Abstract
The ventral hippocampus (vHPC) has long been thought of as the “emotional” hippocampus. Over the past several years, the complexity of vHPC has come to light, highlighting the diversity of cell types, inputs, and outputs that coordinate a constellation of positively and negatively motivated behaviors. Here, we review recent work on how vCA1 contributes to a network that associates external stimuli with internal motivational drive states to promote the selection of adaptive behavioral responses. We propose a model of vHPC function that emphasizes its role in the integration and transformation of internal and external cues to guide behavioral selection when faced with multiple potential outcomes.
Introduction
The ventral hippocampus (vHPC) is a key node in the extended network that generates emotional and motivated behavior. In recent years, there has been growing interest in vHPC function, the mechanisms by which it contributes to specific behaviors, and how its properties differentiate vHPC networks from the well-studied dorsal hippocampal network. This has led to numerous studies delineating the circuits and cell types in vHPC, specifically within the vCA1 subregion, identifying their unique wiring patterns and their diverse functional properties. The complexity of vCA1 has come to light, with distinct components of its structure (cell types, inputs, and outputs) hypothesized to differentially encode features of an explored environment and ongoing internal drive states to promote adaptive behavioral outputs. The field has progressed considerably since lesion and manipulation studies identified the dorsal HPC (dHPC) as a controller of cognitive functions and vHPC as a regulator of unconditioned fear and anxiety responses [1–4], and human studies established corresponding roles in the posterior and anterior HPC [5]. Given the new findings, it is time to refine the current abstract model of vCA1 into one that highlights the rich heterogeneity of the region.
In the following sections, we discuss recent progress and put forward a more mechanistic model for vCA1 function. We propose that vCA1 neurons encode stimuli that have immediate significance for the animal, generating a map that links external stimuli with internal drive states. This is analogous to the well-described properties of assemblies of dCA1 neurons that exhibit location-, stimulus-, and time-specific discharge patterns to generate a map of space ([6,7], among many others). We suggest that ensembles of vCA1 neurons store experiences imbued with the motivation to avoid danger, eat, find mates, and gain or maintain social status. These ensembles of vCA1 neurons are largely anatomically segregated (Box 1) and, therefore, able to route specific information to distinct downstream targets and drive the selection of appropriate adaptive behavioral responses.
BOX 1. Anatomical organization of vCA1.
As described in detail, in this review and elsewhere, vCA1 neurons project to several cortical and subcortical areas implicated in mood/anxiety-related behavior, reward seeking, social approach, and neuroendocrine responses to stress. These include (but are not limited to) outputs to the medial prefrontal cortex (mPFC), nucleus accumbens (NAc), lateral hypothalamus (LH), lateral septum (LS), bed nucleus of the stria terminalis (BNST), and basal and central amygdala (BA, CeA). Retrograde tracing studies and single neuron reconstructions have indicated that these outputs are non-overlapping [9,20,21,71,72]. A recent study using high-throughput sequencing of genetically barcoded neurons (MAPseq) to map the axonal projections of vCA1 neurons found that while many vCA1 neurons show a one-to-one connectivity with downstream areas, as predicted by retrograde tracing, a significant portion of neurons broadcast to multiple downstream areas in a non-random fashion [15]. This indicates that vCA1 contains a mix of single-target neurons and neurons that send highly collateralized outputs. For example, neurons that project to the LS were found by both MAPseq [15], and single neuron tracing studies [71] to send collaterals to multiple downstream areas, including the NAc, BNST, LH, and mPFC. The specialized function of these and other collateralized neurons remains to be fully delineated, but their existence suggests that information in a subclass of vCA1 neurons is relayed to many downstream areas. An active area of research is whether these collateralized neurons exhibit information coding properties that are distinct from those of neurons with a single target. One study addressing this question found that vCA1 neurons with trifurcating (or more) projections to the NAc, mPFC, and amygdala were preferentially activated during sharp-wave ripples and during approach/avoidance and reward learning [8]. Input-output tracing using rabies virus approaches has revealed that vCA1 neurons targeting distinct areas receive similar upstream input, suggesting that vCA1 integrates incoming information and sends it to multiple downstream areas [15]. Some subtle biases to input patterns were found that warrant future investigation, such as the preferential targeting of PVT inputs to vCA1-LH neurons versus vCA1-mPFC/vCA1-BA neurons, and the denser innervation of vCA1-BNST neurons by inputs from the amygdala. A recent study also demonstrated that inhibitory and excitatory input from the amygdala to vHPC can differentially modulate projection neurons to the amygdala, mPFC, and BA [73]. Further investigation is needed to elaborate how different inputs to vCA1 shape the region’s outputs in a projection-specific way. In addition, it is important to build an understanding of how long-range inputs interface with local vCA1 microcircuits to fine-tune the vCA1 output in the control of behavior. As recently described, PV local interneurons preferentially target vCA1-BA output neurons and receive innervation from vCA1-mPFC neurons [74].
