Abstract
Campylobacter jejuni infections are thought to induce antiganglioside antibodies in patients with Guillain-Barré syndrome (GBS) and Miller Fisher syndrome (MFS) by molecular mimicry between C. jejuni lipopolysaccharides (LPS) and gangliosides. We used purified LPS fractions from five Campylobacter strains to induce antiganglioside responses in rabbits. The animals that received injections with LPS from GBS-associated strains developed anti-GM1 and anti-GA1 antibodies. Animals injected with LPS from one MFS-related C. jejuni strain produced anti-GQ1b antibodies. Rabbits that were injected with Penner O:3 LPS had a strong anti-LPS response, but no antiganglioside reactivity was observed. The antiganglioside specificity in the rabbits reflected the specificity in the patients from whom the strains were isolated. In conclusion, our results indicate that an immune response against GBS- and MFS-associated C. jejuni LPS results in antiganglioside antibodies. These results provide strong support for molecular mimicry as a mechanism in the induction of antiganglioside antibodies following infections.
The Guillain-Barré syndrome (GBS) and the Miller Fisher syndrome (MFS) are acute immune-mediated neuropathies and are frequently preceded by an infectious illness. The most frequently identified microorganism is the enteric pathogen Campylobacter jejuni (12, 14, 23). In the majority of patients with Campylobacter-associated GBS and MFS, serum antibodies that react with gangliosides are present (8, 11, 26). The presence of these antibodies is associated with specific clinical symptoms. Anti-GM1 antibodies are associated with motor neuropathy (7, 13, 16), and anti-GQ1b antibodies are associated with oculomotor symptoms (5, 31). Antiganglioside antibodies exert an effect on the neuromuscular junction, indicating that these antibodies may contribute to the neurological deficits (6, 21).
The core oligosaccharides of C. jejuni lipopolysaccharides (LPS) contain ganglioside-like structures, and antiganglioside antibodies from GBS and MFS patients do indeed react with C. jejuni LPS (9, 11, 17, 19). These results suggest that antiganglioside antibodies in neuropathy patients with an antecedent C. jejuni infection are induced through molecular mimicry. Several researchers have addressed this issue in animal studies (6, 24, 32). Wirguin et al. (32) induced anti-GM1 immunoglobulin M (IgM) antibodies in rats following immunization with LPS from the Penner O:19 reference strain and a preimmunization with keyhole limpet hemocyanin. Ritter et al. (24) immunized rabbits with LPS from several C. jejuni strains, resulting in anti-GM2, anti-GD1b, and anti-GM1 IgG antibodies. These two groups used C. jejuni reference strains that were isolated from patients with enteritis without neurological involvement. Goodyear et al. (6) and Ang et al. (1) used GBS-associated C. jejuni strains to induce an antiganglioside response in mice and rabbits, respectively. However, the antiganglioside reactivity in the serum of patients from which these neuropathy-associated strains were cultured was not known, and therefore, the anti-LPS and antiganglioside responses in the animals could not be compared to the responses in neuropathy patients.
The aim of the present study was to investigate whether immunization of rabbits with LPS from GBS- and MFS-associated C. jejuni strains induces cross-reactive antiganglioside and anti-LPS antibodies. Furthermore, we wanted to compare the antiganglioside specificity in the rabbits with the specificity in the patients from whom the C. jejuni strains were isolated.
MATERIALS AND METHODS
Patients and Campylobacter strains.
Strains GB17 and GB18 were isolated from two GBS patients, and strains MF6 and MF8 were isolated from two MFS patients and have been described before as strain A and strain C, respectively (Table 1) (11). For extraction of LPS, bacteria were grown on blood agar plates with 5% sheep blood at 37°C for 48 h under microaerophilic conditions. Cells were scraped from freshly grown plates and washed in phosphate-buffered saline (pH 7.8). LPS were extracted with the hot phenol-water method of Westphal and Jann (28). Combined water phases containing the extracted LPS were dialyzed against water. After ultracentrifugation at 100,000 × g for 2 h, the pellets were freeze-dried and weighed. After resuspension in water, all LPS fractions showed a single dense band migrating at 8 to 15 kDa following electrophoresis on a polyacrylamide gel and silver staining (Novex, San Diego, Calif.), indicating the presence of LPS (2). The antiganglioside and anti-LPS reactivity in the patients from whom the C. jejuni strains were isolated was determined by enzyme-linked immunosorbent assays (ELISA) and confirmed by thin-layer chromatography (TLC) as described previously (Table 2) (10). Results from Penner serotyping of the strains are listed in Table 1. The Penner O:3 serostrain, which is known not to contain a GM1- or GQ1b-like structure was used as a control (3, 18). To confirm the presence of ganglioside-like epitopes, LPS from all five strains were tested by ELISA with a panel of polyclonal GM1-GA1 and GQ1b-GD3-reactive sera, cholera toxin, peanut agglutinin, and a monoclonal antidisialosyl antibody (provided by H. J. Willison, Glasgow, Scotland) (29; C. W. Ang et al., unpublished data).
TABLE 1.
