GIST-T1 cells were washed with PBS and added to fresh DMEM. After 36 h, the scratch recovery of GIST-T1 cells in each compound-treated groups were observed and taken with a Nikon microscope at a magnification of 1000×.
2.10. Invasion Assay
The cell invasion assay was performed using Transwell membrane filter inserts (Corning Costar Corp., Corning, NY, USA). GIST-T1 cells were then suspended in serum-free DMEM at 2 × 106 cells and seeded in the upper chambers. In the lower chambers, the DMEM medium containing 10% FBS was added. After 48 h, the cells migrating down the membrane were stained with crystal violet.
2.11. Soft Agar Assay
Anchorage-independent growth was assessed by determining colony formation on soft agar. GIST-T1 cells in DMEM media containing 0.7% agar were plated at 5,000 cells per well. Cells were treated with each compound for 3 weeks, and the media were changed every 3 days. Colonies were stained using iodonitrotetrazolium chloride (Sigma Aldrich). The average number colonies were counted using ImageJ software (version 1.53e, National Institutes of Health, Bethesda, MD, USA)
2.12. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8.0. Graphpad software, San Diego, CA, USA. All data are reported as average ± standard deviation (S.D.). p < 0.05 was considered to be statistically significant.
3. Results and Discussion
3.1. Synthesis of Thiazolo[5,4-b]pyridine Derivatives
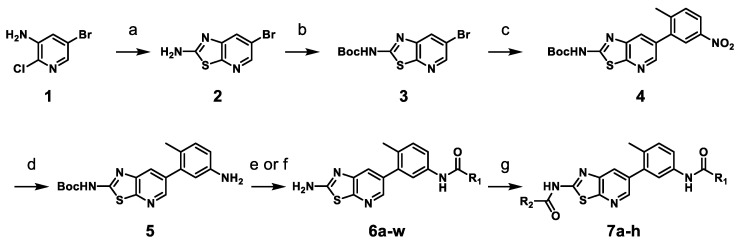
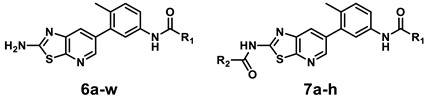
The synthetic route for the thiazolo[5,4-b]pyridine derivatives is described in Scheme 1. To synthesize thiazolo[5,4-b]pyridine scaffold, we carried out the aminothiazole formation using commercially available 3-amino-5-bromo-2-chloropyridine 1 and potassium thiocyanate in 75% yield. The amino group of 2 underwent Boc protection to afford compound 3 in 90% yield. Then, 3 was coupled with 2-methyl-5-nitrophenylboronic acid pinacol ester by Suzuki cross-coupling reaction using Pd(dppf)Cl2 as catalyst to form 4 in 70% yield. Reduction of the nitro group of 4 afforded the key intermediate 5 in 80% yield. The amide formation of aniline 5 with various carboxylic acids, followed by subsequent Boc deprotection yielded 6a–i and 6k–w. Urea formation between aniline 5 and 3-(trifluoromethyl)phenyl isocyanate was followed by subsequent Boc deprotection gave 6j. The primary amino group of selected derivatives was transformed into the corresponding amides 7a–h in 21–38% yields.
Scheme 1.
Synthesis of thiazolo[5,4-b]pyridine derivatives 6a–w and 7a–ha. a Reagent and condition: (a) potassium thiocyanate, HCl, 100 °C, 12 h, 75%; (b) di-tert-butyl dicarbonate, DMAP, THF, rt, 90%; (c) 2-methyl-5-nitrophenylboronic acid pinacol ester, Pd(dppf)Cl2, Na2CO3, DME/H2O, 100 °C, 3 h, 70%; (d) iron powder, NH4Cl, THF/MeOH/H2O, 70 °C, 1 h, 80%; (e) (i) R1-COOH, HATU, DIPEA, DMF, rt, 6 h; (ii) TFA, CH2Cl2, rt, 6 h, 21–65% (over 2 steps); (f) (i) 3-(trifluoromethyl)phenyl isocyanate, TEA, THF, rt; (ii) TFA, CH2Cl2, rt, 6 h, 53% (over 2 steps); (g) R2-COCl, pyridine, CH2Cl2, rt, 3 h, 21–38%.
3.2. Structure-Activity Relationships
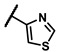
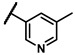
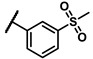
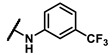
Enzymatic inhibitory activities of all synthesized thiazolo[5,4-b]pyridine derivatives against c-KIT were measured using radiometric biochemical kinase assays (Table 1). We first explored the R1 group of the thiazolo[5,4-b]pyridine scaffold. The results show that only a 3-(trifluoromethyl)phenyl group (6h) resulted in a moderate enzymatic inhibitory activity (IC50 = 9.87 μM) among 6a–j possessing five-membered and six-membered aromatic rings. Molecular docking study revealed that the 3-trifluoromethyl group fits well into the hydrophobic binding pocket [35]. Insertion of methylene group (6i) between the amide and 3-(trifluoromethyl)phenyl group of 6h, and replacement of the amide with urea linkage (6j), as influenced by regorafenib and ripretinib, caused loss of enzymatic inhibitory activities.
Table 1.
Enzymatic inhibitory activities of thiazolo[5,4-b]pyridine derivatives 6a–w and 7a–h against c-KIT.
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | R1 | R2 | IC50 (μM) a | Entry | R1 | R2 | IC50 (μM) a |
| imatinib | - | - | 0.27 | 6p |
|
- | 5.72 |
| sunitinib | - | - | 0.14 | 6q |
|
- | 3.23 |
| 6 a |
|
- | inactiveb | 6r |
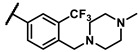
|
- | 0.14 |
| 6b |
|
- | inactiveb | 6s |
|
- | 0.37 |
| 6c |
|
- | inactiveb | 6t |
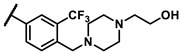
|
- | 1.25 |
| 6d |
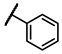
|
- | inactiveb | 6u |
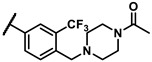
|
- | 4.56 |
| 6e |
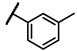
|
- | inactiveb | 6v |
|
- | 0.39 |
| 6f |
|
- | inactiveb | 6w |
|
- | 0.25 |
| 6g |
|
- | inactiveb | 7a |
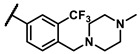
|
cyclohexyl | 1.51 |
| 6h |
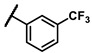
|
- | 9.87 | 7b |
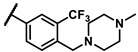
|
phenyl | 0.74 |
| 6i |
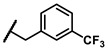
|
- | inactiveb | 7c |
|
methyl | 0.10 |
| 6j |
|
- | inactiveb | 7d |
|
cyclohexyl | inactiveb |
| 6k |
|
- | 4.31 | 7e |
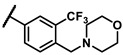
|
methyl | 0.88 |
| 6l |
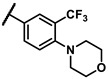
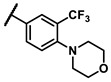
|
- | 1.76 | 7f |
|
methyl | 3.63 |
| 6m |
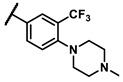
|
- | 2.17 | 7g |
|
methyl | 0.18 |
| 6n |
|
- | Inactive b | 7h |
|
methyl | 0.10 |
| 6o |
|
- | 5.03 | ||||
a Radiometric biochemical kinase assay results. b less than 50% inhibition at a concentration of 10 μM.