Here, we review recent circuit-based studies in rodents that provide support for this important aspect of HPC function. In addition, we discuss how dysfunction in this process may contribute to mood and anxiety disorders. Owing to the focused nature of this review, we will limit our analysis to the primary output of vHPC, the vCA1/vSub subregions.
vCA1 in motivation to avoid
One of the most well-described properties of vCA1 has been its ability to generate representations of innately anxiogenic or fearful environments and and enable an animal to react accordingly. Single-unit electrophysiological recordings and calcium imaging data from rodent models have demonstrated that neurons in vCA1, but not dCA1, have stable responses across anxiety-provoking areas of the elevated plus maze (EPM) and open field test (OFT) [8,9]. In the EPM, activity in vCA1 both correlates with baseline anxiety state and scales with the aversive nature of the cues in the task [9]. In addition, optogenetic silencing of vCA1 in mice during exploration of the anxiety-provoking areas of these assays reduces avoidance [9]. Silencing vHPC, but not dHPC, also impedes an animal’s ability to associate a specific context with a fearful experience, as measured by the context specificity of tone-signaled active avoidance behavior [10]. These data demonstrate a role for vCA1 in encoding salient spatial stimuli, scaling these representations based on anxiety state and the aversiveness of the environment, and transforming these representations into an output signal that can be decoded by a downstream area for appropriate action selection (Figure 1).
Figure 1. Hypothesized model for vCA1 encoding properties and engagement of downstream targets.

First, vCA1 integrates diverse external and internal stimuli and then shapes output signals to engage downstream areas for appropriate behavioral selection. This is a shared feature among a number of approach/avoidance behaviors such as in the elevated plus maze (a), social interaction (b), and food approach/consumption (c). The circuit mechanisms and local computations in vCA1 that integrate inputs to tune output signals remains an area of active investigation.
While vCA1 as a whole is clearly involved in the integration of anxiogenic stimuli and the transformation of this information into appropriate behavior, sub-populations of vCA1 neurons have been implicated in specific aspects of this process. vCA1 cells encoding anxiogenic features of mazes are more abundant in populations projecting to the medial prefrontal cortex (mPFC) or lateral hypothalamus (LH), but not in neurons projecting to the nucleus accumbens (NAc) or the amygdala [8,9]. Optogenetic stimulation of vCA1-LH projection neurons decreases the exploration of anxiogenic portions of the EPM and OFT and drives avoidance in a real-time place preference assay, while inhibition reduces open arm avoidance in the EPM [9]. Similarly, when vHPC inputs to mPFC are inhibited either optogenetically or pharmacologically, open arm avoidance decreases [11,12]. Thus, vHPC projections to mPFC and LH play a role in transforming representations of anxiogenic stimuli into actions that promote avoidance. Furthermore, mPFC neurons that encode anxiety-related information in the EPM also synchronize their firing with theta-frequency (4–12 Hz) oscillations in vHPC [12–14]. Interestingly, vCA1 neurons that project to either mPFC or LH do not tend to collateralize [9,15,16], raising the interesting possibility that these distinct output streams from vCA1 may encode specific features of a fearful environment that drive diverse classes of behavior not captured by the gross assessments used in previous studies. Recent experiments indicate that, even within the projection to mPFC, there exists functional heterogeneity, with deep and superficial layer projection neurons differentially responding to safe versus anxiogenic areas of the EPM [17]. Active areas of investigation include how distinct subclasses of projection neurons encode features of an anxiogenic environment and how local circuits contribute to the transformation of this information into an output signal that is decoded by mPFC and LH to initiate appropriate approach/avoidance decisions.
Unlike the projections to mPFC and LH, vCA1-basal amygdala (BA) projecting neurons do not seem to encode anxiogenic features of anxiety-based assays but do respond to footshocks in a contextual fear conditioning assay and are necessary for encoding context-fear associations [9,18–20]. Meanwhile, vCA1 projections to the central nucleus of the amygdala (CeA) have been implicated in context-dependent fear renewal [20], which has also been shown to recruit vCA1 outputs to prelimbic (PL) and infralimbic cortex (IL) [21]. This leads to the hypothesis that certain vCA1 projections, like vCA1-LH, may link external stimuli with internal drives to avoid more distal/diffuse threats, while other vCA1 projections, like vCA1-amygdala, vCA1-PL, and vCA1-IL, may create relationships between contextual stimuli and internal drives to avoid more proximal and immediate threats. More recently, a role for vHPC has been identified in observational fear learning. This study found that a subset of vHPC neurons responds to a familiar demonstrator mouse during observational fear by reactivating previously learned context-fear ensembles in the BLA [22].
Another area involved in the processing of diffuse threats, the BNST, receives dense input from vCA1 and has been less well studied. High-frequency electrical stimulation of vHPC can induce anxiolysis in rats, an effect blocked by intra-BNST NMDA antagonists [23]. In addition, vCA1 may exert its inhibitory control of the stress response [24] through the BNST; the BNST sends a GABAergic projection to the paraventricular hypothalamic nucleus (PVN) of the hypothalamic-pituitary-adrenal (HPA) axis [25–27]. With respect to other projection outputs, mice susceptible to chronic stress show increased activity in vHPC-NAc projection neurons [28], and individual differences in the activity of this pathway predict vulnerability to stress [29]. In addition, excess corticosterone in adolescence weakens vHPC inputs to the orbitofrontal cortex [30]. Finally, chronic stress produces a reduction in synaptic strength at vHPC-NAc synapses, specifically on D1-receptor containing medium spiny neurons, an effect that can be reversed by chronic antidepressant treatment [31]. These studies are only a few of the many that have attempted to understand how vHPC activity may be impacted in chronic stress, and future functional mapping studies can uncover how stress modulates vCA1 during related behavioral phenotypes, including avoidance and anhedonia [32]. Together, these results demonstrate the dual roles these vHPC projections play in both the integration of information and the translation of this information into a behavioral response.
vHPC and motivation to seek reward
Recent studies have highlighted the role of reward in modifying representations of diverse stimuli in the hippocampus [33,34]. In addition, place-reward representations have been well studied in HPC, and there are many excellent recent reviews on this topic (e.g., [35,36]). Both dHPC and vHPC project to and functionally interact with NAc during navigation for reward [37,38]. In the vHPC, antidromically identified vCA1-NAc projection neurons are activated during the approach to a goal location in maze-based tasks [8].