Clinical characteristics of patients and Penner serotypes of isolated C. jejuni strains
| Patient characteristic or serotype | GB17 | GB18 | MF6 | MF8 |
|---|---|---|---|---|
| Diagnosis | GBS | GBS | MFS | MFS |
| Age (yr) | 54 | 7 | 41 | 22 |
| Gender | Male | Male | Male | Male |
| Prodromal illness | Diarrhea | Vomiting, URTIa | Diarrhea | Diarrhea |
| Maximum clinical deficit | Unable to walk unaided | Bedridden | No limb weakness | No limb weakness |
| Sensory involvement | Paresthesias | No | No | Paresthesias |
| Ataxia | No | No | Yes | Yes |
| Oculomotor weakness | No | No | Yes | Yes |
| Electrophysiological studies | Axonal neuropathy | Not available | Not available | Normal |
| Penner serotype of strain | O:4, O:13, O:64 | O:19 | O:4, O:64 | O:23, O:36 |
URTI, upper respiratory tract infection.
TABLE 2.
Serum antiglycolipid and anti-LPS antibody titers in GBS and MFS patients
| Patient | IgM titer
|
IgG titer
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GA1 | GM1 | GM2 | GD1a | GD1b | GD3 | GQ1b | Homologous LPS | GA1 | GM1 | GM2 | GD1a | GD1b | GD3 | GQ1b | Homologous LPS | |
| GB17 | 200 | —a | — | — | — | — | — | 400 | 25,600 | 100 | — | — | — | 100 | — | 25,600 |
| GB18 | — | 200 | — | — | — | — | — | 800 | 3,200 | 12,800 | — | — | 1,600 | — | — | 51,200 |
| MF6 | — | — | — | — | — | — | — | — | — | — | — | — | 100 | 200 | 1,600 | 200 |
| MF8 | — | — | — | — | 200 | — | — | 200 | — | — | — | — | 200 | 200 | 800 | 800 |
—, <100.
Immunization protocol.
New Zealand White rabbits (2.0 to 2.5 kg) were immunized as described before (1). Two animals were immunized with each LPS. As a control, two animals were injected with adjuvant only, without LPS. Booster injections with the same amount of LPS in incomplete Freund's adjuvant (Difco) were given at days 14, 28, and 42. Blood was collected from the ear vein prior to each immunization. The animals were bled at day 56. The feces of all animals were cultured for C. jejuni prior to immunization and at day 56. This experiment was approved by the animal ethics committee that serves Erasmus University and Dijkzigt University Hospital.
Serology.
Antiganglioside reactivity was detected by ELISA and confirmed by TLC as described before (1). The titer was defined as the highest serum dilution with an optical density at 492 nm of >0.1, corrected for binding to uncoated wells. Anti-LPS reactivity was detected by ELISA and Western blotting as described before (1).
To assess cross-reactivity of anti-LPS antibodies with gangliosides, serum samples from immunized rabbits and from the patients from whom the C. jejuni strains were isolated were incubated with C. jejuni LPS conjugated to Octyl-Sepharose CL4B beads (1). In short, 250 μg of LPS was added to 1 ml of Octyl-Sepharose CL4B in methanol-water (1:1 [vol/vol]) containing 0.1 M KCl and mixed for 1.5 h. After several washes with phosphate-buffered saline, serum samples were incubated with LPS-Sepharose conjugates for 5 h at 4°C. Absorbed serum samples were tested for anti-LPS and antiganglioside reactivity as described above. As controls, serum samples that were processed in an identical way but incubated with unconjugated beads and without beads were included in each experiment.
RESULTS
Clinical data of the GBS and MFS patients are presented in Table 1. Sera from all patients reacted with several glycolipid antigens, although the fine specificity and isotype distribution of the antibodies differed between patients (Table 2). In addition, all patients had antibodies to C. jejuni LPS (Table 2) and protein antigens (data not shown). Detailed electrophysiological data were available only for patients GB17 and MF8. Patient GB17 had an axonal neuropathy, with predominant distal weakness. Motor nerve conduction studies in patient MF8 revealed no abnormalities.
The presence of GM1 and GQ1b-like epitopes on the GBS- and MFS-associated strains was studied with ganglioside-binding ligands. GB18 LPS reacted strongly with cholera toxin, indicating a GM1-like epitope, while MF6 and MF8 LPS showed reactivity with the antidisialosyl antibody, demonstrating a GQ1b-like epitope (data not shown). In addition, both GB17 LPS and MF6 LPS reacted with sera with anti-GM1 and anti-GA1 reactivity and GB17 LPS reacted strongly with peanut agglutinin, a ligand specific for GA1, indicating the presence of GA1-like epitopes in these strains. In contrast, Penner O:3 LPS did not show reactivity to any of the ganglioside-binding ligands (data not shown).
To exclude the possibility that the observed antibody responses resulted from an intercurrent infection of the animals with C. jejuni and not from the immunization procedure, stool cultures for C. jejuni were performed at the start of the immunization procedure and when the animals were bled. Stool cultures remained negative during the immunization period. Furthermore, antiganglioside and anti-LPS reactivity could not be detected in preimmune serum samples. Immunization of rabbits with C. jejuni LPS from neuropathy patients resulted in high-titer anti-LPS and antiglycolipid antibodies, while serum from animals that were immunized with LPS from the O:3 serostrain contained only anti-LPS but no antibodies against any of the purified glycolipids (Table 3). The adjuvant controls did not shown any reactivity to LPS and only a slight elevation of anti-GA1 reactivity (Table 3). All antiglycolipid responses were confirmed by TLC (data not shown).