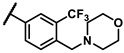
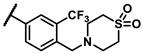
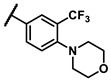
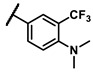
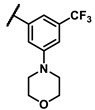
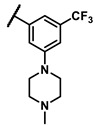
To explore the effects of an additional substituent [36] on the 3-(trifluoromethyl)phenyl group of 6h, we synthesized 6k–o and evaluated enzymatic inhibitory activities. The results reveal that addition of 4-dimethylamino (6k), 4-morpholino (6l), and 4-methylpiperazino (6m) moiety on the para-position of the 3-(trifluoromethyl)phenyl group resulted in 2.3- to 5.6-fold enhanced activities (6k, IC50 = 4.31 μM; 6l, IC50 = 1.76 μM; 6m, IC50 = 2.17 μM) compared with 6h. In contrast, 3-morpholino (6n) and 4-methylpiperazino (6o) on the meta-position of 3-(trifluoromethyl)phenyl group brought about lower enzymatic inhibitory activities (6n, IC50 = inactive; 6o, IC50 = 5.03 μM) relative to 6l and 6m. Therefore, para-substitution led to higher activities than meta-substitution.
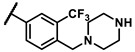
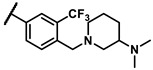
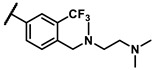
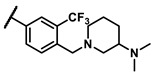
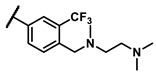
Further investigation of para-substituents of the 3-(trifluoromethyl)phenyl group revealed that 4-morpholinomethyl group (6p) and (1,1-dioxidothiomorpholino)methyl group (6q) caused lower activities relative to 4-morpholino group (6l). Gratifyingly, 6r having 4-((4-methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl group possessed 15.5-fold enhanced activity (IC50 = 0.14 μM) compared to 6m (IC50 = 2.17 μM). The enzymatic inhibitory activity of 6r was comparable to that of sunitinib (IC50 = 0.14 μM) and 1.9-fold higher than that of imatinib (IC50 = 0.27 μM). Encouraged by the promising enzymatic inhibitory activity of 6r, further exploration of the 4-methylpiperazine group on 6r was conducted. Removal of the 4-methyl group (6s) resulted in similar enzymatic inhibitory activity (IC50 = 0.37 μM) but substitution of the 4-methyl group with 2-hydroxyethyl group (6t) and acetyl group (6u) led to decreased activities. The derivatives with 4-(3-(dimethylamino)piperidin-1-yl)methyl group (6v) and 4-((2-(dimethylamino)ethyl)(methyl)amino)methyl group (6w) were less active than 6r. On the basis of these results, the tail group of 6r was selected and the R2 group was further optimized.
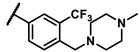
Next, we focused on SAR study for the R2 group. The effects of R2 groups were explored by replacing the primary amino group on 6r with the amide groups (7a–c). The results show that derivatives possessing cyclohexyl amide (7a, IC50 = 1.51 μM) and benzamide (7b, IC50 = 0.74 μM) had 5.3- to 10.8-fold decreased activities relative to 6r (IC50 = 0.14 μM). On the other hand, the activity of 7c having the acetamide group (IC50 = 0.10 μM) was slightly higher than that of 6r. Similarly, among the derivatives 6l, 7d, and 7e, 7e possessing the acetamide group had slightly improved activities (6l, IC50 = 1.76 μM; 7d, IC50 = inactive; 7e, IC50 = 0.88 μM). The additional derivatives having the acetamide group (7f–h) were more active compared with the derivatives possessing the 2-amino group (6p, 6v, and 6w).
We assessed anti-proliferative activities of thiazolo[5,4-b]pyridine derivatives on c-KIT dependent cancer cells such as GIST-T1 and HMC1.2 (Table 2). The GIST-T1 was strongly positive for c-KIT and CD34, indicating that GIST-T1 cells had the characteristics of GIST [37]. The HMC1.2 cells bearing both c-KIT V560G in intracellular juxta-membrane region and c-KIT D816V in the kinase catalytic region caused SCF-independent constitutive activation of c-KIT [38,39]. The anti-proliferative activities of 6r, 6s, 7c, and 7h on GIST-T1 cancer cells were comparable or slightly higher relative to that of imatinib (GI50 = 0.02 μM). On HMC1.2 cells, the activities of 6k, 6o, 6r, 6s, and 7c were higher than that of sunitinib (GI50 = 2.53 μM). It is worth noting that 6r possessed 23.6-fold higher anti-proliferative activity (GI50 = 1.15 μM) on HMC1.2 cells than imatinib (GI50 = 27.10 μM). The SAR results reveal that 6r, 6s, and 7c were able to strongly suppress the proliferation of GIST-T1 and HMC1.2 cancer cells.
Table 2.
Anti-proliferative activities of thiazolo[5,4-b]pyridine derivatives 6a–w and 7a–h against GIST-T1 and HMC1.2 cells.
| Entry | GI50 (μM) a | Entry | GI50 (μM) a | ||
|---|---|---|---|---|---|
| GIST-T1 | HMC1.2 | GIST-T1 | HMC1.2 | ||
| imatinib | 0.02 ± 0.01 | 27.10 ± 3.36 | 6p | 0.66 ± 0.10 | 8.55 ± 2.40 |
| sunitinib | 0.01 ± 0.00 | 2.53 ± 0.35 | 6q | 0.42 ± 0.07 | 15.53 ± 3.97 |
| 6 a | > 50 | Inactive b | 6r | 0.01 ± 0.00 | 1.15 ± 0.96 |
| 6b | 33.05 ± 7.09 | Inactive b | 6s | 0.02 ± 0.00 | 1.33 ± 0.43 |
| 6c | 33.69 ± 3.45 | Inactive b | 6t | 0.12 ± 0.03 | 6.62 ± 0.75 |
| 6d | 16.36 ± 2.73 | 25.18 ± 2.21 | 6u | 0.66 ± 0.08 | 11.31 ± 0.34 |
| 6e | 3.45 ± 0.41 | 11.53 ± 0.62 | 6v | 0.05 ± 0.01 | 6.81 ± 0.67 |
| 6f | 20.94 ± 2.00 | inactive b | 6w | 0.02 ± 0.01 | 4.99 ± 0.43 |
| 6g | 10.84 ± 1.06 | 36.77 ± 10.9 | 7a | 0.08 ± 0.01 | 4.58 ± 0.32 |
| 6h | 0.96 ± 0.10 | 7.26 ± 4.13 | 7b | 0.10 ± 0.01 | 5.22 ± 0.59 |
| 6i | 7.16 ± 0.87 | 9.65 ± 1.06 | 7c | 0.01 ± 0.00 | 1.52 ± 0.43 |
| 6j | 3.31 ± 0.73 | 6.35 ± 0.32 | 7d | 8.13 ± 1.81 | inactive b |
| 6k | 0.18 ± 0.03 | 2.27 ± 0.84 | 7e | 0.23 ± 0.07 | 14.04 ± 10.2 |
| 6l | 0.08 ± 0.01 | 4.34 ± 1.31 | 7f | 0.30 ± 0.06 | 28.39 ± 13.3 |
| 6m | 0.27 ± 0.17 | 3.41 ± 1.49 | 7g | 0.07 ± 0.01 | 4.88 ± 1.18 |
| 6n | 0.55 ± 0.09 | 7.00 ± 2.68 | 7h | 0.02 ± 0.00 | 4.98 ± 0.32 |
| 6o | 0.28 ± 0.08 | 2.36 ± 0.58 | |||
a GI50 means the concentration at which a compound causes half-maximal growth inhibition. b less than 50% inhibition at a concentration of 50 μM.