Independent of place, recent work has identified a role for this vHPC-NAc circuit in connecting the hedonic value of food with the internal drive to seek food (Figure 1). Low-frequency photostimulation of this pathway, but not other inputs to the NAc, increased the palatability of a sugar reward (as measured in licks per bout) and drove preference to a flavor paired with the stimulation, while inhibition reduced innate preference for a high-value reward [39]. In addition to the role vHPC-NAc plays in encoding palatability, vHPC-NAc activity has been implicated in the transition from anticipatory-feeding behavior to consumption. This occurs when the hunger-promoting hormone ghrelin inhibits vHPC-NAc neurons via GHSR1a receptors [40]. In line with this, optogenetic inhibition of vHPC-NAc stimulated greater eating in animals given free access to food [41]. Ghrelin may also exert its effects via the vHPC-LH pathway, where it can attenuate satiety signals by generating an interoceptive energy-deficient state [42]. Ghrelin infusion into vHPC promotes sated sucrose seeking and increases meal size. These effects are driven by vHPC outputs to LH orexin neurons that in turn project to the lateral dorsal tegmental nucleus (LDTg) [42]. These experiments highlight the rich heterogeneity of vHPC, demonstrating that vHPC engages distinct pathways involved in at least two different aspects of feeding: palatability and consumption. Future studies identifying how distinct targets of vHPC modulate these different components of feeding will shed new light on the role of vCA1 in food approach and consumption.
The vHPC has been implicated in modulating adaptive learning in pursuit of reward. For example, a recent study using odor-guided learning found that vCA1 neurons do not represent odorants at baseline and only gain representations after odor-reward learning. Odor-reward learning rapidly reorganized ensembles of neurons in vCA1 and stored these rewarded odor representations for days after learning [33]. In addition, recent work has indicated that these outputs from vCA1 can be modified via novelty—mice that explored a novel arena prior to testing in a reward-guided T-maze showed weakened vHPC-mPFC functional connectivity and were better able to learn the task. This indicates that one function of vCA1 (specifically vCA1-mPFC) is to recognize novelty and use it to modify encoded representations so that animals are better able to successfully seek reward [43].
While these studies indicate that distinct pathways may encode stimuli of specific valences, this specification is most likely plastic. For example, a recent study found that vCA1-BLA and vCA1-NAc projections could drive either preference or avoidance behavior depending on whether the neurons were associated with a positive or negative engram [44]. Such data suggest that these projections do not have fixed roles, and that context is extremely important when understanding the function of vCA1 projections in motivated behavior.
vCA1 in motivation to seek social interaction
In recent years, vHPC has been linked to social behavior, social interaction, social memory, and the representation of social hierarchy [45–48]. An animal may be motivated to approach, avoid, or ignore another animal based on characteristics of both social partners such as sex or relative social standing and by the familiarity or novelty of the other moiety. vCA1 neurons can distinguish between a familiar and novel mouse, and targeted inhibition of either whole vCA1 or CA1-NAc projection neurons (but not vCA1-BLA) reduces discrimination between novel and familiar mice [47]. In addition, vCA1 social memory neurons are reactivated during sharp-wave ripples (SWRs) offline, with a similar temporal pattern to that observed during online social interaction [49]. Interestingly, in a mouse model for autism (Shank3 KO), the number of vCA1 social memory neurons is reduced, and the sequence of neurons reactivated during offline SWRs was disrupted, suggesting a role for vCA1 in social deficits of autism-related mouse models [49]. In rats, vCA1 cells that respond when a rat interacts with a conspecific are sensitive to whisker touch interactions and ultrasonic vocalizations but show little response to an inanimate object [50]. The vCA1-mPFC projection has also been implicated in social behavior—chemogenetic excitation of this pathway or overactivity (in a mouse model of Rett syndrome) produces deficits in discriminating a novel and a familiar mouse [51]. Local silencing of PV-expressing interneurons in vCA1 can impair social discrimination, revealing an important role for local circuit dynamics in vCA1 storage of social memories. CA2 plays a central role in encoding social information [52] and projects to vCA1, creating a local circuit known to be important for social memory [45]. Other ventral hippocampal subregions, such as CA3, have also been linked to social memory [53]. In contrast, the inhibition of dCA1 or the pathway from vCA1 to BLA does not affect social discrimination [47]. Thus, specific pathways from vCA1 to NAc and mPFC are important for integrating external social cues with the internal motivation to interact with conspecifics (Figure 1).
Human studies have hinted at the importance of the hippocampus in social relationships and interactions. In a game where power and affiliation were modeled as two dimensions of social distance, the left hippocampus represented social distance between the participant and virtual characters in the game [54]. This signal was also influenced by the participant’s personality traits—participants who reported less social avoidance and neuroticism showed stronger hippocampal tracking of the relative social standing. Future studies are needed to address the contributions of specific hippocampal subregions to social interaction.
Anterior hippocampus in psychiatric illness and mood
The human hippocampus has been studied for decades in relation to psychiatric illness, and the region plays an important role in the motivations to avoid danger, pursue reward, and seek social interaction, and to the formation of internal models of these three drives. Foundational work has shown decreased overall hippocampal volume in psychiatric mood disorders [55]. More specifically, mood disorders have been associated with a decrease in the number of neurons of the anterior hippocampus (aHPC) which was reversible with antidepressant treatment [56].