TABLE 3.
Serum antiglycolipid and anti-LPS antibody titers in rabbits immunized with C. jejuni LPSa
| Rabbit | C. jejuni strain | IgM titer
|
IgG titer
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GA1 | GM1 | GM2 | GD1a | GD1b | GD3 | GQ1b | Homologous LPS | GA1 | GM1 | GM2 | GD1a | GD1b | GD3 | GQ1b | Homologous LPS | ||
| 1 | GB17 | 800 | 200 | —b | — | 100 | — | — | 400 | 409,600 | — | — | — | — | — | — | 819,200 |
| 2 | GB17 | 800 | — | — | — | — | — | — | 400 | 819,200 | — | — | — | 800 | 200 | — | 3,276,800 |
| 3 | GB18 | 100 | 100 | — | — | — | — | — | 100 | 400 | 51,200 | 200 | — | — | — | — | 12,800 |
| 4 | GB18 | 200 | 200 | — | — | 100 | — | — | 100 | 1,600 | 1,600 | — | — | 100 | — | — | 102,400 |
| 5 | MF6 | 800 | 100 | — | — | — | — | — | 200 | 102,400 | — | — | — | — | — | 400 | 102,400 |
| 6 | MF6 | 1,600 | — | — | — | — | — | 200 | 800 | 25,600 | — | — | — | — | — | — | 3,200 |
| 7 | MF8 | — | 100 | 100 | 100 | — | — | — | 100 | — | — | 800 | — | — | — | — | 200 |
| 8 | MF8 | — | — | 100 | 100 | — | — | — | 100 | — | — | — | — | — | — | — | 800 |
| 9 | Pen O:3 | — | — | — | — | — | — | — | 400 | — | — | — | — | — | — | — | 3,276,800 |
| 10 | Pen O:3 | — | — | — | — | — | — | — | 400 | — | — | — | — | — | — | — | 3,276,800 |
| 11 | CFA | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 12 | CFA | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
Serum samples taken at day 56.
—, <100.
The specificity of antiganglioside antibodies in the rabbits resembled the observed specificity in the patients from whom the strains were cultured. Serum from patient GB17 mainly exhibited antibody reactivity to GA1, as did the rabbits that were immunized with LPS from strain GB17. The other GBS patient, GB18, had a higher anti-GM1 titer than anti-GA1 titer. Sera from the two animals that were immunized with LPS from GB18 displayed the same pattern of antiglycolipid reactivity as that of patient GB18. Both rabbits that were immunized with LPS from MF6 mounted an immune response against GQ1b. One animal had only anti-GQ1b IgM antibodies, while the other had only anti-GQ1b IgG (Table 3). In addition, both animals had high titers of anti-GA1 antibodies, which was not found in the patient from whom MF6 was isolated but was in accordance with the presence of a GA1-like structure in MF6 LPS. Immunization with MF8 LPS did not result in an anti-GQ1b response, although one of the animals had an anti-GM2 IgG response. Anti-LPS reactivity in these two rabbits was also much weaker than that in animals immunized with LPS from other C. jejuni strains.
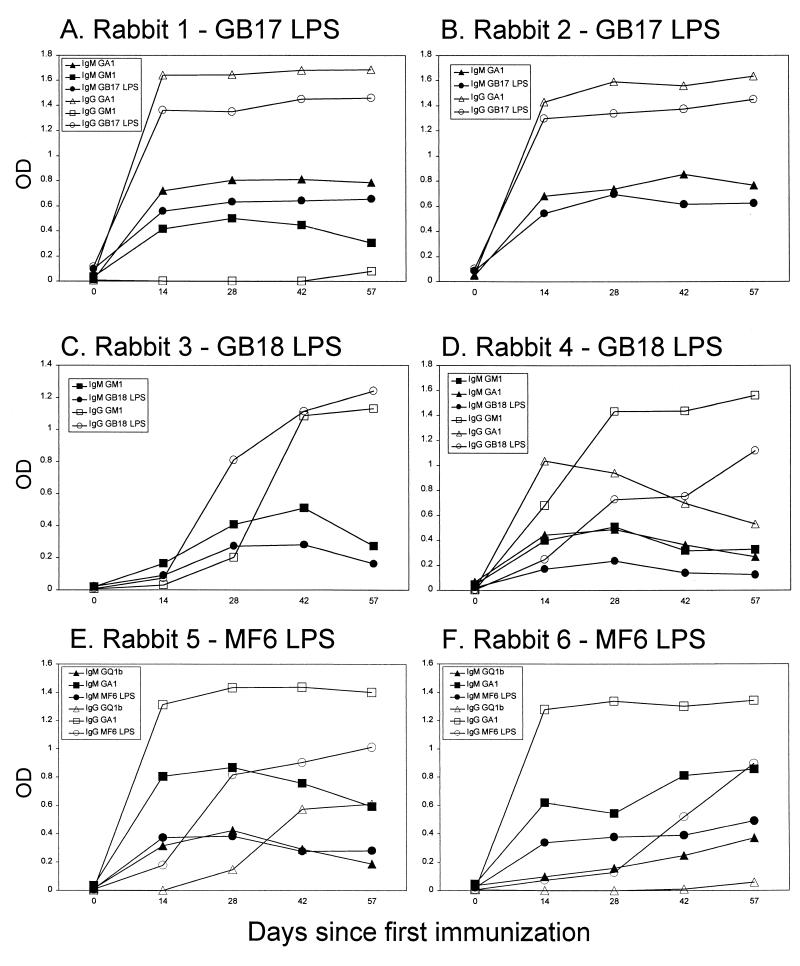
The kinetics of antiglycolipid and anti-LPS antibody responses differed in animals, depending on the source of the LPS. In both animals that were immunized with LPS from GB17, a strong IgG response against GA1 and GB17 LPS could already be observed 2 weeks after the first immunization (Fig. 1A and B). In contrast, animals that were immunized with GB18 and MF6 LPS showed a gradual increase of the antiganglioside and anti-LPS IgG antibody reactivity during the immunization period (Fig. 1C to F). Remarkably, in one animal that was immunized with GB18, the anti-GM1 and anti-GA1 IgG responses showed different kinetics. After an initial increase, the anti-asialo-GM1 reactivity decreased, while the anti-GM1 reactivity continued to increase until the end of the immunization period (Fig. 1D).