Next, we evaluated enzymatic inhibitory activities of 6r, 6s, and 7c against the c-KIT V560G/D816V double mutant (Table 3) [19]. These derivatives displayed 4.9- to 8.0-fold enhanced potency compared to imatinib (IC50 = 37.93 μM) and possessed comparable activities to sunitinib (IC50 = 3.98 μM) against the c-KIT V560G/D816V double mutant. Moreover, anti-proliferative activities of the 6r, 6s, and 7c on c-KIT D816V Ba/F3 cells were evaluated (Table 4). On c-KIT D816V Ba/F3 cells, 6r (GI50 = 1.11 μM), 6s (GI50 = 1.06 μM), and 7c (GI50 = 0.53 μM) had comparable activities to sunitinib (GI50 = 0.58 μM). It is worthwhile to note that 6r, 6s, and 7c possessed higher differential cytotoxicity than sunitinib on c-KIT D816V Ba/F3 cells relative to parental Ba/F3 cells, which implies that 6r, 6s, and 7c would be more selective c-KIT inhibitors relative to sunitinib. These efforts led to the identification of novel thiazolo[5,4-b]pyridine derivatives that possessed enhanced anti-proliferative activities on c-KIT D816V Ba/F3 cells.
Table 3.
Enzymatic inhibitory activities of thiazolo[5,4-b]pyridine derivatives 6r, 6s, and 7c against c-KIT V560G/D816V.
| Entry | IC50 (μM) a |
|---|---|
| c-KIT V560G/D816V | |
| imatinib | 37.93 ± 8.68 |
| sunitinib | 3.98 ± 1.18 |
| 6r | 4.77 ± 0.38 |
| 6s | 7.67 ± 2.64 |
| 7c | 5.07 ± 0.71 |
a ADP-Glo kinase assay.
Table 4.
Anti-proliferative activities of thiazolo[5,4-b]pyridine derivatives 6r, 6s, and 7c against c-KIT D816V Ba/F3 cells and parental Ba/F3 cells.
| Entry | Ba/F3 cells GI50 (μM) a | |
|---|---|---|
| c-KIT D816V | Parental | |
| imatinib | 12.25 ± 2.06 | 17.00 ± 0.20 |
| sunitinib | 0.58 ± 0.29 | 2.72 ± 0.30 |
| 6r | 1.11 ± 0.47 | 8.06 ± 0.57 |
| 6s | 1.06 ± 0.15 | 10.54 ± 3.59 |
| 7c | 0.53 ± 0.06 | 10.52 ± 3.44 |
a GI50 means the concentration at which a compound causes half-maximal growth inhibition.
3.3. Molecular Docking Studies of 6j, 6r, and 7c with c-KIT
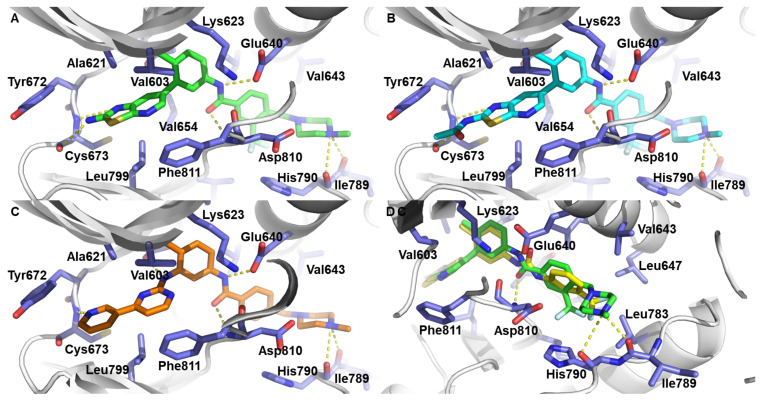
To investigate the binding mode of thiazolo[5,4-b]pyridine derivatives on c-KIT, we performed molecular docking studies of 6j, 6r and 7c using the X-ray co-crystal structure of c-KIT complexed with imatinib (Figure 2). For comparison, the binding mode of imatinib on c-KIT is presented in Figure 2C (PDB code: 1T46) [40]. The results shows that 6r and 7c form a pair of hydrogen bonding with a Cys673 backbone in the hinge region. In addition, 6r and 7c also makes hydrogen bonding with Glu640/Asp810 and Ile789/His790 backbone, and these hydrogen bondings are also observed in the co-crystal structure of imatinib and c-KIT. Moreover, the thiazolo[5,4-b]pyridine moiety of 6r and 7c participates in hydrophobic interactions with Leu799, Val603, Ala621, and Val654. Furthermore, the 3-trifluoromethyl group of 6r and 7c fits well into the hydrophobic binding pocket which is also occupied by ponatinib [35]. However, 6j could not form a hydrogen bonding with Asp810 observed in imatinib and could not fit into the hydrophobic binding pocket occupied by ponatinib. The predicted binding model suggests that 6r and 7c possess inhibitory activity, and 6j is inactive against c-KIT.
Figure 2.
(A,B) Docking model prediction of 6r and 7c on c-KIT (PDB code: 1T46). (C) Binding mode of imatinib on c-KIT (PDB code: 1T46). (D) Superimposition with docking model of 6r and 6j. Protein residues of c-KIT are represented by white cartoon and violet stick. The 6j, 6r, 7c, and imatinib are depicted in yellow, green, cyan, and orange, respectively.