The primate aHPC, while analogous to vHPC in rodents, is typically larger than the posterior hippocampus, features unique extensions like the uncus, and carries more hippocampal commissural connections [57,58]. Bulk sequencing shows transcriptional similarities and functional covariance between aHPC and brain networks active during social and emotional cognition and motivational tasks [59]. Single-nucleus sequencing of the human hippocampus has demonstrated that genes identified in gene-wide association studies and linked to major depression and bipolar disorder are significantly upregulated in the aHPC [60],
Depressed patients show reduced functional connectivity between the anterior/intermediate hippocampus and the insula/NAc, and symptoms of depression are positively correlated with aHPC-NAc connectivity [61]. Similarly, patients diagnosed with post-traumatic stress disorder (PTSD) display alterations in hippocampal functional connectivity. Resting-state functional connectivity of the hippocampus as a whole is not detectably different between PTSD patients and trauma-exposed controls without PTSD. However, separating the anterior and posterior hippocampus reveals differences in anterior-posterior connectivity between the hippocampus and the precuneus and posterior cingulate cortex in trauma-exposed controls, whereas PTSD patients lack these differences [62]. In another study, veterans with PTSD showed an inverse correlation between PTSD symptoms and the anatomical and functional connectivity of the aHPC, with symptoms of hypervigilance being positively associated with reduced anatomical connectivity between the aHPC and the prefrontal cortex [63]. At the functional level, a meta-analysis of fMRI activation in the brains of PTSD patients revealed greater aHPC activity during each of the phases of fear conditioning: conditioning, extinction, and recall [64]. Finally, in a human intracranial EEG study, increased variance in HPC-amygdala coherence at the beta frequency range could predict a worsening in the subjective mood of a patient subset [65].
Together, these studies show predispositions and alterations in aHPC structure and activity that correspond to alterations in human behavior and motivational drives. Future studies linking these changes to actionable biomarkers could be used to pinpoint the early stages of psychiatric disorders when symptoms and changes in hippocampal structure are less pronounced and potentially more treatable.
Conclusion
In recent years, there has been an increasing interest in the vHPC’s role in motivated and emotional behaviors. This paper reviews several important studies that have dissected the principles of the inputs and outputs of vCA1 and highlighted how distinct output streams may represent diverse features of an explored space to drive adaptive behaviors. However, a number of questions remain. First, how ensembles of vCA1 neurons interact to encode divergent behavioral states remains unknown. How these dynamics map on to the well-described anatomy of vCA1 also remains understudied (Figure 2 and Box 1). Second, it is not well understood how emotional state (such as chronic stress, antidepressant treatment, and exercise) impacts the encoding properties of anatomically and functionally defined ensembles described here. Finally, as anxiety is fundamentally a response to diffuse and unknown threats that may elicit harm in the future, it remains important to understand how prospective coding in the vHPC relates to its role in anxiety-related behavior. Functional MRI studies in humans have suggested that the aHPC can recombine details from past experience to construct an imagined future [66]. In dCA1, there is a rapid alternation between the representation of possible future goal locations [67]; in vCA1, future research will determine how prospective coding represents safe versus aversive options. Understanding these phenomena may explain why individuals with mood and anxiety disorders generate less positive and less detailed imagined futures [68–70]. Together, future studies dissecting the cell types, population activity patterns, and behavioral functions of CA1 circuits will undoubtedly enrich our understanding of emotional and motivated states in health and disease.
Figure 2. Functional anatomy of the vCA1 circuit.
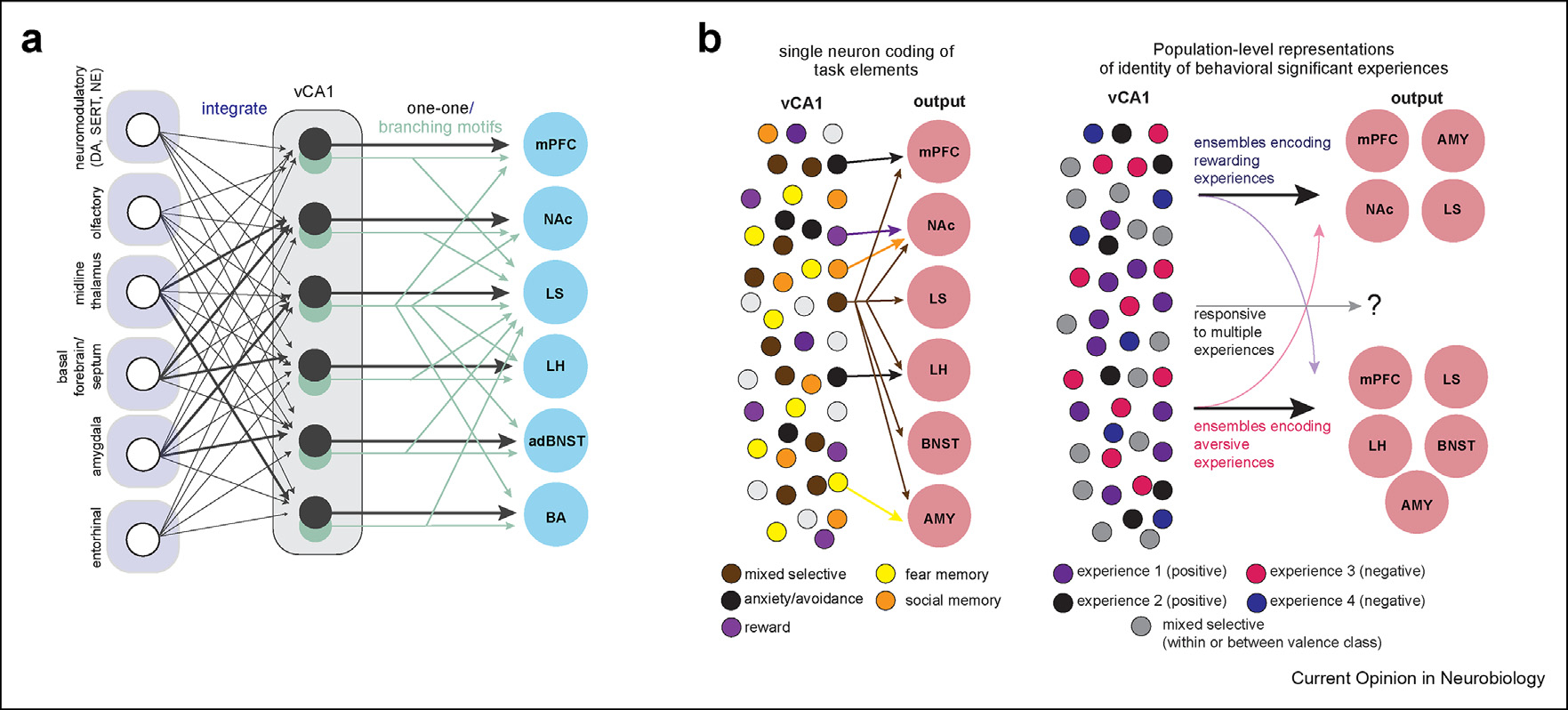