FIG. 1.
Kinetics of anti-LPS and antiglycolipid reactivity in rabbits immunized with C. jejuni LPS. Sequential serum samples were tested for IgM and IgG reactivity against C. jejuni LPS and glycolipids. (A and B) For rabbits 1 and 2, anti-GM1, anti-GA1, and anti-GB17 LPS reactivity and IgM and IgG reactivity rose in parallel and were at high levels after 14 days. (C and D) For rabbits 3 and 4, anti-GM1 and anti-GB18 LPS IgM antibody reactivity decreased after an initial rise, while IgG reactivity continued to rise. The kinetics of anti-GA1 reactivity differed from that of anti-GM1 reactivity in rabbit 4. (E and F) For rabbits 5 and 6, the kinetics of anti-GA1 reactivity differed from that of anti-GM1 reactivity in rabbit 4. The kinetics of anti-GQ1b and anti-MF6 LPS IgM reactivity were similar. The kinetics of anti-GQ1b IgG and anti-GA1 IgG reactivity were different compared to anti-MF6 LPS IgG reactivity. OD, optical density.
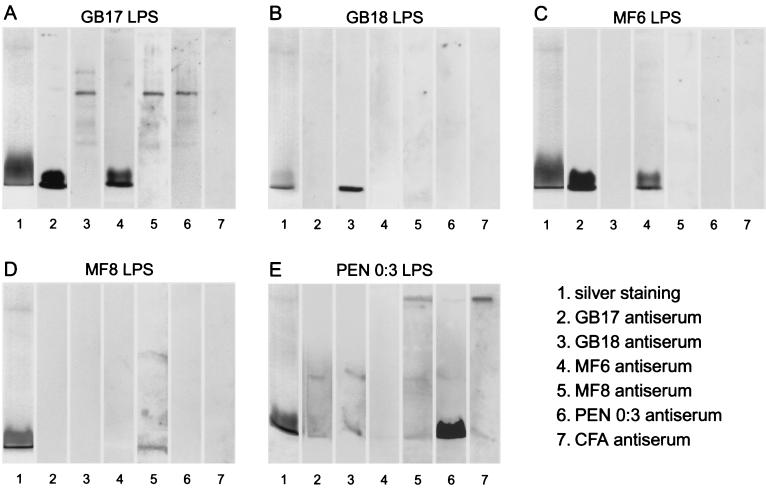
On Western blots, the sera from all LPS-immunized rabbits reacted with the LPS they had been injected with. Silver staining revealed a band that comigrated between 11 and 15 kDa (Fig. 2), consistent with earlier observations for C. jejuni LPS (22). Serum samples predominantly reacted with this low-molecular-weight fraction of the LPS. We did not observe ladder-like patterns as described for C. jejuni (22), although in some instances single bands with higher molecular weights were present. Sera from some rabbits reacted not only with the LPS with which they were immunized but also with LPS from other C. jejuni strains. Serum from animals immunized with MF6 also reacted strongly with LPS from GB17 and vice versa (Fig. 2A, lanes 2 and 4, and C, lanes 2 and 4). Both strains have Penner serotype O:4. Only serum from animals that were immunized with Penner O:3 had a strong response to Penner O:3 LPS (Fig. 2E).
FIG. 2.
Strain-specific anti-LPS responses detected by Western blotting. C. jejuni LPS were subjected to electrophoresis and silver staining (lanes 1) or transferred to nitrocellulose and overlaid with sera from immunized rabbits (lanes 2 to 6). (A) GB17 LPS reacted with serum from a GB17 LPS-immunized animal but also with serum from an MF6 LPS-immunized animal. (B) GB18 LPS reacted only with serum from a GB18 LPS-immunized animal. (C) MF6 LPS reacts with serum from an MF6 LPS-immunized animal but also with serum from a GB17 LPS-immunized animal. (D) MF8 LPS reacted weakly with serum from an MF8-immunized animal. (E) Penner O:3 LPS reacted strongly with serum from a Penner O:3 LPS-immunized animal.