3.4. Kinase Panel Profiling of 6r
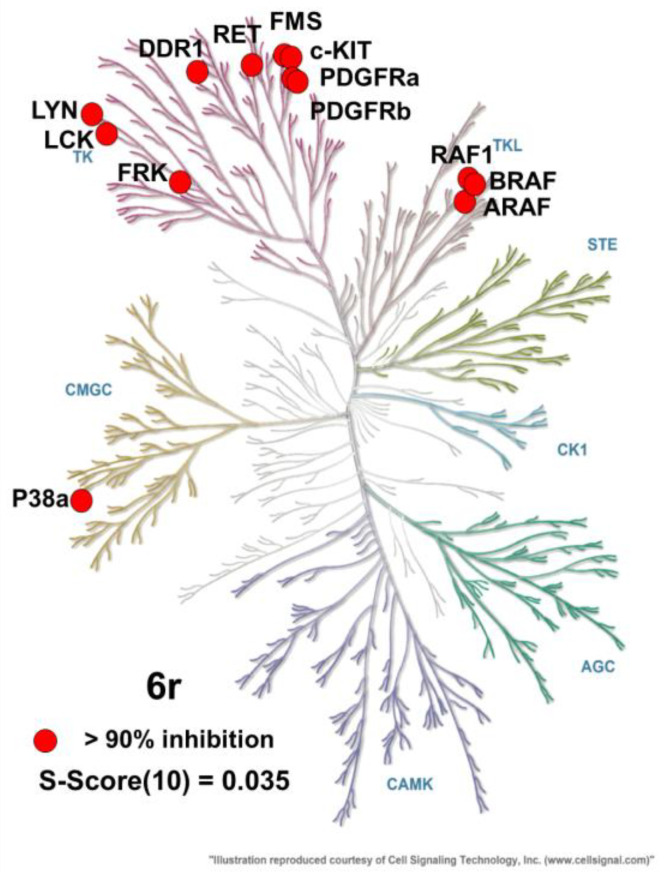
Kinase panel profiling of 6r showed that 13 of 371 kinases (c-KIT, RAF1, DDR1, RET, FMS, FRK, ARAF, LYN, BRAF, LCK, PDGFRa, P38a, PDGFRb) were inhibited more than 90% by 6r (1 μM). The results reveal that 6r possesses reasonable kinase selectivity (Figure 3 and Table S1).
Figure 3.
Kinase panel profiling of 6r. Kinases that are more than 90% inhibited are represented by red circles. Illustration is provided by Cell Signaling Technology, Inc. (www.cellsignal.com, accessed on 3 November 2022) [41].
3.5. Inhibition of c-KIT Signaling in GIST-T1, HMC1.2, and c-KIT D816V Ba/F3 Cells
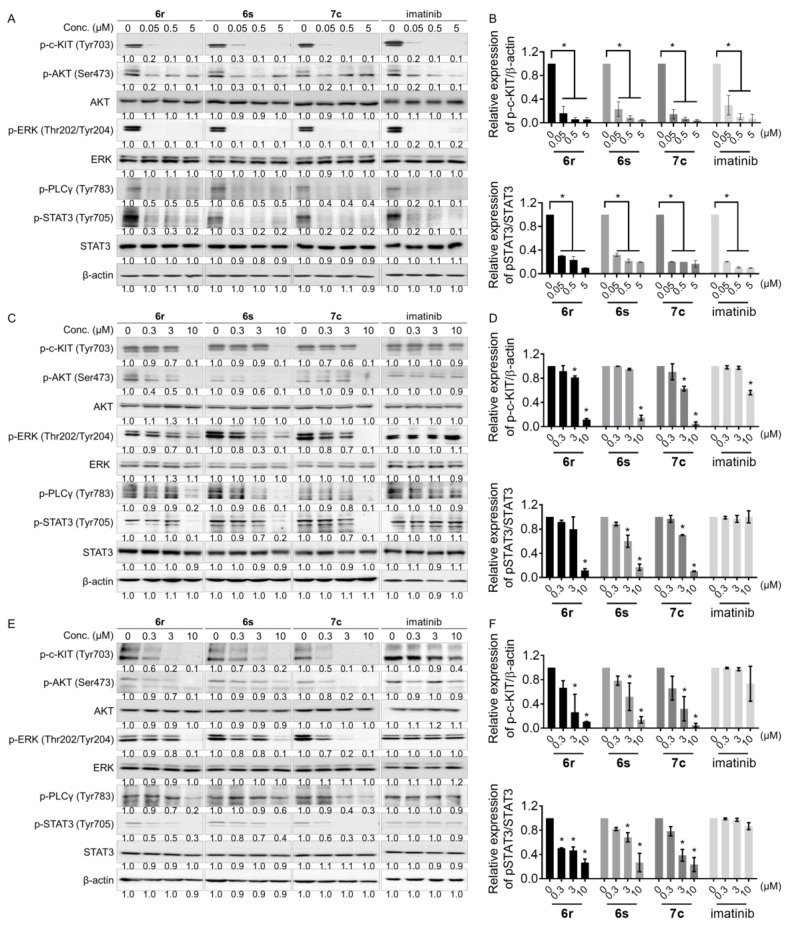
To assess that 6r, 6s, and 7c are capable of inhibiting c-KIT auto-phosphorylation and suppressing downstream signaling, Western blot analysis was assessed using GIST-T1, HMC1.2, and c-KIT D816V Ba/F3 cells. Compound 6r, 6s, 7c, and imatinib remarkably inhibited the levels of phosphorylated c-KIT (Tyr703) and its downstream signaling mediators including p-AKT, p-ERK, p-PLCγ and p-STAT3 in GIST-T1 cells (Figure 4A,B and Figure S1A). On HMC1.2 and c-KIT D816V Ba/F3 cells, 6r, 6s, and 7c at 10 μM concentration completely inhibited c-KIT auto-phosphorylation but not imatinib (Figure 4C–F and Figure S1B,C). These results show that 6r, 6s, and 7c blocked c-KIT downstream signaling associated with their anti-proliferation activities on GIST-T1, HMC1.2, and c-KIT D816V Ba/F3 cells.
Figure 4.
c-KIT and its downstream signaling are inhibited by 6r, 6s, and 7c in (A,B) GIST-T1, (C,D) HMC1.2, and (E,F) c-KIT D816V Ba/F3 cells. Quantification of phosphorylated c-KIT and STAT3 levels by the treatment of 6r, 6s, and 7c in GIST-T1, HMC1.2, and c-KIT D816V Ba/F3 cells. (average ± S.D., n = 3 one-way ANOVA; * p < 0.05).