a. Input-output organization of a subset of vCA1 connections. Modified from Ref. [15]. Based on the analysis of six projection targets of vCA1, it is believed that vCA1 largely integrates input, with some biases in the proportion of upstream input. Output to downstream areas is either via one-to-one connections (black) with a given target or connections that branch to multiple downstream areas (light green). It is important to note that this anatomical map is incomplete, as vCA1 receives input from other areas and sends output to several downstream targets not depicted here, and organizational principles to these areas remain unclear. b. Potential anatomical-functional relationships in vCA1. Left. In this well-studied scenario, single neurons encode diverse stimuli and serve different behavioral functions, and this is parcellated into distinct projection streams. Examples of this scenario include the finding that social memory is encoded in vCA1-NAc projections [47] while vCA1-LH neurons encode anxiety-related stimuli [9]. Right. In an alternate, non-exclusive situation, populations of vCA1 neurons encode behaviorally significant experiences [33], and vCA1 ensembles discriminate based on whether the experience is one that promotes approach or avoidance. Then, these ensembles interact with appropriate downstream areas for action selection. Whether different stimuli of the same valence class are encoded by distinct ensembles of neurons, and how different ensembles that encode different salient stimuli route this information to downstream areas remains an open area of investigation.
Acknowledgements
MAK is supported by NIMH (R01 MH108623, R01 MH111754, R01 MH117961), NIDCD (R01 DC019813) a One Mind Rising Star Award, a Research Grant from HFSP (Ref. No- RGY0072/2019), the Esther A. and Joseph Klingenstein Fund, the Pew Charitable Trusts, the McKnight Memory and Cognitive Disorders Award and The Ray and Dagmar Dolby Family Fund. Mouse images in Figure 1 were created with BioRender.
Footnotes
Conflict of interest statement
None declared.
References
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Fanselow MS, Dong H-W: Are the Dorsal and Ventral Hippocampus functionally distinct structures? Neuron 2010, 65:7, 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kheirbek MA, Drew LJ, Burghardt NS, Costantini DO, Tannenholz L, Ahmari SE, et al. : Differential control of learning and anxiety along the dorsoventral Axis of the dentate gyrus. Neuron 2013, 77:955–968, 10.1016/j.neuron.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kjelstrup KG, Tuvnes FA, Steffenach H-A, Murison R, Moser EI, Moser M-B: Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A 2002, 99:10825–10830, 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strange BA, Witter MP, Lein ES, Moser EI: Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 2014, 15:655–669, 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- 5.Poppenk J, Evensmoen HR, Moscovitch M, Nadel L: Long-axis specialization of the human hippocampus. Trends Cognit Sci 2013, 17:230–240, 10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 6.O’Keefe J, Nadel L: The Hippocampus as a cognitive map. Oxford, UK: Oxford University Press; 1978. [Google Scholar]
- 7.McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser M-B: Path integration and the neural basis of the “cognitive map. Nat Rev Neurosci 2006, 7:663–678, 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- 8.Ciocchi S, Passecker J, Malagon-Vina H, Mikus N, Klausberger T: Brain computation. Selective information routing by ventral hippocampal CA1 projection neurons. Science 2015, 348:560–563, 10.1126/science.aaa3245. [DOI] [PubMed] [Google Scholar]
- 9.Jimenez JC, Su K, Goldberg AR, Luna VM, Biane JS, Ordek G, et al. : Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 2018, 97:670–683, 10.1016/j.neuron.2018.01.016. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oleksiak CR, Ramanathan KR, Miles OW, Perry SJ, Maren S, Moscarello JM: Ventral hippocampus mediates the context-dependence of two-way signaled avoidance in male rats. Neurobiol Learn Mem 2021, 183:107458, 10.1016/j.nlm.2021.107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjaerby C, Athilingam J, Robinson SE, Iafrati J, Sohal VS: Serotonin 1B receptors regulate prefrontal function by gating callosal and hippocampal inputs. Cell Rep 2016, 17:2882–2890, 10.1016/j.celrep.2016.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, et al. : Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron 2016, 89:857–866, 10.1016/j.neuron.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adhikari A, Topiwala MA, Gordon JA: Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron 2011, 71:898–910, 10.1016/j.neuron.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adhikari A, Topiwala MA, Gordon JA: Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 2010, 65:257–269, 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.••. Gergues MM, Han KJ, Choi HS, Brown B, Clausing KJ, Turner VS, et al. : Circuit and molecular architecture of a ventral hippocampal network. Nat Neurosci 2020, 23:1444–1452, 10.1038/s41593-020-0705-8. This study uses novel high-throughput single cell anatomical tracing tools and viral input–output tracing to define the organization of the vCA1 circuit.