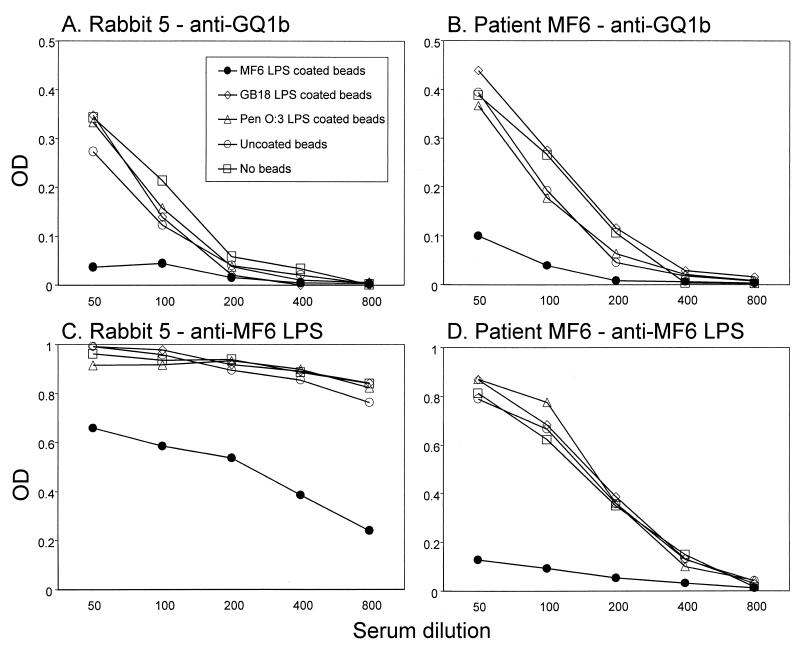
To prove that the anti-LPS antibodies and antiganglioside antibodies are cross-reactive, we conducted a series of depletion experiments with C. jejuni LPS conjugated to Sepharose CL4B beads. The results from experiments on cross-reactive anti-GQ1b and anti-MF6 LPS antibodies are summarized in Fig. 3. Anti-GQ1b and anti-MF6 LPS reactivity in an MF6-immunized rabbit could be inhibited only by incubation of serum with Sepharose beads conjugated to MF6 LPS and not with LPS from GB18 and Penner O:3 (Fig. 3A and C). The observed specific pattern of cross-reactivity was identical to that found in the patient from which the MF6 strain was isolated (Fig. 3B and D). In a similar fashion, cross-reactivity of anti-GA1 and anti-GM1 antibodies in patients GB17 and GB18 and rabbits immunized with corresponding LPS was demonstrated. In the GB17-immunized rabbits, anti-GA1 reactivity could be decreased only by incubation with GB17 LPS and not GB18 LPS, indicating that the anti-GA1 antibodies are specifically induced by the GA1-like GB17 LPS.
FIG. 3.
Identical pattern of cross-reactivity of antiganglioside and anti-LPS antibodies in patients and immunized rabbits. Sera from patient MF6 and from rabbit 5, immunized with MF6 LPS, were incubated with Sepharose CL4B beads that were coated with C. jejuni LPS, and diluted serum samples were tested for residual reactivity against GQ1b and MF6 LPS. The specificities of both human and rabbit sera are identical. Anti-GQ1b reactivity in the rabbit and the patient was reduced only by incubation with MF6 LPS-coated beads (solid circles in panels A and B). The same pattern could be observed when sera were tested for anti-MF6 LPS reactivity (C and D). OD, optical density.
Almost all rabbits tolerated the injections well, did not show any overt signs of weakness or ataxia, and did not lose weight. However, one animal that was immunized with GB18 LPS started losing weight 4 weeks after the first injection. In addition, this animal had fewer spontaneous movements, although there were no signs of muscle weakness. After a few weeks, the animal recovered and gained weight again. Histological examination of the sciatic nerve of this animal, taken 56 days after the first injection, did not show any signs of neuropathy or inflammation.
DISCUSSION
This study demonstrates that immunization of rabbits with LPS from GBS- and MFS-associated C. jejuni strains that contain ganglioside-like structures can induce antiganglioside antibodies, while immunization with the Penner O:3 serostrain did not lead to antiganglioside responses despite a strong anti-LPS response. The specificity of the antiganglioside response in the rabbits reflected the specificity in the patients. This suggests that the immune response in the patients was directed against the same structures as the immune response in the rabbits, thereby giving support for the hypothesis that antiganglioside antibodies can arise through molecular mimicry with ganglioside-like structures of an infectious agent.
The antiganglioside antibody response in the animals was the result of the immunization procedure and not from a intercurrent infection of the animals with C. jejuni, as all animals had negative stool cultures at the beginning and end of the experiment. Furthermore, preimmunization titers of anti-LPS and antiganglioside antibodies were <100. Rabbits that were immunized with only complete Freund's adjuvant (CFA), which contains mycobacterial antigens, did not show a rise in anti-LPS or antiganglioside antibodies. In addition, immunization with O:3 LPS, which does not contain a GM1-like or GQ1b-like structure (3), resulted in a strong anti-LPS response without any antiganglioside reactivity. These results confirm that immunization solely with adjuvants, such as CFA and LPS, does not lead to antiganglioside antibody formation and indicates that these antibodies do not result from polyclonal B-cell stimulation (1).