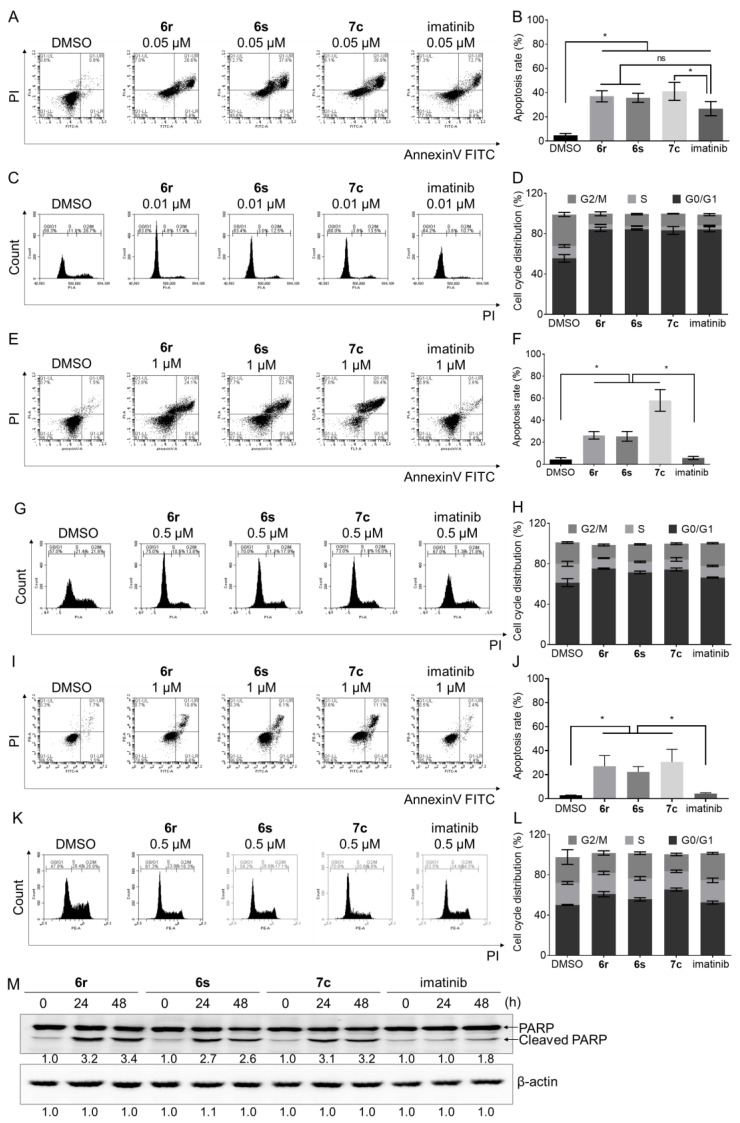
3.6. Induction of Apoptosis and Cell Cycle Arrest in GIST- T1 and HMC1.2 Cells
Apoptosis and cell cycle arrest are important in cancer cell death [42,43]. PI3K/mTOR signaling by mutant c-KIT plays an important role in the tumor cell proliferation and apoptosis in GIST [44,45]. Moreover, c-KIT inhibitor induces apoptosis against mast cells [46]. The effects of 6r, 6s, and 7c on apoptosis induction and cell cycle arrest were assessed in GIST-T1, HMC1.2, and c-KIT D816V Ba/F3 cells. As a result, 6r, 6s, and 7c remarkably induced production of apoptotic cells in GIST-T1 (Figure 5A,B), HMC1.2 (Figure 5E,F), and c-KIT D816V Ba/F3 (Figure 5I,J) cells. It is worth noting that treatment with imatinib (1 μM) for 48 h did not induce apoptosis in HMC1.2 cells (Figure 5E,F). Moreover, induction of apoptosis by 6r, 6s, and 7c was measured using apoptosis markers, which displayed that cleavage of PARP was induced in HMC1.2 cells (Figure 5M and Figure S1D). We performed FACS analysis to determine whether 6r, 6s, 7c, and imatinib induce cell cycle arrest in GIST-T1 and HMC1.2 cells. Treatment with 6r, 6s, 7c, and imatinib (0.01 μM) induced G0/G1 arrest in 25–35% compared to control in GIST-T1 cells (Figure 5C,D). In addition, treatment with 6r, 6s, and 7c (0.5 μM) for 24 h induced G0/G1 arrest more strongly in HMC1.2 and c-KIT D816V Ba/F3 cells compared with imatinib (Figure 5G,H,K,L). Collectively, these results indicate that 6r, 6s, and 7c inhibit proliferation of GIST-T1, HMC1.2, and c-KIT D816V Ba/F3 cells through induction of apoptosis and cell cycle arrest.
Figure 5.
Effects of 6r, 6s, 7c, and imatinib on induction of apoptosis and cell cycle arrest in (A–D) GIST-T1 (0.05 μM), (E–H) HMC1.2 (1 μM), and (I–L) c-KIT D816V Ba/F3 (1 μM) cells. Cells were stainedAnnexinV-FITC and PI. Flow cytometry analysis was assessed to determine the percentage of apoptotic cell. Treatment (24 h) with 6r, 6s, 7c, and imatinib induced G0/G1 arrest in GIST-T1 (0.01 μM), HMC1.2 (0.5 μM), and c-KIT D816V (0.5 μM) Ba/F3 cells. (M) Treatment (24 and 48 h) with 6r, 6s, 7c, and imatinib induced PARP cleavage in HMC1.2 cells. (average ± S.D., n = 3 one-way ANOVA and Tukey’s multiple comparisons test; * p < 0.05. ns: not significant).
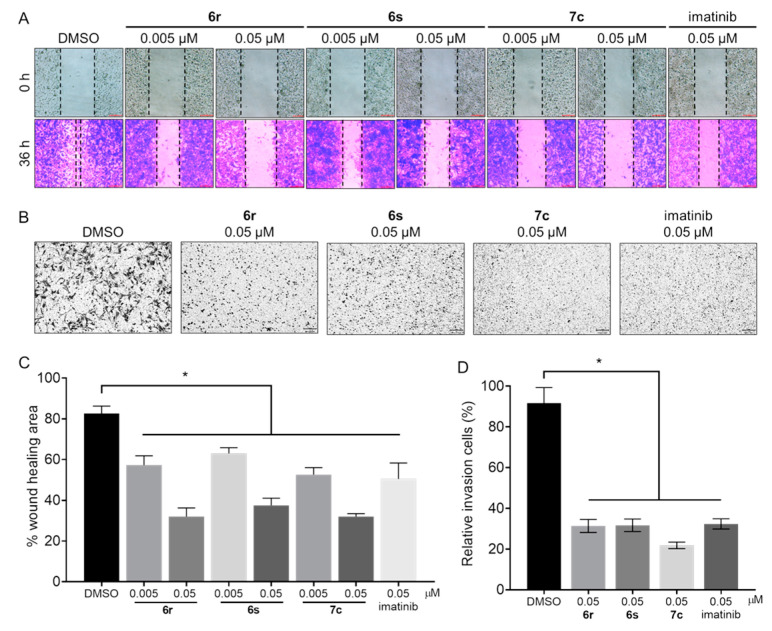
3.7. Suppression of Migration and Invasion in GIST-T1 Cells
c-KIT is related to the migration and invasion of GIST cells [47]. To identify the effects of 6r, 6s, and 7c on migration, we carried out a wound healing assay in the GIST-T1 cells (Figure 6A). Cell migration treated with 6r, 6s, 7c, and imatinib (0.005 μM and 0.05 μM) was decreased by 50–70% compared to that of control (Figure 6C). Moreover, the migration ratio of GIST-T1 cells treated with 6r, 6s, 7c, and imatinib deceased with increasing concentration. We conducted an invasion assay to further test the effects of 6r, 6s, and 7c on invasion (Figure 6B) and found that 6r, 6s, and 7c were capable of significantly suppressing the invasion capability of GIST-T1 cells (Figure 6D).
Figure 6.
Effects of 6r, 6s, 7c, and imatinib on migration and invasion. (A) Cell migration of GIST-T1 cells was inhibited by treatment (36 h) with 6r, 6s, and 7c (0.005 μM and 0.05 μM). (B) Invasion of GIST-T1 cells was inhibited by treatment (48 h) with 6r, 6s, 7c, and imatinib (0.05 μM). (C,D) Percentage wound healing area and relative invasion cells were calculated using ImageJ. (average ± S.D., n = 3 one-way ANOVA and Tukey’s multiple comparisons test; * p < 0.05).