- 16.Wee RWS, MacAskill AF: Biased connectivity of brain-wide inputs to ventral subiculum output neurons. Cell Rep 2020, 30:3644–3654, 10.1016/j.celrep.2020.02.093. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.••. Sánchez-Bellot C, AlSubaie R, Mishchanchuk K, Wee RWS, MacAskill AF: Two opposing hippocampus to prefrontal cortex pathways for the control of approach and avoidance behaviour. Nat Commun 2022, 13:339, 10.1038/s41467-022-27977-7. This study identifies two parallel outputs from vHPC to mPFC that coordinate approach and avoidance in the EPM.
- 18.Graham J, D’Ambra AF, Jung SJ, Teratani-Ota Y, Vishwakarma N, Venkatesh R, et al. : High-frequency stimulation of ventral CA1 neurons reduces amygdala activity and inhibits fear. Front Behav Neurosci 2021, 15:595049, 10.3389/fnbeh.2021.595049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.••. Jimenez JC, Berry JE, Lim SC, Ong SK, Kheirbek MA, Hen R: Contextual fear memory retrieval by correlated ensembles of ventral CA1 neurons. Nat Commun 2020, 11:3492, 10.1038/s41467-020-17270-w. This study demonstrates that populations of vCA1 neurons become correlated with shock-responsive neurons during contextual fear memory encoding.
- 20.Xu C, Krabbe S, Gründemann J, Botta P, Fadok JP, Osakada F, et al. : Distinct hippocampal pathways mediate dissociable roles of context in memory retrieval. Cell 2016, 167:961–972, 10.1016/j.cell.2016.09.051. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q, Jin J, Maren S: Renewal of extinguished fear activates ventral hippocampal neurons projecting to the prelimbic and infralimbic cortices in rats. Neurobiol Learn Mem 2016, 134:38–43, 10.1016/j.nlm.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.••. Terranova JI, Yokose J, Osanai H, Marks WD, Yamamoto J, Ogawa SK, et al. : Hippocampal-amygdala memory circuits govern experience-dependent observational fear. Neuron 2022, 110:1416–1431, 10.1016/j.neuron.2022.01.019. e13. This study identifies a previously unknown role for vHPC-BLA circuits in observational fear learning.
- 23.Glangetas C, Massi L, Fois GR, Jalabert M, Girard D, Diana M, et al. : NMDA-receptor-dependent plasticity in the bed nucleus of the stria terminalis triggers long-term anxiolysis. Nat Commun 2017, 8:14456, 10.1038/ncomms14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson L, Sapolsky R: The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 1991, 12:118–134, 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 25.Cullinan WE, Herman JP, Watson SJ: Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol 1993, 332:1–20, 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- 26.Radley JJ, Sawchenko PE: Evidence for involvement of a limbic paraventricular hypothalamic inhibitory network in hypothalamic-pituitary-adrenal axis adaptations to repeated stress. J Comp Neurol 2015, 523:2769–2787, 10.1002/cne.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radley JJ, Sawchenko PE: A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci Off J Soc Neurosci 2011, 31:9683–9695, 10.1523/JNEUROSCI.6040-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bagot RC, Parise EM, Peña CJ, Zhang H-X, Maze I, Chaudhury D, et al. : Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun 2015, 6:7062, 10.1038/ncomms8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muir J, Tse YC, Iyer ES, Biris J, Cvetkovska V, Lopez J, et al. : Ventral hippocampal afferents to nucleus accumbens encode both latent vulnerability and stress-induced susceptibility. Biol Psychiatr 2020, 88:843–854, 10.1016/j.biopsych.2020.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Barfield ET, Gourley SL: Glucocorticoid-sensitive ventral hippocampal-orbitofrontal cortical connections support goal-directed action - curt Richter Award Paper 2019. Psycho-neuroendocrinology 2019, 110:104436, 10.1016/j.psyneuen.2019.104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LeGates TA, Kvarta MD, Tooley JR, Francis TC, Lobo MK, Creed MC, et al. : Reward behaviour is regulated by the strength of hippocampus–nucleus accumbens synapses. Nature 2018, 564:258–262, 10.1038/s41586-018-0740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia F, Kheirbek MA: Circuit-based biomarkers for mood and anxiety disorders. Trends Neurosci 2020, 43:902–915, 10.1016/j.tins.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.••. Biane JS, Ladow MA, Stefanini F, Boddu SP, Fan A, Hassan S, et al. : Population dynamics underlying associative learning in the dorsal and ventral hippocampus. bioRxiv 2021, 10.1101/2021.11.16.468862. This study shows that vCA1 neurons encode non-spatial stimuli only after behavioral significance has been attached to the stimulus. It also shows that population dynamics in vCA1 rapidly reorganize with odor-reward learning, and that odor representations in vCA1 last for days.