Depletion experiments using LPS-coated Sepharose beads showed that the rabbit anti-LPS and antiganglioside antibodies are cross-reactive. This pattern of cross-reactivity was identical to the pattern that was observed using serum from the patient from which the C. jejuni strains were derived. This serves as further evidence that antiganglioside antibodies in GBS and MFS patients with an antecedent Campylobacter infection are directed against ganglioside-like structures of bacterial antigens.
Two animals that were injected with LPS and MF8 did not produce anti-GQ1b antibodies, although the patient's serum contains anti-GQ1b reactivity and the LPS has a GQ1b-like structure. This may be due to the lack of immunogenicity of the LPS from the MF8 strain in rabbits. These animals showed a weaker anti-LPS response on Western blots than rabbits that were immunized with LPS from other strains. It is improbable that the GQ1b-like epitopes on MF8 LPS were destroyed during extraction and purification, because we confirmed the presence of a GQ1b-like epitope on the LPS with a monoclonal antidisialosyl antibody. Furthermore, we were able to induce an anti-GQ1b response with the MF8 strain in mice (C. W. Ang et al., unpublished observations). Ritter et al. were unable to induce an antiganglioside response in four animals that were immunized with LPS from the O:23 and O:36 serostrains, both having a GM2-like LPS (4, 24). The MF6 strain also has the O:23 serotype, and remarkably, one of the MF6-immunized animals had a moderate anti-GM2 response. There were considerable differences in the kinetics of the antiganglioside responses of strains and isotypes. An explanation for the differences between strains might be that rabbits more easily mount an immune response to GM1-like structures than to GQ1b-like structures. The titers of anti-GM1 and anti-GA1 antibodies were much higher than the titers of anti-GQ1b antibodies. In animals that were immunized with GB18 and MF8 LPS, IgM and IgG responses against LPS and gangliosides differed during the immunization period. IgG responses gradually increased and reached a high titer, while IgM responses remained at the same level or even decreased. This stepwise increase in IgG reactivity suggests a booster effect of the repeated immunizations and T-cell involvement in the response to these nonprotein antigens. In GBS and MFS patients, the IgG response against gangliosides and LPS is of the IgG1 and IgG3 subclasses (10, 20, 30, 33). Unfortunately, lack of information of IgG subclasses in rabbits and lack of specific reagents prohibited further investigation of this issue.
Contrary to our expectations, we observed a difference in the kinetics of anti-LPS and antiganglioside reactivity in some animals. One would expect a simultaneous rise in anti-LPS and antiganglioside reactivity because these antibodies are cross-reactive. There are several explanations for this phenomenon. First, the ELISA systems that detect antiganglioside and anti-LPS reactivity may have different sensitivities, thereby masking an early antiganglioside response that is still below the detection limit. Second, in the early phase of the immunization period, the anti-LPS antibodies may be directed against epitopes on the LPS molecule other than the ganglioside-like oligosaccharide, while the specificity against the core oligosaccharide develops in the latter phase of the immunization period.
During the 56 days of the experiment, none of the animals developed clear clinical signs of neuropathy. One animal that was immunized with GB18 LPS showed remarkable weight loss but had no clinical or histological signs of neuropathy. This lack of clinical symptoms despite the apparent good antibody response against LPS may depend on several factors. First, the animals may have had subclinical nerve damage detectable by electrophysiological examination only (27). Second, the duration of the experiment may have been too short for the animals to develop clinical symptoms. Some galactocerebroside- and GD1b-immunized animals that developed neuropathy had the first clinical symptoms only after several months (15, 25), by which time the animals in our experiment had already been sacrificed. Third, an intact blood-nerve barrier might exclude the antiganglioside antibodies from the nerve, and fourth, species differences in glycolipid composition might render rabbit nerves relatively insensitive to the action of the antiganglioside antibodies.
In conclusion, we have demonstrated an antiganglioside response following immunization of rabbits with LPS from GBS- and MFS-associated C. jejuni strains. The specificity of the antiganglioside antibodies in rabbits is similar to that observed in patients, thereby confirming the role of molecular mimicry in the development of antiganglioside antibodies in patients with postinfectious neuropathy. The pathogenic potential of these anti-LPS and antiganglioside responses remains to be determined.
ACKNOWLEDGMENTS
This work was supported by grants from the Prinses Beatrix Fonds (grant 95–518) and The Netherlands Organization for Scientific Research (grant 940-37-012) to C.W.A.
We thank R. G. C. Hoogendoorn, R. Spruit, A. P. Tio-Gillen, N. Van den Braak and P. Van der Heul for technical assistance; T. M. van Os for preparing the figures; and F Rodgers, Laboratory Centre for Disease Control, Winnipeg, Canada, for serotyping.