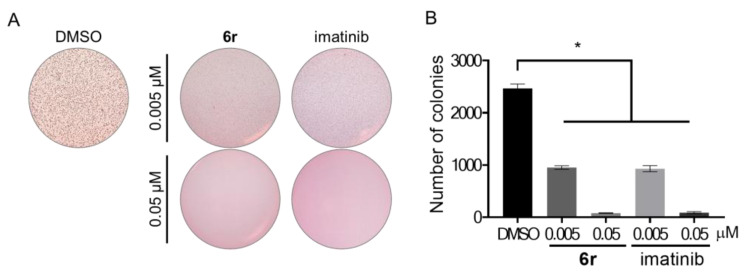
3.8. Suppression of Anchorage-Independent Growth in GIST-T1 Cells
Pharmacological inhibition and knockdown of c-kit decreased the colony growth of GIST cells [48]. A soft agar assay in GIST-T1 cells was performed to evaluate whether 6r blocks anchorage-independent growth (Figure 7). Treatment with two different concentrations of 6r and imatinib for 3 weeks resulted in dramatic inhibition of anchorage-independent growth of the GIST-T1 cells. Notably, incubation of 6r at 0.05 μM completely suppressed anchorage-independent growth compared to control, indicating that blockade of c-KIT by 6r suppresses tumorigenesis of GIST-T1 cells.
Figure 7.
Effects of 6r and imatinib on anchorage-independent growth. (A) GIST-T1 cells were cultured in 0.35% agar for 3 weeks. (B) The number of colonies was counted using ImageJ. (average ± S.D., n = 3 one-way ANOVA and Tukey’s multiple comparisons test; * p < 0.05).
4. Conclusions
Dysregulation of c-KIT is associated with GIST. In order to identify a unique class of c-KIT inhibitors capable of inhibiting clinically relevant c-KIT mutations and overriding imatinib resistance, we designed and synthesized 31 novel thiazolo[5,4-b]pyridine derivatives and carried out structure-activity relationship studies against c-KIT enzyme and c-KIT activated cells. The results of SAR studies show that 6r, 6s, and 7c possessed higher enzymatic and cellular activities than imatinib and these three derivatives were comparable to sunitinib in terms of activities. In particular, 6r hand a 8.0-fold higher enzymatic inhibitory activity against c-KIT V560G/D816V double mutant and 23.6-fold higher anti-proliferative activity on HMC1.2 cells than imatinib, indicating that 6r would be a promising lead for overriding imatinib resistance. It is of note that, compared with sunitinib, 6r had a higher differential cytotoxicity effect on c-KIT D816V Ba/F3 cells relative to parental Ba/F3 cells, which suggests that 6r is more selective c-KIT D816V inhibitor than sunitinib.
The analysis of molecular docking studies suggests that 6r forms hydrogen bonding with a Cys673 backbone, Ile789/His790 backbone, and Glu640/Asp810. Moreover, the 3-trifluoromethyl group of 6r occupies the hydrophobic binding pocket formed by Leu647, Ile653, Leu783, and Ile808. Kinase panel profiling showed that 6r possesses a reasonable kinase selectivity and potent activities against 13 out of 371 kinases including c-KIT.
Western blot analysis revealed that 6r significantly blocks c-KIT auto-phosphorylation and downstream signaling in GIST-T1, HMC1.2, and c-KIT D816V Ba/F3 cells. Compounds 6r, 6s, and 7c induce more significantly apoptosis than imatinib on HMC1.2 cells. In addition, 6r, 6s, and 7c are capable of blocking cell motility and suppressing anchorage-independent growth of GIST-T1 cells. Taken together, the novel thiazolo[5,4-b]pyridine derivatives provide insights into the design of novel c-KIT inhibitors capable of overcoming imatinib resistance.
Abbreviations
GIST, Gastrointestinal stromal tumor; PDGFR, Platelet-derived growth factor receptor; HMC, Human mast cell.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15010143/s1, File S1: Synthesis and chemical characterization; Figure S1: Western blot raw data presented in this study; Table S1: In vitro selectivity profiling of 6r at 1.0 μM against 371 human kinases.
Author Contributions
Y.N., C.K. and S.R. drafted the work. C.K. and J.H. synthesized thiazolo[5,4-b]pyridine derivatives. Y.N., H.C., N.K. and C.S. conducted biological experiments. S.R. and N.D.K. conducted molecular docking study. T.S. conceived and supervised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Taebo Sim is a shareholder of Magicbullettherapeutics Inc.
Funding Statement
This study was financially supported by the National Research Foundation of Korea (NRF) grant (NRF-2021R1A2C3011992), Brain Korea 21 Project, and the KU-KIST Graduate School of Converging Science and Technology Program.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Broudy V.C., Lin N.L., Sabath D.F. The fifth immunoglobulin-like domain of the Kit receptor is required for proteolytic cleavage from the cell surface. Cytokine. 2001;15:188–195. doi: 10.1006/cyto.2001.0907. [DOI] [PubMed] [Google Scholar]
- 2.de Silva C.M., Reid R. Gastrointestinal stromal tumors (GIST): C-kit mutations, CD117 expression, differential diagnosis and targeted cancer therapy with Imatinib. Pathol. Oncol. Res. 2003;9:13–19. doi: 10.1007/BF03033708. [DOI] [PubMed] [Google Scholar]
- 3.Pittoni P., Piconese S., Tripodo C., Colombo M.P. Tumor-intrinsic and-extrinsic roles of c-Kit: Mast cells as the primary off-target of tyrosine kinase inhibitors. Oncogene. 2011;30:757–769. doi: 10.1038/onc.2010.494. [DOI] [PubMed] [Google Scholar]
- 4.Babaei M.A., Kamalidehghan B., Saleem M., Huri H.Z., Ahmadipour F. Receptor tyrosine kinase (c-Kit) inhibitors: A potential therapeutic target in cancer cells. Drug Des. Dev. Ther. 2016;10:2443–2459. doi: 10.2147/DDDT.S89114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali S., Ali S. Role of c-kit/SCF in cause and treatment of gastrointestinal stromal tumors (GIST) Gene. 2007;401:38–45. doi: 10.1016/j.gene.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Corless C.L., Fletcher J.A., Heinrich M.C. Biology of gastrointestinal stromal tumors. J. Clin. Oncol. 2004;22:3813–3825. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 7.Steigen S.E., Eide T.J. Gastrointestinal stromal tumors (GISTs): A review. Apmis. 2009;117:73–86. doi: 10.1111/j.1600-0463.2008.00020.x. [DOI] [PubMed] [Google Scholar]
- 8.Tabone-Eglinger S., Subra F., El Sayadi H., Alberti L., Tabone E., Michot J.-P., Théou-Anton N., Lemoine A., Blay J.-Y., Emile J.-F. KIT mutations induce intracellular retention and activation of an immature form of the KIT protein in gastrointestinal stromal tumors. Clin. Cancer Res. 2008;14:2285–2294. doi: 10.1158/1078-0432.CCR-07-4102. [DOI] [PubMed] [Google Scholar]