- 34.Woods NI, Stefanini F, Apodaca-Montano DL, Tan IMC, Biane JS, Kheirbek MA: The dentate gyrus classifies cortical representations of learned stimuli. Neuron 2020, 107:173–184, 10.1016/j.neuron.2020.04.002. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyberg N, Duvelle É, Barry C, Spiers HJ: Spatial goal coding in the hippocampal formation. Neuron 2022, 110:394–422, 10.1016/j.neuron.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Sosa M, Giocomo LM: Navigating for reward. Nat Rev Neurosci 2021, 22:472–487, 10.1038/s41583-021-00479-z.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sosa M, Joo HR, Frank LM: Dorsal and ventral hippocampal sharp-wave ripples activate distinct nucleus accumbens networks. Neuron 2020, 105:725–741, 10.1016/j.neuron.2019.11.022. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trouche S, Koren V, Doig NM, Ellender TJ, El-Gaby M, Lopes-Dos-Santos V, et al. : A hippocampus-accumbens tripartite neuronal motif guides appetitive memory in space. Cell 2019, 176:1393–1406, 10.1016/j.cell.2018.12.037. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.••. Yang AK, Mendoza JA, Lafferty CK, Lacroix F, Britt JP: Hippocampal input to the nucleus accumbens shell enhances food palatability. Biol Psychiatr 2020, 87:597–608, 10.1016/j.biopsych.2019.09.007. This study shows that modulation of vHPC-NAc activity can impact the palatability of food reward.
- 40.Wee RWS, Mishchanchuk K, AlSubaie R, MacAskill AF: Hippocampal ghrelin signalling informs the decision to eat. Neuroscience 2021, 10.1101/2021.11.05.467326. [DOI] [Google Scholar]
- 41.Reed SJ, Lafferty CK, Mendoza JA, Yang AK, Davidson TJ, Grosenick L, et al. : Coordinated reductions in excitatory input to the nucleus accumbens underlie food consumption. Neuron 2018, 99:1260–1273, 10.1016/j.neuron.2018.07.051. e4. [DOI] [PubMed] [Google Scholar]
- 42.•. Suarez AN, Liu CM, Cortella AM, Noble EE, Kanoski SE: Ghrelin and orexin interact to increase meal size through a descending Hippocampus to hindbrain signaling pathway. Biol Psychiatr 2020, 87:1001–1011, 10.1016/j.biopsych.2019.10.012. This study shows that the vHPC-LH pathway is sensitive to ghrelin, and that ghrelin can modulate satiety signals through the vHPC-LH pathway.
- 43.••. Park AJ, Harris AZ, Martyniuk KM, Chang C-Y, Abbas AI, Lowes DC, et al. : Reset of hippocampal–prefrontal circuitry facilitates learning. Nature 2021, 591:615–619, 10.1038/s41586-021-03272-1. This study demonstrates that novelty resets the vHPC-mPFC circuit via dopamine, which permits future learning.
- 44.Shpokayte M, McKissick O, Yuan B, Rahsepar B, Fernandez FR, Ruesch E, et al. : Hippocampal cells multiplex positive and negative engrams. Neuroscience 2020, 10.1101/2020.12.11.419887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meira T, Leroy F, Buss EW, Oliva A, Park J, Siegelbaum SA: A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat Commun 2018, 9:4163, 10.1038/s41467-018-06501-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montagrin A, Saiote C, Schiller D: The social hippocampus. Hippocampus 2018, 28:672–679, 10.1002/hipo.22797. [DOI] [PubMed] [Google Scholar]
- 47.Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S: Ventral CA1 neurons store social memory. Science 2016, 353:1536–1541, 10.1126/science.aaf7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watarai A, Tao K, Wang M-Y, Okuyama T: Distinct functions of ventral CA1 and dorsal CA2 in social memory. Curr Opin Neurobiol 2021, 68:29–35, 10.1016/j.conb.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 49.••. Tao K, Chung M, Watarai A, Huang Z, Wang M-Y, Okuyama T: Disrupted social memory ensembles in the ventral hippocampus underlie social amnesia in autism-associated Shank3 mutant mice. Mol Psychiatr 2022:1–11, 10.1038/s41380-021-01430-5. This study identifies deficits in online coding and offline reactivation of vCA1 social memory ensembles in a mouse model for autism.