REFERENCES
- 1.Ang C W, Endtz H P, Jacobs B C, Laman J D, De Klerk M A, Van der Meché F G A, Van Doorn P A. Campylobacter jejuni lipopolysaccharides from Guillain-Barré syndrome patients induce IgG anti-GM1 antibodies in rabbits. J Neuroimmunol. 2000;104:133–138. doi: 10.1016/s0165-5728(99)00279-9. [DOI] [PubMed] [Google Scholar]
- 2.Aspinall G O, Fujimoto S, McDonald A G, Pang H, Kurjanczyk L A, Penner J L. Lipopolysaccharides from Campylobacter jejuni associated with Guillain-Barré syndrome patients mimic human gangliosides in structure. Infect Immun. 1994;62:2122–2125. doi: 10.1128/iai.62.5.2122-2125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspinall G O, Lynch C M, Pang H, Shaver R T, Moran A P. Chemical structures of the core region of Campylobacter jejuni O:3 lipopolysaccharide and an associated polysaccharide. Eur J Biochem. 1995;231:570–578. [PubMed] [Google Scholar]
- 4.Aspinall G O, McDonald A G, Raju T S, Pang H, Moran A P, Penner J L. Chemical structures of the core regions of Campylobacter jejuni serotypes O:1, O:4, O:23, and O:36 lipopolysaccharides. Eur J Biochem. 1993;213:1017–1027. doi: 10.1111/j.1432-1033.1993.tb17849.x. [DOI] [PubMed] [Google Scholar]
- 5.Chiba A, Kusunoki S, Obata H, Machinami R, Kanazawa I. Serum anti-GQ1b IgG antibody is associated with ophthalmoplegia in Miller Fisher syndrome and Guillain-Barré syndrome: clinical and immunohistochemical studies. Neurology. 1993;43:1911–1917. doi: 10.1212/wnl.43.10.1911. [DOI] [PubMed] [Google Scholar]
- 6.Goodyear C S, O'Hanlon G M, Plomp J J, Wagner E R, Morrison I, Veitch J, Cochrane L, Bullens R W, Molenaar P C, Conner J, Willison H J. Monoclonal antibodies raised against Guillain-Barré syndrome-associated Campylobacter jejuni lipopolysaccharides react with neuronal gangliosides and paralyze muscle-nerve preparations. J Clin Investig. 1999;104:697–708. doi: 10.1172/JCI6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregson N A, Jones D, Thomas P K, Willison H J. Acute motor neuropathy with antibodies to GM1 ganglioside. J Neurol. 1991;238:447–451. doi: 10.1007/BF00314652. [DOI] [PubMed] [Google Scholar]
- 8.Hao Q, Saida T, Kuroki S, Nishimura M, Nukina M, Obayashi H, Saida K. Antibodies to gangliosides and galactocerebroside in patients with Guillain-Barré syndrome with preceding Campylobacter jejuni and other identified infections. J Neuroimmunol. 1998;81:116–126. doi: 10.1016/s0165-5728(97)00166-5. [DOI] [PubMed] [Google Scholar]
- 9.Ho T W, Hsieh S T, Nachamkin I, Willison H J, Sheikh K, Kiehlbauch J, Flanigan K, McArthur J C, Cornblath D R, McKhann G M, Griffin J W. Motor nerve terminal degeneration provides a potential mechanism for rapid recovery in acute motor axonal neuropathy after Campylobacter infection. Neurology. 1997;48:717–724. doi: 10.1212/wnl.48.3.717. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs B C, Endtz H P, Van der Meché F G A, Hazenberg M P, De Klerk M A, Van Doorn P A. Humoral immune response against Campylobacter jejuni lipopolysaccharides in Guillain-Barré and Miller Fisher syndrome. J Neuroimmunol. 1997;79:62–68. doi: 10.1016/s0165-5728(97)00110-0. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs B C, Hazenberg M P, Van Doorn P A, Endtz H P, Van der Meché F G A. Cross-reactive antibodies against gangliosides and Campylobacter jejuni lipopolysaccharides in patients with Guillain-Barré or Miller Fisher syndrome. J Infect Dis. 1997;175:729–733. doi: 10.1093/infdis/175.3.729. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs B C, Rothbarth P H, Van der Meché F G A, Herbrink P, Schmitz P I M, De Klerk M A, Van Doorn P A. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology. 1998;51:1110–1115. doi: 10.1212/wnl.51.4.1110. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs B C, Van Doorn P A, Schmitz P I M, Tio-Gillen A P, Herbrink P, Visser L H, Hooijkaas H, Van der Meché F G A. Campylobacter jejuni infections and anti-GM1 antibodies in Guillain-Barré syndrome. Ann Neurol. 1996;40:181–187. doi: 10.1002/ana.410400209. [DOI] [PubMed] [Google Scholar]
- 14.Kuroki S, Saida T, Nukina M, Haruta T, Yoshioka M, Kobayashi Y, Nakanishi H. Campylobacter jejuni strains from patients with Guillain-Barré syndrome belong mostly to Penner serogroup 19 and contain beta-N-acetylglucosamine residues. Ann Neurol. 1993;33:243–247. doi: 10.1002/ana.410330304. [DOI] [PubMed] [Google Scholar]
- 15.Kusunoki S, Shimizu J, Chiba A, Ugawa Y, Hitoshi S, Kanazawa I. Experimental sensory neuropathy induced by sensitization with ganglioside GD1b. Ann Neurol. 1996;39:424–431. doi: 10.1002/ana.410390404. [DOI] [PubMed] [Google Scholar]