- 9.Liang J., Wu Y.-L., Chen B.-J., Zhang W., Tanaka Y., Sugiyama H. The C-Kit receptor-mediated signal transduction and tumor-related diseases. Int. J. Biol. Sci. 2013;9:435–443. doi: 10.7150/ijbs.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corless C.L., Barnett C.M., Heinrich M.C. Gastrointestinal stromal tumours: Origin and molecular oncology. Nat. Rev. Cancer. 2011;11:865–878. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 11.Antonescu C.R., Sommer G., Sarran L., Tschernyavsky S.J., Riedel E., Woodruff J.M., Robson M., Maki R., Brennan M.F., Ladanyi M., et al. Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin. Cancer Res. 2003;9:3329–3337. [PubMed] [Google Scholar]
- 12.Vincenzi B., Napolitano A., Fiocco M., Mir O., Rutkowski P., Blay J.-Y., Reichardt P., Joensuu H., Fumagalli E., Gennatas S., et al. Adjuvant imatinib in patients with GIST harboring exon 9 KIT mutations: Results from a multi-institutional european retrospective study. Clin. Cancer Res. 2022;28:1672–1679. doi: 10.1158/1078-0432.CCR-21-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Napolitano A., Vincenzi B. Secondary KIT mutations: The GIST of drug resistance and sensitivity. Br. J. Cancer. 2019;120:577–578. doi: 10.1038/s41416-019-0388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serrano C., Mariño-Enríquez A., Tao D.L., Ketzer J., Eilers G., Zhu M., Yu C., Mannan A.M., Rubin B.P., Demetri G.D., et al. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br. J. Cancer. 2019;120:612–620. doi: 10.1038/s41416-019-0389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J.Q., Bosbach B., Loo J.K., Vitiello G.A., Zeng S., Seifert A.M., Medina B.D., Param N.J., Maltbaek J.H., Rossi F., et al. The V654A second-site KIT mutation increases tumor oncogenesis and STAT activation in a mouse model of gastrointestinal stromal tumor. Oncogene. 2020;39:7153–7165. doi: 10.1038/s41388-020-01489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li B., Wang A., Liu J., Qi Z., Liu X., Yu K., Wu H., Chen C., Hu C., Wang W., et al. Discovery of N-((1-(4-(3-(3-((6,7-dimethoxyquinolin-3-yl)oxy)phenyl)ureido)-2-(trifluoromethyl)phenyl)piperidin-4-yl)methyl)propionamide (CHMFL-KIT-8140) as a highly potent type II inhibitor capable of inhibiting the T670I “Gatekeeper” mutant of cKIT kinase. J. Med. Chem. 2016;59:8456–8472. doi: 10.1021/acs.jmedchem.6b00902. [DOI] [PubMed] [Google Scholar]
- 17.Negri T., Pavan G.M., Virdis E., Greco A., Fermeglia M., Sandri M., Pricl S., Pierotti M.A., Pilotti S., Tamborini E. T670X KIT mutations in gastrointestinal stromal tumors: Making sense of missense. J. Natl. Cancer Inst. 2009;101:194–204. doi: 10.1093/jnci/djn477. [DOI] [PubMed] [Google Scholar]
- 18.Gajiwala K.S., Wu J.C., Christensen J., Deshmukh G.D., Diehl W., DiNitto J.P., English J.M., Greig M.J., He Y.-A., Jacques S.L., et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc. Natl. Acad. Sci. USA. 2009;106:1542–1547. doi: 10.1073/pnas.0812413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma Y., Zeng S., Metcalfe D.D., Akin C., Dimitrijevic S., Butterfield J.H., McMahon G., Longley B.J. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood. 2002;99:1741–1744. doi: 10.1182/blood.V99.5.1741. [DOI] [PubMed] [Google Scholar]
- 20.Hochhaus A., La Rosée P. Imatinib therapy in chronic myelogenous leukemia: Strategies to avoid and overcome resistance. Leukemia. 2004;18:1321–1331. doi: 10.1038/sj.leu.2403426. [DOI] [PubMed] [Google Scholar]
- 21.Lopes L.F., Bacchi C.E. Imatinib treatment for gastrointestinal stromal tumour (GIST) J. Cell Mol. Med. 2010;14:42–50. doi: 10.1111/j.1582-4934.2009.00983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demetri G.D., Reichardt P., Kang Y.-K., Blay J.-Y., Rutkowski P., Gelderblom H., Hohenberger P., Leahy M., Von Mehren M., Joensuu H., et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh C.-N., Chen M.-H., Chen Y.-Y., Yang C.-Y., Yen C.-C., Tzen C.-Y., Chen L.-T., Chen J.-S. A phase II trial of regorafenib in patients with metastatic and/or a unresectable gastrointestinal stromal tumor harboring secondary mutations of exon 17. Oncotarget. 2017;8:44121–44130. doi: 10.18632/oncotarget.17310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lübke J., Naumann N., Kluger S., Schwaab J., Metzgeroth G., Evans E., Gardino A.K., Lengauer C., Hofmann W.-K., Fabarius A., et al. Inhibitory effects of midostaurin and avapritinib on myeloid progenitors derived from patients with KIT D816V positive advanced systemic mastocytosis. Leukemia. 2019;33:1195–1205. doi: 10.1038/s41375-019-0450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grunewald S., Klug L.R., Mühlenberg T., Lategahn J., Falkenhorst J., Town A., Ehrt C., Wardelmann E., Hartmann W., Schildhaus H.-U., et al. Resistance to avapritinib in PDGFRA-driven GIST is caused by secondary mutations in the PDGFRA kinase domain. Cancer Discov. 2021;11:108–125. doi: 10.1158/2159-8290.CD-20-0487. [DOI] [PubMed] [Google Scholar]
- 26.Smith B.D., Kaufman M.D., Lu W.-P., Gupta A., Leary C.B., Wise S.C., Rutkoski T.J., Ahn Y.M., Al-Ani G., Bulfer S.L., et al. Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer cell. 2019;35:738–751. doi: 10.1016/j.ccell.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Serrano C., George S. Gastrointestinal stromal tumor: Challenges and opportunities for a new decade. Clin. Cancer Res. 2020;26:5078–5085. doi: 10.1158/1078-0432.CCR-20-1706. [DOI] [PubMed] [Google Scholar]
- 28.Lu Y., Mao F., Li X., Zheng X., Wang M., Xu Q., Zhu J., Li J. Discovery of potent, selective stem cell factor receptor/platelet derived growth factor receptor alpha (c-KIT/PDGFRα) dual inhibitor for the treatment of imatinib-resistant gastrointestinal stromal tumors (GISTs) J. Med. Chem. 2017;60:5099–5119. doi: 10.1021/acs.jmedchem.7b00468. [DOI] [PubMed] [Google Scholar]
- 29.Wu T.-S., Lin W.-H., Tsai H.-J., Hsueh C.-C., Hsu T., Wang P.-C., Lin H.-Y., Peng Y.-H., Lu C.-T., Lee L.-C., et al. Discovery of conformational control inhibitors switching off the activated c-KIT and targeting a broad range of clinically relevant c-KIT mutants. J. Med. Chem. 2019;62:3940–3957. doi: 10.1021/acs.jmedchem.8b01845. [DOI] [PubMed] [Google Scholar]