- 50.Rao RP, von Heimendahl M, Bahr V, Brecht M: Neuronal responses to conspecifics in the ventral CA1. Cell Rep 2019, 27:3460–3472, 10.1016/j.celrep.2019.05.081. e3. [DOI] [PubMed] [Google Scholar]
- 51.Phillips ML, Robinson HA, Pozzo-Miller L: Ventral hippocampal projections to the medial prefrontal cortex regulate social memory. Elife 2019, 8, e44182, 10.7554/eLife.44182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hitti FL, Siegelbaum SA: The hippocampal CA2 region is essential for social memory. Nature 2014, 508:88–92, 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiang M-C, Huang AJY, Wintzer ME, Ohshima T, McHugh TJ: A role for CA3 in social recognition memory. Behav Brain Res 2018, 354:22–30, 10.1016/j.bbr.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 54.Tavares RM, Mendelsohn A, Grossman Y, Williams CH, Shapiro M, Trope Y, et al. : A map for social navigation in the human brain. Neuron 2015, 87:231–243, 10.1016/j.neuron.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Doherty DCM, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J: A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatr Res 2015, 232:1–33, 10.1016/j.pscychresns.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Boldrini M, Butt TH, Santiago AN, Tamir H, Dwork AJ, Rosoklija GB, et al. : Benzodiazepines and the potential trophic effect of antidepressants on dentate gyrus cells in mood disorders. Int J Neuropsychopharmacol 2014, 17:1923–1933, 10.1017/S1461145714000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeidman P, Maguire EA: Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci 2016, 17:173–182, 10.1038/nrn.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RSJ, et al. : Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci Unit States Am 2000, 97:4398–4403, 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vogel JW, La Joie R, Grothe MJ, Diaz-Papkovich A, Doyle A, Vachon-Presseau E, et al. : A molecular gradient along the longitudinal axis of the human hippocampus informs large-scale behavioral systems. Nat Commun 2020, 11:960, 10.1038/s41467-020-14518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.•. Ayhan F, Kulkarni A, Berto S, Sivaprakasam K, Douglas C, Lega BC, et al. : Resolving cellular and molecular diversity along the hippocampal anterior-to-posterior axis in humans. Neuron 2021, 109:2091–2105, 10.1016/j.neuron.2021.05.003. e6. This study uses single-nuclei RNA sequencing to identify cell-type specific differences along the longitudinal axis of human hippocampus in genes related to mood and psychiatric disease.
- 61.Hu J, Liu J, Liu Y, Wu X, Zhuang K, Chen Q, et al. : Dysfunction of the anterior and intermediate hippocampal functional network in major depressive disorders across the adult lifespan. Biol Psychol 2021, 165:108192, 10.1016/j.biopsycho.2021.108192. [DOI] [PubMed] [Google Scholar]
- 62.Lazarov A, Zhu X, Suarez-Jimenez B, Rutherford BR, Neria Y: Resting-state functional connectivity of anterior and posterior hippocampus in posttraumatic stress disorder. J Psychiatr Res 2017, 94:15–22, 10.1016/j.jpsychires.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdallah CG, Wrocklage KM, Averill CL, Akiki T, Schweinsburg B, Roy A, et al. : Anterior hippocampal dysconnectivity in posttraumatic stress disorder: a dimensional and multimodal approach. Transl Psychiatry 2017, 7:e1045, 10.1038/tp.2017.12. e1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suarez-Jimenez B, Albajes-Eizagirre A, Lazarov A, Zhu X, Harrison BJ, Radua J, et al. : Neural signatures of conditioning, extinction learning, and extinction recall in posttraumatic stress disorder: a meta-analysis of functional magnetic resonance imaging studies. Psychol Med 2020, 50:1442–1451, 10.1017/S0033291719001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirkby LA, Luongo FJ, Lee MB, Nahum M, Van Vleet TM, Rao VR, et al. : An amygdala-Hippocampus subnetwork that encodes variation in human mood. Cell 2018, 175:1688–1700, 10.1016/j.cell.2018.10.005. e14. [DOI] [PubMed] [Google Scholar]
- 66.Addis DR, Schacter DL: The Hippocampus and imagining the future: where do we stand? Front Hum Neurosci 2012, 5:173, 10.3389/fnhum.2011.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.••. Kay K, Chung JE, Sosa M, Schor JS, Karlsson MP, Larkin MC, et al. : Constant sub-second cycling between representations of possible futures in the Hippocampus. Cell 2020, 180:552–567, 10.1016/j.cell.2020.01.014. e25. This study identifies a neural signature in the hippocampus that represents hypothetical future experiences.
- 68.Dere E, Dere D, de Souza Silva MA, Huston JP, Zlomuzica A: Fellow travellers: working memory and mental time travel in rodents. Behav Brain Res 2018, 352:2–7, 10.1016/j.bbr.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 69.Miloyan B, Pachana NA, Suddendorf T: The future is here: a review of foresight systems in anxiety and depression. Cognit Emot 2014, 28:795–810, 10.1080/02699931.2013.863179. [DOI] [PubMed] [Google Scholar]
- 70.Moustafa AA, Morris AN, ElHaj M: A review on future episodic thinking in mood and anxiety disorders. Rev Neurosci 2018, 30:85–94, 10.1515/revneuro-2017-0055. [DOI] [PubMed] [Google Scholar]
- 71.Arszovszki A, Borhegyi Z, Klausberger T: Three axonal projection routes of individual pyramidal cells in the ventral CA1 hippocampus. Front Neuroanat 2014, 8:53, 10.3389/fnana.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim WB, Cho J-H: Synaptic targeting of double-projecting ventral CA1 hippocampal neurons to the medial prefrontal cortex and basal amygdala. J Neurosci 2017, 37:4868–4882, 10.1523/JNEUROSCI.3579-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.•. AlSubaie R, Wee RW, Ritoux A, Mishchanchuk K, Passlack J, Regester D, et al. : Control of parallel hippocampal output pathways by amygdalar long-range inhibition. Elife 2021, 10.7554/eLife.74758. This study shows an inhibitory and excitatory projection from amygdala to vHPC. Inhibitory input targets vHPC-BA neurons and gates placer-ward learning by modulating vHPC-NAc projection neurons.
- 74.Lee S-H, Marchionni I, Bezaire M, Varga C, Danielson N, Lovett-Barron M, et al. : Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron 2014, 82:1129–1144, 10.1016/j.neuron.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]