- 16.Kuwabara S, Yuki N, Koga M, Hattori T, Matsuura D, Miyake M, Noda M. IgG anti-GM1 antibody is associated with reversible conduction failure and axonal degeneration in Guillain-Barré syndrome. Ann Neurol. 1998;44:202–208. doi: 10.1002/ana.410440210. [DOI] [PubMed] [Google Scholar]
- 17.Moran A P. Structure and conserved characteristics of Campylobacter jejuni lipopolysaccharides. J Infect Dis. 1997;176:S115–S121. doi: 10.1086/513781. [DOI] [PubMed] [Google Scholar]
- 18.Moran A P, Rietschel E T, Kosunen T U, Zahringer U. Chemical characterization of Campylobacter jejuni lipopolysaccharides containing N-acetylneuraminic acid and 2,3-diamino-2,3-dideoxy-d-glucose. J Bacteriol. 1991;173:618–626. doi: 10.1128/jb.173.2.618-626.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neisser A, Bernheimer H, Berger T, Moran A P, Schwerer B. Serum antibodies against gangliosides and Campylobacter jejuni lipopolysaccharides in Miller Fisher syndrome. Infect Immun. 1997;65:4038–4042. doi: 10.1128/iai.65.10.4038-4042.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogino M, Orazio N, Latov N. IgG anti-GM1 antibodies from patients with acute motor neuropathy are predominantly of the IgG1 and IgG3 subclasses. J Neuroimmunol. 1995;58:77–80. doi: 10.1016/0165-5728(94)00190-y. [DOI] [PubMed] [Google Scholar]
- 21.Plomp J J, Molenaar P C, O'Hanlon G M, Jacobs B C, Veitch J, Daha M R, Van Doorn P A, Van der Meché F G A, Vincent A, Morgan B P, Willison H J. Miller Fisher anti-GQ1b antibodies: alpha-latrotoxin-like effects on motor endplates. Ann Neurol. 1999;45:189–199. doi: 10.1002/1531-8249(199902)45:2<189::aid-ana9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 22.Preston M A, Penner J L. Characterization of cross-reacting serotypes of Campylobacter jejuni. Can J Microbiol. 1989;35:265–273. doi: 10.1139/m89-040. [DOI] [PubMed] [Google Scholar]
- 23.Rees J H, Soudain S E, Gregson N A, Hughes R A. Campylobacter jejuni infection and Guillain-Barré syndrome. N Engl J Med. 1995;333:1374–1379. doi: 10.1056/NEJM199511233332102. [DOI] [PubMed] [Google Scholar]
- 24.Ritter G, Fortunato S R, Cohen L, Noguchi Y, Bernard E M, Stockert E, Old L J. Induction of antibodies reactive with GM2 ganglioside after immunization with lipopolysaccharides from Campylobacter jejuni. Int J Cancer. 1996;66:184–190. doi: 10.1002/(SICI)1097-0215(19960410)66:2<184::AID-IJC8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 25.Saida T, Saida K, Dorfman S H, Silberberg D H, Sumner A J, Manning M C, Lisak R P, Brown M J. Experimental allergic neuritis induced by sensitization with galactocerebroside. Science. 1979;204:1103–1106. doi: 10.1126/science.451555. [DOI] [PubMed] [Google Scholar]
- 26.Sheikh K A, Nachamkin I, Ho T W, Willison H J, Veitch J, Ung H, Nicholson M, Li C Y, Wu H S, Shen B Q, Cornblath D R, Asbury A K, McKhann G M, Griffin J W. Campylobacter jejuni lipopolysaccharides in Guillain-Barré syndrome: molecular mimicry and host susceptibility. Neurology. 1998;51:371–378. doi: 10.1212/wnl.51.2.371. [DOI] [PubMed] [Google Scholar]
- 27.Thomas F P, Trojaborg W, Nagy C, Santoro M, Sadiq S A, Latov N, Hays A P. Experimental autoimmune neuropathy with anti-GM1 antibodies and immunoglobulin deposits at the nodes of Ranvier. Acta Neuropathol. 1991;82:378–383. doi: 10.1007/BF00296548. [DOI] [PubMed] [Google Scholar]
- 28.Westphal O, Jann K. Bacterial lipopolysaccharides. Extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 29.Willison H J, O'Hanlon G M, Paterson G, Veitch J, Wilson G, Roberts M, Tang T, Vincent A. A somatically mutated human antiganglioside IgM antibody that induces experimental neuropathy in mice is encoded by the variable region heavy chain gene, V1–18. J Clin Invest. 1996;97:1155–1164. doi: 10.1172/JCI118529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willison H J, Veitch J. Immunoglobulin subclass distribution and binding characteristics of anti-GQ1b antibodies in Miller Fisher syndrome. J Neuroimmunol. 1994;50:159–165. doi: 10.1016/0165-5728(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 31.Willison H J, Veitch J, Paterson G, Kennedy P G. Miller Fisher syndrome is associated with serum antibodies to GQ1b ganglioside. J Neurol Neurosurg Psychiatry. 1993;56:204–206. doi: 10.1136/jnnp.56.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wirguin I, Briani C, Suturkova-Milosevic L, Fisher T, Della-Latta P, Chalif P, Latov N. Induction of anti-GM1 ganglioside antibodies by Campylobacter jejuni lipopolysaccharides. J Neuroimmunol. 1997;78:138–142. doi: 10.1016/s0165-5728(97)00095-7. [DOI] [PubMed] [Google Scholar]
- 33.Yuki N, Ichihashi Y, Taki T. Subclass of IgG antibody to GM1 epitope-bearing lipopolysaccharide of Campylobacter jejuni in patients with Guillain-Barré syndrome. J Neuroimmunol. 1995;60:161–164. doi: 10.1016/0165-5728(95)00052-4. [DOI] [PubMed] [Google Scholar]