- 30.Xia L., Zhang Y., Zhang J., Lin S., Zhang K., Tian H., Dong Y., Xu H. Identification of novel thiazolo [5,4-b]pyridine derivatives as potent phosphoinositide 3-kinase inhibitors. Molecules. 2020;25:4630. doi: 10.3390/molecules25204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harling J.D., Deakin A.M., Campos S., Grimley R., Chaudry L., Nye C., Polyakova O., Bessant C.M., Barton N., Somers D., et al. Discovery of novel irreversible inhibitors of interleukin (IL)-2-inducible tyrosine kinase (Itk) by targeting cysteine 442 in the ATP pocket. J. Biol. Chem. 2013;288:28195–28206. doi: 10.1074/jbc.M113.474114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi H.G., Zhang J., Weisberg E., Griffin J.D., Sim T., Gray N.S. Development of ‘DFG-out’ inhibitors of gatekeeper mutant kinases. Bioorg. Med. Chem. Lett. 2012;22:5297–5302. doi: 10.1016/j.bmcl.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okaniwa M., Hirose M., Imada T., Ohashi T., Hayashi Y., Miyazaki T., Arita T., Yabuki M., Kakoi K., Kato J., et al. Design and synthesis of novel DFG-out RAF/vascular endothelial growth factor receptor 2 (VEGFR2) inhibitors. 1. exploration of [5,6]-fused bicyclic scaffolds. J. Med. Chem. 2012;55:3452–3478. doi: 10.1021/jm300126x. [DOI] [PubMed] [Google Scholar]
- 34.Hirose M., Okaniwa M., Miyazaki T., Imada T., Ohashi T., Tanaka Y., Arita T., Yabuki M., Kawamoto T., Tsutsumi S., et al. Design and synthesis of novel DFG-out RAF/vascular endothelial growth factor receptor 2 (VEGFR2) inhibitors: 3. evaluation of 5-amino-linked thiazolo [5,4-d]pyrimidine and thiazolo [5,4-b]pyridine derivatives. Bioorg. Med. Chem. 2012;20:5600–5615. doi: 10.1016/j.bmc.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 35.Garner A.P., Gozgit J.M., Anjum R., Vodala S., Schrock A., Zhou T., Serrano C., Eilers G., Zhu M., Ketzer J., et al. Ponatinib inhibits polyclonal drug-resistant KIT oncoproteins and shows therapeutic potential in heavily pretreated gastrointestinal stromal tumor (GIST) patients. Clin. Cancer Res. 2014;20:5745–5755. doi: 10.1158/1078-0432.CCR-14-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho H., Shin I., Ju E., Choi S., Hur W., Kim H., Hong E., Kim N.D., Choi H.G., Gray N.S., et al. First SAR study for overriding NRAS mutant driven acute myeloid leukemia. J. Med. Chem. 2018;61:8353–8373. doi: 10.1021/acs.jmedchem.8b00882. [DOI] [PubMed] [Google Scholar]
- 37.Taguchi T., Sonobe H., Toyonaga S.-I., Yamasaki I., Shuin T., Takano A., Araki K., Akimaru K., Yuri K. Conventional and molecular cytogenetic characterization of a new human cell line, GIST-T1, established from gastrointestinal stromal tumor. Lab. Investig. 2002;82:663–665. doi: 10.1038/labinvest.3780461. [DOI] [PubMed] [Google Scholar]
- 38.Sundström M., Vliagoftis H., Karlberg P., Butterfield J.H., Nilsson K., Metcalfe D.D., Nilsson G. Functional and phenotypic studies of two variants of a human mast cell line with a distinct set of mutations in the c-kit proto-oncogene. Immunology. 2003;108:89–97. doi: 10.1046/j.1365-2567.2003.01559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furitsu T., Tsujimura T., Tono T., Ikeda H., Kitayama H., Koshimizu U., Sugahara H., Butterfield J.H., Ashman L.K., Kanayama Y. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J. Clin. Investig. 1993;92:1736–1744. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mol C.D., Dougen D.R., Schneider T.R., Skene R.J., Kraus M.L., Scheibe D.N., Snell G.P., Zou H., Sang B.-C., Wilson K.P. Structural basis for the autoinhibition and STI-571 inhibition of c-Kit tyrosine kinase. J. Biol. Chem. 2004;279:31655–31663. doi: 10.1074/jbc.M403319200. [DOI] [PubMed] [Google Scholar]
- 41.Eid S., Turk S., Volkamer A., Rippmann F., Fulle S. KinMap: A web-based tool for interactive navigation through human kinome data. BMC Bioinf. 2017;18:1–6. doi: 10.1186/s12859-016-1433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reyes-Sebastian J., Montiel-Cervantes L.A., Reyes-Maldonado E., Dominguez-Lopez M.L., Ortiz-Butron R., Castillo-Alvarez A., Lezama R.A. Cell proliferation and inhibition of apoptosis are related to c-Kit activation in leukaemic lymphoblasts. Hematology. 2018;23:486–495. doi: 10.1080/10245332.2018.1444564. [DOI] [PubMed] [Google Scholar]
- 43.Chang F., Steelman L.S., Shelton J.G., Lee J.T., Navolanic P.M., Blalock W.L., Franklin R., McCubrey J.A. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (Review) Int. J. Oncol. 2003;22:469–480. doi: 10.3892/ijo.22.3.469. [DOI] [PubMed] [Google Scholar]
- 44.Bauer S., Duensing A., Demetri G.D., Fletcher J.A. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway. Oncogene. 2007;26:7560–7568. doi: 10.1038/sj.onc.1210558. [DOI] [PubMed] [Google Scholar]
- 45.Bosbach B., Rossi F., Yozgat Y., Loo J., Zhang J.Q., Berrozpe G., Warpinski K., Ehlers I., Veach D., Kwok A., et al. Direct engagement of the PI3K pathway by mutant KIT dominates oncogenic signaling in gastrointestinal stromal tumor. Proc. Natl. Acad. Sci. USA. 2017;114:E8448–E8457. doi: 10.1073/pnas.1711449114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juurikivi A., Sandler C., Lindstedt K.A., Kovanen P.T., Juutilainen T., Leskinen M.J., Mäki T., Eklund K.K. Inhibition of c-kit tyrosine kinase by imatinib mesylate induces apoptosis in mast cells in rheumatoid synovia: A potential approach to the treatment of arthritis. Ann. Rheum. Dis. 2005;64:1126–1131. doi: 10.1136/ard.2004.029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Y., Chen J., Weng X., Lin G., Huang Z., Shui H. Establishment of a GIST-T1 gastrointestinal stromal tumour cell line resistant to imatinib mesylate. Oncol. Lett. 2018;15:7589–7594. doi: 10.3892/ol.2018.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J., Zhang Q., Sun X., Xue A., Gao X., Shen K. THZ1 targeting CDK7 suppresses c-KIT transcriptional activity in gastrointestinal stromal tumours. Cell Commun. Signal. 2022;20:138. doi: 10.1186/s12964-022-00928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.